Abstract
Objectives:
To determine the correlation between surgeon radiology assessment and laparoscopic scoring by disease locations in patients with newly diagnosed advanced stage ovarian cancer.
Methods:
Fourteen gynecologic oncology surgeons from a single institution performed a blinded review of pre-operative contrast enhanced CT imaging from patients with advanced stage ovarian cancer. Each of the patients included in this report had also undergone laparoscopic scoring assessment to determine primary resectability using the validated Fagotti scoring method from April 2013 to December 2017. Patients with a predictive index value (PIV) score <8 were offered primary tumor reductive surgery (TRS); those with score ≥8 received NACT. Surgeons were asked to provide anticipated PIV scores based on their blinded review of the antecedent CT imaging. Linear mixed models (LMM) were conducted to calculate the correlation between radiologic and laparoscopic score for surgeons individually, and as a group. Once the model was fit, the inter-class correlation (ICC) and 95% confidence interval was calculated.
Results:
Radiology review was performed on 20 patients with advanced stage ovarian cancer that underwent laparoscopic scoring assessment. The majority had stage IIIC disease (85%) and median laparoscopic PIV score was 9 (range 0–14). Surgeon faculty rank included Assistant Professor (n=5), Associate Professor (n=4), and Professor (n=5). Median surgeon experience during the study period with laparoscopic scoring surgery was 13 cases (range 1–28) and TRS was 22.5 cases (range 2–48). The kappa inter-rater agreement was −0.017 (95% CI 0.023 to −0.005) indicating low inter-rater agreement between radiology review and actual laparoscopic score. The ICC in this model was 0.06 (0.02–0.21) indicating that surgeons do not score the same across all the images. When using a clinical cutoff of PIV of 8, the probability of agreement between radiology and actual laparoscopic score was 0.56 (95% CI: 0.49–0.73). When looking at disease site subscales, the probability of agreement was as follows: peritoneum 0.57 (95% CI 0.51–0.62), diaphragm 0.54 (95% CI 0.48–0.60), mesentery 0.51 (95% CI 0.45–0.57), omentum 0.61 (95% CI 0.55–0.67), bowel 0.54 (95% CI 0.44–0.64), stomach 0.71 (95% CI 0.65–0.76), and liver 0.36 (95% CI 0.31–0.42). Number of laparoscopic scoring cases, TRS cases, or faculty rank was not significantly associated with overall or subscale agreement.
Conclusions:
Surgeon radiology review did not correlate highly with actual laparoscopic scoring assessment findings in patients with advanced stage ovarian cancer. By subscale, the best agreement is seen when evaluating for stomach involvement, and the worst with liver involvement. Our study highlights the utility of laparoscopic scoring assessment to determine resectability over radiology assessment alone.
Keywords: imaging, laparoscopy, ovarian cancer, cytoreduction
Introduction
The approaches by which gynecologic oncology surgeons triage advanced ovarian cancer patients to either primary surgery or neoadjuvant chemotherapy are diverse, inconsistent, and poorly reproducible. Pre-operative computed tomography (CT), serum CA-125, clinical examination and patient factors are amongst the most commonly used non-invasive modalities to triage patients but has been met with variable success and it has been difficult to standardize across surgical practices. [1–5] Accurate assessment of tumor burden and pattern of spread at initial diagnosis is paramount in order to determine ability to achieve optimal tumor cytoreduction and avoid a futile laparotomy.
A laparoscopic-based scoring assessment to determine primary resectability in patients with advanced stage ovarian cancer has been previously reported in the literature. [6–13] Laparoscopic scoring has demonstrated value in achieving an overall high positive predictive value for suboptimal primary tumor cytoreduction [6–8], thereby reducing rates of futile laparotomy at cytoreductive surgery. [9] We have previously reported that incorporating the laparoscopic scoring algorithm validated by Fagotti et al. into our standard triage of newly diagnosed advanced stage ovarian cancer patients resulted in improvement in our complete gross resection (R0) rates at both primary and interval tumor reductive surgeries. Those patients undergoing R0 resection at primary surgery experienced the greatest survival benefit, thus, selecting patients that would achieve the most benefit from an aggressive surgical approach. [14]
Some criticism of laparoscopic assessment of new advanced ovarian cancer patients has been the need for an additional surgical procedure to obtain laparoscopic data, which could lead to delay in primary therapy, or complication risks from the laparoscopic procedure. Thus, a question is raised as to whether a thorough and systematic surgeon review of preoperative imaging can replace laparoscopic scoring assessment. The objective of this study was to determine the correlation between surgeon radiology assessment and laparoscopic scoring by disease sites in patients with newly diagnosed advanced stage ovarian cancer.
Methods
This retrospective imaging review protocol was approved by the University of Texas MD Anderson Cancer Center Institutional Review Board (PA18–0259). Fourteen gynecologic oncology surgeons from a single institution performed a blinded review of contrast enhanced CT imaging from 20 patients with advanced stage ovarian cancer. All patients previously underwent laparoscopic scoring assessment to determine primary resectability at tumor reductive surgery (TRS) using the Fagotti validated scoring algorithm [6] (Supplemental Table 1) from April 2013 to December 2017. These 20 patients were randomly selected from our institutional ovarian cancer database (PA 16–1010) with predictive index value (PIV) scores ranging from 0 to 14 (Supplemental Table 2). The patients with PIV scores <8 were offered primary surgery and those with scores ≥8 received NACT. Surgeons recorded PIV scores based on their blinded review of CT imaging and reports in the anatomic locations evaluated as a part of the Fagotti validated scoring system. Patients were excluded if CT imaging was non-contrast enhanced or was performed outside of MD Anderson to ensure the quality of the imaging was consistent across all patient subjects.
Summary statistics of the radiologic and laparoscopic scores were calculated by surgeon. Box plots were created to graphically depict the scores. The percent agreement (yes/no based on clinical cutoff of 8) was calculated by surgeon and across all surgeons. The kappa inter-rater agreement statistic and corresponding 95% confidence interval were calculated for the radiology scores. Linear mixed models (LMM) were conducted to calculate the correlation between radiology and laparoscopic score for each surgeon and as a group. We regressed laparoscopic score on radiology score. A random intercept was included and our subject effect was the clinician. Once the model was fit, the inter-class correlation (ICC) and 95% confidence interval was calculated. We also fit a model with the image as our subject effect to calculate within image correlations. We then categorized each score as agreement vs. non-agreement based on the pre-defined cutoff of 8 for treatment decision making. Similar generalized linear mixed models (GLMM) were then conducted with agreement (Yes/No) as outcome and clinical and demographic variables of interest as covariates to assess if certain covariates are associated with scoring agreement. Our sample size was chosen to give adequate precision around our estimates of ICC. When the sample size is 280, a two-sided 95% confidence interval (CI) computed using the large sample normal approximation for an ICC based on 14 raters will extend about 0.037 (0.042) from the observed ICC when the expected intra-class correlation is 0.7 (0.3).
All statistical analyses were performed using Stata/MP v15.0 (College Station, TX). Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at MD Anderson. [15]
Results
Radiology review was performed on 20 patients with advanced stage ovarian cancer who underwent laparoscopic scoring assessment. Clinical and demographic data from patients are presented in Table 1. Median age was 65.5 years (range 36–80 years), median BMI was 28.4 kg/m2 (range 21.1–41 kg/m2), and median baseline CA-125 level was 675 U/mL (range 61–12472 U/mL). The majority of patients had stage IIIC disease (85%) and median laparoscopic PIV score was 9 (range 0–14). Surgeon faculty academic rank included Assistant Professor (n=5), Associate Professor (n=4), and Professor (n=5). Median surgeon experience during the study period with laparoscopic scoring surgery was 13 cases (range 1–28 cases) and tumor reductive surgery was 22.5 cases (range 2–48 cases). Following laparoscopic scoring assessment, 5 patients (25%) underwent primary surgery and 15 patients (75%) received NACT. Of those that received NACT, 14 patients did undergo interval TRS and 1 patient was lost to follow-up following the laparoscopic scoring assessment. Majority of patients underwent optimal tumor cytoreduction to no gross residual disease (R0, 79%), compared to optimal <1cm (5%) and suboptimal ≥1cm (16%).
Table 1.
Clinicodemographic data from patient images reviewed
| Patient Characteristic | n=20 |
|---|---|
| Age at diagnosis (years, median) | 65.5 (36–80) |
| BMI (kg/m2, median) | 28.4 (21.1–41) |
| ECOG Performance Status | |
| 0 | 8 (40%) |
| 1 | 9 (45%) |
| 2 | 1 (5%) |
| Unknown | 2 (10%) |
| Baseline CA-125 (U/mL, median) | 675 (61–12472) |
| Stage at diagnosis | |
| IIIC | 17 (85%) |
| IVA | 2 (10%) |
| IVB | 1 (5%) |
| Laparoscopic assessment score (median) | 9 (0–14) |
| Type of TRS | |
| Primary | 5 (26%) |
| Interval | 14 (74%) |
| Unknown* | 1 |
| Remaining tumor size at TRS | |
| R0 | 15 (79%) |
| <1cm | 1 (5%) |
| ≥1 cm | 3 (16%) |
| Unknown* | 1 |
One patient was lost to follow-up following laparoscopic assessment
BMI=body mass index; ECOG=Eastern Cooperative Oncology Group; TRS=tumor reductive surgery; R0=complete gross resection
Summary statistics for each surgeon’s scores based on agreement of the laparoscopic assessment PIV score of <8 or ≥8 are listed in Supplemental Table 3. Average agreement amongst all surgeons for all cases was 55.7%. Agreement by experience level was 51% for Assistant Professor, 70% for Associate Professor, and 56% for Professor level faculty surgeons. Figures 1 and 2 depict agreement and summary scores. The kappa inter-rater agreement was −0.017 (95% CI 0.023 to −0.005) indicating low inter-rater agreement between surgeon radiology review and actual laparoscopic score (Figure 2). To account for the correlations, within images and within surgeons LMMs were conducted to calculate the ICC. When we treat image as the subject effect, our ICC was 0.22 (95% CI: 0.10–0.40). An ICC of zero would imply total agreement between surgeons on the images, thus our ICC indicates mild agreement between surgeons on scoring each image. When treating each surgeon as the subject effect, the ICC in this model was 0.06 (95% CI: 0.02–0.21). An ICC of one would indicate the surgeon tended to score every image the same (i.e. particularly high or low all the time), thus our ICC in this model indicated that surgeons do not score the same across all the images.
Figure 1.
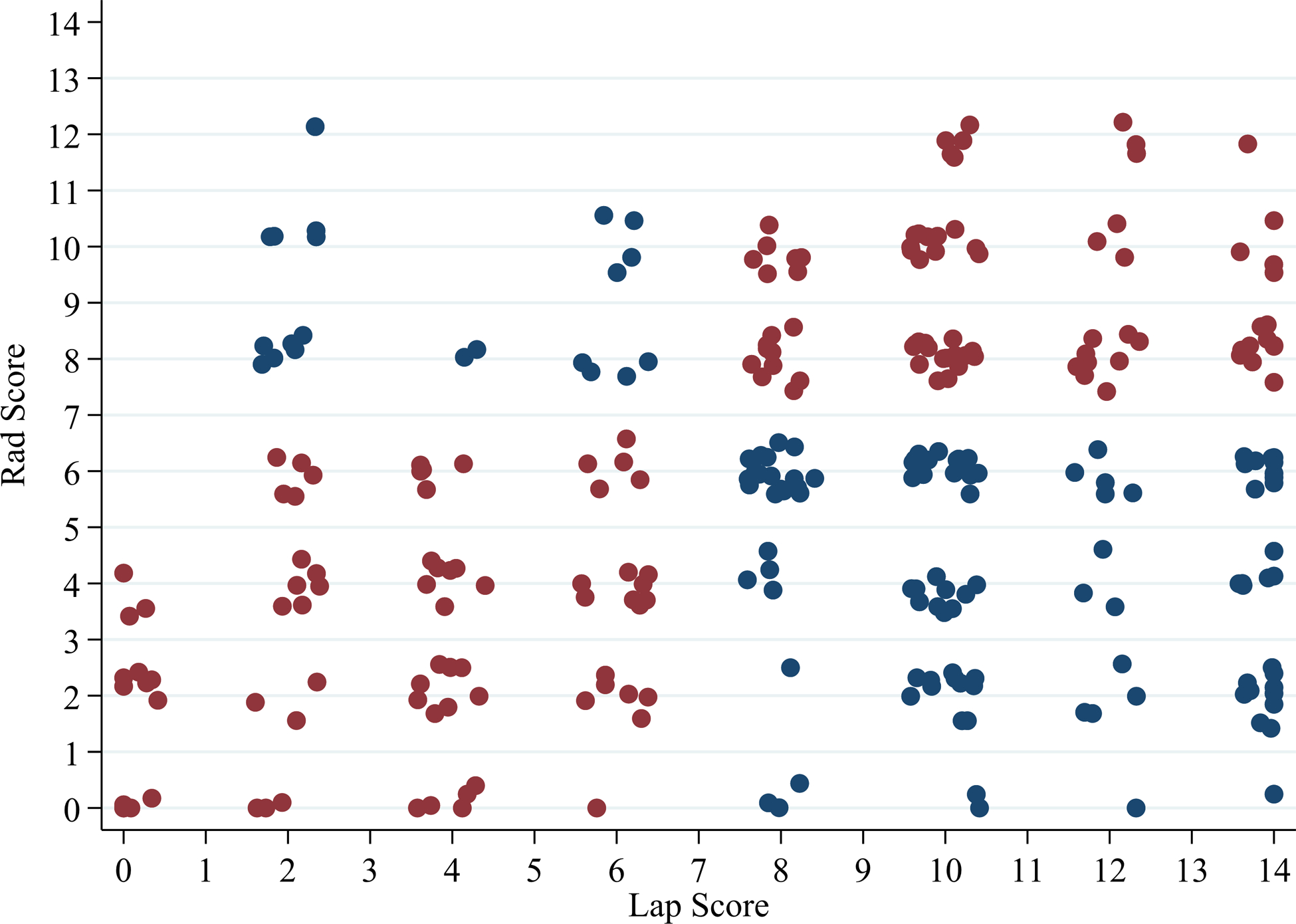
Scatter plot of laparoscopy compared to surgeon radiology scores
Legend: Red Dot=Agreement between Laparoscopy Score and Surgeon Radiology Score; Blue Dot=No agreement between Laparoscopy Score and Surgeon Radiology Score
Figure 2.
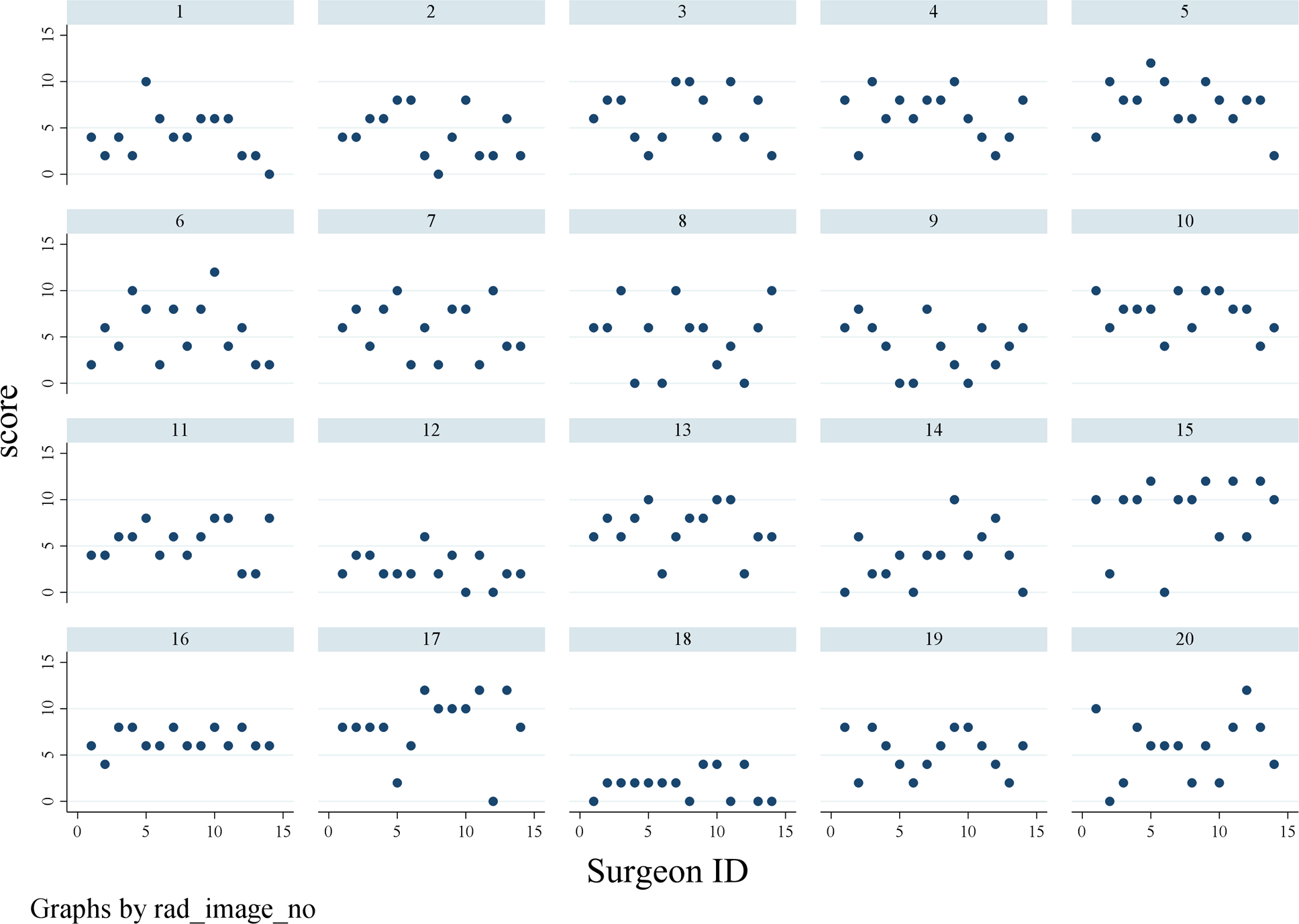
Scatter plot of surgeon radiology score by patient CT imaging
Our next agreement analyzed was defined based on the clinical cutoff of a PIV score of 8. Then GLMMs were conducted to assess whether surgeon academic rank, number of laparoscopic scoring procedures performed by each surgeon, and number of tumor reductive surgery cases performed by each surgeon were associated with agreement (Table 2). When using a clinical cutoff of PIV of 8, the probability of agreement between radiology and actual laparoscopic score was 0.56 (95% CI: 0.49–0.73). The number of laparoscopic scoring cases, tumor reductive surgery cases, and surgeon title was not significantly associated with agreement.
Table 2:
GLMMs assessing factors associated with agreement
| Characteristic | OR | 95% LB | 95% UB | p-value |
|---|---|---|---|---|
| Scope and Score number | 1.00 | 0.97 | 1.03 | 0.865 |
| TRS number | 1.00 | 0.98 | 1.01 | 0.863 |
| Title (ref: Assistant) | 0.113 | |||
| Associate Professor | 2.26 | 1.05 | 4.85 | |
| Professor | 1.22 | 0.74 | 2.03 |
GLMMs=generalized linear mixed models, TRS=tumor reductive surgery
A similar analysis was performed to assess agreement within each disease site subscale (Table 3) within the laparoscopic scoring algorithm. Supplemental Figure 1 includes a scatterplot of radiology and laparoscopic scores by each disease site subscale. Supplemental Table 4a includes the calculations of probability of agreement from the general linear models (GLMs). When evaluating the disease site subscales, the probability of agreement was as follows: peritoneum 0.57 (95% CI 0.51–0.62), diaphragm 0.54 (95% CI 0.48–0.60), mesentery 0.51 (95% CI 0.45–0.57), omentum 0.61 (95% CI 0.55–0.67), bowel 0.54 (95% CI 0.44–0.64), stomach 0.71 (95% CI 0.65–0.76), and liver 0.36 (95% CI 0.31–0.42). Number of laparoscopic scoring cases, TRS cases, or surgeon academic rank was not significantly associated with overall or subscale agreement. Supplemental Table 4b includes the Kappa statistics for the inter-rater agreement on the radiology subscale scores.
Table 3:
Agreement of scores by disease site subscale
| Radiology Score | ||||
|---|---|---|---|---|
| 0 | 2 | |||
| Lap Score | N | % | N | % |
| Peritoneal Carcinomatosis | ||||
| 0 | 54 | 51.92 | 71 | 40.57 |
| 2 | 50 | 48.08 | 104 | 59.43 |
| Diaphragmatic Disease | ||||
| 0 | 69 | 42.33 | 29 | 28.16 |
| 2 | 94 | 57.67 | 74 | 71.84 |
| Mesenteric Disease | ||||
| 0 | 57 | 41.01 | 55 | 39.01 |
| 2 | 82 | 58.99 | 86 | 60.99 |
| Omental Site of Disease | ||||
| 0 | 69 | 64.49 | 71 | 41.04 |
| 2 | 38 | 35.51 | 102 | 58.96 |
| Bowel Infiltration | ||||
| 0 | 24 | 16.00 | 4 | 3.08 |
| 2 | 126 | 84.00 | 126 | 96.92 |
| Stomach Infiltration | ||||
| 0 | 178 | 75.11 | 18 | 62.07 |
| 2 | 59 | 24.89 | 11 | 37.93 |
| Liver Surface | ||||
| 0 | 67 | 28.63 | 3 | 9.38 |
| 2 | 167 | 71.37 | 29 | 90.63 |
An analysis by surgeon was performed comparing the surgeon radiology score and projected triage based on this score (primary surgery or NACT) to the actual laparoscopic score and actual laparoscopic triage results (Supplemental Table 5a–5n). Median percentage agreement in triage results (primary surgery or NACT) was 57.5% and median projected futile laparotomy rate was 42.5%. Two patients did receive an actual laparoscopic PIV score of 6, but based on disease burden based on this assessment were triaged to NACT.
Discussion
Surgeon radiology review did not correlate well with actual laparoscopic scoring assessment findings in patients with advanced stage ovarian cancer, regardless of surgeon experience level. By disease site subscale, the best agreement was noted when evaluating for stomach involvement, and the worst with liver involvement. Our study highlights the utility of laparoscopic scoring assessment to determine resectability over surgeon radiology assessment alone.
Previous studies have shown poor correlation between CT radiographic predictors to findings at the time of tumor reductive surgery and ability to predict suboptimal resection. [1] This study, and others evaluating structured radiology reporting of disease burden by anatomic location by experienced radiologists combined with patient clinical factors, have not been reproducible to predict resection results at the time of tumor cytoreduction in advanced ovarian cancer. [2–4] Implementation of laparoscopy based scoring systems has overcome the limitations of preoperative radiology assessment tools [17], which has led to reduction in unnecessary laparotomy procedures in unresectable cases. [7–9] However, widespread implementation of laparoscopic assessment in new advanced ovarian cancer cases for primary resectability has been met with resistance and controversy.
Our study adds to the limited literature comparing laparoscopic scoring assessment findings to detailed radiographic review by surgeons. There have been recent reports from Ahmed et al. comparing the accuracy of CT and laparoscopy in predicting the peritoneal carcinomatosis index (PCI) score. CT and laparoscopy sensitivity were 94.9%, 98.3%, specificity 86.7%, 80.4%, PPV 97.9 %, 96.8%, NPV 72.2%, 88.8 %, and accuracy 93.8 %, 95.7%, respectively. However, CT diagnostic performance was less accurate than laparoscopy in pelvic and small intestinal regions. The authors concluded that both CT and laparoscopy seem to be effective tools for assessment of peritoneal carcinomatosis using the PCI score. [18–19] There have been no studies reported comparing CT diagnostic performance when compared to laparoscopic scoring using the validated Fagotti scoring algorithm for surgical resectability. There are inherent differences in the PCI, which analyzes tumor size by anatomic location. [20] Multiple previous studies have used the PCI to correlate surgical resectability and its impact on survival in advanced ovarian cancer. [21–24] Although the PCI has been shown to be a validated radiologic assessment tool in advanced cancers with peritoneal spread, studies have suggested its poor utility as a triage test to reliably identify advanced ovarian cancer patients who are likely to have complete cytoreductive surgery. [23] A recent study suggested that selected PCI regions, such as the small intestine with mesentery and hepatoduodenal ligament, are more predictive of complete resection and survival than the entire PCI. [24] This is similar to evaluating the disease distribution in distinct anatomic areas by the Fagotti laparoscopic assessment model, however, there has been no prospective validated correlation between with PCI and laparoscopic assessment in advanced ovarian cancer.
The goal of any triage modality in a patient with newly diagnosed advanced ovarian cancer should be the reproducibility of the method and generalizability to practicing gynecologic oncology surgeons. Thus, the utility of structured radiology reports by specialized gynecologic oncology radiologists may not be practical in a setting that does not have access to this resource. The ability for a gynecologic oncology surgeon to interpret high-quality contrast enhanced CT imaging in order to make their own decisions is important. Our study, which was conducted in large tertiary cancer center, showed that our surgeons’ radiology review did not correlate highly with actual laparoscopic scoring assessment findings in patients with advanced stage ovarian cancer, thus could have altered surgical decision making if radiology review alone would have been used in these cases. Our data supports the high likelihood for futile laparotomy or being inappropriately explored if radiology review would have been used alone.
The strengths of our study are the blinded nature of this correlative study and evaluating a large number of surgeons in a tertiary cancer center with a high volume of advanced ovarian cancer cases. Additional strengths include evaluating surgeons of different experience and rank in their ability to predict laparoscopic score based on CT review, which leads to generalizability of the study findings to gynecologic oncology surgeons in the community. We also chose patient cases of varying degrees of tumor burden in order to evaluate the correlation of CT review to laparoscopy in all disease burden types. Our study is one of the first to evaluate the correlation of CT review with laparoscopy findings by disease site subscale based on the Fagotti algorithm. The weaknesses of the study include the small number of cases selected to review, which could lead to inherent selection bias and possible inaccuracy of the results if more cases were evaluated prospectively. We also elected to not perform a comparison of the performance of a radiologist interpreting CT imaging in comparison to laparoscopy findings as laparoscopic assessment is based on surgical resectability which is difficult to determine from a radiology perspective. Based on our results, we have included a pre-laparoscopy surgeon radiology review and scoring to our quality improvement process at our institution to provide additional prospective cases to report on in the future.
In conclusion, our results suggest that the validated laparoscopic scoring algorithm should be considered the gold standard assessment tool for primary resectability in new advanced ovarian cancer cases. Further prospective studies are needed to determine the utility of novel imaging modalities which may enhance surgeon ability to predict surgical resectability, eliminating the need for laparoscopic scoring assessment.
Supplementary Material
Study Highlights:
Surgeon radiology review did not correlate highly with diagnostic laparoscopy findings.
Correlation between radiology review and laparoscopy did not vary by rank or experience.
Surgeon radiology review best assessed for stomach involvement and worst for liver involvement.
Acknowledgments
This research was in part supported by the MD Anderson Ovarian Cancer Moon Shot and by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant (P30CA016672; used the Clinical Trials Support Resource and the Biostatistics Resource Group), T32 training grant for gynecologic oncologists (T32CA101642), and Ovarian Cancer SPORE funding (CA217685), The National Institute of Health’s National Cancer Institute Grants K08CA234333 and K07CA201013, the Andrew Sabin Family Fellowship, the GOG Foundation Scholar Investigator Award, the Frank McGraw Memorial Chair in Cancer Research, and the American Cancer Society Research Professor Award.
Competing interests
The authors have the following conflicts of interest to disclosure.
Relevant financial activities outside the supported work; NDF: Consultant/Advisory board (Tesaro, BMS/Pfizer); SNW: Consultant (AstraZeneca, Clovis Oncology, GSK/Tesaro, Novartis, Roche/Genentech, Eisai, Merck, Pfizer, Circulogene), research funding (ArQule, AstraZeneca, Clovis Oncology, GSK/Tesaro, Novartis, Roche/Genentech, Bayer, Cotinga Pharmaceuticals); LAM: research funding (AstraZeneca); AAJ: Consultant (Gerson and Lehrman Group, Guidepoint, Iovance, Nuprobe, Simcere, Pact Pharma), research funding (AstraZeneca, BMS, Iovance, Aravive, Pfizer, Immatics USA, Eli Lilly); RLC: Consultant (AstraZeneca, Clovis Oncology, GSK/Tesaro, Novartis, Roche/Genentech, Eisai, Merck, Pfizer, Novocure, Genmab, Gamamab, Oncosec, Tarveda), research funding (AbbVie, Genmab, Merck, AstraZeneca, Clovis Oncology, Roche/Genentech); AKS: Consultant (Merck, Kiyatec), shareholder (Biopath), research funding (M-Trap). The following authors have no disclosures: PB, JAR, PTS, AS, MO, LC, MB, BMF, JKB, PB, BZ, CL
Footnotes
Presented in abstract form at the 2019 American Society of Clinical Oncology Annual Meeting, Chicago, IL
References
- 1.Axtell AE, Lee MH, Bristow RE, et al. Multi-institutional reciprocal validation study of computed tomography predictors of suboptimal primary cytoreduction in patients with advanced ovarian cancer. J Clin Oncol 2007;25:384–9. [DOI] [PubMed] [Google Scholar]
- 2.Suidan RS, Ramirez PT, Sarasohn DM, et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol 2014;134:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suidan RS, Ramirez PT, Sarasohn DM, et al. A multicenter assessment of the ability of preoperative computed tomography scan and CA-125 to predict gross residual disease at primary debulking for advanced epithelial ovarian cancer. Gynecol Oncol 2017;145:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrandina G, Sallustio G, Fagotti A, et al. Role of CT scan-based and clinical evaluation in the preoperative prediction of optimal cytoreduction in advanced ovarian cancer: a prospective trial. Br J Cancer 2009;101:1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aletti GD, Eisenhauer EL, Santillan A, et al. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol Oncol 2011;120:23–8. [DOI] [PubMed] [Google Scholar]
- 6.Fagotti A, Ferrandina G, Fanfani F, et al. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Ann Surg Oncol 2006;13:1156–61 [DOI] [PubMed] [Google Scholar]
- 7.Fagotti A, Ferrandina G, Fanfani F, et al. Prospective validation of laparoscopic predictive model for optimal cytoreduction in advanced ovarian carcinoma. Am J Obstet Gynecol 2008;199:642.e1–6. [DOI] [PubMed] [Google Scholar]
- 8.Fagotti A, Vizzielli G, De Iaco P, et al. A multicentric trial (Olympia-MITO 13) on the accuracy of laparoscopy to assess peritoneal spread in ovarian cancer. Am J Obstet Gynecol 2013;209:462.e1–11. [DOI] [PubMed] [Google Scholar]
- 9.Rutten MJ, van Meurs HS, van de Vrie R, et al. Laparoscopy to predict the result of primary cytoreductive surgery in patients with advanced ovarian cancer: a randomized controlled trial. J Clin Oncol 2017;35:613–621. [DOI] [PubMed] [Google Scholar]
- 10.Fagotti A, Vizzielli G, Fanfani F, et al. Introduction of staging laparoscopy in the management of advanced epithelial ovarian, tubal and peritoneal cancer: impact on prognosis in a single institution experience. Gynecol Oncol 2013;131:341–346. [DOI] [PubMed] [Google Scholar]
- 11.Fagotti A, Vizzielli G, Costantini B, et al. Learning curve and pitfalls of a laparoscopic score to describe peritoneal carcinosis in advanced ovarian cancer. Acta Obstet Gynecol Scand 2011;90:1126–31. [DOI] [PubMed] [Google Scholar]
- 12.Brun J, Rouzier R, Uzan S, Darai E. External validation of a laparoscopic-based score to evaluate resectability of advanced ovarian cancers: Clues for a simplified score. Gynecol Oncol 2008;110:354–59. [DOI] [PubMed] [Google Scholar]
- 13.Petrillo M, Vizzielli G, Fanfani F, et al. Definition of a dynamic laparoscopic model for the prediction of incomplete cytoreduction in advanced epithelial ovarian cancer: proof of concept. Gynecol Oncol 2015;139:5–9. [DOI] [PubMed] [Google Scholar]
- 14.Fleming ND, Nick AM, Coleman RL, et al. Laparoscopic surgical algorithm to triage the timing of tumor reductive surgery in advanced ovarian cancer. Obstet Gynecol 2018;132:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen JM, Sood AK, Coleman RL, et al. Concordance of laparoscopic scoring algorithm with primary surgery findings in advanced ovarian cancer. Gynecol Oncol 2018;151:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van de Vrie R, Rutten MJ, Asseler JD, et al. Laparoscopy for diagnosing resectability of disease in women with advanced ovarian cancer. Cochrane Database Syst Rev 2019. March 23;3:CD009786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdalla Ahmed S, Abou-Taleb H, Ali N, M Badary D. Accuracy of radiologic-laparoscopic peritoneal carcinomatosis categorization in the prediction of surgical outcome. Br J Radiol 2019. August;92(1100):20190163. [E pub 2019 May 24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed SA, Abou-Taleb H, Yehia A, et al. The accuracy of multi-detector computed tomography and laparoscopy in the prediction of peritoneal carcinomatosis index score in primary ovarian cancer. Acad Radiol 2019;26:1650–58. [DOI] [PubMed] [Google Scholar]
- 20.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359–74. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt S, Mueli RA, Achtari C, Prior JO. Peritoneal carcinomatosis in primary ovarian cancer staging: comparison between MDCT, MRI, and 18F-FDG PET/CT. Clin Nucl Med 2015;40:371–7. [DOI] [PubMed] [Google Scholar]
- 22.Mazzei MA, Khader L, Cirigliano A, et al. Accurary of MDCT in the preoperative definition of Peritoneal Cancer Index (PCI) in patients with advanced ovarian cancer who underwent peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Abdom Imaging 2013;38:1422–30. [DOI] [PubMed] [Google Scholar]
- 23.Avesani G, Arshad M, Lu H, et al. Radiological assessment of Peritoneal Cancer Index on preoperative CT in ovarian cancer is related to surgical outcome and survival. Radiol Med 2020;125:770–6. [DOI] [PubMed] [Google Scholar]
- 24.Rosendahl M, Harter P, Bjorn SF, Hogdall C. Specific regions, rather than the entire Peritoneal Carcinosis Index, are predictive of complete resection and survival in advanced epithelial ovarian cancer. Int J Gynecol Cancer 2018;28:316–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


