Abstract
Background
Early psychosis in first-episode psychosis (FEP) and clinical high-risk (CHR) individuals has been associated with alterations in mean regional measures of brain morphology. Examination of variability in brain morphology could assist in quantifying the degree of brain structural heterogeneity in clinical relative to healthy control (HC) samples.
Methods
Structural magnetic resonance imaging data were obtained from CHR (n = 71), FEP (n = 72), and HC individuals (n = 55). Regional brain variability in cortical thickness (CT), surface area (SA), and subcortical volume (SV) was assessed with the coefficient of variation (CV). Furthermore, the person-based similarity index (PBSI) was employed to quantify the similarity of CT, SA, and SV profile of each individual to others within the same diagnostic group. Normative modeling of the PBSI-CT, PBSI-SA, and PBSI-SV was used to identify CHR and FEP individuals whose scores deviated markedly from those of the healthy individuals.
Results
There was no effect of diagnosis on the CV for any regional measure (P > .38). CHR and FEP individuals differed significantly from the HC group in terms of PBSI-CT (P < .0001), PBSI-SA (P < .0001), and PBSI-SV (P = .01). In the clinical groups, normative modeling identified 32 (22%) individuals with deviant PBSI-CT, 12 (8.4%) with deviant PBSI-SA, and 21 (15%) with deviant PBSI-SV; differences of small effect size indicated that individuals with deviant PBSI scores had lower IQ and higher psychopathology.
Conclusions
Examination of brain structural variability in early psychosis indicated heterogeneity at the level of individual profiles and encourages further large-scale examination to identify individuals that deviate markedly from normative reference data.
Keywords: early psychosis, brain morphology, heterogeneity, coefficient of variation, person-based similarity index
Introduction
Schizophrenia is a major mental illness that presents with positive (ie, delusions, hallucinations, disorganization of speech, and behavior) and negative (ie, decreased motivation and diminished expressiveness) symptoms and cognitive impairment.1,2 The syndromal expression of schizophrenia is often preceded by attenuated or time-limited forms of positive symptoms,3 often associated with neurocognitive abnormalities.4–6 Individuals with such presentations are considered clinical high-risk (CHR) cases because they carry a high risk of syndromal transition.7 Neuroimaging has been extensively used to identify alterations in brain organization associated with schizophrenia and CHR states. Associations between structural changes and syndromal schizophrenia primarily involve widespread reductions in cortical thickness (CT) and subcortical volume (SV) that are present as early as at the first-episode psychosis (FEP).8–13 Structural brain findings in CHR studies overlap partially with those observed in syndromal cases and mainly involve reductions in hippocampal/parahippocampal areas, and in medial prefrontal regions.14–17
The findings reported above reflect case-control differences in the mean values of the morphological measures assessed and do not capture inter-individual variability in brain morphology.18–22 For example, a large meta-analysis has found that the degree of variability in morphological measures in patients with schizophrenia was higher in the temporal cortex, the thalamus, and the putamen and lower in the anterior cingulate cortex.18 More recently, there have been attempts to measure the degree of deviation of each patient’s brain structural data against a normative reference dataset. Wolfers et al.21 estimated voxel-level deviation in each patient with schizophrenia as a function of the z-score from the corresponding mean of a group of healthy individuals (HI) who represented the normative reference sample. Kochunov and colleagues22 used a similar approach but with regional measures of CT and SV as the units of analysis. Both studies reported individual-level variations in specific regions in the context of general overlap between individual- and group-level brain structural deviation in schizophrenia.
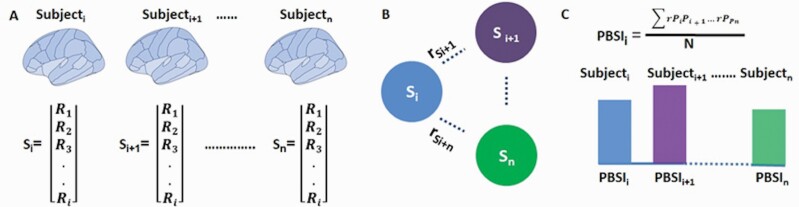
We have previously addressed the issue of inter-individual variability in patients with established diagnosis using two different measures; the coefficient of variation (CV) of regional brain morphometry and the person-based similarity index (PBSI).23–25 The CV is a measure of dispersion that quantifies the degree of variability in relation to the mean of a sample. As prior literature investigated inter-individual variability in schizophrenia at the level of brain regions, the CV was used here to test for the effect of diagnosis on brain regional variance. By contrast, the PBSI does not focus on regional brain measures but provides a personalized estimate of the similarity of the neuroimaging profile of each participant to that of the other members of a study sample (figure 1). The PBSI can be used in two ways; it can quantify the similarity of a patient’s brain imaging profile to that of other patients with the same disorder or it can be used to yield normative estimates by quantifying the similarity of each patient’s profile to that of HI. The former is a measure of within-diagnosis similarity in neuroimaging profiles and the latter is a measure of the normativeness of a patient’s profile. We have previously shown that the PBSI for CT, but not SV, was increased in patients with schizophrenia while the CV did not show case-control differences.25 Application of the PBSI to cortical gyrification measures from an independent sample also demonstrated higher variability in patients with schizophrenia than HI with higher deviation from normative values in patients being associated with greater cognitive deficits.26
Fig. 1.
Computation of the person-based similarity index.
(A) The regional imaging measures (R) of each subject are concatenated into a single vector (S) representing their imaging profile for that measure set; (B) all possible pairwise correlation coefficients between the profile (Si) of each subject and that of the other subjects are calculated; (C) the person-based similarity index (PBSI) of subjecti is the average of all pairwise correlations between subjecti and all other subjects in the same group.
In the current study, we expand this line of inquiry to CHR and FEP individuals to test whether variability in their brain structural profiles and in regional brain measures was higher than that of HI and explore the functional significance of increased variability if present. To achieve this, we leveraged structural magnetic resonance imaging (sMRI) data from CHR and FEP individuals to generate PBSI and CV scores for CT, surface area (SA), and SV; these neuroimaging phenotypes were considered separately on the basis of different genetic determinants,27–29 age-related trajectories,30,31 and our prior findings.23,25
Methods
Sample
The study sample comprised 71 CHR, 72 FEP, and 55 HI. The current analyses are based on previously published MRI data from the same sample.32 The CHR- and FEP individuals were recruited at the Universitären Psychiatrische Kliniken (UPK), University of Basel, Basel, Switzerland. All participants provided written informed consent, and the study was approved by the local ethics committee (Ethikkommission Nordwest-und Zentralschweiz).
All participants were assessed by experienced psychiatrists in a detailed personal interview.
According to the Basel Screening Instrument for Psychosis (BSIP33), the CHR individuals fulfilled one or more of the following criteria: (1) prepsychotic category: attenuated psychotic symptoms (APS) or brief limited intermittent psychotic symptoms (BLIPS) according to criteria by Yung et al.34; (2) genetic risk category: genetic risk in combination with two or more other risk factors such as social decline; (3) unspecific risk category: a certain combination of risk factors according to the BSIP. APS were defined as a score of 2 or 3 on the Brief Psychiatric Rating Scale (BPRS35) hallucination item or a score of 3 or 4 on BPRS items for unusual thought content or suspiciousness over a minimum of 1 week. BLIPS were defined as having a score of 4 or higher on the BPRS hallucination item or a score of 5 or higher on BPRS items for unusual thought content, suspiciousness, or conceptual disorganization, with each symptom lasting <1 week before resolving spontaneously.
FEP individuals fulfilled criteria for acute psychotic disorder according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-1036) or the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV37) but not yet for schizophrenia. Inclusion required a score of 4 or higher on the BPRS hallucination item or a score of 5 or higher on BPRS items for unusual thought content, suspiciousness, or conceptual disorganization. The symptoms must have occurred at least several times a week and persisted for more than 1 week.
HI had no personal lifetime psychiatric disorder and no family history of any psychiatric disorder. No history of a psychiatric illness, head trauma, neurologic illness, serious medical or surgical illness, or substance abuse. All participants were screened to exclude head trauma, neurological or medical disorders, and substance abuse (as defined in the ICD-10), insufficient German language fluency, and IQ < 70, as measured with the Mehrfachwahl-Wortschatz Test Form B (MWT-B).38
In all participants, psychopathology and function were respectively rated with the 24-item BPRS and the Global Assessment of Functioning (GAF37). As used in other studies with CHR and FEP individuals,39–41 and cognitive neuroscience research,42–44 the General Performance Test (Leistungsprüfsystem [LPS], Scale 345) was used to assess nonverbal IQ estimates in terms of fluid (abstract reasoning) intelligence, which resulted from total raw score of correctly solved tasks. The LPS is a standardized, valid, and highly reliable measurement of 14 different subtests, which are related to Thurstone’s primary mental abilities.46 Information about medication was recorded in the clinical samples (table 1 and Supplementary Material). CHR individuals were followed up for a mean (SD) follow-up period of 3.8 (3.2) years. During this period, 16 CHR individuals transitioned to schizophrenia. Thirty CHR individuals have been classified according to the presence of the prepsychotic category (APS and/or BLIPS), 11 according to the mixed category (prepsychotic + genetic risk category), 6 according to genetic risk category, and 8 according to the unspecific risk category. Subgroup information was not available for CHR 16 individuals. FEP patients were not followed up and received routine care within the general psychiatric services.
Table 1.
Characteristics of the Diagnostic Groups
| Healthy Individuals, N = 55 | Clinical High-Risk Individuals, N = 71 | First-Episode Psychosis Individuals, N = 72 | |
|---|---|---|---|
| Demographic features | |||
| Age (y), mean (SD), range | 26 (3.91), 19–39 | 25 (5.36), 18–42 | 26 (6.80), 18–42 |
| Sex (M/F) | 25/30 | 48/23 | 51/21 |
| Years of education, mean (SD) | 14.65 (2.81) | 13.85 (2.57) | 13.21 (2.92) |
| Nonverbal IQ (abstract reasoning), normative values | n/a | 110.72 (14.32) | 108.46 (16.96) |
| Clinical | |||
| BPRS, mean (SD) | 24.16 (0.56) | 37.85 (8.47) | 49.90 (12.81) |
| GAF | 91.92 (4.47) | 60.81 (13.57) | 57.44 (15.69) |
| Duration of untreated illness (DUI), mo, mean (SD) | n/a | n/a | 52.95 (69.20) |
| Duration of untreated psychosis (DUP), mo, mean (SD) | n/a | n/a | 25.29 (28.92) |
| Antipsychotics, N (%) | n/a | 0 | 33 (46) |
| Antidepressants, N (%) | n/a | 22 (31) | 13 (18) |
| Transitions to psychosis, N (%) | n/a | 16 (22.53) | n/a |
| Neuroimaging | |||
| Intracranial volume (ml) | 16,320,49.09 (119,683) | 16,478,57.18 (158,596) | 16,247,55.58 (156,330) |
Note: BPRS, 24-item Brief Psychiatric Rating Scale; GAF, Global Assessment of Functioning; SD, standard deviation.
Information on IQ was available in 43 clinical high-risk and 43 first-episode psychosis individuals.
Neuroimaging
Whole-brain three-dimensional T1-weighted images were acquired in all participants on the same MAGNETOM VERIO 3T scanner (Siemens, Erlangen, Germany) using an identical acquisition sequence (Supplementary Material). Following standard preprocessing, cortical reconstruction based on the Desikan atlas47 and volumetric segmentation of subcortical regions was performed using the Freesurfer 6.0 automated pipeline (https://surfer.nmr.mgh.harvard.edu/fswiki/recon-all/). The steps included removal of non-brain tissue using a hybrid watershed/surface deformation procedure,48 automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures49,50 intensity normalization,51 tessellation of the boundary between the gray and white matter, automated topology correction,52,53 and surface deformation following intensity gradients to optimally place the gray/white matter boundaries and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class. This process yielded an estimate of intracranial volume (ICV), 68 measures of CT, 68 measures of cortical SA, and 14 SV measures (supplementary table S1). Results were visually inspected and statistically evaluated for outliers following standardized ENIGMA protocols for cortical and subcortical structures (http://enigma.ini.usc.edu/protocols/imaging-protocols/) and outlier removal was performed with the code provided by the ENIGMA Consortium (http://enigma.ini.usc.edu/protocols/imaging-protocols/) and continued if the regional value was not in a range of ±3.5 SDs. From the initial dataset, 25 subjects (6 FEP, 14 CHR, and 5 HI) had to be excluded based on poor brain segmentation. Among them, 11 subjects revealed statistical outliers (supplementary table S2). After outlier removal, the final dataset consisted of 71 CHR, 72 FEP, and 55 HI.
Variability in Brain Regional Morphometry in CHR- and FEP Individuals
We calculated the CV for each regional neuroimaging measure separately in the CHR, FEP, and HI. In each group separately, the CV of each brain regional measure was calculated as CV = , where Mean denotes the mean of each regional measure and SD its standard deviation. Statistical differences in regional CV between diagnostic groups were evaluated using the asymptotic test for the equality of CV,54 implemented using the cvequality version 0.2.0 in R-cran.
Computation of PBSIs
PBSI scores were computed separately for cortical thickness (PBSI-CT), cortical surface area (PBSI-SA), and subcortical volumes (PBSI-SV) in accordance with our previous work.23–25 The neuroimaging features used for computing the PBSI-CT comprised 68 measures of CT, the PBSI-SA comprised 68 measures of cortical SA, and the PBS-SV comprised 14 measures of subcortical regions (supplementary table S1). Figure 1 outlines the steps involved in the calculation of PBSI which are the same regardless of the specific imaging phenotype: (i) the neuroimaging features were concatenated to generate the respective person-specific neuroimaging profile; (ii) Spearman’s correlation coefficient ρ were computed between the neuroimaging profile of each individual and the corresponding profiles of the other members of the group of interest. This process generates n–1 coefficients per individual, where n is the total number of individuals in the group; (iii) in each individual, the correlation coefficients generated in the previous step were averaged to yield their PBSI score; a higher PBSI score indicates greater similarity in the neuroimaging profiles of that individual and those of other members of the group. The MATLAB function used to compute the PBSI score is available at: https://www.mathworks.com/matlabcentral/fileexchange/69158-similarityscore.
Within-Group PBSI
Using the procedure described above, we calculated PBSI scores for each imaging phenotype by comparing the neuroimaging profile of each participant to other participants in the same group. For instance, for each CHR individual, we calculated their PBSI score for cortical thickness (CHR-PBSI-CT), surface area (CHR-PBSI-SA), and subcortical volume (CHR-PBSI-SV), each of which quantifies the similarity of that CHR individual to the corresponding profiles of all other CHR individuals. The same has been done for the FEP and healthy control (HC) group separately.
Normative Modeling Using the PBSI
The PBSI scores for CT, cortical SA, and SV of each CHR- and each FEP individual were expressed in z-scores, representing their deviation from the corresponding mean of the HC group. These values are denoted as Z-PBSI-CT, Z-PBSI-SA, and Z-PSBI-SV. Then, we applied a threshold of >1.5 SD to identify CHR- and FEP individuals whose neuroanatomical profile was markedly deviant from that of the healthy group. We used t test to compare total BPRS and IQ scores (abstract reasoning) between CHR and FEP individuals who showed marked deviation in Z-PBSI-CT, Z-PBSI-SA, and Z-PBSI-SV (as defined above) compared with those who did not. The term normative is used here in a narrow sense and simply refers to the data from the HC group.
Statistical Analyses
The PBSI scores for CT, SA, and SV were not normally distributed according to the Shapiro-Wilk test. Accordingly, nonparametric tests were used in the analyses. We conducted the following analyses: (i) we used the Kruskal-Wallis test with Monte Carlo two-tailed P values (which accommodate both smaller samples and non-normal variable distribution) to identify group differences in the mean PBSI scores for each imaging phenotype. Significant results were followed up with pairwise Mann-Whitney U test; (ii) we quantified the contribution of each neuroimaging measure to their respective PBSI by using the leave-one-out approach; this entailed recalculating the PBSI-CT, PBSI-SA, and PBSI-SV scores for each individual within each diagnostic group after leaving out one discrete regional neuroimaging measure at a time. The relationship between nonverbal IQ (abstract reasoning), BPRS, and GAF with the PBSI-CT, PBSI-SA, and PBSI-SV in each clinical group was examined using the Spearman’s correlation coefficient.
The effect of medication on each PBSI score was examined using the Mann-Whitney U test, in which medication was binarised (on/off antipsychotics) and (on/off antidepressants). As shown in table 1, no CHR individual was on antipsychotic medication.
The threshold for statistical significance was adjusted for multiple testing across all analyses using false discovery rate; when uncorrected P values are shown these are denoted as punc.
Results
Sample Characteristics
There were no group differences in age (punc = .12) (and therefore this variable was not modeled as covariate), but there was an over-representation of males in the two clinical groups (χ 2 = 14.00; punc = .001); sex was therefore modeled as a covariate in further analyses. There was a marginal group difference in education (F = 16.50, punc = .06) accounted for by the HI having more years of education than the clinical groups. There were no differences in nonverbal IQ (abstract reasoning) between the two clinical groups (F = 1.39, punc = .24) and no effect of sex (punc = .49) or sex by group interactions (punc = .47). GAF scores were comparable between the clinical groups, which differed from HI (F = 90.46; punc < .0001); there was no effect of sex (punc = .60) or sex by group interaction (punc = .73). All three groups differed from each other in terms of total BPRS scores (F = 106.60, punc < .0001) with no effect of sex (punc = .20) or sex by group interaction (punc = .87).
Variability in Brain Regional Morphometry in CHR- and FEP Individuals
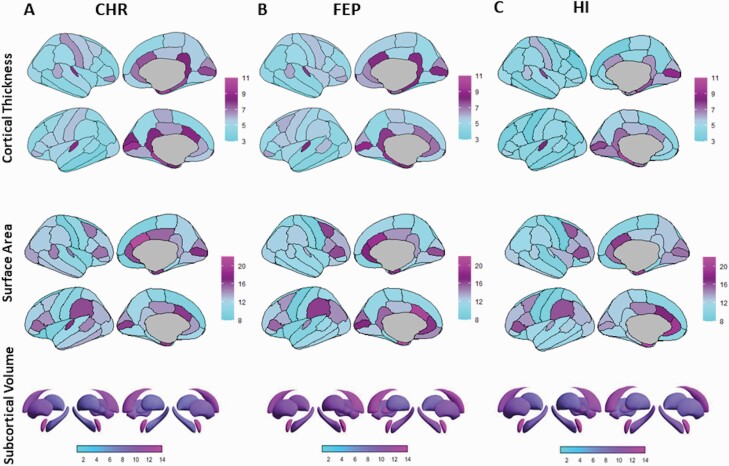
Interrogation of neuroanatomical variability at the level of regional measures of CT, SA, and SV using the CV did not identify any significant differences between the diagnostic groups (all punc > .38) (figure 2). Across diagnostic groups, the highest numerical values were noted for cingulate CT and SA bilaterally and for the volume of the nucleus accumbens, also bilaterally.
Fig. 2.
Regional coefficient of variation in clinical high-risk (CHR), first-episode psychosis (FEP), and healthy individuals.
Panels A, B, and C visualize the coefficient of variation of each regional measure of cortical thickness, surface area, and subcortical volume in CHR-, FEP-, and healthy individuals (HI), respectively; no statistically significant effect of diagnosis was detected across groups.
Within-Group PBSI
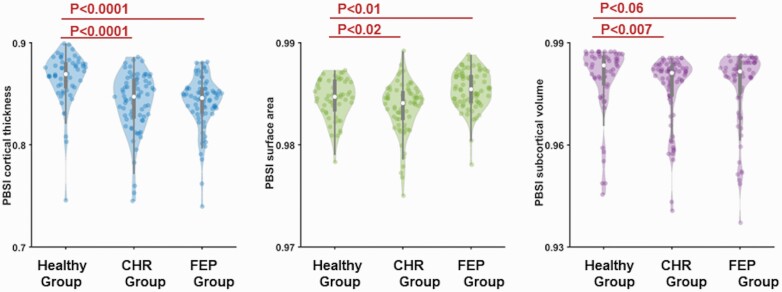
The descriptive statistics are shown in table 1. Outlier detection based at 3 SDs for the corresponding mean for each PBSI in each group identified 5 individuals for PBSI-CT (2 CHR, 2 FEP, and 1 HI), 3 individuals for PBSI-SA (one from each group), and 4 individuals for PBSI-SV (2 CHR, 1 FEP, and 1 HI). Exclusion of these individuals did not alter the results. There was a significant effect of diagnosis on the PBSI scores for CT (Kruskal-Wallis H = 37.46, Monte Carlo P < .0001); pairwise follow-up tests showed that CHR-PBSI-CT and FEP-PBSI-CT were both lower compared with the HI-PBSI-CT (figure 3). There was a significant effect of diagnosis on the PBSI scores for SV (Kruskal-Wallis H = 8.20, Monte Carlo P = .017); pairwise follow-up tests showed that CHR-PBSI-SV and FEP-PBSI-SV were both lower compared with the HI-PBSI-SV (figure 3). The PBSI scores for SA also showed a significant effect of diagnosis (Kruskal-Wallis H = 22.39, Monte Carlo P < .0001); pairwise follow-up tests showed that CHR-PBSI-SV values were lower than HI-PBSI-SA values while FEP-PBSI-SA values were higher than HI-PBSI-SA values (figure 3). In all the above analyses, there was no effect of sex (P > .54) or sex by group interaction (P > .10). Results did not change after removing the six CHR subjects with only a genetic risk.
Fig. 3.
Person-based similarity index (PBSI) for cortical thickness, surface area, and subcortical volume in clinical high-risk (CHR), first-episode psychosis (FEP), and healthy individuals.
Violin plots of the PBSI for cortical thickness, surface area, and subcortical volume in the CHR-, FEP-, and healthy individuals. Statistically significant differences for all PBSI measures were noted between the healthy group and each of the clinical groups.
There were no significant associations between any PBSI score with IQ, BPRS, and GAF (Spearman’s ρ range |.06–.18|, all punc > .10). There was no difference in any of the PBSI scores between those patients who were prescribed psychotropics (either antipsychotics or antidepressants) and those that were not (Mann-Whitney U test; punc > .4).
There were no significant group differences in the regional contributions to either the cortical or subcortical PBSI (supplementary figures S1–S5; supplementary table S3).
Normative Modeling Using the PBSI
Normative PBSI scores inform about the degree to which the neuroanatomical profile of CHR and FEP individuals is similar to that of healthy participants. The two clinical groups did not differ from each other in terms of their normative PBSI scores for CT or SV (Mann-Whitney U test, punc > .73). However, the Z-PBSI-SA was higher in FEP than in CHR individuals (Mann-Whitney U test, punc = .04).
We identified 32 (22%) individuals with deviant Z-PBSI-CT scores (of whom 8 were CHR and 24 were FEP), 12 (8.4%) with deviant Z-PBSI-SA scores (of whom 6 were CHR and 6 were FEP), and 21 (15%) with deviant Z-PBSI-SV scores (of whom 12 were CHR and 9 were FEP); 2 individuals were deviant in both cortical and SA PBSI, 4 individuals had deviant values in both subcortical and CT indices and none for all other combinations.
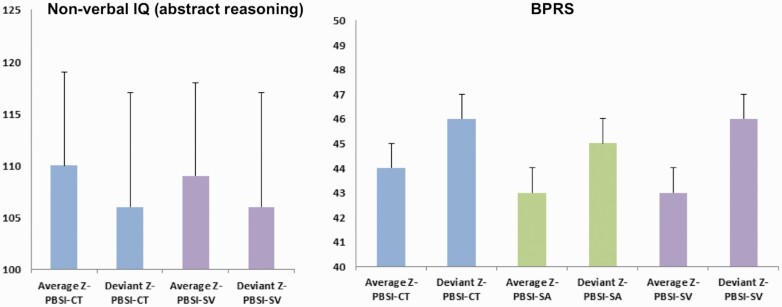
All those with deviant Z-PBSI-CT, Z-PBSI-SA, and Z-PBSI-SV scores had higher total BPRS scores; those with deviant Z-PBSI-CT and Z-PBSI-SV also had lower nonverbal IQ (abstract reasoning) (figure 4). However, the effect size of these differences was small (Cohen’s d ≈ 0.3) and they did not reach statistical significance (punc > .30). CHR individuals who transitioned to psychosis were not over-represented in those with deviant Z-PBSI-CT, Z-PBSI-SA, and Z-PBSI-SV (punc > .60). Also, no risk subgroup was over-represented in the deviant group for CT, SA, and SV (P > .22).
Fig. 4.
Nonverbal IQ (abstract reasoning) and psychopathology as a function of deviation from the normative person-specific similarity index (PBSI) for cortical thickness, surface area, and subcortical volumes.
Left panel: Mean and SD in nonverbal IQ (abstract reasoning) of the clinical high-risk and first-episode psychosis individuals whose PBSI scores for either cortical thickness (PBSI-CT) or subcortical volume (PBSI-SV) deviated by >1.5 SD from the corresponding indices of the healthy group; Right panel: Mean and SD of the total scores of the Brief Psychiatric Rating Scale (BPRS) of the clinical high-risk and first-episode psychosis individuals whose PBSI-CT, or PBSI-SV or PBSI for surface area (PBSI-SA) deviated by >1.5 SD from the corresponding indices of the healthy group; group differences were not statistically significant. Note: PBSI-CT, blue color; PBSI-SA, green color; PBSI-SV, purple color; SD, vertical black line.
Discussion
This study examined for the first time variability in brain morphology in CHR and FEP individuals at the level of each brain regional measure and at the level of brain structural profiles. Regional variation was measured using the CV, a measure of dispersion of the regional morphometric measures within each sample. This measure did not differentiate between diagnostic groups. Variability in brain structural profiles was assessed using the PBSI, which is a personalized estimate of the similarity between the cortical and subcortical profiles of a CHR or FEP individual to that of other individuals in the same group. The PBSI scores for CT and SV were significantly lower in both clinical groups compared with the control sample, indicating greater heterogeneity; this was not the case for cortical SA, where heterogeneity was greater in CHR but lower in FEP individuals. In addition, with respect to the healthy participants who served as a normative reference group, PBSI scores of about 8%–22% individuals with early psychosis showed marked deviation from the control group and a tendency for lower nonverbal IQ (abstract reasoning) and higher psychopathology.
Variability in Brain Regional Morphometry in CHR- and FEP Individuals
Brugger and Howes18 conducted a meta-analysis of 108 studies that reported cortical and SV measures in patients with syndromal schizophrenia and HCs to derive an estimate of group differences in regional variability. They found that the volume of the amygdala, thalamus, putamen, and the temporal cortex showed greater variability in patients while the volume of the anterior cingulate showed greater variability in HCs. A more recent study in a large sample of schizophrenia patients extended this finding by showing increased heterogeneity in frontotemporal thickness and area.19 In the present study, the diagnostic groups did not differ in terms of the CV in any morphometric measure; in addition, both the thickness and SA of the anterior cingulate showed the highest numerical variability across all diagnostic groups. It is possible that diagnostic differences in the CV in individual brain regions are not present in early psychosis but come about later, either because of disease chronicity itself or through the effect of factors associated with chronicity such as medication exposure and poor quality of life. Alternatively, diagnostic differences in brain regional variation may be subtle regardless of disease-stage and can only be reliably detected using meta-analytic methods or large samples.
Within-Group PBSI
CHR and FEP individuals had lower PBSI scores for CT and SV than the control sample. The findings with regards to the PBSI for CT replicate our prior report in two independent samples of patients with syndromal schizophrenia (supplementary figure S6).25 The lower PBSI scores indicate greater dissimilarity in cortical and subcortical profiles amongst CHR and FEP individuals compared with HI. Notably, the PBSI scores for cortical SA were higher than in the healthy group for FEP but not for CHR individuals. The divergence of the PBSI-SA of FEP individuals from the general pattern of lower PBSI scores could be incidental but if true it would indicate that mechanisms leading to greater variability in syndromal psychosis are mainly affecting CT.
The PBSI approach has the advantage of yielding personalized estimates of the degree of similarity of each individual to the remainder of their group and can therefore be used to explore the potential mechanisms that reduce profile similarity. The PBSI index was not related to medication exposure but other potential sources of variability, such as genetic loading for schizophrenia or early environmental adversity, were not examined. As in our previous report,25 the correlation between PBSI scores and symptoms was not significant for any of the imaging phenotypes; this indicates that variability in brain morphology may not be directly linked to symptoms or that the relationship between PBSI and psychopathology cannot be captured by linear models. Larger future studies would be better suited in addressing these questions.
Normative Modeling Using the PBSI
Although the neuroanatomical profiles of CHR and FEP individuals generally showed higher variability than HCs, only a minority had “deviant” PBSI scores. Two prior studies that used normative modeling at the level of regional brain morphology also found that only a small proportion of patients with syndromal schizophrenia had statistically deviant values in reference to normative data.21,22 Here, deviance was most pronounced regarding CT (22%), followed by SV (15%) and SA (8.4%). The pattern on lower nonverbal IQ (abstract reasoning) and higher psychopathology in CHR- and FEP individuals with deviant PBSI scores is interesting and worth further investigation although the small size of the current sample was insufficient to establish statistical significance. We note that in the study of Janssen and colleagues,26 who calculated the PBSI using gyrification data, those individuals with statistically deviant scores also had lower IQ (general cognitive performance) and a more unfavorable prognosis. We infer that disease-related mechanisms seem to increase divergence in brain morphology in psychosis but only a minority of patients differ markedly from HI.
Limitations
The small sample size, although not atypical for single-centre CHR and FEP studies, is an obvious limitation. The results are, however, credible as they resonate with findings in other studies using the PBSI, including studies in schizophrenia patients. We focused on brain morphology as it is arguably one of the most robust neuroimaging phenotypes. However, the methodological approach outlined here could be used to examine variability in a number of other neuroimaging phenotypes that were not covered in this study. Although we described increased variability in the brain structural profiles in early psychosis it was not possible to examine potential etiological associations beyond medication.
Conclusion
We showed that early psychosis is associated with increasing divergence in brain morphology but only a minority of patients differ markedly from HI. Marked divergence in brain morphological profiles, in particular, CT is likely to identify a subgroup of CHR and FEP individuals with worse clinical outcomes such as transition to psychosis or social and occupational impairments. If applied to larger samples, our methodological approach could enable both the identification of genetic and environmental factors that increase morphological brain divergence in early phases of psychosis and the improved characterization of the clinical features, treatment response, and prognosis of CHR and FEP individuals that show marked deviance from normative data.
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
The authors received no specific funding for this work. This study presents a secondary analysis of already acquired data, which was supported by a grant from the Swiss National Science Foundation (3232BO:119382, Dr Borgwardt).
References
- 1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2. Habtewold TD, Rodijk LH, Liemburg EJ, et al. A systematic review and narrative synthesis of data-driven studies in schizophrenia symptoms and cognitive deficits. Transl Psychiatry. 2020;10(1):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70(1):107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Herdt A, Wampers M, Vancampfort D, et al. Neurocognition in clinical high risk young adults who did or did not convert to a first schizophrenic psychosis: a meta-analysis. Schizophr Res. 2013;149(1–3):48–55. [DOI] [PubMed] [Google Scholar]
- 5. Bora E, Lin A, Wood SJ, Yung AR, McGorry PD, Pantelis C. Cognitive deficits in youth with familial and clinical high risk to psychosis: a systematic review and meta-analysis. Acta Psychiatr Scand. 2014;130(1):1–15. [DOI] [PubMed] [Google Scholar]
- 6. Zheng W, Zhang QE, Cai DB, et al. Neurocognitive dysfunction in subjects at clinical high risk for psychosis: a meta-analysis. J Psychiatr Res. 2018;103:38–45. [DOI] [PubMed] [Google Scholar]
- 7. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220–229. [DOI] [PubMed] [Google Scholar]
- 8. Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39(5):1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Erp TG, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Erp TGM, Walton E, Hibar DP, et al. ; Karolinska Schizophrenia Project . Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry. 2018;84(9):644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Radua J, Borgwardt S, Crescini A, et al. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev. 2012;36(10):2325–2333. [DOI] [PubMed] [Google Scholar]
- 12. Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. [DOI] [PubMed] [Google Scholar]
- 13. Vita A, De Peri L, Silenzi C, Dieci M. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res. 2006;82(1):75–88. [DOI] [PubMed] [Google Scholar]
- 14. Fusar-Poli P, Borgwardt S, Crescini A, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35(5):1175–1185. [DOI] [PubMed] [Google Scholar]
- 15. Smieskova R, Fusar-Poli P, Allen P, et al. Neuroimaging predictors of transition to psychosis – a systematic review and meta-analysis. Neurosci Biobehav Rev. 2010;34(8):1207–1222. [DOI] [PubMed] [Google Scholar]
- 16. Ding Y, Ou Y, Pan P, et al. Brain structural abnormalities as potential markers for detecting individuals with ultra-high risk for psychosis: a systematic review and meta-analysis. Schizophr Res. 2019;209:22–31. [DOI] [PubMed] [Google Scholar]
- 17. Andreou C, Borgwardt S. Structural and functional imaging markers for susceptibility to psychosis. Mol Psychiatry. 2020;25(11):2773–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brugger SP, Howes OD. Heterogeneity and homogeneity of regional brain structure in schizophrenia: a meta-analysis. JAMA Psychiatry. 2017;74(11):1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alnæs D, Kaufmann T, van der Meer D, et al. ; Karolinska Schizophrenia Project Consortium . Brain heterogeneity in schizophrenia and its association with polygenic risk. JAMA Psychiatry. 2019;76(7):739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Opel N, Goltermann J, Hermesdorf M, Berger K, Baune BT, Dannlowski U. Cross-disorder analysis of brain structural abnormalities in six major psychiatric disorders: a secondary analysis of mega- and meta-analytical findings from the ENIGMA consortium. Biol Psychiatry. 2020;88(9):678–686. [DOI] [PubMed] [Google Scholar]
- 21. Wolfers T, Doan NT, Kaufmann T, et al. Mapping the heterogeneous phenotype of schizophrenia and bipolar disorder using normative models. JAMA Psychiatry. 2018;75(11):1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kochunov P, Fan F, Ryan MC, et al. Translating ENIGMA schizophrenia findings using the regional vulnerability index: association with cognition, symptoms, and disease trajectory. Hum Brain Mapp. 2020. doi: 10.1002/hbm.25045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doucet GE, Moser DA, Rodrigue A, Bassett DS, Glahn DC, Frangou S. Person-based brain morphometric similarity is heritable and correlates with biological features. Cereb Cortex. 2019;29(2):852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doucet GE, Glahn DC, Frangou S. Person-based similarity in brain structure and functional connectivity in bipolar disorder. J Affect Disord. 2020;276:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doucet G, Lin D, Du Y, et al. Personalized estimates of morphometric similarity in bipolar disorder and schizophrenia. NPJ Schizophr. 2020;6(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Janssen J, Díaz-Caneja C, Alloza C, et al. Dissimilarity in sulcal width patterns in the cortex can be used to identify patients with schizophrenia with extreme deficits in cognitive performance. bioRxiv. 2021;47(2):552–561. doi: 10.1093/schbul/sbaa131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grasby KL, Jahanshad N, Painter JN, et al. The genetic architecture of the human cerebral cortex. Science 2020;367(6484):eaay6690. doi: 10.1126/science.aay6690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wen W, Thalamuthu A, Mather KA, et al. Distinct genetic influences on cortical and subcortical brain structures. Sci Rep. 2016;6:32760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strike LT, Hansell NK, Couvy-Duchesne B, et al. Genetic complexity of cortical structure: differences in genetic and environmental factors influencing cortical surface area and thickness. Cereb Cortex. 2019;29(3):952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frangou S, Modabbernia A, Doucet GE, et al. Cortical thickness trajectories across the lifespan: data from 17,075 healthy individuals aged 3–90 years. bioRxiv. 2020. doi: 10.1101/2020.05.05.077834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dima D, Papachristou E, Modabbernia A, Doucet GE, Agartz I, Aghajani M. Subcortical volume trajectories across the lifespan: data from 18,605 healthy individuals aged 3–90 years. bioRxiv. 2020. doi: 10.1101/2020.05.05.079475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bykowsky O, Harrisberger F, Schmidt A, et al. Association of antidepressants with brain morphology in early stages of psychosis: an imaging genomics approach. Sci Rep. 2019;9(1):8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riecher-Rössler A, Aston J, Ventura J, et al. [The Basel Screening Instrument for Psychosis (BSIP): development, structure, reliability and validity]. Fortschr Neurol Psychiatr. 2008;76(4):207–216. [DOI] [PubMed] [Google Scholar]
- 34. Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172(33):14–20. [PubMed] [Google Scholar]
- 35. Lukoff D, Liberman RP, Nuechterlein KH. Symptom monitoring in the rehabilitation of schizophrenic patients. Schizophr Bull. 1986;12(4):578–602. [DOI] [PubMed] [Google Scholar]
- 36. World Health Organization. International Classification of Mental and Behavioural Disorders (10th Revision). Geneva: World Health Organization; 1992. [Google Scholar]
- 37. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 38. Lehrl S, Triebig G, Fischer B. Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand. 1995;91(5):335–345. [DOI] [PubMed] [Google Scholar]
- 39. Ozgürdal S, Littmann E, Hauser M, et al. Neurocognitive performances in participants of at-risk mental state for schizophrenia and in first-episode patients. J Clin Exp Neuropsychol. 2009;31(4):392–401. [DOI] [PubMed] [Google Scholar]
- 40. Wotruba D, Michels L, Buechler R, et al. Aberrant coupling within and across the default mode, task-positive, and salience network in subjects at risk for psychosis. Schizophr Bull. 2014;40(5):1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ramyead A, Kometer M, Studerus E, et al. Aberrant current source-density and lagged phase synchronization of neural oscillations as markers for emerging psychosis. Schizophr Bull. 2015;41(4):919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schlagenhauf F, Rapp MA, Huys QJ, et al. Ventral striatal prediction error signaling is associated with dopamine synthesis capacity and fluid intelligence. Hum Brain Mapp. 2013;34(6):1490–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Friedel E, Schlagenhauf F, Beck A, et al. The effects of life stress and neural learning signals on fluid intelligence. Eur Arch Psychiatry Clin Neurosci. 2015;265(1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heinzel S, Lorenz RC, Brockhaus WR, et al. Working memory load-dependent brain response predicts behavioral training gains in older adults. J Neurosci. 2014;34(4):1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Horn W. Leistungsprüfung (LPS). Toronto, Canada: Göttingen Verlag für Psychologie; 1983. [Google Scholar]
- 46. Thurstone LL. Primary Mental Abilities. Chicago, IL: University of Chicago Press; 1938. [Google Scholar]
- 47. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 48. Ségonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. [DOI] [PubMed] [Google Scholar]
- 49. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3): 341–355. [DOI] [PubMed] [Google Scholar]
- 50. Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–S84. [DOI] [PubMed] [Google Scholar]
- 51. Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1): 87–97. [DOI] [PubMed] [Google Scholar]
- 52. Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. [DOI] [PubMed] [Google Scholar]
- 53. Ségonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26(4):518–529. [DOI] [PubMed] [Google Scholar]
- 54. Feltz CJ, Miller GE. An asymptotic test for the equality of coefficients of variation from k populations. Stat Med. 1996;15(6):646–658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.