Abstract
Environmental factors were reported to increase the risk of human herpesvirus 8 (HHV-8) transmission. In a population of men who have sex with men (MSM), we found evidence that chemsex was associated with human herpesvirus 8 seropositivity in vivo and that poppers induced HHV-8 virion production in vitro. Our finding may explain the higher HHV-8 transmission in MSM.
Keywords: HHV-8, MSM, HIV PrEP, poppers, chemsex, HHV-8 production
Human herpesvirus 8 (HHV-8) seroprevalence and transmission differ according to world region. In sub-Saharan Africa, more than 50% is infected by HHV-8 with transmission occurring in childhood [1]. In Western countries, HHV-8 seroprevalence is low in the general population (<5%) except in men who have sex with men (MSM), in whom transmission was mainly linked to sexual risk factors such as human immunodeficiency virus (HIV) infection, history of sexually transmitted infections (STIs), and oral-penile or oral-anal contacts [2–5]. The reasons for these disparities are not yet elucidated, but some environmental factors have been highlighted, similar to Epstein-Barr virus–associated cancer development [6, 7]. Indeed, particularly in regions with soils rich in metals such as aluminum, silica, or iron, the risk of transmission, as well as the risk of Kaposi sarcoma development, have been reported to be increased compared to other region [8–11]. Otherwise, epidemiological studies report an increase of STI prevalence in MSM because of changes in sexual behaviors [12, 13]. The new era of HIV preexposure prophylaxis (PrEP) [14] possibly tends to favor sexual practices unprotected by condoms and may contribute to the spate of various STIs not covered by HIV PrEP.
Considering that in MSM, HHV-8 transmission likely occurs during sexual activity and could lead to Kaposi sarcoma in subjects without identifiable context of immunosuppression [15], we conducted a study including HIV-negative PrEP users with high risk of STIs to assess HHV-8 seroprevalence, HHV-8 anal and oral shedding, and risk factors associated with its transmission.
MATERIALS AND METHODS
Between May 2017 and August 2018, we included MSM enrolled in an HIV PrEP program in the Infectious Diseases Department of Pitié-Salpêtrière Hospital. Metadata including age, country of origin, PrEP use duration, number of different partners per month, history of STIs, rate of condom-free anal intercourse, and oral drug use during sex (“chemsex”) were collected during their routine medical visits.
HHV-8 Serological and DNA Analysis
Anal and oral swabs and 1 serum sample were collected for each patient. HHV-8 antibody status was determined by indirect immunofluorescence assay and the quantification of HHV-8 DNA by real-time polymerase chain reaction as previously reported [16].
Drugs Effects on HHV-8 Replication
Twenty-five oral drugs, intravenous drugs, or inhaled pentyl nitrite (poppers), provided by the pharmacological-toxicological department of Raymond Poincaré Hospital, Garches, France, were tested on BC-3 cells (American Type Culture Collection [ATCC], CRL-2277) (Supplementary Table 1).
Cell Viability Assays
The effect of the compounds on BC-3 and BCP-1 (ATCC, CRL-2294) cell viability was assessed using cell viability assay based on the CellTiter-Glo 2.0 assay (Promega). In brief, cells (104 per well) were incubated in a 96-well microculture plate for 3 hours and 48 hours at 37°C with different recreational drugs at 10 µM or with different concentrations of poppers (0.0001%, 0.001%, 0.01%, 0.1%, 0.5%, and 1%). Values were normalized to the untreated cells (control). A Trypan blue (Sigma) exclusion assay was also carried out in triplicate as in the CellTiter-Glo 2.0 assay. The half-maximal inhibitory concentration (IC50) was calculated with CellTiter-Glo data using GraphPad Prism version 6.0 software.
HHV-8 Virion Production
BC-3 cells (104 per well) were incubated with recreational drugs or poppers, as during the cytotoxicity test to obtain the same final concentration, during 48 hours (recreational drugs) or 3 hours, 6 hours, and 24 hours (poppers). Some wells were left untreated as negative control. Supernatant from each well was cleared by centrifugation at 1500 rpm for 5 minutes followed by a second one at 3800 rpm for 30 minutes to remove cell debris [17]. After DNase I treatment and DNA extraction, HHV-8 DNA amplification was performed to quantify HHV-8 virion production; fold change of HHV-8 DNA was determined by dividing HHV-8 DNA concentration in supernatant of each condition by HHV-8 DNA concentration obtained with negative control.
Statistical Analysis
Nonparametric tests were performed, specifically the Mann-Whitney U test and Spearman rank test for quantitative data and Fisher t test for qualitative data.
Ethical Considerations
The study was carried out in accordance with the Declaration of Helsinki. This work was a retrospective noninterventional study with no addition to standard care procedures. Reclassification of biological remnants into research material after completion of the ordered virological tests was approved by the local interventional review board of Pitié-Salpêtrière Hospital. According to the French Public Health Code (CSP Article L.1121–1.1), such protocols are exempted from individual informed consent.
RESULTS
Forty-one PrEP users were enrolled with a median age of 38 (interquartile range [IQR], 29–42) years. The majority (73%) originated from countries with low HHV-8 seroprevalence rates, including 24 (58%) from France. Participants reported a median of 5 (IQR, 2–10]) different partners per month, about 40% (IQR, 20%–72.5%) reported condom-free anal intercourse, and 13 (32%) used recreational oral drugs during sex (Table 1).
Table 1.
Characteristics of the 41 Participants Under HIV Preexposure Prophylaxis, by Human Herpesvirus 8 Status
| Characteristic | Total | HHV-8+ | HHV-8– | P Value |
|---|---|---|---|---|
| No. of participants | 41 (100) | 10 (24) | 31 (76) | |
| Age, y, median (IQR) | 38 (29–42) | 39 (35–42.5) | 36 (26–42) | .29* |
| Native country | ||||
| Countries with low HHV-8 seroprevalence rates (<10%)a | 30 (73) | 6 (60) | 24 (77) | |
| Countries with intermediate HHV-8 seroprevalence rates (10%–20%)b | 9 (22) | 3 (30) | 6 (19) | |
| Countries with high HHV-8 seroprevalence rates (>50%)c | 2 (5) | 1 (10) | 1 (4) | |
| PrEP use duration, mo, median (IQR) | 0 (0–6.1) | 3.05 (0.0–7.1) | 0 (0.0–4.5) | .33* |
| No. of different partners per mo, median (IQR) | 5 (2–10) | 7.5 (4.3–10) | 3.5 (2–9.5) | .24* |
| Percentage of anal intercourse condom-free, median (IQR) | 40 (20–72.5) | 30 (20–62.5) | 50 (20–77.5) | .75* |
| Oral drug use during sex | 13 (32) | 6 (60) | 7 (23) | .048** |
| History of STIs | 26 (63) | 7 (70) | 18 (58) | .71** |
| Bacteriald | 24 (59) | 7 (70) | 17 (55) | |
| Viral (herpes simplex virus) | 1 (2) | 0 | 1 (3) | |
| Condyloma | 7 (17) | 1 (10) | 6 (19) | |
| Concurrent STIs | 7 (17) | 2 (20) | 5 (16) | 1** |
| Bacteriald | 6 (15) | 2 (20) | 4 (13) | |
| Viral (acute hepatitis A) | 1 (2) | 0 (0) | 1 (3) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: HHV-8, human herpesvirus 8; IQR, interquartile range; PrEP, preexposure prophylaxis; STI, sexually transmitted infection.
aWestern Europe, North America, and Asia.
bMediterranean Basin, Eastern Europe, and South America.
cAfrica.
d Treponema pallidum, Mycoplasma genitalium, Chlamydia trachomatis, and Neisseria gonorrhoeae.
*Mann-Whitney U test.
**Fisher t test.
HHV-8 Seropositivity and HHV-8 DNA Shedding
Nine (22%) participants were HHV-8 seropositive, and HHV-8 DNA was detected in 4 (44%) of them in the oral site but never in the anal site. Median HHV-8 DNA viral load was 3.54 (IQR, 2.79–4.06) log10 copies/106 cells.
Among HHV-8–seronegative participants, HHV-8 DNA was not detected in either site except in the oral sample from 1 participant who seroconverted for HHV-8 during the study without clinical signs. In this participant at inclusion, HHV-8 serology was negative and a high HHV-8 DNA viral load (5.37 log10 copies/106 cells) was detected from oral swab. A second serum sample collected 6 months later was HHV-8 seropositive and oral HHV-8 DNA viral load became undetectable. In the peripheral blood compartment, HHV-8 DNA viral load was undetectable at both sampling times.
Overall, HHV-8 seroprevalence was 24% (10/41): 6 originated from countries with a low HHV-8 seroprevalence rate, 2 from Eastern Europe (including the 1 having seroconverted during the study),1 from South America, and 1 from sub-Saharan Africa.
No significant differences were found between HHV-8–positive and –negative participants except drug use during sex (odds ratio, 5.14 [95% confidence interval, 1.21–19.01; P = .048; Table 1).
Effects of Drugs on HHV-8 Virion Production
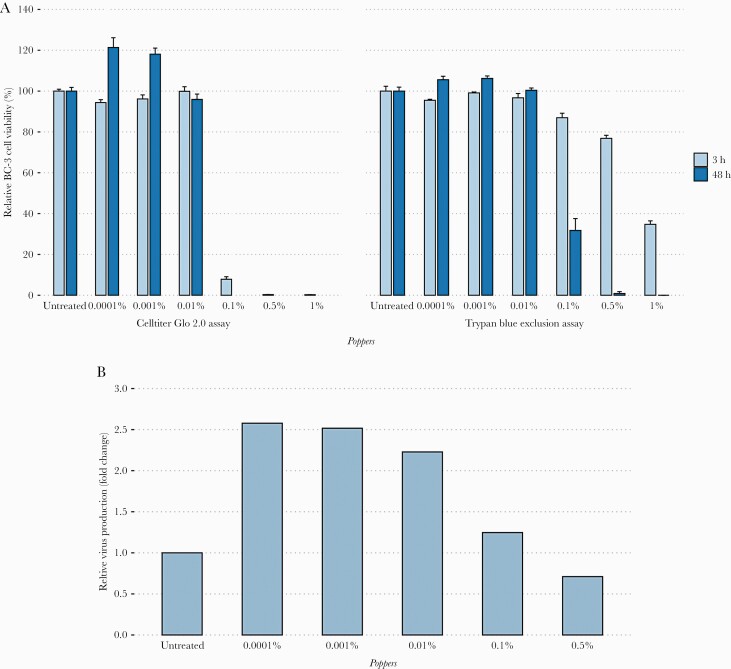
None of the oral and intravenous drugs decreased the viability of BC-3 cells after 48 hours of incubation. However, we observed with the CellTiter-Glo 2.0 assay that poppers reduced BC-3 cell viability in a dose-dependent manner. At 3 hours and 48 hours postincubation, 92%–100% of mortality was detected with poppers from 0.1 to 1%. Remarkably, no cytotoxicity of poppers was observed at 0.01% and below (Figure 1A). Similar results were observed with the BCP-1 cell line (Supplementary Table 2). The IC50 values of poppers against BC-3 and BCP-1 cell lines were 0.072% and 0.11% at 3 hours and 0.014% and 0.024% at 48 hours, respectively. The cytotoxicity of poppers after 3 hours and 48 hours was also confirmed by a Trypan blue exclusion assay (Figure 1A). At this stage, we were not able to determine whether the decline of viability was due to a direct cytotoxicity of the poppers or an induction of HHV-8 virion production leading to cytolysis.
Figure 1.
A, Cytotoxic effects of poppers on BC-3 cell line. HHV-8-infected PEL cell line was incubated with various concentrations of poppers for 3 hours and 48 hours (x-axis). Cell viability was assessed by CellTiter-Glo 2.0 and Trypan blue exclusion assays of triplicate cultures, expressed as a percentage of untreated control (y-axis). Data represent mean ± standard error of the mean for at least 2 independent experiments. B, Human herpesvirus 8 virion production in poppers-treated BC-3 cell line. This graph represents virus production compared to untreated cells (fold change) (y-axis) according to different concentrations of poppers (x-axis). Abbreviation: PEL, primary effusion lymphoma.
Compared to untreated BC-3 cells, an increased quantity of HHV-8 DNA was observed from poppers-treated BC-3 cell supernatant. At 3 hours postincubation, about 2.5-fold more virion particles were detected in the supernatants of BC-3 cells treated with poppers at 0.0001% (2.6-fold), 0.001% (2.5-fold), and 0.01% (2.2-fold), despite no cytotoxic effect of the poppers (Figure 1B). These results showed that poppers induced in vitro HHV-8 reactivation at early time of BC-3 cell incubation, resulting in the production of virus particles (Supplementary Table 3). In contrast, none of the oral or intravenous drugs seemed to affect HHV-8 particle production.
DISCUSSION
Our results confirm that HHV-8 seroprevalence is high in sexually active MSM compared to the general population in Western countries (24% vs <5% according to the literature) [1]. Intermittent but frequent HHV-8 shedding in saliva, occurring without clinical symptoms, probably contributes to HHV-8 transmission. In addition, the use of oral recreational drugs was associated with HHV-8 infection.
Although the exact route of HHV-8 transmission remains unknown in MSM, several sexual risk factors were reported in the literature [2]. In this study, a trend of association between HHV-8 infection and longer PrEP use duration (3.5 vs 0 months, P = .33) or higher number of different partners (7.5 vs 3.5; P = .24) was found. Otherwise, since the number of participants was limited, oral recreational drugs were associated with HHV-8 infection, whereas in the literature mainly the association between HHV-8 infection and injection drug users, and therefore the risk of parenteral HHV-8 transmission, was studied [18–20]. As MSM under HIV PrEP constitute a population at high risk of STIs by frequenting dense sexual networks, chemsex may increase their risky sexual behavior by increasing their number of partners and sexual unprotected intercourse in a restricted environment, as shown for other viral STIs (eg, HIV or hepatitis A) and thus probably contributed to HHV-8 transmission especially when oral HHV-8 shedding occurred.
We also hypothesized that some drugs might influence HHV-8 virion production and promote oral shedding. The evaluation of 25 different drugs, known to be used as recreational drugs, on BC-3 virion production in vitro invalidated this hypothesis. However interestingly, we found that poppers were able to increase release of HHV-8 virus particles in vitro after 3 hours of incubation. In vivo, inhaled poppers may promote HHV-8 oral shedding in the MSM population by stimulating directly and rapidly cells infected by HHV-8. Finally, our results suggest an in vitro mechanism explaining that the use of poppers in MSM was described as an independent risk factor for HHV-8 infection in epidemiological studies [21–23].
The main limitation of our study is the low number of participants, although we report similar results already described in the literature. Furthermore, additional studies exploring HHV-8 viral particle infectivity or lytic gene expression in vitro are required to definitively assess the effect of poppers on the PEL cell line.
In conclusion, this study highlights a high HHV-8 seroprevalence in MSM and HHV-8 shedding in saliva contributing to HHV-8 transmission. Chemsex, which increases sexual risk behavior, and poppers, which stimulate HHV-8 virion production in the oral site, may explain the higher HHV-8 transmission in the MSM population using these drugs.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was funded by Agence Nationale de recherche sur le SIDA et les hépatites virales (ANRS), AC43 Sexually transmitted infection wroking group.
Author contributions. Conception and design of the study: J. P. S., C. K., J. C. A., V. C., A. G. M. Acquisition of the data: R. P., G. M., S. I., L. S., A. S. Analysis of the data: A. J., A. G., V. L., I. A. L. Drafting of significant portion of the manuscript or figures: A. J., A. G., V. C., A. G. M. All authors corrected and approved the final manuscript.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: European Society for Clinical Virology Conference, Athens, Greece, 23–26 September 2018. Poster 293.
References
- 1. Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers 2019; 5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rohner E, Wyss N, Heg Z, et al. HIV and human herpesvirus 8 co-infection across the globe: systematic review and meta-analysis. Int J Cancer 2016; 138:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith NA, Sabin CA, Gopal R, et al. Serologic evidence of human herpesvirus 8 transmission by homosexual but not heterosexual sex. J Infect Dis 1999; 180:600–6. [DOI] [PubMed] [Google Scholar]
- 4. Dukers NH, Renwick N, Prins M, et al. Risk factors for human herpesvirus 8 seropositivity and seroconversion in a cohort of homosexual men. Am J Epidemiol 2000; 151:213–24. [DOI] [PubMed] [Google Scholar]
- 5. Martin JN, Ganem DE, Osmond DH, et al. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med 1998; 338:948–54. [DOI] [PubMed] [Google Scholar]
- 6. Koriyama C, Akiba S, Minakami Y, Eizuru Y. Environmental factors related to Epstein-Barr virus–associated gastric cancer in Japan. J Exp Clin Cancer Res 2005; 24:547–53. [PubMed] [Google Scholar]
- 7. He YQ, Xue WQ, Xu FH, et al. The relationship between environmental factors and the profile of Epstein-Barr virus antibodies in the lytic and latent infection periods in healthy populations from endemic and non-endemic nasopharyngeal carcinoma areas in China. EBioMed 2018; 30:184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simonart T. Role of environmental factors in the pathogenesis of classic and African-endemic Kaposi sarcoma. Cancer Lett 2006; 244:1–7. [DOI] [PubMed] [Google Scholar]
- 9. Pelser C, Dazzi C, Graubard BI, et al. Risk of classic Kaposi sarcoma with residential exposure to volcanic and related soils in Sicily. Ann Epidemiol 2009; 19:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ziegler JL. Endemic Kaposi’s sarcoma in Africa and local volcanic soils. Lancet 1993; 342:1348–51. [DOI] [PubMed] [Google Scholar]
- 11. Simonart T, Noel JC, Andrei G, et al. Iron as a potential co-factor in the pathogenesis of Kaposi’s sarcoma? Int J Cancer 1998; 78:720–6. [DOI] [PubMed] [Google Scholar]
- 12. van de Laar TJ, Richel O. Emerging viral STIs among HIV-positive men who have sex with men: the era of hepatitis C virus and human papillomavirus. Sex Transm Infect 2017; 93:368–73. [DOI] [PubMed] [Google Scholar]
- 13. Chow EPF, Grulich AE, Fairley CK. Epidemiology and prevention of sexually transmitted infections in men who have sex with men at risk of HIV. Lancet HIV 2019; 6:e396–405. [DOI] [PubMed] [Google Scholar]
- 14. Molina JM, Capitant C, Spire B, et al. ANRS IPERGAY Study Group . On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–46. [DOI] [PubMed] [Google Scholar]
- 15. Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol 2019; 58:538–42. [DOI] [PubMed] [Google Scholar]
- 16. Lallemand F, Desire N, Rozenbaum W, et al. Quantitative analysis of human herpesvirus 8 viral load using a real-time PCR assay. J Clin Microbiol 2000; 38:1404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu Y, Black JB, Goldsmith CS, et al. Induction of human herpesvirus-8 DNA replication and transcription by butyrate and TPA in BCBL-1 cells. J Gen Virol 1999; 80 (Pt 1):83–90. [DOI] [PubMed] [Google Scholar]
- 18. Rohner E, Wyss N, Heg Z, et al. HIV and human herpesvirus 8 co-infection across the globe: systematic review and meta-analysis. Int J Cancer 2016; 138:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cannon MJ, Dollard SC, Smith DK, et al. HIV Epidemiology Research Study Group . Blood-borne and sexual transmission of human herpesvirus 8 in women with or at risk for human immunodeficiency virus infection. N Engl J Med 2001; 344:637–43. [DOI] [PubMed] [Google Scholar]
- 20. Fiore JR, Volpe A, Tosatti MA, et al. High seroprevalence of human herpesvirus 8 (HHV-8) in HIV-1-infected pregnant women of southeastern Italy: association with injection drug use and hepatitis C virus infection. J Med Virol 2004; 72:656–60. [DOI] [PubMed] [Google Scholar]
- 21. Pauk J, Huang ML, Brodie SJ, et al. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med 2000; 343:1369–77. [DOI] [PubMed] [Google Scholar]
- 22. Casper C, Carrell D, Miller KG, et al. HIV serodiscordant sex partners and the prevalence of human herpesvirus 8 infection among HIV negative men who have sex with men: baseline data from the EXPLORE Study. Sex Transm Infect 2006; 82:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Casper C, Wald A, Pauk J, et al. Correlates of prevalent and incident Kaposi’s sarcoma-associated herpesvirus infection in men who have sex with men. J Infect Dis 2002; 185:990–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.