Abstract
Background
Family support for adults’ diabetes care is associated with improved self-management and outcomes, but healthcare providers lack structured ways to engage those supporters.
Objective
Assess the impact of a patient-supporter diabetes management intervention on supporters’ engagement in patients’ diabetes care, support techniques, and caregiving experience.
Design
Multivariate regression models examined between-group differences in support-related measures observed as part of a larger trial randomizing participants to a dyadic intervention versus usual care.
Participants
A total of 239 adults with type 2 diabetes and either A1c >8% or systolic blood pressure >160mmHg enrolled with a family supporter.
Intervention
Health coaches provided training on positive support techniques and facilitated self-management information sharing and goal-setting.
Main Measures
Patient and supporter reports at baseline and 12 months of supporter roles in diabetes care and caregiving experience.
Results
At 12 months, intervention-assigned patients had higher odds of reporting increased supporter involvement in remembering medical appointments (AOR 2.74, 95% CI 1.44, 5.21), performing home testing (AOR 2.40, 95% CI 1.29, 4.46), accessing online portals (AOR 2.34, 95% CI 1.29, 4.30), deciding when to contact healthcare providers (AOR 2.12, 95% CI 1.15, 3.91), and refilling medications (AOR 2.10, 95% CI 1.14, 3.89), but not with attending medical appointments or with healthy eating and exercise. Intervention-assigned patients reported increased supporter use of autonomy supportive communication (+0.27 points on a 7-point scale, p=0.02) and goal-setting techniques (+0.30 points on a 5-point scale, p=0.01). There were no differences at 12 months in change scores measuring supporter distress about patients’ diabetes or caregiving burden. Intervention-assigned supporters had significantly larger increases in satisfaction with health system support for their role (+0.88 points on a 10-point scale, p=0.01).
Conclusions
A dyadic patient-supporter intervention led to increased family supporter involvement in diabetes self-management and increased use of positive support techniques, without increasing caregiver stress.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-021-06946-8.
KEY WORDS: diabetes, social support, self-management, caregiving
INTRODUCTION
Over half of adults with diabetes have an unpaid family member or friend (“family supporter”) who regularly assists with diabetes management.1 These family supporters help with essential diabetes management tasks including taking medications and testing home blood glucose as well as more general behaviors such as healthy diet and exercise.1,2 Adults with type 2 diabetes who report help with their diabetes care from a support person have improved medication adherence 3–5 and self-monitoring of blood glucose.6,7 Social support also has been linked to improved clinical outcomes, including glycemic, blood pressure, and lipid control.8 In addition to the benefits of day-to-day self-management support, family supporters can also enhance communication between providers and patients at clinic visits9–11 and through online health portals.12
While general social support can be helpful to people with chronic conditions like diabetes, providing support using specific techniques can enhance the positive impact on patient behaviors. Help that is provided in a way that is “autonomy supportive,” in which supporters acknowledge the patient’s perspectives, provide choices, and affirm patient competence, can bolster patients’ intrinsic motivation and engagement in their self-care.13 Autonomy support from informal supporters is associated with improved glycemic control and more frequent self-monitoring of blood glucose in patients with type 2 diabetes,14 and can reduce the negative impact of patients’ diabetes distress on glycemic control.15 In addition to autonomy support, setting goals and making action plans (i.e., collaborative goal-setting) with others can increase trust and improve glycemic control.16
While recent guidelines from the American Diabetes Association on diabetes self-management education explicitly recommend involvement of family supporters,17 most healthcare teams do not have structured approaches to educate and engage supporters in diabetes care. Many family supporters report not having enough information about the health status of their care recipient and management regimen in order to help effectively.18 To test a new approach to educating and engaging family supporters in adults’ diabetes care, we conducted a randomized controlled trial of the Caring Others Increasing Engagement in Patient Aligned Care Teams (CO-IMPACT) program. This program aimed to improve patients’ diabetes self-management and control through a 12-month dyadic intervention that provided both patients and family supporters with information, monitoring, and behavior change tools. Here, we report the impact of the intervention on family supporters’ roles in patient diabetes care, use of specific support techniques, and caregiving experience.
METHODS
Study Design and Setting
A total of 239 adult patients with type 2 diabetes and high risk for diabetes complications as defined below were recruited along with an unpaid family or friend supporter from two primary care sites within the Veterans Health Administration (VA). Patient-supporter dyads were randomized to receive a multifaceted engagement intervention or enhanced usual care over a 12-month study period. Dyads were randomized 1:1 within strata of those cohabitating vs not. Enhanced usual care for patients with diabetes within the VA includes co-management by a nurse or clinical pharmacist with the primary care provider, with individualized referral to diabetes self-management classes, health psychology services, weight loss programs, and automated telehealth to monitor home testing results. No formal program for engaging supporters in patients’ care is available in these sites. In this study, participants in the enhanced usual care group were also given a handbook with general diabetes management information. Pre-specified primary outcomes of the trial were changes in patient activation as measured by the PAM-13 and UKPDS risk score.19 The present study examines the proximal effects of the intervention on family supporter involvement in patients’ diabetes care and caregiving experience. This study was approved by the VA Ann Arbor Institutional Review Board.
Eligibility Criteria
Patient criteria included a diagnosis of type 2 diabetes, regular primary care at the VA, and risk factors for complications including poor glycemic control (A1c >8%) or blood pressure control (mean outpatient systolic blood pressure >150mmHg over prior 9 months, or if one measurement systolic >160mmHg). Family supporters were adults nominated by the patient who talked with the patient about their health at least twice per month and were not paid to care for the patient. Additional details on participant eligibility and study design can be found in the published study protocol.19
Intervention
Patient-supporter dyads in the intervention arm were invited to receive an initial health coach session, biweekly automated diabetes monitoring calls, and primary care appointment preparation calls and visit summaries. The initial session performed by health coaches taught supporters and patients positive support skills and effective strategies to engage with healthcare providers. Health coaches had degrees in health education and received training in basic diabetes management, autonomy supportive communication, and concepts fundamental to patient activation and action-planning. Biweekly automated calls allowed patients to report information about diabetes management by responding to pre-recorded voice prompts. At the end of each automated call, patients received a tailored message with suggestions to address identified issues and make an action plan. Family supporters received structured summaries of the patient’s call responses with suggested supportive actions, for example, offering the clinic phone number to contact the patient’s primary care team. Healthcare providers did not typically review call responses. Visit preparation calls made by coaches to patients prior to primary care visits focused on identifying concerns to discuss at the visit and encouraged family supporter input. After primary care visits, patients and supporters were provided summaries which included vital signs, relevant lab results, medication regimen, and a summary of diabetes-related issues discussed.
Support Measures
Patients and supporters answered surveys at baseline and 12 months upon study completion. Supporter involvement in 12 specific diabetes management tasks was rated by patients on a 5-point Likert scale where 1 = “None of the time” to 5 = “Almost always.” These questions were developed and tested based on prior studies of patients with diabetes and family supporters (see Appendix 1).20,21 Supporters’ use of specific support techniques was rated by patients in the following areas: autonomy supportive communication (Important Other Climate Questionnaire (IOCQ));13 open communication about chronic illness (Couples’ Illness Communication Scale (CICS));22 and collaborative goal-setting and action-planning (adapted Patient Assessment of Chronic Illness Care (PACIC) subscale).23 Family supporters reported their level of distress about the patient’s diabetes (adapted version of the Problem Areas in Diabetes scale (PAID-5),24 previously used in the DAWN-2 study);25 self-efficacy in helping the patient with disease management (adapted Stanford Chronic Disease Self-Efficacy Scale);26 satisfaction with VA support for their role as a caregiver (single item included in Appendix 1); and general caregiving burden (Modified Caregiver Stain Index (MCSI)).27
Patient and Supporter Characteristics
Characteristics measured at baseline via survey included patient and supporter sociodemographics, patient and supporter relationship and cohabitation, patient use of insulin, and supporter diagnosis of diabetes. Baseline HbA1c was collected via venous sample at enrollment.
Analyses
Dyadic data was analyzed according to group assignment at randomization. Descriptive statistics were used to characterize participants at baseline. For analyses of baseline to 12-month change in family supporter involvement in diabetes management roles, increase in support was defined as ≥1 point increase in the 5-point Likert scale response. Given the standard deviation of these measures at baseline, a 1-point change corresponded to an effect size of 0.7–0.9, which is considered a moderate to large effect.28 Separate logistic regression models assessed the effect of the intervention on supporter involvement. Dyads whose supporters were involved at the highest level (5) in a task at baseline were excluded from these models, as they were unable to improve further over the study period. These dyads are included in descriptive statistics summarizing baseline role involvement, and the percent of dyads excluded from each model for this reason is detailed in Appendix 2. Amount of change in support techniques, supporter distress, and supporter confidence was measured via linear difference at baseline to 12-month scale scores. Separate linear regression models assessed the effect of the intervention on each change score, adjusted for the baseline score. If patients or family supporters did not answer the measure for role involvement or support techniques at baseline or month 12, they were excluded from analysis of that outcome (6–11% of dyads per model). The primary independent variable in all models was intervention versus control group assignment. Model covariates, which were predetermined based on theoretical impact on supporter involvement in diabetes care, included patient age, race/ethnicity, cohabitation with family supporter, baseline insulin use, and HbA1c. Sex of patients and family supporters were not included in models as there was little variation. A significance level of 0.05 was used for all tests; correction for multiple comparisons was not performed as these secondary analyses were hypothesis-generating to assess proximal impacts of this intervention on support types and caregiving experience.29
RESULTS
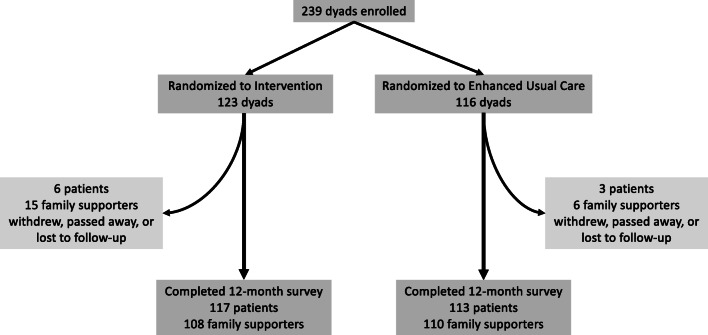
Two hundred and thirty-nine patient-supporter dyads were enrolled in the study; 123 patient-supporter dyads were assigned to the intervention arm and 116 to the control arm (see Fig. 1 for details of dyad attrition). Of the 123 dyads randomized to the intervention, 120 completed the initial coaching session. One-hundred and fourteen patients completed at least one visit preparation call (median of 2 calls completed over the 12-month period). A total of 116 patients completed at least one biweekly automated call with family supporter summary (median of 25 calls completed).
Fig. 1.
Dyad attrition.
Participant Characteristics (Table 1)
Table 1.
Patient and Support Person Baseline Characteristics
| Intervention, N=123 dyads | Control, N=116 dyads | |
|---|---|---|
| Patients | ||
| Age, median6 | 62 (12) | 64 (16.5) |
| Sex = female, n (%) | 6 (4.9%) | 2 (1.7%) |
| White non-Latino(a), n (%) | 88 (72.1%) | 91 (79.1%) |
| College completed, n (%) | 29 (23.6%) | 27 (23.3%) |
| HbA1c, mean6 | 8.4 (1.5) | 8.6 (1.8) |
| Use insulin, n (%) | 78 (63.4%) | 64 (55.2%) |
| Support persons | ||
| Relationship to patient | ||
| Spouse/partner of patient, n (%) | 75 (61.0%) | 70 (60.3%) |
| Friend | 25 (20.33) | 16 (13.79%) |
| Adult child | 9 (7.32%) | 18 (15.52%) |
| Other relative | 14 (11.38%) | 12 (10.34%) |
| Cohabitation with patient, n (%) | 86 (69.9%) | 82 (70.7%) |
| Sex = female, n (%) | 109 (88.6%) | 106 (91.4%) |
| White non-Latino(a), n (%) | 100 (94.3%) | 92 (94.8%) |
| College completed, n (%) | 25 (20.3%) | 30 (25.9%) |
| Diagnosis of diabetes, n (%) | 22 (18.2%) | 24 (20.7%) |
| Support person caregiving experience | ||
| Distress about patient diabetes (adapted PAID-5), mean6; scale range 0–20; higher scores indicate more distress | 6.0 (4.7) | 6.7 (5.1) |
| Self-efficacy for caring for patient’s diabetes (Stanford), mean6; scale range 0–10; higher scores indicate more self-efficacy | 7.6 (1.8) | 7.5 (1.6) |
| Caregiver strain (CSI), mean6, scale range 0–26; higher scores indicate more strain | 3.6 (3.7) | 4.7 (4.4) |
| Satisfaction with VA support for caregivers as reported by support persons, mean6, scale range 1–10; higher scores indicate greater satisfaction | 6.9 (2.9) | 6.7 (3.2) |
As is common in the VA population, patients were primarily male (96.6%) and White, non-Latino (75.5%). Mean age was 64 years and 23.4% completed college. Most (59.4%) used insulin at baseline, and the mean baseline HbA1c was 8.5% (standard deviation 1.6%). Family supporters were predominantly female (90%) and 70.3% lived with the patient. Most family supporters were a spouse/partner (60.7%), followed by friend (17.2%), adult child (11.3%), and other relatives (10.9%). College completion rate was 23.0% for family supporters, and 19.4% of supporters also had a diagnosis of diabetes. Baseline levels of supporter confidence and distress (Table 1) and involvement in diabetes care roles (Table 2) were similar between groups. Mean involvement in diabetes care roles at baseline was typically at level 2 (rarely) and ranged from 1.8 for help navigating the healthcare system to 3.3 for help choosing healthy foods and encouraging exercise.
Table 2.
Baseline Supporter Roles in Patient Diabetes Care and Perceived Quality of Support
| Frequency of supporter involvement (1 never–5 almost always) | Intervention, N=123 dyads mean (SD) | Control, N=116 dyads mean (SD) |
|---|---|---|
| Help remembering to go to appointments | 2.8(1.5) | 3.1 (1.5) |
| Help reviewing home testing results (n=220)* | 2.5 (1.3) | 2.7 (1.4) |
| Help remembering to perform home testing (n=221) | 2.2 (1.3) | 2.6 (1.4) |
| Access patient’s online portal, n (%)† | 20 (16.3%) | 20 (17.2%) |
| Help deciding when to contact healthcare providers about concerns (n=238) | 2.8 (1.4) | 3.0 (1.5) |
| Help remembering to refill medications | 2.2 (1.3) | 2.5 (1.5) |
| Help remembering to take medications | 2.6 (1.4) | 2.9 (1.5) |
| Coming into room for patient’s medical appointments (n=238) | 2.3 (1.6) | 2.2 (1.7) |
| Help with choosing healthy foods | 3.3 (1.3) | 3.4 (1.2) |
| Help navigating healthcare system (n=238) | 1.8 (1.1) | 2.0 (1.3) |
| Help with encouragement to exercise (n=238) | 3.3 (1.3) | 3.4 (1.4) |
| Use of secure messaging/email to contact patient’s providers, n (%)† | 6 (4.8%) | 5 (4.4%) |
| Perceived support quality | ||
| Autonomy supportive communication (IOCQ), scale range 0–7; higher scores indicate greater autonomy support | 5.5 (1.0) | 5.3 (1.1) |
| Discuss health goal-setting (adapted PACIC subscale), scale range 0–5; higher scores indicate more goal-setting | 2.4 (1.0) | 2.2 (1.0) |
| Open communication about chronic illness (CICS), scale range 0–20 (n=234); higher scores indicate more communication | 16.5 (2.9) | 16.0(2.8) |
*n = number of patients with complete data for each measure. Patients who answered that the role was not applicable to them (e.g., they did not do home testing) or opted not to answer the question are not included
†Item ratings provided by the support person, all others provided by the patient
Intervention Effect on Support Roles (Table 3)
Table 3.
Adjusted Intervention Effect on Odds of Increase in Frequency of Support Role from Baseline to 12 Months (Intervention vs. Control Group)
| Support role | AOR* (95% CI) |
|---|---|
| Help remembering to go to appointments (n=177)† | 2.74 (1.44, 5.21) |
| Help reviewing home testing results (n=174) | 2.67 (1.43, 5.01) |
| Help remembering to perform home testing (n=180) | 2.40 (1.29, 4.46) |
| Access patient’s online portal (n=213) | 2.34 (1.29, 4.30) |
| Help deciding when to contact healthcare providers about concerns (n=181) | 2.12 (1.15, 3.91) |
| Help remembering to refill medications (n=193) | 2.10 (1.14, 3.89) |
| Help remembering to take medications (n=178) | 1.92 (1.03, 3.59) |
| Coming into patient’s medical appointments (n=178) | 1.75 (0.86, 3.56) |
| Help with choosing healthy foods (n=173) | 1.62 (0.88, 2.97) |
| Help navigating healthcare system (n=206) | 1.49 (0.84, 2.63) |
| Help with encouragement to exercise (n=161) | 1.18 (0.63, 2.24) |
| Use of secure messaging/email to contact patient’s providers (n=214) | 1.11 (0.35, 3.49) |
*Odds Ratio for intervention group vs. control group from logistic regression adjusted for patient’s age, race, baseline insulin use and baseline HbA1c, and cohabitation with family supporter for outcome of change ≥ 1 point on scale of 1 = none of the time 5 = almost always
†n = number of patients with complete data included in each model. Patients who answered that the role was not applicable to them or started at highest level of support (5/5) were not included in the model
Bold entries are results where p < 0.05
In multivariate regression models, supporters in the intervention group were significantly more likely than controls to increase their involvement in several areas of self-management assistance: deciding when to contact healthcare providers about concerns (AOR 2.12, 95% CI 1.15, 3.91), remembering to go to medical appointments (AOR 2.74, 95% CI 1.44, 5.21), remembering to take (AOR 1.92, 95% CI 1.03, 3.59), refilling medications (AOR 2.10, 95% CI 1.14, 3.89), performing (AOR 2.40, 95% CI 1.29, 4.46) and reviewing (AOR 2.67, 95% CI 1.43, 5.01) home testing results. Family supporters in the intervention group were also more likely to report increased frequency of using the VA online patient portal to view patients’ health information (AOR 2.34, 95% CI 1.29, 4.30) compared to those in the control group. There was no significant association between intervention group and supporter use of email or secure messaging to contact patients’ healthcare providers. There also was no effect of intervention assignment on family supporter assistance with navigating the healthcare system, accompanying patients into exam rooms during clinic appointments, or encouraging healthy eating and exercise .
Change in Support Techniques (Table 4)
Table 4.
Adjusted Intervention Effect on Changes in Perceived Support Quality and Support Person Caregiving Experience from Baseline to 12 Months
| Perceived support quality | Intervention effect* on change over 12 months (95% CI) | p value |
|---|---|---|
| Autonomy supportive communication (IOCQ) (n=221)† | 0.27 (0.05, 0.49) | 0.02 |
| Discuss health goal-setting (adapted PACIC subscale) (n=221) | 0.30 (0.07, 0.52) | 0.01 |
| Open communication about chronic illness (CICS) (n=221) | 0.00 (− 0.58, 0.58) | 0.99 |
| Support person caregiving experience | ||
| Distress about patient diabetes (adapted PAID-5) (n=213) | 0.28 (− 0.65, 1.22) | 0.55 |
| Self-efficacy for caring for patient’s diabetes (Stanford) (n=216) | − 0.02 (− 0.46, 0.42) | 0.92 |
| Caregiver strain (CSI) (n=214) | 0.48 (− 0.38, 1.33) | 0.27 |
| Satisfaction with VA support for caregivers as reported by support persons (n=180) | 0.93 (0.30, 1.55) | 0.01 |
*Coefficient from intervention vs. control group predictor from linear regression models adjusted for patient age, race, baseline insulin use and baseline HbA1c, cohabitation with family supporter, and the baseline score for each respective measure
†n, number of patients with complete data included in each model
Bold entries are results where p < 0.05
In multivariate regression models, intervention assignment was associated with increased autonomy support (additional increase of 0.27 points (p=0.02) in IOCQ score on a 7-point scale) from baseline to month 12 compared to the control group. Intervention assignment was also associated with an increase in collaborative goal-setting (relative increase of 0.30 points (p=0.01) in adapted PACIC score on a 5-point scale) compared to the control group. Intervention assignment was not associated with change in open communication about chronic illness (CICS).
Change in Supporter Caregiving Experience (Table 4)
Intervention assignment was associated with an adjusted relative increase in supporter satisfaction with VA support for their role (+0.88 points on a 10-point scale, p=0.01). There were no significant differences between study arms in change in supporter self-efficacy (p=0.92) or diabetes distress (p=0.55) associated with helping the patient, or overall caregiver strain (p=0.27).
DISCUSSION
With increased recognition of the potential benefits of involving family supporters in diabetes care, finding effective strategies to increase family supporters’ involvement in diabetes management and increasing supporters’ use of evidence-based communication strategies are a key area of focus for diabetes practitioners. We found that a program that provided supporters with training in effective communication for health behavior change, as well as updates on patient self-management concerns and summaries of health information after primary care visits, increased supporters’ active involvement in self-management assistance without negative impacts on supporter distress or strain. This is particularly notable in that the intervention was compared to usual care within a high-resource, well-established primary care medical home setting. As a consequence, the intervention may have even greater impact in other settings with less comprehensive routine care.
Interventions which specifically aim to educate family supporters in self-management assistance have been shown to improve diabetes outcomes.30 A recent evaluation of an intervention focused on goal-setting for health behavior change through phone coaching with text message follow-up for adults with type 2 diabetes and their informal supporters showed that the intervention improved a composite measure of supporter involvement.31 Another study examining the impact of one training session on motivational coaching for patients with type 2 diabetes and supporters followed by automatic sharing of home blood glucose monitoring results with informal supporters demonstrated increased support for blood glucose testing.32 Our study builds on this evidence that brief training of family supporters to promote positive support techniques can increase self-management assistance for patients with type 2 diabetes. In addition, our study provides novel insight into changes in supporter involvement in specific diabetes self-management roles and support qualities.
In this intervention, supporter involvement increased more for self-management tasks that were diabetes-specific (such as taking diabetes medications and checking glucose levels) than for general lifestyle behaviors like healthy eating and exercise. However, supporters had high levels of involvement in lifestyle behaviors at baseline, so the intervention had less potential to increase supporter help with these behaviors. Determining how best to increase effective family involvement in diabetes-specific tasks is particularly important, because prior observational studies have shown that naturally occurring social support for diabetes-specific tasks is not associated with improvements in patient adherence to those behaviors.33 One possible way an intervention can enhance support for self-management tasks is by improving supporter knowledge and confidence in their ability to provide help in these areas, as has been reported among caregivers of patients with heart failure.34 In a study of informal caregiving of adults with heart failure, supporters who received summaries of the self-management problems reported by their care recipients, with education and guidance on how to help, reported increased confidence in their caregiving ability and had increased involvement in disease-specific roles including medication adherence.35,36 Future family interventions for adults with diabetes can focus on increasing supporters’ disease-specific knowledge and involvement in skilled diabetes tasks.
The CO-IMPACT intervention aimed to change not just how much support was provided but how it was provided. In this study, we found significant increases relative to controls in supporter use of autonomy supportive communication and collaborative goal-setting. Importantly, this intervention resulted in increased supporter use of positive techniques after only one in-person training session, suggesting it may be feasible to incorporate effective training on these techniques into diabetes self-management education sessions or healthcare visits. In addition, summaries of self-management concerns identified in automated calls were designed to encourage goal-setting and action-planning between patients and supporters. Prior studies achieving similar changes in these support techniques (e.g., IOCQ and PACIC scores) to those reported in this study have yielded changes in self-management behavior and clinical outcomes. 14,37–39 Therefore, even if supporters provide the same level of support over time, their attempts at helping may be more effective with increased use of these positive support techniques.
Finally, it is important that, despite increased involvement in care recipients’ diabetes management, this intervention did not result in greater diabetes distress or caregiving strain among family supporters. We did not find significant changes in supporter self-efficacy, possibly as a result of high ratings at baseline in both study arms. However, the intervention was associated with increased satisfaction with the health system support for family supporters’ roles, which may be due to meeting supporters’ desire for more information about care recipients’ medical regimen and health status, and expressly involving supporters in preparing for and following up on medical visits. This information and involvement could, in turn, increase caregivers’ confidence and knowledge about how to support the patient.
Limitations
Participants in this study were predominantly male Veterans, and supporters were mostly female. In the general population, women are more likely to serve in the caregiving role than men,40 but results could be different for female care recipients. There was also limited racial, ethnic, and geographic diversity in our sample, which may limit generalizability of results. Because analyses presented here analyzed outcomes according to group assignment, we are unable to evaluate the relative impact on supporter engagement of various levels of intervention involvement.
CONCLUSIONS
This study has important implications for healthcare providers aiming to improve support for their adult patients who are actively managing diabetes. It is possible to improve family level and type of involvement in key aspects of diabetes care. Brief coaching on positive support techniques can be used during healthcare visits or diabetes education sessions. The bulk of this intervention, which included supporters in diabetes monitoring and medical visit information sharing, was carried out remotely over telephone and email, and thus could be delivered to patients and family in rural areas or during restrictions on in-person encounters as have been experienced during the COVID-19 pandemic.
In conclusion, the CO-IMPACT patient and family supporter engagement intervention increased family supporter involvement in several diabetes management behaviors and increased use of positive support techniques, without increasing supporter stress. Approaches to chronic disease management, such as the primary care medical home and diabetes self-management education, can incorporate these mechanisms to engage informal family supporters to maximize benefits from this underutilized and potentially powerful resource to promote self-management behaviors and improve outcomes for patients.
Supplementary Information
(PDF 366 kb)
Acknowledgements
Contributors
None.
Funders
This work was supported by the Veteran’s Health Administration Health Services Research and Development IIR 14–074-1, the NIDDK T32 Research Training in Diabetes and Endocrinology to the University of Pittsburgh 5T32DK007052-45, and the Michigan Center for Diabetes Translational Research (NIH Grant 5P60-DK09292).
Declarations
Conflict of Interest
As noted in the ICJME forms, Dr. Zupa receives salary support through a T32 Training Grant from the NIDDK. Drs. Heisler, Piette, and Rosland received support for this work through a grant from the Michigan Center for Diabetes Translational Research as listed above.
Footnotes
Prior Presentation: An earlier, limited version of these findings was presented as a virtual poster at the 80th Scientific Sessions of the American Diabetes Association, June 12–16, 2020
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosland A-M, Heisler M, Choi H-J, Silveira MJ, Piette JD. Family influences on self-management among functionally independent adults with diabetes or heart failure: do family members hinder as much as they help? Chronic Illness. 2010;6(1):22-33. [DOI] [PMC free article] [PubMed]
- 2.Silliman RA, Bhatti S, Khan A, Dukes KA, Sullivan LM. The care of older persons with diabetes mellitus: families and primary care physicians. Journal of the American Geriatrics Society. 1996;44(11):1314-21. [DOI] [PubMed]
- 3.DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health psychology. 2004;23(2):207-18. [DOI] [PubMed]
- 4.Bouldin ED, Trivedi RB, Reiber GE, et al. Associations between having an informal caregiver, social support, and self-care among low-income adults with poorly controlled diabetes. Chronic illness. 2017;13(4):239–50. doi: 10.1177/1742395317690032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller TA, DiMatteo MR. Importance of family/social support and impact on adherence to diabetic therapy. Diabetes, metabolic syndrome and obesity: targets and therapy. 2013;6:421–26. doi: 10.2147/DMSO.S36368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pamungkas RA, Chamroonsawasdi K, Vatanasomboon P. A systematic review: family support integrated with diabetes self-management among uncontrolled type II diabetes mellitus patients. Behavioral Sciences. 2017;7(3):62–79. doi: 10.3390/bs7030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Y, Nam S, Park S, Shin I-s, Ku BJ. The impact of social support on self-care of patients with diabetes: what is the effect of diabetes type? Systematic review and meta-analysis. The Diabetes Educator. 2017;43(4):396–412. doi: 10.1177/0145721717712457. [DOI] [PubMed] [Google Scholar]
- 8.Strom JL, Egede LE. The impact of social support on outcomes in adult patients with type 2 diabetes: a systematic review. Current Diabetes Reports. 2012;12(6):769–81. doi: 10.1007/s11892-012-0317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff JL, Guan Y, Boyd CM, et al. Examining the context and helpfulness of family companion contributions to older adults’ primary care visits. Patient education and counseling. 2017;100(3):487–94. doi: 10.1016/j.pec.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolff JL, Roter DL. Hidden in plain sight: medical visit companions as a resource for vulnerable older adults. Archives of Internal Medicine. 2008;168(13):1409–1415. doi: 10.1001/archinte.168.13.1409. [DOI] [PubMed] [Google Scholar]
- 11.Rosland A-M, Piette JD, Choi H, Heisler M. Family and friend participation in primary care visits of patients with diabetes or heart failure: patient and physician determinants and experiences. Medical care. 2011;49(1):37–45. doi: 10.1097/MLR.0b013e3181f37d28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed ME, Huang J, Brand R, et al. Communicating through a patient portal to engage family care partners. JAMA Internal Medicine. 2018;178(1):142–44. doi: 10.1001/jamainternmed.2017.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams GC, Lynch MF, McGregor HA, Ryan RM, Sharp D, Deci EL. Validation of the “Important Other” Climate Questionnaire: assessing autonomy support for health-related change. Families, Systems, & Health. 2006;24(2):179–94. doi: 10.1037/1091-7527.24.2.179. [DOI] [Google Scholar]
- 14.Lee AA, Piette JD, Heisler M, Janevic MR, Rosland A-M. Diabetes self-management and glycemic control: the role of autonomy support from informal health supporters. Health psychology. 2019;38(2):122–32. doi: 10.1037/hea0000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee AA, Piette JD, Heisler M, Rosland A-M. Diabetes distress and glycemic control: the buffering effect of autonomy support from important family members and friends. Diabetes care. 2018;41(6):1157–63. doi: 10.2337/dc17-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lafata JE, Morris HL, Dobie E, Heisler M, Werner RM, Dumenci L. Patient-reported use of collaborative goal setting and glycemic control among patients with diabetes. Patient education and counseling. 2013;92(1):94–99. doi: 10.1016/j.pec.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powers MA, Bardsley JK, Cypress M, et al. Diabetes self-management education and support in adults with type 2 diabetes: a consensus report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. The Science of Diabetes Self-Management and Care. 2021;47(1):54–73. doi: 10.1177/0145721720987936. [DOI] [PubMed] [Google Scholar]
- 18.Lee AA, Piette JD, Heisler M, Janevic MR, Langa KM, Rosland A-M. Family members’ experiences supporting adults with chronic illness: A national survey. Families, Systems, & Health. 2017;35(4):463–73. doi: 10.1037/fsh0000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosland A-M, Piette JD, Trivedi R, et al. Engaging family supporters of adult patients with diabetes to improve clinical and patient-centered outcomes: study protocol for a randomized controlled trial. Trials. 2018;19(1):394–410. doi: 10.1186/s13063-018-2785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosland A-M, Heisler M, Trivedi R, Gaudioso S, Fennelly J, Piette J. Improving diabetes management by engaging family supporters in the patient-centered medical home: a pilot intervention study. [Conference presentation abstract]. Academy Health Annual Research Meeting, Minneapolis, MN. June 15, 2015. https://academyhealth.confex.com/academyhealth/2015arm/meetingapp.cgi/Paper/2810.
- 21.Janevic MR, Piette JD, Ratz DP, Kim HM, Rosland AM. Correlates of family involvement before and during medical visits among older adults with high-risk diabetes. Diabetic Medicine. 2016;33(8):1140–48. doi: 10.1111/dme.13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arden-Close E, Moss-Morris R, Dennison L, Bayne L, Gidron Y. The Couples’ Illness Communication Scale (CICS): development and evaluation of a brief measure assessing illness-related couple communication. British Journal of Health Psychology. 2010;15(3):543–59. doi: 10.1348/135910709X476972. [DOI] [PubMed] [Google Scholar]
- 23.Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the patient assessment of chronic illness care (PACIC). Medical care. 2005:436-44. [DOI] [PubMed]
- 24.McGuire B, Morrison T, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53(1):66–9. doi: 10.1007/s00125-009-1559-5. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs Burns K, Nicolucci A, Holt RIG, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2™): cross-national benchmarking indicators for family members living with people with diabetes. Diabetic Medicine. 2013;30(7):778–88. doi: 10.1111/dme.12239. [DOI] [PubMed] [Google Scholar]
- 26.Lorig K, Stewart A, Ritter P, Lynch J, Gonzalez V, Laurent D.Outcome measures for health education and other health care interventions. Sage; 1996.
- 27.Sullivan MT. Caregiver strain index (CSI) Home Healthcare Now. 2003;21(3):197–198. doi: 10.1097/00004045-200303000-00024. [DOI] [PubMed] [Google Scholar]
- 28.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Medical Care. 1989;27(3):S178–89. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 29.Althouse AD. Adjust for multiple comparisons? It’s not that simple. The Annals of thoracic surgery. 2016;101(5):1644–45. doi: 10.1016/j.athoracsur.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Baig AA, Benitez A, Quinn MT, Burnet DL. Family interventions to improve diabetes outcomes for adults. Annals of the New York Academy of Sciences. 2015;1353(1):89–112. doi: 10.1111/nyas.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayberry LS, Berg CA, Greevy RA, et al. Mixed-Methods Randomized Evaluation of FAMS: a Mobile Phone-Delivered Intervention to Improve Family/Friend Involvement in Adults’ Type 2 Diabetes Self-Care. Annals of Behavioral Medicine. 2020. 10.1093/abm/kaaa041 [DOI] [PMC free article] [PubMed]
- 32.Roblin DW. The potential of cellular technology to mediate social networks for support of chronic disease self-management. Journal of health communication. 2011;16(sup1):59–76. doi: 10.1080/10810730.2011.596610. [DOI] [PubMed] [Google Scholar]
- 33.Rosland AM, Piette JD, Lyles CR, et al. Social support and lifestyle vs. medical diabetes self-management in the diabetes study of Northern California (DISTANCE) Ann Behav Med. 2014;48(3):438–47. doi: 10.1007/s12160-014-9623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trivedi RB, Slightam C, Nevedal A, et al. Comparing the barriers and facilitators of heart failure management as perceived by patients, caregivers, and clinical providers. Journal of Cardiovascular Nursing. 2019;34(5):399–409. doi: 10.1097/JCN.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 35.Piette JD, Striplin D, Marinec N, Chen J, Aikens JE. A randomized trial of mobile health support for heart failure patients and their informal caregivers: impacts on caregiver-reported outcomes. Medical care. 2015;53(8):692–99. doi: 10.1097/MLR.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piette JD, Striplin D, Marinec N, et al. A mobile health intervention supporting heart failure patients and their informal caregivers: a randomized comparative effectiveness trial. Journal of medical Internet research. 2015;17(6):e142. doi: 10.2196/jmir.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorin AA, Powers TA, Koestner R, Wing RR, Raynor HA. Autonomy support, self-regulation, and weight loss. Health Psychology. 2014;33(4):332–9. doi: 10.1037/a0032586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baron CE, Smith TW, Baucom BR, et al. Relationship partner social behavior and continuous positive airway pressure adherence: the role of autonomy support. In: American Psychological Association; 2020. pp. 325–34. [DOI] [PubMed] [Google Scholar]
- 39.Vermunt NPCA, Harmsen M, Westert GP, Olde Rikkert MGM, Faber MJ. Collaborative goal setting with elderly patients with chronic disease or multimorbidity: a systematic review. BMC Geriatrics. 2017;17(1):167–79. doi: 10.1186/s12877-017-0534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.AARP and National Alliance for Caregiving. Caregiving in the United States 2020. Washington, DC: AARP. May 2020. 10.26419/ppi.00103.001.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 366 kb)