Abstract
Macrolide resistance was found in 39.5% of 3626 nonduplicate Streptococcus pneumoniae isolates from adult ambulatory and inpatient settings at 329 US hospitals (2018–2019). Macrolide resistance was significantly higher for respiratory vs blood isolates and ambulatory vs inpatient settings. Despite geographic variation, S. pneumoniae macrolide resistance was >25% in most regions.
Keywords: antibiotic resistance, community-acquired pneumonia, epidemiology, macrolides, Streptococcus pneumoniae
Streptococcus pneumoniae remains a highly virulent pathogen [1, 2] despite reductions in invasive pneumococcal disease following the widespread implementation of pneumococcal conjugate vaccination [3, 4]. S. pneumoniae is the most common bacterial etiology for community-acquired pneumonia (CAP) [5, 6], a disease that results in over 1 million emergency department visits [7] and an estimated 700 000 to 1.5 million hospitalizations annually in the United States [8, 9]. The mortality rate for patients hospitalized with CAP is ~10% [10]; although the number of deaths has decreased with the advent of pneumococcal vaccination, the mortality rate has not [3]. Resistance to commonly used antibiotics has complicated the management of pneumococcal infections [11].
Because of the significant health care burden associated with S. pneumoniae, the US Centers for Disease Control and Prevention (CDC) designated drug-resistant S. pneumoniae a serious threat [12]. Macrolides have long been an important component of empiric CAP therapy, but increasing resistance has diminished effectiveness and prompted a change in American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) guidelines for CAP treatment. Although macrolide monotherapy is still considered an option for initial treatment of outpatients with suspected CAP and no comorbid conditions, the ATS/IDSA 2019 update specifies that this therapy should only be used if local pneumococcal resistance is <25% [11].
In 2018, the CDC Active Bacterial Core Surveillance (ABCs) reported a nationwide S. pneumoniae macrolide nonsusceptibility rate of 28.8% [13] based on isolates cultured from normally sterile sites, primarily blood. The ABCs’ surveillance area includes 10 states; for some states, only a specific metropolitan region is included. Data from other surveillance programs may provide additional insights into nationwide resistance and resistance in S. pneumoniae isolated from respiratory cultures to complement the CDC findings.
We used microbiological laboratory data from a large US hospital database to determine the prevalence of macrolide-resistant S. pneumoniae isolated from blood or respiratory cultures in hospitalized and ambulatory patients throughout the United States.
METHODS
Study Design
This retrospective cohort study was based on de-identified microbiological results from adult patients with a positive S. pneumoniae blood or respiratory culture evaluated between October 2018 and September 2019 at 329 US facilities in the BD Insights Research Database (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) [14–16], which provides diverse geographic and demographic representation across the United States. Evaluations of geographic distribution were based on US Census geographic regions and zip code tabulation areas. The primary objective was to determine the proportion of S. pneumoniae isolates resistant to macrolides in blood and respiratory cultures.
The study data set was approved as a limited, de-identified data set for retrospective analysis and was exempted from patient consent by the New England Institutional Review Board (Wellesley, MA, USA).
Microbiology and Susceptibility Testing
Nonduplicate S. pneumoniae isolates, defined as the first isolate of a species from the same source per 30-day period, were obtained from blood or respiratory cultures. Isolates from each source were considered separately, and isolates from the same source within 30 days were included if they differed by >1 susceptibility result. Antimicrobial susceptibility test (AST) data were obtained from S. pneumoniae–positive cultures.
Assessment of macrolide resistance was based on facility reports using commercial panels and local laboratory breakpoints. Resistance to any member of the class (azithromycin, clarithromycin, or erythromycin) was considered macrolide resistance.
Statistical Analysis
The data were analyzed descriptively. Macrolide resistance rates were compared by use of the chi-square test, with P values <.05 indicating statistical significance. All analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Nationwide S. pneumoniae Macrolide Resistance
Our primary analyses included 3626 S. pneumoniae isolates with AST results from blood (n = 1591; 43.9%) or respiratory (n = 2035; 56.1%) cultures collected from 329 US inpatient or ambulatory care facilities. The overall rate of macrolide resistance in S. pneumoniae isolates was 39.5% (Table 1). The resistance rate in respiratory isolates (47.3%) was significantly higher than the rate in blood isolates (29.6%; P < .0001). Isolates obtained from ambulatory settings had a significantly higher rate of macrolide resistance compared with isolates from inpatients (45.3% vs 37.8%; P < .001).
Table 1.
S. pneumoniae Macrolide Resistance Rates by Setting and US Census Region
| % Resistant (No. Tested) | ||||
|---|---|---|---|---|
| Setting or Region | No. of Facilities | Blood Isolates | Respiratory Isolates | All Isolates |
| Total | 329a | 29.6 (1591) | 47.3 (2035) | 39.5 (3626) |
| Inpatient | 313 | 28.2 (1211) | 45.2 (1587) | 37.8 (2798) |
| Ambulatory | 231 | 33.9 (380) | 54.9 (448) | 45.3 (828) |
| Census region (states) | ||||
| West North Central (IA, KS, MN, MO, ND, NE, SD) | 12 | 52.1 (48) | 55.0 (131) | 54.2 (179) |
| South Atlantic (DE, DC, FL, GA, MD, NC, SC, VA, WV) | 40 | 30.3 (145) | 60.8 (199) | 48.0 (344) |
| East South Central (AL, KY, MS, TN) | 49 | 38.0 (229) | 55.6 (252) | 47.2 (481) |
| West South Central: (AR, LA, OK, TX) | 71 | 35.6 (455) | 48.5 (643) | 43.2 (1098) |
| East North Central: (IL, IN, MI, OH, WI) | 56 | 29.0 (217) | 49.7 (320) | 41.3 (537) |
| Middle Atlantic (NJ, NY, PA) | 50 | 28.3 (191) | 39.8 (236) | 34.7 (427) |
| Pacific (AK, CA, OR, WA) | 36 | 13.2 (257) | 25.3 (190) | 18.3 (447) |
| New England (CT, MA, ME, NH, RI, VT) | 5 | 4.0 (25) | 25.0 (52) | 18.2 (77) |
| Mountain (AZ, CO, ID, MT, NM, NV, UT, WY) | 10 | 4.2 (24) | 33.3 (12) | 13.9 (36) |
aFacilities could provide both inpatient and ambulatory services.
Geographic Differences in S. pneumoniae Macrolide Resistance
Statistically significant differences (P < .0001) were observed in macrolide resistance in different US Census regions (Table 1). The overall highest rate was observed in the West North Central region (54.2%), which also had the highest resistance rate in blood isolates (52.1%), followed by South Atlantic (48.0% overall), which had the highest resistance rate in respiratory isolates (60.8%). Regions with overall S. pneumoniae macrolide resistance rates <25% were Mountain (13.9%), New England (18.2%), and Pacific (18.3%). Even in these regions, however, the rates of macrolide resistance in respiratory isolates were ≥25% (33.3%, 25.0%, and 25.3%, respectively).
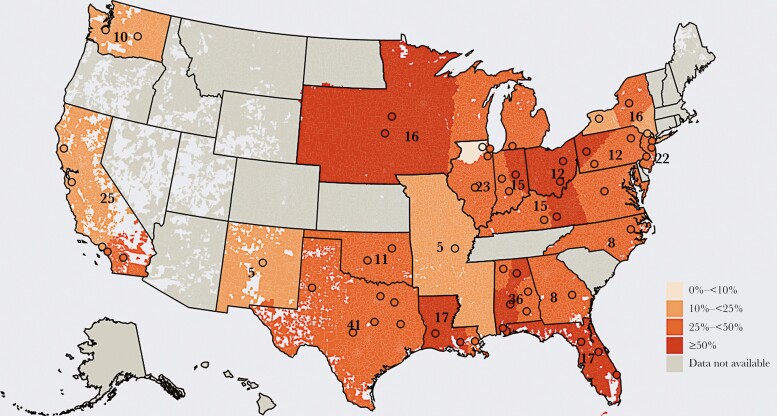
Analysis of geographic distribution by zip codes (Figure 1) identified subregional and within-state differences. For instance, California showed higher macrolide resistance in the southern part of the state, and Pennsylvania showed higher resistance rates in the western part of the state.
Figure 1.
Geographic distribution of S. pneumoniae macrolide resistance rates by zip code. The data represent 3464 isolates collected from 314 facilities between October 2018 and September 2019.a Shaded circles show the geographic centroid for each geographic cluster, and numbers indicate the total number of included hospitals at the state level. aFacilities with <5 isolates were not included, which resulted in slight differences between the numbers shown here and in Table 1. Data were aggregated into geographic clusters of ≥5 hospitals from ≥2 integrated delivery networks; the geographic centroid for each cluster is represented by a shaded circle. Zip code tabulation areas were attributed a rate based on that area’s proximity to the nearest cluster’s geographic centroid. Within each state, the number of hospitals in each cluster is distributed equally, and the total number of hospitals at the state level is labeled on the map. Data for contiguous states each containing <5 hospitals were aggregated (IA, NE, SD, MN, WI, MI; KY, WV, MD, DC, VA; MS, AR, MO).
DISCUSSION
Our analyses of 3626 S. pneumoniae blood and respiratory isolates from US facilities reveal a high burden of macrolide resistance. Overall, 39% of S. pneumoniae isolates were resistant to macrolides; this rate increased to 47% for respiratory isolates. The rate of macrolide resistance was higher in ambulatory patients than in admitted patients, suggesting that outpatient macrolide resistance is common. Although geographic differences were observed, most regions had S. pneumoniae macrolide resistance rates that exceeded the 25% threshold for use of macrolide monotherapy as recommended by ATS/IDSA guidelines for outpatients with suspected CAP, and all regions had >25% macrolide resistance in S. pneumoniae respiratory isolates.
The overall S. pneumoniae macrolide resistance rates in our study are in line with rates from large-scale US or North American studies from previous years, including a 48.4% azithromycin resistance rate in 2014 [17], a 43.8% azithromycin nonsusceptibility rate in 2015–2016 [18], and a 46.6% erythromycin nonsusceptibility rate in 2016 [19]. The rate of resistance in blood isolates (29.6%) is similar to the 2018 rate reported by the CDC ABC surveillance system for isolates from sterile sites (28.8%). These data on the high rates of macrolide resistance may explain recent findings of high failure rates (21%) with macrolide monotherapy in outpatient CAP, resulting in increased mortality and health care costs [20].
Higher antimicrobial resistance rates in respiratory compared with blood cultures have been observed in previous studies of CAP, including studies focused solely on S. pneumoniae in CAP [21, 22] and a study of patients with pneumonia or other causes of respiratory failure associated with any bacterial pathogen [23]. These studies observed higher resistance rates for a broad range of CAP therapies in respiratory vs blood isolates [22, 23] as well as for nonpneumococcal pathogens, such as methicillin-resistant Staphylococcus aureus and gram-negative bacteria [23]. A recent study by Haessler et al. noted that patients with pneumonia/respiratory failure and positive respiratory cultures had different baseline characteristics from those with positive blood cultures, suggesting that resistance in isolates from different cultures sites is associated with distinct multifactorial risk factors or patient phenotypes [23]. We agree with these authors that the source of the isolate should be included in future prediction models of antibiotic resistance. In addition, in contrast to respiratory isolates, which are primarily identified in patients with CAP, blood culture isolation of pneumococcus can involve CAP, meningitis, or contiguous infections from patients with higher-risk comorbidities such as asplenia, HIV infection, or other immunocompromised states [24, 25]. These clinical situations would ideally also be delineated in future analyses to further assess risk factors for pneumococcal bacteremia, but were beyond the scope of the current study.
Our data support the need for ongoing surveillance of CAP epidemiology and resistance profiles. These efforts are particularly important given changes in azithromycin prescriptions during the coronavirus disease 2019 pandemic [26]. Current ATS/IDSA recommendations reserve urine antigen tests and blood/sputum cultures for patients with severe disease and those with empiric treatment or a history of methicillin-resistant Staphylococcus aureus (MRSA) or P. aeruginosa [11]. While the need for resource optimization is well taken, the prospect of reserving culture and urine antigen tests for patients with severe disease may hamper surveillance efforts and limit information on epidemiologic resistance patterns in patients with less severe disease, potentially impacting appropriate therapy. The risk of losing valuable culture data is further compounded by the increasing use of urine antigen testing. Without adequate AST data, future CAP guidelines may fall behind clinical needs and current resistance profiles, as seen with the advent of molecular diagnostics for gonorrhea, chlamydia, and Mycoplasma genitalium in sexually transmitted disease guidelines [27, 28]. We propose a balance of antimicrobial susceptibility results coupled with more convenient molecular diagnostics to optimize the guidance of appropriate empiric therapy, much like the Gonococcal Isolate Surveillance System enacted by the CDC for similar reasons [29].
In addition to macrolide monotherapy, other recommended empiric treatments for outpatients with suspected CAP include amoxicillin or doxycycline for patients with no comorbidities or risk factors for MRSA or Pseudomonas aeruginosa, and broader spectrum regimens (a respiratory fluoroquinolone or combination therapy with a beta-lactam plus macrolide or doxycycline) for patients with comorbidities or MRSA/P. aeruginosa risk factors [11]. Increasing resistance in nonvaccine pneumococcal serotypes [30–32] and potential adverse effects associated with fluoroquinolones, particularly in elderly patients [33], may limit the use of these therapies. New antibiotics for CAP may provide options for enhanced empiric therapy with reduced resistance [34]. In addition, antimicrobial stewardship programs in the outpatient setting have recently been supported by The Joint Commission [35] and may provide an infrastructure to counteract unnecessary macrolide use, which may help curtail macrolide resistance over time [36].
The limitations of our study include underrepresentation of certain geographic regions. The results represent culture-positive isolates and not confirmed invasive infections. Macrolide resistance was based on local microbiology practices at each facility and not standardized across facilities. Selection bias due to a higher likelihood of performing cultures in more severely ill patients is a potential issue for all microbiologic surveillance studies and may have increased estimates of resistance.
Our findings document the high rates of macrolide-resistant S. pneumoniae throughout the United States and suggest that, in most parts of the country, clinicians should consider alternatives to macrolide monotherapy as empiric therapy for suspected CAP. Ongoing surveillance efforts are required to track trends in resistance.
Acknowledgments
The authors wish to thank John Murray, MPH (Becton, Dickinson & Company, Franklin Lakes, NJ, USA), for his dedicated contribution of database management for this study and Kennedy Shurke (Becton, Dickinson & Company, Franklin Lakes, NJ, USA) for development of Figure 1. Medical writing support was provided by Sharon L. Cross, PhD, Fusion MD Medical Science Network, Inc., Montreal, Canada, with funding from Becton, Dickinson & Company.
Financial support. This work was supported by a grant from Nabriva Therapeutics US, Inc. (King of Prussia, PA, USA).
Potential conflicts of interest. V.G. and K.C.Y. are employees of Becton, Dickinson & Company, which was contracted by Nabriva Therapeutics to conduct the study, and own stock in Becton, Dickinson & Company. J.S. and S.P.G. are employees of Nabriva and hold stock in Nabriva Therapeutics.
Patient consent. This study does not include factors necessitating patient consent. The study data set was approved as a limited, de-identified data set for retrospective analysis and was exempted from patient consent by the New England Institutional Review Board (Wellesley, MA, USA).
References
- 1.Brooks LRK, Mias GI. Streptococcus pneumoniae’s virulence and host immunity: aging, diagnostics, and prevention. Front Immunol 2018; 9:1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infection in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018; 18:1191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013; 369:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klugman KP, Black S. Impact of existing vaccines in reducing antibiotic resistance: primary and secondary effects. Proc Natl Acad Sci U S A 2018; 115:12896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander E, Goldberg L, Das AF, et al. Oral lefamulin vs moxifloxacin for early clinical response among adults with community-acquired bacterial pneumonia. The LEAP 2 randomized clinical trial. JAMA 2019; 322:1661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carugati M, Aliberti S, Sotgiu G, et al. ; GLIMP Collaborators . Bacterial etiology of community-acquired pneumonia in immunocompetent hospitalized patients and appropriateness of empirical treatment recommendations: an international point-prevalence study. Eur J Clin Microbiol Infect Dis 2020; 39:1513–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services. National Hospital Ambulatory Medical Care Survey: 2017 emergency department summary tables. Available at: https://www.cdc.gov/nchs/data/nhamcs/web_tables/2017_ed_web_tables-508.pdf. Accessed September 2020.
- 8.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project, National Inpatient Sample, 2017. Available at: https://www.hcup-us.ahrq.gov/faststats/NationalDiagnosesServlet. Accessed September 2020.
- 9.Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis 2017; 651806–12. [DOI] [PubMed] [Google Scholar]
- 10.Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014; 371:1619–28. [DOI] [PubMed] [Google Scholar]
- 11.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Available at: www.cdc.gov/DrugResistance/Biggest-Threats.html. Accessed September 2020.
- 13.Centers for Disease Control and Prevention. Active Bacterial Core Surveillance report, Emerging Infections Program Network, Streptococcus pneumoniae, 2018. 2018. Available at: https://www.cdc.gov/abcs/reports-findings/survreports/spneu18.pdf. Accessed September 2020.
- 14.Tabak YP, Zilberberg MD, Johannes RS, et al. Attributable burden of hospital-onset Clostridium difficile infection: a propensity score matching study. Infect Control Hosp Epidemiol 2013; 34:588–96. [DOI] [PubMed] [Google Scholar]
- 15.McCann E, Srinivasan A, DeRyke CA, et al. Carbapenem-nonsusceptible gram-negative pathogens in ICU and non-ICU settings in US hospitals in 2017: a multicenter study. Open Forum Infect Dis 2018; 5:ofy241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabak YP, Srinivasan A, Yu K, et al. Hospital-level high-risk antibiotic use in relation to hospital-associated Clostridioides difficile infections: retrospective analysis of 2016–2017 data from US hospitals. Infect Control Hosp Epidemiol 2019; 40:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keedy K, Li J, Nenninger A, et al. Antibiotic susceptibility of Streptococcus pneumoniae in the US in 2014. Paper presented at: ID Week 2016, Oct. 26–30, 2016, New Orleans, LA, US. Available at https://idsa.confex.com/idsa/2016/webprogram/Paper60288.html. Accessed September 2020. [Google Scholar]
- 18.Sader HS, Mendes RE, Le J, et al. Antimicrobial susceptibility of Streptococcus pneumoniae from North America, Europe, Latin America, and the Asia-Pacific region: results from 20 years of the SENTRY Antimicrobial Surveillance Program (1997–2016). Open Forum Infect Dis 2019; 6(Suppl 1):S14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller MA, Mendes RE, Duncan LR, Flamm RK, Sader HS. In vitro activities of ceftaroline and comparators against Streptococcus pneumoniae isolates from U.S. hospitals: results from seven years of the AWARE surveillance program (2010 to 2016). Antimicrob Agents Chemother 2018; 62:e01555-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tillotson G, Lodise T, Classi P, Mildvan D, McKinnell JA. Antibiotic treatment failure and associated outcomes among adult patients with community-acquired pneumonia in the outpatient setting: a real-world US insurance claims database study. Open Forum Infect Dis 2020; 7:ofaa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins SG, Farrell DJ. Increase in pneumococcus macrolide resistance, United States. Emerg Infect Dis 2009; 15:1260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golden AR, Baxter MR, Davidson RJ, et al. Comparison of antimicrobial resistance patterns in Streptococcus pneumoniae from respiratory and blood cultures in Canadian hospitals from 2007–16. J Antimicrob Chemother 2019; 74(Suppl 4):iv39–47. [DOI] [PubMed] [Google Scholar]
- 23.Haessler S, Lindenauer PK, Zilberberg MD, et al. Blood cultures versus respiratory cultures: 2 different views of pneumonia. Clin Infect Dis 2020; 71:1604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buie KA, Klugman KP, von Gottberg A, et al. Gender as a risk factor for both antibiotic resistance and infection with pediatric serogroups/serotypes, in HIV-infected and -uninfected adults with pneumococcal bacteremia. J Infect Dis 2004; 189:1996–2000. [DOI] [PubMed] [Google Scholar]
- 25.van Aalst M, Lötsch F, Spijker R, et al. Incidence of invasive pneumococcal disease in immunocompromised patients: a systematic review and meta-analysis. Travel Med Infect Dis 2018; 24:89–100. [DOI] [PubMed] [Google Scholar]
- 26.Vaduganathan M, van Meijgaard J, Mehra MR, et al. Prescription fill patterns for commonly used drugs during the COVID-19 pandemic in the United States. JAMA 2020; 323:2524–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Low N, Unemo M, Skov Jensen J, et al. Molecular diagnostics for gonorrhoea: implications for antimicrobial resistance and the threat of untreatable gonorrhoea. PLoS Med 2014; 11:e1001598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaydos CA, Manhart LE, Taylor SN, et al. Molecular testing for Mycoplasma genitalium in the United States: results from the AMES prospective multicenter clinical study. J Clin Microbiol 2019; 57:e01125-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hook EW, Shafer W, Deal C, et al. CDC Grand Rounds: the growing threat of multidrug-resistant gonorrhea. MMWR Morb Mortal Wkly Rep 2013; 62:103–6. [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur R, Pham M, Yu KOA, Pichichero ME. Rising pneumococcal antibiotic resistance in the post 13-valent pneumococcal conjugate vaccine era in pediatric isolates from a primary care setting. Clin Infect Dis 2021; 72:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corcoran M, Mereckiene J, Cotter S, Murchan S, Cunney R, Humpreys H. Invasive Streptococcus pneumoniae infections and vaccine failures in children in Ireland from the postvaccine era from 2007 to 2018. Pediatr Infect Dis J 2020; 39:339–44. [DOI] [PubMed] [Google Scholar]
- 32.González-Díaz A, Càmara J, Ercibengoa M, et al. Emerging non-13-valent pneumococcal conjugate vaccine (PCV13) serotypes causing adult invasive pneumococcal disease in the late-PCV13 period in Spain. Clin Microbiol Infect 2020; 26:753–9. [DOI] [PubMed] [Google Scholar]
- 33.Sakoulas G. Adverse effects of fluoroquinolones: where do we stand now? NEJM Journal Watch. 13 February 2019. Available at: https://www.jwatch.org/na48248/2019/02/13/adverse-effects-fluoroquinolones-where-do-we-stand. Accessed September 2020. [Google Scholar]
- 34.Kollef MH, Betthauser KD. New antibiotics for community-acquired pneumonia. Curr Opin Infect Dis 2019; 32:169–75. [DOI] [PubMed] [Google Scholar]
- 35.The Joint Commission. R3 report issue 23: antimicrobial stewardship in ambulatory health care. 20 June 2019. Available at: https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/r3_23_antimicrobial_stewardship_amb_6_14_19_final2.pdf. Accessed September 2020.
- 36.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315:1864–73. [DOI] [PubMed] [Google Scholar]