Abstract
Schizophrenia-spectrum disorders (SSD) are associated with increased inflammatory markers, both in brain and periphery. Augmentation with drugs that lower this pro-inflammatory status may improve clinical presentation. Simvastatin crosses the blood-brain barrier, has anti- inflammatory and neuroprotective effects and reduces metabolic syndrome. In this study, we investigated if 12 months of simvastatin augmentation can improve symptoms and cognition in patients with early SSD. This double-blind placebo-controlled trial included 127 SSD patients across the Netherlands, <3 years after their diagnosis. From these, 119 were randomly assigned 1:1 to simvastatin 40 mg (n = 61) or placebo (n = 58), stratified for sex and study site. Primary outcomes were symptom severity and cognition after 12 months of treatment. Depression, symptom subscores, general functioning, metabolic syndrome, movement disorders, and safety were secondary outcomes. Intention to treat analyses were performed using linear mixed models and ANCOVA. No main effect of simvastatin treatment was found on total symptom severity after 12 months of treatment as compared to placebo (X2(1) = 0.01, P = .90). Group differences varied over time (treatment*time X2(4) = 11.2; P = .025), with significantly lower symptom severity in the simvastatin group after 6 months (mean difference = −4.8; P = .021; 95% CI: −8.8 to −0.7) and at 24 months follow-up (mean difference = −4.7; P = .040; 95% CI: −9.3 to −0.2). No main treatment effect was found for cognition (F(1,0.1) = 0.37, P = .55) or secondary outcomes. SAEs occurred more frequently with placebo (19%) than with simvastatin (6.6%). This negative finding corroborates other large scale studies on aspirin, minocycline, and celecoxib that could not replicate positive findings of smaller studies, and suggests that anti-inflammatory augmentation does not improve the clinical presentation of SSD.
Keywords: schizophrenia, symptoms, simvastatin, inflammation, RCT
Introduction
Patients with schizophrenia-spectrum disorders (SSD) have urgent unmet needs, as current treatment is far from optimal. First, not all patients achieve remission using standard treatments.1 Current treatment options also have little effect on negative and cognitive symptoms.2 Finally, mortality is much higher in SSD patients than in healthy peers.3 While suicide is partly responsible for the increased mortality, the largest part stems from cardiovascular deaths due to the high prevalence of metabolic syndrome.3 Antipsychotic treatment targets increased striatal dopamine synthesis, which is a well-known, albeit not ubiquitous, feature of schizophrenia pathology. In recent years, other pathophysiological mechanisms have (partly) been elucidated, which may provide new leads for therapeutic strategies.4 Immune dysregulation is one of these leads, although it is not a well-established etiological factor for SSD, given several negative results.5–7 Nevertheless, increased levels of the pro-inflammatory cytokines IL-6, TNFα, IFN, IL-1, and acute-phase protein CRP, have repeatedly been reported in early-stage SSD patients.4 In postmortem brain tissue, the expression of pro-inflammatory genes was also increased.8 Such immune mechanisms mediate the interaction between neurons and glial cells, regulating neurodevelopmental processes such as synaptic pruning and neurogenesis, as well as neurotransmission.9 Maternal bacterial infection and childhood infections, important risk factors for schizophrenia,10,11 elicit an immune response, which may result in functional changes that are retained into adulthood.9 Indeed, a meta-analysis showed increased activity of microglia cells in schizophrenia,12 but a later review could not replicate this finding.13 Application of anti-inflammatory drugs may correct this immune activation and enable normal neurodevelopment.14
Several studies targeted this increased pro-inflammatory status in SSD, with heterogeneous results.15 Multiple studies opted for minocycline, a broad-spectrum antibiotic that crosses the blood-brain barrier and decreases microglia activation.16 In 10 studies, this drug was administered (100–300 mg) for 2–12 months with an overall effect size of 0.4 (P = .007).15 However, the largest study found a significant negative effect of minocycline.17 Potential deleterious actions of minocycline were also demonstrated in animal models18 and patient-derived organoids using high doses,19 urging the need for safer and more tolerable anti-inflammatory drugs. Like minocycline, simvastatin crosses the blood-brain barrier. Importantly, statins lower cardiovascular mortality, an effect not only caused by its cholesterol lowering actions.20 Reduced cholesterol levels deplete the cholesterol-rich membrane domains known as lipid rafts, which affect cellular signaling,21 translating into neuroprotective effects: improved blood-flow, reduced coagulation, reduced oxidative damage and reduced inflammatory status.22 As simvastatin is used chronically by millions of people world-wide, its long-term effects are well-known. Moreover, simvastatin reduces metabolic syndrome, a pivotal factor increasing mortality in SSD. A study in patients with multiple sclerosis showed that 24 months of simvastatin use slowed down progression of cognitive dysfunction and loss of cerebral gray matter.23 Three small studies provided 40 mg of statins (ie, simvastatin and pravastatin) to SSD patients for 824 to 1225,26 weeks. Although the mean weighted effect size of these studies was 0.5, significance was not reached due to low power.15
In this study, we provided simvastatin 40 mg/d to SSD patients for 12 months and investigated the effects on symptom severity and cognitive decline. We included only recent-onset patients, as this group is reported to benefit most from augmentation with anti-inflammatory drugs.27 To be robust against false-negative findings, we recruited a relatively large group of 127 patients.
Methods
Design and Registration
This is a double-blind randomized placebo-controlled multi-center trial. Trial registration: ClinicalTrails.gov:NCT01999309; EudraCT-number:2013-000834-36.
Participants
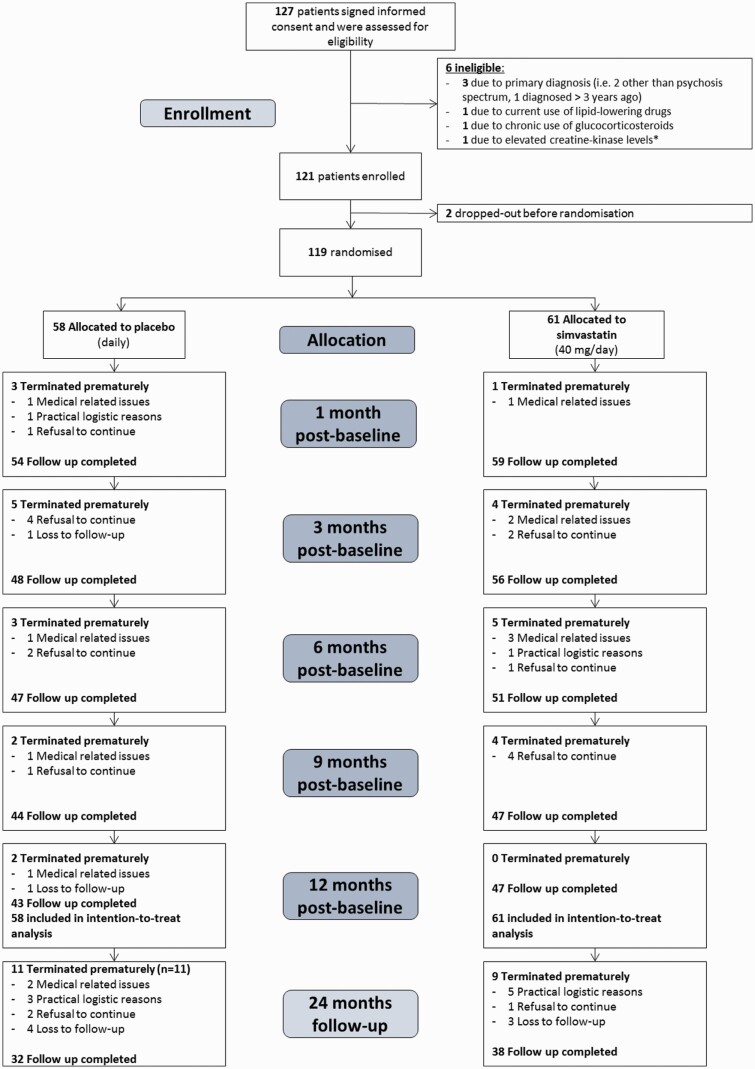
Eligible patients were 18–50 years of age, with a DSM-IV diagnosis of schizophrenia, schizoaffective, schizophreniform disorder (295.x) or psychotic disorder not otherwise specified (298.9), figure 1.28 First psychosis onset was no longer than 3 years ago. Patients were recruited from Dutch inpatient and outpatient settings between November 2013 and February 2019. Study procedures and instruments are described per visit by Begemann et al28.
Fig. 1.
Trial profile. *In case of familial risk for muscular disorders or previously experienced muscle toxicity when taking medication similar to simvastatin, creatine kinase (CK) levels will be checked (as recommended by the Dutch Farmacotherapeutisch Kompas).
Power Analysis
Following our unplanned interim analyses (at n = 97) to reevaluate our power analysis and recruitment target, performed by a blinded independent researcher, we adjusted our power analysis using the standard deviation of 14.3 instead of 20 (based on our previous RCT), while the expected difference in mean total PANSS scores remained at 7.5 (based on previous studies).17,29,30 Correlation between PANSS baseline and 12 months was corrected to 0.6 instead of 0.4.28 Dropout rate of 24% was also lower than the anticipated 30%,28 resulting in an adjusted power calculation of n = 120.
Randomization and Masking
A web-based randomization table was generated by the Julius Center (Utrecht, the Netherlands). Patients were randomized 1:1 to either simvastatin or placebo, using block-randomization (blocks of 4) with stratification for study site and sex. Randomization codes were not available to the study staff. Emergency unblinding was only allowed in case of serious concerns about patient safety. The data management group was not involved in patient recruitment. All study staff and patients were blind to treatment allocation. Study drugs were manufactured by ACE pharmaceuticals (Zeewolde, the Netherlands), being simvastatin 40 mg or placebo, with indistinguishable appearance, shape, smell, mass, and taste. Drug accountability was performed by documenting drug shipment, storage conditions, drug dispense, return, and destruction.
Procedures
Participants visited the research center 8 times during 2 years. Medical history and medication use were checked to (re)assess in- and exclusion criteria. Blood was screened for cholesterol and when levels exceeded >8 mmol/L at any visit, patients were excluded as they had a clinical indication to start statin treatment. To prevent the possibility of unblinding due to the cholesterol-lowering properties of simvastatin, raters did not have access to cholesterol results. During participation, patients continued their regular (antipsychotic) medication treatment. The institutional review board of the University Medical Center Utrecht approved the study (NL43806.041.13; Clinicaltrials.gov: NCT01999309).
Primary Outcomes
Symptom severity was evaluated using the Positive and Negative Syndrome Scale (PANSS)31 by trained and certified raters. Neurocognitive functioning was assessed with the Brief Assessment of Cognition in Schizophrenia (BACS).32 The PANSS and BACS total score at the end of 12 months of treatment time point were defined as the primary outcomes.
Secondary Outcomes
We evaluated PANSS positive, negative, and general subscales at all time points as secondary outcomes. General functioning was assessed using the Global Assessment of Functioning scale (GAF).33 The presence and severity of movement disorders was evaluated using St. Hans Rating Scale for extrapyramidal syndromes (SHRS)34 and the Barnes Akathisia Rating Scale (BARS).35 Depressive symptoms were rated using the Calgary Depression Scale for Schizophrenia (CDSS).36 Metabolic syndrome was assessed by measuring blood pressure, waist circumference, fasting glucose, triglycerides, and HDL-C and was defined as having 3 or more symptoms (American Heart Association/National Heart, Lung, and Blood Institute).37
Safety
Patients were asked if they experienced any side effects or health problems since the last visit, with special focus on myalgia and dark-colored urine. We compared incidences (number and % of subjects with at least one occurrence) of serious adverse events (SAEs) and adverse events (AE) between both groups (eg, hospitalizations).
Statistical Analysis
Statistical analyses were performed using IBM SPSS for Windows (version 25). Continuous primary and secondary outcomes were analyzed with a linear mixed model for repeated measurements. To model the effect of simvastatin, we included time point, treatment, sex and study site as fixed factors, age and baseline scores as covariates and subject as random intercept factor. To evaluate whether group differences varied over time, we also assessed the time*treatment interaction effect. When significant, group differences were compared at the individual time points. Full analyses are shown in supplementary material, p1. An SP_POWER structure was used to model the residual covariance matrix. Validity of the model (ie, distributional assumptions, homoscedasticity) was assessed with residual analyses. Moreover, sensitivity analyses were conducted. Considering the assumption of random missing data, we used logistic regression to investigate the association between trial drop-out and baseline patient characteristics. Moreover, we repeated the mixed model analyses by adding completion of the treatment phase as a fixed factor (ie, categorical variable indicating trial completion or drop-out) and interaction terms (ie, treatment*completer status, time*completer status, treatment*time*completer status). We also compared the individuals who adequately adhered to simvastatin treatment to those who did not. As simvastatin 40 mg directly decreases lipid levels, especially LDL-cholesterol levels, we used >20% reduction of LDL-cholesterol (compared to baseline) throughout the study as a reflection of adequate treatment adherence.38 Analysis of covariance (ANCOVA) was applied for group differences in cognitive performance at 6 and 12 months of treatment, including baseline score as a covariate. BACS total composite score was calculated by converting raw test scores of the subtasks into z-scores and averaging these standardized scores. Post-hoc analyses were conducted for the 6 individual subtasks and follow-up measurements 24 months post-baseline. ANCOVA was applied to evaluate depressive symptoms (CDSS), correcting for baseline severity, age and sex. Mixed models were used to analyze PANSS subscales and GAF, as described above, also including follow-up measurements 24 months post-baseline. Metabolic syndrome was analyzed using the linear mixed models described above, with a logit link function for the dichotomous endpoint. For movement disorders, incidence and mean scores were presented per group per time point. Moreover, incidences of SAEs and AEs were presented per group and tested with chi-square analyses. The significance level for all statistical tests was P<.050, 2-tailed.
Results
Demographics
Participants were recruited between November 2013 and February 2019, when the project end date was reached and the required number of participants was met. A total of 127 patients signed informed consent of which 119 were randomized (figure 1). Ninety subjects completed the total treatment duration of 12 months. The follow-up 1 year after the end of treatment was completed by 70 subjects. Baseline demographic characteristics are summarized in table 1. The simvastatin group was comparable to the placebo group for age, sex, education, diagnosis and time since first SSD diagnosis. Both groups had similar levels of symptom severity on PANSS total, PANSS negative and PANSS general, but the simvastatin group had higher baseline PANSS positive scores. The mixed model analysis corrected for this difference.
Total Symptom Severity
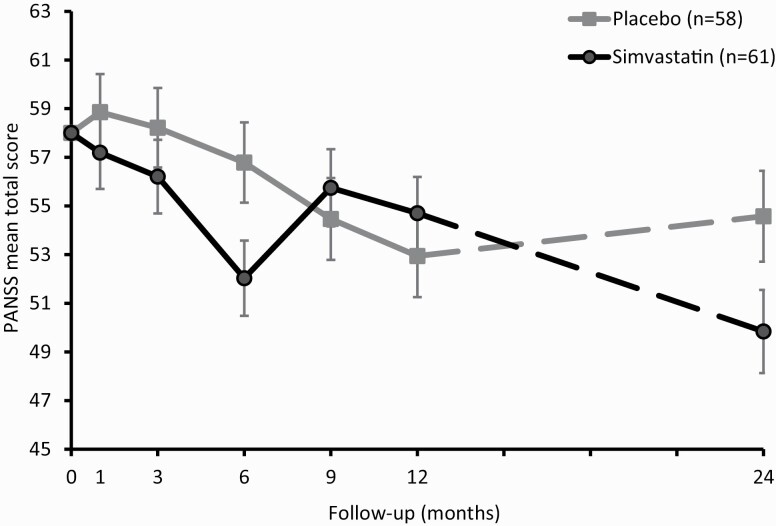
The linear mixed-effects model of the total PANSS score showed no main effect of simvastatin after 12 months of treatment (X2(1) = 0.01, P = .90) (see supplementary material, p1 and p2 for change scores from baseline). The interaction between treatment*time was significant (X2(4) = 11.2, P = .025), indicating that group differences in symptom severity differed over time. The simvastatin group scored significantly lower on total PANSS scores at 6 months (mean difference = −4.8, P = .021, 95% CI: −8.8 to −0.73; figure 2) and at 24 months post-baseline (mean difference = −4.7, P = .040, 95% CI: −9.3 to −0.2).
Fig. 2.
Mean symptom severity (PANSS total score) for the simvastatin and placebo group. Means are estimated by a linear mixed model and displayed with standard errors. Data are displayed per visit for the active treatment phase (0–12 mo; continuous line) and follow up phase (24 mo; dashed line).
Cognitive Functioning
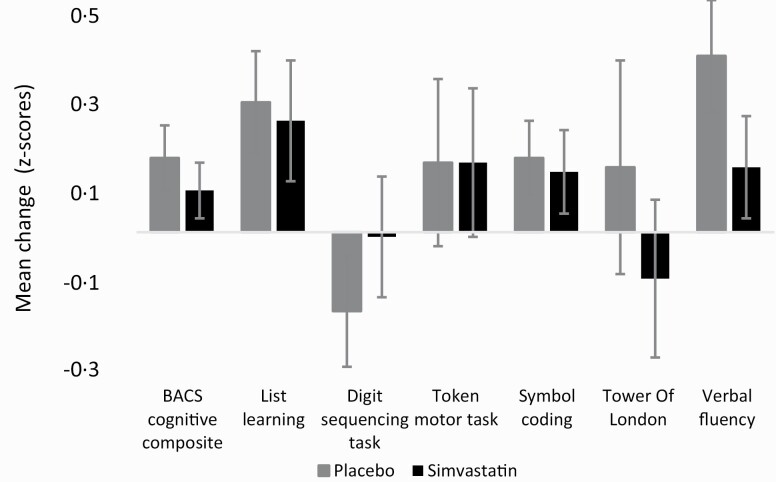
The simvastatin group did not differ from placebo in cognitive performance (BACS composite score) after 12 months (F(1,0.1) = 0.4, P = .55). Post-hoc analyses did not show effects for any of the cognitive subtasks (P-values ≥ .21) (figure 3, supplementary material, p2).
Fig. 3.
Mean change of cognitive functioning during the active treatment phase (0–12 mo). Mean Z-scores with standard errors are displayed for the composite cognition score and for the 6 subtasks of the BACS.
Secondary Outcomes
None of the secondary outcomes showed a significant main effect for treatment at 12 months. The treatment*time interaction was only significant for PANSS general subscores (X2(5) = 13.7, P = .017), which was lower in the simvastatin group at 6 months (mean difference = −2.7, P = .017, 95% CI: −4.9 to −0.5). When evaluating general functioning (GAF), the main treatment effect was not significant (X2(5) = 1.0, P = .97). There was a significant time*treatment interaction effect (X2(5) = 11.0, P = .052), with higher general functioning in the simvastatin group at 24 months (mean difference = 4.9, P = .048, 95% CI: 0.04 to 9.8). ANCOVA showed that the simvastatin group had lower depressive symptoms (CDSS) than the placebo group at 6 months (F(1,38.4) = 5.5, P = .021). Movement disorders were largely absent in both groups, except for Parkinsonism (supplementary material, p6). Metabolic syndrome did not show a significant time*treatment interaction effect (X2(6) = 1.5, P = .96).
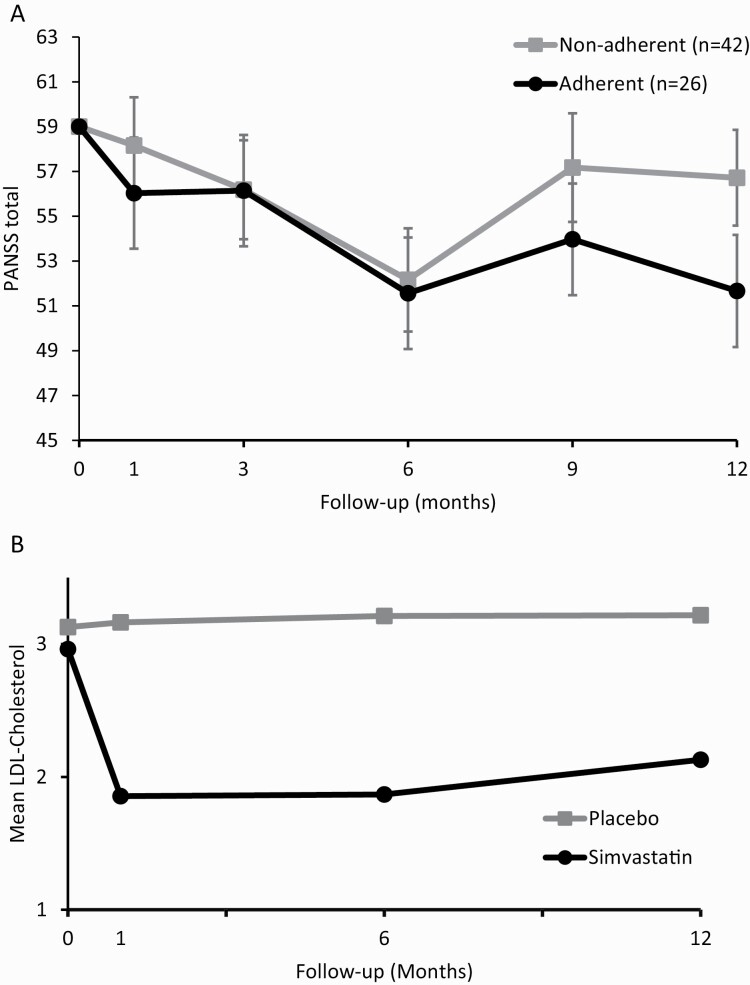
Trial drop-out was not associated with age, sex, education, treatment group, duration of illness, antipsychotic dose, GAF or PANSS scores (supplementary material, p1). Adding completion of the treatment phase and the respective interaction effects to the linear mixed model, did not improve model fit (X2(10) = 7.5, P = .68). When comparing adherent participants (ie, >20% reduction of LDL-cholesterol at all measurements)38 to non-adherent participants within the simvastatin group, no significant main effect of adherence group (X2(1) = 0.5, P = .49) and no interaction effect (time*adherence group) were found (X2(4) = 3.0, P = .56) (figure 4a and 4b).
Fig. 4.
(a) Mean symptom severity (PANSS total score) for the adherent and non-adherent participants within the simvastatin group Means are estimated by a linear mixed model, with standard error. Data are displayed per visit for the active treatment phase (0–12 mo). Adherence to study medication (simvastatin) was defined as >20% reduction of LDL-cholesterol, compared to baseline, throughout the study. (b) Mean LDL-cholesterol levels for the simvastatin and placebo group. Means are depicted, with standard error. Data are displayed per visit for the active treatment phase (0–12 mo).
Laboratory Assay
There was a significant decrease in total cholesterol (F(2.7,216.4) = 26.0, P = .000), LDL cholesterol (F(2.5,188.8) = 28.9, P < .001; figure 4a) and non-HDL cholesterol (F(2.4,128.6) = 20.1, P < .001) in the simvastatin group over time compared to placebo (supplementary material). There was no significant main effect or time*treatment interaction effect for HDL cholesterol levels (F(3.2) = 0.83, P = .48).
Safety Assessments
During the treatment phase, the number of individuals experiencing SAE(s) was higher in the placebo group compared to simvastatin (11 [19%] vs. 4 [6.6%], respectively; X2(1) = 4.2, P = .042). The incidences were similar during the follow-up phase (4 [12.5%] vs. 6 [15.8%], X2(1) = 0.2, P = .70). Two adverse events (AEs) were specifically screened for during the treatment phase. Myalgia was reported by 13 (21%) participants in the simvastatin group and 13 (22%) in the placebo group. Dark-colored urine was reported by 4 patients in the simvastatin group (7%) and by 7 (12%) in the placebo group (supplementary material). In total, the number of individuals reporting AE(s) was similar in both groups during the treatment phase (50 [86.2%] vs 57 [93.4%], respectively; X2(1) = 1.7, P = .19) and follow-up (5 [15.6%] vs. 12 [31.6%], X2(1) = 2.4, P = .12).
Discussion
We investigated the efficacy of simvastatin 40 mg/d augmentation in 127 patients with early-stage SSD. Our predefined primary outcome: symptom severity and cognition at 12 months treatment was negative. Previous studies with statins had a shorter treatment duration,39 which could explain the different findings. At 6 months, both total and general symptom severity were significantly lower in the simvastatin group. The simvastatin group also had lower PANSS total scores when evaluating 24 months post-baseline measurements. Regarding secondary outcomes, general functioning (GAF) was marginally better functioning in the simvastatin group at 24 months (P = .052). No treatment effect was found for depression, presence or severity of metabolic syndrome or presence of movement disorders. SAEs occurred more frequently with placebo (17/27 SAEs) than with simvastatin use (10/27).
This indicates that simvastatin augmentation is not an effective way to clinical presentation of early-phase SSD patients. There are several reasons why we could not replicate initial positive effects of statins.24–26 A complicating factor, especially in studies with a long treatment phase, is adherence. Treatment adherence in our sample was lower in the second half of the treatment as monitored by LDL cholesterol levels. However, our comparison between completers and non-completers did not reveal significant differences. Furthermore, as is often the case in randomized controlled trials, participants had relatively high education and showed few cognitive deficits compared to a more general sample with SDD, possibly inducing ceiling effects in cognition.
The most obvious reason for the present negative findings is that lowering the increased pro-inflammatory status in SSD is not an effective treatment for total symptom severity or cognition. This corroborates recent large-scale studies on minocycline,17 celecoxib,29 and aspirin30 that could not replicate previous smaller RCTs showing positive effects of anti-inflammatory augmentation. There is a general trend in scientific developments in which initial publications on new prospectives first feature small studies with positive findings which spark hope, followed by a later wave of larger studies with negative findings that temper that hope.40 Perhaps we are now in that second wave regarding anti-inflammatory augmentation.
Beneficial effects of simvastatin have been reported for other brain disorders, including stroke, multiple sclerosis, Parkinson’s disease, and Alzheimer’s disease, although the latter association is much disputed.41 In multiple sclerosis, simvastatin slowed down cognitive decline,23 a finding that we could not replicate for SSD. In contrast to multiple sclerosis and neurodegenerative disorders, cognitive deficits in SDD are generally stable.42 Also, the pro-inflammatory status of the brain in schizophrenia is much more subtle compared to multiple sclerosis and neurodegenerative disorders. Apparently, statins are not able to ameliorate stable and subtle cognitive impairment.
Despite several unfunded claims in lay press,43 statins are safe and generally well tolerated,44 which was also demonstrated in this study. Although many statin users complain of muscle ache, this is rarely observed in double-blind RCTs, as rhabdomyolysis and statin-induced myopathy are rare.44 In this trial, myopathy and dark colored urine were at least as common in the placebo group, demonstrating strong nocebo effects for these complaints. Use of statins, especially in individuals with metabolic syndrome, is associated with protection from cardiovascular events: the most important cause of excess mortality in SSD, especially in men.3 In this trial, simvastatin significantly lowered cholesterol without a decrease in metabolic syndrome severity, due to our ethical requirement to exclude patients with hypercholesterolemia at any time point. Yet, for SSD patients with increased lipids, statins are an effective and well tolerated solution. As metabolic syndrome is common, SSD patients need statin therapy at a younger age than mentally healthy peers.45
Limitations
A clear limitation was treatment adherence, which decreased during the long study duration, especially after the first 6 months. The population of early-onset SSD may not have been optimal, as metabolic syndrome and movement disorders were largely absent.
In conclusion, we could not demonstrate a positive effect of simvastatin 40 mg/d on symptom severity and cognition at 12 months. This study, together with other recent large studies with negative findings,17,29,30 imply that anti-inflammatory augmentation may not be beneficial for symptom severity and cognition in schizophrenia.
Funding
This work was supported by the Stanley Medical Research Institute (grant number: 12T-008) and the Dutch Research Council (NWO; grant number: 40-00812-98-12154). The sponsors were not involved in the collection, analysis and interpretation of data, nor in writing of the report and the decision to submit the paper for publication.
Supplementary Material
Acknowledgments
I.E.S., S.G., S.K., S.V., C.B., K.G., R.B., L.D.W., R.S.K., and M.J.H.B. declare to have no competing interest related to this study. S.B. is director of Psynova Neurotech Ltd and Psyomics Ltd. I.E.S. designed the study. I.E.S., L.D.W., and R.S.K. obtained funding. I.E.S., S.G., S.K., R.S.K., and M.J.H.B. coordinated the study. W.V., R.B., M.P., S.W., S.V., K.G., and N.B. were involved in patient recruitment and local coordination. I.E.S., S.G., S.K., C.B., and M.J.H.B. analyzed and interpreted the data. I.E.S., S.G., S.K., and M.J.H.B. drafted the article. All authors participated in the critical revision of the article and approved the final article.
References
- 1. Levi L, Bar Haim M, Burshtein S, et al. Duration of untreated psychosis and response to treatment: an analysis of response in the OPTiMiSE cohort. Eur Neuropsychopharmacol. 2020;32:131–135. [DOI] [PubMed] [Google Scholar]
- 2. Bucci P, Mucci A, van Rossum IW, et al. Persistent negative symptoms in recent-onset psychosis: relationship to treatment response and psychosocial functioning. Eur Neuropsychopharmacol. 2020;34:76–86. [DOI] [PubMed] [Google Scholar]
- 3. Sommer IE, Tiihonen J, van Mourik A, Tanskanen A, Taipale H. The clinical course of schizophrenia in women and men-a nation-wide cohort study. npj Schizophr. 2020;6(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fond G, Lançon C, Korchia T, Auquier P, Boyer L. The role of inflammation in the treatment of schizophrenia. Front Psychiatry. 2020;11:160. doi: 10.3389/fpsyt.2020.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birnbaum R, Weinberger DR. A genetics perspective on the role of the (neuro)immune system in schizophrenia. Schizophr Res. 2020;217:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Mierlo HC, Schot A, Boks MPM, de Witte LD. The association between schizophrenia and the immune system: review of the evidence from unbiased ‘omic-studies’. Schizophr Res. 2020;217:114–123. [DOI] [PubMed] [Google Scholar]
- 7. Snijders GJLJ, Zuiden W, Sneeboer MAM, et al. A loss of mature microglial markers without immune activation in schizophrenia. Glia. 2021:glia.23962. doi: 10.1002/glia.23962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Kesteren CF, Gremmels H, de Witte LD, et al. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry. 2017;7(3):e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7(3):272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee YH, Cherkerzian S, Seidman LJ, et al. Maternal bacterial infection during pregnancy and offspring risk of psychotic disorders: variation by severity of infection and offspring sex. Am J Psychiatry. 2020;177(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nielsen PR, Meyer U, Mortensen PB. Individual and combined effects of maternal anemia and prenatal infection on risk for schizophrenia in offspring. Schizophr Res. 2016;172(1-3):35–40. [DOI] [PubMed] [Google Scholar]
- 12. Marques TR, Ashok AH, Pillinger T, et al. Neuroinflammation in schizophrenia: meta-analysis of in vivo microglial imaging studies. Psychol Med. 2019;49(13):2186–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plavén-Sigray P, Matheson GJ, Coughlin JM, et al. Meta-analysis of the glial marker TSPO in psychosis revisited: reconciling inconclusive findings of patient-control differences. Biol Psychiatry. 2021;89(3):e5–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maas DA, Vallès A, Martens GJM. Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Transl Psychiatry. 2017;7(7):e1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol Med. 2019;49(14):2307–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watabe M, Kato TA, Monji A, Horikawa H, Kanba S. Does minocycline, an antibiotic with inhibitory effects on microglial activation, sharpen a sense of trust in social interaction? Psychopharmacology (Berl). 2012;220(3):551–557. [DOI] [PubMed] [Google Scholar]
- 17. Deakin B, Suckling J, Barnes TRE, et al. ; BeneMin Study team . The benefit of minocycline on negative symptoms of schizophrenia in patients with recent-onset psychosis (BeneMin): a randomised, double-blind, placebo-controlled trial. Lancet Psychiatry. 2018;5(11):885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inta D, Lang UE, Borgwardt S, Meyer-Lindenberg A, Gass P. Microglia activation and schizophrenia: lessons from the effects of minocycline on postnatal neurogenesis, neuronal survival and synaptic pruning. Schizophr Bull. 2017;43:493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sellgren CM, Gracias J, Watmuff B, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 2019;22(3):374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castilla Guerra L, del Carmen Fernández Moreno M, López Chozas JM, Jiménez Hernández MD. Statins in stroke prevention: what an internist should know. Eur J Intern Med. 2008;19(1):8–14. [DOI] [PubMed] [Google Scholar]
- 21. van der Most PJ, Dolga AM, Nijholt IM, Luiten PG, Eisel UL. Statins: mechanisms of neuroprotection. Prog Neurobiol. 2009;88(1):64–75. [DOI] [PubMed] [Google Scholar]
- 22. Pearson TA, Ballantyne CM, Veltri E, et al. Pooled analyses of effects on C-reactive protein and low density lipoprotein cholesterol in placebo-controlled trials of ezetimibe monotherapy or ezetimibe added to baseline statin therapy. Am J Cardiol. 2009;103(3):369–374. [DOI] [PubMed] [Google Scholar]
- 23. Chan D, Binks S, Nicholas JM, et al. Effect of high-dose simvastatin on cognitive, neuropsychiatric, and health-related quality-of-life measures in secondary progressive multiple sclerosis: secondary analyses from the MS-STAT randomised, placebo-controlled trial. Lancet Neurol. 2017;16(8):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tajik-Esmaeeli S, Moazen-Zadeh E, Abbasi N, et al. Simvastatin adjunct therapy for negative symptoms of schizophrenia: a randomized double-blind placebo-controlled trial. Int Clin Psychopharmacol. 2017;32(2):87–94. [DOI] [PubMed] [Google Scholar]
- 25. Vincenzi B, Stock S, Borba CP, et al. A randomized placebo-controlled pilot study of pravastatin as an adjunctive therapy in schizophrenia patients: effect on inflammation, psychopathology, cognition and lipid metabolism. Schizophr Res. 2014;159(2-3):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaudhry IB, Husain N, Drake R, et al. Add-on clinical effects of simvastatin and ondansetron in patients with schizophrenia stabilized on antipsychotic treatment: pilot study. Ther Adv Psychopharmacol. 2014;4(3):110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. García-Bueno B, Bioque M, Mac-Dowell KS, et al. Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull. 2014;40(2):376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Begemann MJH, Schutte MJL, Slot MIE, et al. Simvastatin augmentation for recent-onset psychotic disorder: A study protocol. BBA Clin. 2015;4:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rappard F, Mueller N. Celecoxib add-on therapy does not have beneficial antipsychotic effects over risperidone alone in schizophrenia. Neuropsychopharmacology. 2004;29(S1):S222. [Google Scholar]
- 30. Weiser M, Zamora D, Levi L, et al. Adjunctive aspirin vs. placebo in patients with schizophrenia: results of two randomized controlled trials [published online ahead of print January 22, 2021]. Schizophr Bull. 2021;47(4):1068–1076. doi: 10.1093/schbul/sbaa198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 32. Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2-3):283–297. [DOI] [PubMed] [Google Scholar]
- 33. Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF). Br J Psychiatry. 1995;166(5):654–659. [DOI] [PubMed] [Google Scholar]
- 34. Gerlach J, Korsgaard S, Clemmesen P, et al. The St. Hans Rating Scale for extrapyramidal syndromes: reliability and validity. Acta Psychiatr Scand. 1993;87(4):244–252. [DOI] [PubMed] [Google Scholar]
- 35. Barnes TRE. A Rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672–676. [DOI] [PubMed] [Google Scholar]
- 36. Addington D, Addington J, Maticka-tyndale E. Assessing depression in schizophrenia: the Calgary depression scale. Br J Psychiatry. 1993;163(S22):39–44. [PubMed] [Google Scholar]
- 37. Grundy SM. Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol. 2005;25(11):2243–2244. [DOI] [PubMed] [Google Scholar]
- 38. Weng TC, Yang YH, Lin SJ, Tai SH. A systematic review and meta-analysis on the therapeutic equivalence of statins. J Clin Pharm Ther. 2010;35(2):139–151. [DOI] [PubMed] [Google Scholar]
- 39. Shen H, Li R, Yan R, et al. Adjunctive therapy with statins in schizophrenia patients: a meta-analysis and implications. Psychiatry Res. 2018;262:84–93. [DOI] [PubMed] [Google Scholar]
- 40. Ioannidis JPA. Why most published research findings are false. PLoS Med. 2005;2(8):e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fracassi A, Marangoni M, Rosso P, et al. Statins and the brain: more than lipid lowering agents? Curr Neuropharmacol. 2017;17(1):59–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Javitt DC. Current and emergent treatments for symptoms and neurocognitive impairment in schizophrenia. Curr Treat Options Psychiatry. 2014;1(2):107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Feintuch S. Statins: The Pros and Cons. 2019. https://www.healthline.com/health/high-cholesterol/statins-pros-cons. Accessed January 19, 2021.
- 44. Thompson PD, Panza G, Zaleski A, Taylor B. Statin-associated side effects. J Am Coll Cardiol. 2016;67(20):2395–2410. [DOI] [PubMed] [Google Scholar]
- 45. Blackburn R, Osborn D, Walters K, Nazareth I, Petersen I. Statin prescribing for prevention of cardiovascular disease amongst people with severe mental illness: cohort study in UK primary care. Schizophr Res. 2018;192:219–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.