ABSTRACT
Purpose
The Health state descriptive system includes standardized self-administered instruments for measuring Health-Related Quality of Life (HRQoL) respectively among adolescents, and children. The objectives of the current study were: (1) to translate and adapt the pediatric-adolescent version 16D and 17D from English into French (Canada), (2) to demonstrate their feasibility in pediatric conditions.
Methods
The translation methodology combined forward and back translations, and cognitive debriefing with eight adolescents and eight children. Four bilingual translators were involved in the process. We administered the translated versions to two clinical samples, being treated for Primary immunodeficiency (PID, n = 48, aged 14.1 years, 20 girls), and having recovered from pediatric Acute Lymphoblastic Leukemia (ALL, n = 153, aged 14.7 years, 77 girls).
Results
Cognitive debriefing indicated that that the instructions, items, and response options were clear, easy to understand, and easy to answer. Adjustments were made for clarity. Translated versions were highly usable (measurement completion >90%). HRQoL levels were high for both samples (range 0.85–0.96). Participants reported lower levels if they were adolescents, particularly if they were girls. Older boys with PID reported a lower HRQoL than their counterparts with a history of ALL. PID and ALL patients mainly reported issues with discomfort and pain, concentration/learning, physical appearance, and psychological distress and sleeping, although to a different degree.
Conclusion
The French-language versions of the 16D and 17D are easy to administer and may be used to identify problematic domains. Greater availability of translated versions of short evaluation tools may facilitate broader uptake of screening practices in pediatric care.
KEYWORDS: Childhood leukemia, health status, primary immunodeficiency, Quality of Life, pediatrics
Introduction
Medical advances and improvements of treatments have led to increased survival rates among children living with a chronic health condition (Haverman, Limperg, Young, Grootenhuis, & Klaassen, 2017). Chronic health conditions are often associated with long-term sequalae which impact health-related quality of life (HRQoL) (Haverman et al., 2017). Consequently, assessment of HRQoL is an essential component of caring for children and adolescents with chronic health conditions. Over the past several years, an increasing number of studies have measured HRQoL in pediatric populations (Choo et al., 2019; López-Bastida et al., 2019; Ohnemus et al., 2020; Shull, Ediger, Hill, & Schroedl, 2019). HRQoL has been defined as the impact of a specific illness, injury, health condition, and medical treatment on the subjective evaluation of one’s own status and well-being (Drotar, 2004). Although it has been common practice to ask informants (e.g. caregivers) about the status of a child, the research has consistently shown discrepancies between informants (e.g. caregivers and youth) (Abate et al., 2018). Measuring HRQoL directly from child report can be challenging as tools must be short, age-specific, and easy to respond to. Several pediatric generic HRQoL inventories are now available, including the Pediatric Quality of Life Inventory (PedsQL), the Child Health Questionnaire (CHQ), and the Health state descriptive system (Apajasalo et al., 1996a, 1996b; Haverman et al., 2017; Lemmon, Huffstetler, & Reeve, 2020; Sintonen, 2001).
The Health state descriptive system includes the 15D (Sintonen, 2001) 16D (Apajasalo et al., 1996a) and 17D (Apajasalo et al., 1996b), which are generic, comprehensive, and standardized self-administered instruments for measuring HRQoL respectively among adults (16+ years), adolescents (12–15 years), and children (8–11 years). These instruments are structured around the same core domains but include some domains specific to age ranges. They provide a description of health status, each assessing 15, 16, or 17 health domains. Each dimension comprises one item with five options varying in severity. The 15D, 16D, and 17D can each be used as a single index measure or as a profile measure. The single index score (0–1 scale) is calculated from the health state descriptive system by using a set of population-based preference or utility weights (Sintonen, 2001). The reference used is from the original European samples (Apajasalo et al., 1996a, 1996b). We provide utility weights used in supplementary files to this report. A detailed description of the valuation system is available in a publicly available report (Sintonen, 1995). The single index score represents the overall HRQoL. The maximum score is 1 (full HRQoL, no problems on any dimension) and the minimum score is 0 (being dead). The domains measures for young people are mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, school and hobbies, friends, physical appearance, discomfort and symptoms, depression, vitality (16D and 17D), mental function, distress (16D) anxiety, ability to concentrate, and learning ability and memory (17D).
The tools from the Health state descriptive system have been used in multiple studies, to monitor HRQoL in drug clinical research (Huoponen et al., 2020; Ojala, Sintonen, Roine, Strandberg, & Schalin-Jantti, 2020), following surgery (Ho et al., 2020; Vannas, Farkkila, Sintonen, Aberg, & Isoniemi, 2020), during rehabilitation (Roine et al., 2020) and in various conditions such as asthma (Ilmarinen et al., 2019; Rutishauser, Sawyer, & Bowes, 1998), diabetes (Karamanakos et al., 2019), renal transplantation (Qvist et al., 2004), cancer (Toija, Kettunen, Leidenius, Vainiola, & Roine, 2019), and mental illness (Granö, Karjalainen, Suominen, & Roine, 2011). The adult version (15D) is already available in multiple languages including French. Yet, the 16D and 17D have been only available in English, Finnish, Swedish (16D and 17D), and Norwegian (16D only). To make this system fully available to French-speaking patients and their caregivers, French versions of the 16D and 17D are needed. As pediatrics sample sizes are often small, it is also particularly beneficial to collect new data to further document HRQoL levels that could be used as reference levels for this population.
Objectives
The objectives of the current study were: (1) to translate and adapt the 16D and 17D from English into French (Canada), and (2) to demonstrate the feasibility of their use in various clinical conditions, providing item responses and score description from two samples of young patients with Primary Immunodeficiency (PID) and youth in aftercare for cancer.
Methods
Translation process
We used a standardized approach based on the World Health Organization (WHO) process of translation and adaptation of instruments, which has been widely used in patient-reported outcome research (WHO, 2015). The same process was used for the 16D and the 17D. We contacted the authors (Drs. Sintonen and Apajasalo) to obtain their approval and guidance and involved four bilingual translators familiar with the use of patient-reported outcomes and working in a pediatric research hospital. All translators were graduate students in our hospital research center. Their mother tongue was French (ML and SB) and English (LG and WB) (see acknowledgments). Each step was checked for quality by the original developers of the instruments, and they approved the final version.
Forward translation
The first step was to produce two independent forward translations performed by two independent translators who were French native speakers and fluent in English (ML and SB). In this work, the focus was put on cross-cultural relevance and understandability, rather than on linguistic/literal equivalence. We used the following main criteria for the translation: simplicity, clarity, and natural language. The instruments needed to be easily understood by pediatric patients so technical language was avoided. The two forward translations were then compared. Discrepancies were discussed between the two translators (ML and SB) and the project manager (ER) until a consensus emerged to produce a first consensus French version v1 (Tables S1 and S2).
Back translation
Using the same approach, the French version v1 was then back-translated into English by two independent bilingual translators whose native language was English and were bilingual (LG and WB). The two back translations were compared to one another and to the original English version and discrepancies were resolved the same way. Differences between the original version and the back translations or between the two back translations were discussed to explore if important cross-cultural or conceptual issues were raised. Following this detailed comparison, we made adjustments to produce a second consensus French version v2.
Cognitive testing
We then pretested the French version v2 with 16 respondents. For each instrument, the eight respondents were evenly distributed across gender (16D: four boys/four girls; 17D: five boys/three girls) and condition (16D: four cancer/four healthy; 17D: three cancer/five healthy). Mean ages for the 16D and 17D respondents were 14.2 and 9.7 years respectively. All were French (Canada) native speakers. Demographic characteristics for this pretest sample are available in Table S3. For cognitive testing we adopted a procedure used in the development of the pediatric item bank of the PROMIS (Irwin, Varni, Yeatts, & DeWalt, 2009). The questionnaires were self-administered in the presence of a member of the research team (PB). We then organized individual interviews to assessed item comprehension. Participants were asked if the questions, instructions, and responses options were clear, easy to understand, easy to answer, and if there were any wording they found difficult to understand, in line with the practice of cognitive interview. The respondents were then asked to give their general appreciation of the questionnaire. Based on the respondents’ comments, final adjustments were made to the French version of the 16D and 17D questionnaires. This led to the final translated version (supplementary material). For this anonymous cognitive interview study, we obtained a waiver from the Sainte-Justine UHC Research Ethics Board and respected the Helsinki convention.
Application to two clinical samples
We administered the translated versions of the 16D and 17D to two clinical samples, one being treated for PID, the other having recovered from pediatric cancer (Acute Lymphoblastic Leukemia (ALL)). These samples are drawn from larger studies (Abate et al., 2018; Anestin et al., 2018; Boulet-Craig et al., 2018; Lamore et al., 2020; Marcoux et al., 2017; Pépin et al., 2017; Sultan et al., 2017). Full sample description data are available in Tables S4-S5. Descriptions of participants’ flow are available in the original study reports (Abate et al., 2018; Anestin et al., 2018; Boulet-Craig et al., 2018; Lamore et al., 2020; Marcoux et al., 2017; Pépin et al., 2017; Sultan et al., 2017). As we did not have hypotheses we did not calculate the required samples size. Written informed consent/assent was obtained from all participants and both studies were approved by the Sainte-Justine UHC Research Ethics Board (PID study: #2014-584, 3771; ALL study: #2013-479, 3607).
PID sample (N = 48)
Participants were children and adolescents with PID. Eligible families were approached by telephone by a research coordinator and invited to participate in the study. Children and parents were asked to complete the questionnaire independently at home and mail them back to the research team as part of a larger Quality of Life study (Sultan et al., 2017). Twenty-seven adolescents (17 boys, 10 girls) completed the 16D questionnaire and 21 children (11 boys, 10 girls) completed the 17D questionnaire. 16D respondents had a mean age of 17.1 years (SD = 3.0) and 17D respondents 10.2 years (SD = 1.6). Mean time since diagnosis was 7.2 years (SD = 4.2) for 16D respondents and 5.5 years (SD = 2.5) for 17D respondents. The majority of participants were on subcutaneous administration of immunoglobulin (SCIG) treatment (96.3% of 16D respondents, 90.5% of 17D respondents).
ALL sample (N = 153)
Participants were childhood ALL survivors. Participants were contacted by telephone by a research coordinator. Recruitment was organized at the long-term follow-up clinic of Sainte-Justine UHC as part of a larger study on late effects (Abate et al., 2018; Anestin et al., 2018; Boulet-Craig et al., 2018; Lamore et al., 2020; Marcoux et al., 2017; Pépin et al., 2017). During the child's research visit at the clinic, participants were asked to complete a questionnaire on site or, if not possible, at home and return it by mail. The 16D and 17D questionnaires were completed respectively by 124 adolescents (60 boys, 64 girls) and 29 children (16 boys, 13 girls). The 16D respondents had a mean age of 15.6 years (SD = 1.6) and 17D respondents were on average 10.9 years (SD = 1.3). Mean time since diagnosis was 11.1 years (SD = 2.7) for 16D respondents and 7.8 years (SD = 1.4) for 17D respondents. The majority of participants had a standard risk status (66.9% of 16D respondents, 69.0% of 17D respondents) and had not received radiotherapy (63.7% of 16D respondents, 75.9% of 17D respondents). All participants were on a Dana Farber Cancer Institute (DFCI) treatment protocol (Silverman et al., 2010).
Ethics statement
For the anonymous cognitive interview study, we obtained a waiver from the Sainte-Justine UHC Research Ethics Board. For the application to clinical sample, a written informed consent/assent was obtained from all participants and both studies were approved by the Sainte-Justine UHC Research Ethics Board (PID study: #2014-584, 3771; ALL study: #2013-479, 3607). The research complies with the declaration of Helsinki.
Results
16D. Translation
Forward translation
The forward translation was conducted without major difficulties and the two independent translations were very similar. In the reconciliation process, of the 84 sentences, there were 25 (29.8%) that were the same, 42 (50.0%) where one of the two translations was selected (13 and 29 for first and second translator respectively), 13 (15.5%) which were the result of a combination of the two translations, and there were 4 (4.8%) sentences where the project manager (ER) made suggestions of change. Suggestions were discussed between the project manager and the two translators and were all accepted. In rare cases, we had to initiate a discussion with translators when some literal translation did not make sense in French (see Table S1). In order to produce clear and natural-sounding translations, we made adjustments to a number of items. For instance, in items 5 and 9, ‘normal speech’ was translated as ‘when someone speaks normally’ (items 5); ‘my speech’ was translated as ‘when I speak’ (item 9); ‘disjointed’ was translated as ‘incoherent’ (item 9); ‘difficulty understanding my speech’ was translated as ‘difficulty understanding when I speak’ (item 9). Another instance was item 6 (‘full use of sleeping pills’). Translators were not sure that ‘full use’ and ‘sleeping pills’ would be easily understood by adolescents. In that case, the team decided to keep the item as it was and check for its understanding during cognitive testing.
Back translation
The two independent versions were similar. In the validation of the back translations, of the 84 sentences, no changes were necessary for 59 (70.2%) sentences and the original translation was used. The back translations were identical for 36 (42.9%) sentences and nearly identical for 23 (27.4%) sentences to the original English version. Twenty-five (29.8%) sentences needed a discussion between the project manager and the two translators for further clarification to finally accept the original translation. No modifications were suggested. For some items, the wording and syntax used in the back translations were different as compared to the original version, but the meaning was the same. Consequently, no modifications were made at this step.
Cognitive testing
All participants mentioned that the instructions, items, and response options were clear, easy to understand, and easy to answer. In five cases, items were found difficult to understand for some participants and we proceeded with final adjustments to the French version (Table 1). Item 2 was understood by all participants, but three of them suggested that the item would be clearer by reformulating ‘without a guide’ by ‘without help’. As for Items 6 and 11, two participants found some words were difficult, namely ‘sleeping pills’ and ‘interfere’. To deal with this issue we added ‘sleep medicines’ (médicaments pour dormir) to specify ‘sleeping pills’. For item 11, a participant suggested to change ‘does not interfere with’ by ‘has no impact on’ because ‘interfere’ could be difficult to understand for some adolescents. Finally, many participants did not understand the word ‘catheter’ from item 15 and ‘melancholic’ from item 16. We added ‘tubes’ to specify ‘catheter’ (item 15) and we replaced ‘melancholic’ by ‘unhappy’ (malheureux) which is much easier to understand in this age range (item 16). Prompts and full results from the cognitive testing are available in supplementary material online.
Table 1.
Pretest of the translated version of the 16D (8 adolescents aged 13–16 years).
| Translated item into French | Respondents’ comments on translated version | Modifications and final version in French |
|---|---|---|
|
Item 2, Option 4 I cannot read books and TV text, even with glasses, but I can see well enough to walk without a guide. Je ne peux pas lire de livres et le texte à la télévision, même avec des lunettes, mais je peux voir assez bien pour marcher sans un guide. |
P2, P3, P7: understood the question but would reformulate: ‘without a guide’ could be replaced by ‘without help’ | ‘Without a guide’ seemed to be a little confusing. We suggested replacing ‘without a guide’ by ‘without help’. Je ne peux pas lire de livres et le texte à la télévision, même avec des lunettes, mais je peux voir assez bien pour marcher sans aide. |
|
Item 6, Option 4 I have great problems with sleeping, e.g. I have to take sleeping pills often or every night, or I usually wake at night or too early in the morning. J’ai de gros problèmes de sommeil, par exemple je dois prendre des somnifères souvent ou chaque nuit ou je me réveille habituellement la nuit ou trop tôt le matin. |
P1, P2: ‘Sleeping pills’: did not understand | Two participants did not understand ‘somnifères’. We suggested adding ‘médicaments pour dormir’. Both mean ‘sleeping pills’. J’ai de gros problèmes de sommeil, par exemple je dois prendre des somnifères (médicaments pour dormir) souvent ou chaque nuit ou je me réveille habituellement la nuit ou trop tôt le matin. |
|
Item 11, Option 1 My state of health does not interfere with going to school or having hobbies. Mon état de santé n’interfère pas avec le fait d’aller à l’école ou d’avoir des loisirs. |
P1, P5: ‘interfere’: did not understand P4: understood the question but would reformulate: ‘My state of health has no impact on going to school or having hobbies’ |
‘Interfere’ was hard to understand. We suggested changing ‘interfere’ by ‘has no impact on’. Mon état de santé n’a aucun impact sur le fait d’aller à l’école ou d’avoir des loisirs. |
|
Item 15, Option 4 I have serious problems with my bladder or bowels, e.g. frequent ‘accidents’, or need for enemas or catheters. J’ai de sérieux problèmes avec ma vessie ou mes intestins, par exemple avoir des ‘accidents’ fréquents ou avoir besoin de lavements ou de cathéters. |
P1-P5: ‘catheter’: did not understand P2, P3, P6: ‘enemas’: did not understand |
‘Enemas’ and ‘catheters’ seemed to be hard to understand. If children have never had this problem, it is normal that they do not know what it is. We suggested adding ‘tubes’ next to ‘catheters’. ‘Tubes’ is easy to understand and it should help to clarify the meaning of the sentence. J’ai de sérieux problèmes avec ma vessie ou mes intestins, par exemple avoir des ‘accidents’ fréquents ou avoir besoin de lavements ou de cathéters (tubes). |
|
Item 16, Option 1 I do not feel at all sad, melancholic or depressed. Je ne me sens pas du tout triste, mélancolique ou déprimé(e). |
P1, P3, P6, P7: ‘melancholic’: did not understand | ‘Melancholic’ seemed to be hard to understand. We suggested changing ‘mélancolique’ (melancholic) by ‘malheureux’ (unhappy). ‘Malheureux’ is much easier to understand than ‘mélancolique’. Je ne me sens pas du tout triste, malheureux(se) ou déprimé(e). |
17D. Translation
Forward translation
The two forward translations were similar. In the reconciliation process, of the 107 sentences, there were 56 (52.3%) that were the same, 42 (39.3%) where one of the two translations was selected (22 and 20 for first and second translator respectively), 5 (4.7%) which were the result of a combination of the 2 translations, and there were 4 (3.8%) sentences where the project manager made suggestions of change. Suggestions were discussed between the project manager and the two translators and were all accepted (Table S2). Some expressions were very clear in English but a literal translation made no sense in French. In order to produce clear and natural-sounding translations, some adjustments were made to items 2 and 11: ‘normal speech’ was translated as ‘when someone speaks normally’ (items 2); ‘completely happy’ was translated as ‘very happy’ (item 11).
Back translation
Here too, the back translation was conducted without major difficulties. The two back translations were similar. In the validation of the back translations, of the 107 sentences, no changes were necessary for 80 (74.8%) sentences and the original translation was used. The back translations were identical for 57 (53.3%) sentences or nearly identical for 23 (21.5%) sentences to the original English version. Twenty-seven (25.2%) sentences needed a discussion between the project manager and the two translators for further clarification to finally accept the original translation. For some items, the words and the syntax used in the back translations were different, as compared to the original version but the meaning was the same. As with the 16D, we opted to make no modifications at this step.
Cognitive testing
All participants mentioned that the instructions, the items, and response options were clear, easy to understand, and easy to respond. Six items presented some issues for some participants and final adjustments were made to the French version (Table 2). For items 1, 10 and 12, we changed the wording so it would be easier to understand for children: we changed ‘guide’ for ‘accompanying person’ (accompagnateur, item 1), we changed ‘frightened’ for ‘fear’ (peur) and ‘tensed’ for ‘nervous’ (nerveux, item 10), we changed ‘hobbies’ for ‘activities’ (activités, item 12). For item 2, two participants did not understand the meaning of ‘the latter’ (celui-ci) so we reworded the sentence for more clarity. For item 6, none of the participants understood ‘catheter’ so we added ‘tubes’ to specify ‘catheter’. Prompts and full results from the cognitive testing are available in supplementary material.
Table 2.
Pretest of the translated version of the 17D (8 children aged 8–11 years).
| Translated item into French | Respondents’ comments on translated version | Modifications and final version in French |
|---|---|---|
|
Item 1, Option 4 I cannot see writing even with glasses, but I can see well enough to walk around without a guide. Je ne peux pas voir ce qui est écrit même avec des lunettes, mais je peux voir assez bien pour me promener sans un guide. |
P1, P4: understood the question but would reformulate P3, P6: ‘without a guide’: did not understand |
‘Without a guide’ seemed to be a little confusing. We suggested replacing ‘guide’ by ‘accompagnateur’. Je ne peux pas voir ce qui est écrit même avec des lunettes, mais je peux voir assez bien pour me promener sans un accompagnateur. |
|
Item 2, Option 3 I need a hearing aid, but I can hear well with it. J’ai besoin d’un appareil auditif, mais je peux bien entendre avec celui-ci. |
P1, P7: ‘celui-ci’: did not understand | Two participants did not understand the meaning of ‘celui-ci’ so they had difficulty to understand the meaning of the sentence. We suggested changing the wording so the sentence will be much easier to understand. J’ai besoin d’un appareil auditif pour bien entendre. |
|
Item 6, Option 4 I often have ‘accidents’, or I need a catheter or medicine to help me go to the toilet. J’ai souvent des ‘accidents’ ou j’ai besoin d’un cathéter ou de médicaments pour m’aider à aller aux toilettes |
P1-P8: ‘catheter’: did not understand | The word ‘catheter’ seemed to be hard to understand. If children have never had this problem, it is normal that they do not know what it is. We suggested adding ‘tubes’ next to ‘catheters’. ‘Tubes’ is easy to understand and it should help to clarify the meaning of the option. J’ai souvent des ‘accidents’ ou j’ai besoin d’un cathéter (tube) ou de médicaments pour m’aider à aller aux toilettes. |
|
Item 10 (Question) Do you feel scared or tense? Te sens-tu effrayé(e) ou tendu(e)? |
P1, P6: ‘scared’ and ‘tense’: did not understand | ‘Scared’ and ‘tense’ seemed to be hard to understand. We suggested changing ‘effrayé’ by ‘peur’. Both words mean ‘scared’ and ‘peur’ is much easier to understand than ‘effrayé’. We suggested changing ‘tendu’ by ‘nerveux’. Both words mean ‘tense’ and ‘nerveux’ is much easier to understand than ‘tendu’. As-tu peur ou es-tu nerveux(se)? |
|
Item 12 (Question) Does your state of health make it difficult to go to school or have hobbies? Est-ce que ton état de santé rend difficile le fait d’aller à l’école ou d’avoir des loisirs? |
P1, P5, P6: ‘hobbies’: did not understand | ‘Hobbies’ seemed to be hard to understand. We suggested changing ‘loisirs’ by ‘activités’. Both words mean ‘hobbies’ and ‘activités’ is much easier to understand than ‘loisirs’. Est-ce que ton état de santé rend difficile le fait d’aller à l’école ou de faire des activités? |
|
Item 14, Option 4 My thoughts are always jumping from one thing to another, and I can’t really concentrate much. Mes pensées sautent toujours d’une chose à l’autre et je ne peux pas vraiment me concentrer beaucoup. |
P3: understood the question but would reformulate | A participant suggested that we reformulate the sentence to make it more understandable. Mes pensées sautent toujours d’une chose à l’autre et je ne peux presque pas me concentrer. |
|
Item 14, Option 5 I’m so restless that I can’t concentrate for a moment. Je suis si agité(e) que je ne peux pas me concentrer pendant un moment. |
P3: understood the question but would reformulate | A participant suggested that we reformulate the sentence to make it more understandable. Je suis si agité(e) que je ne peux pas me concentrer du tout. |
Application to clinical samples
Feasibility of the 16D and 17D inventories
The PID original sample included 67 participants. Of those, 49 were in the targeted age range to complete the 16D or 17D inventory. The other ones were too young (0–7 years). Of these 49, 48 (98%) managed to complete the questionnaire on their own. The ALL sample included 387 participants. Of those, 167 were eligible to complete the 16D or 17D. The other ones were older and completed the adult form (15D). Of the 167 eligible, 153 (92%) managed to complete the questionnaire on their own and returned the forms to the research team. There were no missing values for both samples. These figures speak for an excellent feasibility of the 16D-17D in their age ranges. No issues were raised among clinical participants as to the use of these surveys. This is also shown by the absence of missing values.
Item responses and score description
Summary HRQoL indices were very high, ranging 0.85–0.93 for the 16D and 0.90–0.96 for the 17D (Tables 3 and 4). When describing the D16 score we found that seven individuals had the highest functioning in all dimensions, and 0 had the lowest functioning. Min–Max were 0.57–1.00 with Mean and SD 0.91 ± 0.09. When describing the D17 score we found that five individuals had the highest functioning in all dimensions, and 0 had the lowest functioning. Min–Max were 0.70–1.00 with Mean and SD 0.93 ± 0.07. Although the measures are slightly different across age groups (16D vs. 17D), non overlapping confidence intervals suggested that HRQoL levels were significantly lower in adolescents than in children with ALL (0.88 vs. 0.93 for girls; 0.93 vs. 0.96 for boys). This was not the case for the PID group. When examining differences across genders, we observed lower scores for adolescent ALL girls than ALL boys (16D, 0.88 vs. 0.93). These differences were not observed in PID or in younger samples using the 17D. Finally, we observed little differences across conditions except that the 16D index was significantly lower for PID boys than ALL survivor boys (0.85 vs. 0.93). We observed no difference across conditions in younger samples, when using the 17D (Tables 3 and 4). When running multivariate regression models predicting for summary HRQoL indices, and introducing age (8–11 vs. 12–15 years), gender, and condition as predictors, we found a lower HRQoL being uniquely associated with adolescent age (p = 0.001), female gender (p = 0.002), and PID condition (p < 0.001). These factors explained 14% of the variance in HRQoL (p < 0.001).
Table 3.
Means and standard deviations for 16D individual items and the single index score.
| 16D items | ALL survivors (n = 124) | PID patients (n = 27) | ||||||
|---|---|---|---|---|---|---|---|---|
| Girls | Boys | Girls | Boys | |||||
| n = 64 | n = 60 | n = 10 | n = 17 | |||||
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | Mean (SD) | 95% CI | Mean (SD) | 95% CI | |
| 1 Vitality | 1.70 (0.90) | 1.48–1.93 | 1.48 (0.75) | 1.29–1.68 | 2.10 (1.20) | 1.24–2.96 | 1.71 (0.77) | 1.31–2.10 |
| 2 Vision | 1.53 (0.62) | 1.38–1.69 | 1.20 (0.40) | 1.10–1.30 | 1.40 (0.52) | 1.03–1.77 | 1.47 (0.62) | 1.15–1.79 |
| 3 Breathing | 1.47 (0.62) | 1.31–1.62 | 1.15 (0.36) | 1.06–1.24 | 1.70 (1.25) | 0.80–2.60 | 1.71 (1.05) | 1.17–2.24 |
| 4 Distress | 2.27 (1.16) | 1.98–2.55 | 1.82 (0.95) | 1.57–2.06 | 1.80 (1.03) | 1.06–2.54 | 1.88 (0.86) | 1.44–2.32 |
| 5 Hearing | 1.13 (0.33) | 1.04–1.21 | 1.13 (0.34) | 1.04–1.22 | 1.20 (0.63) | 0.75–1.65 | 1.24 (0.44) | 1.01–1.46 |
| 6 Sleeping | 1.98 (0.83) | 1.78–2.19 | 1.38 (0.64) | 1.22–1.55 | 2.20 (0.79) | 1.64–2.76 | 1.65 (0.70) | 1.29–2.01 |
| 7 Eating | 1.03 (0.18) | 0.99–1.08 | 1.02 (0.13) | 0.98–1.05 | 1.00 (0.00) | 1.00–1.00 | 1.12 (0.33) | 0.95–1.29 |
| 8 Discomfort/symptoms | 1.55 (0.83) | 1.34–1.76 | 1.32 (0.62) | 1.16–1.48 | 2.30 (1.25) | 1.40–3.20 | 1.71 (0.77) | 1.31–2.10 |
| 9 Speech | 1.28 (0.45) | 1.17–1.39 | 1.32 (0.47) | 1.20–1.44 | 1.20 (0.42) | 0.90–1.50 | 1.47 (0.80) | 1.06–1.88 |
| 10 Physical appearance | 2.02 (1.03) | 1.76–2.27 | 1.72 (0.83) | 1.50–1.93 | 1.80 (1.03) | 1.06–2.54 | 1.53 (0.94) | 1.04–2.01 |
| 11 School and hobbies | 1.11 (0.32) | 1.03–1.19 | 1.13 (0.43) | 1.02–1.24 | 1.70 (0.82) | 1.11–2.29 | 1.65 (1.12) | 1.07–2.22 |
| 12 Mobility | 1.02 (0.13) | 0.98–1.05 | 1.02 (0.13) | 0.98–1.05 | 1.50 (1.08) | 0.73–2.27 | 1.06 (0.24) | 0.93–1.18 |
| 13 Friends | 1.05 (0.21) | 0.99–1.10 | 1.02 (0.13) | 0.98–1.05 | 1.10 (0.32) | 0.87–1.33 | 1.59 (1.00) | 1.07–2.10 |
| 14 Mental function | 1.22 (0.45) | 1.11–1.33 | 1.18 (0.43) | 1.07–1.29 | 1.00 (0.00) | 1.00–1.00 | 1.41 (0.71) | 1.05–1.78 |
| 15 Elimination | 1.19 (0.43) | 1.08–1.30 | 1.05 (0.29) | 0.98–1.12 | 1.40 (0.70) | 0.90–1.90 | 1.41 (0.62) | 1.09–1.73 |
| 16 Depression | 1.58 (0.81) | 1.38–1.78 | 1.33 (0.63) | 1.17–1.50 | 1.70 (0.95) | 1.02–2.38 | 1.59 (0.62) | 1.27–1.91 |
| Single index score | 0.88 (0.08) | 0.86–0.90 | 0.93 (0.07) | 0.91–0.94 | 0.85 (0.08) | 0.79–0.90 | 0.85 (0.12) | 0.79–0.91 |
Notes: ALL, acute lymphoblastic leukemia; PID, primary immunodeficiency; SD, standard deviation; CI, confidence interval.
Table 4.
Means and standard deviations for 17D individual items and the single index score.
| 17D items | ALL survivors (n = 29) | PID patients (n = 21) | ||||||
|---|---|---|---|---|---|---|---|---|
| Girls | Boys | Girls | Boys | |||||
| n = 13 | n = 16 | n = 10 | n = 11 | |||||
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | Mean (SD) | 95% CI | Mean (SD) | 95% CI | |
| 1 Vision | 1.31 (0.48) | 1.02–1.60 | 1.19 (0.40) | 0.97–1.40 | 1.40 (0.52) | 1.03–1.77 | 1.18 (0.41) | 0.91–1.45 |
| 2 Hearing | 1.00 (0.00) | 1.00–1.00 | 1.06 (0.25) | 0.93–1.20 | 1.10 (0.32) | 0.87–1.33 | 1.27 (0.65) | 0.84–1.71 |
| 3 Mobility | 1.00 (0.00) | 1.00–1.00 | 1.00 (0.00) | 1.00–1.00 | 1.10 (0.32) | 0.87–1.33 | 1.36 (1.21) | 0.55–2.17 |
| 4 Eating | 1.00 (0.00) | 1.00–1.00 | 1.00 (0.00) | 1.00–1.00 | 1.00 (0.00) | 1.00–1.00 | 1.09 (0.30) | 0.89–1.29 |
| 5 Sleeping | 1.77 (0.93) | 1.21–2.33 | 1.19 (0.40) | 0.97–1.40 | 1.60 (0.70) | 1.10–2.10 | 1.36 (0.51) | 1.02–1.70 |
| 6 Elimination | 1.23 (0.60) | 0.87–1.59 | 1.19 (0.54) | 0.90–1.48 | 1.40 (0.70) | 0.90–1.90 | 1.09 (0.30) | 0.89–1.29 |
| 7 Breathing | 1.23 (0.44) | 0.97–1.50 | 1.19 (0.40) | 0.97–1.40 | 1.30 (0.48) | 0.95–1.65 | 1.45 (1.21) | 0.64–2.27 |
| 8 Discomfort/symptoms | 1.31 (0.48) | 1.02–1.60 | 1.25 (0.45) | 1.01–1.49 | 2.10 (1.20) | 1.24–2.96 | 1.45 (0.69) | 0.99–1.92 |
| 9 Vitality | 1.08 (0.28) | 0.91–1.24 | 1.00 (0.00) | 1.00–1.00 | 1.70 (0.82) | 1.11–2.29 | 1.36 (0.51) | 1.02–1.70 |
| 10 Anxiety | 1.85 (1.21) | 1.11–2.58 | 1.25 (0.45) | 1.01–1.49 | 1.40 (0.52) | 1.03–1.77 | 1.36 (0.51) | 1.02–1.70 |
| 11 Physical appearance | 1.46 (0.66) | 1.06–1.86 | 1.44 (0.81) | 1.00–1.87 | 1.80 (0.92) | 1.14–2.46 | 1.82 (0.75) | 1.31–2.32 |
| 12 School and hobbies | 1.08 (0.28) | 0.91–1.24 | 1.00 (0.00) | 1.00–1.00 | 1.20 (0.42) | 0.90–1.50 | 1.36 (0.51) | 1.02–1.70 |
| 13 Friends | 1.08 (0.28) | 0.91–1.24 | 1.00 (0.00) | 1.00–1.00 | 1.30 (0.48) | 0.95–1.65 | 1.36 (0.92) | 0.74–1.98 |
| 14 Ability to concentrate | 2.31 (0.95) | 1.74–2.88 | 1.75 (0.86) | 1.29–2.21 | 2.10 (0.88) | 1.47–2.73 | 1.91 (0.54) | 1.55–2.27 |
| 15 Learning ability/memory | 1.54 (0.52) | 1.22–1.85 | 1.44 (0.81) | 1.00–1.87 | 1.60 (0.97) | 0.91–2.29 | 1.64 (0.67) | 1.18–2.09 |
| 16 Speech | 1.08 (0.28) | 0.91–1.24 | 1.19 (0.40) | 0.97–1.40 | 1.00 (0.00) | 1.00–1.00 | 1.45 (0.69) | 0.99–1.92 |
| 17 Depression | 1.00 (0.00) | 1.00–1.00 | 1.00 (0.00) | 1.00–1.00 | 1.10 (0.32) | 0.87–1.33 | 1.09 (0.30) | 0.89–1.29 |
| Single index score (17D score) | 0.93 (0.05) | 0.90–0.96 | 0.96 (0.04) | 0.94–0.98 | 0.90 (0.09) | 0.83–0.96 | 0.91 (0.08) | 0.85–0.96 |
Notes: ALL, acute lymphoblastic leukemia; PID, primary immunodeficiency; SD, standard deviation; CI, confidence interval.
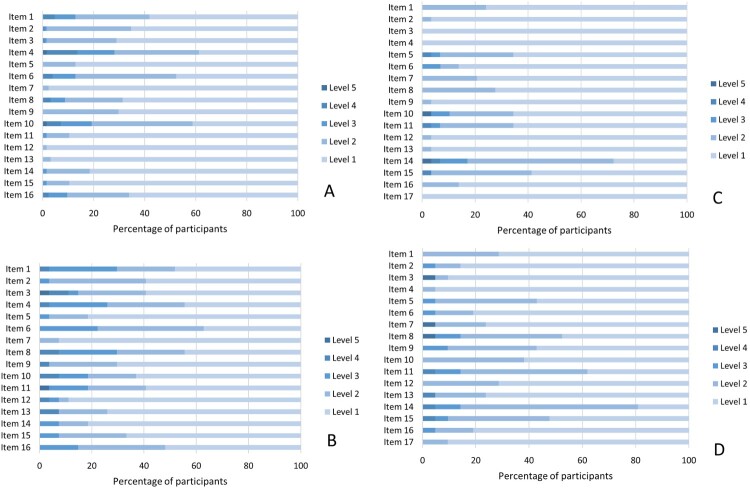
When examining the percent of endorsement in each subsample (levels 2–5, Figure 1), we found the most frequently reported issues to be in the ALL samples: distress (61%), physical appearance (59%), sleeping (52%) (16D), and ability to concentrate (72%), learning/memory (41%), physical appearance and sleeping (34%) (17D). In the PID sample items most endorsed were discomfort (56%), distress (56%), vitality (52%) (16D), and ability to concentrate (81%), physical appearance (62%), discomfort (52%) (17D). These rankings suggest that, across conditions, distress was most common among the older ones, whereas concerns on the ability to concentrate and physical appearance are frequent among the younger ones. They also suggest that, across ages, sleeping difficulties were more common in the ALL groups whereas discomfort, pain, and symptoms were more frequent in the context of PID (Figure 1 and Tables S6-S7).
Figure 1.
. Frequencies of participants’ responses for 16D (panels A-B), and 17D (panels C-D) individual items and options in 2 groups with Primary Immunodeficiency (PID) (n = 48) and survivors of Acute Lymphoblastic Leukemia (ALL) (n = 153). Note. Panel A Frequencies for 16D individual items for ALL. Panel B Frequencies for 16D individual items for PID. Panel C Frequencies for 17D individual items for ALL. Panel D Frequencies for 17D individual items for PID. Levels 1 to 5 refer to levels of impairment of function. Level 1: no impairment, Level 5: severe impairment. Frequency tables of individual responses to each item are available in supplementary material to this article.
When looking at items mean scores, one item showed particularly higher scores, item 14 ability to concentrate (17D). Non overlapping confidence intervals suggested that this item exceeded the levels of 12 other items in ALL girls (mean = 2.31/5) and seven other items in the group of ALL boys (mean = 1.75). Values exceeded that of five other items for the girls with PID (mean = 2.10) and four other items for boys with PID (mean = 1.91). When looking at the 16D alone (adolescent sample), items with the highest impairments were item 4 distress, item 10 physical appearance and item 6 sleeping. The examination of confidence intervals revealed that item 4 distress had higher levels than 13 other items in ALL survivors (both genders). The item 10 physical appearance had higher levels than 11 items in the same sample (both genders). As for item 6 sleeping, its level exceeded that of 12 other items in ALL girls. When exploring association patterns with Kendall τ correlation coefficients, we found the 16D index to be most closely associated with the items depression, vitality, distress, breathing, discomfort and symptoms (median τ = 0.53), and the 17D index to be most closely associated with the items distress, discomfort and symptoms, concentration, vitality, learning and memory (median τ = 0.49). These results suggest that variability on distress, discomfort and symptoms, and vitality had an impact on indices from the health state descriptive system in the studied samples.
A full dataset is provided as a supplementary file to this article to elicit further research on the instruments.
Discussion
General summary. This study translated two versions of the Health state descriptive system designed for children and adolescents aged 8–15 into French (Canada), the 16D and 17D (Apajasalo et al., 1996a, 1996b). We used a standardized method widely used in patient-reported outcome research (WHO, 2015). The translated versions were found to be equivalent to the original English versions. The French versions of the 16D and 17D were also highly feasible to administer in two young clinical samples, with measurement completion greater than 90% across samples and measures. The French youth versions of the 16D and 17D provide an important contribution to the literature as they support efforts to address health disparities and improve equitable access to screening practices across pediatric populations. Greater availability of French versions of screening tools may facilitate broader uptake of screening practices in pediatric care. This is particularly important for youth with chronic illnesses, where psychosocial screening is a recommended practice (Kazak et al., 2015), given the risk of youth experiencing psychosocial sequelae related to their illness (Pinquart, 2020).
Disease specific summary. We found that summary HRQoL indices were high for both samples. Overall indices suggested a somewhat lower HRQoL level in adolescent samples, particularly in girls, confirming previous observations (Abate et al., 2018; Jean & Syrjala, 2017; Pépin et al., 2017). As for differences across conditions, it was notable that older boys with PID reported a lower HRQoL than their counterparts with a history of ALL. In PID, the most frequently reported difficulties were issues with discomfort and pain, concentration, being concerned with one’s physical appearance, and psychological distress. In ALL survivors, the main difficulties were again issues with concentration and learning, experiencing psychological distress, being preoccupied with one’s physical appearance, and sleeping difficulties. It is noteworthy that psychological distress and physical appearance were endorsed across participants, highlighting potential targets for trans-diagnostic intervention. Indeed, identifying and addressing psychosocial needs may be particularly relevant in these populations.
Limitations. Several possible limitations may be noted. First, we recognize that regardless of how carefully they worked, translators were not professional translators. Second, this study only included PID and ALL youth, therefore findings cannot be generalized to other pediatric chronic illness populations. Nonetheless, the inclusion of these samples is an important contribution to the literature, given the novelty of the use of the French 16D and 17D and the similarities and discrepancies found across groups. Third, we did not obtain caregiver report as part of this study, as caregiver version is not yet available with the 16D/17D. Therefore, concordance between youth and caregiver report on the present versions of the 16D and 17D remains to be determined (Abate et al., 2018). Future research should develop and evaluate caregiver versions to help with fatigued or vulnerable patients. Finally, the calculation of indices was done by using utility weights from European samples. Future research should collect Canadian references for these instruments.
Future directions. The findings from this study contribute to a growing body of resources available to support HRQoL monitoring as a clinical practice across pediatric patients, including expanding access across language populations. The French versions of the 16D and 17D are easy to administer and may be used to better identify the HRQoL domains impacted in French-speaking youth within pediatric care settings. These tools may complement the range of instruments already available in French, such as the HRQoL utility measure for pre-school children (HuPS) and the Health Utility Index-2 and -3 (HUI) (Chen & Ratcliffe, 2015; Poder et al., 2021). Future investigations should consider providing psychometric analyzes (e.g. Item-response theory), including our dataset made publicly available as supplementary file. The present study reports on the initial stage of the scale translation process. Future research will need to focus on demonstrating validity, reliability, temporal stability and sensitivity to change. The descriptive findings from the samples supports the need for regular psychosocial monitoring and highlight specific domains affected in PID and ALL youth. Future interventions could target these domains as many intervention modalities are now available for children and adolescents to support attention/concentration issues (Zeng, Cheng, & Chan, 2016), sleep quality (Zhou and Recklitis, 2020), psychological distress and self-esteem/body image (Jean & Syrjala, 2017). Ultimately, better identification through accessible and equitable screening practices may lead to more targeted interventions in order to support the optimal psychosocial functioning of youth with chronic illnesses.
Acknowledgements
We are most grateful to the following individuals who helped in the translation process and collecting the data: Pauline Berthélémy, Laurence Bertout, Sarah Bérubé, Willow Burns, Hélène Decaluwe, Renée Dicaire, Simon Drouin, Lucie Gouveia, Maja Krajinovic, Martin Lamothe, Marie-Claude Levasseur, Sarah Lippé, Philippe Robaey. Special thanks to Philippe Robaey who initially attracted our attention on the 15D-16D-17D system.
Funding Statement
This work was supported by the following institutions: Sainte Justine University Hospital Center to Dr Serge Sultan, CSL Behring (investigator-initiated research project 2015) to Dr Elie Haddad, and C17 Council, Garron Family Cancer Centre of the Toronto Hospital for Sick Children, Canadian Cancer Society, Institute of Cancer Research, Fonds de Recherche du Québec-Santé, Pediatric Oncology Group of Ontario, Canadian Cancer Research Society, to Drs. Daniel Sinnett, Maja Krajinovic, and Caroline Laverdière.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abate, C., Lippé, S., Bertout, L., Drouin, S., Krajinovic, M., Rondeau, É., … Sultan, S. (2018). Could we use parent report as a valid proxy of child report on anxiety, depression, and distress? A systematic investigation of father-mother-child triads in children successfully treated for leukemia. Pediatric Blood & Cancer, 65(2), e26840. [DOI] [PubMed] [Google Scholar]
- Anestin, A. S., Lippé, S., Robaey, P., Bertout, L., Drouin, S., Krajinovic, M., … Sultan, S. (2018). Psychological risk in long-term survivors of childhood acute lymphoblastic leukemia and its association with functional health status: A PETALE cohort study. Pediatric Blood & Cancer, 65, e27356. [DOI] [PubMed] [Google Scholar]
- Apajasalo, M., Rautonen, J., Holmberg, C., Sinkkonen, J., Aalberg, V., Pihko, H., … Sintonen, H. (1996a). Quality of life in pre-adolescence: A 17-dimensional health-related measure (17D). Quality of Life Research, 5(6), 532–538. [DOI] [PubMed] [Google Scholar]
- Apajasalo, M., Sintonen, H., Holmberg, C., Sinkkonen, J., Aalberg, V., Pihko, H., … Rautonen, J. (1996b). Quality of life in early adolescence: A sixteen-dimensional health-related measure (16D). Quality of Life Research, 5(2), 205–211. [DOI] [PubMed] [Google Scholar]
- Boulet-Craig, A., Robaey, P., Laniel, J., Bertout, L., Drouin, S., Krajinovic, M., … Lippé, S. (2018). DIVERGT screening procedure predicts general cognitive functioning in adult long-term survivors of pediatric acute lymphoblastic leukemia: A PETALE study. Pediatric Blood & Cancer, 65(9), e27259. [DOI] [PubMed] [Google Scholar]
- Chen, G., & Ratcliffe, J. (2015). A review of the development and application of generic multi-attribute utility instruments for paediatric populations. Pharmacoeconomics, 33(10), 1013–1028. [DOI] [PubMed] [Google Scholar]
- Choo, C. C., Chew, P. K. H., Tan, P., et al. (2019). Health-related quality of life in pediatric patients with Leukemia in Singapore: A cross-sectional pilot study. International Journal of Environmental Research and Public Health, 16(12), 2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drotar, D. (2004). Validating measures of pediatric health status, functional status, and health-related quality of life: Key methodological challenges and strategies. Ambulatory Pediatrics, 4(4 Suppl), 358–364. [DOI] [PubMed] [Google Scholar]
- Granö, N., Karjalainen, M., Suominen, K., & Roine, M. (2011). Poor functioning ability is associated with high risk of developing psychosis in adolescents. Nordic Journal of Psychiatry, 65(1), 16–21. [DOI] [PubMed] [Google Scholar]
- Haverman, L., Limperg, P. F., Young, N. L., Grootenhuis, M. A., & Klaassen, R. J. (2017). Paediatric health-related quality of life: What is it and why should we measure it? Archives of Disease in Childhood, 102(5), 393–400. [DOI] [PubMed] [Google Scholar]
- Ho, K. W., Pong, G., Poon, W. C., Chung, K. Y., Kwok, Y. Y., & Chiu, K. H. (2020). Progression of health-related quality of life of patients waiting for total knee arthroplasty. Journal of Evaluation in Clinical Practice, 27(1), 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huoponen, S., Eberl, A., Räsänen, P., Roine, R. P., Sipponen, T., Arkkila, P., & Blom, M. (2020). Health-related quality of life and costs of switching originator infliximab to biosimilar one in treatment of inflammatory bowel disease. Medicine (Baltimore), 99(2), e18723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmarinen, P., Juboori, H., Tuomisto, L. E., Niemela, O., Sintonen, H., & Kankaanranta, H. (2019). Effect of asthma control on general health-related quality of life in patients diagnosed with adult-onset asthma. Scientific Reports, 9(1), 16107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin, D. E., Varni, J. W., Yeatts, K., & DeWalt, D. A. J. H. (2009). Cognitive interviewing methodology in the development of a pediatric item bank: A patient reported outcomes measurement information system (PROMIS) study. Outcomes QoL, 7(1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean, C., & Syrjala, K. (2017). Anxiety and depression in cancer survivors. Medical Clinics of North America, 101(6), 1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanakos, G., Costa-Pinel, B., Gilis-Januszewska, A., Velickiene, D., Barrio-Torrell, F., Cos-Claramunt, X., … Herder, C. (2019). The effectiveness of a community-based, type 2 diabetes prevention programme on health-related quality of life. The DE-PLAN study. PLoS One, 14(10), e0221467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak, A. E., Abrams, A. N., Banks, J., Christofferson, J., DiDonato, S., Grootenhuis, M. A., … Kupst, M. J. (2015). Psychosocial assessment as a standard of care in pediatric cancer. Pediatric Blood & Cancer, 62(S5), S426–S459. [DOI] [PubMed] [Google Scholar]
- Lamore, K., Bourdeau, C., Alos, N., et al. (2020). Contributing factors of unmet needs among young adult survivors of childhood acute lymphoblastic leukemia with comorbidities. Journal of Adolescent Young Adult Oncology. 10.1089/jayao.2020.0090 [DOI] [PubMed] [Google Scholar]
- Lemmon, M. E., Huffstetler, H. E., & Reeve, B. B. (2020). Measuring health-related quality of life in pediatric neurology. Journal of Child Neurology, 35(10), 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bastida, J., López-Siguero, J. P., Oliva-Moreno, J., Vázquez, L. A., Aranda-Reneo, I., Reviriego, J., … Perez-Nieves, M. (2019). Health-related quality of life in type 1 diabetes mellitus pediatric patients and their caregivers in Spain: An observational cross-sectional study. Current Medical Research and Opinion, 35(9), 1589–1595. [DOI] [PubMed] [Google Scholar]
- Marcoux, S., Drouin, S., Laverdière, C., Alos, N., Andelfinger, G. U., Bertout, L., … Sinnett, D. (2017). The PETALE study: Late adverse effects and biomarkers in childhood acute lymphoblastic leukemia survivors. Pediatric Blood & Cancer, 64(6), e26361. [DOI] [PubMed] [Google Scholar]
- Ohnemus, D., Neighbors, K., Rychlik, K., Venick, R. S., Bucuvalas, J. C., Sundaram, S. S., … Alonso, E. M. (2020). Health-related quality of life and cognitive functioning in pediatric liver transplant recipients. Liver Transplantation, 26(1), 45–56. [DOI] [PubMed] [Google Scholar]
- Ojala, A. K., Sintonen, H., Roine, R. P., Strandberg, T. E., & Schalin-Jantti, C. (2020). Impaired breathing, sleeping, vitality, and depression, and negative impact of L-T4 treatment characterize health-related quality of life in older people with stable CVD. Aging Clinical and Experimental Research, 32(10), 2041–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pépin, A. J., Lippé, S., Krajinovic, M., Laverdière, C., Michon, B., Sinnett, D., & Sultan, S. (2017). How to interpret high levels of distress when using the distress thermometer in the long-term follow-up clinic? A study with acute lymphoblastic leukemia survivors. Pediatric Hematology and Oncology, 34(3), 131–135. [DOI] [PubMed] [Google Scholar]
- Pinquart, M. (2020). Health-related quality of life of young people with and without chronic conditions. Journal of Pediatric Psychology, 45(7), 780–792. [DOI] [PubMed] [Google Scholar]
- Poder, T. G., Guertin, J. R., Touré, M., Pratte, G., Gauvin, C., Feeny, D., … Camden, C. (2021). Canadian French translation and linguistic validation of the health-related quality of life utility measure for pre-school children. Expert Review of Pharmacoeconomics & Outcomes Research, 1–7. 10.1080/14737167.2021.1895754 [DOI] [PubMed] [Google Scholar]
- Qvist, E., Närhi, V., Apajasalo, M., Rönnholm, K., Jalanko, H., Almqvist, F., & Holmberg, C. (2004). Psychosocial adjustment and quality of life after renal transplantation in early childhood. Pediatric Transplantation, 8(2), 120–125. [DOI] [PubMed] [Google Scholar]
- Roine, E., Sintonen, H., Kellokumpu-Lehtinen, P.-L., Penttinen, H., Utriainen, M., Vehmanen, L., … Saarto, T. (2020). Health-related quality of life of breast cancer survivors attending an exercise intervention study: A five-year follow-up. In Vivo, 34(2), 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser, C., Sawyer, S. M., & Bowes, G. (1998). Quality-of-life assessment in children and adolescents with asthma. European Respiratory Journal, 12(2), 486–494. [DOI] [PubMed] [Google Scholar]
- Shull, M. H., Ediger, T. R., Hill, I. D., & Schroedl, R. L. (2019). Health-related quality of life in newly diagnosed pediatric patients With celiac disease. Journal of Pediatric Gastroenterology & Nutrition, 69(6), 690–695. [DOI] [PubMed] [Google Scholar]
- Silverman, L. B., Stevenson, K. E., O'Brien, J. E., et al. (2010). Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000). Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK, 24(2), 320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sintonen, H. (1995). The 15D measure of health related quality of life. II. Feasibility, reliability and validity of its valuation system. Melbourne: National Centre for Health Program Evaluation. [Google Scholar]
- Sintonen, H. (2001). The 15D instrument of health-related quality of life: Properties and applications. Annals of Medicine, 33(5), 328–336. [DOI] [PubMed] [Google Scholar]
- Sultan, S., Rondeau, É, Levasseur, M.-C., Dicaire, R., Decaluwe, H., & Haddad, É. (2017). Quality of life, treatment beliefs, and treatment satisfaction in children treated for primary immunodeficiency with SCIg. Journal of Clinical Immunology, 37(5), 496–504. [DOI] [PubMed] [Google Scholar]
- Toija, A. S., Kettunen, T. H., Leidenius, M. H. K., Vainiola, T. H. K., & Roine, R. P. A. (2019). Effectiveness of peer support on health-related quality of life in recently diagnosed breast cancer patients: A randomized controlled trial. Supportive Care in Cancer, 27(1), 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannas, M., Farkkila, M., Sintonen, H., Aberg, F., & Isoniemi, H. (2020). Health-related quality of life before and after liver transplantation in patients with primary sclerosing cholangitis. Scandinavian Journal of Gastroenterology, 55(3), 347–353. [DOI] [PubMed] [Google Scholar]
- WHO . (2015). World health organization process of translation and adaptation of instruments. http://www.who.int/substance_abuse/research_tools/translation/en/
- Zeng, Y., Cheng, A. S., & Chan, C. C. H. (2016). Meta-analysis of the effects of neuropsychological interventions on cognitive function in non–central nervous system cancer survivors. Integrative Cancer Therapies, 15(4), 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, E. S., & Recklitis, C. J. J. (2020). Internet-delivered insomnia intervention improves sleep and quality of life for adolescent and young adult cancer survivors. Pediatric Blood & Cancer, 67(9), e28506. [DOI] [PubMed] [Google Scholar]