Abstract
We describe the synthesis and characterization of a new class of oligomers built from a terphenyl-based amino acid. These oligomeric amides are of interest because the adoption of specific conformations could potentially be driven by coordinated formation of inter-residue hydrogen bonds and aromatic interactions. Although high-resolution structural data have proven inaccessible, circular dichroism and nuclear magnetic resonance studies suggest that the new oligomers fold concomitantly with discrete self-association in chloroform.
Helices are the most common form of secondary structure in folded proteins, and helical motifs dominate higher-order structure in nucleic acids. This prevalence has motivated many efforts to identify non-natural oligomers that adopt helical conformations.1–5 Synthetic, helix-forming foldamers have tended to rely on amide connections between subunits,6–8 although alternative linkages have been explored.9 In most systems the backbone linkage contains both an H-bond donor and an H-bond acceptor, as exemplified by secondary carboxamides, but backbones that lack H-bond donors7,10,11 or that lack H-bonding groups altogether12–16 have also been evaluated.
Two classes of helix-forming foldamers can be identified among those with inter-subunit linkages that offer H-bond donor and acceptor sites (secondary amides or ureas). One class contains sp3 atoms within the subunits, while the other class contains exclusively sp2 atoms within the subunits (aromatic oligo-amides).1–3,5,17–19 In the α-helix, which can be seen as the prototype of the sp3 class, H-bonds occur between C=O and N-H groups in adjacent turns. Comparable inter-turn H-bond connectivity is seen in foldamers that contain β-, γ- and δ-amino acid residues with saturated backbones.20–23 In contrast, aromatic oligo-amides display intra-turn H-bonds.17 In these systems, stacking of aromatic surfaces, rather than H-bonding, represents the dominant interaction between adjacent helical turns. Huc et al. have explored oligomers with alternation of α-amino acid residues and aromatic amino acid residues;24 the results suggest that inter-turn stacking and inter-turn H-bonding may compete with rather than reinforce one another in these systems.25 β-Peptides and oligoureas assemble into helix bundles.18,20,26–29 The multiple non-covalent attractions possible in synthetic foldamer helices (H-bonds, aromatic stacking) open the door to diverse modes of intermolecular interaction.25,30–34
Here we explore a new approach to combining sp2 and sp3 carbons within an oligomeric backbone in a search for novel conformational behavior. The amino acid we employ contains a single sp3 carbon that is attached to an ortho-terphenyl unit (Fig. 1). Most prior studies of aromatic oligo-amide foldamers have featured subunits engineered to favor planarity, based on fusion of aromatic rings and/or formation of small-ring H-bonds.2,17,35 In contrast, ortho-terphenyl must deviate from planarity despite containing exclusively sp2 carbon atoms to minimize internal repulsions.36 We hypothesized that if the new oligomers adopt helical conformations, then rotation about arylaryl bonds might allow the ortho-terphenyl units to modulate their shape to optimize non-covalent attractions between adjacent turns, potentially allowing both H-bonds and aromatic-aromatic interactions. Although we have not been able to obtain high-resolution structural insight for the new oligomers, data from circular dichroism (CD) and nuclear magnetic resonance (NMR) measurements in nonpolar solvents suggest that these oligomers can fold concomitantly with self-association in a process driven by H-bonding.
Figure 1.

Structure of terphenyl oligomers.
Synthesis of the new o-terphenyl-based amino acid (Tph) necessary for the oligo-amide series shown in Figure 1 involved successive Suzuki couplings (Scheme S1). Homochiral Tph oligomers were prepared via standard solution-phase coupling reactions involving N-Boc derivatives (Scheme S2). We use the notation R-Tph-N for these oligomers, where N refers to the number of Tph subunits.
CD provided evidence that a discrete conformation is adopted by longer R-Tph oligomers dissolved in chloroform at room temperature. Figure 2 shows CD data in terms of mean residue ellipticity (MRE), which normalizes the CD intensities for number of Tph subunits and concentration. At 0.1 mM and 25 °C, R-Tph-6, R-Tph-8, and R-Tph-10 display a distinctive CD signature, with a strong minimum at 258 nm and a strong maximum at 299 nm that has a shoulder at lower wavelength (Fig. 2A). The signature becomes more intense as the R-Tph oligomer is lengthened. Under these conditions, R-Tph-2 and R-Tph-4 display a weak CD signature with a different pattern, including a minimum at 282 nm and a maximum at 247 nm.
Figure 2.
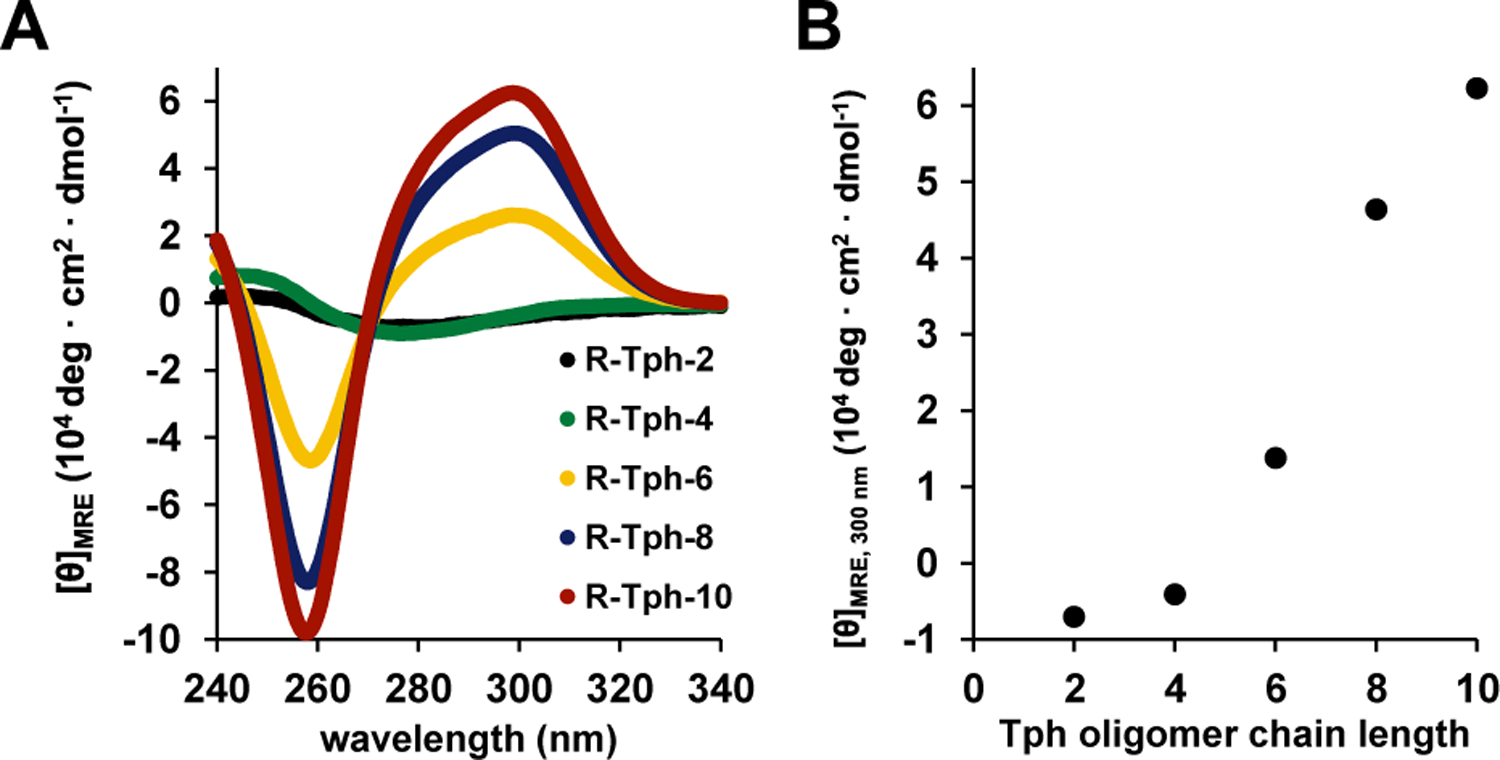
Circular dichroism of terphenyl oligomers (0.1 mM, 25 °C, CDCl3). (A) Full spectra of different chain lengths (B) Length-dependent cooperativity of folding. Ellipticity was converted to mean residue ellipticity (MRE) to account for different oligomer lengths.
The CD signature of R-Tph-8 maintains the characteristic shape but loses intensity slightly when the sample is diluted from 0.1 mM to 0.03 mM (Fig. S1). The concentration-dependence of mean residue ellipticity observed for R-Tph-8 indicates that the phenomenon giving rise to the strong CD signal requires intermolecular association. The strong CD signatures in Fig. 2A indicate that the longer Tph oligomers adopt a specific conformation (or possibly a set of conformations) in the associated state. The fact that CD signatures become more intense with length suggests that increasing Tph oligomer length leads to more avid self-association.
Comparing MRE values among 0.1 mM samples (Fig. 2B) indicates that the folded/self-associated state displays length-dependent cooperativity,37 i.e., that this state becomes more highly populated (more stable) as the number of subunits increases. Such cooperativity is evident for α-helix formation38 and for helical β-peptides39 and meta-phenylacetylene oligomers.13
If the self-association of Tph oligomers involves enthalpically favorable intermolecular contacts, such as H-bonds or aromatic-aromatic interactions, then lower temperatures should encourage association. Indeed, at −10 °C, the CD signature of 0.1 mM R-Tph-4 resembles those of the longer oligomers at 0.1 mM and 25 °C (Fig. 3A) in terms of shape, although the intensity is much lower than observed for longer oligomers. As the temperature is raised, the CD spectrum gradually evolves into the different signature noted above for R-Tph-4 at room temperature (Fig. 2A). The presence of an isodichroic point at 271 nm suggests that this change in CD signature corresponds to a two-state transformation at the molecular level. The CD signature of 0.1 mM R-Tph-4 at 25 °C is observed for 1 mM R-Tph-2 at −10 °C (not shown), leading us to conclude that this signature represents an unfolded and monomeric state of R-Tph oligomers in chloroform. Higher temperatures are required to induce unfolding/dissociation of longer oligomers at a given concentration (Fig. 3B), and this unfolding/dissociation is reversible (Fig. S4).
Figure 3.

Circular dichroism of terphenyl oligomers at 0.1 mM in CDCl3. (A) R-Tph-4 from −10 to 90 °C; note that the vertical scale matches Fig. 2A. (B) Rate of thermal denaturation of terphenyl oligomers. Ellipticity was converted to mean residue ellipticity (MRE) to account for different oligomer lengths.
Fig. 3B shows the effect of temperature on MRE at 300 nm for four Tph oligomers. Collectively, these data suggest that the fully unfolded/dissociated state for these oligomers is favored by elevated temperatures. R-Tph-4 is fully unfolded/dissociated over most of the temperature range, while R-Tph-10 retains at least some structure even at the highest temperature. The data for the three longer oligomers suggest that two thermally driven structural transitions occur over the accessible temperature range, with MRE more sensitive to temperature variation at higher temperatures. Variable-temperature CD data for R-Tph-8 are indistinguishable for 0.1 mM and 0.05 mM samples, but further dilution to 0.03 mM shows a slight diminution in normalized intensity at lower temperatures (Fig. S5). This observation suggests that self-association of R-Tph-8 is maximal at 0.05 mM in this temperature range and begins to diminish upon dilution to 0.03 mM.
Addition of methanol to a chloroform solution of R-Tph-8 suppresses the CD signature characteristic of the folded/associated state (Fig. 4A). As the methanol proportion is increased incrementally to 50 vol %, the CD spectrum evolves toward the weak signature we have assigned to the unfolded/dissociated state. The presence of an isodichroic point at 271 nm in the variable-methanol CD series suggests that methanol-induced denaturation is a two-state process. The effect of added methanol, a strong H-bond donor and acceptor, suggests that the folded/associated state of Tph oligomers in chloroform is stabilized by H-bonds formed between backbone amide groups; the H-bonds could be intramolecular, intermolecular or both.
Figure 4.

(A) Methanol-dependent denaturation of R-Tph-8 shown by circular dichroism (0.1 mM, 25 °C). Data are shown as percent methanol in CDCl3. Ellipticity was converted to mean residue ellipticity (MRE). Aromatic and amide N-H region of 1H-NMR spectra of oligomers (10 mM, room temperature, 500 MHz, CDCl3).
We turned to NMR)spectroscopy to gain further insights on the folding/association behavior of R-Tph oligomers. Comparison of the 1H NMR spectra of R-Tph oligomers at 10 mM in chloroform (room temperature) shows that resonances are sharp for R-Tph-2, slightly broadened for R-Tph-4 and quite broad for the longer oligomers (Fig. 4B). For R-Tph-6, R-Tph-8 and R-Tph-10, the envelope of aromatic resonances is shifted upfield relative to the envelope for R-Tph-2 and R-Tph-4. Particularly noteworthy is the appearance of resonances between 6.3 and 6.8 ppm for these longer oligomers. These upfield NMR signals suggest that aromatic groups from different Tph residues are near one another and cause shielding of some ring protons, as might be expected upon folding and/or intermolecular association. Broad resonances above 8.5 ppm are observed for only the longer three Tph oligomers in chloroform. Two-dimensional NMR experiments (COSY and HSQC) revealed that the hydrogen atoms responsible for these broad, downfield resonances couple to the CH signals at 5.28 ppm and are not bonded to carbon (Fig. S19). These hydrogen atoms can be fully exchanged with deuterium (Fig. S15). We assign these broad resonances to amide NH units engaged in H-bonds. The 1H NMR spectrum of 10 mM R-Tph-4 shares some features evident in 1H NMR spectra of the longer oligomers, which suggests that R-Tph-4 may be partially folded/associated under these conditions.
In DMSO, a solvent that is a strong H-bond acceptor, the 1H NMR spectra of all five Tph oligomers are nearly identical, with sharp aromatic resonances clustered between 6.8 and 7.8 ppm (Fig. S17). These sharp aromatic resonances, present at consistent chemical shifts regardless of oligomer length, suggest that all oligomers are unassociated and unfolded in DMSO, supporting the hypothesis that H-bonds between and/or within terphenyl oligomers are necessary for the folding/association detected in chloroform.
To probe the self-association of the Tph oligomers, we conducted diffusion NMR studies at room temperature in CDCl3. Diffusion coefficients were measured for R-Tph-2, R-Tph-4 and R-Tph-8 at different concentrations and normalized to an internal standard (Table S1). The normalized diffusion coefficient of R-Tph-2 did not vary significantly between 0.1 and 50 mM, indicating that the size of the species does not change over this concentration range. Based on this result and CD data discussed above, we conclude that R-Tph-2 is unassociated throughout this concentration range. The normalized diffusion coefficient of R-Tph-8 did not vary significantly between 0.03 and 5 mM. Based on this result and CD data discussed above, we conclude that R-Tph-8 is fully folded/associated throughout this concentration range. The relative diffusion coefficient of R-Tph-4 varied significantly between 0.1 and 50 mM, and we conclude that the folding/association state of R-Tph-4 changes substantially over this concentration range, which is consistent with variations in the NMR spectrum of R-Tph-4 over this range (Fig. S24).
Based on CD data and NMR chemical shift data, we conclude that R-Tph-4 is monomeric at 0.1 mM in chloroform at room temperature. This conclusion can be used to estimate the approximate particle volume of R-Tph-4 at higher concentrations, based on normalized diffusion coefficients (SI). This analysis suggests that volume increases, relative to the species at 0.1 mM, by approximately 1.2-fold at 1 mM, 1.4-fold at 10 mM and 2.4-fold at 50 mM. The non-integer values of these volumes suggest that equilibration between or among the structured and unstructured states is rapid relative to the NMR timescale. Moreover, these data indicate that the self-association involves a small number of molecules and may lead to a discrete oligomeric stoichiometry. This conclusion is supported by the invariant diffusion coefficient measured for R-Tph-8 over a ~170-fold change in concentration in chloroform. Dynamic light-scattering measurements of R-Tph-8 at 10 mM in CHCl3 suggested a single species with a radius of 1.6 nm and polydispersity of 0.2 nm, consistent with an oligomeric stoichiometry (See SI).
To explore the hypothesis that R-Tph-8 does not form stable helical structures as a single molecule, we investigated the conformational behavior of an isolated R-Tph-8 molecule in chloroform through a series of enhanced sampling molecular dynamics simulations between 200 K and 350 K (details in SI). The simulation results were consistent with the hypothesis that an isolated R-Tph-8 molecule does not adopt a fully helical conformation in chloroform. Our simulations focused exclusively on isolated molecules, and they therefore cannot provide insight on possible self-association.
Our data suggest that homochiral oligomers of a new terphenyl-derived amino acid can undergo a coordinated folding and self-association process in a nonpolar solvent. The folded/associated state appears to be stabilized by H-bonds between backbone amide groups, intramolecular, intermolecular or both. Because all subunits are identical in the Tph oligomers, we propose that the folded state is helical, which would allow each subunit to adopt a similar local conformation. Future work will be directed toward elucidating the folded/associated structure at high resolution and computationally investigating the stability of self-associated structures.
Supplementary Material
ACKNOWLEDGMENT
This research was supported in part by the US National Science Foundation (CHE-1904940). NMR spectrometers were supported by NIH grant S10 OD012245, NSF grant CHE-1048642, and a generous gift from Paul J. and Margaret M. Bender. Mass spectrometers were supported by NIH grant 1S10 OD020022 and a generous gift from Paul J. and Margaret M. Bender. CD spectrometers were supported by NIH grant R01GM061238. Dynamic light-scattering data were obtained at the University of Wisconsin–Madison Biophysics Instrumentation Facility, which was established with support from the University of Wisconsin – Madison and grants BIR-9512577 (NSF) and S10 RR13790 (NIH). TLF acknowledges support from a GAANN fellowship. Computational work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering (MSE) Division, under Award Number DE-SC0018651 (computational studies). This work also utilized computational resources from the University of Colorado Boulder Research Computing Group, which is supported by the National Science Foundation (awards ACI-1532235 and ACI-1532236), the University of Colorado Boulder, and Colorado State University. BPC was supported in part by a graduate fellowship from the NSF (DGE-1747503) and a Biotechnology Training Grant from NIGMS (T32 GM008349).
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental details, characterization data, additional CD and NMR spectra, molecular dynamics simulation details (PDF)
GROMACS input files (.zip)
The authors declare no competing financial interest.
REFERENCES
- (1).John EA; Massena CJ; Berryman OB Helical Anion Foldamers in Solution. Chem. Rev 2020, 120 (5), 2759–2782. 10.1021/acs.chemrev.9b00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Ferrand Y; Huc I Designing Helical Molecular Capsules Based on Folded Aromatic Amide Oligomers. Acc. Chem. Res 2018, 51 (4), 970–977. 10.1021/acs.accounts.8b00075. [DOI] [PubMed] [Google Scholar]
- (3).Hill DJ; Mio MJ; Prince RB; Hughes TS; Moore JS A Field Guide to Foldamers. Chem. Rev 2001, 101 (12), 3893–4011. 10.1021/cr990120t. [DOI] [PubMed] [Google Scholar]
- (4).Gong B Hollow Crescents, Helices, and Macrocycles from Enforced Folding and Folding-Assisted Macrocyclization. Acc. Chem. Res 2008, 41 (10), 1376–1386. 10.1021/ar700266f. [DOI] [PubMed] [Google Scholar]
- (5).Gellman SH Foldamers: A Manifesto. Acc. Chem. Res 1998, 31 (4), 173–180. 10.1021/ar960298r. [DOI] [Google Scholar]
- (6).George KL; Horne WS Foldamer Tertiary Structure through Sequence-Guided Protein Backbone Alteration. Acc. Chem. Res 2018, 51 (5), 1220–1228. 10.1021/acs.accounts.8b00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Laursen JS; Engel-Andreasen J; Olsen CA β-Peptoid Foldamers at Last. Acc. Chem. Res 2015, 48 (10), 2696–2704. 10.1021/acs.accounts.5b00257. [DOI] [PubMed] [Google Scholar]
- (8).Teng P; Ma N; Cerrato DC; She F; Odom T; Wang X; Ming LJ; Van der Vaart A; Wojtas L; Xu H; Cai J Right-Handed Helical Foldamers Consisting of De Novo D-AApeptides. J. Am. Chem. Soc 2017, 139 (21), 7363–7369. 10.1021/jacs.7b03007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Violette A; Averlant-Petit MC; Semetey V; Hemmerlin C; Casimir R; Graft R; Marraud M; Briand JP; Rognan D; Guichard GN, N′-Linked Oligoureas as Foldamers: Chain Length Requirements for Helix Formation in Protic Solvent Investigated by Circular Dichroism, NMR Spectroscopy, and Molecular Dynamics. J. Am. Chem. Soc 2005, 127 (7), 2156–2164. 10.1021/ja044392b. [DOI] [PubMed] [Google Scholar]
- (10).Butterfoss GL; Yoo B; Jaworski JN; Chorny I; Dill KA; Zuckermann RN; Bonneau R; Kirshenbaum K; Voelz VA De Novo Structure Prediction and Experimental Characterization of Folded Peptoid Oligomers. Proc. Natl. Acad. Sci. U. S. A 2012, 109 (36), 14320–14325. 10.1073/pnas.1209945109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Urushibara K; Yamada T; Yokoyama A; Mori H; Masu H; Azumaya I; Kagechika H; Yokozawa T; Tanatani A Development of Helical Aromatic Amide Foldamers with a Diphenylacetylene Backbone. J. Org. Chem 2020, 85 (4), 2019–2039. 10.1021/acs.joc.9b02758. [DOI] [PubMed] [Google Scholar]
- (12).Hartley CS Folding of Ortho-Phenylenes. Acc. Chem. Res 2016, 49 (4), 646–654. 10.1021/acs.accounts.6b00038. [DOI] [PubMed] [Google Scholar]
- (13).Nelson JC; Saven JG; Moore JS; Wolynes PG Solvophobically Driven Folding of Nonbiological Oligomers. Science 1997, 277 (5333), 1793–1796. 10.1126/science.277.5333.1793. [DOI] [PubMed] [Google Scholar]
- (14).Hua Y; Flood AH Click Chemistry Generates Privileged CH Hydrogen-Bonding Triazoles: The Latest Addition to Anion Supramolecular Chemistry. Chem. Soc. Rev 2010, 39 (4), 1262–1271. 10.1039/b818033b. [DOI] [PubMed] [Google Scholar]
- (15).Angelo NG; Arora PS Nonpeptidic Foldamers from Amino Acids: Synthesis and Characterization of 1,3-Substituted Triazole Oligomers. J. Am. Chem. Soc 2005, 127 (49), 17134–17135. 10.1021/ja056406z. [DOI] [PubMed] [Google Scholar]
- (16).Varni AJ; Fortney A; Baker MA; Worch JC; Qiu Y; Yaron D; Bernhard S; Noonan KJT; Kowalewski T Photostable Helical Polyfurans. J. Am. Chem. Soc 2019, 141 (22), 8858–8867. 10.1021/jacs.9b01567. [DOI] [PubMed] [Google Scholar]
- (17).Zhang DW; Zhao X; Hou JL; Li ZT Aromatic Amide Foldamers: Structures, Properties, and Functions. Chem. Rev 2012, 112 (10), 5271–5316. 10.1021/cr300116k. [DOI] [PubMed] [Google Scholar]
- (18).Rinaldi S The Diverse World of Foldamers: Endless Possibilities of Self-Assembly. Molecules 2020, 25 (14), 3276. 10.3390/molecules25143276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Zhang DW; Zhao X; Li ZT Aromatic Amide and Hydrazide Foldamer-Based Responsive Host-Guest Systems. Acc. Chem. Res 2014, 47 (7), 1961–1970. 10.1021/ar5000242. [DOI] [PubMed] [Google Scholar]
- (20).Gopalan RD; Del Borgo MP; Mechler AI; Perlmutter P; Aguilar MI Geometrically Precise Building Blocks: The Self-Assembly of β-Peptides. Chem. Biol 2015, 22 (11), 1417–1423. 10.1016/j.chembiol.2015.10.005. [DOI] [PubMed] [Google Scholar]
- (21).Altmayer-Henzien A; Declerck V; Farjon J; Merlet D; Guillot R; Aitken DJ Fine Tuning of β-Peptide Foldamers: A Single Atom Replacement Holds Back the Switch from an 8-Helix to a 12-Helix. Angew. Chemie Int. Ed 2015, 54 (37), 10807–10810. 10.1002/anie.201504126. [DOI] [PubMed] [Google Scholar]
- (22).Fanelli R; Berta D; Földes T; Rosta E; Atkinson RA; Hofmann HJ; Shankland K; Cobb AJA Organocatalytic Access to a cis-Cyclopentyl-γ-Amino Acid: An Intriguing Model of Selectivity and Formation of a Stable 10/12-Helix from the Corresponding γ/α-Peptide. J. Am. Chem. Soc 2020, 142 (3), 1382–1393. 10.1021/jacs.9b10861. [DOI] [PubMed] [Google Scholar]
- (23).Vasudev PG; Chatterjee S; Narayanaswamy S; Padmanabhan B Structural Chemistry of Peptides Containing Backbone Expanded Amino Acid Residues: Conformational Features of β, γ, and Hybrid Peptides. Chem. Rev 2011, 111 (2), 657–687. 10.1021/cr100100x. [DOI] [PubMed] [Google Scholar]
- (24).Kudo M; Maurizot V; Kauffmann B; Tanatani A; Huc I Folding of a Linear Array of α-Amino Acids within a Helical Aromatic Oligoamide Frame. J. Am. Chem. Soc 2013, 135 (26), 9628–9631. 10.1021/ja404656z. [DOI] [PubMed] [Google Scholar]
- (25).Hu X; Mandal PK; Kauffmann B; Huc I Hybrid Sequences That Express Both Aromatic Amide and α-Peptidic Folding Features. Chempluschem 2020, 85 (7), 1580–1586. 10.1002/cplu.202000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Collie GW; Pulka-Ziach K; Lombardo CM; Fremaux J; Rosu F; Decossas M; Mauran L; Lambert O; Gabelica V; Mackereth CD; Guichard G Shaping Quaternary Assemblies of Water-Soluble Non-Peptide Helical Foldamers by Sequence Manipulation. Nat. Chem 2015, 7 (11), 871–878. 10.1038/nchem.2353. [DOI] [PubMed] [Google Scholar]
- (27).Lombardo CM; Collie GW; Pulka-Ziach K; Rosu F; Gabelica V; Mackereth CD; Guichard G Anatomy of an Oligourea Six-Helix Bundle. J. Am. Chem. Soc 2016, 138 (33), 10522–10530. 10.1021/jacs.6b05063. [DOI] [PubMed] [Google Scholar]
- (28).Kulkarni K; Habila N; Del Borgo MP; Aguilar MI Novel Materials from the Supramolecular Self-Assembly of Short Helical β3-Peptide Foldamers. Front. Chem 2019, 7, 1–12. 10.3389/fchem.2019.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Eom JH; Gong J; Kwon S; Jeon A; Jeong R; Driver RW; Lee HS A Hollow Foldecture with Truncated Trigonal Bipyramid Shape from the Self-Assembly of an 11-Helical Foldamer. Angew. Chemie Int. Ed 2015, 54 (45), 13204–13207. 10.1002/anie.201504248. [DOI] [PubMed] [Google Scholar]
- (30).Le Bailly BAF; Clayden J Dynamic Foldamer Chemistry. Chem. Commun 2016, 52 (27), 4852–4863. 10.1039/c6cc00788k. [DOI] [PubMed] [Google Scholar]
- (31).Marafon G; Crisma M; Moretto A Intrinsically Photoswitchable α/β Peptides toward Two-State Foldamers. Angew. Chemie Int. Ed 2018, 57 (32), 10217–10220. 10.1002/anie.201806035. [DOI] [PubMed] [Google Scholar]
- (32).Berl V; Huc I; Khoury RG; Krische MJ; Lehn JM Interconversion of Single and Double Helices Formed from Synthetic Molecular Strands. Nature 2000, 407 (6805), 720–723. 10.1038/35037545. [DOI] [PubMed] [Google Scholar]
- (33).Bao C; Gan Q; Kauffmann B; Jiang H; Huc I A Self-Assembled Foldamer Capsule: Combining Single and Double Helical Segments in One Aromatic Amide Sequence. Chem. - A Eur. J 2009, 15 (43), 11530–11536. 10.1002/chem.200900877. [DOI] [PubMed] [Google Scholar]
- (34).Liu Y; Parks FC; Sheetz EG; Chen CH; Flood AH Polarity-Tolerant Chloride Binding in Foldamer Capsules by Programmed Solvent-Exclusion. J. Am. Chem. Soc 2021, 143 (8), 3191–3204. 10.1021/jacs.0c12562. [DOI] [PubMed] [Google Scholar]
- (35).Zhao Y; Connor AL; Sobiech TA; Gong B Effects of Oligomer Length, Solvents, and Temperature on the Self-Association of Aromatic Oligoamide Foldamers. Org. Lett 2018, 20 (17), 5486–5489. 10.1021/acs.orglett.8b02438. [DOI] [PubMed] [Google Scholar]
- (36).Mathew S; Crandall LA; Ziegler CJ; Hartley CS Enhanced Helical Folding of Ortho-Phenylenes through the Control of Aromatic Stacking Interactions. J. Am. Chem. Soc 2014, 136 (47), 16666–16675. 10.1021/ja509902m. [DOI] [PubMed] [Google Scholar]
- (37).Stone MT; Heemstra JM; Moore JS The Chain-Length Dependence Test. Acc. Chem. Res 2006, 39 (1), 11–20. 10.1021/ar0501267. [DOI] [PubMed] [Google Scholar]
- (38).Pace CN; Scholtz JM A Helix Propensity Scale Based on Experimental Studies of Peptides and Proteins. Biophys. J 1998, 75 (1), 422–427. 10.1016/S0006-3495(98)775290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Cheng RP; Gellman SH; Degrado WF β-Peptides : From Structure to Function. Chem. Rev 2001, 101 (10), 3219–3232. 10.1021/cr000045i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


