Abstract
Due to their superior tolerability and efficacy, β-lactams are the most potent and prescribed class of antibiotics in the clinic. The emergence of resistance to those antibiotics, mainly due to the production of bacterial enzymes called β-lactamases, has been partially solved by the introduction of β-lactamase inhibitors, which restore the activity of otherwise obsolete molecules. This solution is limited because currently available β-lactamase inhibitors only work against serine β-lactamases, whereas metallo-β-lactamases continue to spread, evolve, and confer resistance to all β-lactams, including carbapenems. Furthermore, the increased use of antibiotics to treat secondary bacterial pneumonia in severely sick patients with COVID-19 might exacerbate the problem of antimicrobial resistance. In this Personal View, we summarise the main advances accomplished in this area of research, emphasise the main challenges that need to be solved, and the importance of research on inhibitors for metallo-B-lactamases amidst the current pandemic.
The scourge of antimicrobial resistance
β-lactam antibiotics are the cornerstones of antimicrobial chemotherapy. However, antimicrobial resistance against these life-saving drugs is a major public health problem worldwide. The most concerning problem in antimicrobial resistance involves carbapenem-resistant Gram-negative bacteria. In the past decade, the so-called superbugs (eg, multidrug-resistant Klebsiella pneumoniae, carbapenem-resistant Acinetobacter baumannii, and multidrug-resistant Pseudomonas aeruginosa) have gained even more notoriety due to their intrinsic abilities to cause life-threatening disease in older people (older than 60 years) and immunocompromised hosts. These pathogens are responsible for more than 540 000 infections and nearly 14 000 deaths annually in the USA alone.1 Regrettably, the situation is not expected to improve, since current estimates predict that antimicrobial resistance will be the main cause of death in 2050 (10 million deaths per year),2 threatening even the simplest medical procedure.
The current COVID-19 pandemic might be aggravating this scenario in the future. Most deaths associated with the influenza pandemic of 1918 were caused by subsequent bacterial pneumonia,3 and secondary bacterial infections were also reported in the 2009 swine influenza pandemic,4 during the 2003 severe acute respiratory syndrome outbreak,5 and during the 2012 Middle East respiratory syndrome outbreak.6 In the current COVID-19 pandemic, sentinel reports showed that secondary infections were present in up to 30 % of critically ill patients7 and these infections were shown to markedly decrease the survival of patients with COVID-19.8 The bacterial species more frequently isolated are Mycoplasma pneumoniae, Staphylococcus aureus, Legionella pneumophila, Haemophilus spp, Klebsiella spp, P aeruginosa, Chlamydia spp, Streptococcus pneumoniae, and A baumannii. Notably, infections with antibiotic-resistant S aureus, K pneumoniae, P aeruginosa, or A baumannii have been reported in patients with COVID-19 in intensive care units.8
Understandably, as inpatients with SARS-CoV-2 infection often exhibit symptoms undistinguishable from hospital-acquired and ventilator-associated pneumonia, empirical treatments with broad-spectrum antibiotics are administered in many cases.9 A 2020 literature review by Fattorini and colleagues8 reported that 476 (88·3%) of 539 patients with COVID-19 were treated with broad-spectrum antibiotics, including expanded-spectrum cephalosporins (eg, ceftriaxone, ceftazidime, and cefepime), quinolones, and carbapenems. Consequently, antibiotic use has substantially increased in many settings.9 With the spread of the pandemic, hospitals around the world have seen an increase of patients infected with COVID-19. This situation has demanded major adjustments to health-care systems and infrastructure, and especially to the infection control and antimicrobial stewardship programmes.10 Unfortunately, less robust health-care systems, such as those in many Latin American and Asian countries where antimicrobial resistance rates are already perilously high11, 12 and antimicrobial stewardship programmes are just beginning to be implemented,13 are adjusting their response to the pandemic at varying degrees.14, 15, 16 Regrettably, these circumstances create a so-called perfect storm for an accelerated evolution of antimicrobial resistance.17, 18
Carbapenemases: a threat to be faced and the intricacies of metallo-β-lactamases
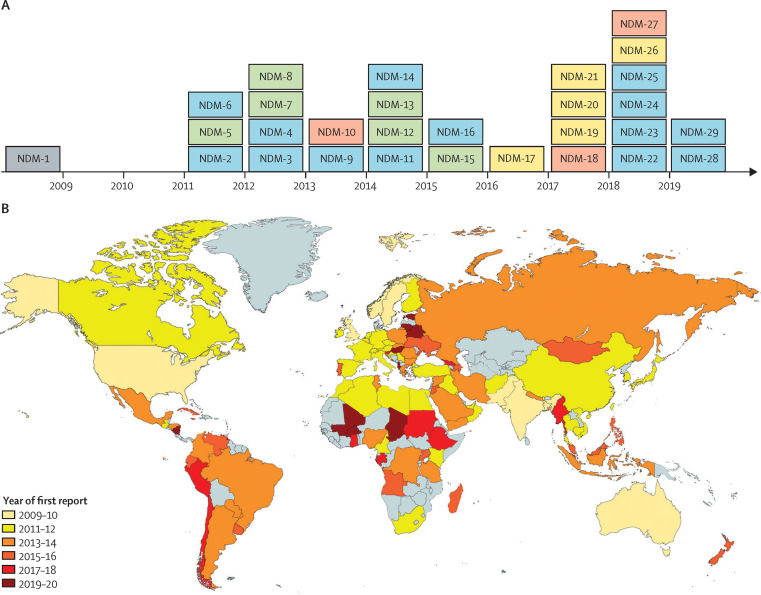
Resistance to carbapenems among Gram-negative bacteria is primarily due to the production of carbapenemases, which inactivate these life-saving drugs. Bacteria achieve these challenging chemical reactions with two types of enzymes: one based on a serine residue (serine β-lactamases) or another using one or two zinc ions (metallo-β-lactamases). Some serine β-lactamases (such as K pneumoniae carbapenemases [KPC] and oxacillinase-48 [OXA-48-like] carbapenemases) can hydrolyse carbapenems, whereas all metallo-β-lactamases are carbapenemases. Metallo-β-lactamases are of particular interest and concern given several factors: (1) their ability to hydrolyse and provide resistance to virtually all β-lactam antibiotics; (2) the unavailability of clinically useful metallo-β-lactamase inhibitors; (3) the rapid pace at which new variants are isolated (figure 1A ); (4) the transferability of their encoding genes; and (5) their ubiquity, because they are isolated from nosocomial and environmental sources.19, 20
Figure 1.
Evolution and dissemination of NDM-1 carbapenemase
(A) Timeline of appearance of NDM variants. Number of amino acid substitutions compared with NDM-1: one (blue), two (green), three (yellow), and five (red). (B) Global dissemination of NDM carbapenemases. Grey sections indicate areas with no reports of NDM carbapenemase. NDM=New Delhi metallo-β-lactamase.
Metallo-β-lactamases are further divided into three subclasses (B1, B2, and B3), based primarily on their metal content and different active site features. Most metallo-β-lactamases that have been identified so far belong to subclass B1, among which imipenemase (IMP), Verona imipenemase (VIM), and New Delhi metallo-β-lactamase (NDM) families are the three most common metallo-β-lactamases found in clinical isolates.21 Subclass B2 has the fewest members and includes enzymes produced by different species of Aeromonas, such as A hydrophila, A veronii, and Serratia fonticola (the enzyme are commonly named in the literature as CphA, ImiS, and Sfh-I, respectively). Subclass B3 includes L1 of Stenotrophomonas maltophilia, an emerging multidrug-resistant bacterium in patients who are severely immunocompromised. This microorganism is associated with respiratory tract infections in patients who are chronically ill, who have cancer, or who have cystic fibrosis, and for whom antibiotic treatment options are limited.22
The β-lactamase genes (bla genes) encoding subclass B1 metallo-β-lactamases are largely plasmid-borne and are of greater clinical relevance compared with metallo-β-lactamase subclasses B2 and B3, and other B1 enzymes that are not plasmid borne. This characteristic means subclass B1 metallo-β-lactamases can be transferred between bacterial strains via these mobile genetic elements. IMP-type β-lactamases were identified in 1991 in Japan23 and are still the predominant metallo-β-lactamases in southeast Asia, where they can be found among P aeruginosa, A baumannii, and different species of Enterobacterales.20 However, VIM-type β-lactamases were discovered in 1997 in Italy24 and, until 2017, were the predominant metallo-β-lactamases in Europe, especially in Mediterranean countries.25 VIM-2-like β-lactamases are associated mostly with P aeruginosa, whereas VIM-1-like β-lactamases (eg, VIM-4) are frequently reported in strains of Enterobacterales.26 Lastly, the first documented case of infection caused by NDM-producing bacteria occurred in 2008, when the bla NDM gene was detected in K pneumoniae and Escherichia coli strains from a patient returning to Sweden from India.27 Since then, bla NDM has disseminated globally, and it is considered endemic in the Indian subcontinent and the Middle East (figure 1B). Notably, NDM is currently the predominant metallo-β-lactamase in Europe.28 The bla NDM gene has been reported in several families of Enterobacterales as well as in other Gram-negative bacteria, such as Vibrio cholerae, Pseudomonas spp, and A baumannii.20, 25, 29 NDM-producing microorganisms can cause life-threatening infections; therefore, that fact that these microorganisms are actively disseminating outside the health-care system is a matter of concern.30, 31
NDM-1 is unique in being a membrane-bound protein, by contrast with other metallo-β-lactamases. This cellular localisation favours its secretion and dissemination in outer membrane vesicles.32 Outer membrane vesicles are spherical portions of the outer membrane that protrude and detach from growing cells in response to a wide variety of environments.33, 34 NDM-1 is selectively secreted in an active form into outer membrane vesicles by different bacteria, therefore being protected against the action of extracellular proteases.32 These outer membrane vesicles possess potent carbapenemase activity that helps with titrating the amount of available antibiotic at the infection site and can protect neighbouring bacteria otherwise susceptible to antibiotics.35, 36 Furthermore, the finding of a plasmid containing the bla NDM-1 gene in outer membrane vesicles from A baumannii 37 has also suggested an active role of vesicles in direct gene transfer.36 Despite that, the effects of outer membrane vesicles in the dissemination of resistance is controversial, and this area deserves attention. Notably, NDM-1 is also tailored to be expressed by all bacterial hosts highlighted as Priority One by WHO,26, 38 by contrast with other host-specific metallo-β-lactamases. The rapid evolution (figure 1A) and spread (figure 1B) of NDM presents a threat that requires urgent action.
Two main strategies are currently used to counteract resistance due to β-lactamases. The first involves the synthesis of new β-lactam compounds refractory to hydrolysis by these enzymes (eg, cefiderocol). The second strategy is the development of β-lactamase inhibitors, which can be paired with the antibiotics and formulated as a single product.39 In this way, β-lactamase inhibitors prolong the usefulness of otherwise obsolete β-lactam antibiotics. Currently, clinical inhibitors approved for metallo-β-lactamases are not available. Metallo-β-lactamases do not share an evolutionary relationship with serine β-lactamases, because these β-lactamases possess different structures, active sites, and catalytic mechanisms.
The first generation of β-lactamase inhibitors introduced in the clinic includes mechanistic-based inhibitors—ie, so-called suicide inactivators with a β-lactam structure (eg, clavulanic acid, tazobactam, and sulbactam; table ). Suicide inactivators are β-lactam compounds that are hydrolysed by the serine β-lactamases but remain bound to the active site serine residue, thereby inactivating the enzyme. None of these compounds are markedly active against the current carbapenemases. The second generation of β-lactamase inhibitors corresponds to non-β-lactam-based compounds—the diazabicyclooctanones—such as avibactam40, 41 and relebactam.42 These compounds act as reversible inhibitors of class A carbapenemases (eg, KPC-2) and extended-spectrum β-lactamases (eg, CTX-M), class C cephalosporinases (eg, AmpC, as commonly referred to in the literature, from Enterobacter cloacae complex and P aeruginosa), and some class D, notably the OXA-48 carbapenemase. However, none of these compounds effectively inactivate metallo-β-lactamases in a clinically meaningful way. In 2017, the approval of vaborbactam in combination with meropenem defined a third generation of β-lactamase inhibitors: boronate compounds, designed as transition state analogues. Vaborbactam is a potent inhibitor of serine β-lactamases, especially KPC-2, but is not active against metallo-β-lactamases.43 Overall, there are six approved serine β-lactamase inhibitors in different combinations with antibiotics, but none of these inhibitors work against metallo-β-lactamases (table).
Table.
Clinically available serine β-lactamase inhibitors and metallo-β-lactamase inhibitors in clinical trials
| Year of FDA approval | Clinical trial phase | β-lactam partner | Formulation | Usage | Inhibitor type | Inhibition mechanism |
Inhibition profile of the inhibitor |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine β-lactamases |
Metallo-β-lactamases |
||||||||||||
| Class A (extended-spectrum β-lactamase) | Class A (KPC) | Class C | Class D | Subclass B1 | Subclass B3 | ||||||||
| Clavulanic acid | 1984 | .. | Amoxicillin | Augmentin | Wide* | First generation | Suicide inhibitor, β-lactam analogue | Yes | No | No | No | No | No |
| Sulbactam | 1987 | .. | Ampicillin | Unasyn | Wide† | First generation | Suicide inhibitor, β-lactam analogue | Yes | No | No | No | No | No |
| Tazobactam | 1993 | .. | Piperacillin | Zosyn | Wide‡ | First generation | Suicide inhibitor, β-lactam analogue | Yes | No | No | No | No | No |
| Tazobactam | 2014 | .. | Ceftolozane | Zerbaxa | cIAIs and cUTIs | First generation | Suicide inhibitor, β-lactam analogue | Yes | No | No | No | No | No |
| Avibactam | 2015 | .. | Ceftazidime | Avycaz | cIAIs and cUTIs | Second generation | Reversible inhibitor, DBO type | Yes | Yes | Yes | Yes | No | No |
| Avibactam | .. | Phase 3 (NCT03580044) | Aztreonam | .. | To be determined§ | Second generation | Reversible inhibitor, DBO type | Yes | Yes | Yes | Yes | Yes | Yes |
| Vaborbactam | 2017 | .. | Meropenem | Vabomere | cUTIs | Third generation | β-lactam transition state analogue, boronate type | Yes | Yes | Yes | Yes | No | No |
| Relebactam | 2019 | .. | Imipenem and cilastatin¶ | Recarbrio | cUTIs and cIAIs | Second generation | Reversible inhibitor, DBO type | Yes | Yes | Yes | No | No | No |
| Taniborbactam | .. | Phase 3 (NCT03840148) | Cefepime | .. | cUTIs | Third generation | β-lactam transition state analogue, boronate type | Yes | Yes | Yes | Yes | Yes‖ | No |
| QPX7728 | .. | Phase 1 (NCT04380207) | .. | .. | .. | Third generation | β-lactam transition state analogue, boronate type | Yes | Yes | Yes | Yes | Yes‖ | No |
FDA=US Food and Drug Administration. cIAI=complicated intra-abdominal infection. cUTI=complicated urinary tract infection. DBO=diazabicyclooctanone.
Clavulanic acid is used to treat a wide variety of bacterial infections, among them: lower respiratory tract infections, acute bacterial otitis media, sinusitis, skin and skin structure infections, and cUTIs.
Sulbactam is used to treat a wide variety of bacterial infections, among them: gynaecological infections, bone and joint infections, cIAIs, and skin and skin structure infections.
Tazobactam with piperacillin is used to treat a wide variety of bacterial infections, among them: cIAIs, skin and skin structure infections, gynaecological infections, community-acquired pneumonia, and nosocomial pneumonia.
The phase 3 study will determine the efficacy against cIAIs, nosocomial pneumonia (including hospital-acquired pneumonia and ventilator associated pneumonia), cUTIs, or bloodstream infections due to metallo-β-lactamase-producing Gram-negative bacteria.
Relebactam is in combination with imipenem and cilastatin.
IMP-type metallo-β-lactamases are weakly inhibited.
Challenges for the development of metallo-β-lactamase inhibitors
The development of an efficient metallo-β-lactamase inhibitor that is active against the three subclasses is a challenging task. The clinically available serine β-lactamase inhibitors target the catalytic serine residue that cleaves the β-lactam ring. Metallo-β-lactamases do not possess this serine residue in their active site. Instead, a water molecule activated by the zinc ions pursues β-lactam hydrolysis. As a result of this fundamental difference in the catalytic mechanism, none of the currently available serine β-lactamase inhibitors are active against metallo-β-lactamases. Furthermore, the active sites of serine β-lactamases and metallo-β-lactamases present different sizes and topologies. Serine β-lactamases have a narrow and deep catalytic site. By contrast, the active site in metallo-β-lactamases is in a shallow groove, with only a few contact points to bind the inhibitor or substrate. An additional challenge in achieving metallo-β-lactamase inhibition resides on their large structural diversity, which involves low homology among active site residues and different Zn2+ content. Lastly, we note that, unlike serine β-lactamases, which are exclusively bacterial enzymes, metallo-β-lactamases belong to a superfamily of metalloproteins with diverse biological functions beyond β-lactam hydrolysis. This superfamily, designated as the metallo-hydrolase/oxidoreductase superfamily, groups more than 30 000 genes distributed in all three domains of life, Archaea, Bacteria, and Eukarya.44, 45 Members of this superfamily share a common protein fold that results in all of them having similar active sites. This characteristic explains why L-captopril, an inhibitor of the angiotensin-converting enzyme, can inhibit metallo-β-lactamases. Therefore, another challenge is to achieve a fine balance to develop effective broad-spectrum metallo-β-lactamase inhibitors that are still selective enough to avoid toxicity due to inhibition of off-target enzymes.
New perspectives in the development of metallo-β-lactamase inhibitors
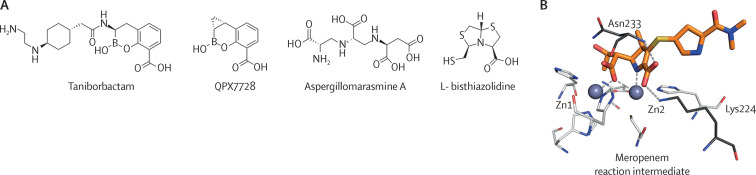
Taniborbactam46 and QPX772847, 48 are novel boronate compounds that are effective against most of the B1 metallo-β-lactamases (figure 2A ). Both compounds are currently in phase 3 and phase 1 clinical trials, respectively, giving some hope (table). The development of boronate-based inhibitors, although promising, is still in its infancy. There are limitations in the scope of currently available boronates, as well as an incomplete understanding of their mechanism of inhibition and spectrum of action. What makes some boronates potent metallo-β-lactamase inhibitors and others not? Why do boronates inhibit some B1 metallo-β-lactamases (eg, the IMP-type metallo-β-lactamases) less efficiently? Why are boronates not active against B3 metallo-β-lactamases, such as L1 metallo-β-lactamase? Expanding this knowledge will enable the design of potent boronate molecules able to be cross-class metallo-β-lactamase inhibitors.
Figure 2.
Metallo-β-lactamase inhibitors and reaction intermediate structures
(A) Chemical structure of some metallo-β-lactamase inhibitors: the boronates, taniborbactam and QPX7728; the chelator, Aspergillomarasmine A; and the bisthiazolidine, L- bisthiazolidine. (B) Reaction intermediate of meropenem hydrolysis bound to New Delhi metallo-β-lactamase-1.
Metallo-β-lactamases can also be inactivated by metal chelators that remove the essential zinc ions, such as Aspergillomarasmine A (figure 2A),49 or metal-based compounds that replace the zinc ions with other metals that gives rise to an inactive variant. The latter situation is the case of colloidal bismuth subcitrate,50 a compound used for the treatment of Helicobacter pylori infections. The use of chelators is appealing because these agents mimics a natural defence mechanism in vertebrate hosts that is triggered by bacterial infections, which consists of a massive metal sequestration by metal-binding proteins, such as calprotectin.51, 52, 53 However, exposure to this environment of metal starvation has led to the selection of metallo-β-lactamases that have developed a higher zinc binding affinity, thus being able to escape the action of chelators.54, 55 Additionally, a major concern regarding the use of chelators is their low specificity because these agents can also target many other metalloproteins. In this regard, Aspergillomarasmine A seems to be more selective and less toxic in animal infections than other chelators. However, the clinical efficacy of Aspergillomarasmine A remains to be determined. A limitation of concern is its inactivity against some metallo-β-lactamases of the B1 subclass, such as São Paolo metallo-1 (SPM-1), Adelaide imipenemase (AIM), or IMP-1, with high affinity towards zinc.56
Remarkably, despite the different active site topology and Zn2+ content among the three metallo-β-lactamase subclasses, a shared mechanism of hydrolysis of carbapenems has been elucidated.57 The zinc ions in metallo-β-lactamases activate a water molecule, which is responsible for the cleavage of the β-lactam ring. After this step, a central reaction intermediate with a negative charge is formed. This intermediate is bound to the active site by interaction with the metal centre and some conserved residues, such as Asn233 and Lys224 (figure 2B). Finally, this intermediate species receives a proton from a water molecule before being released from the active site. Notably, this intermediate is not present in the reaction of carbapenem hydrolysis that is catalysed by serine β-lactamases. The elucidation of this common catalytic mechanism is of major relevance to advance in the metallo-β-lactamase inhibitor design, given that novel strategies inspired by the mechanism are possible.58 A representative example of this novel approach is the family of bisthiazolidines, which mimic the β-lactam antibiotics and are active against the three subclasses of metallo-β-lactamases (figure 2A).59 It is also necessary to elucidate which is the action of these new compounds on other cellular targets in bacteria.
The present prospects offers hope, but further efforts are needed in understanding how metallo-β-lactamases can be inhibited and, also importantly, how metallo-β-lactamases can escape the action of novel inhibitors by means of new mutations. Future developments should also consider that tebipenem, the first oral carbapenem, is in phase 3 clinical trials.60, 61 Therefore, the development of oral β-lactamase inhibitors to be paired with this antibiotic would be a major step forward in the fight against bacterial resistance.
As made evident by the COVID-19 pandemic, densely populated urban settlements characterised by poor hygiene, contaminated water, and close proximity of domestic animals favours the dissemination of antimicrobial resistance.62 This finding corresponds to cities, where 90% of the urban population growth is expected to take place. Global travel and food transportation will soon recover; however, the growing threat of antimicrobial resistance will not diminish. As bla genes become more and more widespread, vulnerable populations will probably bear the burden of untreatable infections. The wealth of information that has been gathered during the past 5 years in understanding metallo-β-lactamases (particularly NDM-1) should now be translated into practical solutions to counteract antimicrobial resistance.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health to RAB and AJV (award number R01AI100560). This work was also supported, in part, by funds and/or facilities provided by the Cleveland Department of Veterans Affairs (award number 1I01BX001974) to RAB from the Biomedical Laboratory Research & Development Service of the Veterans Affairs Office of Research and Development, and the Geriatric Research, Education, and Clinical Center VISN 10. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
RAB and AJV conceived the idea for this Personal View and wrote the Personal View. MFM and M-AR did the literature search, analysed and discussed the data, made the figures, and also assisted in writing the Personal View. All authors discussed and approved the final version of the Personal View.
References
- 1.CDC . Centers for Disease Control and Prevention; Atlanta, GA: 2019. Antibiotic resistance threats in the United States. [Google Scholar]
- 2.O'Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2014. http://www.jpiamr.eu/wp-content/uploads/2014/12/AMR-Review-Paper-Tackling-a-crisis-for-the-health-and-wealth-of-nations_1-2.pdf
- 3.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017;8 doi: 10.3389/fmicb.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilder-Smith A, Green JA, Paton NI. Hospitalized patients with bacterial infections: a potential focus of SARS transmission during an outbreak. Epidemiol Infect. 2004;132:407–408. doi: 10.1017/s0950268803001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Memish ZA, Perlman S, Van Kerkhove MD, Zumla A. Middle East respiratory syndrome. Lancet. 2020;386:995–1007. doi: 10.1016/S0140-6736(19)33221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudoignon E, Caméléna F, Deniau B, et al. Bacterial pneumonia in COVID-19 critically ill patients: a case series. Clin Infect Dis. 2020;72:905–906. doi: 10.1093/cid/ciaa762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fattorini L, Creti R, Palma C, Pantosti A. Bacterial coinfections in COVID-19: an underestimated adversary. Ann Ist Super Sanita. 2020;56:359–364. doi: 10.4415/ANN_20_03_14. [DOI] [PubMed] [Google Scholar]
- 9.Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis Clin Infect Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis MW, McManus D, Koff A, et al. Re-purposing antimicrobial stewardship tools in the electronic medical record for the management of COVID-19 patients. Infect Control Hosp Epidemiol. 2020;41:1335–1337. doi: 10.1017/ice.2020.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Betancur JC, Appel TM, Esparza G, et al. Update on the epidemiology of carbapenemases in Latin America and the Caribbean. Expert Rev Anti Infect Ther. 2021;19:197–213. doi: 10.1080/14787210.2020.1813023. [DOI] [PubMed] [Google Scholar]
- 12.Sader HS, Castanheira M, Arends SJR, Goossens H, Flamm RK. Geographical and temporal variation in the frequency and antimicrobial susceptibility of bacteria isolated from patients hospitalized with bacterial pneumonia: results from 20 years of the SENTRY Antimicrobial Surveillance Program (1997–2016) J Antimicrob Chemother. 2019;74:1595–1606. doi: 10.1093/jac/dkz074. [DOI] [PubMed] [Google Scholar]
- 13.Cox JA, Vlieghe E, Mendelson M, et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect. 2017;23:812–818. doi: 10.1016/j.cmi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Acosta LD. Capacidad de respuesta frente a la pandemia de COVID-19 en América Latina y el Caribe. Rev Panam Salud Publica. 2020;44:1. doi: 10.26633/RPSP.2020.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia PJ, Alarcón A, Bayer A, et al. COVID-19 response in Latin America. Am J Trop Med Hyg. 2020;103:1765–1772. doi: 10.4269/ajtmh.20-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezequiel GE, Jafet A, Hugo A, et al. The COVID-19 pandemic: a call to action for health systems in Latin America to strengthen quality of care. Int J Qual Heal Care. 2020;2020:1–2. doi: 10.1093/intqhc/mzaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reardon S. Antibiotic treatment for COVID-19 complications could fuel resistant bacteria. 2020. https://www.sciencemag.org/news/2020/04/antibiotic-treatment-covid-19-complications-could-fuel-resistant-bacteria
- 18.Antimicrobial resistance in the age of COVID-19. Nat Microbiol. 2020;5:779. doi: 10.1038/s41564-020-0739-4. [DOI] [PubMed] [Google Scholar]
- 19.Tooke CL, Hinchliffe P, Bragginton EC, et al. β-lactamases and β-lactamase inhibitors in the 21st century. J Mol Biol. 2019;431:3472–3500. doi: 10.1016/j.jmb.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bush K, Bradford PA. Epidemiology of β-lactamase-producing pathogens. Clin Microbiol Rev. 2020;33:e00047–e00119. doi: 10.1128/CMR.00047-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bush K. The ABCD's of β-lactamase nomenclature. J Infect Chemother. 2013;19:549–559. doi: 10.1007/s10156-013-0640-7. [DOI] [PubMed] [Google Scholar]
- 22.Chang YT, Lin CY, Chen YH, Hsueh PR. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol. 2015;6:893. doi: 10.3389/fmicb.2015.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauretti L, Riccio ML, Mazzariol A, et al. Cloning and characterization of blaVIM, a new integron-borne metallo-beta-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob Agents Chemother. 1999;43:1584–1590. doi: 10.1128/aac.43.7.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mojica MF, Bonomo RA, Fast W. B1-Metallo-β-lactamases: where do we stand? Curr Drug Targets. 2016;17:1029–1050. doi: 10.2174/1389450116666151001105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López C, Ayala JA, Bonomo RA, González LJ, Vila AJ. Protein determinants of dissemination and host specificity of metallo-β-lactamases. Nat Commun. 2019;10 doi: 10.1038/s41467-019-11615-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in Gram-negative bacteria. Clin Infect Dis. 2019;69(suppl 7):S521–S528. doi: 10.1093/cid/ciz824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu W, Feng Y, Tang G, Qiao F, McNally A, Zong Z. NDM metallo-β-lactamases and their bacterial producers in health care settings. Clin Microbiol Rev. 2019;32:e00115–e00118. doi: 10.1128/CMR.00115-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills MC, Lee J. The threat of carbapenem-resistant bacteria in the environment: Evidence of widespread contamination of reservoirs at a global. Environ Pollut. 2019;255 doi: 10.1016/j.envpol.2019.113143. [DOI] [PubMed] [Google Scholar]
- 31.Köck R, Daniels-Haardt I, Becker K, et al. Carbapenem-resistant Enterobacteriaceae in wildlife, food-producing, and companion animals: a systematic review. Clin Microbiol Infect. 2018;24:1241–1250. doi: 10.1016/j.cmi.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 32.González LJ, Bahr G, Nakashige TG, Nolan EM, Bonomo RA, Vila AJ. Membrane anchoring stabilizes and favors secretion of New Delhi metallo-β-lactamase. Nat Chem Biol. 2016;12:516–522. doi: 10.1038/nchembio.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13:605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwechheimer C, Kulp A, Kuehn MJ. Modulation of bacterial outer membrane vesicle production by envelope structure and content. BMC Microbiol. 2014;14:324. doi: 10.1186/s12866-014-0324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rumbo C, Fernández-Moreira E, Merino M, et al. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob Agents Chemother. 2011;55:3084–3090. doi: 10.1128/AAC.00929-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatterjee S, Mondal A, Mitra S, Basu S. Acinetobacter baumannii transfers the blaNDM-1 gene via outer membrane vesicles. J Antimicrob Chemother. 2017;72:2201–2207. doi: 10.1093/jac/dkx131. [DOI] [PubMed] [Google Scholar]
- 37.Kim SW, Lee JS, Bin PS, et al. The importance of porins and β-lactamase in outer membrane vesicles on the hydrolysis of β-lactam antibiotics. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21082822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO WHO publishes list of bacteria for which new antibiotics are urgently needed. 2017. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed
- 39.Bush K, Bradford PA. Interplay between β-lactamases and new β-lactamase inhibitors. Nat Rev Microbiol. 2019;17:295–306. doi: 10.1038/s41579-019-0159-8. [DOI] [PubMed] [Google Scholar]
- 40.Coleman K. Diazabicyclooctanes (DBOs): a potent new class of non-β-lactam β-lactamase inhibitors. Curr Opin Microbiol. 2011;14:550–555. doi: 10.1016/j.mib.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 41.Ehmann DE, Jahić H, Ross PL, et al. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc Natl Acad Sci USA. 2012;109:11663–11668. doi: 10.1073/pnas.1205073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blizzard TA, Chen H, Kim S, et al. Discovery of MK-7655, a β-lactamase inhibitor for combination with Primaxin®. Bioorganic Med Chem Lett. 2014;24:780–785. doi: 10.1016/j.bmcl.2013.12.101. [DOI] [PubMed] [Google Scholar]
- 43.Hecker SJ, Reddy KR, Totrov M, et al. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs class A serine carbapenemases. J Med Chem. 2015;58:3682–3692. doi: 10.1021/acs.jmedchem.5b00127. [DOI] [PubMed] [Google Scholar]
- 44.Murzin AG, Brenner SE, Hubbard T, Chothia C. SCOP: a structural classification of proteins database for the investigation of sequences and structures. J Mol Biol. 1995;247:536–540. doi: 10.1006/jmbi.1995.0159. [DOI] [PubMed] [Google Scholar]
- 45.Daiyasu H, Osaka K, Ishino Y, Toh H. Expansion of the zinc metallo-hydrolase family of the beta-lactamase fold. FEBS Lett. 2001;503:1–6. doi: 10.1016/s0014-5793(01)02686-2. [DOI] [PubMed] [Google Scholar]
- 46.Liu B, Trout REL, Chu GH, et al. Discovery of taniborbactam (VNRX-5133): a broad-spectrum serine- and metallo-β-lactamase inhibitor for carbapenem-resistant bacterial infections. J Med Chem. 2020;63:2789–2801. doi: 10.1021/acs.jmedchem.9b01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsivkovski R, Totrov M, Lomovskaya O. Biochemical characterization of QPX7728, a new ultrabroad-spectrum beta-lactamase inhibitor of serine and metallo-beta-lactamases. Antimicrob Agents Chemother. 2020;64:e00130–e00150. doi: 10.1128/AAC.00130-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hecker SJ, Reddy KR, Lomovskaya O, et al. Discovery of cyclic boronic acid QPX7728, an ultrabroad-spectrum inhibitor of serine and metallo-β-lactamases. J Med Chem. 2020;63:7491–7507. doi: 10.1021/acs.jmedchem.9b01976. [DOI] [PubMed] [Google Scholar]
- 49.King AM, Reid-Yu SA, Wang W, et al. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature. 2014;510:503–506. doi: 10.1038/nature13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang R, Lai TP, Gao P, et al. Bismuth antimicrobial drugs serve as broad-spectrum metallo-β-lactamase inhibitors. Nat Commun. 2018;9:439. doi: 10.1038/s41467-018-02828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zygiel EM, Nolan EM. transition metal sequestration by the host-defense protein calprotectin. Annu Rev Biochem. 2018;87:621–643. doi: 10.1146/annurev-biochem-062917-012312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol. 2010;14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corbin BD, Seeley EH, Raab A, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:96. doi: 10.1126/science.1152449. 65. [DOI] [PubMed] [Google Scholar]
- 54.Bahr G, Vitor-Horen L, Bethel CR, Bonomo RA, González LJ, Vila AJ. Clinical evolution of New Delhi metallo-β-lactamase (NDM) optimizes resistance under Zn(II) deprivation. Antimicrob Agents Chemother. 2017;62:e01849–e01917. doi: 10.1128/AAC.01849-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng Z, Thomas PW, Ju L, et al. Evolution of New Delhi metallo-β-lactamase (NDM) in the clinic: effects of NDM mutations on stability, zinc affinity, and mono-zinc activity. J Biol Chem. 2018;293:12606–12618. doi: 10.1074/jbc.RA118.003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rotondo CM, Sychantha D, Koteva K, Wright GD. Suppression of β-lactam resistance by Aspergillomarasmine A is influenced by both the metallo-β-lactamase target and the antibiotic partner. Antimicrob Agents Chemother. 2020;64:e01386–e01419. doi: 10.1128/AAC.01386-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lisa M-N, Palacios AR, Aitha M, et al. A general reaction mechanism for carbapenem hydrolysis by mononuclear and binuclear metallo-β-lactamases. Nat Commun. 2017;8:538. doi: 10.1038/s41467-017-00601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palacios AR, Rossi M-A, Mahler GS, Vila AJ. Metallo-β-lactamase inhibitors inspired on snapshots from the catalytic mechanism. Biomolecules. 2020;10:854. doi: 10.3390/biom10060854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hinchliffe P, González MM, Mojica MF, et al. Cross-class metallo-β-lactamase inhibition by bisthiazolidines reveals multiple binding modes. Proc Natl Acad Sci USA. 2016;113:e3745–e3754. doi: 10.1073/pnas.1601368113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jain A, Utley L, Parr TR, Zabawa T, Pucci MJ. Tebipenem, the first oral carbapenem antibiotic. Expert Rev Anti Infect Ther. 2018;16:513–522. doi: 10.1080/14787210.2018.1496821. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi R, Konomi M, Hasegawa K, Morozumi M, Sunakawa K, Ubukata K. In vitro activity of tebipenem, a new oral carbapenem antibiotic, against penicillin-nonsusceptible Streptococcus pneumoniae. Antimicrob Agents Chemother. 2005;49:889–894. doi: 10.1128/AAC.49.3.889-894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nadimpalli ML, Marks SJ, Montealegre MC, et al. Urban informal settlements as hotspots of antimicrobial resistance and the need to curb environmental transmission. Nat Microbiol. 2020;5:787–795. doi: 10.1038/s41564-020-0722-0. [DOI] [PubMed] [Google Scholar]