Abstract
Introduction
Recent network-based analyses suggest that schizophrenia symptoms are intricately connected and interdependent, such that central symptoms can activate adjacent symptoms and increase global symptom burden. Here, we sought to identify key clinical and neurobiological factors that relate to symptom organization in established schizophrenia.
Methods
A symptom comorbidity network was mapped for a broad constellation of symptoms measured in 642 individuals with a schizophrenia-spectrum disorder. Centrality analyses were used to identify hub symptoms. The extent to which each patient’s symptoms formed clusters in the comorbidity network was quantified with cluster analysis and used to predict (1) clinical features, including illness duration and psychosis (positive symptom) severity and (2) brain white matter microstructure, indexed by the fractional anisotropy (FA), in a subset (n = 296) of individuals with diffusion-weighted imaging (DWI) data.
Results
Global functioning, substance use, and blunted affect were the most central symptoms within the symptom comorbidity network. Symptom profiles for some patients formed highly interconnected clusters, whereas other patients displayed unrelated and disconnected symptoms. Stronger clustering among an individual’s symptoms was significantly associated with shorter illness duration (t = 2.7; P = .0074), greater psychosis severity (ie, positive symptoms expression) (t = −5.5; P < 0.0001) and lower fractional anisotropy in fibers traversing the cortico-cerebellar-thalamic-cortical circuit (r = .59, P < 0.05).
Conclusion
Symptom network structure varies over the course of schizophrenia: symptom interactions weaken with increasing illness duration and strengthen during periods of high positive symptom expression. Reduced white matter coherence relates to stronger symptom clustering, and thus, may underlie symptom cascades and global symptomatic burden in individuals with schizophrenia.
Keywords: psychosis, symptom network, diffusion-weighted imaging, fractional anisotropy
Introduction
Schizophrenia is a complex mental health disorder, whose course is marked by gradual deterioration in psychopathology and brain structure. No curative treatment exists, and first-line antipsychotic treatments do not relieve the full scope of symptoms and functional sequalae.
Diagnostic classification of schizophrenia currently relies on categorical typologies, whereby symptoms are equally weighted and considered manifestations of an underlying illness. However, it is increasingly clear that symptoms carry differing degrees of importance in terms of their impact on overall illness and may derive from numerous antecedents.1,2 Recent studies have leveraged graph theory to reconceptualize symptoms as an interactive comorbidity network, rather than representing independent entities.3 In this way, a collection of symptoms are represented as nodes (network elements) and their local interactions as edges.4,5 This model suggests that psychiatric disorders reflect a causal interplay between multiple symptoms, whereby symptoms form meaningful associations and interactions with each other.
Network analysis has been used to identify the relative importance of symptoms in schizophrenia (ie, their degree of connectedness with other symptoms). Preliminary network studies identify negative symptoms and functioning levels as central to overall clinical profiles in schizophrenia.6,7 Thus, amelioration of negative symptoms—a longstanding pharmacotherapy challenge8,9—could potentially protect against global symptom cascades and improve overall illness states.10,11 However, it is unclear whether the centrality of negative symptoms and general functioning is conserved in comorbidity networks comprising broader symptom constellations.
In addition to key symptoms/nodes, global network features may also bear on general psychopathology. In this regard, higher psychosis symptom severity and risk of psychotic relapse was observed in schizophrenia subgroups with stronger connectivity between multiple different symptoms (ie, higher symptom connectedness)—for example, by way of reinforcing maladaptive feedback loops.12 These findings raise the important question of whether symptom structures remain stable or vary over the course of illness.
Changes across the course of illness are well established with respect to brain structure and function in schizophrenia. Numerous magnetic resonance imaging (MRI) studies report structural brain alterations in schizophrenia, which progress over the course of illness, particularly in white matter microstructure.13–15 These alterations may relate to aspects of psychopathology16,17 and cognitive or functional decline.18–20 However, precise brain-symptom associations remain to be established due to mixed findings. This may be due in part to a complex interplay between symptoms that confound direct relationships between neuroimaging measures and discrete symptoms. Accounting for symptom interplay by way of a network approach may thus, lead to more realistic, and clinically meaningful brain-symptom associations in schizophrenia.
This study builds on previous symptom network studies by examining key clinical and neurobiological factors that could relate to symptom network architecture in a large schizophrenia cohort. We consider a broad constellation of symptoms to construct a symptom comorbidity network. Each network node represents a particular symptom and edges (ie, connections) are drawn between comorbid symptoms (ie, symptoms that frequently co-occur among individuals). We quantify the extent to which each patient’s set of symptoms form clusters in the comorbidity network and test whether the extent to which an individual’s symptoms are interconnected associates with illness duration, psychosis severity, and regional white matter microstructure. We hypothesized that symptom clustering varies as a function of illness course in schizophrenia and relates to diffusion-indexed white matter microstructure. More specifically, we postulated that individuals whose symptoms were interconnected to form clusters would show higher psychosis (ie, positive) symptom severity12,21 and lower white matter fractional anisotropy, compared to individuals with symptoms that were disconnected from each other.
Methods
Participants
This study included data from 668 individuals diagnosed with a schizophrenia-spectrum disorder, drawn from the Australian Schizophrenia Research Bank (ASRB).22 Participants had an established diagnosis of schizophrenia (n = 451), schizophreniform (n = 2), schizoaffective disorder (n = 86), delusional disorder (n = 18) or psychotic disorder not otherwise specified (n = 85). Diagnoses were confirmed by trained clinical assessment officers using the Diagnostic Interview for Psychosis (DIP).23 Exclusion criteria included a history of brain injury or neurological brain disorder; an intelligence quotient (IQ) below 70; and/or a diagnosed movement disorder. Approval for data analysis was provided by the Melbourne Health Human Research Ethics Committee (MHREC: 2010.250) and written informed consent was obtained from all participants, according to the Declaration of Helsinki.
Clinical Assessments
Clinical assessment data were collected across 5 Australian sites. Detailed clinical assessment protocols, including quality control measures undertaken to ensure inter-rater and inter-site reliability are described elsewhere.22 The Wechsler Test of Adult Reading24 (3 items) was used to assess premorbid IQ and the following 4 scales were used to assess current cognitive ability: (1) the Wechsler Abbreviated Scale of Intelligence25 (3 items), (2) the Controlled Oral Word Association Test26 (3 items); (3) the Repeatable Battery for Assessment of Neuropsychological Status27 (12 items); and, (4) the Letter Number Sequencing28 (1 item). Psychopathology (e.g., positive, negative, and depressive symptoms) and substance abuse/dependence were assessed with the Diagnostic Interview for Psychosis (DIP)23,29 (82 items) and the Scale for Assessment of Negative Symptoms30 (22 items). We note that substance use items were included in the construction of an overall symptom network due to known interactions between drug use and core schizophrenia symptoms.3,31,32 General functioning was assessed with the Global Assessment of Functioning scale33 (GAF; 1 item). Psychosis (positive symptom) severity was measured by summing the positive symptom items in the DIP scale. Refer to supplementary table 1 for a complete list of symptom items (126 in total) and corresponding scales used in this study.
Symptom Comorbidity Network Construction
Symptom items measured for each subject formed a 648 (subjects) × 159 (symptoms) matrix. Missing data were handled iteratively: first, subject rows or symptom columns with >30% missing values were removed and second, remaining missing elements were imputed using probabilistic principal component analysis (PPCA).34 This resulted in a 642 (subjects) by 126 (symptoms) matrix used to build the symptom comorbidity network. Symptom scores were quantile normalized (into z-scores) and cognition and function-related symptom items were inverted (1/raw symptom score) so that higher values signified poor performance/functional deterioration, in accord with the remaining symptom items.
The overall symptom network was represented with a symmetric M by M correlation matrix, where M denoted the number of symptom items (ie, nodes). The Pearson correlation coefficient35 between each pair of symptoms was computed across all patients (n = 642).5 The correlation between a pair of symptoms provided a continuous measure of comorbidity and quantified the extent to which the symptoms co-occurred in the cohort. While complementary measures of comorbidity are available, including partial correlation36 and information-theoretic measures,37 they mandate specific data assumptions and add complexity to the construction process. Correlation coefficients below a fixed threshold (r = .2, which yielded the high betweenness across all symptoms) were set to zero to remove weak symptom correlations from the network.
In post hoc analyses, symptom networks were mapped separately for each of the 5 acquisition sites (Melbourne, Brisbane, Sydney, Perth, Newcastle) and for each sex. This enabled the effect of sex and the confound of site to be investigated. Consistency between pairs of sites and between males and females was assessed by computing the Pearson correlation coefficient across the set of unique elements in the symptom networks. Consistency in symptom centrality measures was assessed in the same way. Furthermore, an overall symptom network was constructed using least absolute shrinkage and selection operator (LASSO) to evaluate network stability against an alternative approach to network construction.
Symptom Network Organization and Centrality
Graph-theoretic analyses were performed to identify the most central (hub) symptoms and to test for evidence of modular organization. These analyses were undertaken using functionality provided in the Brain Connectivity Toolbox (BCT).38 While nodes reflect distinct elements of a network, individual symptom items may be highly correlated, particularly those derived from the same assessment scale. Therefore, Modularity was used to decompose the symptom comorbidity network into distinct modules, where each module contains more strongly related symptoms relative to the rest of the whole network. To this end, the Louvain (gamma = 0.4) was used to maximize the number of within-group edges, and minimize the number of between-group edges.39,40 We systematically selected the gamma for which there was no module comprising only one symptom. Subsequently, 3 measures of node centrality were used to identify influential symptom nodes within each symptom module and across the entire network.2Betweenness quantified the portion of all shortest paths in the network that contain a given node. Nodes with high betweenness are considered bridges or gatekeepers, as they participate in many shorter paths.2Closeness quantified the topological distance of a given node to all the other nodes in the network by inverting the summed distance between the node of interest and all other nodes in the network. Degree quantified the fraction of edges connected to a given node and is taken to reflect how influential the given node is.2 For the overall group symptom network, correlation coefficients below a fixed threshold (r = .2, which yielded the highest betweenness across all symptoms) were set to zero to remove weak symptom correlations from the network. However, network centrality measures (described below) were computed by averaging across multiple correlation thresholds (supplementary figure 1).
Symptom Clustering Analysis
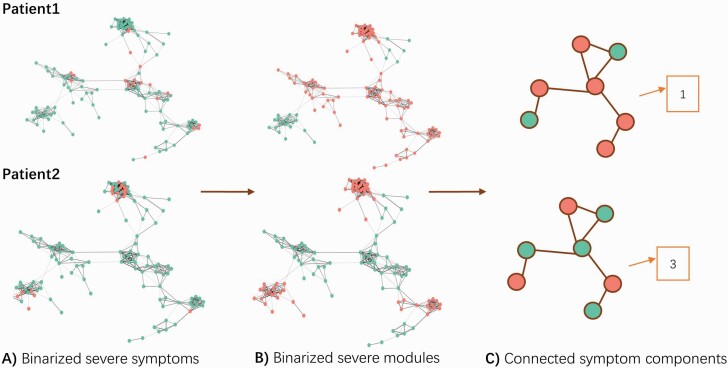
The extent to which each patient’s most prominent symptoms were interconnected in the symptom comorbidity network was determined using cluster analysis. Patients were ranked according to symptom severity for each symptom item, yielding a ranked list for each item. For each patient, the top 15% of ranked symptom items (repeated at thresholds of 10% and 20%) were identified and projected onto the symptom comorbidity network. We tested whether the top 15% (repeated at thresholds of 10% and 20%) of ranked items for each subject formed interconnected clusters between the predefined modules (figure 1A). For each subject, a symptom module was considered active if at least one of its constituent symptoms was ranked as severe (ie, among the top 15% of ranked items for that particular subject; figure 1B). Note that the symptom comorbidity network and modules remained the same for all subjects: only the set of active modules varied between individuals. The degree of symptom clustering was quantified by the number of connected components among active symptom modules (figure 1C), where fewer components signify higher symptom clustering.1,10,21,41
Fig. 1.
Measuring the degree of clustering intrinsic to individual symptom profiles. The degree of symptom clustering was measured by (A) delineating severe symptoms (red circles) and non-severe symptoms (green circles). (B) Modules containing a severe symptom were designated as “active symptom modules” (red modules) and modules without severe symptoms were designated as “non-active symptom modules” (green modules). (C) The degree of symptom clustering was obtained by computing the number of connected components among the active symptom modules. Patient 1 displays high symptom clustering as all active modules are connected (forming a total of one connected component), whereas Patient 2 displays weaker symptom clustering, as their active symptom modules formed 3 connected components (ie, their symptoms are more disconnected/segregated compared to Patient 1).
Exploring the Neural Basis of Symptom Networks With White Matter Anisotropy
Diffusion-weighted images (DWIs) were acquired in a subset (n = 296) of individuals with a Siemens Avanto 1.5-Tesla system (Siemens) across 5 different sites in Australia.22 The same model of MRI scanner and acquisition sequences were used at each site and no scanner upgrades were performed during the study lifetime. A retrospective harmonization procedure was performed on raw DWI data to remove nonlinear site/scanner effects.42,43
Processing procedures for DWI data were completed for a prior study42 and are described in supplementary material. Fractional anisotropy (FA) maps were generated by fitting a single-diffusion tensor to the harmonized and processed DWIs. Tract-based spatial statistics (TBSS)44 was employed to skeletonize FA maps and mean values were extracted from a subset of white matter regions comprising the JHU white matter atlas (48 regions). Statistical inference was performed on the resulting skeletonized regions-of-interest.
Statistical Modeling
A general linear model was used to examine the impact of symptom clustering (ie, the number of connected components among active symptom modules) on illness duration and psychosis symptom severity. The number of active symptom modules was included as a covariate to remove bias due to systematic variation across individuals in the number of active symptom modules.
10-Fold Cross Validation.
A 10-fold cross-validation framework was used to examine the stability of associations between symptom clustering with illness duration and psychosis symptom severity. To this end, the sample was randomly partitioned into 10 equal-sized subsamples. In each fold, a single subsample was left out and symptom comorbidity and modules were mapped using the remaining set of subjects. The left-out subjects were then projected onto the network to determine their number of active modules. This was repeated for each subsample. The entire 10-fold cross-validation process was repeated 1000 times, each time using a different allocation of subjects to each sub-sample. We report the proportion of cross-validations for which a significant association was identified.
Canonical Correlation Analysis.
Canonical correlation analysis (CCA) was used to examine possible relationships between symptom network clustering and regional white matter FA. To this end, symptom clustering represented a vector (296 subjects x 1 value representing the number of connected components) and regional FA measures were represented as a matrix (296 subjects × 48 regions). Potential confounding effects of age, the square of age, and sex were regressed from each factor (symptom clustering and regional FA) and the residuals were used as inputs to the CCA. One million permutations were used to evaluate statistical significance, with P < .05 considered significant (reflecting the proportion of randomized data that were greater than the observed data).
Results
Participants and Symptoms
Six subjects were removed due to high missing data rates, resulting in a total of 642 subjects (sex: 433 [67.45%] males; age range = 18–65 years, mean [SD] age = 39.46 [10.77] years). Patient characteristics are shown in table 1. Of 159 symptom items, 33 were removed due to high missing data rates, resulting in 126 variables used for network construction (supplementary table 1). Resulting data were recorded as a 642 (subjects) × 126 (symptoms) matrix, which was fed into PPCA for missing data imputation.
Table 1.
Schizophrenia Cohort Characteristics
| N = 642 | |
| Males, n (%) | 433 (67.45) |
| Age, mean (SD) | 39.46 (10.77) |
| Duration of illness in years, mean (SD) | 15.83 (10.00) |
| Site | n (%) |
| Sydney | 123 (19.16) |
| Melbourne | 148 (23.05) |
| Brisbane | 163 (25.39) |
| Perth | 161 (25.08) |
| Newcastle | 47 (7.32) |
| Diagnosis | n (%) |
| Schizophrenia | 451 (70.25) |
| Schizophreniform disorder | 2 (0.31) |
| Schizoaffective disorder, depressed type | 52 (8.10) |
| Schizoaffective disorder, bipolar type | 34 (5.30) |
| Delusional disorder | 18 (2.80) |
| Psychotic disorder NOS | 85 (13.24) |
Symptom Network Organization
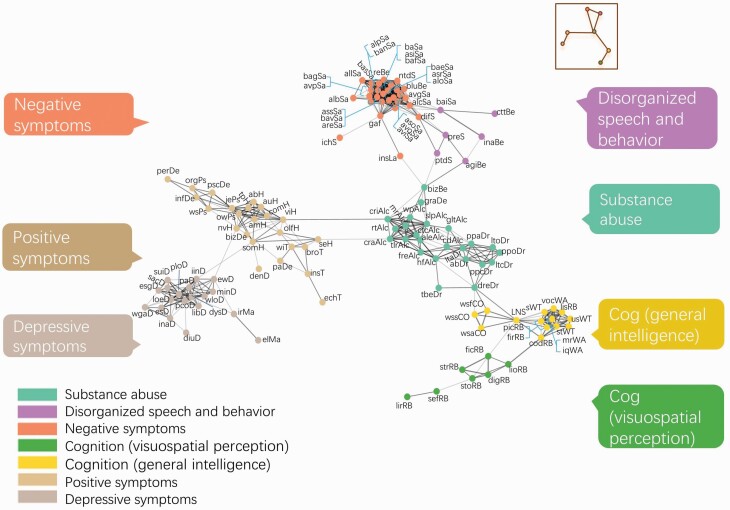
Symptom co-occurrence (comorbidity) was quantified between 7875 pairs of symptoms and summarized in terms of a 126 by 126 correlation matrix (supplementary figure 2). The symptom comorbidity network was partitioned into 7 non-overlapping modules (figure 2), which distinguished broad symptom domains: negative symptoms, positive symptoms, depressive symptoms, disorganized speech and behavior, substance use, cognition 1 (general intelligence) and cognition 2 (visual and spatial perception). As shown, “global functioning (gaf)” clustered together with negative symptoms and formed a bridge to “bizarre behavior (bizBe)” within the substance use domain. The substance use domain was central to the network, connecting positive, cognitive and disorganized symptoms. The centrality of substance use items was confirmed in a secondary analysis in which these items were removed (supplementary figure 3). Conversely, depressive symptoms were peripheral to the network, forming connections only with positive symptom nodes via links between hallucinatory symptoms (positive symptom module) and sleep symptoms (depressive symptom module). Notably, the positive symptom module was directly linked to the substance use module via hallucinatory symptoms and alcohol dependence symptoms. Network structure was found to be stable: closeness and degree were significantly correlated across all study sites (supplementary tables 2–4) and between males and females (supplementary table 5), and was conserved using an alternative LASSO approach to measure symptom comorbidity (supplementary table 6). Betweenness was less stable than closeness and degree, which may be due to several multi-hop paths utilizing a common connection. Omission of this common connection can thus result in marked change in betweenness. This requires further investigation.
Fig. 2.
Symptom comorbidity network in schizophrenia. Circles represent nodes (symptoms) and lines represent associations between symptom pairs: thicker lines indicate stronger correlations. The network layout is force-directed, whereby distant variables are weakly correlated and adjacent variables are strongly correlated. Modularity analysis yielded 7 symptom modules: negative symptoms (peach), positive symptoms (brown), depressive symptoms (gray), disorganized speech and behavior (violet), substance use (mint), cognition—intelligence (yellow), and cognition—visuospatial perception (green). Refer to supplementary table 1 for specific item names and items removed from the network.
Symptom Centrality
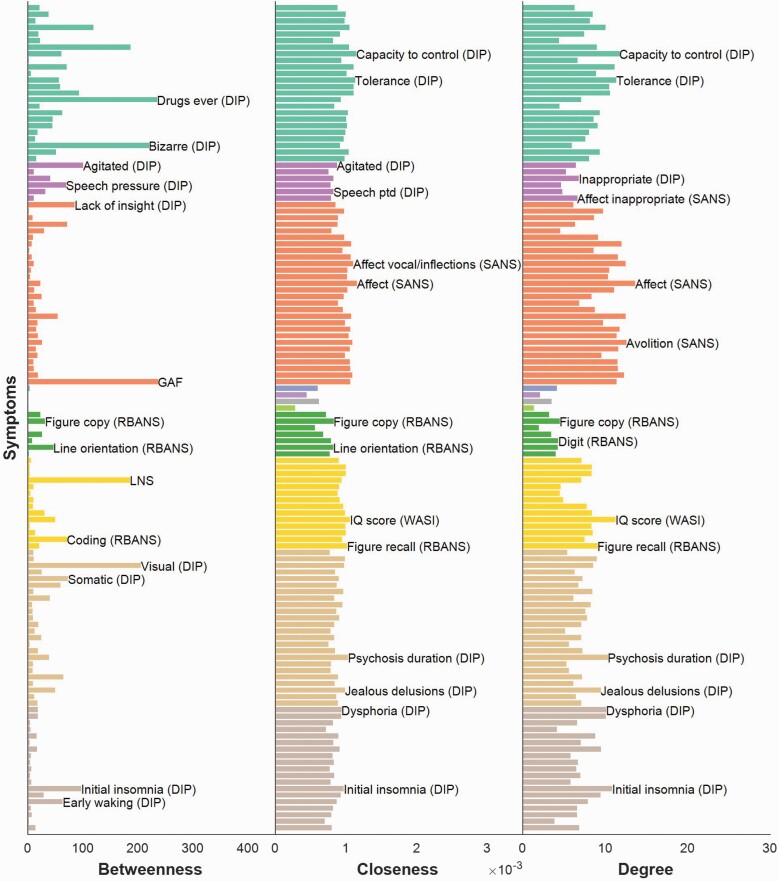
Network centrality analyses provided insight into the extent of comorbidity associated with each symptom item (figure 3). The most central symptoms were found within the negative symptom and substance use modules. In particular, symptoms with the highest betweenness were global functioning and lifetime drug use, followed closely by bizarre behavior and visual hallucinations. The closeness and degree measures revealed strikingly similar patterns, with blunted affect (within the negative symptom domain) displaying the highest closeness and degree, followed by alcohol use items (capacity to control alcohol use, alcohol tolerance, alcohol craving, and alcohol related withdrawal problems). Collectively, substance use, negative symptoms and global functioning exhibited high centrality across all 3 indices. Symptoms with high betweenness tended to form bridges between distinct modules.
Fig. 3.
Symptom centrality in schizophrenia. The relative importance of symptoms in the overall network was quantified by the betweenness, closeness, and degree, respectively. For each parameter, symptom names are given for the top 2 most central symptoms within each module. Nodes are colored according to their superordinate module (refer to figure 1 for color legend). “Global functioning (GAF),” “lack of insight,” “bizarre behavior” and “drugs ever used” showed the highest betweenness values. Closeness and degree revealed a strikingly similar pattern of symptom importance, with “blunted affect” (negative symptom module), and “capacity to control alcohol intake” reflecting the most central symptoms across both parameters.
Similar within-module centralities were also observed in closeness and degree. For example, the capacity to regulate alcohol intake and alcohol tolerance were highly central within the substance use module according to both closeness and degree. For betweenness, endorsement of drugs ever taken and bizarre behavior were identified as most central within the substance use module.
Symptom Clusters Associate With Clinical Course and Psychotic Symptom Severity
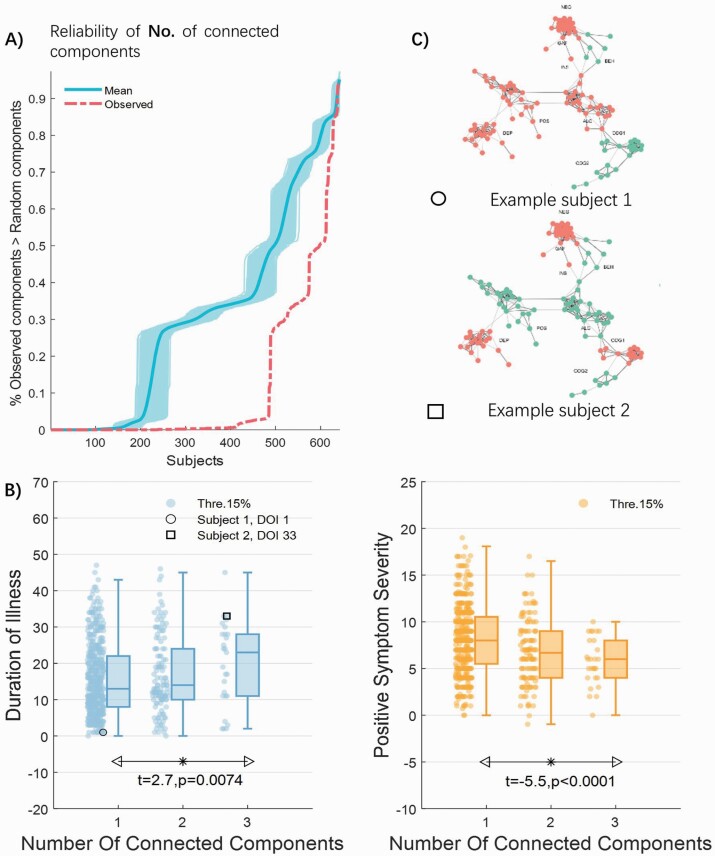
Following the approach exemplified in figure 1, the symptom profiles of many patients (76.32%) formed a single tightly interconnected cluster within the symptom comorbidity network. This suggests that the symptom comorbidity network accurately captured symptom interactions found in most individuals. The symptom profiles of the remaining patients formed either 2 (19.47%) or 3 (4.21%) distinct clusters. The extent to which symptom profiles formed clusters in the symptom network was significantly greater than attributable to chance (figure 4A). The presence of multiple symptom clusters does not necessarily indicate a greater symptom burden compared to a single cluster. Multiple clusters could potentially be remnants of an initially larger single cluster that has been broken down by effective treatment.
Fig. 4.
Association between symptom clustering and clinical course in schizophrenia. (A) The observed extent to which symptom profiles formed clusters in the network (red dashed line) was significantly greater than attributable to chance—the number of connected components computed from randomly generated networks (1000 permutations). The dark blue line and light blue shading represents the mean and 95% confidence intervals, respectively). (B) The degree of symptom clustering (number of connected components) increases as a function of illness duration and decreases as a function of psychosis (positive symptom) severity. * denotes a significant group difference. (C) An example younger subject with a shorter illness duration (circle) displays 1 connected component (ie, tightly clustered symptoms). Conversely, an example older subject with a longer illness duration (square) displays 3 connected components (ie, weakly clustered symptoms).
As hypothesized, both illness duration and psychosis symptom severity were significantly associated with interindividual variation in the number of symptom clusters (1, 2, or 3 connected components). Specifically, GLM analysis detected a linear increase in the number of connected components as a function of illness duration (t = 2.7, P = .0074; Cohen’s d = 0.12; figure 4B; see supplementary table 7 for post hoc comparisons), after controlling for the number of active symptom modules. Conversely, GLM analysis detected a linear decrease in the number of connected components as a function of psychosis symptom severity (t = −5.5; P < .0001; Cohen’s d = 0.21; figure 4B; see supplementary table 7 for post hoc comparisons), after controlling for the number of active symptom modules. All results were robust against site differences (supplementary material), as well as across lower (10%) and higher (20%) thresholds to delineate severe symptoms intrinsic to each subject’s interconnectivity profile (Supplementary Table 8). The association between duration of illness and the number of connected components was significant against chance in 42.4% out of 1000 repetitions of 10-fold cross validation; whereas the association between psychosis severity and number of connected components was significant against chance in 96.8% out of 1000 repetitions of 10-fold cross-validation, see supplementary figure 4).
Symptom Networks and White Matter Microstructure
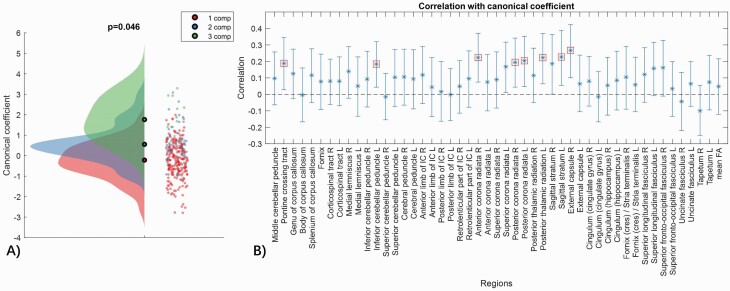
Figure 5 presents results from the CCA. There was an overall positive association between the symptom clustering and regional FA measures (r = .59, P = .047, bootstrap = 5000). Therefore, in general, higher symptom clustering associated with lower FA levels. A total of 8 white matter regions comprising the cortico-cerebellar-thalamic-cortical circuit, significantly contributed to the observed covariation between symptom profiles and white matter anisotropy. The specific regions included the pontine crossing tract (cortico-ponto-cerebellar fibers), as well as the bilateral posterior corona radiata (main cortical projection fibers) and the left inferior cerebellar peduncle (olivocerebellar fibers), retrolenticular (or retrolentiform) internal capsule (parieto- and occipito-pontine fibers), posterior thalamic radiations (thalamocortical fibers), and sagittal stratum (corticosubcortical fibers).
Fig. 5.
Associations between symptom network connectivity and white matter microstructure. (A) A significant mode of covariation was found between the number of connected components (one value per subject) and the composite of regional fractional anisotropy (FA) measures (48 values per subject). Datapoints represent the canonical coefficient for each individual subject (n = 296), with respect to the number of components. (B) Correlations (r) between regional FA and the mode of population covariation with the set of 48 FA measures and mean FA. Error bars indicate 95% confidence intervals estimated with bootstrapping (5000 bootstrapped samples). Regions that significantly contributed to the mode of covariation are marked with red squares.
Discussion
We studied emergent properties of a schizophrenia symptom comorbidity network, as well as relationships with clinical features and cortical white matter anisotropy—frequently reported as reduced in schizophrenia.13,14 We observed weaker symptom clustering in older individuals with longer illness durations, suggesting a pattern of gradual symptom decoupling over the course of illness. In contrast, stronger clustering of an individual’s symptom profile coincided with higher psychotic (positive symptom) severity and lower white matter anisotropy. Our findings suggest that strong symptom interactions manifest in psychotic illness states—the neural basis of which relates to impaired white matter circuitry.
In accord with prior work and previous dimensional symptom models,20,45,46 the symptom comorbidity network naturally clustered into broad symptom domains, with negative symptoms and general functioning situated most centrally. Global functioning was more closely linked to negative symptoms than to positive symptoms. This is perhaps unsurprising, as the resolution of positive symptoms does not necessarily translate into functional recovery.47 Furthermore, individuals with treatment-resistant schizophrenia often exhibit persistent and severe negative symptoms stemming from both primary and secondary sources (eg, neuroleptic side effects, or depression)48 that can affect functional outcomes more so than positive symptoms.49 As such, interventions that target central negative symptoms, including alogia and speech difficulties, may lead to improved functional recovery.
Extending previous work, we also identify substance use as central within the symptom network. The rate of lifelong substance abuse in individuals with schizophrenia is high (ranging between 40% and 70%),50 the effects of which are known to exacerbate existing positive, negative, cognitive symptoms, as well as social functioning.31 This may explain our finding that positive and cognitive symptoms indirectly relate to functioning via substance abuse symptoms. In particular, substance abuse may serve to exacerbate core cognitive and psychotic symptoms, which in turn contributes to functional decline. Longitudinal studies that track temporal relationships between substance abuse of various substances, positive symptoms and cognitive decline are required to test this hypothesis. Furthermore, richer substance use phenotyping in future study designs could help towards disentangling the effects of distinct substances on symptom network organization and dynamics.
Another key finding is that mood symptoms, such as depression and mania, interact indirectly with positive symptoms via sleep-related symptoms, facilitated by direct links between hallucinations and insomnia/early waking. Cumulative evidence supports a role for sleep disturbance in positive symptoms. This is consistent with evidence that sleep-arousal cycle disturbances are more prevalent in schizophrenia patients with severe positive symptoms51 and that insomnia is linked to psychotic experiences.52 Collectively, the symptom comorbidity network mapped in this study mirrors previously observed symptom dynamics, providing strong support for a network approach to gain insights into the complex nature of co-occurring symptoms and symptom clusters in schizophrenia. Our findings add to the body of evidence that management of schizophrenia requires a combined approach that targets not only positive symptoms, but also negative symptoms, sleep and substance abuse problems.53
Beyond describing microscopic symptom (node) effects, the present study examined macroscopic (systems-level) network effects and was the first to evaluate positive symptom severity and illness duration in relation to clustering intrinsic to individual symptom profiles. We observed a positive relationship between the degree of symptom clustering and illness duration, indicating that symptom interactions may vary over the course of illness. In our cohort, which is mostly comprised of out-patients that respond well to antipsychotic medication (table 1), network decoupling may reflect a pattern of symptom improvement owing to successful treatment. It is alternatively possible that symptom network decoupling manifests with advancing age and is associated with typical or accelerated aging processes in brain structure and function. Regardless of the mechanisms, weak symptom interactions between modules may serve to sustain illness, as local symptom improvements are less likely to produce carryover effects in other symptom modules, leading to cumulative symptom burden within distinct modules.
On the other side of the coin, higher clustering among symptoms was associated with greater psychosis (positive) symptom severity. This finding coheres with prior work linking stronger symptom connectivity to psychosis onset21 and treatment resistance.41 Together, these findings implicate greater symptom interactions during periods of active psychosis (high positive symptom expression) or psychotic relapse. From a network perspective, targeting central symptoms during active psychosis may effectively remove key nodes, thereby breaking apart the network into smaller symptom subnetworks to reduce global feedback loops caused by strong symptom interactions.
The biology underlying symptom interactions may relate to alterations in white matter. This is the first study to describe brain-symptom network relationships, and our results suggest that densely connected symptoms associated with reduced white matter anisotropy. This finding provides support for the longstanding disconnection hypothesis of schizophrenia, which ascribes the diverse symptom profile of schizophrenia to alterations in brain connectivity.54,55 White matter fibers in the brain serve to facilitate communication between brain regions that coordinate multiple brain functions.56 Intuitively then, reduced white matter connectivity between functionally distinct brain regions could reflect the neural basis for activating comorbidities between distinct symptom modules. The regional pattern of significant FA associations (ie, brain regions that significantly contributed to the mode of covariation) implicated the cortico-cerebellar-thalamic-cortical circuit (CCTCC). A burgeoning of structural and functional imaging studies provide evidence for CCTCC disruption in schizophrenia,57–60 which is proposed to result in diverse sensory, behavioral and cognitive disturbance due to difficulties in processing and coordinating mental functions.61–63
Our findings are subject to the following limitations. Symptom networks from cross-sectional data are inherently limited by their undirected nature. The cross-sectional findings here provide a strong basis for follow-up longitudinal studies to assess temporal relations between distinct symptoms, and synergistic effects across time. Furthermore, it is challenging to disentangle the effects of illness duration and age, which are highly correlated (r = .78, P = 1.12e-134), and in turn, controlling for age in illness duration (and vice versa) is inappropriate. Lastly, this study employed the Diagnostic Interview for Psychosis (DIP) to assess for the presence of psychosis symptoms. While the DIP represents a reliable and valid scale for measuring psychosis symptoms,23 statistical correspondence with alternative scales, such as the Positive and Negative Syndrome Scale (PANSS), has not been examined. Therefore, future work is needed to test whether the current symptom network findings can be reproduced with alternative psychosis symptom scales.
Conclusions
Negative symptoms, general functioning and substance use are central among a schizophrenia symptom comorbidity network. The strength of symptom interactions is dynamic and relates to aspects of illness course: weaker clustering relates to prolonged illness duration and stronger clustering relates to higher psychosis (positive symptom) severity. Higher symptom clustering was linked to decreased white matter anisotropy, suggesting that symptom network properties may aid in our quest to understand the biological underpinnings of schizophrenia.
Supplementary Material
Acknowledgments
We gratefully acknowledge all participants for making this study possible. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
This study used samples and data from the Australian Schizophrenia Research Bank (ASRB), funded by a National Health and Medical Research Council (NHMRC) Enabling Grant (386500; Carr V, Schall U, Scott R, Jablensky A, Mowry B, Michie P, Catts S, Henskens F, Pantelis C, Loughland C), and the Pratt Foundation, Ramsay Health Care, the Viertel Charitable Foundation, and the Schizophrenia Research Institute, using an infrastructure grant from the NSW Ministry of Health. This study was supported by an Australian National Health and Medical Research Council (NHMRC) Investigator Grant (1175754 to M.A.D.), Senior Research Fellowship (1136649 to A.Z.) Senior Principal Research Fellowship (1105825 to C.P.).
References
- 1. Wigman JT, de Vos S, Wichers M, van Os J, Bartels-Velthuis AA. A transdiagnostic network approach to psychosis. Schizophr Bull. 2017;43(1):122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bringmann LF, Elmer T, Epskamp S, et al. What do centrality measures measure in psychological networks? J Abnorm Psychol. 2019;128(8):892–903. [DOI] [PubMed] [Google Scholar]
- 3. Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. 2013;9:91–121. [DOI] [PubMed] [Google Scholar]
- 4. Newman M. Networks. Oxford, UK: Oxford University Press; 2018. [Google Scholar]
- 5. Costantini G, Epskamp S, Borsboom D, Perugini M, Mõttus R, Waldorp LJ, Cramer AOJ. State of the aRt personality research: a tutorial on network analysis of personality data in R. Journal of Research in Personality 2015;54:13–29. [Google Scholar]
- 6. Chang WC, Wong CSM, Or PCF, et al. Inter-relationships among psychopathology, premorbid adjustment, cognition and psychosocial functioning in first-episode psychosis: a network analysis approach. Psychol Med. 2019:1–9. [DOI] [PubMed] [Google Scholar]
- 7. Galderisi S, Rucci P, Kirkpatrick B, et al. ; Italian Network for Research on Psychoses . Interplay among psychopathologic variables, personal resources, context-related factors, and real-life functioning in individuals with schizophrenia: a network analysis. JAMA Psychiatry. 2018;75(4):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murphy BP, Chung YC, Park TW, McGorry PD. Pharmacological treatment of primary negative symptoms in schizophrenia: a systematic review. Schizophr Res. 2006;88(1–3):5–25. [DOI] [PubMed] [Google Scholar]
- 9. Mäkinen J, Miettunen J, Isohanni M, Koponen H. Negative symptoms in schizophrenia: a review. Nord J Psychiatry. 2008;62(5):334–341. [DOI] [PubMed] [Google Scholar]
- 10. Strauss GP, Esfahlani FZ, Kirkpatrick B, et al. Network analysis reveals which negative symptom domains are most central in schizophrenia vs bipolar disorder. Schizophr Bull. 2019;45(6):1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Strauss GP, Zamani Esfahlani F, Sayama H, et al. Network analysis indicates that avolition is the most central domain for the successful treatment of negative symptoms: evidence from the roluperidone randomized clinical trial. Schizophr Bull. 2020;46(4):964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borsboom D. A network theory of mental disorders. World Psychiatry. 2017;16(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Biase MA, Cropley VL, Baune BT, et al. White matter connectivity disruptions in early and chronic schizophrenia. Psychol Med. 2017;47(16):2797–2810. [DOI] [PubMed] [Google Scholar]
- 14. Cropley VL, Klauser P, Lenroot RK, et al. Accelerated gray and white matter deterioration with age in schizophrenia. Am J Psychiatry. 2017;174(3):286–295. [DOI] [PubMed] [Google Scholar]
- 15. Cetin-Karayumak S, Di Biase MA, Chunga N, et al. White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol Psychiatry. 2020;25:3208–3219. doi: 10.1038/s41380-019-0509-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Samartzis L, Dima D, Fusar-Poli P, Kyriakopoulos M. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J Neuroimaging. 2014;24(2):101–110. [DOI] [PubMed] [Google Scholar]
- 17. Yang X, Cao D, Liang X, Zhao J. Schizophrenia symptomatic associations with diffusion tensor imaging measured fractional anisotropy of brain: a meta-analysis. Neuroradiology. 2017;59(7):699–708. [DOI] [PubMed] [Google Scholar]
- 18. de Boer JN, van Hoogdalem M, Mandl RCW, et al. Language in schizophrenia: relation with diagnosis, symptomatology and white matter tracts. npj Schizophr. 2020;6(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63(5):512–518. [DOI] [PubMed] [Google Scholar]
- 20. Chen J, Patil KR, Weis S, et al. ; Pharmacotherapy Monitoring and Outcome Survey (PHAMOUS) Investigators . Neurobiological divergence of the positive and negative schizophrenia subtypes identified on a new factor structure of psychopathology using non-negative factorization: an international machine learning study. Biol Psychiatry. 2020;87(3):282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt A, Hauke DJ, Das T, Lang U, Riecher-Rössler A, Borgwardt S, Palaniyappan L. Increased Symptom Consolidation Preceding Transition to Psychosis: A Phenomenological Network Study (July 31, 2019). Available at SSRN: https://ssrn.com/abstract=3429923 or 10.2139/ssrn.3429923 [DOI]
- 22. Loughland C, Draganic D, McCabe K, et al. Australian Schizophrenia Research Bank: a database of comprehensive clinical, endophenotypic and genetic data for aetiological studies of schizophrenia. Aust N Z J Psychiatry. 2010;44(11):1029–1035. [DOI] [PubMed] [Google Scholar]
- 23. Castle DJ, Jablensky A, McGrath JJ, et al. The diagnostic interview for psychoses (DIP): development, reliability and applications. Psychol Med. 2006;36(1):69–80. [DOI] [PubMed] [Google Scholar]
- 24. Wechsler D. Wechsler Test of Adult Reading: WTAR. New York, NY: Psychological Corporation; 2001. [Google Scholar]
- 25. Wechsler D. Manual for the Wechsler Abbreviated Intelligence Scale (WASI). San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 26. Spreen O. Neurosensory Center Comprehensive Examination for Aphasia. Victoria: University of Victoria; 1977. [Google Scholar]
- 27. Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). San Antonio, TX: Psychological Corporation; 1998. [Google Scholar]
- 28. Wechsler D. The Wechsler Adult Intelligence Scale. 3rd ed. London: Psychological Corporation; 1998. [Google Scholar]
- 29. van Erp TGM, Walton E, Hibar DP, et al. ; Karolinska Schizophrenia Project . Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) consortium. Biol Psychiatry. 2018;84(9):644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39(7):784–788. [DOI] [PubMed] [Google Scholar]
- 31. Seddon JL, Birchwood M, Copello A, et al. Cannabis use is associated with increased psychotic symptoms and poorer psychosocial functioning in first-episode psychosis: a report from the UK National EDEN study. Schizophr Bull. 2016;42(3):619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Winklbaur B, Ebner N, Sachs G, Thau K, Fischer G. Substance abuse in patients with schizophrenia. Dialogues Clin Neurosci. 2006;8(1):37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. American Psychiatric Association. Diagnostic Criteria from dsM-iV-tr. Washington, DC: American Psychiatric Pub; 2000. [Google Scholar]
- 34. Ilin A, Raiko T. Practical approaches to principal component analysis in the presence of missing values. J Mach Learn Res.. 2010;11:1957–2000. [Google Scholar]
- 35. Gibbons J. Nonparametric Statistical Inference, 2nd edn. New York, NY: M. Dekker; 2003. [Google Scholar]
- 36. Lauritzen SL. Graphical Models. Vol. 17. Oxford, UK: Clarendon Press; 1996. [Google Scholar]
- 37. Strauss GP, Esfahlani FZ, Galderisi S, et al. Network analysis reveals the latent structure of negative symptoms in schizophrenia. Schizophr Bull. 2019;45(5):1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. [DOI] [PubMed] [Google Scholar]
- 39. Newman ME. Modularity and community structure in networks. Proc Natl Acad Sci U S A. 2006;103(23):8577–8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reichardt J, Bornholdt S. Statistical mechanics of community detection. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;74(1 Pt 2):016110. [DOI] [PubMed] [Google Scholar]
- 41. van Rooijen G, Isvoranu AM, Kruijt OH, et al. ; GROUP investigators . A state-independent network of depressive, negative and positive symptoms in male patients with schizophrenia spectrum disorders. Schizophr Res. 2018;193:232–239. [DOI] [PubMed] [Google Scholar]
- 42. Lv J, Di Biase M, Cash RFH, et al. Individual deviations from normative models of brain structure in a large cross-sectional schizophrenia cohort. Mol Psychiatry. 2020. doi: 10.1038/s41380-020-00882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ning L, Bonet-Carne E, Grussu F, et al. Cross-scanner and cross-protocol multi-shell diffusion MRI data harmonization: algorithms and results. Neuroimage. 2020;221:117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. [DOI] [PubMed] [Google Scholar]
- 45. Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137(1-3):246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van der Gaag M, Hoffman T, Remijsen M, et al. The five-factor model of the Positive and Negative Syndrome Scale II: a ten-fold cross-validation of a revised model. Schizophr Res. 2006;85(1–3):280–287. [DOI] [PubMed] [Google Scholar]
- 47. Best MW, Law H, Pyle M, Morrison AP. Relationships between psychiatric symptoms, functioning and personal recovery in psychosis. Schizophr Res. 2020;223:112–118. [DOI] [PubMed] [Google Scholar]
- 48. Peralta V, Cuesta MJ, Martinez-Larrea A, Serrano JF. Differentiating primary from secondary negative symptoms in schizophrenia: a study of neuroleptic-naive patients before and after treatment. Am J Psychiatry. 2000;157(9):1461–1466. [DOI] [PubMed] [Google Scholar]
- 49. Abdin E, Chong SA, Vaingankar JA, et al. Trajectories of positive, negative and general psychopathology symptoms in first episode psychosis and their relationship with functioning over a 2-year follow-up period. PLoS One. 2017;12(11):e0187141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tekin Uludağ Y, Güleç G. Prevalence of substance use in patients diagnosed with schizophrenia. Noro Psikiyatr Ars. 2016;53(1):4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Afonso P, Brissos S, Figueira ML, Paiva T. Schizophrenia patients with predominantly positive symptoms have more disturbed sleep-wake cycles measured by actigraphy. Psychiatry Res. 2011;189(1):62–66. [DOI] [PubMed] [Google Scholar]
- 52. Reeve S, Sheaves B, Freeman D. The role of sleep dysfunction in the occurrence of delusions and hallucinations: a systematic review. Clin Psychol Rev. 2015;42:96–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Galletly C, Castle D, Dark F, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry. 2016;50(5):410–472. [DOI] [PubMed] [Google Scholar]
- 54. Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30(2):115–125. [DOI] [PubMed] [Google Scholar]
- 55. Friston KJ, Frith CD, Fletcher P, Liddle PF, Frackowiak RS. Functional topography: multidimensional scaling and functional connectivity in the brain. Cereb Cortex. 1996;6(2):156–164. [DOI] [PubMed] [Google Scholar]
- 56. Fields RD. White matter matters. Sci Am. 2008;298(3):42–49. [PubMed] [Google Scholar]
- 57. Sears LL, Andreasen NC, O’Leary DS. Cerebellar functional abnormalities in schizophrenia are suggested by classical eyeblink conditioning. Biol Psychiatry. 2000;48(3):204–209. [DOI] [PubMed] [Google Scholar]
- 58. Ho BC, Mola C, Andreasen NC. Cerebellar dysfunction in neuroleptic naive schizophrenia patients: clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biol Psychiatry. 2004;55(12):1146–1153. [DOI] [PubMed] [Google Scholar]
- 59. Hamoda HM, Makhlouf AT, Fitzsimmons J, et al. Abnormalities in thalamo-cortical connections in patients with first-episode schizophrenia: a two-tensor tractography study. Brain Imaging Behav. 2019;13(2):472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Forsyth JK, Bolbecker AR, Mehta CS, et al. Cerebellar-dependent eyeblink conditioning deficits in schizophrenia spectrum disorders. Schizophr Bull. 2012;38(4):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Andreasen NC, O’Leary DS, Cizadlo T, et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A. 1996;93(18):9985–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–218. [DOI] [PubMed] [Google Scholar]
- 63. Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46(7):908–920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.