Abstract
In the presence of normal left ventricular systolic function, the development of left ventricular thrombus has seldom been documented in hypercoagulable conditions. Echocardiographic results of left ventricular thrombi can be differentiated from those found in cardiac tumors such as myxoma. Many other organs, including the central and peripheral nervous systems, as well as the cardiac and vascular systems, have recently been linked to thromboembolic events in coronavirus disease 2019 (COVID-19). It not only exacerbates acute pancreatitis but often predisposes, in fact, to a novel prothrombotic state in patients with pre-existing comorbid conditions such as chronic calcific pancreatitis. We report an unusual correlation of two prothrombotic disorders – COVID-19 and acute on chronic pancreatitis contributing to the development of thrombus in a normal left ventricle.
<Learning objective: Intra-cardiac thrombus in a normal functioning left ventricle has not been described in severe SARS-CoV-2 infection. The triple association of COVID-19, acute pancreatitis, and left ventricular thrombus is an extremely rare and fatal disorder. Echocardiography is an easy bedside tool to diagnose intra-cardiac left ventricular thrombus formation in COVID-19 patients with high D-dimer values.>
Keywords: Thromboembolism, COVID-19, Chronic pancreatitis, Left ventricular function
Introduction
Common causes of left ventricular thrombus (LVT) formation include scarred culprit-vessel territories secondary to non-reperfused myocardial infarction (MI), non-ischemic cardiomyopathy, non-compaction of LV, endomyocardial fibrosis, and long-standing arrhythmias. However, in the context of large MIs associated with apical akinesia or dyskinesia, LV aneurysms are frequently predisposed to forming LVT [1]. The hypercoagulable or inflammatory disorder can rarely predispose to the formation of LVT [2]. Infection with coronavirus disease 2019 (COVID-19) is associated with a remarkably hypercoagulable state that increases the risk of the early development of LVT in the setting of MI or underlying prethrombotic conditions [1].
We discuss a case in which the amalgamation of prothrombotic states, COVID-19, and chronic calcific pancreatitis predisposed to the formation of a large LVT in a normally functioning left ventricle.
Case report
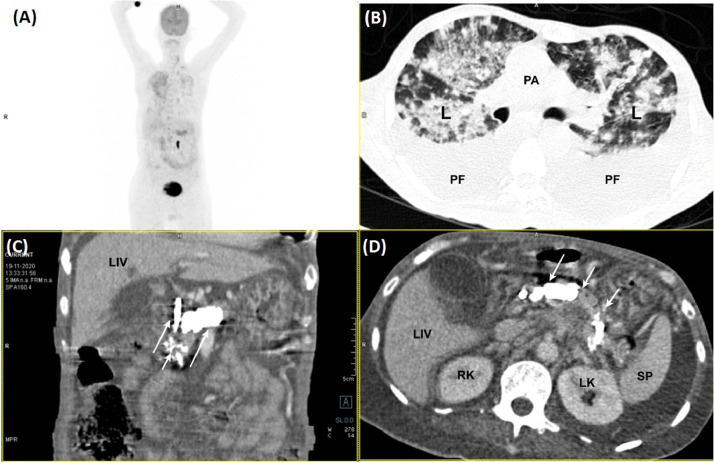
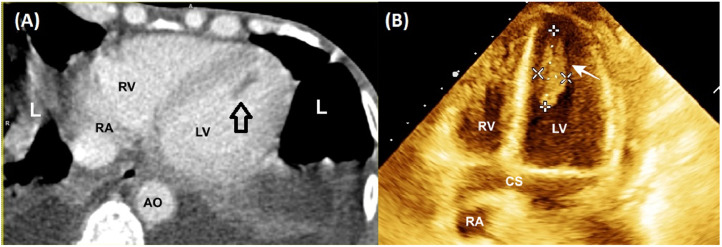
A 56-year-old male, with chronic calcific pancreatitis (CCP), presented with fever, abdominal pain, dry cough, and dyspnea for 7 days. The abdominal pain was mostly limited to the epigastric region with radiation to the back. The vital parameters recorded pulse rate of 122/min, blood pressure of 102/70 mmHg, room air saturation of 80% and, temperature of 39.05 °C. The laboratory parameters revealed low hemoglobin (8 gm/L), thrombocytopenia (100,000 cells/microlitres), leucocytosis (14,500 cells/microliters), increased levels of serum lipase (1023 U/L), serum amylase (884 U/L), interleukin-6 (65 pg/mL), D-dimer (6548 ng/mL), and serum creatinine (1.6 mg/dL). Thoracic-images with whole-body positron-emission-tomography with computed-tomography (PET-CT) (Fig. 1A) revealed extensive bilateral ground-glass opacification, septal thickening with areas of consolidations, and pleural-effusion (Fig. 1B, C). The abdominal-sections showed intraductal calcifications (Fig. 1C, D) and heterogeneous increase of 18F-fluorodeoxyglucose uptake in the pancreas (Fig. 1D); features suggestive of CCP. The CT images of the LV in the long-axis show hypodense linear filling defects at the LV apex (Fig. 2A). The echocardiography revealed normal LV systolic function with no regional-wall-motion abnormality and a large LV mobile thrombus (3.21 × 1.16 cm) (Fig. 2B; Video 1). Considering the high risk for any cardiac surgery, he was managed medically using low molecular weight heparin, enoxaparin. The patient's condition deteriorated, prompting endotracheal intubation. Despite specific COVID-19 treatment, including plasma therapy, remdesivir, dexamethasone, and other supportive therapy, he died of multi-organ failure.
Fig. 1.
(A–D) Coronal PET maximum intensity projection image detects a diffuse and heterogeneous increase of FDG uptake in the pancreas (A). Axial contrast-enhanced CT slice of the thorax. There are bilateral patchy ground-glass and consolidative changes (B). Axial contrast-enhanced CT image shows intraductal calcifications associated with dilatation of main pancreatic duct (C) & (D).
Abbreviations: CT = computed tomography; FDG = fluorodeoxyglucose; LIV= Liver; L = Lung; LK = left kidney; PET = positron emission tomography; PA = pulmonary artery; PF = pleural effusion; RK = right kidney; SP = spleen.
Fig. 2.
Images of conventional 64-row MSCT of the patient in diastolic long axis show a low-density shadow of filling defects at the LV apex (Arrow; A). Four chamber views in 3D echocardiography of heart showing Intra-ventricular thrombus (3.21 x 1.16 cm) (B).
Abbreviations: AO= aorta; CS = coronary sinus; LV = left ventricle; L = lung; MSCT = multi-slice computed tomography; RA= right atrium; RV= right ventricle; 3D = 3-dimensional.
Discussion
It is exceptionally unusual for a thrombus to develop in a normal functioning LV. To date in the literature, there were few cases reported of LVT with normal LV function [[3], [4], [5]]. In most cases, the associated procoagulant state is responsible for the development of LVT. The presence of COVID-19 increases the risk of thrombus formation [1].
Multiorgan involvement of the COVID-19 infection is a well-established fact [5]. Pancreatic manifestations of COVID-19 have been rare and subject of great interest due to its association with high morbidity and mortality. Recent data from across the world suggest acute pancreatitis as one of the gastrointestinal manifestations of COVID-19. Acute pancreatic damage by COVID-19 can be caused directly by viral activity or secondary to enzyme disturbances in the background of chronic pancreatitis. This is confirmed by the fact that angiotensinogen-converting enzyme-2 receptor on the pancreatic islet cells acts as a receptor for severe acute respiratory syndrome–coronavirus-2 (SAR-CoV2) to bind and, as a result, invasion of the gland is possible as the virus can spread from the duodenal epithelium to the pancreatic duct and then to the acinar and islet cells [6,7]. Furthermore, pancreatic inflammation is related to a prothrombotic condition and endothelial dysfunction, all of which facilitate and accelerate atherothrombosis growth [8].
Microvascular ischemia secondary to inflammation and the prothrombotic state has earlier been hypothesized as a cause of platelet aggregation by triggering blotchy areas of endocardial fibrosis and the development of thrombi on such endomyocardial sites [3]. We did not investigate coronary anatomy since there was no substantiation of the acute coronary syndrome, with normal electrocardiography findings, normal biomarker levels, and no history of chest pain.
Hyper-inflammatory processes, hypoxia, diffuse intravascular coagulation, and immobilization are also possible causes of these vascular thrombotic events in COVID-19. Hypoxemia can increase the hypercoagulable state even further [9]. Hypoxia-inducible transcription factors (HIFs) can trigger platelets and coagulation factors specifically, enhancing tissue factor expression, raising plasminogen activator inhibitor-1, and suppressing the endogenous anticoagulant protein, all of which contribute to hypercoagulability condition [10].
Conclusion
Rarely, LVT can occur in patients with normal systolic function. In all these patients, rapid echocardiography must be done. Clinicians must suspect thrombus as a differential diagnosis of every cardiac mass visualized on echocardiography. This unusual association of COVID-19 pneumonia, pancreatitis, and LV-thrombus with normal LV function is exceptional and poses a high risk of death.
Consent from patient/patient's attender
As the corresponding author, I declare that there is no financial or non-financial conflict/competing of interests. This manuscript has not been submitted to any journal before for publication as a part or complete version. I give complete consent and rights to the journal for its publication. Informed consent was obtained from the participant included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Financial and non-financial relations
There is no source of funding for this article.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jccase.2021.06.010.
Appendix. Supplementary materials
Video 1 Four-chamber apical view in 3-dimensional echocardiography showing left ventricular large mobile thrombus attached to the apex of the left ventricle.
References
- 1.Jadhav K.P., Jariwala P. Intra-cardiac thrombus in COVID-19 pandemic – case series and review. Eur J Cardiovasc Med. 2020;VI(I) doi: 10.5083/ejcm20424884.180. http://www.healthcare-bulletin.com/journals/cardiovascular-medicine/the-european-journal-of-cardiovascular-medicine/details/article/intra-cardiac-thrombus-in-covid-19-pandemic-case-series-and-review/ Nov 23 Available from: [DOI] [Google Scholar]
- 2.de-Madaria E., Capurso G. COVID-19, and acute pancreatitis: examining the causality. Nat Rev Gastroenterol Hepatol. 2021;18:3–4. doi: 10.1038/s41575-020-00389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma A.K., Alam M., Rosman H.S., Brymer J., Keith F. Systemic embolization from thrombus in normal left ventricles. Chest. 1988;93:441–442. doi: 10.1378/chest.93.2.441. [DOI] [PubMed] [Google Scholar]
- 4.Vaganos S.A., Fox K.R., Kitchen J.G. Left ventricular thrombus in the absence of detectable heart disease. Chest. 1989;96:426–427. doi: 10.1378/chest.96.2.426. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos G., Nunez A., Grodman R., Mamadova A., Rahmany Z., El-Eshmawi A., Hao Y., Gala B. Massive mobile left ventricular thrombus in a patient with normal left ventricular systolic function. CASE. 2019;3:277–279. doi: 10.1016/j.case.2019.07.007. (Phila) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Mazrouei SS, GA Saeed, Al Helali A.A. COVID-19-associated acute pancreatitis: a rare cause of acute abdomen. Radiol Case Rep. 2020;15:1601–1603. doi: 10.1016/j.radcr.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inamdar S., Benias P.C., Liu Y., Sejpal D.V., Satapathy S.K., Trindade A.J., Northwell COVID-19 Research Consortium Prevalence, risk factors, and outcomes of hospitalized patients with coronavirus disease 2019 presenting as acute pancreatitis. Gastroenterology. 2020;159:2226–2228. doi: 10.1053/j.gastro.2020.08.044. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherata D.A., Donoiu I., Mirea O., Diaconu R., Istratoaie O. Right atrium floating thrombus and bilateral pulmonary embolism in a patient with pancreatic pseudocyst. J Cardiol Cases. 2018;18:57–59. doi: 10.1016/j.jccase.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Carranza M., Salazar D.E., Troya J., Alcázar R., Peña C., Aragón E., Domínguez M., Torres J., Muñoz-Rivas N. Aortic thrombus in patients with severe COVID-19: review of three cases. J Thromb Thrombolysis. 2021;51:237–242. doi: 10.1007/s11239-020-02219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frantzeskaki F., Armaganidis A., Orfanos S.E. Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation. Respiration. 2017;93:212–225. doi: 10.1159/000453002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1 Four-chamber apical view in 3-dimensional echocardiography showing left ventricular large mobile thrombus attached to the apex of the left ventricle.