Abstract
Background
Matrix metalloproteinase 9 (MMP-9), an extracellular network protease implicated in glutamatergic signaling, may be part of the pathophysiology of schizophrenia spectrum disorders (SSD).
Methods
We performed a systematic review in PubMed/Embase until July 15, 2020, conducting a random-effects meta-analysis of studies comparing MMP-9 blood levels in SSD vs healthy controls (HCs) and psychiatric controls (PCs), calculating between-group differences in standardized mean differences (SMDs) ± 95% confidence intervals (CIs). Meta-regression analyses included sex, age, illness duration, antipsychotic dose, and Positive and Negative Syndrome Scale (PANSS) total/subscales. Subgroup analyses included first-episode patients (FEP) vs non-FEP, each vs HCs and vs PCs, and blood sample type. Study quality was assessed using the Newcastle-Ottawa scale.
Results
Four, five, and two trials were rated as high, fair, and low quality. In 11 studies (n = 1443), 643 patients (age = 36.7 ± 14.1 years, females = 42.9%) were compared with HCs (n = 631), with 4 studies including also 169 PCs. MMP-9 levels were higher in SSD vs HCs (SMD = 0.52, 95%CI = 0.20–0.85, P = .002), but not in PCs vs HCs (n = 132, after removing one implausible outlier [SMD = 0.33, 95%CI = −0.16 to 0.85, P = .082]). MMP-9 differences between SSD and HCs were associated with higher PANSS total (coefficient = 0.02, 95%CI = 0.01–0.02, P < .001), PANSS positive (coefficient = 0.08, 95%CI = 0.02–0.13, P = .006), and PANSS general scores (coefficient = 0.02, 95%CI = 0.01–0.03, P < .001). MMP-9 level differences vs HCs did not vary significantly between FEP (n = 103, SMD = 0.44, 95%CI = 0.15–0.72, P = .71) and non-FEP patients (n = 466, SMD = 0.59, 95%CI = 0.38–0.80; P = .34) (FEP vs non-FEP: P = .39). In four high-quality studies, MMP-9 levels remained significantly higher in SSD vs HCs (SMD = 0.82, 95%CI = 0.03–1.61).
Conclusions
Findings suggest MMP-9 upregulation in SSD, requiring further validation and understanding of related pathways.
Keywords: matrix metalloproteinase 9 (MMP-9), oxidative stress, schizophrenia spectrum disorders (SSD), neuroimmunology, glutamatergic transmission
Introduction
Increasingly available data on the pathogenesis of schizophrenia focus on neuroinflammatory processes.1 In this context, the extracellular network, which consists of a large cluster of macromolecules and enzymes, such as matrix metalloproteinase (MMPs), involved in central nervous system (CNS) signaling but also neuronal morphology, has been investigated.2 Hence, a main hypothesis suggests alterations in the extracellular network in patients with schizophrenia spectrum disorders (SSD) compared with healthy controls (HCs).3 Suggested pathways have included genetic correlates affecting the expression of MMP-9,4 which is the best-characterized MMP in CNS.5 Accordingly, rodent models proposed an association between psychosocial stress and schizophrenia-like behaviors in mice with decreased MMP-9 activity.6 Evidence for other proteases are not available, but methods combining in vivo, in situ, and immunolocalization techniques reported that MMP-9 contributes to hippocampal gelatinolytic activity,7 which highly interacts with postsynaptic N-methyl-d-aspartate (NMDA) receptors.7 Additionally, MMP-9 is expressed in neurons in the cerebellum and the cortex.8,9 Nevertheless, a recent meta-analysis did not report any preponderance of the MMP-9 gene functional polymorphism in patients with schizophrenia.10 Moreover, the effects of MMP-9 genetic polymorphisms on peripheral (blood) MMP-9 levels have been barely investigated in patients with schizophrenia, with only one trial reporting no differences for serum MMP-9 concentrations in patients with different MMP-9 polymorphisms.11
On the other hand, an early trial using a battery of biomarkers reported elevated MMP-9 blood levels in patients with schizophrenia12; authors discussed this finding in light of the role of MMP-9 in modulating synaptic plasticity. Some of the later trials contrasted this pattern, reporting nonsignificant differences,13,14 whereas other studies replicated it.15,16 Additionally, emerging data provided insight into potential moderators or associations of blood MMP-9 levels, such as sex, smoking and metabolic status, cognitive performance, and psychopathological features, as well as antipsychotic treatment effects.11,16 Specifically, age and sex did not seem to affect differences for MMP-9 levels between patients and HCs.11 However, two trials suggested that smokers with schizophrenia had higher MMP-9 levels compared with nonsmokers11,14; this difference has been reported also in HCs and may be related to white blood cell count increase in smokers.17 Current evidence does not allow conclusions on antipsychotic treatment effects on MMP-9 levels, as comparisons have included antipsychotic-free patients vs HCs, but not vs medicated patients.15,16 Nevertheless, MMP-9 levels did not differ between first-episode patients (FEP) and HCs,13 although an earlier trial reported higher MMP-9 levels in high-risk individuals converting to a psychotic disorder compared with HCs.3 Regarding psychopathological correlates, Positive and Negative Syndrome Scale (PANSS) ratings were positively associated with MMP-9 levels in 44 outpatients,11 a finding which was neither replicated in a sample of 40 unmedicated males16 nor 22 clozapine-treated patients with treatment-resistant schizophrenia.18 Lastly, MMP-9 levels were negatively associated with cognitive performance in the combined sample of patients with schizophrenia and HCs, but not in the patients with schizophrenia separately,15 potentially due to lack of statistical power.
Despite the emerging data, the understanding of alteration patterns for MMP-9 and its potential clinical utility remains limited. Thus, the purpose of this study was to systematically review and meta-analyze data on blood measures of MMP-9 in adults with SSD and assess potential moderators of MMP-9 blood levels.
Methods
The study was conducted with the use of MOOSE (Meta-analysis Of Observational Studies in Epidemiology) guidelines for observational studies19 and was registered with PROSPERO (registration number CRD42020189153). Studies of peripheral (serum or plasma) MMP-9 levels in patients with SSD were identified by searching Embase and Medline, using the following search terms: metalloproteinase AND (psych* OR schiz* OR mental). Databases were searched last on July 15, 2020 for publications, without language restriction since data inception. References from identified studies were hand-searched for additional studies.
Inclusion and Exclusion Criteria
Type of Studies. Included were observational studies reporting on MMP-9 blood concentrations in adults with SSD versus HCs or psychiatric controls (PCs), regardless of the setting. PCs were individuals with psychiatric diagnoses other than SSD or at clinical high-risk of psychosis. Case reports were excluded.
Types of Participants. Adult patients of both sexes with SSD were included. There were no restrictions with regard to treatment setting, illness duration, and dosage or duration of antipsychotic treatment. Antipsychotic-naïve, antipsychotic-free as well as patients receiving antipsychotic treatment were considered.
Comparator. HCs and PCs.
Types of Exposure. Diagnosis of SSD regardless of the assessment method in patients.
Outcomes. The primary outcome was defined as standardized mean difference (SMD) for blood concentrations of MMP-9 between patients with SSD vs HCs or PCs. Meta-regression analyses assessing the effects of age, percentage males, percentage smokers, antipsychotic daily dose (in chlorpromazine [CPZ] equivalents), illness duration, and psychopathological rating scale scores were performed.
Selection of eligible studies was independently performed by two authors (G.S. and R.d.F.). In case of doubt, papers were discussed and consensus was reached. As consensus was reached in all cases, no additional co-author was involved.
Data Extraction
Two authors (M.N. and R.d.F.) independently extracted data regarding sample sizes, demographic characteristics, psychopathological ratings, daily antipsychotic dosages, illness duration, types of MMP-9 blood samples, and blood MMP-9 levels (mean and SD). Before data entry, values were converted to the same unit for each parameter and weighted means for covariates were computed based on means of subgroups. When data were not provided, authors were contacted. When data for means and variance measures were provided only in figures, the WebPlotDigitlizer (version 4.2 for Windows) was used to extract data.
Quality of Studies
The modified version of the Newcastle-Ottawa scale for cross-sectional studies was used for quality assessment20; we removed the item “representativeness of the exposed cohort,” that we judged to be related to applicability, and added ascertainment of diagnosis of SSD as described elsewhere.21
Statistical Analysis
We used a random-effects model for our primary outcome, given the potential heterogeneity related to patient populations, analytical methods, and the inherently large variability of the laboratory variables. Results were summarized using SMD and 95% confidence intervals (CI) and were presented in forest plots. The heterogeneity variance parameter (τ2) was calculated using the DerSimonian-Laird estimator.22 When more than one cohort was reported in one study, they were considered separately. We also calculated the I-square (I2) statistic as a measure of the proportion of variability that can be attributed to heterogeneity.23 Thereafter, the effects of demographic and clinical parameters were assessed in a meta-regression analysis.24 Subgroup analyses included FEP or non-FEP vs HCs and PCs and type of sample (plasma vs serum); the latter was performed, as the measurement of MMP-9 levels may be very sensitive to pre-analytic variables, with high MMP-9 levels being contained in white blood cells and platelets that are released during blood clotting.17 Further, a sensitivity analysis including high-quality studies was conducted. Last, we examined the potential of publication bias using funnel plots and Egger’s test.25 All analyses were performed using the meta package in R.26
Results
The electronic database search yielded 1959 articles from Medline, 1026 from Embase, and one from the full-text reviewed articles’ reference lists. After removing duplicates, 1241 unique articles remained. After exclusion of 1192 articles based on title and abstract review, 49 articles were full-text screened, leading to rejection of 38 papers due to reviews (n = 18), lack of MMP-9 blood levels (n = 12), samples with diagnoses other than SSD (n = 5), posters with overlapping data (n = 2), and failure to provide data for mean MMP-9 levels per study group (n = 1). Ultimately, 11 studies fulfilled all inclusion criteria and were used for data extraction (supplementary figure 1). One of these 11 studies separately provided data for seven pairs of cohorts (patients with schizophrenia vs HCs),15 of which six were included individually in our analysis, except for one of the cohorts of treatment-resistant schizophrenia patients, as this cohort had been described in a previous publication,18 that was also included in our meta-analysis.
Study and Patient Characteristics
We meta-analyzed 11 studies with 1443 individuals (mean age = 35.7 ± 14.3 years, 34.5% females) including 643 patients with SSD (age = 36.7 ± 14.1 years, females = 42.9%, mean illness duration = 18.4 ± 10.1 years, mean daily CPZ equivalent antipsychotic dose in the SSD group: 209.5 ± 552.2 mg/day) who were compared with 631 HCs, with 4 studies containing also a third arm of 169 PCs (major depressive disorder: n = 78, “mood disorders”: n = 21, clinical high risk for psychosis: n = 70; table 1). SSD, PC, and HC groups were matched for age and sex in 15 comparisons, but only five cohorts were also matched for smoking status (table 1).
Table 1.
Characteristics of Included Studies (in Chronological Order)
| Groups Matched For: | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, y | Total n | Group | n | Age (SD), y | %♀ | Age | Sex | Smoking | Duration of Illness (SD), y | CPZE Dosage (SD) | PANSS Total (SD) | PANSS Positive (SD) | PANSS Negative (SD) | PANSS General (SD) | MMP-9 Levels (SD), ng/ml | Quality |
| Schennach- Wolff, 2012 | 153 | SSD | 37 | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | 143.0 (101.0) | Poor |
| Healthy controls | 38 | NP | NP | NA | NA | NA | NA | NA | NA | 54.0 (48.0) | ||||||
| MDD | 78 | NP | NP | NP | NP | NP | NP | NP | NP | 135.0 (141.0)a | ||||||
| Schwarz, 2012 | 130 | Paranoid SZ | 71 | 31.0 (10.0) | 40.8 | √ | √ | √ | NP | 0.0 (0.0) | NP | 23.0 (6.0) | 23.0 (8.0) | NP | 19.84 (15.64) | High |
| Healthy controls | 59 | 30.0 (8.0) | 47.4 | NA | NA | NA | NA | NA | NA | 14.0 (3.75) | ||||||
| Yamamori, 2013 | 44 | TRS | 22 | 38.1 (13.2) | 45.4 | √ | √ | √ | 17.2 (11.1) | 897.2 (260.0) | 101.4 (NP) | 23.0 (4.6) | 25.5 (5.5) | 52.9 (9.6) | 43.0 (22.4) | High |
| Healthy controls | 22 | 38.1 (12.9) | 45.4 | NA | NA | NA | NA | NA | NA | 29.8 (17.4) | ||||||
| Niitsu, 2014 | 115 | 44 residual SZ 19 paranoid SZ |
63 | 35.9 (8.2) | 58.7 | √ | √ | √ | 9.1 (7.3) | 323.9 (184.2) | 45.0b | NP | NP | NP | 700.9 (330.8) | High |
| Healthy controls | 52 | 34.9 (7.3) | 51.9 | NA | NA | NA | NA | NA | NA | 672.5 (378.4) | ||||||
| Devanarayanan, 2015 | 80 | Schizophrenia | 40 | 28.3 (6.7) | 0.0 | √ | √ | No | 3.6 (3.7) | 0.0 (0.0) | 79.8 (12.9) | 23.0 (4.7) | 21.3 (6.5) | 35.5 (5.7) | 38.4 (10.3) | Fair |
| Healthy controls | 40 | 26.6 (4.5) | 0.0 | NA | NA | NA | NA | NA | NA | 30.5 (8.7) | ||||||
| Shibasaki, 2016 | 74 | 3 SZ 5 Catatonic SZ 4 Paranoid 1Undifferentiated |
13 | 46.9 (15.1) | 53.8 | √ | √ | No | 15.5 (12.1) | 1325.2 (813.5)c | 96.0b,c | NP | NP | NP | 657.1 (272.9)c,d | Fair |
| Healthy controls | 40 | 54.2 (13.9) | 65.0 | NA | NA | NA | NA | NA | NA | 478.6 (217.1)c,d | ||||||
| Mood disorders | 21 | 58.5 (15.4) | 71.4 | 7.4 (8.4) | NA | NA | NA | NA | NA | 578.9 (260.6)a,c,d | ||||||
| Ali, 2017 | 94 | Schizophrenia | 44 | 25.1 (4.0) | 22.7 | √ | √ | √ | 2.0 (1.0– 3.0)e | NP | 152.1 (20.6) | 33.0 (4.3) | 28.3 (5.9) | 90.9 (13.3) | 89.47 (62.2) | High |
| Healthy controls | 50 | 26.1 (3.9) | 36.0 | NA | NA | NA | NA | NA | NA | 3.5 (2.1) | ||||||
| Jeffries, 2018 | 107 | FEP | 32 | 19.2 (3.7) | 30.3 | √ | √ | No | NP | NP | NP | NP | NP | NP | 179.0 (263.01) | Fair |
| Healthy controls | 35 | 20.0 (4.5) | 34.0 | NA | NA | NA | NA | NA | NA | 115.4 (69.03) | ||||||
| Clinical high-risk | 40 | 19.5 (4.6) | 37.5 | NA | NA | NA | NA | NA | NA | 107.56 (56.4) | ||||||
| Arabska, 2019 | 96 | Paranoid SZ | 64 | 49.0 (8.2) | 54.7 | √ | √ | No | NPf | 668.1 (315.8) | NP | NP | NP | NP | 456.8 (278.4) | Fair |
| Healthy controls | 32 | 51.0 (8.9) | 46.9 | NA | NA | NA | NA | NA | NA | 341.5 (162.4) | ||||||
| He, 2019 | 89 | FEP | 30 | 19.6 (3.7) | 30.0 | √ | √ | No | NP | NP | NP | NP | NP | NP | 11.5 (1.0) | Poor |
| Healthy controls | 29 | 20.4 (1.1) | 41.4 | NA | NA | NA | NA | NA | NA | 13.7 (1.3) | ||||||
| Clinical high-risk | 30 | 19.8 (4.2) | 30.0 | NA | NA | NA | NA | NA | NA | 15.5 (1.2) | ||||||
| Kudo, 2020 (Osaka-1) | 67 | SZ | 32 | 57.3 (11.9) | 65.6 | √ | √ | No | 20.5 (15.1) | 608.8 (505.4) | 72.7 (25.5) | 18.4 (7.3) | 17.5 (7.4) | 36.8 (12.7) | 38.4 (20.0) | Fair |
| Healthy controls | 35 | 54.5 (5.9) | 57.1 | NA | NA | NA | NA | NA | NA | 22.2 (10.1) | ||||||
| Kudo, 2020 (Osaka-2) | 79 | SZ | 39 | 38.0 (12.3) | 48.7 | √ | √ | No | 11.4 (9.9) | 562.6 (398.3) | 79.0 (23.1) | 17.1 (5.6) | 19.6 (5.6) | 42.2 (13.1) | 27.6 (18.8) | |
| Healthy controls | 40 | 37.8 (12.2) | 50.0 | NA | NA | NA | NA | NA | NA | 19.3 (7.7) | ||||||
| Kudo, 2020 (Osaka-3) | 80 | SZ | 40 | 41.4 (11.8) | 50.0 | √ | √ | No | NP | NP | NP | NP | NP | NP | 50.9 (53.7) | |
| Healthy controls | 40 | 42.3 (12.2) | 50.0 | NA | NA | NA | NA | NA | NA | 19.1 (9.3) | ||||||
| Kudo, 2020 (Drug-free) | 77 | SZ | 37 | 35.5 (13.1) | 37.8 | √ | √ | √ | 9.5 (7.4) | 0.0 (0.0) | 79.9 (25.8) | 20.1 (6.5) | 18.4 (6.8) | 41.4 (14.6) | 31.3 (15.8) | |
| Healthy controls | 40 | 35.5 (12.3) | 42.5 | NA | NA | NA | NA | NA | NA | 21.9 (10.5) | ||||||
| Kudo, 2020 (Tokushima) | 79 | SZ | 40 | 55.2 (7.2) | 50.0 | √ | √ | No | NP | NP | NP | NP | NP | NP | 43.5 (29.6) | |
| Healthy controls | 39 | 52.2 (7.2) | 51.3 | NA | NA | NA | NA | NA | NA | 44.5 (23.2) | ||||||
| Kudo, 2020 (Chiba) | 79 | SZ | 39 | 31.8 (5.7) | 50.0 | √ | √ | No | 6.9 (4.2) | NP | NP | NP | NP | NP | 37.7 (24.8) | |
| Healthy controls | 40 | 32.4 (6.2) | 50.0 | NA | NA | NA | NA | NA | NA | 29.7 (19.5) | ||||||
| Total | 1443 | SSD | 643 | 36.7 (14.1) | 42.9 | Yes (for all 3): 5 No/NP: 11 |
10.6 (10.2) | 209.5 (552.2) | 95.0 (38.1) | 22.8 (7.5) | 22.1 (7.6) | 51.1 (24.0) | 171.3 (277.6) | High:4 Fair: 5 Poor: 2 |
||
| Healthy controls | 631 | 36.7 (14.0) | 44.7 | NA | NA | NA | NA | NA | NA | 127.6 (243.6) | ||||||
| PC | 169 | 28.6 (18.4) | 42.8 | 7.4 (8.4) | NA | NA | NA | NA | NA | 162.4 (211.7) | ||||||
Note: ♀, females; √, yes; CPZE, chlorpromazine equivalents; FEP, first-episode patients; MDD, major depressive disorder; MMP-9, matrix metalloproteinase 9; NA, not applicable; NP, not provided; PANSS, Positive and Negative Syndrome Scale; PC, psychiatric controls; SSD, schizophrenia spectrum disorders; SZ, schizophrenia; TRS, treatment-resistant schizophrenia. Quality assessments were based on the Newcastle-Ottawa scale for cross-sectional studies.
aData were not included in the analysis as we focused on patients with schizophrenia spectrum disorders, but are provided here for reasons of completeness.
bAssessments included the Brief Psychiatric Reporting Scales (BPRS); BPRS values were converted to PANSS using equivalents published elsewhere.27
cAuthors provided data before and after electroconvulsive therapy (ECT). Here we provide pre-ECT data.
dData extracted from figures using a WebPlotDigitlizer (version 4.2 for Windows).
eMedian values.
fAuthors provided treatment duration (15.2 ± 8.7 years) instead of illness duration.
Primary Outcome
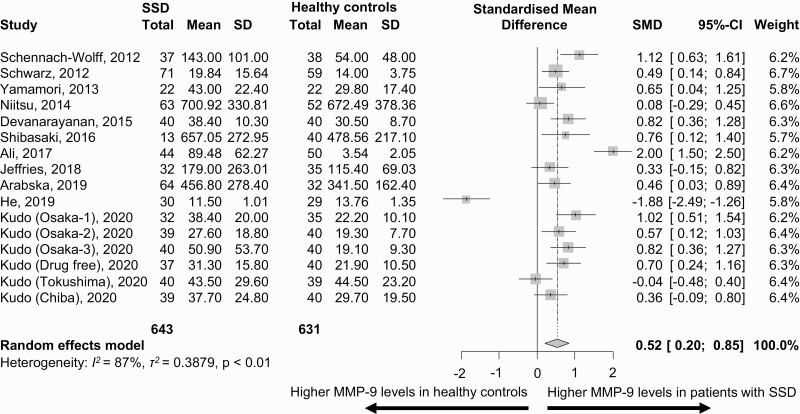
In 11 studies (n = 1443) including 643 patients with SSD and 631 HCs, MMP-9 blood levels were significantly higher in patients compared with HCs (SMD = 0.52, 95%CI = 0.20–0.85, P = .002) (figure 1). Heterogeneity was large (I2 = 87.3%, τ2 = 0.39).
Fig. 1.
Matrix metalloproteinase-9 (MMP-9) blood levels (ng/ml) in patients with schizophrenia spectrum disorders (SSD) vs healthy controls.
Meta-Regression Analyses
We did not observe effects for illness duration (estimated coefficient 0.02, 95%CI = −0.02, 0.07, P = .29), PANSS negative scores (estimated coefficient 0.08, 95%CI: −0.02, 0.17, P = .14), CPZ equivalents (estimated coefficient 0.01, 95%CI = −0.01, 0.01, P = .72), age (estimated coefficient 0.01, 95%CI = −0.02, 0.04, P = .37), sex (estimated coefficient −0.21, 95%CI = −2.25, 1.83, P = .84). Conversely, the higher MMP-9 levels in SSD vs HCs was significantly moderated by higher PANSS positive (estimated coefficient 0.08, 95%CI = 0.02, 0.13, P = .006), PANSS general (estimated coefficient 0.02, 95%CI = 0.01, 0.03, P < .001), and PANSS total scores (estimated coefficient 0.02, 95%CI = 0.01, 0.02, P < .001) (table 2).
Table 2.
Meta-Regression Analyses
| Coefficient | Lower 95%CI | Upper 95%CI | P Value | |
|---|---|---|---|---|
| Sex | −0.21 | −2.25 | 1.83 | .84 |
| Age | 0.01 | −0.02 | 0.04 | .37 |
| CPZE | 0.01 | −0.01 | 0.01 | .72 |
| Illness duration | 0.02 | −0.02 | 0.07 | .29 |
| PANSS positive | 0.08 | 0.02 | 0.13 | .006 |
| PANSS negative | 0.07 | −0.02 | 0.17 | .14 |
| PANSS general | 0.02 | 0.01 | 0.03 | <.001 |
| PANSS total | 0.02 | 0.01 | 0.02 | <.001 |
Note: CI, confidence interval; CPZE, chlorpromazine equivalents; PANSS, Positive and Negative Syndrome Scale.
Subgroup Analyses
In our subgroup analysis, we did not include two trials with implausible results.11,13 In one study, values provided as SDs were unusually small and more likely to reflect SEs, resulting in an implausible outlier SMD of 1.9; authors were contacted for clarification, but we received no response.13 Despite being rated of high quality, another study reported very large intergroup differences without any overlap, resulting in an implausible SMD of 2.011; likewise, authors were contacted, but we did not receive any response.
MMP-9 Blood Levels FEP and Non-FEP vs HCs and PCs
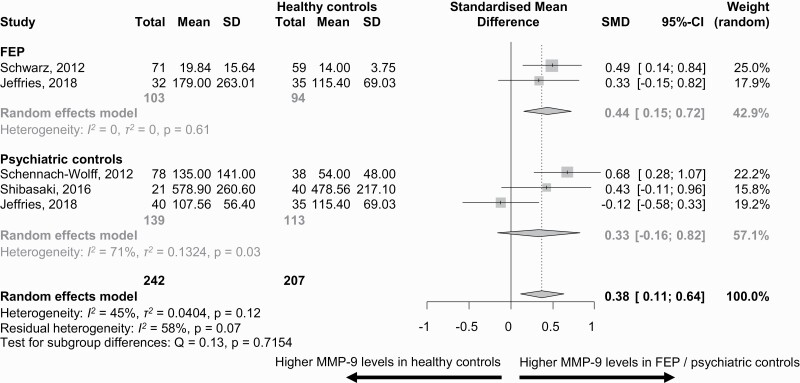
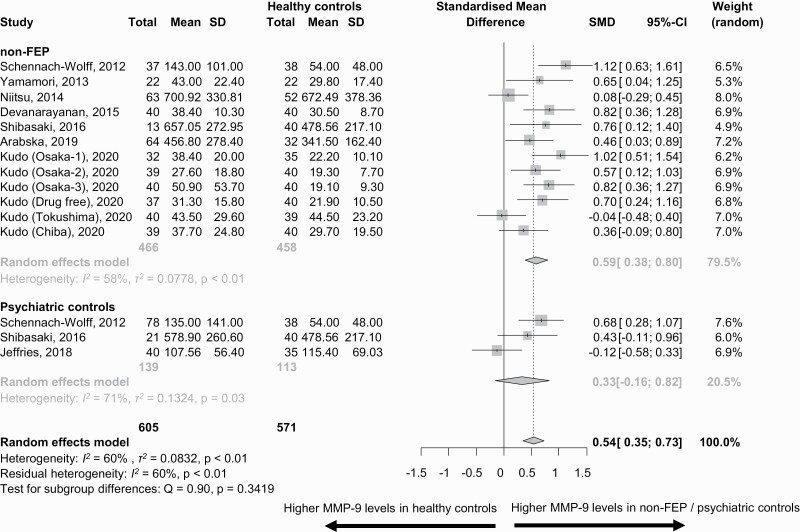
MMP-9 levels were higher in FEP vs HCs (k = 2 studies, n = 103, SMD = 0.44, 95%CI = 0.15, 0.72, between groups, P = .71), and in non-FEP vs. HCs (k = 12, n = 466, SMD = 0.59, 95%CI = 0.38, 0.80, P = .34) (FEP vs non-FEP: P = .39), but not in PCs vs HCs (n = 139, SMD = 0.33, 95%CI = −0.16, 0.82, P = .71) (figures 2 and 3).
Fig. 2.
Matrix metalloproteinase-9 (MMP-9) blood levels (ng/ml) in patients with first-episode patients (FEP) and psychiatric controls vs healthy controls.
Fig. 3.
Matrix metalloproteinase-9 (MMP-9) blood levels (ng/ml) in patients who were not first-episode patients (non-FEP) and psychiatric controls vs healthy controls.
MMP-9 Blood Levels in Studies Using Serum vs Plasma Samples
In nine trials, plasma levels of MMP-9 were higher in patients with SSD compared with HCs (n = 318, SMD = 0.60, 95%CI = −0.07, 0.83), with moderate heterogeneity (I2 = 56.5%, τ2 = 0.08). In five trials using serum samples, higher MMP-9 levels in patients with SSD vs HCs were observed (n = 251, SMD = 0.48, 95%CI = 0.22, 0.74). However, no differences between studies using vs serum samples were observed (P = .50). Heterogeneity was moderate (I2 = 45.7%, τ2 = 0.04) (table 3).
Table 3.
Subgroup and Sensitivity Analyses
| k | N | SMD (95% CI) | P Value | I 2 (%) | |
|---|---|---|---|---|---|
| Fixed effects model | 16 | 1274 | 0.53 (0.41, 0.65) | <.001 | 87.3 |
| Exclusion of two trialsa | 14 | 1121 | 0.56 (0.38, 0.74) | <.001 | 51.7 |
| Studies using plasma samplesa | 9 | 647 | 0.60 (0.36, 0.85) | <.001 | 56.5 |
| Studies using serum samplesa | 5 | 474 | 0.48 (0.22, 0.74) | <.001 | 45.7 |
| High-quality studies | 4 | 392 | 0.82 (0.03, 1.61) | .04 | 92.2 |
| High- and fair-quality studies | 14 | 1140 | 0.63 (0.38, 0.88) | <.001 | 75.8 |
Sensitivity Analyses
In a sensitivity analysis including the four trials that were rated as high quality (which were also the trials that controlled for smoking status), MMP-9 blood levels were significantly higher in patients with SSD than HCs (n = 191, SMD = 0.82, 95%CI = 0.03, 1.61, P = .042). Heterogeneity remained large (I2 = 92.2%, τ2 = 0.59) (table 3).
Publication Bias
Neither the visual inspection of funnel plots (supplementary figure 2) nor the Egger’s test results (P = .96) revealed signs of publication bias.
Quality Assessment
Lack of critical information included assessment methods for diagnosis of SSD, matching processes for smoking status, and sample size estimation. Of studies included for the primary outcome, four were rated as high, five as fair, and two as poor quality (supplementary table 1).
Discussion
As the theoretical framework on neuroinflammatory processes underpinning SSD is gaining attention, the search for specific alteration patterns of immune system proteins in patients is ongoing. This meta-analysis provides evidence of higher MMP-9 levels in patients with SSD vs HCs. When including only high-quality studies, the same pattern of findings remained. These results may be considered in light of the role of MMP-9 in the interplay of hippocampal gelatinolytic and postsynaptic NMDA activity,7 although the relationship between peripheral and central MMP-9 levels remains unclear. However, if we assume that peripheral levels reflect MMP-9 levels in the CNS, elevated MMP-9 levels may be linked to NMDA hypofunction.28 In the context of deficits regarding NMDA signaling, alterations of MMP-9 may be a proxy for redox dysregulation.1,8,11,29 Specifically, rodent models highlighted the key role of MMP-9 in the loop linking oxidative stress and neuroinflammatory processes29; this model displayed that early inhibition of MMP-9 activation allowed normalization of neuronal development. In a case-control study of antipsychotic-free patients with schizophrenia, MMP-9 serum levels were negatively correlated with total antioxidant status.16 Therefore, excess MMP-9 levels in patients with schizophrenia may represent an overpotentiation of the loop linking oxidative stress and neuroinflammation. In other words, the increased release of MMP-9 may be linked to a cytokine secretion cascade that may perpetuate the oxidative stress process.29 Alternative peripheral measures of antioxidant defense activity, such as glutathione levels, have previously reported deficits in patients with schizophrenia.30 Therefore, the elevated MMP-9 levels may reflect altered patterns of antioxidant activity.
Of specific interest are the psychopathological correlates of the elevated MMP-9 levels. In the meta-regression analysis, higher PANSS total, and general and positive subscales were associated with higher MMP-9 levels. Administering the PANSS does not provide specific assessments of cognition.31 However, a trial including cognitive scales reported a negative correlation between MMP-9 levels and general cognitive and memory performance in the pooled sample of patients with schizophrenia and HCs.15 It could also be hypothesized that the larger differences for MMP-9 levels between HCs and SSD subjects with higher PANSS general ratings may have masked higher cognitive deficits.32 Specifically, a study investigating seven SSD cohorts reported negative associations between MMP-9 levels and several cognitive scales ratings,15 i.e. subjects with higher MMP-9 levels had lower scores in several intelligence quotient, memory, attention, and concentration assessments. Likewise, one study reported a negative association between MMP-9 serum levels and fluency test ratings.33 In alignment with these findings, a more recent study reported higher MMP-9 levels in patients with lower fluency and language test ratings.34 Therefore, MMP-9 alterations may possibly identify an endophenotype in SSD characterized by cognitive dysfunction. However, future studies are needed to test this hypothesis further. The role of cognition ties in well with the theoretical framework linking MMP-9 alterations, NMDA signaling, and cognitive symptoms.35
Additionally, in samples with higher PANSS positive scales ratings, larger elevations in MMP-9 levels in SSD vs HCs were observed. This finding suggests that MMP-9 upregulation may be related to positive symptoms, although the included data reflect cross-sectional assessments. To interpret this finding, we may consider previous evidence linking positive symptoms with deficits in glutamatergic neurotransmission.36 Although the direction of the association is unclear, it needs to be examined whether MMP-9 central and peripheral upregulation is involved in the pathophysiological mechanisms linking positive symptoms and altered neurotransmitter plasticity. An interplay of positive and cognitive symptoms in the grounds of hippocampal hyperactivity can be also proposed.37 Volumetric research has also connected positive symptoms with alterations in hippocampal subfields.38 The hippocampus is richly innervated with neurons, which are structurally and physiologically regulated by the MMP-9.39
Our subgroup analyses yielded numerically smaller differences in FEP vs HCs than non-FEP vs HCs. Likewise, we did not find a significant effect for illness duration in our meta-regression, yet, this may have been related to the inclusion of mainly patients with chronic illness and deserves further research. When assessing separately trials using plasma vs serum samples, MMP-9 differences were slightly higher in studies using plasma samples. Assessment of MMP-9 may be very sensitive to pre-analytic variables, as high MMP-9 levels are contained in white blood cells and platelets released during blood clotting.17 The use of different types of samples may also have contributed to the large heterogeneity of our findings and, ideally, future studies would always assess and report MMP-9 levels in serum and plasma to allow for full comparability across studies.
Results of this study need to be interpreted within its limitations. First, the heterogeneity of the included observational trials poses the most severe limitation of our findings and justified our decision to perform random-effects model. A major driver of the heterogeneity may have been the sample type; therefore, meta-analytically combining studies using serum and plasma samples for MMP-9 may not be recommended, although future meta-analyses of peripheral markers may need to make methodological choices based on the individual properties of the target marker and the available literature on comparability between different sample types. For example, a previous meta-analysis of peripheral brain-derived neurotrophic factor levels in major depression reported different patterns for studies using serum and plasma samples,40 highlighting at least the need to compare results from different sample sources. Second, our findings refer to a potential role of confounders that were insufficiently assessed in the available studies. For example, only a small number of study groups were matched for smoking status; nevertheless, our sensitivity analysis of high-quality studies, including only samples matching for smoking status, replicated elevated MMP-9 levels in patients with SSD compared with HCs. Third, including more patients with recent-onset illness may have provided a better account of MMP-9 peripheral alterations as function of illness course. Fourth, information on antipsychotic treatment duration could have provided more sophisticated insight into treatment effects. Sixth, our power to assess the specificity of MMP-9 alterations for SSD was limited by the few studies and small samples with PCs. Nevertheless, as PCs received psychopharmacological treatment, this fact reduces the likelihood that reported differences for MMP-9 are driven by treatment effects. However, future studies should control for medication differences. Seventh, since none of the studies assessed cognition, we could not investigate a potential interaction between elevated MMP-9 levels and cognitive dysfunction. Finally, we were unable to test the hypothesis that increased MMP-9 levels in SSD provide a link to increased oxidative stress and inflammation, as such data were not concurrently assessed in the available studies.
In summary, results of this first meta-analysis of MMP-9 blood levels in SSD suggest a potential role for MMP-9 upregulation in pathophysiological processes underlying schizophrenia. Patients with SSD had higher MMP-9 levels compared with HCs and higher MMP-9 levels were associated with higher PANSS total and general and positive subscale ratings. Prospective studies focusing on the dynamic course of the interaction between MMP-9 alterations and psychopathological features are required to shed additional light on the temporal fashion of this interplay in patients with SSD. Future research on MMP-9 as potential biomarker also needs to put more emphasis on unraveling the role of relevant clinical and biological factors, such as cognitive performance correlates, various psychopathology components within SSD, duration of antipsychotic-exposure, measures of oxidative stress and inflammation, and analytical method variables. A better understanding of these factors and of biological underpinnings of MMP-9 dysregulation could contribute to improving knowledge about the neuroimmunological aspects of schizophrenia.
Supplementary Material
Acknowledgments
Authors are particularly grateful to Dr. Diana O. Perkins, Department of Psychiatry, University of North Carolina, Chapel Hill, NC, USA, Dr. Ryota Hashimoto, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan and Peter Zill, Psychiatric Genetics and Neurochemistry, Psychiatric Clinic, Ludwig-Maximilians-University, Munich, Germany for providing valuable information about their studies.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interest
Drs. G.S., R.d.F., and Miss M.N. have nothing to disclose.
Dr. C.U.C. has been a consultant and/or advisor to or has received honoraria from Alkermes, Allergan, Angelini, Boehringer-Ingelheim, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka. He served on a Data Safety Monitoring Board for Boehringer-Ingelheim, Lundbeck, Rovi, Supernus, and Teva. He received royalties from UpToDate and grant support from Janssen and Takeda. He is also a shareholder of LB Pharma.
Dr. S.L. has received personal fees from Angelini, Böhringer Ingelheim, Gedeon, Richter UK, Janssen, Johnson & Johnson, Lundbeck, LTS Lohmann, Merck, Otsuka, Recordati, Sanofi-Aventis, Sandoz, Sunovion, and TEVA.
Dr. J.M.K. has been a consultant for or received honoraria from Alkermes, Dainippon Sumitomo, Eli Lilly, Forum, Allergan, Genentech, H. Lundbeck, Intracellular Therapies, Janssen Pharmaceutica, Johnson and Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Otsuka, Pierre Fabre, Reviva, Roche, Sunovion, Takeda, and Teva. He has received grant support from Otsuka, Lundbeck, and Janssen. He has participated in advisory boards for Alkermes, Dainippon Sumitomo, Intracellular Therapies, Lundbeck, Neurocrine, Otsuka, Pierre Fabre, Takeda, and Teva. He is a Shareholder in Vanguard Research Group and LB Pharmaceuticals, Inc.
Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. No informed consent was necessary for this type of research.
Authorship Contributions
Participated in research design: G.S., R.d.F., M.N., S.L., C.U.C., and J.M.K.
Performed data analysis: G.S. and R.d.F.
Wrote or contributed to the writing and critical revisions of the manuscript: G.S., R.d.F., M.N., S.L., C.U.C., and J.M.K.
References
- 1. Bitanihirwe BKY, Woo TW. A conceptualized model linking matrix metalloproteinase-9 to schizophrenia pathogenesis. Schizophr Res. 2020;218:28–35. [DOI] [PubMed] [Google Scholar]
- 2. Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13(11):743–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeffries CD, Perkins DO, Fournier M, et al. Networks of blood proteins in the neuroimmunology of schizophrenia. Transl Psychiatry. 2018;8(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rybakowski JK, Skibinska M, Kapelski P, Kaczmarek L, Hauser J. Functional polymorphism of the matrix metalloproteinase-9 (MMP-9) gene in schizophrenia. Schizophr Res. 2009;109(1–3):90–93. [DOI] [PubMed] [Google Scholar]
- 5. Lepeta K, Kaczmarek L. Matrix metalloproteinase-9 as a novel player in synaptic plasticity and schizophrenia. Schizophr Bull. 2015;41(5):1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vafadari B, Mitra S, Stefaniuk M, Kaczmarek L. Psychosocial stress induces schizophrenia-like behavior in mice with reduced MMP-9 activity. Front Behav Neurosci. 2019;13:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilczynski GM, Konopacki FA, Wilczek E, et al. Important role of matrix metalloproteinase 9 in epileptogenesis. J Cell Biol. 2008;180(5):1021–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dwir D, Giangreco B, Xin L, et al. MMP9/RAGE pathway overactivation mediates redox dysregulation and neuroinflammation, leading to inhibitory/excitatory imbalance: a reverse translation study in schizophrenia patients. Mol Psychiatry. 2020;25(11):2889–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yong VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci. 2005;6(12):931–944. [DOI] [PubMed] [Google Scholar]
- 10. Xia QR, Zhang C, Liang J, Xu YY. The association of functional polymorphism of matrix metalloproteinase-9 gene (rs3918242) with schizophrenia: a meta-analysis. Int J Psychiatry Clin Pract. 2019;23(3):207–214. [DOI] [PubMed] [Google Scholar]
- 11. Ali FT, Abd El-Azeem EM, Hamed MA, Ali MAM, Abd Al-Kader NM, Hassan EA. Redox dysregulation, immuno-inflammatory alterations and genetic variants of BDNF and MMP-9 in schizophrenia: pathophysiological and phenotypic implications. Schizophr Res. 2017;188:98–109. [DOI] [PubMed] [Google Scholar]
- 12. Domenici E, Willé DR, Tozzi F, et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS One. 2010;5(2):e9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He Y, Yuan L, Li Z, et al. Plasma protein levels of brain-derived neurotrophic factor pathways and their association with cognitive performance in patients with clinical high risk for psychosis and first episode psychosis. Schizophr Res. 2019;206:460–461. [DOI] [PubMed] [Google Scholar]
- 14. Arabska J, Margulska A, Strzelecki D, Wysokiński A. Does metabolic status affect serum levels of BDNF and MMP-9 in patients with schizophrenia? Nord J Psychiatry. 2019;73(8):515–521. [DOI] [PubMed] [Google Scholar]
- 15. Kudo N, Yamamori H, Ishima T, et al. Plasma levels of matrix metalloproteinase-9 (MMP-9) are associated with cognitive performance in patients with schizophrenia. Neuropsychopharmacol Rep. 2020;40(2):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Devanarayanan S, Nandeesha H, Kattimani S, Sarkar S. Relationship between matrix metalloproteinase-9 and oxidative stress in drug-free male schizophrenia: a case control study. Clin Chem Lab Med. 2016;54(3):447–452. [DOI] [PubMed] [Google Scholar]
- 17. Snitker S, Xie K, Ryan KA, et al. Correlation of circulating MMP-9 with white blood cell count in humans: effect of smoking. PLoS One. 2013;8(6):e66277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamamori H, Hashimoto R, Ishima T, et al. Plasma levels of mature brain-derived neurotrophic factor (BDNF) and matrix metalloproteinase-9 (MMP-9) in treatment-resistant schizophrenia treated with clozapine. Neurosci Lett. 2013;556:37–41. [DOI] [PubMed] [Google Scholar]
- 19. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 20. Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schoretsanitis G, Nikolakopoulou A, Guinart D, Correll CU, Kane JM. Iron homeostasis alterations and risk for akathisia in patients treated with antipsychotics: a systematic review and meta-analysis of cross-sectional studies. Eur Neuropsychopharmacol. 2020;35:1–11. [DOI] [PubMed] [Google Scholar]
- 22. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Meta-regression. In: Borenstein M, Hedges LV, Higgins JP, Rothstein HR, eds. Introduction to Meta-Analysis. West Sussex, UK: John Wiley & Sons; 2009: 187–203. [Google Scholar]
- 25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwarzer G, Carpenter JR, Rücker G.. Meta-Analysis with R. Heidelberg: Springer; 2015. [Google Scholar]
- 27. Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology 2006;31:2318–2325. [DOI] [PubMed] [Google Scholar]
- 28. Jackson MF. Epigenetic mechanism links NMDA receptor hypofunction and cognitive deficits in schizophrenia to D2 receptors. Biol Psychiatry. 2020;87(8):692–694. [DOI] [PubMed] [Google Scholar]
- 29. Perkins DO, Jeffries CD, Do KQ. Potential roles of redox dysregulation in the development of schizophrenia. Biol Psychiatry. 2020;88(4):326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raffa M, Barhoumi S, Atig F, Fendri C, Kerkeni A, Mechri A. Reduced antioxidant defense systems in schizophrenia and bipolar I disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):371–375. [DOI] [PubMed] [Google Scholar]
- 31. Nielsen RE, Lindström E, Telléus GK, Levander S. Is the PANSS cognitive scale measuring cognition? Nord J Psychiatry. 2014;68(8):573–578. [DOI] [PubMed] [Google Scholar]
- 32. Ehmann TS, Khanbhai I, Macewan GW, et al. Neuropsychological correlates of the PANSS Cognitive Factor. Psychopathology. 2004;37(5):253–258. [DOI] [PubMed] [Google Scholar]
- 33. Niitsu T, Ishima T, Yoshida T, et al. A positive correlation between serum levels of mature brain-derived neurotrophic factor and negative symptoms in schizophrenia. Psychiatry Res. 2014;215(2):268–273. [DOI] [PubMed] [Google Scholar]
- 34. Keshri N, Nandeesha H, Rajappa M, Menon V. Matrix metalloproteinase-9 increases the risk of cognitive impairment in schizophrenia. Nord J Psychiatry. 2020:1–5. [DOI] [PubMed] [Google Scholar]
- 35. Roza C, Campos-Sandoval JA, Gómez-García MC, Peñalver A, Márquez J. Lysophosphatidic acid and glutamatergic transmission. Front Mol Neurosci. 2019;12:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tamminga C. Glutamatergic aspects of schizophrenia. Br J Psychiatry Supplement. 1999;37:12–15. [PubMed] [Google Scholar]
- 37. Zierhut K, Bogerts B, Schott B, et al. The role of hippocampus dysfunction in deficient memory encoding and positive symptoms in schizophrenia. Psychiatry Res. 2010;183(3):187–194. [DOI] [PubMed] [Google Scholar]
- 38. Kühn S, Musso F, Mobascher A, Warbrick T, Winterer G, Gallinat J. Hippocampal subfields predict positive symptoms in schizophrenia: first evidence from brain morphometry. Transl Psychiatry. 2012;2:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci U S A. 2008;105(49):19520–19525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bocchio-Chiavetto L, Bagnardi V, Zanardini R, et al. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry. 2010;11(6):763–773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.