Abstract
Aims
Identify the prevalence, risk factors and outcomes of lower extremity ischemic complications.
Methods
A systematic review was conducted by searching PubMed and SCOPUS databases for SARS-CoV-2, COVID-19 and peripheral arterial complications.
Results
Overall 476 articles were retrieved and 31 articles describing 133 patients were included. The mean age was 65.4 years. Pain and gangrene were the most common presentation. Hypertension (51.3%), diabetes (31.9%) and hypercholesterolemia (17.6%) were associated co-morbidities. Overall, 30.1% of patients died and amputation was required in 11.8% patients.
Conclusions
COVID-19 patients with diabetes or hypertension are susceptible for lower limb complications and require therapeutic anti-coagulation.
Keywords: COVID-19, Diabetes, Gangrene, Heparin, Peripheral arterial disease, SARS-CoV2
Introduction
The global pandemic of COVID-19 has stirred the scientific community not only because of the scale of infection affecting millions of individuals but also because of the varied presentations involving multiple organ systems. Though, COVID-19 characteristically involves the respiratory system causing acute respiratory distress syndrome (ARDS) even more distinctive is the complications of COVID-19 pertaining to the vascular system. COVID-19 is associated with cytokine storm that precipitates disseminated intravascular coagulation and thrombotic microangiopathy involving the medium and small size vessels.[1] Multiple thrombotic complications and presentations have been ascribed to COVID-19 mainly acute coronary syndrome, pulmonary thromboembolism, stroke, mesenteric ischemia, renal artery thrombosis and peripheral arterial disease (PAD) [2].
Involvement of the peripheral vasculature is relatively uncommon but there has been a surge in reported cases of peripheral gangrene after COVID-19 infection [2,3]. The cutaneous changes in COVID-19 secondary to arterial and venous thrombotic events manifest as gangrene of the extremities. The risk factors for peripheral gangrene in COVID-19 may be directly related to SARS-CoV-2 infection or secondary to cytokine storm, disseminated intravascular coagulation, hypercoagulability, thrombotic microangiopathy, use of inotropes in critically ill patients, cold antigen induced auto-immune phenomenon and complement activation or worsening of pre-existing diabetic peripheral vascular disease [4]. Peripheral gangrene in COVID-19 is more likely in patients with prior endothelial dysfunction secondary to hypertension or diabetes [4]. Patients with diabetes and foot complications are known to have poor survival and limb outcomes in the presence of co-existing peripheral arterial disease [5]. Studies have shown that patients with acute arterial thromboembolic lower limb complications due to COVID-19 are likely to have higher mortality (around 50%) compared to compared to similar patients without COVID-19 [6]. Therefore, we performed a systematic review of the reported cases of peripheral gangrene in COVID-19 patients, co-existing comorbidities, specific treatment given, and outcomes of limb amputations or death.
Methods
We conducted a literature search in the electronic database of PubMed central and SCOPUS using MeSH terms “COVID-19”; SARS-CoV-2″AND “gangrene”, “peripheral gangrene”, “peripheral arterial disease”. The words were used interchangeably for articles published in any language from Jan 2020 until June 5, 2021. Two authors conducted an independent search for case reports, case series, intervention studies, original articles reporting peripheral gangrene as outcome in COVID-19 patients. Papers that included patients who became COVID-19 positive after the occurrence of peripheral ischemia were excluded from the analysis. All articles retrieved were collated, duplicates were removed and final list prepared. In addition, reference list of the included articles were checked for additional cases. The demographic characteristics of patient population, symptom onset and duration, risk factors for peripheral arterial disease other than COVID-19 like the presence of diabetes, hypertension, hyperlipidaemia, coronary artery disease, and smoking status were noted. The duration of hospital stays, treatment offered for peripheral gangrene and outcomes in the form of limb amputation, mortality and reasons for mortality were noted.
Results
Overall, 474 articles that described ischemic complications in COVID-19 patients were retrieved from PUBMED and SCOPUS. After removing duplicates, the title and abstract of 424 publications were studied. We further excluded publications that were unrelated to peripheral gangrene but focused on gangrene of other organs example. intestine, Fournier gangrene etc. Of the selected publications, 76 were review articles, commentaries or editorial and were excluded (Fig. 1 ). Finally, 31 articles describing 133 patients with peripheral gangrene in COVID-19 were included for analysis as shown in Table 1 [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]]. The mean age of the subjects was 65.4 years with 81 males and 35 females (gender was not mentioned for 17 subjects). Mean duration of symptoms before hospital presentation was 7.4 days. Pain, paraesthesia, and gangrene of the affected extremity were the most common symptoms in addition to the COVID-19 related symptoms of fever, cough and respiratory complaints. Other presentations related to peripheral extremities included swelling of leg, acrocyanosis, limb weakness, asthenia and ischemic ulcer. Majority of the articles did not mention the time from SARS-CoV-2 positivity to the onset of gangrene.
Fig. 1.
PRISMA flowchart depicting records screened and study included for data synthesis.
Table 1.
Characteristics of subjects with COVID-19 and peripheral arterial complications.
| NAME OF THE AUTHOR | PLACE | NO. OF PATIENTS | AGE | GENDER |
COMORBIDITY |
DURATION OF SYMPTOMS (IN DAYS) | TREATMENT |
OUTCOME | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | HTN | CAD | OBESITY | Others | ANTIBIOTICS | ANTIVIRAL | ANTICOAGULANT | OTHER | ||||||||
| 1 | Zhang et al. [7] | CHINA | n = 7 | 71 | F | – | – | – | – | – | 11 | – | – | Y (n = 6) | – | 5 DEATH 2 IMPROVED |
| 63 | F | – | – | – | – | – | 13 | – | – | – | ||||||
| 59 | M | Y | Y | – | – | – | 11 | – | – | – | ||||||
| 49 | M | – | – | – | – | – | 7 | – | – | – | ||||||
| 56 | M | Y | Y | Y | – | – | 16 | – | – | – | ||||||
| 65 | M | – | Y | – | – | Cerebral Infarction | 13 | – | – | – | ||||||
| 56 | F | – | – | – | – | – | 3 | – | – | – | ||||||
| 2 | Novara et al. [8] | ITALY | n = 1 | 78 | F | – | Y | Y | – | Diverticulosis, Brady Arrhythmia | – | – | Y | Y | Amiodarone | DEATH |
| 3 | Alonso et al. [9] | SPAIN | n = 24 | AGE-44-78 Mean = 62.4 |
F:M 7:17 |
Y n = 7 | Y n = 15 | Y n = 8 | Y n = 8 | Dyslipidemia, Cancer, Autoimmune Disease Etc |
– | Y | Y | Y | Interferon, Glucocorticoids, Tocilizumab, Cyclosporine, Colchicine |
3 DEATH 21 IMPROVED |
| 4 | Mathilde et al. [10] | GERMANY | n = 1 | 73 | F | Y | Y | – | Y | Peripheral Arteriosclerosis, Pulmonary Disease, Lichen Simplex Chronicus |
– | Y | – | Y | – | IMPROVED |
| 5 | Khalid et al. [11] | UAE | n = 1 | 41 | M | Y | – | – | – | – | 14 | – | Y | Y | Tocilizumab, Interferon Beta |
AMPUTATION |
| 6 | Bamgboje et al. [12] | USA | n = 1 | 61 | M | – | Y | – | – | – | 14 | Y | Y | Y | – | IMPROVED |
| 7 | Singh et al. [13] | India | n = 1 | 64 | F | – | – | – | – | Venous Insufficiency, Vertigo, Migraine Headaches, Hypothyroidism, Tobacco Abuse |
– | Y | Y | Y | Apixaban | IMPROVED |
| 8 | Ramachandran et al. [14] | India | n = 1 | 44 | M | Y | – | – | – | – | 3 | Y | – | Y | Npwti, Pirfenidone |
AMPUTATION |
| 9 | Shubhra et al. [15] | India | n = 1 | 65 | M | – | – | – | – | – | 10 | Y | – | Y | Aspirin, Cilastazole, Inj. Pentoxifylline |
DEATH |
| 10 | Chaudhary et al. [16] | India | n = 1 | 8 | M | – | – | – | – | Red Eyes And Generalized Erythematous Rash | 7 | Y | IVIG, Methylprednisolone, Prednisone, Ceftriaxone, Aspirin |
IMPROVED | ||
| 11 | Adekiigbe et al. [17] | USA | n = 1 | 47 | M | Y | – | – | – | Chronic Back Pain | 10 | y | Y | Azithromycin, Ceftriaxone, Apixaban, Methylprednisolone, |
AMPUTATION | |
| 12 | Baccellieri et al. [18] | Italy | n = 1 | 67 | M | – | Y | – | Y | – | 5 | Y | IMPROVED | |||
| 13 | Chun et al. [19] | USA | n = 1 | 51 | M | – | – | – | – | Congenital Tricuspid Atresia, Pulmonary Stenosis | 2 | Y | AMPUTATION | |||
| 14 | Sores et al. [20] | Brazil | n = 1 | 67 | M | Y | Y | – | – | Smoker | – | Y | Y | Y | Corticosteroid | DEATH |
| 15 | Qian et al. [21] | China | n = 1 | 53 | M | – | – | – | – | – | 9 | Y | Y | Y | IMPROVED | |
| 16 | Martino et al. [22] | Italy | n = 1 | 86 | F | – | – | – | – | Acute Coronary Syndrome | – | Y | AMPUTATION | |||
| 17 | Ilonzo et al. [23] | USA | n = 4 | 62 | M | Y | Y | – | – | CKD, Smoker, Chronic Pulmonary Disease | 2 | Y | AMPUTATION | |||
| 79 | M | – | Y | – | – | Gastroesophagal Reflux | 14 | Y | AMPUTATION | |||||||
| 69 | F | Y | Y | – | – | Hyperlipdemia | 2 | Y | AMPUTATION | |||||||
| 89 | F | – | – | – | – | CKD, Atrial Fibrillation |
– | Y | AMPUTATION | |||||||
| 18 | Valle et al. [24] | Spain | n = 3 | – | – | – | – | – | – | – | 17 | Y | IMPROVED | |||
| – | – | – | – | – | – | – | 24 | Y | IMPROVED | |||||||
| – | – | – | – | – | – | – | 28 | Y | IMPROVED | |||||||
| 19 | Mascia et al. [25] | Italy | n = 14 | AGE-65-81 Mean = |
F:M – |
Y n = UK | Y n = UK | Y n = UK | Y n = UK | CKD, Smoking, Dyslipidemia | – | Y | DEATHS n = 2 IMPROVED n = 1 UKNOWN n = 11 |
|||
| 20 | Etkin et al. [26] | USA | n = 49 | AGE-58-75 Mean = |
F:M 12:37 |
Y n = 17 | Y n = 26 | Y n = 8 | Y n = 28 | CKD | Mean: 6 | Y | DEATHS n = 21 IMPROVED n = 25 UKNOWN n = 3 |
|||
| 21 | Perini et al. [27] | Italy | n = 2 | 53 | M | – | – | – | – | – | 7 | Y | DEATH n = 1 IMPROVED n = 1 |
|||
| 37 | M | – | – | – | – | – | Y | |||||||||
| 22 | Maureree et al. [28] | USA | n = 1 | 60 | M | – | Y | – | Y | – | 10 | Y | IMPROVED | |||
| 23 | Borrelli et al. [29] | Italy | n = 2 | 54 | M | – | – | – | – | Dyslipidemia | 1 | Y | Clopidogrel, Aspirin |
IMPROVED | ||
| 58 | M | Y | Y | – | – | – | 1 | Y | IMPROVED | |||||||
| 24 | Singh et al. [30] | USA | n = 3 | 71 | F | – | – | – | – | Parkinson, Dementia, Depression | – | Y | DEATH | |||
| 70 | M | – | Y | – | – | – | – | Y | IMPROVED | |||||||
| 70 | F | Y | Y | – | – | – | 7 | Y | IMPROVED | |||||||
| 25 | Kathryn et al. [31] | USA | n = 2 | 70 | F | – | – | – | – | – | 7 | Y | Apixaban | DEATH | ||
| 43 | M | – | Y | – | Y | Hyperlipidemia | 7 | Y | Y | IMPROVED | ||||||
| 26 | Veyre et al. [32] | France | n = 1 | 24 | M | – | – | – | – | – | – | Y | Aspirin | IMPROVED | ||
| 27 | Khattab et al. [33] | Egypt | n = 3 | 75 | F | – | Y | – | – | Atrial Fibrillation | – | Y | Catecholamine | DEATH | ||
| 76 | F | Y | Y | – | – | – | – | Y | IMPROVED | |||||||
| 73 | F | – | – | – | – | Non-Hodgkin Lymphoma | – | Y | DEATH | |||||||
| 28 | Ali et al. [34] | USA | n = 1 | 74 | M | Y | – | – | – | – | Y | Argatroban | AMPUTATION | |||
| 29 | Muhammed et al. [35] | UK | n = 1 | 49 | M | – | – | – | – | – | Y | Y | Aspirin, Atorvastatin |
IMPROVED | ||
| 30 | Patel et al. [36] | USA | n = 1 | 73 | M | – | Y | – | – | Smoker | Y | DEATH | ||||
| 31 | Showers et al. [37] | USA | n = 1 | 63 | F | Y | Y | – | – | Charcot Foot, Asthma | Y | Y | Aspirin, Atorvastatin, , Methylprednisolone, |
AMPUTATION | ||
CAD: Chronic Artery Disease; CKD: Chronic Kidney Disease; DM: Diabetes mellitus; HTN: Hypertension; IVIG: Intravenous immunoglobulin.
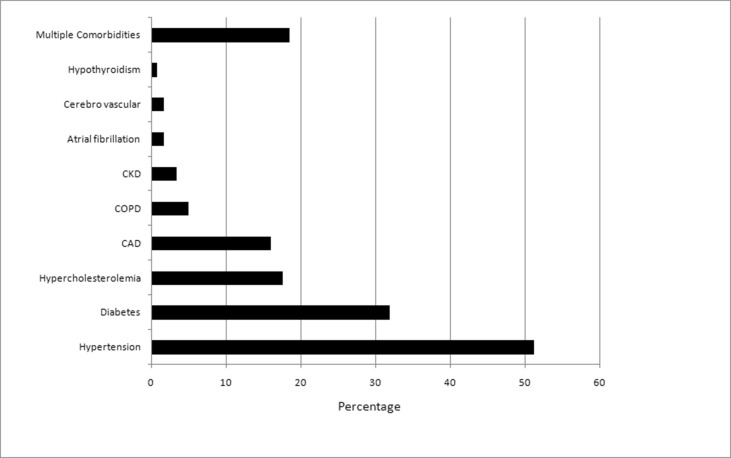
Details of pre-existing co-morbidities were available for 119 patients; hypertension was the most common associated co-morbidity present in 61 patients (51.3%), followed by diabetes in 38 (31.9%), hypercholesterolemia in 21 (17.6%), prior CAD in 19 (16.0%), COPD in 6 (5.0%), chronic kidney disease in 4 (3.4%), atrial fibrillation and prior stroke in 2 subjects each and hypothyroidism (0.84%) in one patient (Fig. 2 ). Anticoagulants were added to the COVID specific treatment for peripheral ischemia in 78.9% (n = 105) of patients. Heparin was the most prescribed anti-coagulant (n = 98), followed by dual anticoagulants (apixaban along with heparin) in 5 patients, warfarin only and apixaban only in one patient each. Overall, 30.1% of patients (n = 40) died during the hospital stay. COVID related ARDS and multiorgan failure (n = 26, 65%) were the most common cause of death followed by acute coronary event (n = 9, 22.5%) followed by invasive aspergillosis, pulmonary thromboembolism, stroke, terminal ileal perforation and intestinal bleeding in one patient each. All deaths were ascribed to severe COVID-19 illness. Amputation of the affected digit/limb was required in 11 of the 93 surviving of participants (11.8%).
Fig. 2.
Pre-existing co-morbidities in patients with COVID-19 and peripheral arterial complications.
Discussion
We analyzed the prevalence, presentation and outcomes of peripheral vascular complications in people with COVID-19. Although millions of people are afflicted with COVID-19 globally, peripheral extremity complications are uncommon. Lower limb pain and gangrene are the most frequent presentations amongst those with peripheral arterial complications. More than two-third of patients had risk factor for peripheral arterial disease including hypertension and diabetes. Almost one-third of the patients died and one in ten required limb amputations during the illness suggesting a poor prognosis.
COVID-19 is associated with a prothrombotic state and various thrombotic events predominantly involving the pulmonary and coronary vasculature in critically ill patients [38,39]. The thrombotic events in COVID-19 may manifest as pulmonary thromboembolism and acute coronary events. The incidence of clinically manifest thrombotic events is much higher in SARS-CoV-2 infection as compared to other respiratory infections such as acute influenza or other viral infections [40]. However, autopsy studies have shown that alveolar microthrombi are nine times more common in COVID-19 patients [41]. The risk of arterial thrombotic events in COVID-19 correlates with the severity of the illness as most of the events are described in critically ill patients. The risk of thrombotic events prevails in COVID-19 patients despite thrombo-prophylaxis with heparin or low molecular weight heparin that is routinely administered to all admitted patients [39]. A good correlation has been found between systemic markers of inflammation like D-dimer, fibrinogen levels and risk of thrombosis in COVID-19 [42]. However, a study by Tan et al. found a similar incidence of venous thromboembolic episodes in COVID-19 and non-COVID-19 patients admitted during the COVID-19 pandemic and no correlation between D-dimer or fibrinogen and thromboembolic events.39The coronary, pulmonary and venous thromboembolism are found to be more common than arterial thrombosis in COVID-19 which is testimony to very few cases of peripheral arterial manifestations in the literature. Peripheral arterial disease may manifest as acute lower limb pain, paraesthesias, livido reticularis, gangrene, or asymptomatic chilblain like lesions. We found that pain in the affected extremity and gangrene were the most common presenting features of peripheral arterial involvement in COVID-19 patients.
Thromboembolic risk in COVID-19 seems to be a systemic phenomenon secondary to disseminated intravascular coagulation as highlighted by markedly increased levels of inflammatory cytokineslike Il-6 and TNF-a. Also, there is a consistently increased level of fibrinogen, D-dimer, factor VIII, von Willebrand factor (vWF), and decreased antithrombin leading to a prothrombotic milieu in COVID-19. It is known that immobilized patients with critical illness are at heightened risk of thromboembolism, and COVID-9 further heightens the risk owing to a unique hypercoagulable mileu through a profound pro-inflammatory state [43]. It is proposed that viral entry into pneumocytes incites an inflammatory response that sets off a cascade of thrombosis initially localised to pulmonary vasculature and subsequent systemic response. COVID-19 is associated with endothelial injury as SARS-CoV-2 docking sites are the ACE2 receptor present on endothelial cells. It is known that SARS-CoV2 docks through its spike protein on to angiotensin-converting enzyme (ACE-2) present on the cell membrane and enters the cells. ACE-2 degrades angiotensin –II (Ang-II) and depletion of ACE-2 after binding of SARS-CoV2 to ACE-2 is associated with excess Ang-II. Ang-II binds to the Angiotensin receptor −1 and exacerbates the hypercoagulable state by increasing cytokine levels and induction of plasminogen activator inhibitor 1 (PAI-1) expression on endothelial cells. People with hypertension, diabetes and prior cardiovascular disease have reduced expression of ACE-2 that additionally contributes to high Ang-II levels in COVID-19. In addition, it has been proposed that heightened activation of monocytes and complement system confirmed by histopathological demonstration of pauci-inflammatory vasculitis with complement deposits in the affected vessel may also contribute to thrombotic microangiopathy [41]and peripheral gangrene in COVID-19.
We found that almost two-thirds of patients with reported peripheral arterial complications in COVID-19 had background hypertension, diabetes or dyslipidaemia. The risk of thrombotic peripheral arterial complications is increased manifold in patients with pre-existing endothelial dysfunction like hypertension and diabetes [44]. Diabetes (odds ratio of 2.72) and smoking (odds ratio of 1.88) are considered as the strongest risk factor for PAD [45]. It is known that diabetes being a pro-inflammatory state contributes to endothelial dysfunction, abnormal vascular smooth muscle cell (VSMC) migration into the intima layer of vessels, decreased endothelial nitric oxide synthase (eNOS) activity and platelet dysfunction that adds to hypercoagulability of COVID-19.[46] Almost one-fifth to one-third of people with diabetes have PAD that is related to the duration and severity of diabetes [47]. People with uncontrolled diabetes are more susceptible to severe COVID-19, requiring hospitalisation, thus increased likelihood of detection of peripheral arterial complications. We noticed that 10% of the subjects required limb amputation over a short duration of hospital stay and almost one fourth of the patients with COVID-19 identified in the present systematic review died due to acute coronary events that may be or not related to COVID-19. This emphasises the need for heightened screening for thrombotic complications amongst hospitalised patients with diabetes and COVID-19.
We found that almost all the patients were on therapeutic anticoagulation in the form of subcutaneous heparin (most frequent). Considering increased thrombotic risk in COVID-19, prophylactic or therapeutic anticoagulation is routinely prescribed in clinical practice. Though, the doses and duration of anticoagulation were inadequately described amongst the reported cases suggesting lack of consensus. Similarly, there is controversy regarding the prophylactic or therapeutic use of anticoagulation especially for people with co-morbidities like diabetes. The risk of thrombotic complications persists despite appropriate prophylactic anticoagulation with increased thrombotic events especially in people with diabetes, though less frequent in those receiving therapeutic doses of anticoagulants [48]. On the other hand, there is a risk of fatal bleeding episodes on higher or therapeutic anticoagulation which require careful evaluation. However, a recent study found reduced rate of thrombotic complications without bleeding risk with therapeutic anticoagulation of LMWH (dose.
(100 IU/kg/12 h SC) or (UFH (500 IU/kg/24 h) [49]. Also, a systematic review found a slightly reduced mortality in patients of COVID-19 receiving therapeutic anticoagulation [50]. Thus, people with heightened risk of thrombotic complications like diabetes may be offered therapeutic anticoagulation immediately on hospitalisation with severe COVID-19 (Table 2 ).
Table 2.
Clinical management of pro-thrombotic state in COVID-19.
| Thromboprophylaxis and COVID-19 |
|---|
| 1. Consider thromboprophylaxis∗ in |
| •Acutely ill hospitalized patients with COVID-19 |
| •Critically ill patients with COVID-19 |
| ∗Contraindicated in those with active bleeding and platelet count less than 25 × 109/L |
|
|
|
|
|
|
| 4. Routine ultrasound for detection of DVT is not required unless clinically indicated |
In conclusion, COVID-19 is a unique thrombo-inflammatory condition and patients with background diabetes or hypertension are more susceptible for lower limb complications due to peripheral arterial disease presenting as gangrene. The outcomes of COVID-19 with peripheral arterial complications are poor in terms of limb preservation and mortality. Considering the heightened risk of peripheral thrombotic complications in COVID- 19, therapeutic anticoagulation must be considered. Future studies are urgently needed to assess such treatments to reduce amputation and mortality.
References
- 1.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W., Ni Z., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sameni F., Hajikhani B., Yaslianifard S., Goudarzi M., Owlia P., Nasiri M.J., et al. COVID-19 and skin manifestations: an overview of case reports/case series and meta-analysis of prevalence studies. Front Med (Lausanne) 2020;7:573188. doi: 10.3389/fmed.2020.573188. 2020 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han H., Yang L., Liu R., Wu K.L., Li Z., Liu X.H., et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116e20. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 5.Rastogi A., Goyal G., Kesavan R., Bal A., Kumar H., Mangalanandan, et al. Long term outcomes after incident diabetic foot ulcer: multicenter large cohort prospective study (EDI-FOCUS investigators) epidemiology of diabetic foot complications study. Diab Res Clin Pract. 2020 doi: 10.1016/j.diabres.2020.108113. [DOI] [PubMed] [Google Scholar]
- 6.Hemingway J., Emanuels D., Aarabi S., Quiroga E., Tran N., Starnes B., et al. Safety of transfer, type of procedure, and factors predictive of limb salvage in a modern series of acute limb ischemia. J Vasc Surg. 2019;69:1174e9. doi: 10.1016/j.jvs.2018.08.174. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y., Cao W., Xiao M., Li Y.J., Yang Y., Zhao J., et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. ZhonghuaXue Ye Xue Za Zhi. 2020 Mar 28:41. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. Chinese. [DOI] [PubMed] [Google Scholar]
- 8.Novara E., Molinaro E., Benedetti I., Bonometti R., Lauritano E.C., Boverio R. Severe acute dried gangrene in COVID-19 infection: a case report. Eur Rev Med Pharmacol Sci. 2020 May;24(10):5769–5771. doi: 10.26355/eurrev_202005_21369. [DOI] [PubMed] [Google Scholar]
- 9.Alonso M.N., Mata-Forte T., García-León N., Vullo P.A., Ramirez-Olivencia G., Estébanez M., et al. Incidence, characteristics, laboratory findings and outcomes in acro-ischemia in COVID-19 patients. Vasc Health Risk Manag. 2020 Nov 24;16:467–478. doi: 10.2147/VHRM.S276530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen M.B., Lund M.L., Jacobsen S., Kümler T., Simonsen S., Ravn P. [Acral ischaemia with multiple microthromboses and imminent gangrene in a 73-year-old woman with COVID-19] Ugeskr Laeger. 2020 Jun 22;182(26):V05200379. [PubMed] [Google Scholar]
- 11.Alattar K.O., Subhi F.N., Saif Alshamsi A.H., Eisa N., Shaikh N.A., Mobushar J.A., et al. COVID-19-associated leukocytoclastic vasculitis leading to gangrene and amputation. ID Cases. 2021;24 doi: 10.1016/j.idcr.2021.e01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bamgboje A., Hong J., Mushiyev S., Pekler G. A 61-Year-Old Man with SARS-CoV-2 Infection and venous thrombosis presenting with painful swelling and gangrene of the lower limb consistent with phlegmasia cerulea dolens. Am J Case Rep. 2020 Dec 16;21 doi: 10.12659/AJCR.928342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S., Zuwasti U., Haas C. Coronavirus-associated coagulopathy: lessons from SARS-CoV1 and MERS-CoV for the current SARS-CoV2 pandemic. Cureus. 2020 Nov 3;12(11) doi: 10.7759/cureus.11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandran R., Vasudevan Pillai A., Raja S., Sailesh S. Axillary artery thrombosis resulting in upper limb amputation as a COVID-19 sequela. BMJ Case Rep. 2021 Jan 26;14(1) doi: 10.1136/bcr-2020-240981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shubhra S., Yadav A., Sardana K., Goila A.K. Unilateral deep vein thrombosis with gangrene involving the ascending aorta with sepsis and pulmonary thromboembolism-a pertinent cutaneous marker of severity of COVID-19. J Cosmet Dermatol. 2021 May 12 doi: 10.1111/jocd.14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaudhary H., Mohan M., Jain A., Kumar V., Takia L., Sudhakar M., et al. Acral gangrene: ugly cousin of "COVID toes" in multi-system inflammatory syndrome in children associated with SARS-COV-2? Pediatr Infect Dis J. 2021 May 3 doi: 10.1097/INF.0000000000003181. [DOI] [PubMed] [Google Scholar]
- 17.Adekiigbe R., Ugbode F., Seoparson S., Katriyar N., Fetterman A. A 47-year-old hispanic man who developed cutaneous vasculitic lesions and gangrene of the toes following admission to hospital with COVID-19 pneumonia. Am J Case Rep. 2020 Oct 1;21 doi: 10.12659/AJCR.926886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baccellieri D., Bilman V., Apruzzi L., Monaco F., D'Angelo A., Loschi D., et al. A case of covid-19 patient with acute limb ischemia and heparin resistance. Ann Vasc Surg. 2020 Oct;68:88–92. doi: 10.1016/j.avsg.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chun T.T., Jimenez J.C., Pantoja J.L., Moriarty J.M., Freeman S. Phlegmasia cerulea dolens associated with acute coronavirus disease 2019 pneumonia despite supratherapeutic warfarin anticoagulation. J Vasc Surg Cases Innov Tech. 2020 Dec;6(4):653–656. doi: 10.1016/j.jvscit.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soares R.A., Vedovello R.S., de Medeiros S.C.G., Nunes C.Z., Sian C.A., Jorge P.D.M. Covid-19 diagnosis in a patient with critical limb ischemia: complications and clinical outcomes [Diagnóstico de covid-19 empaciente com isquemiacrítica do membro: complicações e desfechosclínicos] Jornal Vascular Brasileiro. 2020;19:1–6. doi: 10.1590/1677-5449.200071. art. no. e20200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian S.Z., Pan J.Y. COVID-19 with limb ischemic necrosis. J Cardiothorac Vasc Anesth. 2020 Oct;34(10):2846–2847. doi: 10.1053/j.jvca.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sena G., Gallelli G. An increased severity of peripheral arterial disease in the COVID-19 era. J Vasc Surg. 2020 Aug;72(2):758. doi: 10.1016/j.jvs.2020.04.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilonzo N., Kumar S., Borazan N., Hansen T., Rao A., Lantis J., et al. Endotheliitis in coronavirus disease 2019-positive patients after extremity amputation for acute thrombotic events. Ann Vasc Surg. 2021 Apr;72:209–215. doi: 10.1016/j.avsg.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suarez-Valle A., Fernandez-Nieto D., Diaz-Guimaraens B., Dominguez-Santas M., Carretero I., Perez-Garcia B. Acro-ischaemia in hospitalized COVID-19 patients. J Eur Acad Dermatol Venereol. 2020 Sep;34(9):e455–e457. doi: 10.1111/jdv.16592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mascia D., Kahlberg A., Melloni A., Rinaldi E., Melissano G., Chiesa R. Single-Center vascular hub experience after 7 weeks of COVID-19 pandemic in lombardy (Italy) Ann Vasc Surg. 2020 Nov;69:90–99. doi: 10.1016/j.avsg.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Etkin Y., Conway A.M., Silpe J., Qato K., Carroccio A., Manvar-Singh P., et al. Acute arterial thromboembolism in patients with COVID-19 in the New York city area. Ann Vasc Surg. 2021 Jan;70:290–294. doi: 10.1016/j.avsg.2020.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perini P., Nabulsi B., Massoni C.B., Azzarone M., Freyrie A. Acute limb ischaemia in two young, non-atherosclerotic patients with COVID-19. Lancet. 2020 May 16;395(10236):1546. doi: 10.1016/S0140-6736(20)31051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurera A.H., Vu J.H., Rehring T.F., Layman P.F., Johnson S.P. Acute limb ischemia in minimally symptomatic SARS-CoV-2 infection. J Vasc Interv Radiol. 2020 Dec;31(12):2150–2153. doi: 10.1016/j.jvir.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borrelli M.P., Buora A., Scrivere P., Sponza M., Frigatti P. Arterial thrombotic sequalae after covid-19: mind the gap. Ann Vasc Surg. 2021 May 1;(21) doi: 10.1016/j.avsg.2021.04.009. S0890-5096. 00356-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh B., Aly R., Kaur P., Gupta S., Vasudev R., Virk H.S., et al. COVID-19 infection and arterial thrombosis: report of three cases. Annals Vascular Surgery. 2021;70:314–317. doi: 10.1016/j.avsg.2020.08.115. ISSN 0890-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz K., Wolf J.M. Digital ischemia in COVID-19 patients: case report. J Hand Surg Am. 2020 Jun;45(6):518–522. doi: 10.1016/j.jhsa.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veyre F., Poulain-Veyre C., Esparcieux A., Monsarrat N., Aouifi A., Lapeze J., Chatelard P. Femoral arterial thrombosis in a young adult after nonsevere COVID-19. Ann Vasc Surg. 2020 Nov;69:85–88. doi: 10.1016/j.avsg.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khattab K., Kempa A.T., Atas R., Asani H., Ehab A. Peripheral ischemic limb necrosis (Acro-ischemia) associated with severe COVID-19 patients (COVID-19 limbs): a report of three cases. Lung India. 2021 Mar;38(Supplement):S58–S60. doi: 10.4103/lungindia.lungindia_470_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ali Z., Ullah W., Saeed R., Ashfaq A., Lashari B. Acute COVID-19 induced fulminant systemic vascular thrombosis: a novel entity. Int J Cardiol Heart Vasc. 2020 Oct;30:100620. doi: 10.1016/j.ijcha.2020.100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muhammad K., Tantawy T.G., Makar R.R., Olojugba O. Successful catheter-directed thrombolysis for acute lower limb ischemia secondary to COVID-19 infection. Ann Vasc Surg. 2021 Feb;71:103–111. doi: 10.1016/j.avsg.2020.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel P., Yu Y., Zia S., Padberg F., Curi M., Huang J. Systemic thrombolysis as initial treatment of COVID-19 associated acute aortoiliac and lower extremity arterial thrombosis. Ann Vasc Surg. 2021 Jan;70:297–301. doi: 10.1016/j.avsg.2020.08.083. Epub 2020 Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Showers C.R., Nuovo G.J., Lakhanpal A., Siegel C.H., Aizer J., Elreda L., et al. A covid-19 patient with complement-mediated coagulopathy and severe thrombosis. Pathobiology. 2021;88(1):28–36. doi: 10.1159/000512503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020 Jul;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan C.W., Tan J.Y., Wong W.H., Chiong M.A., Ng I.M., Conceicao E.P., et al. Clinical and laboratory features of hypercoagulability in COVID-19 and other respiratory viral infections amongst predominantly younger adults with few comorbidities. Sci Rep. 2021;11:1793. doi: 10.1038/s41598-021-81166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA. 2020 Aug 25;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020 Jul 9;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertoletti L., Bikdeli B., Zuily S., Blondon M., Mismetti P. Thromboprophylaxis strategies to improve the prognosis of COVID-19. Vascul Pharmacol. 2021 Jun 4:106883. doi: 10.1016/j.vph.2021.106883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abou-Ismail M.Y., Diamond A., Kapoor S., Arafah Y., Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May 2;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fowkes F.G., Rudan D., Rudan I., Aboyans V., Denenberg J.O., McDermott M.M., et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013 Oct 19;382(9901):1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 46.Pomero F., Di Minno M.N.D., Fenoglio L., Ageno W., Dentali F. Is diabetes a hypercoagulable state? A critical appraisal. Acta Diabetol. 2015;52:1007–1016. doi: 10.1007/s00592-015-0746-8. [DOI] [PubMed] [Google Scholar]
- 47.Marso S.P., Hiatt W.R. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006 Mar 7;47(5):921–929. doi: 10.1016/j.jacc.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 48.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M., et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020 Jul;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helms J., Severac F., Merdji H., Schenck M., Clere-Jehl R., Baldacini M., et al. CRICS TRIGGERSEP group (clinical research in intensive care sepsis trial group for global evaluation research in sepsis). Higher anticoagulation targets and risk of thrombotic events in severe COVID-19 patients: bi-center cohort study. Ann Intensive Care. 2021 Jan 25;11(1):14. doi: 10.1186/s13613-021-00809-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wijaya I., Andhika R., Huang I. The use of therapeutic-dose anticoagulation and its effect on mortality in patients with COVID-19: a systematic review. Clin Appl Thromb Hemost. 2020 Jan-Dec;26 doi: 10.1177/1076029620960797. 1076029620960797. [DOI] [PMC free article] [PubMed] [Google Scholar]