Abstract
Self-disturbances such as an anomalous perception of one’s own body boundary are central to the phenomenology of schizophrenia (SZ), but measuring the spatial parameters of the hypothesized self–other boundary has proved to be challenging. Peripersonal space (PPS) refers to the immediate zone surrounding the body where the self interacts physically with the environment; the space that corresponds to hypothesized self–other boundary. PPS is represented by enhanced multisensory integration and faster reaction time (RT) for objects near the body. Thus, multisensory RT tasks can be used to estimate self–other boundary. We aimed to quantify PPS in SZ using an immersive virtual reality visuotactile RT paradigm. Twenty-four participants with SZ and 24 demographically matched controls (CO) were asked to detect tactile vibration while watching a ball approaching them, thrown by either a machine (nonsocial condition) or an avatar (social condition). Parameters of PPS were estimated from the midpoint of the spatial range where the tactile RT decreased most rapidly (size) and the gradient of the RT change at this midpoint (slope). Overall, PPS was smaller in participants with SZ compared with CO. PPS slope for participants with SZ was shallower than CO in the social but not in nonsocial condition, indicating an increased uncertainty of self–other boundary across an extended zone in SZ. Social condition also increased false alarms for tactile detection in SZ. Clinical symptoms were not clearly associated with PPS parameters. These findings suggest the context-dependent nature of weakened body boundary in SZ and underscore the importance of reconciliating objective and subjective aspects of self-disturbances.
Keywords: self-disturbance, multisensory processing, social distance, self–other distinction
Introduction
Weakened or porous sense of self is a fundamental feature of schizophrenia (SZ).1–3 First-person accounts4,5 and empirical evidence2,6,7 suggest that blurred self–other differentiation and an unstable sense of bodily self in SZ are phenomenologically salient and interfere with social functioning.2,3,8,9 For adaptive interpersonal interactions to occur, it is necessary to have a tacit understanding of one’s own body as a unified entity with fixed boundaries that allows one to distinguish self from other. Yet, despite the importance of bodily self-disturbance and its negative impact on social outcome, parameters of self-other boundary have not been extensively examined. Disrupted self–other distinction may be understood as the manifestation of abnormal representation of the body in relation to the regions of space that mark the perceptual border between self and others.3,10–14 This buffer zone that immediately surrounds the body is known as the peripersonal space (PPS) where the physical interaction between the self and objects in the environment occurs.7,15,16 To achieve the everyday miracle of moving through the world without colliding with objects or people while protecting ourselves from potential harm, the brain continuously integrates multisensory exteroceptive and proprioceptive signals to predict the self-location with respect to the environment.17,18,19,20 This ability to estimate the PPS, the buffer zone between self and other, is closely related to our capacity for self–other distinction.21,22 Importantly, our experience of direct physical contact with objects including social agents occurs in the PPS.23–25 Thus, PPS is the core spatial component of self-consciousness that allows us to act upon the world and represents a protective zone such that when PPS boundaries are breached, alarm signals may be triggered.19
Neurophysiological studies of nonhuman primates indicate that PPS is encoded by multisensory neurons in the ventral premotor26–28 and the posterior parietal cortex.29,30 In humans, PPS-related multisensory processing is associated with the frontoparietal cortical network.31–34 Multisensory neurons integrate inputs from tactile stimulation of specific parts of the body (eg, hand, face, or abdomen) with auditory or visual stimuli when they are presented close to the same body part. Thus, the estimated size of the PPS is thought to be directly related to the receptive fields of these multisensory neurons.32 These neurons do not respond when the exteroceptive stimuli are presented far away from the PPS.24,35–37 Thus, it is possible to infer the limits of the self-space from the firing patterns of these neurons in frontoparietal regions. In other words, spatially sensitive coding represents the PPS in the brain.24,28,35
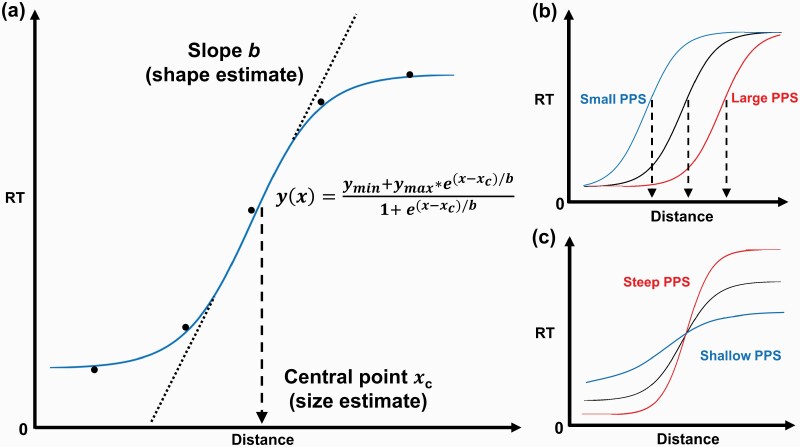
Within the PPS, auditory, visual, and tactile integration occurs much faster than in the extrapersonal space. Therefore, adaptive facilitation of multisensory integration that occurs within the PPS boundary can be leveraged to measure the extent of the PPS.31–33 A typical multisensory method to estimate the PPS involves asking participants to detect a tactile stimulation (eg, vibration) on a specific body part while a task-irrelevant auditory or visual stimulus is presented as approaching the participant. Tactile reaction time (RT) drops sharply when the stimulus is perceived to be near the body (ie, entering the PPS) due to the facilitation of multisensory integration within the PPS. This RT facilitation is optimally fitted to a sigmoidal function, and the size (ie, central point) and shape (ie, slope) of the PPS can be estimated from the sigmoid function by identifying the inflection point where multisensory facilitation occurs32,38,39 (figure 1). This approach, grounded in neurophysiology, offers an implicit route to quantify self–other boundary by estimating the parameters of PPS from low-level multisensory-motor input. Importantly, the size and the slope of the PPS are malleable, depending on the types of environment or interaction. Modification of the PPS has been observed with respect to anxiety40 and interoceptive accuracy,41 as well as the types of interaction.33,35,36,42–45
Fig. 1.
Estimating PPS from the sigmoidal fitting of visuotactile RTs. (a) Sigmoidal fitting of the mean tactile RTs. The mean RTs are represented by 5 black dots. The blue line represents the sigmoidal function fitted to data. The dotted line represents the slope and the dashed line represents the central point. (b) Schematic representation of the PPS sizes (xc) from small (blue) to large (red). (c) Schematic representation of the PPS slopes (b) ranging from shallow (blue) to steep (red). PPS, peripersonal space; RT, reaction time.
The existing literature on the PPS of SZ based on the multisensory approach described above is sparse. An auditory-tactile RT study using dynamic approaching sounds and tactile vibration found contracted PPS and a steeper gradient of slope in individuals with SZ compared with controls (CO).46 In contrast, a visuotactile RT task using LED lights and tactile vibration found no group difference in PPS parameters.47 These divergent outcomes might stem from the differences in the sensory modality of the tasks but how auditory vs visual inputs lead to these discrepancies remains to be understood.
Space surrounding the self has also been investigated with social cognitive paradigms such as the stop-distance task that explicitly requires participants to indicate preferred interpersonal distance when another person approaches them.48–50 Interpersonal distance increases under threat and decreases in friendly interactions. Stop-distance studies indicate increased preferred interpersonal distance in SZ51–56 and this effect is associated with negative symptoms.53,55,57 Moreover, increased variability of social distance judgment is associated with disorganized symptoms of SZ.58
Seemingly inconsistent findings in the literature may stem from differences in how self–other boundary is conceptualized and measured. Differences in the sensory modality of the approaching stimuli (eg, auditory vs visual) or task demands (eg, sensory RT vs social decision-making) may tap into different aspects of the spatial self. Clearly defined PPS is essential for making fast, adaptive decisions to approach or withdraw (ie, expand or contract protective space).25 The plasticity of the PPS in response to fast changes in the environment helps protect the self as we go about our daily lives.42 The interpersonal distance regulation as assessed by stop-distance paradigms depends upon multiple factors including past experiences, personality traits, culture, and social–emotional factors.59 Larger preferred social distance in SZ53 may be driven by differential effects of social stressors or social stimuli on individuals with SZ compared with CO.60–63
Clinical relevance of preferred interpersonal distance to SZ is easy to grasp. A larger social distance between self and others may indicate discomfort and anxiety.53 Indeed, preference for greater physical distance from others is associated with poor social functioning and negative symptoms in SZ.53,55 Positive symptoms such as paranoia could also play a role in interpersonal distance regulation but effects of paranoia are nuanced. Preferred interpersonal distance increases under paranoid threat but decreases when one experiences paranoid power through grandiose delusions.64 In both cases, altered interpersonal space is nevertheless associated with social difficulties, which could lead to isolation. In turn, perceived social isolation has been implicated in bodily self-aberrations10,65,66 and related psychotic symptoms.45,63,65,67
Clinical and social relevance of multisensory-motor PPS is also significant. The PPS can be conceptualized as a buffer zone that allows us to avoid collisions with external objects and agents as we move through the environment.25,42 When external objects or people breach this protective zone, alarm signals are thought to be generated to protect the body. Faulty PPS estimation could lead to unwanted contact with the world and contribute to a heightened perception of threat. Moreover, unclear self–other boundary could interfere with the capacity to respond adaptively to approaching agents or objects by over-estimating or under-estimating the self–other distance. Over time, such difficulties with distinguishing self from other in space are likely to play an important role in social outcome.3,68
In this study, we examined the nature of self–other boundary alterations in SZ by estimating the size and the shape of PPS with a visuotactile RT task.22 By simulating the social and nonsocial environment in virtual reality (VR), we also sought to elucidate the potential impact of social agents on PPS. A recent study that used a nonsocial visuotactile RT paradigm reported comparable PPS for individuals with SZ and CO.47 Accordingly, we expected similar PPS parameters for individuals with SZ and CO when they interact with objects. However, we expected a group difference between individuals with SZ and CO in PPS to emerge under a simulated interpersonal condition. Lastly, we explored the relationship between clinical and psychological variables and PPS.
Methods
Participants
Twenty-four participants with SZ were recruited from a community mental health center in Nashville, United States. All were taking antipsychotic medication. Twenty-five age-matched CO were recruited from the Nashville area. The Structured Clinical Interview for DSM-569 was administered to verify the diagnosis of SZ and to confirm that CO had no history of DSM-5 disorders. Exclusion criteria for both groups were history of head injury or seizures, neurological diseases, substance use or abuse, and IQ level below 85. All participants provided written informed consent and were paid, as approved by the Vanderbilt Institutional Review Board. Demographic and clinical information is summarized in table 1.
Table 1.
Demographic and Clinical Information
| SZ (n = 24) | CO (n = 24) | Test | P value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Demographic | ||||
| Age (years) | 48.42 (9.24) | 47.96 (9.38) | t = 0.17 | .87 |
| Gender M/F | 13/11 | 11/13 | χ 2 = 0.33 | .56 |
| Education (years) | 12.83 (1.71) | 15.83 (2.04) | t = −5.53 | <.01 |
| Cognitive | ||||
| IQ (NART) | 103.13 (8.00) | 111.16 (8.48) | t = −3.37 | <.01 |
| Clinical | ||||
| Years of illness | 27.04 (9.70) | |||
| No. of hospitalization | 11.50 (21.92) | |||
| CPZ equivalent dose | 311.31 (209.54) | N/A | ||
| BPRS | 18.83 (11.12) | |||
| SAPS | 36.29 (13.64) | |||
| SANS | 22.67 (19.37) | |||
| SPQ | ||||
| Total | 10.75 (11.44) | N/A | ||
| Positive | 4.25 (5.72) | |||
| Negative | N/A | 5.50 (5.70) | ||
| Disorganized | 2.21 (2.69) |
Note: NART, North American Adult Reading Test70; CPZ, chlorpromazine equivalent dose (mg/day)71; BPRS, Brief Psychiatric Rating Scale72; SAPS, Scale for the Assessment of Positive Symptoms73; SANS, Scale for the Assessment of Negative Symptoms74; SPQ, Schizotypal Personality Questionnaire.75; SZ, participants with schizophrenia.
Clinical and Psychological Assessments
The Brief Psychiatric Rating Scale72 and the Scales for the Assessment of Positive Symptoms (SAPS)73 and Negative Symptoms (SANS)74 were conducted to assess the severity of symptoms in SZ. Schizotypal Personality Questionnaire75 was administered to assess schizotypy in CO. Revised version of Green Paranoid Thought Scale (R-GPTS)74 was administered to all participants to assess ideas of reference and persecution.77 Lifetime self-disturbances were assessed with the Body Disturbance Inventory (BODI),65 which yields frequency, distress, and vividness scores of bodily disturbances. The UCLA Loneliness Scale78 was used to measure perceived social isolation. Intelligence was estimated using the North American Adult Reading Test—revised.70
Procedure for the PPS Experiment
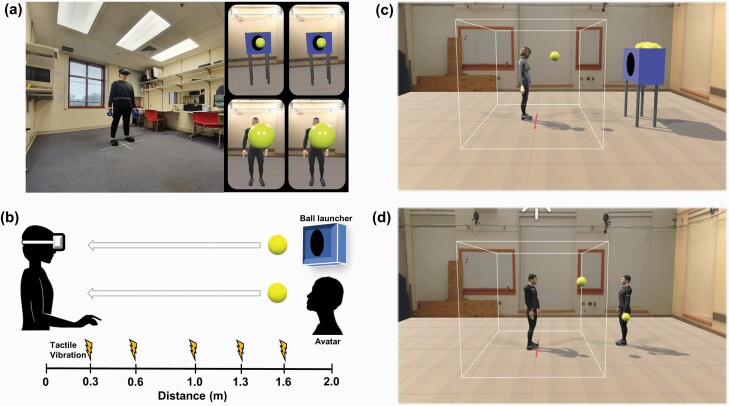
A well-established visuotactile RT task43 was administered in immersive VR to incorporate the visual context of social interactions. After informed consent and psychological assessments, participants were given instructions for the PPS experiment. Before starting the task, participants stood at the center of the room (marked on the floor by red tape) and put VR tracker devices on their heads, bodies, and feet. The Steam VR software (Valve Corporation) was run to check the signal strength on all 8 sensors (ie, the headset: HTC Vive, 2160 × 1200, 1080 × 1200 per eye, FOV 110° diagonally; the belt, 2 handheld controllers, both shoes, and 2 base station cameras). Participants were asked to press a trigger on one handheld controller to be calibrated to the self-avatar (Orion-IKINEMA) to achieve full immersion in the VR environment, and familiarized themselves. They were instructed to stand at the red tape at the center of the VR environment during the PPS task to fix the self-location. Presentation of stimuli and recording of responses were implemented by Unity. After practice trials, the experiment began.
At each trial, participants were asked to respond as quickly as possible to tactile stimulation (vibration) on their hands while watching a ball approaching them. The ball was thrown at them either by a ball launcher (nonsocial condition) or an avatar (social condition). The ball always approached the participant at 75 cm/s, which approximates the average human walking speed.31 The duration of the ball’s complete trajectory was 2600 ms and the distance between the participant and the ball launcher or avatar was fixed at 2 m in VR. After each trial, the screen cleared and there was a 2000 ms fixation time.
Tactile vibrations were delivered when the ball was at one of 5 distances in a randomized order: 0.3 m (D1), 0.6 m (D2), 1 m (D3), 1.3 m (D4), or 1.6 m (D5). Participants responded to the tactile vibration by pulling the trigger. In contrast to the previous study43 in which the ball continued moving even after reaching the participant, in this study, the ball disappeared immediately when the participant pulled the trigger. If participants pulled the trigger before the vibration, it was recorded as a commission error (false alarm). There were 100 trials (50 trials per condition). The order of the presentation of conditions was counterbalanced (see figure 2 for the material and procedures).
Fig. 2.
The visuotactile RT task in VR: stimuli and procedure. (a) PPS experiment setup (left) and what the participant sees in VR (upper-right for the non-social condition, and lower-right for the social condition). (b) Overview of the experimental procedure. (c) VR environment for the non-social condition. The participant stands at the red line. (d) VR environment for the social condition. The participant stands at the red line. PPS, peripersonal space; RT, reaction time; VR, virtual reality.
Estimation of PPS Parameters
Mean RT to tactile stimulation was collected at different distances (D1–D5). The RT data were optimally fitted to a sigmoidal function31,43,46,79 following the equation,
x represents the distance at which the vibration was delivered (ie, independent variable). y represents the predicted RT (ie, dependent variable). The ymin and ymax values were assigned a priori as parameters denoting the minimum and maximum RT of each individual dataset, respectively, and saturated at the lower and upper bounds of the sigmoidal function.31,43,46xc is the value of the abscissa where
which is equivalent to the central point of the sigmoid; this allows us to estimate the PPS size.32,79 The gradient of the PPS slope at xc is represented by b, capturing the multisensory facilitation effect in RTs; this estimate is a measure of the (un)certainty of the PPS boundary.12 Note that the slope estimate (b) is inverted in the formula; a larger b value represents a shallower (ie, fuzzier or diffuse) PPS boundary and vice versa. Both xc and b are not fixed; they change with the shape of the sigmoid (see figure 1 and supplementary material for further details).
Data Analysis
Participants sometimes made anticipatory responses (pressing the trigger before the vibration presentation). These trials were not included in the mean RTs for computing PPS parameters but instead, we classified them as false alarms or commission errors. Commission errors were defined as the % of the trials where participants pressed the trigger before the vibration was presented. Multivariate analysis of variance (MANOVA) was conducted to test for group differences in commission errors and the effect of social vs nonsocial conditions.
MANOVA was also conducted to compare PPS parameters (ie, the central point, xc; and the slope, b, respectively) between groups and conditions. After examining the main effects and interactions, Tukey’s post hoc tests were conducted. Correlations were computed to explore relationships between PPS estimates and clinical or psychological variables. False discovery corrected alpha (P < .05) was applied to minimize Type I errors.80 All analyses were conducted using SPSS 23.0.
Results
Data from one CO were excluded because of the very low goodness of fit (R2 < 0.3; see supplementary material for the computation of R2) resulting in a dataset of 24 participants with SZ and 24 CO. For further details, please refer to supplementary tables 1–7.
Commission Errors
There was a significant main effect of diagnosis (F1, 46 = 3.95, P < .05) with increased false alarm rate in participants with SZ than CO regardless of condition (supplementary figure 1). There was no main effect of the condition (F1, 46 = 0.33, P = .57) but there was a significant diagnosis-by-condition interaction (F1, 46 = 4.20, P = .04). Contrast analyses showed an elevated commission error rate in participants with SZ (M = 6.67%, SD = 7.61) compared with that in CO (M = 2.33%, SD = 4.57) for the social (F1, 46 = 6.95, P = .011) condition but there was no group difference in the nonsocial condition (F1, 46 = 0.01, P = .91.
Computation of PPS Variables From Tactile RTs
PPS Central Point (xc) and Shallowness (b) Estimates.
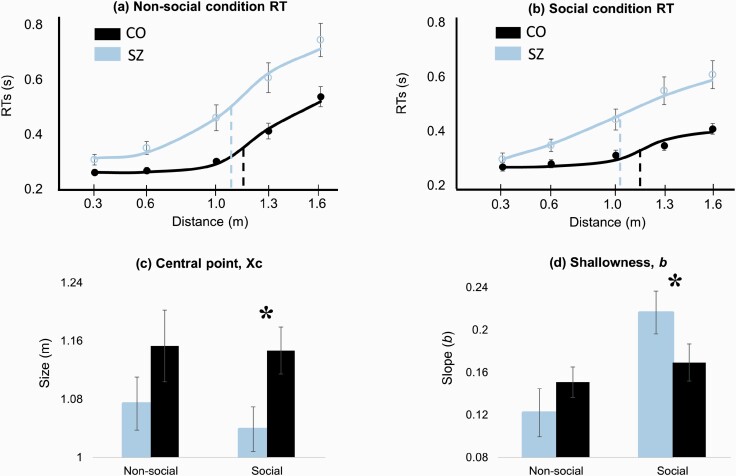
We computed the central point (xc) and slope (b) for participants with SZ and CO for both social and nonsocial conditions. Figure 3 displays the PPS size and slope in participants with SZ and CO for both conditions. Larger xc indicates larger PPS size, while larger b indicates shallower PPS slope.
Fig. 3.
PPS size and slope estimates for SZ and CO groups. Error bar: ±SE (standard error). (a) Fitted RT data for the nonsocial condition. xc for CO (black dashed line) > xc for SZ (blue dashed line). (b) Fitted RT data for the social condition. The difference between xc for CO (black dashed line) and xc for SZ (blue dashed line) is larger than in the nonsocial condition. The slope at xc for SZ is flatter than that for CO. (c) Group difference in xc is significant for the social condition but not for the nonsocial condition. (d) Shallowness of the slope is represented by b at xc. PPS slope is shallower for SZ than CO in the social condition. Note that the b is inverted in the sigmoidal function. PPS, peripersonal space; RT, reaction time; SZ, participants with schizophrenia; CO, controls.
Size of the PPS (Central Point, xc).
There was a significant main effect of diagnosis (F1, 46 = 4.82, P = .03); PPS size was smaller in participants with SZ than in CO overall. There was no main effect of condition (F1, 46 = 0.40, P = .53) or diagnosis-by-condition interaction (F1, 46 = 0.20, P = .66). Although the diagnosis-by-condition interaction was not significant, PPS size in participants with SZ (M = 1.04 m, SD = 0.16) was significantly smaller than CO (M = 1.15 m, SD = 0.15) in the social condition (F1, 46 = 5.90, P = .019) but not in the nonsocial condition (F1, 46 = 1.67, P = .20).
Gradient of the PPS Slope (b).
There was no main effect of diagnosis (F1, 46 = 0.42, P = .52). The main effect of condition (F1, 46 = 9.29, P = .004) and the diagnosis-by-condition interaction (F1, 46 = 6.02, P = .018) were significant. The b was similar across the group in the nonsocial condition (F1, 46 = 1.16, P = .29) but the group difference was significant in the social condition (F1, 46 = 4.29, P = .04) with the mean b values of 0.22 (SD = 0.09) for participants with SZ and 0.16 (SD = 0.10) for CO.
Relationships Among PPS, Symptoms, and Psychological Measures
All correlations were corrected for false discovery.77 PPS parameters were not significantly correlated with overall clinical symptoms in SZ or schizotypy in CO (supplementary material) except for a significant association of hallucination subscore of SAPS and PPS slope in the social condition, such that steeper gradient was associated with the severity of hallucinations (r = −0.47, P = .02).
Participants with SZ scored higher than CO on BODI (t = 2.28, P = .03), GPTS-r (t = 5.70, P < .01), and Loneliness (t = 2.47, P = .02). But PPS parameters were not associated with overall BODI, GPTS-r, or Loneliness scores (supplementary material). However, in the nonsocial condition, PPS size of participants with SZ was associated with increased loneliness (r = −0.46, P = .03) and negative symptoms (r = −0.51, P < .01).
Discussion
We investigated spatial aspects of the self to further understand the nature of self-disturbances in SZ. The concept of PPS allows for a systematic investigation of the self–other boundary in relation to social contexts. The present study utilized a visuotactile task in immersive VR to estimate the size and slope of the PPS, associated with the extent of the uncertainty of the self–other boundary.
The PPS size was contracted in individuals with SZ overall. Thus, the border between the self and the extrapersonal space seems to be pulled closer toward the body in individuals with SZ than in CO. Smaller size of the PPS in individuals with SZ is consistent with the reports of contracted PPS from an audio-tactile RT study46 and reduced personal space in a VR-based social interaction experiment81 but somewhat inconsistent with the findings of a recent study that used a nonsocial visuotactile task and found no group differences in the PPS size.47 The latter study used an array of LED lights to induce an illusion of an approaching or receding stimulus (ie, apparent motion), whereas our current experiment simulated an immersive experience of interacting with the environment. It is interesting that under more ecologically valid simulation conditions individuals with SZ showed altered PPS whereas under a laboratory environment devoid of any social context,47 there was no group difference in PPS parameters. However, further research is needed to disentangle the effects of task conditions.
Given the importance of social context in self-processing, and based on past findings,12,82 we expected the PPS variables to change in response to a social agent. We observed a shallower PPS slope in individuals with SZ compared with CO only in the social condition, which indicates a broader zone of uncertainty where the self–other boundary is ambiguous in individuals with SZ (figure 4). A shallower gradient of PPS from self to other is consistent with a weakened, diffuse, and more variable representation of the bodily self.12 Furthermore, shallower PPS slope in the social condition, which implies an extended zone of ill-defined self–other border, is consistent with the proneness to keep others at bay as a safety strategy, as documented by studies of preferred interpersonal distance in individuals with SZ.53,55
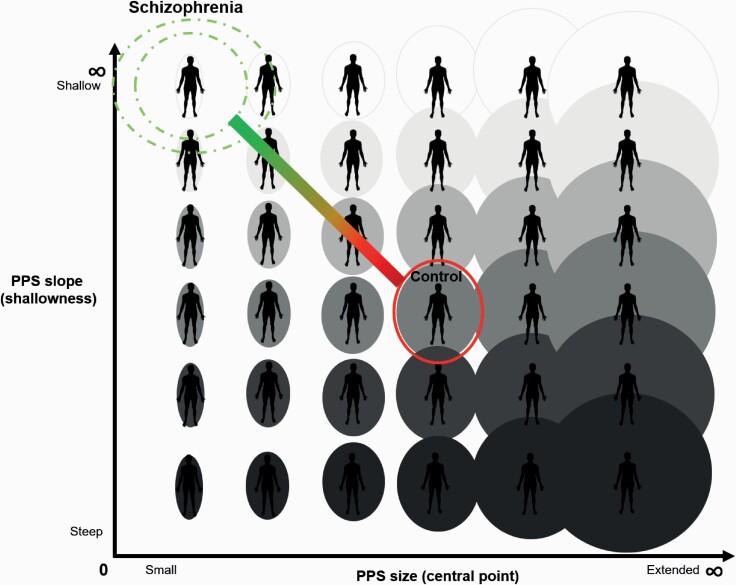
Fig. 4.
Schematic representation of PPS in individuals with schizophrenia. Ellipses or circles around the body represent the size of the PPS along the x-axis. The gradient of the PPS slope is represented by shading along the y-axis. Darker shades correspond to steeper slopes and more distinct self–other boundary. Lighter shades correspond to shallower gradient from self to other (fuzzier self–other boundary). CO participants are placed at the center of the figure. Small PPS size (xc) and shallow slope (b) of the SZ group are represented at the top left. This figure may be used to visualize hypothesized PPS for other neuropsychiatric conditions associated with anomalous self–other boundary, such as autism (eg, small size and steep gradient of the slope). PPS, peripersonal space; SZ, schizophrenia; CO, controls.
In contrast to our results, Di Cosmo et al46 found a steeper PPS slope in individuals with SZ using an auditory-tactile RT task. A steeper slope suggests a faster transition from self-space to the external world and a clear self–other boundary in individuals with SZ, at least in the auditory modality. Well-defined self–other boundary in the auditory domain may be interpreted in the context of auditory hallucinations. Auditory hallucinations are associated with the tendency toward attributing and locating self-generated experiences to extrapersonal space (ie, beyond PPS). It is possible that repeated experience of auditory hallucination may help define the PPS because the “voices” provide spatial cues as to where the self-boundary is. Although Di Cosmo et al46 did not examine the impact of hallucination of auditory-tactile PPS in their study, our results, albeit preliminary, hint at this possibility.
Psychotic symptoms, such as hallucinations, have been proposed to stem from multisensory perceptual incoherence13,83 but what is the nature of the relationship between hallucinations and PPS? In healthy participants, transient sensory deprivation results in a shallower PPS slope and an increased tendency to report hallucination or self-disorganization.84 Thus, temporary induction of a fuzzy self–other boundary increases the likelihood of experiencing “felt presence” or attributing self-generated actions to “other” in healthy participants. In contrast, within the SZ group, we found an association of steeper PPS slope with increased severity of hallucinations. Thus, in chronic SZ, felt presence or auditory hallucinations might serve to define and sharpen the self–other boundary because “voices” and felt presence are perceived to belong to the “other” located in the extrapersonal space. In other words, self–other border may be experienced more clearly when voices or felt presence as social agents provide spatial cues to define “others.” However, this conjecture has not been empirically tested. Future studies are needed to disentangle the complex relationships among self-disturbances, social behavior, and clinical syndromes.
Beyond hallucinations, PPS slope and size were not associated with the overall severity of clinical symptoms as assessed by SANS and SAPS. This lack of relationship may be partly due to the long illness duration of our participants. Self-disturbances are clearly evident in the prodromal phase and predict future psychosis2,3,45,68,85 as well as functional outcome,8,86–89 but with increasing chronicity, the potential association between self-disturbances and DSM-based clinical symptoms may weaken. If clinical symptoms fluctuate over time but self-disturbances remain stable, correlations between the symptoms and PPS would also wax and wane. Furthermore, people acquire adaptive coping skills over time that help mitigate the negative impact of hallucinations90 and self-disturbances.3,68 Thus, while bodily self-disturbances may be present throughout the course of illness, many learn to live with these experiences. Therefore, it may be important to examine PPS across all phases of SZ to elucidate the relationships among symptom severity, coping skills, and self-disturbance.
Lastly, the importance of social context was observed in the patterns of commission errors. Social condition tended to elicit more commission errors in individuals with SZ compared with CO. As the virtual ball approaches, the participant’s anticipation of possible contact22,91 may elicit a preparatory reaction (eg, attempting to catch the ball).42,92–95 The presence of a social “other” (especially a stranger) who throws an object into the protective zone of PPS might trigger an expectation of negative consequences of physical contact and thus increase the likelihood of anticipatory false alarms.19,84,96
There are caveats. First, the adjustment of RT using baseline performance was not applied in our study. Some investigators measure baseline RTs from unimodal trials to account for the facilitation effect from multisensory processing and the temporal expectancy effect.22,43,97 However, to reduce the temporal expectancy effect, we made the ball disappear immediately after the participant responded to the vibration, and we counted all pre-vibration responses (false alarms) as commission errors. In future studies, both approaches could be applied. Second, our social condition was rudimentary, consisting of an athletic male avatar throwing a ball at the participant. Richer representation of interactional partners and scenarios in VR could help elucidate the flexibility of PPS in response to social situations. Third, qualitative and phenomenological measures were beyond the scope of this study, but future studies should strive to integrate the subjective and objective aspects of self-disturbances. Nevertheless, our findings of altered PPS representation in SZ are consistent with the broader literature bridging the gap between incoherent multisensory processing and self-disturbances..3,13,83,98–100
To summarize, we simulated social and nonsocial environment in immersive VR to investigate the size and the shape of the self–other boundary in individuals with SZ. PPS was plastic and responsive to the nature of the interactional environment. The size of the PPS was contracted in individuals with SZ overall, but the behavioral and clinical implications of reduced PPS size remain to be fully elucidated. Individuals with SZ also experienced increased uncertainty of the extent of the self–other boundary in the social condition, as indicated by shallower PPS slope. Incoherent and blurry bodily self-boundary could undermine adaptive interactions with the external world. Difficulties with self–other distinction in space are salient features of SZ but rarely targeted for intervention. Systematic efforts to specify multisensory roots of self-disturbances could pave the way for integrating body-centered approaches to treatments and bring us closer to elucidating the etiology of schizophrenia.
Supplementary Material
Acknowledgments
We would like to thank Lénie Torregrossa, Taylor Griffith, Hafsah Diakhate, and Andrea Prada for their invaluable assistance throughout this project. We are also immensely grateful to Dr Clara Humpston for her comments.
Conflict of Interest Statement
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
This work was supported by the Gertrude Conaway Vanderbilt Endowment and the Vanderbilt Kennedy Center Director’s Strategic Grant.
References
- 1. Bleuler E. Dementia Praecox oder gruppe der Schizophrenien. In: Aschaffenburg G, ed. Handbuch Der Psychiatrie. Leipzig, Germany: Deuticke; 1911. [Google Scholar]
- 2. Nelson B, Thompson A, Yung AR. Basic self-disturbance predicts psychosis onset in the ultra high risk for psychosis “prodromal” population. Schizophr Bull. 2012;38(6):1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophr Bull. 2003;29(3):427–444. [DOI] [PubMed] [Google Scholar]
- 4. Deegan G. Discovering recovery. Psychiatr Rehabil J. 2003;26(4):368–376. [DOI] [PubMed] [Google Scholar]
- 5. Kean C. Silencing the self: schizophrenia as a self-disturbance. Schizophr Bull. 2009;35(6):1034–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alderson-Day B, Woods A, Moseley P, et al. . Voice-hearing and personification: characterizing social qualities of auditory verbal hallucinations in early psychosis. Schizophr Bull. 2020;47(1):228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thakkar KN, Nichols HS, McIntosh LG, Park S. Disturbances in body ownership in schizophrenia: evidence from the rubber hand illusion and case study of a spontaneous out-of-body experience. PLoS One. 2011;6(10):e27089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haug E, Lien L, Raballo A, et al. . Selective aggregation of self-disorders in first-treatment DSM-IV schizophrenia spectrum disorders. J Nerv Ment Dis. 2012;200(7):632–636. [DOI] [PubMed] [Google Scholar]
- 9. van der Weiden A, Prikken M, van Haren NE. Self-other integration and distinction in schizophrenia: a theoretical analysis and a review of the evidence. Neurosci Biobehav Rev. 2015;57:220–237. [DOI] [PubMed] [Google Scholar]
- 10. Benson TL, Park S. Increased plasticity of bodily self-experience in individuals who may carry latent liability for schizophrenia. Schizophr Res. 2019;207:58–62. [DOI] [PubMed] [Google Scholar]
- 11. Brent BK, Seidman LJ, Thermenos HW, Holt DJ, Keshavan MS. Self-disturbances as a possible premorbid indicator of schizophrenia risk: a neurodevelopmental perspective. Schizophr Res. 2014;152(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Noel JP, Cascio CJ, Wallace MT, Park S. The spatial self in schizophrenia and autism spectrum disorder. Schizophr Res. 2017;179:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Postmes L, Sno HN, Goedhart S, van der Stel J, Heering HD, de Haan L. Schizophrenia as a self-disorder due to perceptual incoherence. Schizophr Res. 2014;152(1):41–50. [DOI] [PubMed] [Google Scholar]
- 14. Blanke O, Metzinger T. Full-body illusions and minimal phenomenal selfhood. Trends Cogn Sci. 2009;13(1):7–13. [DOI] [PubMed] [Google Scholar]
- 15. Rizzolatti G, Fadiga L, Fogassi L, Gallese V. The space around us. Science. 1997;277(5323):190–191. [DOI] [PubMed] [Google Scholar]
- 16. Graziano MS, Cooke DF. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia. 2006;44(6):845–859. [DOI] [PubMed] [Google Scholar]
- 17. Makin TR, Holmes NP, Brozzoli C, Rossetti Y, Farnè A. Coding of visual space during motor preparation: approaching objects rapidly modulate corticospinal excitability in hand-centered coordinates. J Neurosci. 2009;29(38):11841–11851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sambo CF, Forster B, Williams SC, Iannetti GD. To blink or not to blink: fine cognitive tuning of the defensive peripersonal space. J Neurosci. 2012;32(37):12921–12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Haan AM, Smit M, Van der Stigchel S, Dijkerman HC. Approaching threat modulates visuotactile interactions in peripersonal space. Exp Brain Res. 2016;234(7):1875–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salomon R, Noel JP, Łukowska M, et al. . Unconscious integration of multisensory bodily inputs in the peripersonal space shapes bodily self-consciousness. Cognition. 2017;166:174–183. [DOI] [PubMed] [Google Scholar]
- 21. Blanke O. Multisensory brain mechanisms of bodily self-consciousness. Nat Rev Neurosci. 2012;13(8):556–571. [DOI] [PubMed] [Google Scholar]
- 22. Serino A, Noel JP, Mange R, et al. . Peripersonal space: an index of multisensory body–environment interactions in real, virtual, and mixed realities. Front ICT. 2018;4:31. [Google Scholar]
- 23. Blanke O, Slater M, Serino A. Behavioral, neural, and computational principles of bodily self-consciousness. Neuron. 2015;88(1):145–166. [DOI] [PubMed] [Google Scholar]
- 24. Cléry J, Guipponi O, Wardak C, Ben Hamed S. Neuronal bases of peripersonal and extrapersonal spaces, their plasticity and their dynamics: knowns and unknowns. Neuropsychologia. 2015;70:313–326. [DOI] [PubMed] [Google Scholar]
- 25. de Vignemont F, Iannetti GD. How many peripersonal spaces? Neuropsychologia. 2015;70:327–334. [DOI] [PubMed] [Google Scholar]
- 26. Rizzolatti G, Scandolara C, Matelli M, Gentilucci M. Afferent properties of periarcuate neurons in macaque monkeys. II. Visual responses. Behav Brain Res. 1981;2(2):147–163. [DOI] [PubMed] [Google Scholar]
- 27. Graziano MS, Hu XT, Gross CG. Visuospatial properties of ventral premotor cortex. J Neurophysiol. 1997;77(5):2268–2292. [DOI] [PubMed] [Google Scholar]
- 28. Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G. Coding of peripersonal space in inferior premotor cortex (area F4). J Neurophysiol. 1996;76(1):141–157. [DOI] [PubMed] [Google Scholar]
- 29. Avillac M, Denève S, Olivier E, Pouget A, Duhamel JR. Reference frames for representing visual and tactile locations in parietal cortex. Nat Neurosci. 2005;8(7):941–949. [DOI] [PubMed] [Google Scholar]
- 30. Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J Neurophysiol. 1998;79(1):126–136. [DOI] [PubMed] [Google Scholar]
- 31. Canzoneri E, Magosso E, Serino A. Dynamic sounds capture the boundaries of peripersonal space representation in humans. PLoS One. 2012;7(9):e44306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Serino A, Noel JP, Galli G, et al. . Body part-centered and full body-centered peripersonal space representations. Sci Rep. 2015;5:18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teneggi C, Canzoneri E, di Pellegrino G, Serino A. Social modulation of peripersonal space boundaries. Curr Biol. 2013;23(5):406–411. [DOI] [PubMed] [Google Scholar]
- 34. Grivaz P, Blanke O, Serino A. Common and distinct brain regions processing multisensory bodily signals for peripersonal space and body ownership. Neuroimage. 2017;147:602–618. [DOI] [PubMed] [Google Scholar]
- 35. Cléry J, Guipponi O, Odouard S, Pinède S, Wardak C, Ben Hamed S. The prediction of impact of a looming stimulus onto the body is subserved by multisensory integration mechanisms. J Neurosci. 2017;37(44):10656–10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Làdavas E, Serino A. Action-dependent plasticity in peripersonal space representations. Cogn Neuropsychol. 2008;25(7–8):1099–1113. [PubMed] [Google Scholar]
- 37. Macaluso E, Maravita A. The representation of space near the body through touch and vision. Neuropsychologia. 2010;48(3):782–795. [DOI] [PubMed] [Google Scholar]
- 38. Ferri F, Costantini M, Huang Z, et al. . Intertrial variability in the premotor cortex accounts for individual differences in peripersonal space. J Neurosci. 2015;35(50):16328–16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Serino A. Variability in multisensory responses predicts the self-space. Trends Cogn Sci. 2016;20(3):169–170. [DOI] [PubMed] [Google Scholar]
- 40. Sambo CF, Iannetti GD. Better safe than sorry? The safety margin surrounding the body is increased by anxiety. J Neurosci. 2013;33(35):14225–14230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ardizzi M, Ferri F. Interoceptive influences on peripersonal space boundary. Cognition. 2018;177:79–86. [DOI] [PubMed] [Google Scholar]
- 42. Bufacchi RJ, Iannetti GD. An action field theory of peripersonal space. Trends Cogn Sci. 2018;22(12):1076–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pellencin E, Paladino MP, Herbelin B, Serino A. Social perception of others shapes one’s own multisensory peripersonal space. Cortex. 2018;104:163–179. [DOI] [PubMed] [Google Scholar]
- 44. Serino A. Peripersonal space (PPS) as a multisensory interface between the individual and the environment, defining the space of the self. Neurosci Biobehav Rev. 2019;99:138–159. [DOI] [PubMed] [Google Scholar]
- 45. Chapman LJ, Chapman JP, Raulin ML. Body-image aberration in Schizophrenia. J Abnorm Psychol. 1978;87(4):399–407. [DOI] [PubMed] [Google Scholar]
- 46. Di Cosmo G, Costantini M, Salone A, et al. . Peripersonal space boundary in schizotypy and schizophrenia. Schizophr Res. 2018;197:589–590. [DOI] [PubMed] [Google Scholar]
- 47. Noel JP, Failla M, Quinde-Zlibut J, et al. . Visual-tactile spatial multisensory interaction in adults with autism and schizophrenia. Front Psychiatry. 2020;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hayduk LA. Personal space: where we now stand. Psychol Bull. 1983;94(2):293–335. [Google Scholar]
- 49. Cartaud A, Ruggiero G, Ott L, Iachini T, Coello Y. Physiological response to facial expressions in peripersonal space determines interpersonal distance in a social interaction context. Front Psychol. 2018;9:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Quesque F, Ruggiero G, Mouta S, Santos J, Iachini T, Coello Y. Keeping you at arm’s length: modifying peripersonal space influences interpersonal distance. Psychol Res. 2017;81(4):709–720. [DOI] [PubMed] [Google Scholar]
- 51. Deus V, Jokić-Begić N. Personal space in schizophrenic patients. Psychiatr Danub. 2006;18(3–4):150–158. [PubMed] [Google Scholar]
- 52. Duke MP, Mullens MC. Preferred interpersonal distance as a function of locus of control orientation in chronic schizophrenics, nonschizophreic patients, and normals. J Consult Clin Psychol. 1973;41(2):230–234. [DOI] [PubMed] [Google Scholar]
- 53. Holt DJ, Boeke EA, Coombs G 3rd, et al. . Abnormalities in personal space and parietal-frontal function in schizophrenia. Neuroimage Clin. 2015;9:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Horowitz MJ, Duff DF, Stratton LO. Body-buffer zone; exploration of personal space. Arch Gen Psychiatry. 1964;11:651–656. [DOI] [PubMed] [Google Scholar]
- 55. Park SH, Ku J, Kim JJ, et al. . Increased personal space of patients with schizophrenia in a virtual social environment. Psychiatry Res. 2009;169(3):197–202. [DOI] [PubMed] [Google Scholar]
- 56. Srivastava P, Mandal MK. Proximal spacing to facial affect expressions in schizophrenia. Compr Psychiatry. 1990;31(2):119–124. [DOI] [PubMed] [Google Scholar]
- 57. Nechamkin Y, Salganik I, Modai I, Ponizovsky AM. Interpersonal distance in schizophrenic patients: relationship to negative syndrome. Int J Soc Psychiatry. 2003;49(3):166–174. [DOI] [PubMed] [Google Scholar]
- 58. Delevoye-Turrell Y, Vienne C, Coello Y. Space boundaries in schizophrenia. Soc Psychol 2011;42(3):193–204. [Google Scholar]
- 59. Geraets CNW, van Beilen M, Pot-Kolder R, Counotte J, van der Gaag M, Veling W. Social environments and interpersonal distance regulation in psychosis: a virtual reality study. Schizophr Res. 2018;192:96–101. [DOI] [PubMed] [Google Scholar]
- 60. Collip D, Nicolson NA, Lardinois M, Lataster T, van Os J, Myin-Germeys I; G.R.O.U.P . Daily cortisol, stress reactivity and psychotic experiences in individuals at above average genetic risk for psychosis. Psychol Med. 2011;41(11):2305–2315. [DOI] [PubMed] [Google Scholar]
- 61. Haralanova E, Haralanov S, Beraldi A, Möller HJ, Hennig-Fast K. Subjective emotional over-arousal to neutral social scenes in paranoid schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2012;262(1):59–68. [DOI] [PubMed] [Google Scholar]
- 62. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. [DOI] [PubMed] [Google Scholar]
- 63. Kim J, Park S, Blake R. Perception of biological motion in schizophrenia and healthy individuals: a behavioral and FMRI study. PLoS One. 2011;6(5):e19971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schoretsanitis G, Kutynia A, Stegmayer K, Strik W, Walther S. Keep at bay! Abnormal personal space regulation as marker of paranoia in schizophrenia. Eur Psychiatry. 2016;31:1–7. [DOI] [PubMed] [Google Scholar]
- 65. Benson TL, Brugger P, Park S. Bodily self-disturbance in schizophrenia-spectrum populations: introducing the Benson et al. Body Disturbances Inventory (B-BODI). Psych J. 2019;8(1):110–121. [DOI] [PubMed] [Google Scholar]
- 66. Michael J, Park S. Anomalous bodily experiences and perceived social isolation in schizophrenia: an extension of the social deafferentation hypothesis. Schizophr Res. 2016;176(2–3):392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Park S, Nasrallah HA. The varieties of anomalous self experiences in schizophrenia: splitting of the mind at a crossroad. Schizophr Res. 2014;152(1):1–4. [DOI] [PubMed] [Google Scholar]
- 68. Parnas J, Handest P. Phenomenology of anomalous self-experience in early schizophrenia. Compr Psychiatry. 2003;44(2):121–134. [DOI] [PubMed] [Google Scholar]
- 69. First MB, Williams JBW, Karg RS, Spitzer RL.. Structured Clinical Interview For DSM-5—Research Version (SCID-5 For DSM-5, Research Version; SCID-5-RV). Arlington, VA: American Psychiatric Association; 2015. [Google Scholar]
- 70. Blair JR, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol. 1989;3(2):129–136. [Google Scholar]
- 71. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. [DOI] [PubMed] [Google Scholar]
- 72. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24(1):97–99. [PubMed] [Google Scholar]
- 73. Andreasen NC. Scale for the assessment of positive symptoms (SAPS). Group. 1984;17(2):173–180. [Google Scholar]
- 74. Freeman D, Loe BS, Kingdon D, et al. . The revised Green et al., Paranoid Thoughts Scale (R-GPTS): psychometric properties, severity ranges, and clinical cut-offs. Psychol Med. 2021;51(2):244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry. 1989;155(S7):49–52. [PubMed] [Google Scholar]
- 76. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17(4):555–564. [DOI] [PubMed] [Google Scholar]
- 77. Green CE, Freeman D, Kuipers E, et al. . Measuring ideas of persecution and social reference: the Green et al. Paranoid Thought Scales (GPTS). Psychol Med. 2008;38(1):101–111. [DOI] [PubMed] [Google Scholar]
- 78. Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66(1):20–40. [DOI] [PubMed] [Google Scholar]
- 79. Serino A, Canzoneri E, Marzolla M, di Pellegrino G, Magosso E. Extending peripersonal space representation without tool-use: evidence from a combined behavioral-computational approach. Front Behav Neurosci. 2015;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 81. Veling W, Brinkman WP, Dorrestijn E, van der Gaag M. Virtual reality experiments linking social environment and psychosis: a pilot study. Cyberpsychol Behav Soc Netw. 2014;17(3):191–195. [DOI] [PubMed] [Google Scholar]
- 82. Noel JP, Pfeiffer C, Blanke O, Serino A. Peripersonal space as the space of the bodily self. Cognition. 2015;144:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stanghellini G, Broome M, Fernandez AV, Raballo A.. The Oxford Handbook of Phenomenological Psychopathology. New York, NY: Oxford University Press; 2019. [Google Scholar]
- 84. Noel JP, Park HD, Pasqualini I, et al. . Audio-visual sensory deprivation degrades visuo-tactile peri-personal space. Conscious Cogn. 2018;61:61–75. [DOI] [PubMed] [Google Scholar]
- 85. Raballo A, Pappagallo E, Dell’ Erba A, et al. . Self-disorders and clinical high risk for psychosis: an empirical study in help-seeking youth attending community mental health facilities. Schizophr Bull. 2016;42(4):926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nordgaard J, Handest P, Vollmer-Larsen A, Sæbye D, Pedersen JT, Parnas J. Temporal persistence of anomalous self-experience: a 5years follow-up. Schizophr Res. 2017;179:36–40. [DOI] [PubMed] [Google Scholar]
- 87. Raballo A, Preti A. The self in the spectrum: a closer look at the temporal stability of self-disorders in schizophrenia. Psychopathology. 2018;51(4):285–289. [DOI] [PubMed] [Google Scholar]
- 88. Svendsen IH, Øie MG, Møller P, Nelson B, Haug E, Melle I. Basic self-disturbances independently predict recovery in psychotic disorders: a seven year follow-up study. Schizophr Res. 2019;212:72–78. [DOI] [PubMed] [Google Scholar]
- 89. Værnes TG, Røssberg JI, Møller P. Anomalous self-experiences are strongly associated with negative symptoms in a clinical high-risk for psychosis sample. Compr Psychiatry. 2019;93:65–72. [DOI] [PubMed] [Google Scholar]
- 90. Thomas N, Hayward M, Peters E, et al. . Psychological therapies for auditory hallucinations (voices): current status and key directions for future research. Schizophr Bull. 2014;40(suppl 4):S202–S212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kandula M, Hofman D, Dijkerman HC. Visuo-tactile interactions are dependent on the predictive value of the visual stimulus. Neuropsychologia. 2015;70:358–366. [DOI] [PubMed] [Google Scholar]
- 92. Brozzoli C, Gentile G, Ehrsson HH. That’s near my hand! Parietal and premotor coding of hand-centered space contributes to localization and self-attribution of the hand. J Neurosci. 2012;32(42):14573–14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Brozzoli C, Ehrsson HH, Farnè A. Multisensory representation of the space near the hand: from perception to action and interindividual interactions. Neuroscientist. 2014;20(2):122–135. [DOI] [PubMed] [Google Scholar]
- 94. Cardellicchio P, Sinigaglia C, Costantini M. Grasping affordances with the other’s hand: a TMS study. Soc Cogn Affect Neurosci. 2013;8(4):455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fini C, Costantini M, Committeri G. Sharing space: the presence of other bodies extends the space judged as near. PLoS One. 2014;9(12):e114719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Noel JP, Blanke O, Magosso E, Serino A. Neural adaptation accounts for the dynamic resizing of peripersonal space: evidence from a psychophysical-computational approach. J Neurophysiol. 2018;119(6):2307–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kandula M, Van der Stoep N, Hofman D, Dijkerman HC. On the contribution of overt tactile expectations to visuo-tactile interactions within the peripersonal space. Exp Brain Res. 2017;235(8):2511–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Stevenson RA, Park S, Cochran C, et al. . The associations between multisensory temporal processing and symptoms of schizophrenia. Schizophr Res. 2017;179:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ferri F, Nikolova YS, Perrucci MG, et al. . A neural “Tuning Curve” for multisensory experience and cognitive-perceptual schizotypy. Schizophr Bull. 2017;43(4):801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Northoff G, Sandsten KE, Nordgaard J, Kjaer TW, Parnas J. The self and its prolonged intrinsic neural timescale in schizophrenia. Schizophr Bull. 2020;47(1):170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.