Summary
Massive vaccination offers great promise for halting the global COVID-19 pandemic. However, the limited supply and uneven vaccine distribution create an urgent need to optimize vaccination strategies. We evaluate SARS-CoV-2-specific antibody responses after Sputnik V vaccination of healthcare workers in Argentina, measuring IgG anti-spike titers and neutralizing capacity after one and two doses in a cohort of naive or previously infected volunteers. By 21 days after receiving the first dose of the vaccine, 94% of naive participants develop spike-specific IgG antibodies. A single Sputnik V dose elicits higher antibody levels and virus-neutralizing capacity in previously infected individuals than in naive ones receiving the full two-dose schedule. The high seroconversion rate after a single dose in naive participants suggests a benefit of delaying administration of the second dose to increase the number of people vaccinated. The data presented provide information for guiding public health decisions in light of the current global health emergency.
Keywords: Sputnik V, SARS-CoV-2 vaccination, seroconversion, COVIDAR IgG, neutralizing antibodies, COVID-19, WHO SARS-CoV-2 antibody International Standard
Graphical abstract
Highlights
First dose of Sputnik V results in 94% seroconversion rate in naive individuals
A second dose greatly increases antibody titers and neutralizing capacity
One dose in seropositive individuals elicits higher titers than two doses in naive
There is no evident benefit of using a second dose in previously infected individuals
Rossi et al. provide data on antibody responses to Sputnik V vaccine in naive and previously infected volunteers. This study shows a high seroconversion rate following the first dose in naive individuals. In seropositive participants, a single dose of Sputnik V elicits a fast and robust antibody response without apparent benefit from a second dose.
Introduction
Sputnik V (Gam-COVID-Vac) is a combined vector vaccine based on recombinant adenovirus (rAd) type 26 and rAd5.1 A two-dose protocol displays 91.6% efficacy against coronavirus disease 2019 (COVID-19).2 In the context of the current pandemic, an important question is whether administration of a single Sputnik V dose would achieve a greater public health benefit than a two-dose protocol, allowing protection of a larger population more quickly. The vaccine developed by AstraZeneca, also a two-dose, single rAd-vectored vaccine, demonstrated 76.0% (59.3%–85.9%) efficacy after a single standard dose,3 supporting a longer interval between the two doses. In the case of the mRNA vaccines mRNA-1273 (Moderna) and BNT162b2 (Pfizer), individuals with prior infection may acquire sufficient immunity after a single dose, with no apparent benefit of a two-dose protocol.4,5
Results and discussion
We evaluated antibody responses and viral neutralizing capacity in participants receiving one and two doses of Sputnik V, with or without prior severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection (N = 227 and N = 62, respectively). Plasma samples were taken on three occasions: before vaccination (baseline), 21 days after the first dose, and 21 days after the second dose. SARS-CoV-2 spike immunoglobulin G (IgG) was measured using a previously described enzyme-linked immunosorbent assay by titration6 and quantification with the WHO International Standard for comparing data from different laboratories.7 Virus-neutralizing antibodies were evaluated using a wild-type (WT) SARS-CoV-2 and a pseudotyped vesicular stomatitis virus (VSV) spike-expressing GFP.8
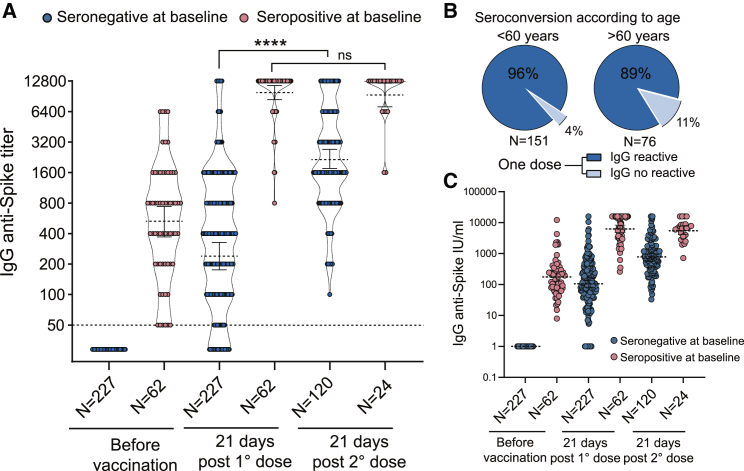
After the first dose of Sputnik V, 94% of seronegative participants at baseline showed a positive SARS-CoV-2 IgG response with a geometric mean titer (GMT) of 244 (95% confidence interval [CI] 180–328) (Figure 1A). Segregation of participants by age showed seroconversion in 96% and 89% of individuals younger or older than 60 years old, respectively (Figure 1B). After the second dose, 100% of participants showed seroconversion, with a GMT of 2,148 (95% CI 1,742–2,649).
Figure 1.
Immune response to SARS-CoV-2 Sputnik V vaccine
(A) Quantitative SARS-CoV-2 spike antibody titers for 288 participants, with or without prior infection (indicated as seropositive or seronegative at baseline, respectively). Measurements were performed before vaccination (baseline), 21 days after the first dose, and 21 days after the second dose. Geometric means with 95% confidence intervals are shown. The Mann-Whitney U test was used to compare at various time points antibody titers. Statistical significance is shown with the following notations: ∗∗∗∗p < 0.0001; ns, not significant.
(B) Seroconvertion after one dose in participants older or younger than 60 years.
(C) Quantification of antibody levels by WHO International Standard.
Participants with SARS-CoV-2 antibodies at baseline developed high antibody titers within 21 days of receiving the first vaccine dose. The GMTs were 531 (95% CI 380–742) before and 9,850 (95% CI 8,460–11,480) after the first dose, showing a 19-fold increase in specific antibody levels. By contrast, IgG anti-spike titers after one or two doses were not significantly different: GMTs of 9,850 (95% CI 8,460–11,480) and 9,590 (95% CI 7,410–12,408) after one and two doses, respectively.
As reference for comparison among laboratories, IgG levels were expressed as international units (IUs) after normalization with the WHO International Standard for anti-SARS-CoV-2 antibody (Figure 1C). The GM of IgG concentrations in IU/mL was 104.2 (95% CI 92.3–157.5) after the first dose and 787.8 (95% CI 626.0–936.2) after the second dose in naive individuals, and 181.1 (95% CI 105.2–243.7), 6,356 (95% CI 5,409–14,763), and 5,609 (95% CI 3,621–8,496) for baseline, one and two doses, respectively, in previously infected individuals.
Notably, antibody titers after one dose in participants with preexisting immunity were significantly higher than those in naive vaccinees receiving one or even two doses (p < 0.0001, two-tailed Mann-Whitney test). GMTs for previously infected participants after one dose were 40- and 4.6-fold higher than those for naive individuals receiving one or two doses, respectively (Figure 1A). This highlights the robust response to the vaccination of previously infected individuals, suggesting that naturally acquired immunity may be enhanced sufficiently by a single dose, in agreement with recent studies using mRNA vaccines.4,5
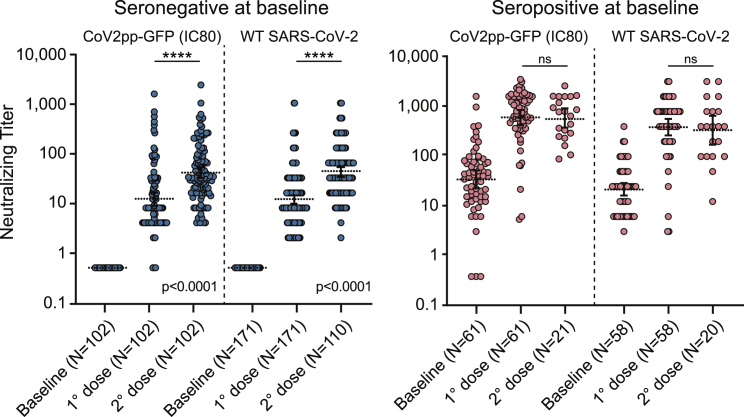
Neutralizing capacity was evaluated by measuring antibody neutralizing titers for seronegative and seropositive individuals at baseline, using the WT SARS-CoV-2 or CoV2pp GFP pseudovirus infection. For the WT SARS-CoV-2, neutralization titers were defined as the highest serum dilution without any cytopathic effect on the monolayer, and for the pseudotyped virus antibody, concentration for 80% inhibition of infection (IC80) was determined (see Figure 2, and for 50% inhibition of infection IC50, see Figure S1). At 21 days after administration of the first dose, neutralizing antibodies were detected in 90% of naive participants (seronegative at baselines), with GM neutralizing titers of 12 (95% CI 10–14) with WT SARS-CoV-2 (Figure 2, left panel). Neutralizing GMTs after the complete two-dose vaccine protocol in naive individuals was 42 (95% CI 33–53) (Figure 2, left panel), which was higher than that observed for convalescent individuals (seropositive at baseline) before vaccination, which was 28 (95% CI 23–40) (Figure 2, right panel). Notably, a robust increase in neutralizing antibody activity was observed after a single vaccine dose in participants with prior infection. In this regard. neutralizing GMTs were 500 (95% CI 341–732) and 782 (95% CI 557–1,099) following infections with the WT and pseudotype virus, respectively (Figure 2, right panel), indicating that a single dose of the vaccine triggers a large production of neutralizing antibodies in convalescent individuals, which did not increase after a second dose.
Figure 2.
Neutralizing capacity with and without prior SARS-CoV-2 infection after one and two doses of Sputnik V vaccine
Neutralizing titers were measured by 80% inhibition for the pseudotyped virus (CoV2pp GFP) in 232 participants. For WT SARS-CoV-2, neutralization titer was defined as the highest serum dilution without any cytopathic effect on the monolayer. Titers at baseline and 21 days after one or two doses are shown. Left and right panels display data from individuals who were seronegative or seropositive at baseline, as indicated at top, respectively. Geometric means with 95% confidence intervals are shown in the dashed lines. The Mann-Whitney U test was used to compare at various time points antibody titers. Statistical significance is shown with the following notations: ∗∗∗∗p < 0.0001; ns, not significant.
Conclusions
This study provides new data about antibody responses to Sputnik V vaccine in SARS-CoV-2 naive and previously infected volunteers. We observed a high seroconversion rate following a single vaccine dose in naive individuals. A global 94% of vaccinees elicited specific anti-spike antibody responses, with 90% displaying WT virus-neutralizing capacity. Importantly, a single dose of Sputnik V vaccine caused a fast and robust immune response in seropositive participants, with neutralizing titers that exceeded those found in seronegative participants who received two doses. Although a protective role of anti-spike antibodies against COVID-19 has been reported, the level of protection required for a beneficial outcome during infection is uncertain, and further efficacy studies combined with quantitative information on levels of anti-spike antibodies are needed to define the minimum threshold required for protection. Presentation of our data in IUs allows comparison of measurements obtained using different technologies, helping to define antibody levels associated with protection after vaccination. Evidence based on quantitative information will guide vaccine deployment strategies in the face of worldwide vaccine supply restrictions.
Limitations of study
This study shows high humoral responses after Sputnik V administration using a cohort of 288 volunteers from over 200,000 vaccinated healthcare workers. However, an efficacy study has not been carried out yet and will be necessary to assess vaccine protection in the population and to define correlates with antibody titers. In addition, follow-up studies are necessary to evaluate the duration of the immune response.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| SARS-CoV-2 | Gift from Dr. Sandra Gallegos | 2019 hCoV-19/Argentina/PAIS-G0001/2020 |
| VSV-eGFP-SARS-CoV-2 | Gift from Dr. Sean Whelan8 | N/A |
| Biological samples | ||
| Patient serum set | This study | https://dx.doi.org/10.17632/5bjwph8xkr.1 |
| Chemicals, peptides, and recombinant proteins | ||
| DMEM high glucose | Thermo Fisher | Cat# D5796 |
| DPBS powder, no calcium, no magnesium, 10x1L | Thermo Fisher | Cat# 21600010 |
| Trypsin, 0.05% (1X) with EDTA 4Na, liquid | Thermo Fisher | Cat# 25300-054 |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Fisher | Cat# 15140-122 |
| Paraformaldehyde | Sigma Aldrich | Cat# 30525-89-4 |
| DAPI, FluoroPure grade | Thermo Scientific | Cat# D21490 |
| Critical commercial assays | ||
| SARS-CoV-2 antibody ELISA (IgG) Kit | Laboratorio LEMOS6 | COVIDAR IgG |
| Deposited data | ||
| Dataset uploaded to Medeley | This study | https://dx.doi.org/10.17632/5bjwph8xkr.1 |
| Experimental models: Cell lines | ||
| Vero E6 | ATCC | Cat# CRL-1586 |
| 293T ACE2/TMPRSS2 | Gift from Dr. Benhur Lee | N/A |
| Software and algorithms | ||
| GraphPad Prism V8.0 | GraphPad | https://www.graphpad.com |
| Image Acquired: InCell Analyzer 2200 | GE Life Sciences | 7.1-16402 |
| Image Analyses: InCell Analyzer 1000 Workstation | GE Life Sciences | 3.7.3 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Andrea V. Gamarnik (agamarnik@leloir.org.ar).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Human subjects and samples
This study monitors the humoral immune response over time in health care workers immunized with Sputnik V vaccine. Study enrollment started in January 2021 and is ongoing. Ethical approval was obtained from the central committee of the Ministry of Health of Buenos Aires and all participants provided written informed consent prior to collection of data and specimen (Cod#2021-00983502). Blood was collected by venipuncture into SST tubes (BD Sciences) for serum and stored at −20°C. All specimens were de-identified prior to processing and antibody testing for all serum specimens.
Cell lines
Vero E6 cells (ATCC) and 293T ACE2/TMPRSS2 cells, kindly provided by Dr. Benhur Lee, were cultured at 37°C in 5% CO2 in Dulbecco’s Modified Eagle’s high glucose medium (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS) (GIBCO).
Recombinant VSV
Viral stocks (VSV-eGFP-SARS-CoV-2), generated in Sean Whelan laboratory,8 were amplified in our laboratory using 293T ACE2/TMPRSS2 cells at an MOI of 0.01 in Dulbecco’s Modified Eagle’s medium containing 2% FBS at 37°C. Viral supernatants were harvested upon extensive cytopathic effect and GFP positive cells. The media was clarified by centrifugation at 1,000 x g for 5 min. Viral stocks were titrated by fluorescence forming units per milliliter (UFF/ml) in Vero cell line. Aliquots were maintained at −80°C.
SARS-CoV-2
SARS-CoV-2 strain 2019 hCoV-19/Argentina/PAIS-G0001/2020 was obtained from Dr. Sandra Gallegos (InViV working group). Virus was passaged in Vero E6 cells. Work with SARS-CoV-2 was approved by the INBIRS Institutional Biosafety Committee at Biosafety level 3 with negative pressure.
Method details
SARS-CoV-2 antibody ELISA
Antibodies to SARS-CoV-2 spike protein were detected using an established two step ELISA previously described.6 This assay has plates coated with a mixture of spike and the receptor binding domain (RBD). For end point titrations, samples were serially diluted in IgG SARS-CoV-2 negative serum or fetal bovine serum (FBS) from 1/50 to 1/12800. The SARS-CoV-2 antibody concentration of each sample expressed in International Units/mL (UI/mL)7 was calculated by extrapolation of the OD 450 nm value on a calibration curve. For construction of the calibration curve, we determined OD 450 nm of serial dilutions of the WHO International Standard for anti-SARS-CoV-2 immunoglobulin. The linear range used was 0.2-1.5 OD 450nm. Therefore, we performed serial dilutions of the samples in order to find conditions where the OD 450nm of each sample fit adequately in the linear range.
SARS-CoV 2 neutralization assay
Serum samples were heat-inactivated at 56°C for 30min and serial dilutions from 1/2 to 1/8192 were incubated for 1hs at 37°C in the presence of WT SARS-CoV-2 (hCoV-19/Argentina/PAIS-G0001/2020, GISAID Accession ID: EPI_ISL_499083) in DMEM 2% FBS. Fifty l of the mixtures were then deposited over Vero cells monolayers for an hour at 37°C (MOI = 0.01). Infectious media was removed and replaced for DMEM 2% FBS. After 72 hs, cells were fixed with PFA 4% (4°C 20min) and stained with crystal violet solution in methanol. The cytopathic effect (CPE) of the virus on the cell monolayer was assessed visually, if even a minor damage to the monolayer (1-2 «plaques») was observed in the well, this well was considered as a well with a manifestation of CPE. Neutralization titer was defined as the highest serum dilution without any CPE in two of three replicable wells.
Pseudovirus neutralization assay
Neutralization assays were carried out with SARS-CoV-2 pseudotyped particles (CoV2pp-GFP), generated in Sean Whelan laboratory.8 CoV2pp-GFP carries vesicular stomatitis virus as viral backbone, bearing the E gene in place of its G glycoprotein (VSV-eGFP-SARS-CoV-2), and expresses full length wild-type spike from Wuhan on its envelope. Vero cells were used for these assays. Cells were maintained with DMEM high glucose with 10% FBS and were seeded in a 96-well plate the day before infection. Patient sera were heat inactivated at 56°C for 30 minutes and serially diluted in DMEM high glucose medium. Serum neutralizations were performed by first diluting the inactivated sample 2-folds and continuing with a 2-fold serial dilution. A pre-titrated amount of pseudotyped particles was incubated with a 2-fold serial dilution of patient sera for 1 h at 37°C prior to infection. Subsequently, cells were fixed in 4% formaldehyde containing 2 μg/mL DAPI nuclear stain (Invitrogen) for 1 hour at room temperature, and fixative was replaced with PBS. Images were acquired with the InCell 2000 Analyzer (GE Healthcare) automated microscope in both the DAPI and FITC channels to visualize nuclei and infected cells (i.e., eGFP-positive cells), respectively (4X objective, 4 fields per well, covering the entire well). Images were analyzed using the Multi Target Analysis Module of the InCell Analyzer 2000 Workstation Software (GE Healthcare). GFP-positive cells were identified in the FITC channel using the top-hat segmentation method and subsequently counted within the InCell Workstation software. Absolute inhibitory concentrations (absIC) values were calculated for all patient sera samples by modeling a 4-parameter logistic (4PL) regression with GraphPad Prism 8. The 4PL model describes the sigmoid-shaped response pattern. For clarity, it is assumed that the response can be expressed so that the slope increases as the concentration increase. Absolute inhibitory concentration (absIC) was calculated as the corresponding point between the 0% and 100% assay controls. Eighty % inhibition were defined by the controls for all the samples on the same plate. For example, the absIC80 would be the point at which the curve matches inhibition equal to exactly 80% of the 100% assay control relative to the assay minimum. Furthermore, 50% inhibition was also calculated by modeling a 4-parameter logistic (4PL) regression with GraphPad Prism 8.
Quantification and statistical analysis
All statistical tests and plots were performed using GraphPad Prism 8.0 software. Comparisons of the antibody titers at various time points were made using the Mann Whitney U test in Figures 1A, 2, and S1. Statistical significance is shown in the figure legends with the following notations: ∗∗∗∗p < 0.0001; ns: not significant. Geometric means with 95% confidence intervals were calculated in all time points. In all figures “N” represents the number of human serum sample tested.
Acknowledgments
The authors are grateful to the Sean Whelan laboratory for providing the VSV-eGFP-SARS-CoV-2 pseudovirus and advice on neutralization assays and Dr. Ana Fernandez Sesma for helpful discussions. This work was supported by NIH (NIAID) R01.AI095175, PICT-2017-1717, and PICT-2015-2555 (to A.V.G.) and by Fundación Williams and the National Ministry of Science, Technology, and Innovation of Argentina. We are grateful to the following institutions and staff who contributed to the sample collection: Biobanco de Enfermedades Infecciosas: Alejandro Czernikier, Yanina Ghiglione, Denise Giannone, María L. Polo, Florencia Quiroga, Gabriela Turk, and Belen Vecchione; HIEAC “San Juan de Dios”: Andrea Gatelli, Sofia Di Bella, Agustina Martinez, Martina Ferioli, Francisco Echeverria, Ramiro Agüero, Ana Caproli, and Karina Gil; HIGA “Dr. Rodolfo Rossi”: Claudia Varela, Ángeles Baridon, Soledad Ocampo, Emanuel Zapata, Melina Cancela, and Verónica Forneris; HIGA “San Martín”: Sebastián Gutiérrez, María Maxwell, Rosario Marcó, Cecilia Zolorzano, Micaela Nieva, and Claudia Conta; HIGA “Evita”: Silvina Olivera, Isabel Desimone, and Alejandra Musto; HIGA “Dr. Pedro Fiorito”: Aime Balanzino, Katherina Prost, Miriam Pereiro, Eliana Correa, Noelia Portillo, Cynthia Leguizamon, and Alicia Quetglas; HIGA “San Roque”: Mariana Artazcoz, Agustina Venturi Grossi, Rosana Toro, Anabella Masci, and Sofía Padín; HAC “El Cruce- Néstor Kirchner”: Mabel Skrypnik, Blanca Guevara, Virginia Aniasi, and Alan Estigarribia.
Author contributions
Conceptualization, A.H.R., J.G., G.D., M.P., N.K. and A.V.G.; general coordination, M.P., A.H.R., A.A.J., and A.V.G.; collection of serum samples and clinical data, D.P., A. Rima, C.E., R.E., P.G., S.M., M.Z., Y.L., and N.L.; production of SARS-CoV-2 antibody ELISA (IgG) Kit (COVIDAR), D.S.O., L.S., M.M.G.L.L., H.M.P., G.S.C.N., D.A., J.J.C., and J.C.; IgG anti-spike titer determination, D.S.O., L.S., N.R., C.I.G., S.D.W., L.Y.R., M.G.B., M.J.d.L., C.E.H., S.S., L.B., A. Ríos, M.S.T.C., Y.L., and N.L.; determination of neutralizing titers using WT SARS-CoV-2 virus, A.V., I.M., and J.G.; determination of neutralizing titers using CoV2pp GFP pseudotyped virus, D.S.O., L.S., M.M.G.L.L., D.A., and S.O.R.; data curation and analysis, D.S.O., L.S., S.O.R., J.J.C., M.Y., and A.V.G.; writing – original draft, M.M.G.L.L., D.S.O., L.S., S.O.R., M.Y., and A.V.G.; funding acquisition, A.V.G.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. We worked to ensure gender balance in the recruitment of human subjects. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
Published: July 13, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2021.100359.
Supplemental information
Data and code availability
Datasets generated in this study have been uploaded to https://data.mendeley.com at https://dx.doi.org/10.17632/5bjwph8xkr.1.
References
- 1.Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatulin A.I., Shcheblyakov D.V., Dzharullaeva A.S., Grousova D.M., Erokhova A.S., Kovyrshina A.V., Botikov A.G. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., Gam-COVID-Vac Vaccine Trial Group Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung I.F.N., Poland G.A. Single-dose Oxford-AstraZeneca COVID-19 vaccine followed by a 12-week booster. Lancet. 2021;397:854–855. doi: 10.1016/S0140-6736(21)00528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., Bermúdez-González M.C., Bielak D.A., Carreño J.M., Chernet R.L. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. N. Engl. J. Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebinger J.E., Fert-Bober J., Printsev I., Wu M., Sun N., Prostko J.C., Frias E.C., Stewart J.L., Van Eyk J.E., Braun J.G. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ojeda D.S., Gonzalez Lopez Ledesma M.M., Pallarés H.M., Costa Navarro G.S., Sanchez L., Perazzi B., Villordo S.M., Alvarez D.E., Echavarria M., Oguntuyo K.Y., BioBanco Working Group Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLoS Pathog. 2021;17:e1009161. doi: 10.1371/journal.ppat.1009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristiansen P.A., Page M., Bernasconi V., Mattiuzzo G., Dull P., Makar K., Plotkin S., Knezevic I. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397:1347–1348. doi: 10.1016/S0140-6736(21)00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Case J.B., Rothlauf P.W., Chen R.E., Liu Z., Zhao H., Kim A.S., Bloyet L.M., Zeng Q., Tahan S., Droit L. Neutralizing Antibody and Soluble ACE2 Inhibition of a Replication-Competent VSV-SARS-CoV-2 and a Clinical Isolate of SARS-CoV-2. Cell Host Microbe. 2020;28:475–485.e5. doi: 10.1016/j.chom.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets generated in this study have been uploaded to https://data.mendeley.com at https://dx.doi.org/10.17632/5bjwph8xkr.1.