Abstract
Objectives
The coronavirus disease 2019 (COVID-19) pandemic has rapidly spread all over the world. Lung ultrasound (LUS) has emerged as a useful tool for diagnosing many respiratory diseases. The prognostic role of LUS in COVID-19 patients has not yet been established.
Methods
Several databases were searched on 09 April 2021. The difference in LUS score between the death and survival groups, and the relationship between LUS score and COVID-19 severity were both assessed.
Results
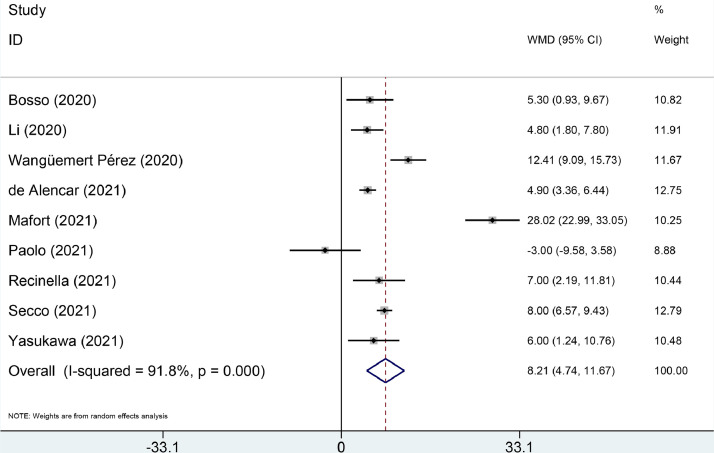
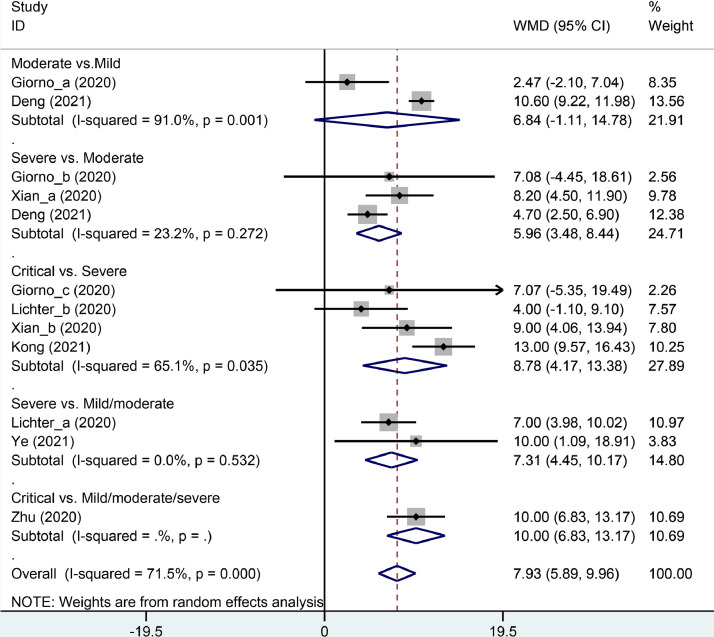
The LUS score was significantly higher in the death group compared with the survival group (weighted mean difference (WMD) = 8.21, 95% CI: 4.74–11.67, P < 0.001), which was confirmed by trial sequential analysis. Those with mild/moderate, severe and critical COVID-19 had a progressively higher LUS score (critical vs. severe: WMD = 8.78, 95% CI: 4.17–13.38; P < 0.001; critical vs. mild/moderate/severe: WMD = 10.00, 95% CI: 6.83–13.17, P < 0.001; severe vs. moderate: WMD = 5.96, 95% CI: 3.48–8.44, P < 0.001; severe vs. mild/moderate: WMD = 7.31, 95% CI: 4.45–10.17, P < 0.001).
Conclusions
The LUS score was associated with mortality and severity of COVID-19. The LUS score might be a risk stratification tool for COVID-19 patients.
Keywords: COVID-19, Lung ultrasound score, Mortality, Severity, Meta-analysis
Introduction
Global coronavirus disease-19 (COVID-19) broke out at the end of 2019 (Zhu et al., 2020). COVID-19 has rapidly spread all over the world, causing a pandemic within a short period due to its transmission dynamics. By the end of January 2021, more than one hundred million COVID-19 cases were confirmed in 215 countries, causing just under two million deaths (Bajaba et al., 2021).
The clinical manifestation spectrum of infection with COVID-19 ranges from mild self-limited disease to severe pneumonia (Hatmi, 2021). The mortality rate differs by countries and the infection rate is still rising, around 1%–5% (Kwok et al., 2021). However, the patients who are required to be transferred to intensive care units (ICU) have suffered from a very high mortality rate (45%) (Brandao Neto et al., 2021).
Since polymerase chain reaction analysis became the gold standard for diagnosing COVID-19, doctors devoted more time and effort to the studies regarding risk stratification or prediction about COVID-19. Recent studies have revealed that early prediction might assist clinicians in stratifying risk and initiate early therapy for patients with serious conditions (Gong et al., 2020). However, due to the serious economic burden caused by the COVID-19 pandemic, there is a critical need for a low-cost tool to stratify risk.
In recent decades, lung ultrasound (LUS) has emerged as a useful and non-invasive tool for both adult and pediatric patients, enabling rapid evaluation of many chest conditions. This popularity is due to several advantages of the method such as low cost, rapidity, lack of ionizing radiation, availability at the bedside, and repeatability of the method (Iovine et al., 2021). LUS can been performed for: diagnosis and follow-up of pediatric lung infectious diseases (namely bronchiolitis and pneumonia) and lung complications (such as pneumothorax, pleural effusion and lung abscess); diagnosis of respiratory distress syndrome (Ma et al., 2020); diagnosis and follow-up of pulmonary edema; diagnosis of thoracic trauma and early detection of signs of child abuse (Iovine et al., 2021); diagnosis of pneumothorax (Fei et al., 2021); and assessing and predicting acute heart failure (Johannessen et al., 2021). LUS has become part of the basic knowledge of physicians caring for critically ill patients in emergency units and ICUs (Mayo et al., 2019; Mojoli et al., 2019).
After COVID-19 broke out, the use of LUS became common practice. In the first half of 2020, most studies focused on LUS diagnostic capacity, while few evaluated its prognostic value (Kameda et al., 2021). Some studies used the LUS score to predict the mortality of COVID-19 patients (Bosso et al., 2020). Others found that the LUS score may correlate with the severity of COVID-19 (Giorno et al., 2020). However, the results have been inconsistent, and small sample sizes can affect the strength of previous evidence. Therefore, this meta-analysis of studies was conducted with the aim of providing a more comprehensive summary of currently available research to explore the association of LUS score with mortality and severity of COVID-19.
Materials and methods
This meta-analysis was conducted according to PRISMA guidelines. The protocol was registered (number: CRD42021241307) online (https://www.crd.york.ac.uk/PROSPERO).
Electronic literature search
Two independent investigators (GS and WQ) independently searched PubMed, MEDLINE, Embase, Scopus, and Cochrane library from database inception to 09 April 2021, to identify the relevant studies. No limits were applied for language. The search keywords included “COVID-19” and “lung ultrasound score”. The details of the search strategy are shown in the Supplementary file S1. At the same time, the article references were read to find any potential literature that may have met the criteria.
Study selection and exclusion
Two researchers (GS and WQ) independently screened the titles and abstracts for eligibility. Full papers were assessed to confirm disagreement in existence according to the exclusion criteria by the two researchers. Disagreements were discussed and resolved by involving a third reviewer (XW) for adjudication.
Original studies were eligible if they met the following criteria: (i) observational studies with death as the research endpoint, or studies comparing LUS score in different severities of COVID-19 patients; (ii) quantitative assessment of LUS score using 12-zone/0–36 score protocol, which is the most widely used; (iii) COVID-19 cases could be classified as mild, moderate, severe, or critical, according to the Guidelines for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Infection by the National Health Commission (Lin and Li, 2020).
Studies were ineligible if they: (i) were reviews, abstracts, letters, or case reports/series; (ii) did not report the data necessary for calculating the mean and standard deviation of the LUS score; (iii) did not mention the death/survival outcome or severity of COVID-19; or (iv) were animal studies. If there were several publications from the same study, the study with the most cases and relevant information was included.
The extracted data included the first author of the study, year of publication, country, group and participant number, gender, age, LUS time, and study design. The LUS scores were extracted and stored in the excel sheet. Numeric data were gathered directly from tables, or were inferred by digitizing the figure with GetData Graph Digitizer 2.26 when presented in graphs only (Li et al., 2017). Data extraction was performed independently by two of the reviewers (GS and XW). Disagreements were discussed and resolved by involving a third reviewer (XY) for adjudication.
The quality assessment was performed by the Newcastle-Ottawa Scale (NOS) assessment tool. Two researchers (GS and WQ) assessed the involved articles separately. Disagreements were solved by a third researcher (XW).
Statistical analysis
The pooled effects were presented as the weighted mean difference (WMD) with 95% confidence intervals (CIs). Heterogeneity was assessed using the I 2 statistic. If there was no heterogeneity (P > 0.1 or I 2 < 50%), a fixed-effects model was used to estimate the pooled WMD; otherwise, a random-effects model was utilized. Subgroup analyses were conducted based on “Country”, “Male percentage”, “Mean age”, “Patient source”, “LUS time”, and “Study design” when heterogeneity existed. Sensitivity analyses were directed to assess the influence of the individual study on the overall estimate. The symmetry of a funnel plot was analyzed to evaluate possible small sample effects and Begg's and Egger's tests were used to evaluate publication bias in the included studies. A P-value < 0.1 was considered statistically significant for asymmetry. Statistical analyses were performed using Stata (version 14.0; Stata Corp, College Station, TX, USA). Trial sequential analysis (TSA, version 0.9.5.10 Beta) was conducted according to a previous paper (Song et al., 2020).
Results
Description of the included studies
A total of 117 potentially relevant publications were identified and reviewed from five databases: 114 from PubMed, 38 from MEDLINE, 89 from Embase, 28 from Scopus, and five from Cochrane library (Figure 1 ). After application of the inclusion and exclusion criteria, 16 studies were identified (Bosso et al., 2020; de Alencar et al., 2021; Deng et al., 2021; Giorno et al., 2020; Kong et al., 2021; Li et al., 2020; Lichter et al., 2020; Mafort et al., 2021; Paolo et al., 2021; Recinella et al., 2021; Secco et al., 2021; Wangüemert Pérez et al., 2020; Xian et al., 2020; Yasukawa et al., 2021; Ye et al., 2021; Zhu et al., 2021). The baseline characteristics of the included studies are shown in Table 1 . All studies were published in these two years. Studies were conducted in Italy, China, Spain, Brazil, USA, and Israel. A total of 1541 patients with COVID-19 were included. In the studies with death as the endpoint, most (77.8%, 7/9) conducted LUS within 24 hours after admission. Seven prospective studies and nine retrospective studies were identified. The Newcastle-Ottawa Scale (NOS) scores ranging 7-9 indicated that there was no low-quality study involved.
Figure 1.
Flow-chart of study selection.
LUSS, lung ultrasound score
Table 1.
The baseline characteristics of the included studies.
| No | Study | Year | Country | Group | N | Sex (male%) | Age (years) | LUS time | Study design | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bosso | 2020 | Italy | Death | 12 | 75.0 | 71.7±12.3 | Other* | Retrospective study | 9 |
| Survivor | 14 | 64.3 | 61.1±16.5 | |||||||
| 2 | Li | 2020 | China | Death | 12 | 66.7 | 63.6±19.1 | Within 24 hours after admission | Prospective study | 9 |
| Survivor | 36 | 69.4 | 66.1±12.9 | |||||||
| 3 | Wangüemert Pérez | 2020 | Spain | Death | 10 | 44.4 | 82.4±9.9 | Within 24 hours after admission | Retrospective study | 8 |
| Survivor | 35 | |||||||||
| 4 | de Alencar | 2021 | Brazil | Death | 61 | 58.3 | 60.0±15.6 | Within 24 hours after admission | Prospective study | 9 |
| Survivor | 109 | |||||||||
| 5 | Mafort | 2021 | Brazil | Death | 3 | 31.8 | 40.0±11.9 | Other* | Retrospective study | 8 |
| Survivor | 444 | |||||||||
| 6 | Paolo | 2021 | Italy | Death | 10 | 75.0 | 69.0±11.9 | Within 24 hours after admission | Prospective study | 9 |
| Survivor | 18 | |||||||||
| 7 | Recinella | 2021 | Italy | Death | 11 | 36.4 | 90.0±9.6 | Within 24 hours after admission | Prospective study | 8 |
| Survivor | 26 | 57.7 | 80.5±13.7 | |||||||
| 8 | Secco | 2021 | Italy | Death | 79 | 67.1 | 77.0±16.5 | Within 24 hours after admission | Prospective study | 9 |
| Survivor | 165 | 67.9 | 60.0±16.8 | |||||||
| 9 | Yasukawa | 2021 | USA | Death | 9 | 61.9 | 57.9±14.2 | Within 24 hours after admission | Prospective study | 9 |
| Survivor | 96 | |||||||||
| 10 | Giorno | 2020 | Brazil | Mild | 18 | 77.8 | 1.9±4.0 | Other* | Retrospective study | 9 |
| Moderate | 8 | 50.0 | 0.3±2.7 | |||||||
| Severe | 3 | 37.5 | 2.4±2.7 | |||||||
| Critical | 5 | |||||||||
| 11 | Lichter | 2020 | Israel | Mild/moderate | 75 | 57.3 | 64.2±21.0 | Within 24 hours after admission | Prospective study | 9 |
| Severe | 31 | 67.7 | 72.3±13.0 | |||||||
| Critical | 14 | 71.4 | 72.5±24.0 | |||||||
| 12 | Xian | 2020 | China | Moderate | 15 | 54.8 | 60.0±14.3 | Other* | Retrospective study | 8 |
| Severe | 11 | |||||||||
| Critical | 5 | |||||||||
| 13 | Zhu | 2020 | China | Critical | 16 | 50.0 | 64.8±11.6 | Other* | Retrospective study | 9 |
| Non-critical | 32 | 56.3 | 62.0±14.3 | |||||||
| 14 | Deng | 2021 | China | Mild | 6 | 100.0 | 29.0±3.0 | Other* | Retrospective study | 8 |
| Moderate | 29 | 100.0 | 30.0±3.7 | |||||||
| Severe | 4 | 100.0 | 30.0±2.2 | |||||||
| 15 | Kong | 2021 | China | Severe | 63 | 39.7 | 63.4±12.2 | Within 24 hours after admission | Retrospective study | 9 |
| Critical | 33 | 60.6 | 69.2±12.7 | |||||||
| 16 | Ye | 2021 | China | Severe | 11 | 72.7 | 67.2±15.9 | Other* | Retrospective study | 7 |
| Mild/moderate | 12 | 33.3 | 53.5±13.5 |
LUS, lung ultrasound; NOS, Newcastle-Ottawa Scale
lung ultrasound exams were performed as early as possible after admission and being diagnosed with COVID-19.
Association of LUS score with mortality risk of COVID-19 and subgroup analysis
The LUS score was significantly higher in the death group compared with the survival group (WMD = 8.21, 95% CI: 4.74–11.67, P < 0.001, Figure 2 ). Subgroup analysis was conducted to investigate the possible sources of heterogeneity of death/survival (I 2 = 91.8%, P < 0.001) (Table 2 ). After being stratified by patient source, heterogeneity was slightly decreased. Subgroup analysis indicated that significant results were observed in most of the subgroup analyses.
Figure 2.
Forest plots for lung ultrasound score between the death and survival groups of COVID-19 patients.
Table 2.
Subgroup analyses of lung ultrasound score between the death and survival groups.
| Number of studies | Test of difference |
Test of Heterogeneity |
|||
|---|---|---|---|---|---|
| WMD (95% CI) | P value | I2 (%) | P value | ||
| Country | |||||
| Europe | 5 | 6.73 (3.21–10.25) | <0.001 | 79.1 | 0.001 |
| China | 1 | 4.80 (1.81–7.80) | 0.002 | - | - |
| Brazil | 2 | 16.33 (-6.33–33.99) | 0.158 | 98.7 | <0.001 |
| USA | 1 | 6.00 (1.24–10.76) | 0.014 | - | - |
| Male percentage | |||||
| > 50% | 7 | 5.46 (3.53–7.39) | <0.001 | 65.5 | 0.008 |
| < 50% | 2 | 20.10 (4.80–35.39) | 0.010 | 96.1 | <0.001 |
| Mean age | |||||
| > 65 years | 6 | 6.44 (3.50–9.39) | <0.001 | 78.1 | <0.001 |
| < 65 years | 3 | 12.84 (-0.20–25.88) | 0.054 | 97.3 | <0.001 |
| Patient source | |||||
| Emergency department | 3 | 6.25 (3.79–8.70) | <0.001 | 76.9 | 0.013 |
| Intensive care unit | 2 | 1.47 (-6.09–9.04) | 0.702 | 77.6 | 0.035 |
| General ward | 3 | 8.77 (4.48–13.06) | <0.001 | 67.0 | 0.048 |
| Outpatient | 1 | 28.02 (22.99–33.05) | <0.001 | - | - |
| LUS time | |||||
| Within 24 hours after admission | 7 | 6.33 (3.93–8.72) | <0.001 | 79.9 | <0.001 |
| Other | 2 | 16.62 (-5.64–38.89) | 0.143 | 97.8 | <0.001 |
| Study design | |||||
| Prospective study | 6 | 5.43 (3.27–7.59) | <0.001 | 71.0 | 0.004 |
| Retrospective study | 3 | 15.16 (3.57–26.75) | 0.010 | 95.6 | <0.001 |
CI, confidence interval; WMD, weighted mean difference
Association of LUS score with the severity of COVID-19
Those with mild/moderate, severe and critical COVID-19 had a progressively higher LUS score (critical vs. severe: WMD = 8.78, 95% CI: 4.17–13.38, P < 0.001; critical vs. mild/moderate/severe: WMD = 10.00, 95% CI: 6.83–13.17, P < 0.001; severe vs. moderate: WMD = 5.96, 95% CI: 3.48–8.44, P < 0.001; and severe vs. mild/moderate: WMD = 7.31, 95% CI: 4.45–10.17, P < 0.001; Figure 3 ). However, the LUS score was similar in the moderate group compared with the mild group (WMD = 6.84, 95% CI: -1.11–14.78, P = 0.092, Figure 3).
Figure 3.
Subgroup analysis of weighted mean difference (WMD) in lung ultrasound score between different severities of COVID-19.
Sensitivity analysis, publication bias and TSA
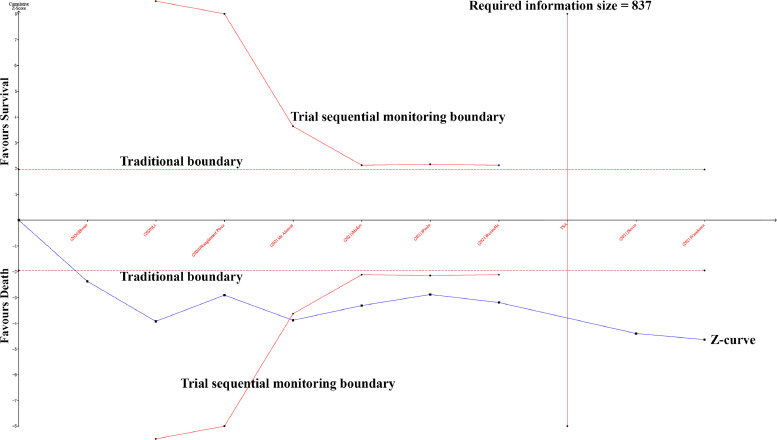
To evaluate the robustness of the results, sensitivity analyses were performed by sequentially removing each study. No apparent change occurred when an individual study was omitted. No publication bias was observed in evaluation of the funnel plots for the death and survival groups, and this finding was confirmed by Begg's (P = 0.602) and Egger's (P = 0.588) tests. No publication bias was revealed in evaluation of the funnel plots for LUS score with severity of COVID-19, and this finding was confirmed by Begg's (P = 0.837) and Egger's (P = 0.348) tests. The cumulative Z-curve passed both the traditional boundary and trial sequential monitoring boundary, suggesting sufficient evidence of such a difference between the death and survival groups (Figure 4 ).
Figure 4.
Comparison of the lung ultrasound score between death and survival groups by trial sequential analysis.
Discussion
This is the first meta-analysis to comprehensively summarize the association of LUS score with mortality and severity of COVID-19. This meta-analysis found that the LUS score was a potential prognostic index for mortality. Additionally, the LUS score was a risk stratification and monitoring tool for COVID-19, which was confirmed by sensitivity analysis, publication bias test and TSA.
Since the COVID-19 outbreak, the number of confirmed cases has increased exponentially. Although quarantine and facemask use have been reported as effective preventive measures (Hantoko et al., 2021), some countries are facing second and third waves of the COVID-19 pandemic. Hospitals are currently overcrowded. A serious problem that is arising is how to quickly and easily evaluate the severity of COVID-19, which could efficiently allocate medical resources. Meanwhile, rapid and accurate prediction of clinical adverse outcomes is central to the management of global outbreaks of infection. Stratification by this predicted tool, most commonly for death, can help doctors make treatment-related decisions.
Numerous studies have explored several prognostic factors for adverse outcomes of COVID-19, including: male sex, old age, severe obesity, hypertension, diabetes mellitus, and cigarette smoking (Hatmi, 2021). Other studies have used hematological and biochemical indices, such as C-reactive protein, IL-6 and D-dimer (Kotru et al., 2021), as alternative predictive tools. Radiologists have measured computed tomography (CT) scores to predict composite adverse outcomes (Xu et al., 2020). However, CT may not be suitable for critically ill patients because of the risks of patient transport and infecting others. The ionizing radiation of CT is a concern for children and pregnant women. The current COVID-19 pandemic has caused serious economic burden, and CT examination has been overburdened. Safe usage of CT scanners to image COVID-19 patients is also logistically challenging and can overwhelm the available resources (Chan et al., 2020).
Ultrasound has several advantages such as its ease of sterilization, low cost and absence of radiation. In recent years, LUS has emerged as an accurate diagnostic tool for respiratory diseases. LUS is also an excellent monitoring tool. During the pandemic, LUS has been applied to diagnose, monitor and follow-up cases with COVID-19. New ultrasound technologies, such as portable pocket-sized ultrasound or 5G-based robot-assisted remote ultrasound, may play an important role in emergency units and ICUs in the future of anti-COVID-19 (Ye et al., 2021).
A sonographer performs the specific LUS protocol then calculates the LUS score, which is defined as the sum of the scores of each exam zone by measuring lung aeration loss (Yin et al., 2019). Although there is no consensus about the most efficient and accurate LUS protocol, 12-zone/0–36 score is the most widely used protocol in COVID-19 patients. Previous studies have found that there were similarities between different protocols: Wangüemert Pérez et al. found that the results of 8-zone/0–24 score protocol were similar to the 12-zone/0–36 score protocol in their study (Wangüemert Pérez et al., 2020); Ramos et al. revealed that the results of the 8-zone/0–24 score protocol were similar to the 14-zone/0–42 score protocol in their cohort of COVID-19 patients (Ramos Hernández et al., 2021).
The current results revealed that survivors had a lower LUS score than the deaths. The explanation is that LUS could allow a semi-quantitative estimation of the extravascular lung water and, indirectly, of the blood oxygenation (Zong et al., 2020). A lower LUS score means less impaired aeration of the lung in COVID-19 patients (Bosso et al., 2020). The reason that the LUS score is associated with mortality of COVID-19 is that the LUS score has been found to be associated with several proven COVID-19 risk factors, including: CT severity score, C-reactive protein, IL-6, D-dimer, and PaO2/FiO2. The LUS score has been significantly positively correlated with the CT severity score (Deng et al., 2020; Nouvenne et al., 2020; Zhu et al., 2021), C-reactive protein (Møller-Sørensen et al., 2020), IL-6 (Rojatti et al., 2020), and D-dimer (Perrone et al., 2020). However, the LUS score has been significantly negatively correlated with PaO2/FiO2 (Bosso et al., 2020; Li et al., 2020; Perrone et al., 2020; Rojatti et al., 2020; Secco et al., 2021). PaO2/FiO2 has been found to be lower in COVID-19 patients, particularly those with the worst outcomes (Grasselli et al., 2020). PaO2/FiO2 can be considered as a global index of tissue aeration. All these correlations illustrate that a high LUS score is a potential risk factor for COVID-19 patients.
In the studies in this meta-analysis, some found that the LUS score was associated with other adverse outcomes, including the need for respiratory support, hospitalization, or ICU admission (de Alencar et al., 2021; Yasukawa et al., 2021); more evidence is needed to confirm these findings. Meanwhile, the LUS score was also associated with the severity of COVID-19. According to the results of this meta-analysis, a higher LUS score means a more severe COVID-19 condition. Recently, three studies already used the LUS score as a risk stratification tool for COVID-19 patients (Ji et al., 2020; Lichter et al., 2020; Rubio-Gracia et al., 2021). Several of the involved papers proposed their cut-off value of the LUS score for mortality and severity of COVID-19 (de Alencar et al., 2021; Li et al., 2020; Lichter et al., 2020; Recinella et al., 2021; Secco et al., 2021; Zhu et al., 2021). The cut-off values for predicting survival were 13 (Secco et al., 2021), 16 (de Alencar et al., 2021), 17 (Recinella et al., 2021), 18 (Lichter et al., 2020), and 22.5 (Li et al., 2020), respectively. The cut-off value for predicting critical COVID-19 was 7, with a sensitivity of 80.8% and specificity of 95.8% (Zhu et al., 2021). The application of the LUS score as a risk stratification tool needs to be discussed and verified by experts in the future.
Limitations
There were several limitations to this study: first, most studies were single-center. Single race and the small sample size cannot be ignored. These disadvantages could reduce the credibility of the conclusion of this study. Second, all LUS exams in the involved studies were performed as early as possible (most within 24 hours after admission); however, some studies did not mention the specific details of the LUS exam time. Third, some underlying confounders may not be adjustable in the involved studies, such as the therapy for COVID-19 and comorbidities in each group.
Conclusion
The LUS score was associated with mortality and severity of COVID-19. The LUS score has the potential to be a risk stratification tool for COVID-19 patients.
Acknowledgments
Conflict of interest
None declare.
Ethical approval
Not applicable.
Funding
None declare.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.06.026.
Appendix. Supplementary materials
References
- Bajaba S., Mandurah K., Yamin M. A framework for pandemic compliant higher education national system. Int J Inf Technol. 2021:1–8. doi: 10.1007/s41870-021-00629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosso G., Allegorico E., Pagano A., Porta G., Serra C., Minerva V., et al. Lung ultrasound as diagnostic tool for SARS-CoV-2 infection. Intern Emerg Med. 2020:1–6. doi: 10.1007/s11739-020-02512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandao Neto R.A., Marchini J.F., Marino L.O., Alencar J.C.G., Lazar Neto F., Ribeiro S., et al. Mortality and other outcomes of patients with coronavirus disease pneumonia admitted to the emergency department: A prospective observational Brazilian study. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0244532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.C.X., Kwok K.Y., Ma J.K.F., Wong Y.C. Radiology and COVID-19. Hong Kong Med J. 2020;26(4):286–288. doi: 10.12809/hkmj205102. [DOI] [PubMed] [Google Scholar]
- de Alencar J.C.G., Marchini J.F.M., Marino L.O., da Costa Ribeiro S.C., Bueno C.G., da Cunha V.P., et al. Lung ultrasound score predicts outcomes in COVID-19 patients admitted to the emergency department. Ann Intensive Care. 2021;11(1):6. doi: 10.1186/s13613-020-00799-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q., Cao S., Wang H., Zhang Y., Chen L., Yang Z., et al. Application of quantitative lung ultrasound instead of CT for monitoring COVID-19 pneumonia in pregnant women: a single-center retrospective study. BMC Pregnancy Childbirth. 2021;21(1):259. doi: 10.1186/s12884-021-03728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q., Zhang Y., Wang H., Chen L., Yang Z., Peng Z., et al. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single-center study. Acad Radiol. 2020;27(10):1363–1372. doi: 10.1016/j.acra.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Q., Lin Y., Yuan T.M. Lung Ultrasound, a Better Choice for Neonatal Pneumothorax: A Systematic Review and Meta-analysis. Ultrasound Med Biol. 2021;47(3):359–369. doi: 10.1016/j.ultrasmedbio.2020.11.011. [DOI] [PubMed] [Google Scholar]
- Giorno E.P.C., De Paulis M., Sameshima Y.T., Weerdenburg K., Savoia P., Nanbu D.Y., et al. Point-of-care lung ultrasound imaging in pediatric COVID-19. Ultrasound J. 2020;12(1):50. doi: 10.1186/s13089-020-00198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Ou J., Qiu X., Jie Y., Chen Y., Yuan L., et al. A Tool for Early Prediction of Severe Coronavirus Disease 2019 (COVID-19): A Multicenter Study Using the Risk Nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71(15):833–840. doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantoko D., Li X., Pariatamby A., Yoshikawa K., Horttanainen M., Yan M. Challenges and practices on waste management and disposal during COVID-19 pandemic. J Environ Manage. 2021;286 doi: 10.1016/j.jenvman.2021.112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatmi Z.N. A Systematic Review of Systematic Reviews on the COVID-19 Pandemic. SN Compr Clin Med. 2021:1–18. doi: 10.1007/s42399-021-00749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovine E., Nenna R., Bloise S., La Regina D.P., Pepino D., Petrarca L., et al. Lung Ultrasound: Its Findings and New Applications in Neonatology and Pediatric Diseases. Diagnostics (Basel) 2021;11(4):652. doi: 10.3390/diagnostics11040652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L., Cao C., Gao Y., Zhang W., Xie Y., Duan Y., et al. Prognostic value of bedside lung ultrasound score in patients with COVID-19. Crit Care. 2020;24(1):700. doi: 10.1186/s13054-020-03416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen O., Myhre P.L., Omland T. Assessing congestion in acute heart failure using cardiac and lung ultrasound - a review. Expert Rev Cardiovasc Ther. 2021;19(2):165–176. doi: 10.1080/14779072.2021.1865155. [DOI] [PubMed] [Google Scholar]
- Kameda T., Mizuma Y., Taniguchi H., Fujita M., Taniguchi N. Point-of-care lung ultrasound for the assessment of pneumonia: a narrative review in the COVID-19 era. J Med Ultrason (2001) 2021;48(1):31–43. doi: 10.1007/s10396-020-01074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong S., Wang J., Li Y., Tian Y., Yu C., Zhang D., et al. Value of Bedside Lung Ultrasound in Severe and Critical COVID-19 Pneumonia. Respir Care. 2021;66(6):920–927. doi: 10.4187/respcare.08382. [DOI] [PubMed] [Google Scholar]
- Kotru S., Klimuntowski M., Ridha H., Uddin Z., Askhar A.A., Singh G., et al. Electrochemical sensing: A prognostic tool in the fight against COVID-19. Trends Analyt Chem. 2021;136 doi: 10.1016/j.trac.2021.116198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok K.O., Huang Y., Tsoi M.T.F., Tang A., Wong S.Y.S., Wei W.I., et al. Epidemiology, clinical spectrum, viral kinetics and impact of COVID-19 in the Asia-Pacific region. Respirology. 2021;26(4):322–333. doi: 10.1111/resp.14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Qin A., Yang X., Zhou S., Luo Y., Zhu F., et al. Findings and Prognostic Value of Lung Ultrasonography in Coronal Virus Disease 2019(COVID-19) Pneumonia. Shock. 2020 doi: 10.1097/SHK.0000000000001700. Accessed November 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.M., Kang M.T., Wu S.S., Meng B., Sun Y.Y., Wei S.F., et al. Studies using concentric ring bifocal and peripheral add multifocal contact lenses to slow myopia progression in school-aged children: a meta-analysis. Ophthalmic Physiol Opt. 2017;37(1):51–59. doi: 10.1111/opo.12332. [DOI] [PubMed] [Google Scholar]
- Lichter Y., Topilsky Y., Taieb P., Banai A., Hochstadt A., Merdler I., et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020;46(10):1873–1883. doi: 10.1007/s00134-020-06212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Li T.S. [Interpretation of "Guidelines for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Infection by the National Health Commission (Trial Version 5)"] Zhonghua Yi Xue Za Zhi. 2020;100(11):805–807. doi: 10.3760/cma.j.cn112137-20200205-00199. [DOI] [PubMed] [Google Scholar]
- Ma H., Yan W., Liu J. Diagnostic value of lung ultrasound for neonatal respiratory distress syndrome: a meta-analysis and systematic review. Med Ultrason. 2020;22(3):325–333. doi: 10.11152/mu-2485. [DOI] [PubMed] [Google Scholar]
- Mafort T.T., Rufino R., da Costa C.H., da Cal M.S., Monnerat L.B., Litrento P.F., et al. One-month outcomes of patients with SARS-CoV-2 infection and their relationships with lung ultrasound signs. Ultrasound J. 2021;13(1):19. doi: 10.1186/s13089-021-00223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo P.H., Copetti R., Feller-Kopman D., Mathis G., Maury E., Mongodi S., et al. Thoracic ultrasonography: a narrative review. Intensive Care Med. 2019;45(9):1200–1211. doi: 10.1007/s00134-019-05725-8. [DOI] [PubMed] [Google Scholar]
- Mojoli F., Bouhemad B., Mongodi S., Lichtenstein D. Lung Ultrasound for Critically Ill Patients. Am J Respir Crit Care Med. 2019;199(6):701–714. doi: 10.1164/rccm.201802-0236CI. [DOI] [PubMed] [Google Scholar]
- Møller-Sørensen H., Gjedsted J., Lind Jørgensen V., Lindskov Hansen K. COVID-19 Assessment with Bedside Lung Ultrasound in a Population of Intensive Care Patients Treated with Mechanical Ventilation and ECMO. Diagnostics (Basel) 2020;10(7):447. doi: 10.3390/diagnostics10070447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouvenne A., Zani M.D., Milanese G., Parise A., Baciarello M., Bignami E.G., et al. Lung Ultrasound in COVID-19 Pneumonia: Correlations with Chest CT on Hospital admission. Respiration. 2020;99(7):617–624. doi: 10.1159/000509223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolo P., Ilaria V., Francesco Z., Edoardo F., Nicolò S., Giulio A., et al. Patients in intensive care unit for COVID-19 pneumonia: the lung ultrasound patterns at admission and discharge. An observational pilot study. Ultrasound J. 2021;13(1):10. doi: 10.1186/s13089-021-00219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone T., Soldati G., Padovini L., Fiengo A., Lettieri G., Sabatini U., et al. A New Lung Ultrasound Protocol Able to Predict Worsening in Patients Affected by Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. J Ultrasound Med. 2020 doi: 10.1002/jum.15548. Accessed November 6, 2020. [DOI] [PubMed] [Google Scholar]
- Ramos Hernández C., Botana Rial M., Pazos Area L.A., Núñez Fernández M., Pérez Fernández S., Rubianes González M., et al. [Lung Ultrasound to Predict Unfavorable Progress in Patients Hospitalized for COVID-19] Arch Bronconeumol. 2021;57(Suppl 1):47–54. doi: 10.1016/j.arbres.2020.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recinella G., Marasco G., Tufoni M., Brizi M., Evangelisti E., Maestri L., et al. Clinical Role of Lung Ultrasound for the Diagnosis and Prognosis of Coronavirus Disease Pneumonia in Elderly Patients: A Pivotal Study. Gerontology. 2021;67(1):78–86. doi: 10.1159/000512209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojatti M., Regli I.B., Zanforlin A., Ferretti E., Falk M., Strapazzon G., et al. Lung Ultrasound and Respiratory Pathophysiology in Mechanically Ventilated COVID-19 Patients-an Observational Trial. SN Compr Clin Med. 2020:1–8. doi: 10.1007/s42399-020-00536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Gracia J., Giménez-López I., Garcés-Horna V., López-Delgado D., Sierra-Monzón J.L., Martínez-Lostao L., et al. Point-of-care lung ultrasound assessment for risk stratification and therapy guiding in COVID-19 patients. A prospective non-interventional study. Eur Respir J. 2021 doi: 10.1183/13993003.04283-2020. Accessed February 25, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secco G., Delorenzo M., Salinaro F., Zattera C., Barcella B., Resta F., et al. Lung ultrasound presentation of COVID-19 patients: phenotypes and correlations. Intern Emerg Med. 2021:1–11. doi: 10.1007/s11739-020-02620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G., Qiao W., Liu K., Yu X. Epicardial adipose tissue in patients with chronic kidney disease: a meta-analysis study and trial sequential analysis. Int Urol Nephrol. 2020;52(12):2345–2355. doi: 10.1007/s11255-020-02575-y. [DOI] [PubMed] [Google Scholar]
- Wangüemert Pérez A.L., Figueira Gonçalves J.M., Hernández Pérez J.M., Ramallo Fariña Y., Del Castillo Rodriguez J.C. Prognostic value of lung ultrasound and its link with inflammatory biomarkers in patients with SARS-CoV-2 infection. Respir Med Res. 2020;79 doi: 10.1016/j.resmer.2020.100809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian J., Lu W., Li R., Zhang S., Huang M., Su Z. [The clinical value of ultrasound in the assessment of the severity of COVID-19] Chinese Journal of Ultrasonography. 2020;29(7):559–563. [Google Scholar]
- Xu P.P., Tian R.H., Luo S., Zu Z.Y., Fan B., Wang X.M., et al. Risk factors for adverse clinical outcomes with COVID-19 in China: a multicenter, retrospective, observational study. Theranostics. 2020;10(14):6372–6383. doi: 10.7150/thno.46833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa K., Minami T., Boulware D.R., Shimada A., Fischer E.A. Point-of-Care Lung Ultrasound for COVID-19: Findings and Prognostic Implications From 105 Consecutive Patients. J Intensive Care Med. 2021;36(3):334–342. doi: 10.1177/0885066620988831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R., Zhou X., Shao F., Xiong L., Hong J., Huang H., et al. Feasibility of a 5G-Based Robot-Assisted Remote Ultrasound System for Cardiopulmonary Assessment of Patients With Coronavirus Disease 2019. Chest. 2021;159(1):270–281. doi: 10.1016/j.chest.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W., Zou T., Qin Y., Yang J., Li Y., Zeng X., et al. Poor lung ultrasound score in shock patients admitted to the ICU is associated with worse outcome. BMC Pulm Med. 2019;19(1):1. doi: 10.1186/s12890-018-0755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Zhao X., Wang T., Wang Z., Guo F., Xue H., et al. Ultrasonic Characteristics and Severity Assessment of Lung Ultrasound in COVID-19 Pneumonia in Wuhan, China: A Retrospective. Observational Study. Engineering (Beijing) 2021;7(3):367–375. doi: 10.1016/j.eng.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H.F., Guo G., Liu J., Bao L.L., Yang C.Z. Using lung ultrasound to quantitatively evaluate pulmonary water content. Pediatr Pulmonol. 2020;55(3):729–739. doi: 10.1002/ppul.24635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.