Abstract
The development of novel, non-invasive techniques and standardization of protocols to assess microvascular dysfunction have elucidated the key role of microvascular changes in the evolution of cardiovascular (CV) damage, and their capacity to predict an increased risk of adverse events. These technical advances parallel with the development of novel biological assays that enabled the ex vivo identification of pathways promoting microvascular dysfunction, providing novel potential treatment targets for preventing cerebral-CV disease. In this article, we provide an update of diagnostic testing strategies to detect and characterize microvascular dysfunction and suggestions on how to standardize and maximize the information obtained from each microvascular assay. We examine emerging data highlighting the significance of microvascular dysfunction in the development CV disease manifestations. Finally, we summarize the pathophysiology of microvascular dysfunction emphasizing the role of oxidative stress and its regulation by epigenetic mechanisms, which might represent potential targets for novel interventions beyond conventional approaches, representing a new frontier in CV disease reduction.
Keywords: Microvascular disease, Oxidative stress, Epigenetics, Clinical manifestations
Graphical Abstract
Introduction
The microcirculation, constituted by pre-arterioles (lumen diameter 100–500 µm), arterioles (lumen diameter <100 µm), capillaries, and venules, is responsible for most part of the resistance to flow that modulates blood pressure (BP) and tissue perfusion. As such, the microvascular structures have key roles in regulating systemic haemodynamics, tissue oxygenation and nutrition, transport of mediators, exchanges of gases and metabolites to and from tissues.1 Over the years, microvascular dysfunction has been recognized as an early marker of cardiovascular (CV) disease and a key feature of several clinical manifestations (see the paragraph ‘Historical Perspectives’ in the Supplementary material online, Addendum).
This has led to the development of novel methods and refinement of old techniques for the assessment of microcirculation. Reference values to identify microvascular dysfunction are now being generated and have the potential to promote harmonization in the interpretation of the results between different laboratories worldwide. In parallel, advances have been made in the understanding of the biological processes regulating microvascular function, including cross-talk between inflammation, oxidative stress, and cellular ageing pathways.
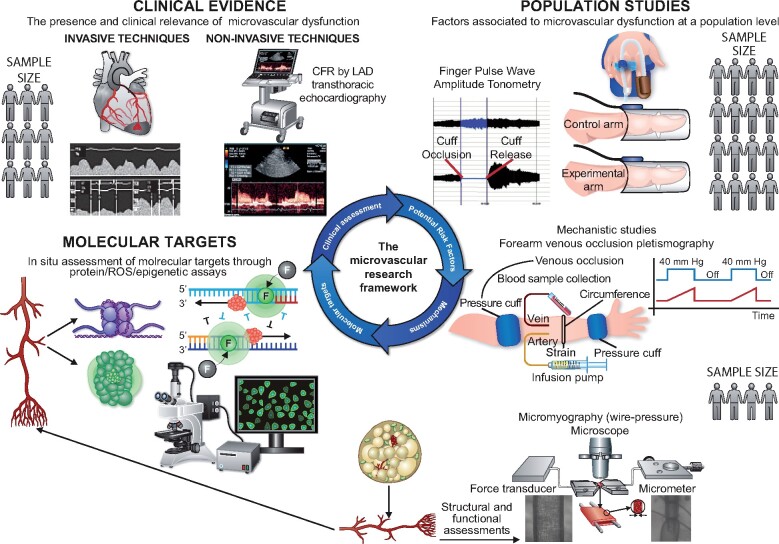
In this article, we discuss how coronary and peripheral microcirculation can be investigated, the strengths and limitations of each method, and the reference values that could be used for a correct interpretation of the results. We also provide recent advances on the molecular mechanisms underpinning microvascular dysfunction, with particular emphasis on the role of epigenetic alterations regulating inflammation, oxidative stress, and cellular ageing pathways. We conclude that a more integrated approach to microvascular research should be adopted in the future, whereby central and peripheral assessment methods should be more frequently combined given their capacity to provide important complementary information. Such an integrated approach has the potential to overcome current limitations in the development of microvascular active drugs (Take home figure).
Take home figure.
The importance of an integrated approach to microvascular research. The potential contribution of microvascular dysfunction to a specific diseased condition or clinical presentation is suspected and confirmed through central microvascular assessment methods (for example, by estimating the coronary flow reserve by left anterior descending transthoracic echocardiography). Subsequently, non-invasive, reproducible peripheral microvascular assessment techniques might help the identification of potential risk factors associated with microvascular dysfunction in the specific diseased condition. Mechanistic studies can be used to confirm the impact of the putative risk factor and the specific pathways through which it induces microvascular dysfunction. Finally, in situ studies performed on isolated arteries can be used to identify novel potential therapeutic targets. The use of drugs directed against such targets requires clinical validation, closing the circle of the microvascular research framework.
Methods for assessing coronary microvascular function
Coronary microcirculation can be assessed using invasive and non-invasive techniques. Although each method has its advantages and limitations (Table 1), it is now clear that patients may present different types of coronary microvascular dysfunction (CMD), highlighting the necessity of a full assessment of coronary microvascular function.
Table 1.
Comparative summary of the strength, weakness, reproducibility and operator-dependency of each method used to assess coronary microvascular function in clinical practice
| Availability | Repeatability | Costs | Risks | Reproducibility and operator dependance | CMV dilatation | CMV constriction | |
|---|---|---|---|---|---|---|---|
| Invasive methodsa | +/− | − | −− | +/−− | + | +++ | +++ |
| TTDE | +++ | +++ | +++ | +++ | +/− | ++ | +/− |
| MCE | +/− | +/− | + | + | +/− | ++ | +/− |
| PET | − | − | − | +/− | ++ | +++ | +/− |
| CMR | + | +/− | − | + | ++ | +++ | +/− |
(−) = poor for the item; (+) = sufficient for the item; (++) = good for the item; (+++) = very good for the item; (+/−) = between sufficient/poor.
CMR, cardiac magnetic resonance; CMV, coronary microvascular; IIM, invasive intracoronary methods; MCE, myocardial contrast echocardiography; PET, positron emission tomography; TTDE, transthoracic Doppler echocardiography.
Include intracoronary Doppler recording of coronary blood flow velocity and thermodilution method (see text for discussion).
Invasive assessment of coronary microvascular function
Nearly half of patients undergoing coronary angiography for appropriate indications have normal coronary arteries (NCAs) or non-significant coronary artery disease, as defined by the absence of any stenosis ≥50% of lumen diameter and/or an abnormal fractional flow reserve (FFR <0.80). Most of these patients has microvascular angina (MVA), i.e. angina caused by abnormalities of coronary microcirculation.2–5
Coronary microvascular function in the catheterization laboratory can be assessed by intracoronary vascular reactivity tests, that can be performed with high reproducibility and safety.6 , 7 The evaluation usually begins with the assessment of coronary flow reserve (CFR), the maximal dilator response to an arteriolar vasodilator, usually adenosine. Furthermore, the response to acetylcholine (Ach) is also assessed. In normal vessels Ach at lower doses causes vasodilatation through release of nitric oxide (NO) by the endothelium; at higher doses, however, it also exerts direct constrictor effects on small coronary arteries via muscarinic receptors on vascular smooth muscle cells (VSMCs), which might occur at lower doses in dysfunctional vessels.8 , 9 Accordingly, an abnormal coronary microvascular response to Ach may result from endothelial dysfunction, an increased reactivity of VSMCs, or both.
Coronary flow reserve
CFR in the cath lab can be assessed by measuring coronary blood flow (CBF) velocity by an intracoronary Doppler flow wire placed in the studied coronary artery [usually the left anterior descending (LAD) artery, Supplementary material online, Figure S1] or using a temperature sensor-tipped guidewire (CFRthermo) (see the Supplementary material online, Addendum). Both CFRDoppl and CFRthermo have been shown to predict clinical events10 , 11 and, therefore, in the absence of large comparative studies, both methods can be used to measure CFR. In a recent small study, CFRDoppl showed a better correlation, compared to CFRthermo, with CFR assessed by positron emission tomography (PET), in particular in arteries with no-flow-limiting stenosis. Poor quality traces that did not allow a reliable assessment of CFR were, however, more frequent with the Doppler than the thermodilution method.12 An advantage of the thermodilution method is that it allows a simultaneous assessment of FFR to exclude or confirm the presence of haemodynamically significant epicardial stenosis.
Adenosine-induced CFR is considered normal when >2.5. Although some differences in cut-offs may be found among individual studies, there is now large consensus that an adenosine CFR <2.0 should be considered as definitely demonstrative of an impaired dilatation of coronary microcirculation.13 Values between 2.0 and 2.5 are of borderline clinical significance.
Index of microcirculatory resistance
Coronary microcirculatory function can also be tested by obtaining the index of microcirculatory resistance (IMR) with the pressure-thermodilution guidewire catheter (see above and the Supplementary material online, Addendum). IMR is a measure of coronary microvascular resistance during hyperaemia (usually obtained with adenosine). Assuming a zero pressure in the coronary vein/right atrium, IMR is calculated with the formula: IMR = P d × TmnHyp, where P d is the mean coronary pressure in the distal part of the epicardial artery and TmnHyp the Tmn during hyperaemia.
IMR has been shown to be independent of epicardial vascular function and reproducible. In STEMI patients, IMR provides important prognostic information regarding the post-MI recovery of ventricular function.14 , 15 Despite reasonable prediction of events and outcomes following PCI,16 , 17 there are no guideline-based recommendations for its regular utilization in the catheterization laboratory at present.
Acetylcholine testing
To assess the microvascular response to Ach, this endothelial agonist is infused at increasing doses while CBF velocity is measured by Doppler guidewire. The effect is expressed as the ratio of average peak velocity (APV) of CBF during Ach administration over basal APV. A concomitant assessment of the epicardial response to Ach is usually done, to exclude the occurrence of epicardial spasm. Further details on Ach test can be found in the Supplementary material online, Addendum.
Result interpretation and cut-offs of acetylcholine-induced changes in coronary blood flow
The coronary microvascular response to Ach is considered normal when CBF increases by >50% compared to baseline,2 , 4 , 9 , 18 , 19 in absence of significant epicardial constriction. Thus, an increase lower than 50% indicates impaired coronary microvascular dilatation, but some patients show CBF reduction, which suggests an abnormal coronary microvascular constrictive response. Coronary microvascular spasm (CMS) can also be diagnosed when angina and ischaemic electrocardiogram (ECG) changes occur during Ach test in the absence of significant epicardial spasm.13 , 20
Non-invasive assessment of coronary microcirculation
In angina patients in whom flow-limiting coronary stenoses have been excluded by angiography and by FFR, coronary microvascular dilator function can be assessed non-invasively by various methods able to provide estimates of coronary/myocardial blood flow at rest and during maximal hyperaemia. In all cases, adenosine (140 µg/kg/min intravenously) or dipyridamole (0.56–0.84 mg intravenously) CFR is usually measured and criteria for normal/abnormal values are the same of those indicated for the invasive assessment, while an increased reactivity to constrictor stimuli, as indicated by a critical reduction of CBF in response to their administration, cannot adequately be assessed by non-invasive methods due to the impossibility to exclude with certainty an involvement of epicardial vessels in the constrictor response.
Transthoracic Doppler echocardiography of the LAD coronary artery is a largely available and potentially widely applicable method to assess CFR,21 but its use can be limited by technical issues and operator variability.22 Myocardial contrast echocardiography, on the other hand, is a promising more objective ultrasound method to assess CFR, but it has been used in only few studies until now and requires further validation.23 , 24
PET and cardiac magnetic resonance (CMR) are the most reliable methods for non-invasive assessment of coronary microvascular dilation as they allow valuable measurements of global and regional myocardial blood flow. Both methods have been validated in animal models by microspheres blood flow studies, providing data for physiological classification and diagnosis of perfusion abnormalities.25 Compared to PET, CMR shows a higher spatial resolution, lack of ionizing radiations and guarantees an assessment of cardiac structure and function (Table 1). However, the limited availability, high costs and long execution times of these methods, make them poorly suitable for serial assessment of CMV function (Table 1). Further limitations include radiation exposure for PET and, for CMR, contraindication to gadolinium use in patients with glomerular filtration rate <30 mL/min, claustrophobia, arrhythmias and implanted devices.26
Methods of assessing peripheral microvascular function/structure
The most validated and largely used techniques to measure microvascular function and/or structure in the peripheral microcirculation include forearm plethysmography, micromyography, finger plethysmography, and laser-Doppler flowmetry (LDF). Practical recommendations for the selection of the most appropriate peripheral vascular technique based on their strengths and limitations, as well as the research question to be addressed, are provided in Table 2 and in the Supplementary material online, Addendum.
Table 2.
Strengths and weakness of the most commonly used central and peripheral microvascular assessment methods
| Strengths | Weakness | Reproducibility | Inter-observer variability | Recommended | Not recommended | |
|---|---|---|---|---|---|---|
| Micromyography |
|
|
The intra-assay coefficient of variation of the media cross-sectional area calculation is 10.4% (6 vessels, 10 measurements in each vessel in a single session)27 | The interassay coefficient of variation is 11.2% (6 vessels, 10 measurements in each vessel performed in 2 sessions by 2 different observers)27 | Studies requiring:
|
|
| Forearm venous plethysmography |
|
|
Within-subjects coefficient of variation of baseline forearm blood flow ratio is around 20%.28 , 29 Between-day reproducibility of hyperaemic vascular resistance is around 10%.30 |
Not available | Studies requiring:
|
Studies requiring:
|
| Finger plethysmography |
|
|
The interclass correlation for repeated assessments is reported between 0.6131 and 0.74.32 | Not available | Studies requiring:
|
Studies requiring:
|
NO, nitric oxide.
Forearm plethysmography
This technique allows measurements in forearm blood flow (FBF) changes by venous plethysmography before and after the infusion of endothelium-dependent and -independent vasoactive substances into a cannulated brachial artery.33 The absence of systemic effects of the vasoactive drugs being tested is guaranteed by the lack of FBF changes in the contralateral forearm and the constant rate of arterial inflow.34 , 35 Details are reported in the Supplementary material online, Addendum.
Venous plethysmography can also provide indirect information on the structure of small resistance arteries by measuring the maximum post-ischaemic flow. This is obtained by occlusion of the brachial artery of the dominant arm, followed by dynamic exercise (see the Supplementary material online, Addendum file for further details). Once the arterial occlusion is rapidly removed, the venous occlusion is maintained. The increased volume of the forearm is proportional to the maximal arterial flow capacity. The minimum vascular resistances are obtained by dividing the mean BP for the maximum arterial flow. This measure correlates well with more direct measurements of resistance vessel structure, such as the media-to-lumen ratio (MLR) obtained with micromyography,36 and is not influenced by sympathetic tone or administration of vasodilators.37
Cut-off of forearm blood flow and minimum forearm vascular resistance
Although some studies have documented a relationship between forearm endothelial function and risk of CV events,38 , 39 at present clear cut-off values for abnormal FBF and minimum vascular resistance are not available. Accordingly, the use of this technique remains mainly limited to mechanistic studies.
Micromyography
This technique is performed in two major variants: wire and perfusion-pressure micromyography. Both methods enable the assessment of both functional and structural characteristics of isolated resistance arterioles (100–300-µm lumen diameter), obtained from subcutaneous tissue biopsies from the glutaeal or anterior abdominal region.40–43 After isolation, vessels are cannulated with stainless steel wires (wire micromyograph) or slipped into two glass microcannulae contained in a pressurized chamber (pressurized myograph). Under the direct visualization of the vessel by a microscope connected to a computer, the endothelium-dependent and -independent relaxations are assessed by measuring the dilatory responses to cumulative doses of endothelial agonists (i.e. Ach) and sodium nitroprusside, respectively.43 , 44 Details on the recommended protocols are reported in the Supplementary material online, Addendum.
The micromyography is the most widely used approach to evaluate microvascular structural changes. The total wall thickness, as well as the internal diameter of the vessels, can be evaluated by computer-assisted video analysers.44 These data inform on the severity and the pattern of vascular remodelling induced by CV risk factor exposure. Given its high sensitivity and specificity, MLR is the most commonly used index of resistance artery remodelling, as it is independent from the vessels’ dimensions,45 , 46 with no difference between wire or pressure micromyography.45 , 47 , 48 Conversely, media thickness and internal diameters are dependent on vessel dimensions;45 therefore, comparisons between such parameters need caution.
Cut-off of media-to-lumen ratio
Age- and sex-specific percentiles of MLR in a healthy population have been recently provided and should be used to discriminate healthy subjects from those with early or advanced microvascular remodelling (Supplementary material online, Table S1).40 Moreover, a cut-off value of 0.098 has been shown in two studies to distinguish between people with vs. those without CV events.49 , 50 In contrast, no studies have documented a prognostic role for endothelial function, as evaluated by micromyography.
Finger plethysmography
This technique provides observer-independent measures of endothelial function using peripheral arterial tonometry (PAT). The technique captures beat-to-beat recordings of the finger arterial pulse wave amplitude with pneumatic probes.51 A pressure cuff is placed on one forearm and inflated to a supra-systolic pressure to produce 5 min of ischaemia followed by reactive hyperaemia. The pneumatic probe applied to the fingertip of the same arm records the increase in arterial blood volume, which is reflected by an increase in pulsatile arterial column changes. The derived reactive hyperaemia index (RHI) is considered a marker of endothelial function. Details of the recommended protocol are reported in the Supplementary material online, Addendum.
An inverse relation between RHI and multiple CV risk factors has been described,51–54 and a systematic meta-analysis has documented that the RHI is as effective as flow-mediated dilation in predicting CV events.55 However, it has been objected that RHI does not reflect only endothelial function, as augmentation of the pulse amplitude after reactive hyperaemia involves changes in flow that are only partly (50%) dependent on NO availability.56 In addition, a poor correlation has been reported between PAT and flow-mediated dilation in various clinical settings.57 , 58
Cut-off of reactive hyperaemia index
Early studies have associated an RHI of >2.1 with a low risk of CV events,59 , 60 while values <1.5 have been associated with an increased risk of CV events,61 particularly in subjects at moderate-to-high CV risk or with angina symptoms. Other studies have confirmed that the cut-off value of RHI that independently discriminate between subjects at high or low risk for CV events ranges between 1.60 and 1.75.62 , 63
Laser-Doppler flowmetry
LDF provides a non-invasive real-time quantification of relative changes in skin microcirculation. It is based on light-beating spectroscopy and the detection of a Doppler shift of the laser light backscattered from moving red blood cell in cutaneous microcirculation.64 , 65 The relative modifications in skin blood flow in response to endothelial stimuli [usually post-occlusive reactive hyperaemia (PORH), local thermal hyperaemia, Ach, or sodium nitroprusside iontophoresis] are considered. LDF shows a good reproducibility (coefficient of variation <10%).66 Early studies suggested that PORH area is mainly driven by the activation of sensory nerves and myogenic response.67 In turn, more recent reports have suggested that time to half before and after hyperaemia (TH2) may represent a reliable marker of endothelial function.68 , 69
Local thermal hyperaemia approach leads to a temperature-dependent sustained increase in skin cutaneous flow. The plateau is mostly mediated by NO and endothelium-derived hyperpolarizing factor (EDHF),70 while the initial rapid phase relies predominantly on local sensory nerves.71 The contributions of neural reflex, NO, and EDHF vary according to the target temperature and rate of heating. A rapid heating to 39°C seems to cause an almost exclusively NO-dependent hyperaemia.72
Iontophoresis can be used to test the influence on the LDF signal of vasoactive compounds. Ach and sodium nitroprusside might be used to generate endothelium-dependent and -independent vasodilatation, respectively. Some authors, however, showed that a large part of the Ach-related increase in blood flow is mediated by prostanoids with only a modest attenuation by NO synthase inhibition.73 Others, instead, confirmed that Ach‐mediated vasodilation is highly NO dependent, showing also an important interactions of NO with prostaglandins.74 Of note, methacholine seems to induce a more specific NO-dependent vasodilation.75
Cut-off of laser-Doppler flowmetry
The LDF does not provide measures of absolute perfusion values. Measurements are expressed as arbitrary perfusion unit (PU) or in millivolts (1 PU = 10 mV). To account for BP variations, most authors present results as cutaneous vascular conductance, dividing blood flow by mean BP values (mV/mm Hg).76 The non-specific responses to iontophoresis and different methods used to acquire and elaborate the results make difficult the identification of normal values for LDF; furthermore, it remains unclear whether LDF measurements have any additional value in predicting CV events.
Molecular mechanisms of microvascular disease: oxidative stress and inflammation drive microvascular ageing
Reactive oxygen species (ROS) and the inflammatory response can be considered final common pathways leading to microvascular dysfunction consequent to CV risk factor exposure. Increasing evidence supports a tight and highly dynamic regulation of inflammatory and redox-signalling pathways induced by inflammatory, metabolic, and haemodynamic changes at the level of the microcirculation. Many of these pathways are also involved in the control of cellular ageing and apoptosis and, in animal experiments, their modulation can induce substantial changes in the lifespan, ultimately supporting a relevant contribution of an inflammageing process to the evolution of microvascular dysfunction.
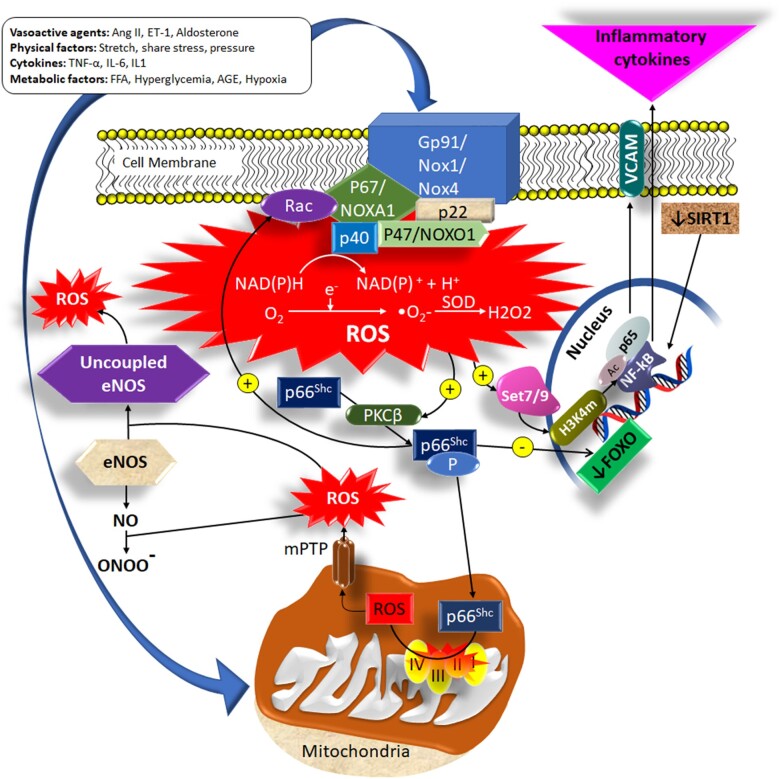
The mitochondrial adaptor p66Shc has emerged as a pivotal modulator of mitochondrial ROS production, inflammation, and vascular aging.77 Several CV risk factors have been shown to induce p66Shc up-regulation, leading to premature ROS accumulation, senescence, and endothelial dysfunction in mice and humans77 (Figure 1). Genetic deletion of p66Shc prevents age-dependent endothelial dysfunction and extends lifespan by 30% in mice.78–80 Activation of Arginase II (Arg II), the enzyme that metabolizes the eNOS substrate l-arginine to urea and l-ornithine, also represents an important link between CV risk factors and microvascular aging. Arg II activity is increased in patients with type 2 diabetes (T2DM) and hypertension, where accounts for reduced l-arginine availability, decreased NO production, and endothelial dysfunction.81 It also triggers vascular senescence in VSMCs and endothelial cells.82 Arginase inhibition restores endothelial-dependent vasorelaxation in small arteries of obese patients, although this effect is attenuated in ageing due to a high pro-oxidant environment sustained by hyperactivation of the NADPH oxidase (Nox).41 , 83
Figure 1.
Major pathways leading to ROS-mediated microvascular dysfunction. Nox isoforms and mitochondria represent major systems accounting for the dynamic regulation of ROS production within the microvascular wall. The activation of Nox leads to ROS production and triggers p66Shc phosphorylation and translocation within the mitochondria, where it further promotes ROS generation by altering the function of the mitochondria respiratory chain and opening the mitochondria permeability transition pore. In turn, p66Shc activation stimulates the activity of Nox, thus generating a vicious cycle that amplifies ROS production. The increased concentration of intracellular ROS promotes the transformation of NO in peroxynitrite radicals and uncouples the eNOS, switching its activity from a NO to a ROS producing enzyme. Epigenetic modifications commonly detected in ageing, such as a reduced SIRT1 expression, sustain a further increase in intracellular oxidative stress, reducing the expression of antioxidant enzymes (secondary to FOXO inhibition) and promoting pro-inflammatory cytokine production through activation of the NF-kB and expression of adhesion molecules. NO, nitric oxide; NoX, NADPH oxidase; ROS, reactive oxygen species.
The family of Nox enzymes is another major source of microvascular ROS. Clinical conditions associated to the perturbation of microvascular haemodynamic and metabolic homeostasis are characterized by increased vascular reactivity and overexpression of different Nox isoforms, which has been implicated in the development of an early microvascular ageing phenotype.41 , 84 Furthermore, inflammatory cytokines [i.e. tumour necrosis factor alpha (TNF-α) and interleukin-6] are often elevated in patients with cardiometabolic disturbances and associate with the augmented activity of Nox and vascular aging.85 Sirtuins (a family of nicotinamide adenine dinucleotide-dependent proteins) represent another example of the connection between oxidative stress and ageing pathways at the level of the microcirculation. Age-related loss of SIRT1 leads to functional deficits, diminishes stress response, reduces capacity for migration/proliferation, and increases senescence by modulating several downstream effectors including LKB1, AMPK, and FOXO.86 Moreover, microRNA-217 suppresses SIRT1-dependent eNOS functionality and triggers endothelial senescence.87
The increased ROS generation at the level of the microvascular wall is also a potent activator of the inflammatory response. ROS activate mitogen-activated protein kinases (ERK1/2, p38MAPK, and JNK), tyrosine kinases, and tyrosine phosphatases, which control vascular cell proliferation, migration, hypertrophy, and inflammation.88–90 Important downstream redox targets are transcription factors (NF-kB, AP-1 and HIF-1) and pro-inflammatory genes, critically involved in chemokine and cytokine production, recruitment, and activation of inflammatory and immune cells, which promote microvascular inflammation and fibrosis in response to CV risk factors.89 Indeed, human studies showed an inverse relationship between peripheral microvascular structural changes and circulating Treg lymphocytes.91 In addition, subpopulations of circulating T lymphocytes are associated with microvascular oxidative stress in peripheral human arteries.91
Epigenetic regulation of microvascular function
Epigenetic changes or mutations are emerging as key mechanisms underpinning the dynamic ability of the microcirculation to modulate inflammation, oxidative stress, and ageing signalling in response to changes in the local metabolic and haemodynamic environment.92 Some of most intensively studied epigenetic changes involved in oxidative stress-mediated microvascular dysfunction relate to the p66Shc gene. Epigenetic remodelling of p66Shc promoter by DNA methylation and histone modifications is involved in obesity and diabetes-induced oxidative stress, endothelial dysfunction, and vascular hyperglycaemic memory, the phenomenon responsible for persistent oxidative stress and vascular dysfunction despite the achievement of an optimal glycaemic control.93–95 Reduced DNA methylation and increased methylation of histone at lysine 4 (H3K4) were found on the promoter of TNFα, a pro-inflammatory cytokine involved in obesity and hypertension-related microvascular dysfunction.96 , 97 Similarly, activation of the methyltransferase SUV39H1 in VSMCs from diabetic mice was found to regulate the pro-inflammatory transcription factor NF-kB p65.98 Furthermore, mono-methylation of histone 3 at lysine 4 (H3K4me1) by the methyltransferase SETD7 fosters endothelial inflammation, senescence, and vascular dysfunction via up-regulation of NF-kB p65 and p53.99 Besides the modifications in chromatin, several miRNAs (miR-92, miR-126, miR-195, miR 26a, and miR-155) are implicated in microvascular dysfunction by targeting relevant pathways involved in endothelial cell and VSMC damage (i.e. eNOS, RhoA, Smad1, Sirt1, Bcl-2).100 The importance of these epigenetic discoveries is supported by the recent development of epigenetic pharmacological intervention. The histone deacetylase inhibitor Vorinostat has shown to prevent NF-kB activation, eNOS uncoupling, and vascular oxidative stress in diabetic mice.101 Similarly, histone deacetylase blockade by Trichostatin A and sodium butyrate prevented TNF-α transcription while promoting angiogenic response.102 Apabetalone—an epigenetic regulator targeting bromodomain and extra-terminal proteins—modulates vascular inflammation and reduces CV events.103 Also, miRs targeting, by mimics or antagomiRs, is a promising epigenetic therapy currently tested in clinical trials.104 , 105
Clinical implications of microvascular dysfunction
Coronary microcirculation
Many studies have shown that CMD can be responsible for both chronic and acute myocardial ischaemic syndromes.106 , 107 The microvascular origin of ischaemic symptoms should be suspected in patients with the evidence of myocardial ischaemia at non-invasive investigation who show no obstructive stenosis (usually ≥50%) and no other specific causes of ischaemia (e.g. epicardial spasm, intracoronary thrombus, coronary dissection) at coronary angiography, but it is definitely demonstrated by confirming the presence of CMD.13
MVA [and asymptomatic myocardial ischaemia with non-obstructed coronary arteries (INOCAs)] may occur in the context of various heart diseases,108 but it also represents the main or specific cardiac issue for many patients (primary MVA). Stable MVA, mainly related to efforts, is the most typical clinical presentation.106 Both an impairment of coronary microvascular dilatation and an increased coronary microvascular constriction have been demonstrated and may variably contribute to the symptoms in these patients.109–112
The initial treatment of stable MVA is based on traditional anti-ischaemic drugs (beta-blockers, calcium antagonists, nitrates). These, however, fail to obtain a satisfactory control of angina symptoms in a sizeable proportion of patients.113 , 114 Thus, several additional drugs (e.g. ranolazine, xanthines, ivabradine, trimetazidine) and non-pharmacological therapies (e.g. spinal cord stimulation, enhanced external counterpulsation) have been proposed and may help achieve significant angina relief.115 , 116 Importantly, the recent CorMicA trial has shown that, in patients with angina but non-obstructive CAD, pharmacological therapy guided by the results of invasive provocative testing, aimed to identify the pathophysiological mechanism(s) responsible for the symptoms, may result in better symptom control, as compared with empiric choices.117
Prognosis of stable MVA is debated. Studies including patients with NCAs have consistently shown good clinical outcome.113 , 114 , 118 , 119 Some reports, however, have described increased rates of clinical events in angina patients with non-obstructive coronary stenoses120 , 121 or a relation between CMD and events.9 , 122 , 123 The interpretation of these results is complicated by the recruitment of heterogeneous populations, in whom the role of CMD as an independent causal factor was not assessed appropriately. Furthermore, the relation of CMD with outcome was not consistent among studies.9 , 122–125 Thus, whether CMD adds prognostic information in patients with non-significant stenosis requires further assessment.126
Most recently, CMS has been suggested to be a cause of the clinical picture of acute coronary syndrome (ACS) with non-obstructed coronary arteries at angiography, who constitute 10–15% of those admitted with a diagnosis of ACS and include patients with myocardial infarction with non-obstructed coronary arteries (MINOCAs).13 , 106 , 127 The mechanisms of these ACSs, however, are heterogeneous, and the prevalence of CMS-related ACSs remains to be established.128 In a study, 16.3% of 80 MINOCA patients129 had evidence of CMS on Ach testing, which was associated with a worse clinical outcome. However, most patients with events also had epicardial spasm and, therefore, the exact prognostic role of CMS in this context also remains to be better defined.
No established treatment for either acute INOCA or MINOCA is available at present, due to the different pathophysiological mechanisms responsible for the syndrome and the lack of clinical trials. Some benefits on CV events have been reported in a retrospective study by statins, beta-blockers, and inhibitors of the renin–angiotensin system,130 but calcium antagonists are expected to be the main treatment for recurrent angina episodes related to CMS.131
Finally, recent data suggest that CMD may be involved in the mechanisms responsible for heart failure with preserved left ventricular ejection fraction,132 although prospective studies relating CMD to the development of this clinical condition are still lacking.
Peripheral microcirculation
Some studies showed that peripheral microvascular endothelial dysfunction might be a powerful predictor of CV events in both subjects at high or low CV risk.38 , 39 In particular, the RHI and the hyperaemic velocity (considered an indirect marker of microvascular function) confirmed that the severity of endothelial dysfunction at the forearm microcirculation can predict the risk of future CV events.61 , 133 , 134
Microvascular structural changes also provide reliable prognostic information. Structural alterations in the subcutaneous vascular district are, indeed, a good reflection of those detectable in target organs, including heart135 and brain.136 This might lead to impaired organ flow reserve and, as such, could account for the faster progression of organ damage and the increased CV risk of diabetic and hypertensive patients.137 , 138 An increased MLR is also a powerful and independent predictor of adverse CV outcome.49 , 50 , 139
There is evidence suggesting that interventions on advanced microvascular disease burden are less effective than early treatments.140 Indeed, individuals with high MLR remain at increased CV risk despite normalization of BP values.141 Instead, early CV interventions have significant impact on peripheral artery remodelling,142–144 potentially leading to improved tissue perfusion and regression of organ damage.142 Also, more aggressive interventions are needed to reduce the burden of microvascular remodelling in patients with advanced disease.145
Conclusions
The increasing use of invasive and non-invasive coronary and peripheral microvascular assessment methods has unravelled the role of microvascular dysfunction in several CV disease manifestations. In parallel, a better understanding of the biological mechanisms involved in microvascular dysfunction has shown that regulation of the microvascular phenotype is highly dynamic and tightly regulated by local levels of inflammation and oxidative stress. Epigenetic modifications are emerging as key mechanisms of post-natal microvascular adaptation to different environmental factors and have highlighted the substantial overlap between inflammatory, oxidative stress, metabolic and cellular ageing pathways in the regulation of the microcirculation phenotype. Despite these advancements, no microvascular active drugs are available at present. This is related to several factors, including the difficulties in creating large datasets of microvascular data acquired using standardized protocols and the limited integration of the data obtained from central and peripheral microvascular methods. The refinement of microvascular assessment techniques and standardization of their protocols, combined with the availability of specific prognostic cut-off values, provide now a unique opportunity to move microvascular research from the bench to a clinical setting, potentially allowing the earlier identification of patients at high CV risk. Furthermore, the adoption of a more integrated approach to microvascular research in the future, whereby central and peripheral microvascular assessments are performed as part of the same study, has the potential to accelerate the identification of novel and disease-specific therapeutic targets and the development of drugs that could be used to prevent the evolution and clinical consequences of microvascular disease.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
F.P. is the recipient of an H.H. Sheikh Khalifa bin Hamad Al Thani Foundation Assistant Professorship at the Faculty of Medicine, University of Zurich.
Funding
British Heart Foundation Chair award (CH/12/4/29762 to R.M.T.).
Conflict of interest: none declared.
Contributor Information
Stefano Masi, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy; Institute of Cardiovascular Science, University College London, London, UK.
Damiano Rizzoni, Clinica Medica, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy; Division of Medicine, Istituto Clinico Città di Brescia, Brescia, Italy.
Stefano Taddei, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy.
Robert Jay Widmer, Division of Cardiovascular Diseases, Mayo Clinic College of Medicine, Rochester, MN, USA.
Augusto C Montezano, Institute of Cardiovascular & Medical Sciences, BHF Glasgow Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
Thomas F Lüscher, Heart Division, Royal Brompton and Harefield Hospital and Imperial College, London, UK; Center for Molecular Cardiology, University of Zürich, Zürich, Switzerland.
Ernesto L Schiffrin, Department of Medicine and Lady Davis Institute, Sir Mortimer B. Davis-Jewish General Hospital, McGill University, Montreal, QC, Canada.
Rhian M Touyz, Institute of Cardiovascular & Medical Sciences, BHF Glasgow Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
Francesco Paneni, Center for Molecular Cardiology, University of Zürich, Zürich, Switzerland; Department of Cardiology, University Heart Center, University Hospital Zurich, Zürich, Switzerland; Department of Research and Education, University Hospital Zurich, Zürich, Switzerland.
Amir Lerman, Division of Cardiovascular Diseases, Mayo Clinic College of Medicine, Rochester, MN, USA.
Gaetano A Lanza, Department of Cardiovascular and Thoracic Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy.
Agostino Virdis, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy.
References
- 1. Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation 2008;118:968–976. [DOI] [PubMed] [Google Scholar]
- 2. Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 2010;56:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin EA, Prasad A, Rihal CS, Lerman LO, Lerman A. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J Am Coll Cardiol 2010;56:1840–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015;8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 5. Yoshino S, Cassar A, Matsuo Y, Herrmann J, Gulati R, Prasad A, Lennon RJ, Lerman LO, Lerman A. Fractional flow reserve with dobutamine challenge and coronary microvascular endothelial dysfunction in symptomatic myocardial bridging. Circ J 2014;78:685–692. [DOI] [PubMed] [Google Scholar]
- 6. Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, Azarbal B, Petersen J, Sharaf B, Handberg E, Shufelt C, Kothawade K, Sopko G, Lerman A, Shaw L, Kelsey SF, Pepine CJ, Merz CN. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv 2012;5:646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ong P, Athanasiadis A, Borgulya G, Vokshi I, Bastiaenen R, Kubik S, Hill S, Schaufele T, Mahrholdt H, Kaski JC, Sechtem U. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation 2014;129:1723–1730. [DOI] [PubMed] [Google Scholar]
- 8. Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med 1986;315:1046–1051. [DOI] [PubMed] [Google Scholar]
- 9. Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000;101:948–954. [DOI] [PubMed] [Google Scholar]
- 10. Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam CW, Shin ES, Koo BK. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol 2016;67:1158–1169. [DOI] [PubMed] [Google Scholar]
- 11. Meuwissen M, Chamuleau SA, Siebes M, de Winter RJ, Koch KT, Dijksman LM, van den Berg AJ, Tijssen JG, Spaan JA, Piek JJ. The prognostic value of combined intracoronary pressure and blood flow velocity measurements after deferral of percutaneous coronary intervention. Catheter Cardiovasc Interv 2008;71:291–297. [DOI] [PubMed] [Google Scholar]
- 12. Everaars H, de Waard GA, Driessen RS, Danad I, van de Ven PM, Raijmakers PG, Lammertsma AA, van Rossum AC, Knaapen P, van Royen N. Doppler flow velocity and thermodilution to assess coronary flow reserve: a head-to-head comparison with [(15)O]H2O PET. JACC Cardiovasc Interv 2018;11:2044–2054. [DOI] [PubMed] [Google Scholar]
- 13. Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Bairey Merz CN; Coronary Vasomotion Disorders International Study Group. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J 2017;38:2565–2568. [DOI] [PubMed] [Google Scholar]
- 14. De Maria GL, Cuculi F, Patel N, Dawkins S, Fahrni G, Kassimis G, Choudhury RP, Forfar JC, Prendergast BD, Channon KM, Kharbanda RK, Banning AP. How does coronary stent implantation impact on the status of the microcirculation during primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction? Eur Heart J 2015;36:3165–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Payne AR, Berry C, Doolin O, McEntegart M, Petrie MC, Lindsay MM, Hood S, Carrick D, Tzemos N, Weale P, McComb C, Foster J, Ford I, Oldroyd KG. Microvascular resistance predicts myocardial salvage and infarct characteristics in ST-elevation myocardial infarction. J Am Heart Assoc 2012;1:e002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fearon WF, Low AF, Yong AS, McGeoch R, Berry C, Shah MG, Ho MY, Kim HS, Loh JP, Oldroyd KG. Prognostic value of the index of microcirculatory resistance measured after primary percutaneous coronary intervention. Circulation 2013;127:2436–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGeoch R, Watkins S, Berry C, Steedman T, Davie A, Byrne J, Hillis S, Lindsay M, Robb S, Dargie H, Oldroyd K. The index of microcirculatory resistance measured acutely predicts the extent and severity of myocardial infarction in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2010;3:715–722. [DOI] [PubMed] [Google Scholar]
- 18. Lim SH, Flammer AJ, Yoon MH, Lennon RJ, Gulati R, Mathew V, Rihal CS, Lerman LO, Lerman A. The long-term effect of coronary stenting on epicardial and microvascular endothelial function. Circ Cardiovasc Interv 2012;5:523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Widmer RJ, Lerman A. Endothelial dysfunction and cardiovascular disease. Glob Cardiol Sci Pract 2014;2014:291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN, Bairey Merz CN; Coronary Vasomotion Disorders International Study Group. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 21. Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near-normal coronary arteries. Am J Cardiol 2009;103:626–631. [DOI] [PubMed] [Google Scholar]
- 22. Lanza GA, Camici PG, Galiuto L, Niccoli G, Pizzi C, Di Monaco A, Sestito A, Novo S, Piscione F, Tritto I, Ambrosio G, Bugiardini R, Crea F, Marzilli M; Gruppo di Studio di Fisiopatologia Coronarica e Microcircolazione SIdC. Methods to investigate coronary microvascular function in clinical practice. J Cardiovasc Med 2013;14:1–18. [DOI] [PubMed] [Google Scholar]
- 23. Galiuto L, Sestito A, Barchetta S, Sgueglia GA, Infusino F, La Rosa C, Lanza G, Crea F. Noninvasive evaluation of flow reserve in the left anterior descending coronary artery in patients with cardiac syndrome X. Am J Cardiol 2007;99:1378–1383. [DOI] [PubMed] [Google Scholar]
- 24. Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL, Nihoyannopoulos P. Contrast echocardiography: evidence-based recommendations by European Association of Echocardiography. Eur J Echocardiogr 2008;10:194–212. [DOI] [PubMed] [Google Scholar]
- 25. Gould KL, Johnson NP. Coronary physiology beyond coronary flow reserve in microvascular Angina: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:2642–2662. [DOI] [PubMed] [Google Scholar]
- 26. Camici PG, d’Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol 2015;12:48–62. [DOI] [PubMed] [Google Scholar]
- 27. Rizzoni D, Porteri E, Castellano M, Bettoni G, Muiesan ML, Muiesan P, Giulini SM, Agabiti-Rosei E. Vascular hypertrophy and remodeling in secondary hypertension. Hypertension 1996;28:785–790. [DOI] [PubMed] [Google Scholar]
- 28. Petrie JR, Ueda S, Morris AD, Murray LS, Elliott HL, Connell JM. How reproducible is bilateral forearm plethysmography? Br J Clin Pharmacol 1998;45:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walker HA, Jackson G, Ritter JM, Chowienczyk PJ. Assessment of forearm vasodilator responses to acetylcholine and albuterol by strain gauge plethysmography: reproducibility and influence of strain gauge placement. Br J Clin Pharmacol 2001;51:225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mathiassen ON, Buus NH, Olsen HW, Larsen ML, Mulvany MJ, Christensen KL. Forearm plethysmography in the assessment of vascular tone and resistance vasculature design: new methodological insights. Acta Physiol (Oxf) 2006;188:91–101. [DOI] [PubMed] [Google Scholar]
- 31. Brant LC, Barreto SM, Passos VM, Ribeiro AL. Reproducibility of peripheral arterial tonometry for the assessment of endothelial function in adults. J Hypertens 2013;31:1984–1990. [DOI] [PubMed] [Google Scholar]
- 32. McCrea CE, Skulas-Ray AC, Chow M, West SG. Test-retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vasc Med 2012;17:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol 2001;52:631–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Junejo RT, Ray CJ, Marshall JM. Cuff inflation time significantly affects blood flow recorded with venous occlusion plethysmography. Eur J Appl Physiol 2019;119:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barendsen GJ, Venema H, van den Berg J. Semicontinuous blood flow measurement by triggered venous occlusion plethysmography. J Appl Physiol 1971;31:288–291. [DOI] [PubMed] [Google Scholar]
- 36. Rosei EA, Rizzoni D, Castellano M, Porteri E, Zulli R, Muiesan ML, Bettoni G, Salvetti M, Muiesan P, Giulini SM. Media: lumen ratio in human small resistance arteries is related to forearm minimal vascular resistance. J Hypertens 1995;13:341–7. [PubMed] [Google Scholar]
- 37. Rizzoni D, Aalkjaer C, De Ciuceis C, Porteri E, Rossini C, Rosei CA, Sarkar A, Rosei EA. How to assess microvascular structure in humans. High Blood Press Cardiovasc Prev 2011;18:169–177. [DOI] [PubMed] [Google Scholar]
- 38. Heitzer T, Schlinzig T, Krohn K, Meinertz T, MüNzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001;104:2673–2678. [DOI] [PubMed] [Google Scholar]
- 39. Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 2001;104:191–196. [DOI] [PubMed] [Google Scholar]
- 40. Bruno RM, Grassi G, Seravalle G, Savoia C, Rizzoni D, Virdis A; Study Group on Micro, Macrocirculation of the Italian Society of Hypertension. Age- and sex-specific reference values for media/lumen ratio in small arteries and relationship with risk factors. Hypertension 2018;71:1193–1200. [DOI] [PubMed] [Google Scholar]
- 41. Masi S, Colucci R, Duranti E, Nannipieri M, Anselmino M, Ippolito C, Tirotta E, Georgiopoulos G, Garelli F, Nericcio A, Segnani C, Bernardini N, Blandizzi C, Taddei S, Virdis A. Aging modulates the influence of arginase on endothelial dysfunction in obesity. Arterioscler Thromb Vasc Biol 2018;38:2474–2483. [DOI] [PubMed] [Google Scholar]
- 42. Masi S, Georgiopoulos G, Chiriaco M, Grassi G, Seravalle G, Savoia C, Volpe M, Taddei S, Rizzoni D, Virdis A. The importance of endothelial dysfunction in resistance artery remodelling and cardiovascular risk. Cardiovasc Res 2020;116:429–437. [DOI] [PubMed] [Google Scholar]
- 43. Virdis A, Santini F, Colucci R, Duranti E, Salvetti G, Rugani I, Segnani C, Anselmino M, Bernardini N, Blandizzi C, Salvetti A, Pinchera A, Taddei S. Vascular generation of tumor necrosis factor-alpha reduces nitric oxide availability in small arteries from visceral fat of obese patients. J Am Coll Cardiol 2011;58:238–247. [DOI] [PubMed] [Google Scholar]
- 44. Virdis A, Savoia C, Grassi G, Lembo G, Vecchione C, Seravalle G, Taddei S, Volpe M, Rosei EA, Rizzoni D. Evaluation of microvascular structure in humans: a ‘state-of-the-art’ document of the Working Group on Macrovascular and Microvascular Alterations of the Italian Society of Arterial Hypertension. J Hypertens 2014;32:2120–2129; discussion 2129. [DOI] [PubMed] [Google Scholar]
- 45. Schiffrin EL, Deng LY. Structure and function of resistance arteries of hypertensive patients treated with a beta-blocker or a calcium channel antagonist. J Hypertens 1996;14:1247–1255. [DOI] [PubMed] [Google Scholar]
- 46. Endemann DH, Pu Q, De Ciuceis C, Savoia C, Virdis A, Neves MF, Touyz RM, Schiffrin EL. Persistent remodeling of resistance arteries in type 2 diabetic patients on antihypertensive treatment. Hypertension 2004;43:399–404. [DOI] [PubMed] [Google Scholar]
- 47. Falloon BJ, Stephens N, Tulip JR, Heagerty AM. Comparison of small artery sensitivity and morphology in pressurized and wire-mounted preparations. Am J Physiol 1995;268:H670–8. [DOI] [PubMed] [Google Scholar]
- 48. Schiffrin EL, Hayoz D. How to assess vascular remodelling in small and medium-sized muscular arteries in humans. J Hypertens 1997;15:571–584. [DOI] [PubMed] [Google Scholar]
- 49. De Ciuceis C, Porteri E, Rizzoni D, Rizzardi N, Paiardi S, Boari GE, Miclini M, Zani F, Muiesan ML, Donato F, Salvetti M, Castellano M, Tiberio GA, Giulini SM, Agabiti Rosei E. Structural alterations of subcutaneous small-resistance arteries may predict major cardiovascular events in patients with hypertension. Am J Hypertens 2007;20:846–852. [DOI] [PubMed] [Google Scholar]
- 50. Mathiassen ON, Buus NH, Sihm I, Thybo NK, Morn B, Schroeder AP, Thygesen K, Aalkjaer C, Lederballe O, Mulvany MJ, Christensen KL. Small artery structure is an independent predictor of cardiovascular events in essential hypertension. J Hypertens 2007;25:1021–1026. [DOI] [PubMed] [Google Scholar]
- 51. Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J 2003;146:168–174. [DOI] [PubMed] [Google Scholar]
- 52. Kuvin JT, Mammen A, Mooney P, Alsheikh-Ali AA, Karas RH. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med 2007;12:13–16. [DOI] [PubMed] [Google Scholar]
- 53. Mahmud FH, Earing MG, Lee RA, Lteif AN, Driscoll DJ, Lerman A. Altered endothelial function in asymptomatic male adolescents with type 1 diabetes. Congenit Heart Dis 2006;1:98–103. [DOI] [PubMed] [Google Scholar]
- 54. Mahmud FH, Van Uum S, Kanji N, Thiessen-Philbrook H, Clarson CL. Impaired endothelial function in adolescents with type 1 diabetes mellitus. J Pediatr 2008;152:557–562. [DOI] [PubMed] [Google Scholar]
- 55. Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc 2015;4:e002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol 2006;101:545–548. [DOI] [PubMed] [Google Scholar]
- 57. Weisrock F, Fritschka M, Beckmann S, Litmeier S, Wagner J, Tahirovic E, Radenovic S, Zelenak C, Hashemi D, Busjahn A, Krahn T, Pieske B, Dinh W, Dungen HD. Reliability of peripheral arterial tonometry in patients with heart failure, diabetic nephropathy and arterial hypertension. Vasc Med 2017;22:292–300. [DOI] [PubMed] [Google Scholar]
- 58. Nil M, Schafer D, Radtke T, Saner H, Wilhelm M, Eser P. Reproducibility of peripheral arterial tonometry measurements in male cardiovascular patients. Eur J Clin Investig 2014;44:1065–1071. [DOI] [PubMed] [Google Scholar]
- 59. Matsuzawa Y, Svedlund S, Aoki T, Guddeti RR, Kwon TG, Cilluffo R, Widmer RJ, Nelson RE, Lennon RJ, Lerman LO, Gao S, Ganz P, Gan LM, Lerman A. Utility of both carotid intima-media thickness and endothelial function for cardiovascular risk stratification in patients with angina-like symptoms. Int J Cardiol 2015;190:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shechter M, Matetzky S, Prasad M, Goitein O, Goldkorn R, Naroditsky M, Koren-Morag N, Lerman A. Endothelial function predicts 1-year adverse clinical outcome in patients hospitalized in the emergency department chest pain unit. Int J Cardiol 2017;240:14–19. [DOI] [PubMed] [Google Scholar]
- 61. Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J 2010;31:1142–1148. [DOI] [PubMed] [Google Scholar]
- 62. Matsue Y, Suzuki M, Nagahori W, Ohno M, Matsumura A, Hashimoto Y, Yoshida K, Yoshida M. Endothelial dysfunction measured by peripheral arterial tonometry predicts prognosis in patients with heart failure with preserved ejection fraction. Int J Cardiol 2013;168:36–40. [DOI] [PubMed] [Google Scholar]
- 63. Matsue Y, Yoshida K, Nagahori W, Ohno M, Suzuki M, Matsumura A, Hashimoto Y, Yoshida M. Peripheral microvascular dysfunction predicts residual risk in coronary artery disease patients on statin therapy. Atherosclerosis 2014;232:186–190. [DOI] [PubMed] [Google Scholar]
- 64. Fabregate-Fuente M, Arbeitman CR, Cymberknop LJ, Bara-Ledesma N, Arriazu-Galindo M, Martin-Fernandez L, Armentano RL, Saban-Ruiz J. Characterization of microvascular post occlusive hyperemia using laser Doppler flowmetry technique in subjects with cardiometabolic disorders. Conf Proc IEEE Eng Med Biol Soc 2019;2019:514–517. [DOI] [PubMed] [Google Scholar]
- 65. Neubauer-Geryk J, Hoffmann M, Wielicka M, Piec K, Kozera G, Brzeziński M, Bieniaszewski L. Current methods for the assessment of skin microcirculation: part 1. Postepy Dermatol Alergol 2019;36:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Roustit M, Blaise S, Millet C, Cracowski JL. Reproducibility and methodological issues of skin post-occlusive and thermal hyperemia assessed by single-point laser Doppler flowmetry. Microvasc Res 2010;79:102–108. [DOI] [PubMed] [Google Scholar]
- 67. Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol 2007;585:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Davida L, Pongracz V, Mohamed EA, Szamosi S, Szucs G, Vancsa A, Timar O, Csiki Z, Vegh E, Soltesz P, Szekanecz Z, Kerekes G. A prospective, longitudinal monocentric study on laser Doppler imaging of microcirculation: comparison with macrovascular pathophysiology and effect of adalimumab treatment in early rheumatoid arthritis. Rheumatol Int 2020;40:415–424. [DOI] [PubMed] [Google Scholar]
- 69. Cankar K, Finderle Z, Strucl M. The effect of alpha-adrenoceptor agonists and L-NMMA on cutaneous postocclusive reactive hyperemia. Microvasc Res 2009;77:198–203. [DOI] [PubMed] [Google Scholar]
- 70. Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol 2012;590:3523–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV-1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol 2010;588:4317–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Choi PJ, Brunt VE, Fujii N, Minson CT. New approach to measure cutaneous microvascular function: an improved test of NO-mediated vasodilation by thermal hyperemia. J Appl Physiol 2014;117:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol 2005;563:965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Medow MS, Glover JL, Stewart JM. Nitric oxide and prostaglandin inhibition during acetylcholine-mediated cutaneous vasodilation in humans. Microcirculation 2008;15:569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fujii N, McGinn R, Halili L, Singh MS, Kondo N, Kenny GP. Cutaneous vascular and sweating responses to intradermal administration of ATP: a role for nitric oxide synthase and cyclooxygenase? J Physiol 2015;593:2515–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci 2006;27:503–508. [DOI] [PubMed] [Google Scholar]
- 77. Cosentino F, Francia P, Camici GG, Pelicci PG, Volpe M, LüScher TF. Final common molecular pathways of aging and cardiovascular disease: role of the p66Shc protein. Arterioscler Thromb Vasc Biol 2008;28:622–628. [DOI] [PubMed] [Google Scholar]
- 78. Francia P, Delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, LüScher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation 2004;110:2889–2895. [DOI] [PubMed] [Google Scholar]
- 79. Shi Y, Savarese G, Perrone-Filardi P, Lüscher TF, Camici GG. Enhanced age-dependent cerebrovascular dysfunction is mediated by adaptor protein p66Shc. Int J Cardiol 2014;175:446–450. [DOI] [PubMed] [Google Scholar]
- 80. Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 1999;402:309–313. [DOI] [PubMed] [Google Scholar]
- 81. Mahdi A, Kovamees O, Pernow J. Improvement in endothelial function in cardiovascular disease - Is arginase the target? Int J Cardiol 2020;301:207–214. [DOI] [PubMed] [Google Scholar]
- 82. Paneni F, Costantino S, Cosentino F. Molecular pathways of arterial aging. Clin Sci (Lond) 2015;128:69–79. [DOI] [PubMed] [Google Scholar]
- 83. Shemyakin A, Kovamees O, Rafnsson A, Bohm F, Svenarud P, Settergren M, Jung C, Pernow J. Arginase inhibition improves endothelial function in patients with coronary artery disease and type 2 diabetes mellitus. Circulation 2012;126:2943–2950. [DOI] [PubMed] [Google Scholar]
- 84. Bruno RM, Duranti E, Ippolito C, Segnani C, Bernardini N, Di Candio G, Chiarugi M, Taddei S, Virdis A. Different impact of essential hypertension on structural and functional age-related vascular changes. Hypertension 2017;69:71–78. [DOI] [PubMed] [Google Scholar]
- 85. Paneni F, Diaz Canestro C, Libby P, Luscher TF, Camici GG. The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol 2017;69:1952–1967. [DOI] [PubMed] [Google Scholar]
- 86. Winnik S, Auwerx J, Sinclair DA, Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J 2015;36:3404–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G, Lauro R, Federici M. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation 2009;120:1524–1532. [DOI] [PubMed] [Google Scholar]
- 88. Xu Q, Huff LP, Fujii M, Griendling KK. Redox regulation of the actin cytoskeleton and its role in the vascular system. Free Radic Biol Med 2017;109:84–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012;24:981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tabet F, Schiffrin EL, Touyz RM. Mitogen-activated protein kinase activation by hydrogen peroxide is mediated through tyrosine kinase-dependent, protein kinase C-independent pathways in vascular smooth muscle cells: upregulation in spontaneously hypertensive rats. J Hypertens 2005;23:2005–2012. [DOI] [PubMed] [Google Scholar]
- 91. De Ciuceis C, Agabiti-Rosei C, Rossini C, Airo P, Scarsi M, Tincani A, Tiberio GAM, Piantoni S, Porteri E, Solaini L, Duse S, Semeraro F, Petroboni B, Mori L, Castellano M, Gavazzi A, Agabiti-Rosei E, Rizzoni D. Relationship between different subpopulations of circulating CD4+ T lymphocytes and microvascular or systemic oxidative stress in humans. Blood Press 2017;26:237–245. [DOI] [PubMed] [Google Scholar]
- 92. Handy DE, Castro R, Loscalzo J. Epigenetic modifications: basic mechanisms and role in cardiovascular disease. Circulation 2011;123:2145–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Costantino S, Paneni F, Virdis A, Hussain S, Mohammed SA, Capretti G, Akhmedov A, Dalgaard K, Chiandotto S, Pospisilik JA, Jenuwein T, Giorgio M, Volpe M, Taddei S, Luscher TF, Cosentino F. Interplay among H3K9-editing enzymes SUV39H1, JMJD2C and SRC-1 drives p66Shc transcription and vascular oxidative stress in obesity. Eur Heart J 2019;40:383–391. [DOI] [PubMed] [Google Scholar]
- 94. Costantino S, Paneni F, Battista R, Castello L, Capretti G, Chiandotto S, Tanese L, Russo G, Pitocco D, Lanza GA, Volpe M, Luscher TF, Cosentino F. Impact of glycemic variability on chromatin remodeling, oxidative stress, and endothelial dysfunction in patients with type 2 diabetes and with target HbA1c levels. Diabetes 2017;66:2472–2482. [DOI] [PubMed] [Google Scholar]
- 95. Paneni F, Mocharla P, Akhmedov A, Costantino S, Osto E, Volpe M, Luscher TF, Cosentino F. Gene silencing of the mitochondrial adaptor p66(Shc) suppresses vascular hyperglycemic memory in diabetes. Circ Res 2012;111:278–289. [DOI] [PubMed] [Google Scholar]
- 96. Sullivan KE, Reddy AB, Dietzmann K, Suriano AR, Kocieda VP, Stewart M, Bhatia M. Epigenetic regulation of tumor necrosis factor alpha. Mol Cell Biol 2007;27:5147–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Virdis A, Duranti E, Rossi C, Dell’Agnello U, Santini E, Anselmino M, Chiarugi M, Taddei S, Solini A. Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: role of perivascular adipose tissue. Eur Heart J 2015;36:784–794. [DOI] [PubMed] [Google Scholar]
- 98. Brasacchio D, Okabe J, Tikellis C, Balcerczyk A, George P, Baker EK, Calkin AC, Brownlee M, Cooper ME, El-Osta A. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes 2009;58:1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Okabe J, Orlowski C, Balcerczyk A, Tikellis C, Thomas MC, Cooper ME, El-Osta A. Distinguishing hyperglycemic changes by Set7 in vascular endothelial cells. Circ Res 2012;110:1067–1076. [DOI] [PubMed] [Google Scholar]
- 100. Zhang Y, Sun X, Icli B, Feinberg MW. Emerging roles for microRNAs in diabetic microvascular disease: novel targets for therapy. Endocr Rev 2017;38:145–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Advani A, Huang Q, Thai K, Advani SL, White KE, Kelly DJ, Yuen DA, Connelly KA, Marsden PA, Gilbert RE. Long-term administration of the histone deacetylase inhibitor vorinostat attenuates renal injury in experimental diabetes through an endothelial nitric oxide synthase-dependent mechanism. Am J Pathol 2011;178:2205–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Paneni F, Costantino S, Battista R, Castello L, Capretti G, Chiandotto S, Scavone G, Villano A, Pitocco D, Lanza G, Volpe M, Luscher TF, Cosentino F. Adverse epigenetic signatures by histone methyltransferase Set7 contribute to vascular dysfunction in patients with type 2 diabetes mellitus. Circ Cardiovasc Genet 2015;8:150–158. [DOI] [PubMed] [Google Scholar]
- 103. Nicholls SJ, Ray KK, Johansson JO, Gordon A, Sweeney M, Halliday C, Kulikowski E, Wong N, Kim SW, Schwartz GG. Selective BET protein inhibition with apabetalone and cardiovascular events: a pooled analysis of trials in patients with coronary artery disease. Am J Cardiovasc Drugs 2018;18:109–115. [DOI] [PubMed] [Google Scholar]
- 104. Poller W, Dimmeler S, Heymans S, Zeller T, Haas J, Karakas M, Leistner DM, Jakob P, Nakagawa S, Blankenberg S, Engelhardt S, Thum T, Weber C, Meder B, Hajjar R, Landmesser U. Non-coding RNAs in cardiovascular diseases: diagnostic and therapeutic perspectives. Eur Heart J 2018;39:2704–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Landmesser U, Poller W, Tsimikas S, Most P, Paneni F, Luscher TF. From traditional pharmacological towards nucleic acid-based therapies for cardiovascular diseases. Eur Heart J 2020; [DOI] [PubMed] [Google Scholar]
- 106. Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation 2010;121:2317–2325. [DOI] [PubMed] [Google Scholar]
- 107. Ford TJ, Corcoran D, Padmanabhan S, Aman A, Rocchiccioli P, Good R, McEntegart M, Maguire JJ, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sattar N, Hsu LY, Arai AE, Oldroyd KG, Touyz RM, Davenport AP, Berry C. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. Eur Heart J 2020;41:3239–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 109. Bottcher M, Botker HE, Sonne H, Nielsen TT, Czernin J. Endothelium-dependent and -independent perfusion reserve and the effect of L-arginine on myocardial perfusion in patients with syndrome X. Circulation 1999;99:1795–1801. [DOI] [PubMed] [Google Scholar]
- 110. Chauhan A, Mullins PA, Taylor G, Petch MC, Schofield PM. Effect of hyperventilation and mental stress on coronary blood flow in syndrome X. Br Heart J 1993;69:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lanza GA, Buffon A, Sestito A, Natale L, Sgueglia GA, Galiuto L, Infusino F, Mariani L, Centola A, Crea F. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol 2008;51:466–472. [DOI] [PubMed] [Google Scholar]
- 112. Ohba K, Sugiyama S, Sumida H, Nozaki T, Matsubara J, Matsuzawa Y, Konishi M, Akiyama E, Kurokawa H, Maeda H, Sugamura K, Nagayoshi Y, Morihisa K, Sakamoto K, Tsujita K, Yamamoto E, Yamamuro M, Kojima S, Kaikita K, Tayama S, Hokimoto S, Matsui K, Sakamoto T, Ogawa H. Microvascular coronary artery spasm presents distinctive clinical features with endothelial dysfunction as nonobstructive coronary artery disease. J Am Heart Assoc 2012;1:e002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kaski JC, Rosano GM, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol 1995;25:807–814. [DOI] [PubMed] [Google Scholar]
- 114. Lamendola P, Lanza GA, Spinelli A, Sgueglia GA, Di Monaco A, Barone L, Sestito A, Crea F. Long-term prognosis of patients with cardiac syndrome X. Int J Cardiol 2010;140:197–199. [DOI] [PubMed] [Google Scholar]
- 115. Lanza GA, Parrinello R, Figliozzi S. Management of microvascular angina pectoris. Am J Cardiovasc Drugs 2014;14:31–40. [DOI] [PubMed] [Google Scholar]
- 116. Boari GE, Rizzardi N, de Ciuceis C, Platto C, Paiardi S, Porteri E, Paini A, Salvetti M, Muiesan ML, Rizzoni D, Rosei EA. Determinants of the structure of resistance-sized arteries in hypertensive patients. Blood Press 2008;17:204–211. [DOI] [PubMed] [Google Scholar]
- 117. Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sidik N, McCartney P, Corcoran D, Collison D, Rush C, McConnachie A, Touyz RM, Oldroyd KG, Berry C. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol 2018;72:2841–2855. [DOI] [PubMed] [Google Scholar]
- 118. Radico F, Zimarino M, Fulgenzi F, Ricci F, Di Nicola M, Jespersen L, Chang SM, Humphries KH, Marzilli M, De Caterina R. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease: a systematic review and meta-analysis. Eur Heart J 2018;39:2135–2146. [DOI] [PubMed] [Google Scholar]
- 119. Lanza GA, Filice M, De Vita A, Lamendola P, Villano A, Spera F, Golino M, Rota E, Argiro A, Crea F. Primary stable microvascular angina: a long-term clinical follow-up study. Circulation 2017;135:1982–1984. [DOI] [PubMed] [Google Scholar]
- 120. Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med 2009;169:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 122. Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002;106:653–658. [DOI] [PubMed] [Google Scholar]
- 123. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, Shaw LJ, Pepine CJ, Ahmed B. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol 2019;73:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA; National Heart Lung, and Blood Institute. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:722–725. [DOI] [PubMed] [Google Scholar]
- 126. Lanza GA, Crea F, Kaski JC. Clinical outcomes in patients with primary stable microvascular angina: is the jury still out? Eur Heart J Qual Care Clin Outcomes 2019;5:283–291. [DOI] [PubMed] [Google Scholar]
- 127. Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, Lerman A, Cushman M, Kumbhani DJ, Arslanian-Engoren C, Bolger AF, Beltrame JF; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation 2019;139:e891–e908. [DOI] [PubMed] [Google Scholar]
- 128. Lanza GA, Careri G, Stazi A, Villano A, De Vita A, Aurigemma C, Crea F. Clinical spectrum and outcome of patients with non-ST-segment elevation acute coronary syndrome and no obstructive coronary atherosclerosis. Circ J 2016;80:1600–1606. [DOI] [PubMed] [Google Scholar]
- 129. Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Camma G, Lanza GA, Crea F. Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J 2018;39:91–98. [DOI] [PubMed] [Google Scholar]
- 130. Lindahl B, Baron T, Erlinge D, Hadziosmanovic N, Nordenskjold A, Gard A, Jernberg T. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation 2017;135:1481–1489. [DOI] [PubMed] [Google Scholar]
- 131. Beltrame JF, Turner SP, Leslie SL, Solomon P, Freedman SB, Horowitz JD. The angiographic and clinical benefits of mibefradil in the coronary slow flow phenomenon. J Am Coll Cardiol 2004;44:57–62. [DOI] [PubMed] [Google Scholar]
- 132. Crea F, Bairey Merz CN, Beltrame JF, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Camici PG; Coronary Vasomotion Disorders International Study Group. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J 2017;38:473–477. [DOI] [PubMed] [Google Scholar]
- 133. Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation 2011;123:163–169. [DOI] [PubMed] [Google Scholar]
- 134. Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, Matsubara J, Sumida H, Kaikita K, Kojima S, Nagayoshi Y, Yamamuro M, Izumiya Y, Iwashita S, Matsui K, Jinnouchi H, Kimura K, Umemura S, Ogawa H. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol 2010;55:1688–1696. [DOI] [PubMed] [Google Scholar]
- 135. Rizzoni D, Palombo C, Porteri E, Muiesan ML, Kozakova M, La Canna G, Nardi M, Guelfi D, Salvetti M, Morizzo C, Vittone F, Rosei EA. Relationships between coronary flow vasodilator capacity and small artery remodelling in hypertensive patients. J Hypertens 2003;21:625–631. [DOI] [PubMed] [Google Scholar]
- 136. Rizzoni D, De Ciuceis C, Porteri E, Paiardi S, Boari GE, Mortini P, Cornali C, Cenzato M, Rodella LF, Borsani E, Rizzardi N, Platto C, Rezzani R, Rosei EA. Altered structure of small cerebral arteries in patients with essential hypertension. J Hypertens 2009;27:838–845. [DOI] [PubMed] [Google Scholar]
- 137. Agabiti-Rosei E, Rizzoni D. Microvascular structure as a prognostically relevant endpoint. J Hypertens 2017;35:914–921. [DOI] [PubMed] [Google Scholar]
- 138. Heagerty AM. Predicting hypertension complications from small artery structure. J Hypertens 2007;25:939–940. [DOI] [PubMed] [Google Scholar]
- 139. Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation 2003;108:2230–2235. [DOI] [PubMed] [Google Scholar]
- 140. Rossi GP, Bolognesi M, Rizzoni D, Seccia TM, Piva A, Porteri E, Tiberio GA, Giulini SM, Agabiti-Rosei E, Pessina AC. Vascular remodeling and duration of hypertension predict outcome of adrenalectomy in primary aldosteronism patients. Hypertension 2008;51:1366–1371. [DOI] [PubMed] [Google Scholar]
- 141. Buus NH, Mathiassen ON, Fenger-Gron M, Praestholm MN, Sihm I, Thybo NK, Schroeder AP, Thygesen K, Aalkjaer C, Pedersen OL, Mulvany MJ, Christensen KL. Small artery structure during antihypertensive therapy is an independent predictor of cardiovascular events in essential hypertension. J Hypertens 2013;31:791–797. [DOI] [PubMed] [Google Scholar]
- 142. Buus NH, Bottcher M, Jorgensen CG, Christensen KL, Thygesen K, Nielsen TT, Mulvany MJ. Myocardial perfusion during long-term angiotensin-converting enzyme inhibition or beta-blockade in patients with essential hypertension. Hypertension 2004;44:465–470. [DOI] [PubMed] [Google Scholar]
- 143. Schiffrin EL, Deng LY, Larochelle P. Effects of a beta-blocker or a converting enzyme inhibitor on resistance arteries in essential hypertension. Hypertension 1994;23:83–91. [DOI] [PubMed] [Google Scholar]
- 144. Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation 2000;101:1653–1659. [DOI] [PubMed] [Google Scholar]
- 145. Savoia C, Touyz RM, Endemann DH, Pu Q, Ko EA, De Ciuceis C, Schiffrin EL. Angiotensin receptor blocker added to previous antihypertensive agents on arteries of diabetic hypertensive patients. Hypertension 2006;48:271–277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.