Abstract
Background
Cirrhosis is an end-stage liver disease and is reported as an independent risk factor for cryptococcosis. Information about cryptococcosis in patients with cirrhosis remains sparse.
Methods
Human immunodeficiency virus–uninfected patients with cryptococcosis and cirrhosis admitted to Huashan Hospital from July 2005 to June 2020 were reviewed. Efficacy and safety of antifungal treatments, clinical outcome, and prognostic factors of mortality were evaluated.
Results
A total of 49 cryptococcosis patients with cirrhosis were included. Sites of infection involved central nervous system (n = 38), lung (n = 21), bloodstream (n = 11), skin (n = 1), and bone (n = 1). Nine patients (18.4%) had pulmonary cryptococcosis alone. Viral hepatitis B infection (57.1%) was the most common cause of cirrhosis. Patients with decompensated cirrhosis (Child-Pugh class B and C) were more likely to have extrapulmonary cryptococcosis than those with compensated cirrhosis (90.7% vs 64.7%; P = .049). In patients with cryptococcal meningitis (CM), 7 were treated with amphotericin B with/without flucytosine, 5 with amphotericin B plus fluconazole with/without flucytosine, and 12 with fluconazole with/without flucytosine. Fluconazole (>400 mg/day) was well tolerated and only 1 patient had a mild adverse drug reaction. At 1-year follow-up, all patients treated with fluconazole with or without flucytosine survived, whereas the mortality rate was 14.3%–20.0% in the remaining groups. In addition, Child-Pugh class C cirrhosis (hazard ratio [HR], 7.555 [95% confidence interval {CI}, 1.393–40.971]) and time to diagnosis >120 days (HR, 18.619 [95% CI, 2.117–163.745]) were independent factors for 1-year mortality in patients with CM.
Conclusions
Severity of cirrhosis was associated with developing extrapulmonary cryptococcosis and mortality in CM. Early diagnosis and intervention of cryptococcosis are key for outcome.
Keywords: cryptococcosis, cryptococcal meningitis, extrapulmonary cryptococcosis, liver cirrhosis, pulmonary cryptococcosis
This study shows a higher rate of extrapulmonary cryptococcosis and higher mortality of cryptococcal meningitis in patients with more advanced cirrhosis, which calls on early recognition and timely intervention of cryptococcosis in patients with cirrhosis.
Cirrhosis has been regarded as an end-stage liver disease that causes 1.16 million deaths per year worldwide, remaining a great public health challenge globally [1]. Infection is one of the major complications that may increase mortality in cirrhosis by 4 times and is associated with progression of disease and poor outcome [2]. Bacterial infections are the most common; however, 4.0%–10.3% of cases of cirrhosis are also complicated by invasive fungal infections, mainly caused by Candida or Aspergillus, presenting as peritonitis, fungemia, and pulmonary infection [3–5]. Notably, cirrhosis also increases the susceptibility to Cryptococcus and is reported as an independent risk factor for cryptococcosis [6].
Cryptococcus neoformans and Cryptococcus gattii are important causes of cryptococcosis [7]. The respiratory tract serves as the most important portal of entry and the infection has been recognized to disseminate through the bloodstream, with the central nervous system being the most common site of dissemination [8]. The life-threatening cryptococcal meningitis (CM) causes 15% of AIDS-related deaths, and the fatality in human immunodeficiency virus (HIV)–uninfected cases approaches 30% in some areas [9, 10]. Additionally, cryptococcosis could occur in both those with HIV/AIDS and natural or iatrogenic immunosuppression, but also in immunocompetent individuals [7]. In China, HIV-unrelated CM predominates and the annual incidence is 2-fold higher than that in HIV-infected patients [11].
It is noteworthy that cryptococcal disease in patients with cirrhosis often remains clinically silent or undiagnosed, so data are quite limited. In the present study, we retrospectively reviewed a series of cryptococcosis in patients with cirrhosis with the aim to evaluate the demographic, clinical characteristics, and outcome of cryptococcosis in this population.
PATIENTS AND METHODS
Study Population and Data Resources
A total of 49 HIV-uninfected patients with cryptococcosis and cirrhosis who were admitted to Huashan Hospital, Fudan University, during July 2005 to June 2020 were included in this study. Demographic, clinical, and analytical data were retrieved from the electronic medical record system in Huashan Hospital during hospitalization and regular follow-ups in outpatient clinic. Variables collected included demographics, underlying liver diseases and other predisposing factors, sites of involvement, clinical characteristics of cryptococcosis, antifungal therapies, and outcomes. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were fully applied to ensure the reporting of the study (Supplementary Data) [12].
Patient Consent Statement
This study was approved by the medical ethics committee of Huashan Hospital, Fudan University. Informed consent was waived since no interventions were performed and patient information was protected. Patient care was conducted in compliance with the Declaration of Helsinki.
Definitions
Proven CM was diagnosed meeting any of the following criteria: (i) positive culture or ink smear of Cryptococcus from cerebrospinal fluid (CSF); (ii) positive cryptococcal antigen (CrAg) in the CSF; or (iii) positive histopathological findings of brain tissue yielding 5–10 μm encapsulated yeasts according to the European Organization for Research and Treatment in Cancer and Mycoses Study Group (EORTC/MSG) criteria [13]. A proven diagnosis of pulmonary cryptococcosis (PC) was made through Cryptococcus detected by histopathology or tissue culture. A minor modified probable diagnosis of PC required all of the following criteria being met: (i) positive results of CrAg in serum; (ii) radiological examination showing lesions in lung; and (iii) improved clinical symptoms, CrAg titer, and radiology after antifungal treatment according to previous study [14]. Bloodstream infection was diagnosed only if Cryptococcus was positive by blood culture. Serum and CSF CrAg tests mentioned above were performed using the latex agglutination test (IMMY Inc, Norman, Oklahoma) before 2013, and then switched to lateral flow assay (IMMY) due to its availability.
The diagnosis of cirrhosis was made by either (i) liver biopsy or (ii) a combination of clinical manifestations, biochemical test, imaging, and/or endoscopy findings [15]. Decompensated status was defined when a patient was scored with Child-Pugh class B or C cirrhosis [16].
Efficacy and Safety Assessment
The efficacy of antifungal treatment was evaluated at the end of induction therapy. A response to antifungal treatment was defined as success (complete or partial response) or failure (stable response, progression, or death) after a comprehensive assessment of survival, clinical symptoms and signs, radiology, and mycological evidence based on the EORTC/MSG criteria [17]. Two-week, 10-week, and 1-year mortality were also calculated. Safety was assessed on the basis of the severity and causality evaluation for adverse drug reactions (ADRs) existing during antifungal therapy. Grades 1 through 5 were used to describe an increasing severity of ADRs according to the criteria published by the National Cancer Institute [18]. For causality assessment, ADRs were categorized as in certain, probable, or possible relationship with antifungal treatments based on the World Health Organization Collaborating Center for International Drug Monitoring, the Uppsala Monitoring Center causality assessment system [19].
Statistical Analysis
Continuous variables with normal distribution were described as mean and standard deviation and compared by t test. Nonnormally distributed continuous variables were expressed as median (range) and compared with Mann-Whitney U test. Categorical variables were reported as number and proportions, which were compared between groups using χ 2 test or Fisher exact test, as appropriate. Categorical variables across ordered groups were accessed using a nonparametric test for trend. Cox proportional hazards were used to identify independent prognostic factors using variables with P value of < .20 in univariate analysis. Kaplan-Meier survival curves were drawn for people in different Child-Pugh classification and compared by log-rank test. A P value of < .05 was considered statistically significant. All statistics were performed with Stata/SE version 15.0 software (StataCorp, College Station, Texas).
RESULTS
Demographics and Basic Characteristics
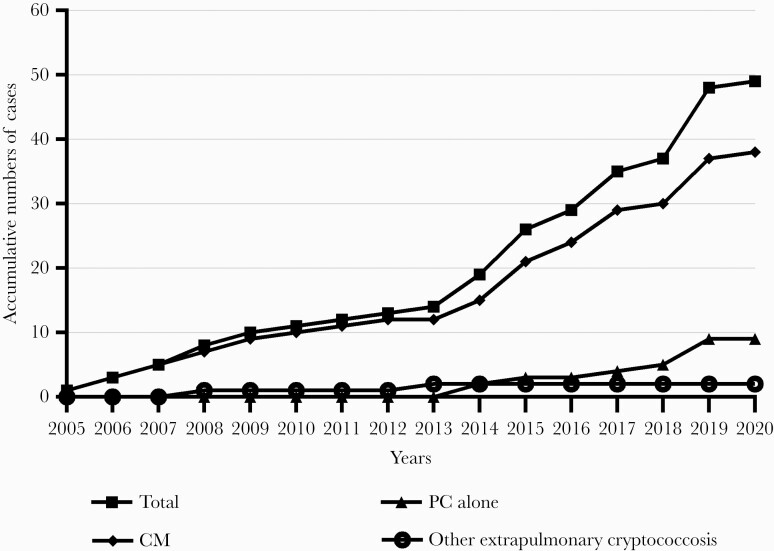
Among the 49 cryptococcosis patients with cirrhosis included in this study during the 15 years, more than half of the cryptococcosis cases (n = 30 [61.2%]) were diagnosed during the latest 5 years (Figure 1). The median age was 54 (range, 29–75) years, with 55.1% males. Of the 49 patients, 9 (18.4%) had PC alone, while the other 40 patients had extrapulmonary cryptococcal infections including meningitis (n = 38 [77.6%]) and other sites’ infections (n = 2 [4.1%]). Besides cirrhosis, 1 or more predisposing factors were found in 32 patients (65.3%), among which glucocorticoid or immunosuppressant administration accounted for the highest proportion (24.5%) (Table 1).
Figure 1.
Dynamics of cryptococcosis diagnosed in patients with cirrhosis over 15 years. Abbreviations: CM, cryptococcal meningitis; PC, pulmonary cryptococcosis.
Table 1.
Basic Characteristics of 49 Cryptococcosis Patients With Cirrhosis
| Variables | Total (N = 49) |
|---|---|
| Age, y, median (range) | 54 (29–75) |
| Gender, male | 27 (55.1) |
| Etiology of cirrhosisa | |
| Viral hepatitis B infection | 28 (57.1) |
| Autoimmune liver disease | 13 (26.5) |
| Schistosomiasis | 3 (6.1) |
| Alcohol | 2 (4.1) |
| Wilson disease | 1 (2.0) |
| Drug-induced liver injury | 1 (2.0) |
| Cryptogenic cirrhosis | 3 (6.1) |
| Child-Pugh classification | |
| A | 17 (34.9) |
| B | 18 (36.7) |
| C | 14 (28.6) |
| Concurrent predisposing factors | 21 (65.3) |
| Glucocorticoid or immunosuppressant therapy | 12 (24.5) |
| Autoimmune disease | 11 (22.4) |
| Diabetes mellitus | 10 (20.4) |
| Splenectomy | 9 (18.4) |
| Solid organ tumor | 5 (10.2) |
| Hematological malignancy | 3 (6.1) |
| Past history of chemotherapy or radiotherapy | 3 (6.1) |
| Multisite cryptococcal infectionsb | 19 (38.8) |
| Groups by sites of infection | |
| Pulmonary cryptococcosis alone | 9 (18.3) |
| Cryptococcal meningitis | 38 (77.6) |
| Other extrapulmonary cryptococcosis | 2 (4.1) |
Data are presented as No. of patients (%) unless otherwise indicated.
Abbreviations: CM, cryptococcal meningitis; PC, pulmonary cryptococcosis.
aTwo patients with CM had 2 etiologies of cirrhosis at the same time.
bInfectious sites involved central nervous system (n = 38), lung (n = 21), bloodstream (n = 11), skin (n = 1), and bone (n = 1).
Clinical Manifestations of Cryptococcosis With Cirrhosis
Symptoms and Signs of Cryptococcosis
For the 9 patients with PC alone, a majority (78%) were afebrile. Cough was observed in 5 patients (55.6%), followed by shortness of breath and chest pain each in 1 patient (11.1%). Among the 38 CM patients, diagnoses were made at a median of 29 (range, 3–336) days after symptom onset in 37 cases, while the remaining 1 presented no obvious symptom. Fever and headache were the most frequent symptoms in CM patients, accounting for 92.1% each. Other manifestations included vomiting (34.2%), altered mental status (34.2%), visual (21.1%) and auditory disturbance (7.9%), dyskinesia (18.4%), epilepsy (7.9%), and sensory disorder (2.6%). Fever appeared in both of the remaining 2 patients who had other extrapulmonary cryptococcosis.
Etiology and Severity of Cirrhosis
Etiologies of cirrhosis varied, and viral hepatitis B infection (57.1%) and autoimmune diseases (26.5%) were most commonly seen (Table 1). Based on Child-Pugh classification, patients were evaluated with class A (34.9%), B (36.7%), and C (28.6%) cirrhosis. Of note, patients who had decompensated cirrhosis (Child-Pugh class B and C) were more likely to develop extrapulmonary cryptococcosis (90.6% vs 64.7% in decompensated and compensated cirrhosis, respectively; P = .049), despite no significant correlation being found between risk of dissemination and severity of cirrhosis (proportions of extrapulmonary cryptococcosis: 64.7% vs 94.4% vs 85.7% in patients with Child-Pugh class A, B, and C, respectively; P for trend = .112).
Laboratory Examinations
Lumbar puncture was performed in 36 of 38 patients with CM before antifungal treatment. Nine patients (39.1%) had an opening pressure >300 mm H2O. CSF examination showed elevated white blood cell count in 28 (90.3%; median, 73.5/mm3 [range, 10–756]), elevated protein in 31 (91.2%; median, 1329 mg/L [range, 610–9673]), and reduced glucose in 30 (90.1%; median, 1.65 mmol/L [range, <.56–4.2]). CSF culture was positive for Cryptococcus in 75.9% (22/29), and 64.7% (22/34) had positive ink smear results. CSF CrAg test was documented in 27 CM patients at baseline, with all being positive and a median of 1:1280 (1:10 to >1:1280). Baseline serum CrAg result was available in 33 patients (67.3%), and all except 1 had positive (97.0%) results ranging from 1:5 to >1:1280 (median: 1:640). The only patient (3.0%) with negative CrAg titer was a proven case of PC based on histopathology. Serum CrAg titers trended to be higher in patients with CM than those with PC alone (median titer, 1:1280 vs 1:160; P = .087).
Radiological Findings.
All of the 21 patients who had lung involvement of cryptococcosis presented multiple lesions according to chest computed tomography (CT) scan. Patches were most common features (n = 14), followed by nodules (n = 13) and streaks (n = 7). Chest CT scan also showed pleural effusion in 9, pulmonary cavity in 2, atelectasis in 3, and pleural incrassation in 1. Meanwhile, 27 of 34 (79.4%) cases with CM on whom cranial imaging was performed at baseline had abnormal results either by magnetic resonance imaging or CT scan. Meningeal enhancement was presented in 36.8% (7/19). Parenchymal lesions appeared in 21 patients, with 95.2% multiple. Additionally, 7 patients were documented with enlarged ventricle.
Efficacy and Safety of Initial Antifungal Treatments
All patients received antifungal treatment. Efficacy of initial therapy was evaluated in 46 patients because 3 CM patients died acutely within 1 week after the initiation of antifungal therapy. As shown in Table 2, the total successful response rate was 65.2%, while CM and PC patients achieved response rates of 57.1% and 88.9%, respectively.
Table 2.
Efficacy of Initial Antifungal Treatment for Cryptococcosis of Different Sites
| Initial Antifungal Treatment | Median Duration (range), d | CR | PR | Stable | Progression | Death | Successful Response Rate, % |
|---|---|---|---|---|---|---|---|
| Total (n = 46) | 26 (7–350) | 1 | 29 | 9 | 6 | 1 | 65.2 |
| CM (n = 35) | 21 (7–142) | 1 | 19 | 9 | 5 | 1 | 57.1 |
| AmB ± FC (n = 7)a | 26 (7–127) | 0 | 7 | 0 | 0 | 0 | 100.0 |
| FCZ ± FC (n = 12) | 43 (7–142) | 1 | 6 | 4 | 1 | 0 | 58.3 |
| AmB + FCZ ± FC (n = 5)b | 32 (14–77) | 0 | 4 | 1 | 0 | 0 | 80.0 |
| Other regimens (n = 11)c | 18 (7–73) | 0 | 2 | 4 | 4 | 1 | 18.2 |
| PC alone (n = 9) | 88 (11–350) | 0 | 8 | 0 | 1 | 0 | 88.9 |
| FCZ (n = 7) | 91 (14–350) | 0 | 7 | 0 | 0 | 0 | 100.0 |
| Other regimens (n = 2)d | 19.5 (11–28) | 0 | 1 | 0 | 1 | 0 | 50.0 |
| Other extrapulmonary cryptococcosis (n = 2) | 28 (27–28) | 0 | 2 | 0 | 0 | 0 | 100.0 |
| AmB (n = 1) | 28 | 0 | 1 | 0 | 0 | 0 | 100.0 |
| FCZ + FC (n = 1) | 27 | 0 | 1 | 0 | 0 | 0 | 100.0 |
Abbreviations: ±, with or without; AmB, amphotericin B; CM, cryptococcal meningitis; CR, complete response; FC, flucytosine; FCZ, fluconazole; PC, pulmonary cryptococcosis; PR, partial response.
aMedian daily and accumulative dose of AmB in this group was 0.38 (range, 0.33–0.50) mg/kg and 1636 (range, 224–3354) mg, respectively.
bMedian daily and accumulative dose of AmB in this group was 0.39 (range, 0.35–0.45) mg/kg/day and 1483 (range, 26–5704) mg, respectively.
cOther regimens administered in patients with CM included involvement of itraconazole (n = 5), voriconazole (n = 3), and liposomal AmB–based treatment (n = 2); 1 case was not available.
dOther regimens administered in patients with PC included 1 patient with liposomal AmB plus fluconazole, and another with voriconazole.
For CM patients, 7 patients who received amphotericin B with/without flucytosine therapy all achieved partial response, while successful response rates were 80.0% in 5 patients receiving combination therapy of amphotericin B plus fluconazole with/without flucytosine, and 58.3% in 12 patients treated with fluconazole with/without flucytosine. No significant difference was found among these 3 groups (P = .119). The daily dose of fluconazole included 800 mg (n = 5), 400 mg (n = 5), and 200 mg (n = 2), with successful response rates of 80%, 40%, and 50%, respectively (P = .747). No significant difference of successful response rate was discovered among CM patients with different severity of cirrhosis (successful response rate: 63.6% vs 43.8% vs 66.7% in patients with Child-Pugh class A, B, and C cirrhosis, respectively; P = .344).
In 9 patients having PC alone, trend of better efficacy in fluconazole than other regimens was shown (successful response rate: 100% vs 50.0%, respectively; P = .222). The 2 patients with other extrapulmonary cryptococcosis were initially treated with either amphotericin B or fluconazole, both having successful response.
A total of 10 patients (30.3%; 6 in amphotericin B with or without flucytosine regimen, 3 in amphotericin B plus fluconazole with or without flucytosine regimen, and 1 in fluconazole with or without flucytosine regimen, respectively) were observed with ADRs during antifungal treatment. ADRs were significantly less frequent in the fluconazole with/without flucytosine group than in amphotericin B with/without flucytosine group (5.0% vs 75.0%, respectively; P < .001) or combination therapy of amphotericin B plus fluconazole with/without flucytosine group (5.0% vs 60.0%, respectively; P = .016) (Table 3). One patient who received amphotericin B plus fluconazole therapy developed grade 4 ADR (hypokalemia, probable case), and underwent modification of antifungal treatment. No other grade 3 or 4 ADR was identified.
Table 3.
Safety Profiles of Initial Treatments
| ADR Category | AmB ± FC (n = 8) | AmB + FCZ ± FC (n = 5) | FCZ ± FC (n = 20) | P Value (AmB ± FC vs FCZ ± FC) | P Value (AmB + FCZ ± FC vs FCZ ± FC) |
|---|---|---|---|---|---|
| Patients with any ADR | 6 (75.0) | 3 (60.0) | 1 (5.0) | <.001 | .016 |
| Elevated serum creatinine | 3 (37.5) | 2 (40.0) | 1 (5.0) | .058 | .091 |
| Elevated ALP | 1 (12.5) | 0 (0.0) | 0 (0.0) | .286 | |
| Elevated GGT | 0 (0.0) | 1 (20.0) | 0 (0.0) | .200 | |
| Hypokalemia | 2 (25.0) | 1 (20.0) | 0 (0.0) | .074 | .200 |
| Altered mental status | 1 (12.5) | 0 (0.0) | 0 (0.0) | .286 | |
| Rash | 0 (0.0) | 1 (20.0) | 0 (0.0) | .200 | |
| Drug fever | 0 (0.0) | 1 (20.0) | 0 (0.0) | .200 |
Values are number of patients (percentage).
Abbreviations: ±, with or without; ADR, adverse drug reaction; ALP, alkaline phosphatase; AmB, amphotericin B; FC, flucytosine; FCZ, fluconazole; GGT, gamma-glutamyl transferase.
Outcome
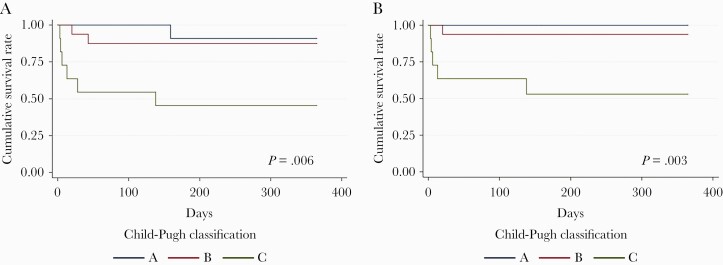
Mortality rates were 8.2% (4/49) at 2 weeks, 16.3% (8/49) at 10 weeks, and 20.4% (10/49) at 1 year (Table 4). For patients with CM, there was no significant difference of 1-year mortality among 3 therapeutic groups (0% for fluconazole with or without flucytosine vs 14.3% for amphotericin B with or without flucytosine vs 20.0% for amphotericin B plus fluconazole with or without flucytosine; P = .239). However, Kaplan-Meier curves demonstrated a significantly low 1-year survival rate in Child-Pugh class C patients with CM compared with Child-Pugh class A (P = .017 in all-cause survival; P = .112 in cause-specific survival of CM) or class B (P = .013 in all-cause survival; P = .012 in cause-specific survival of CM) patients (Figure 2).
Table 4.
Profiles of 10 Patients With Cryptococcal Meningitis or Pulmonary Cryptococcosis Who Died During 1-Year Follow-up
| No. | Age | Gender | Etiology of Cirrhosis | Child-Pugh Classification | Concurrent Predisposing Factors | Site of Infection | Antifungal Treatment | Time of Follow-up, d | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | F | HBV | C | None | CNS | ICZ × 3 d | 3 | CM |
| 2 | 74 | F | PBC | C | RA, glucocorticoid therapy, diabetes mellitus | CNS | FCZ | 4 | Multiple organ dysfunction syndrome, CM |
| 3 | 74 | F | AIH | C | Splenectomy, glucocorticoid therapy | CNS, lung | FCZ | 6 | Multiple sites of cryptococcal infection |
| 4 | 66 | F | PBC | C | Sjogren syndrome | CNS | AmB + ICZ + FC × 3 d FCZ + FC × 5 d FCZ × 5 d |
13 | DIC secondary to cryptococcosis |
| 5 | 60 | M | HBV | B | None | CNS, bloodstream | ICZ × 17 d Liposomal AmB + ICZ × 3d |
20 | Cerebral herniation |
| 6 | 47 | F | HBV | C | Connective tissue disease | CNS, bloodstream | VCZ × 19 d FCZ × 9 d |
28 | DIC secondary to bacterial infection |
| 7 | 65 | F | AIH + PBC | C | AIHA, glucocorticoid therapy | Lung | FCZ × 31 d ICZ × 4 d |
35 | Secondary Aspergillus infection, hepatic, and respiratory failure |
| 8 | 67 | M | HBV | B | Splenectomy, hepatic tumor | CNS | AmB + FCZ × 14 d FCZ × 31 d |
45 | Decompensated cirrhosis, hepatic cancer |
| 9 | 70 | M | HBV | C | Diabetes mellitus | CNS | AmB × 38 d FCZ × 100 d |
138 | Cerebral herniation, AKI |
| 10 | 58 | M | HBV | A | None | CNS | Liposomal AmB × 25 d Liposomal AmB + VCZ × 30 d AmB × 9 d AmB + FCZ × 72 d FCZ × 81 d |
217 | Bacterial pneumonia |
Abbreviations: AIH, autoimmune hepatitis; AIHA, autoimmune hemolytic anemia; AKI, acute kidney injury; AmB, amphotericin B; CM, cryptococcal meningitis; CNS, central nervous system; DIC, diffuse intravascular coagulation; F, female; FC, flucytosine; FCZ, fluconazole; HBV, hepatitis B virus; ICZ, itraconazole; M, male; PBC, primary biliary cholangitis; PC, pulmonary cryptococcosis; RA, rheumatic arthritis; VCZ, voriconazole.
Figure 2.
Kaplan-Meier curve of 1-year survival of 38 patients with cryptococcal meningitis (CM). All-cause survival (A) and cause-specific survival (B) of CM both present statistically significant differences among Child-Pugh class A, B, and C cirrhosis (P = .006 in all-cause survival and P = .003 in cause-specific survival of CM).
Factors Associated With 1-Year Mortality of CM
Nine of 38 (23.7%) patients with CM had died by 1-year follow-up. Univariate analysis revealed that 1-year mortality was significantly high in patients with age >65 years (P = .010) and Child-Pugh class C cirrhosis (P = .009). Multivariate analysis was constructed using the following variables: age >65 years, male, time to diagnosis >120 days, Child-Pugh C cirrhosis, and initial therapy of fluconazole with or without flucytosine. Independent predictive factors associated with 1-year mortality in patients with CM were Child-Pugh class C cirrhosis (hazard ratio [HR], 7.555 [95% confidence interval {CI}, 1.393–40.971]; P = .019) and time to diagnosis >120 days (HR, 18.619 [95% CI, 2.117–163.745]; P = .008) (Table 5).
Table 5.
Univariate and Multivariate Analysis of Factors Associated With 1-Year Mortality in Patients With Cirrhosis and Cryptococcal Meningitis (n = 38)
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Factor | Survived (n = 29) | Died (n = 9) | OR (95% CI) | P Value | HR (95% CI) | P Value |
| Age >65 y | 4 (13.9) | 5 (55.6) | 7.813 (1.073–57.660) | .010 | 1.623 (.394–6.691) | .503 |
| Male sex | 20 (69.0) | 4 (44.4) | 0.360 (.058–2.178) | .146 | 0.665 (.146–3.026) | .598 |
| Time to diagnosis >120 d | 1 (3.4) | 2 (22.2) | 8.000 (.342–488.179) | .134 | 18.619 (2.117–163.745) | .008 |
| Child-Pugh classification | ||||||
| A | 10 (34.5) | 1 (11.1) | 0.238 (.005–2.303) | .237 | … | |
| B | 14 (48.3) | 2 (22.2) | 0.306 (.027–2.051) | .254 | … | |
| C | 5 (17.2) | 6 (66.7) | 9.600 (1.366–75.089) | .009 | 7.555 (1.393–40.971) | .019 |
| Etiology of cirrhosis | ||||||
| HBV infection | 17 (58.6) | 6 (66.7) | 1.412 (.238–10.380) | 1.000 | … | |
| Autoimmune liver disease | 6 (20.7) | 3 (33.3) | 1.917 (.236–12.617) | .655 | … | |
| Concurrent predisposing factors | 17 (58.6) | 6 (66.7) | 1.412 (.238–10.380) | 1.000 | … | |
| Autoimmune disease | 5 (17.2) | 3 (33.3) | 2.400 (.283–16.713) | .363 | … | |
| Glucocorticoid or immunosuppressant therapy | 6 (20.7) | 2 (22.2) | 1.095 (.089–8.215) | 1.000 | … | |
| Baseline symptoms | ||||||
| Fever | 27 (93.1) | 8 (88.9) | 0.593 (.028–39.280) | 1.000 | … | |
| Mild fever (37.2°C–38°C) | 6 (20.7) | 2 (22.2) | 1.095 (.089–8.215) | 1.000 | … | |
| Moderate fever (38.1°C–39°C) | 16 (55.2) | 3 (33.3) | 0.406 (.056–2.417) | .447 | … | |
| Severe fever (>39°C) | 5 (17.2) | 3 (33.3) | 2.400 (.283–16.713) | .363 | … | |
| GCS score <15 | 8 (27.6) | 5 (55.6) | 3.281 (.532–20.618) | .226 | … | |
| Epilepsy | 2 (6.9) | 1 (11.1) | 1.688 (.025–35.994) | 1.000 | … | |
| Vomiting | 8 (27.6) | 5 (55.6) | 3.281 (.532–20.618) | .226 | … | |
| Baseline cranial imaging abnormalitya | 22 (84.6) | 5 (62.5) | 0.303 (.038–2.843) | .315 | … | |
| Meningeal enhancement | 7 (41.2) | 0 (0.0) | … | .509 | … | |
| Multiple parenchyma damage | 16 (61.5) | 4 (50.0) | 0.625 (.094–4.244) | .689 | … | |
| Ventricular enlargement | 4 (15.4) | 3 (37.5) | 3.300 (.352–26.610) | .315 | … | |
| Baseline laboratory testsb | ||||||
| Opening pressure of lumbar puncture >300 mm H2O | 7 (41.2) | 2 (33.3) | 0.714 (.052–6.870) | 1.000 | … | |
| CSF culture | 18 (78.3) | 4 (66.7) | 0.556 (.058–8.006) | .612 | … | |
| CSF ink smear | 17(63.0) | 5 (71.4) | 1.471 (.190–17.997) | 1.000 | … | |
| CSF protein >1000 mg/L | 16 (59.3) | 5 (71.4) | 1.719 (.223–20.837) | .682 | … | |
| CSF glucose <1.1 mmol/L | 5 (18.5) | 2 (33.3) | 2.200 (.153–20.860) | .584 | … | |
| Serum CrAg titer >1:1280 | 5 (29.4) | 2 (33.3) | 1.200 (.082–12.082) | 1.000 | … | |
| CSF CrAg titer >1:1280 | 6 (37.5) | 3 (50.0) | 1.667 (.162–16.602) | .655 | … | |
| Initial therapyc | ||||||
| AmB ± FC | 6 (20.7) | 1 (16.7) | 0.767 (.139–9.176) | 1.000 | … | |
| AmB + FCZ ± FC | 4 (13.8) | 1 (16.7) | 1.250 (.021–16.900) | 1.000 | … | |
| FCZ ± FC | 12 (41.4) | 0 (0.0) | … | .074 | … | 1.000 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AmB, amphotericin B; CI, confidence interval; CrAg, cryptococcal antigen; CSF, cerebrospinal fluid; FC, flucytosine; FCZ, fluconazole; GCS, Glasgow Coma Scale; HBV, hepatitis B virus; HR, hazard ratio; OR, odds ratio.
aCranial imaging was performed in 34 patients, while 19 of them underwent enhanced cranial magnetic resonance imaging or computed tomography.
bOpening pressure of lumbar puncture was recorded in 23 patients. CSF culture, ink smear, protein, glucose, and CrAg titer were available in 29, 34, 34, 33, and 23 patients, respectively. Quantitative CrAg test performed in serum was documented in 22 patients.
cA total of 35 patients were included, as the other 3 patients died acutely within 1 week after diagnosis.
DISCUSSION
Patients with cirrhosis are uniquely susceptible to Cryptococcus. Of HIV-uninfected cryptococcosis, 12 of 166 cases (7.2%) in US series were concurrent with cirrhosis and the main etiology was viral hepatitis C infection and alcohol misuse [20, 21]. In China, where a cryptococcosis rate of 9.1%–9.7% has been reported, of which hepatitis B virus (HBV) infection was the most common cause for cirrhosis [10, 22, 23]. In our present study, we showed an average age of 54 years with male predominance, in keeping with previous study. Of note, the etiologies of cirrhosis comprised not only HBV infection and autoimmune liver diseases as the majority, but also schistosomiasis, alcohol abuse, Wilson disease, and drug-induced liver injury. This diversity indicated that the specific etiologies for cirrhosis may not be the ultimate cause for the susceptibility toward cryptococcosis, though HBV infection has been reported to be a predisposing factor [24–26]. Instead, the cirrhosis-associated defects in host immune defenses, including progressive impaired cell-mediated immunity and deteriorating immune surveillance function, better explain the susceptible state to Cryptococcus [27].
Decompensated liver disease was an important risk factor for Cryptococcus dissemination. Baddley et al retrospectively reviewed 166 patients with PC and found that cirrhosis was independently associated with a 5.3-fold higher rate of extrapulmonary dissemination [20]. Lin et al also conducted a case-control study to distinguish risk factors for cryptococcosis, of which decompensated cirrhosis was associated with 8.5- and 23.8-fold increased risks for CM and cryptococcemia, respectively [28]. Additionally, in a multicenter study of 112 patients with cirrhosis and cryptococcosis during 2000–2014, 91.5% were in a decompensated stage (Child-Pugh class B and C) and 76.8% were disseminated cases [21]. We presented Child-Pugh class A cirrhosis in 34.9% and infection sites of PC alone in 18.3%, CM in 77.6%, and other extrapulmonary cryptococcosis in 4.1%. Based on these findings, our study first revealed that most patients with decompensated cirrhosis (Child-Pugh class B and C) have extrapulmonary cryptococcosis, the proportion of which was significantly higher than those with compensated cirrhosis (90.7% vs 64.7%; P = .049). Meanwhile, a dynamic trend indicated that extrapulmonary cryptococcosis increased as cirrhosis progressed (P for trend = .112). Moreover, we observed that cirrhotic patients with CM presented with higher serum CrAg titers than those with PC alone (1:1280 vs 1:160; P = .087), also implying the relationship between cirrhosis and dissemination. Recently, Sun et al highlighted the “filtration function” of Kupffer cells in the liver to reduce fungal dissemination to target organs, proposing complement C3 in the capture of circulating yeast cells through complement receptor of the immunoglobulin superfamily signaling [29]. Furthermore, Kupffer cells could rapidly engulf C. neoformans and efficiently limit fungal growth, which also reduced the risk of secondary dissemination. Thus, we speculated that decompensated cirrhosis with impaired Kupffer cell function may contribute to the higher rate of Cryptococcus dissemination and higher fungal burden. In our study, growing proportions of PC alone had been witnessed in recent years, which may ascribe to the improved understanding of disease and progressed diagnostic technique for cryptococcal infections such as CrAg lateral flow assay [30]. More routinely, lumbar puncture in PC and rapid diagnostic tests may facilitate earlier diagnosis of dissemination and even improve prognosis, as delayed diagnosis predicted high 1-year mortality in CM patients (HR, 18.619 [95% CI, 2.117–163.745]; P = .008). The risk of dissemination and poor outcome strongly raised the importance of timely recognition of cryptococcosis and intervention.
In terms of treatment, updated guidelines have recommended amphotericin B in combination with flucytosine as initial therapy for non-HIV-infected and non–solid organ transplant patients with CM, followed by fluconazole as consolidation and maintenance therapy [31]. However, amphotericin B is known for severe adverse drug reactions such as liver and renal dysfunction, anemia, leukemia, and thrombocytopenia, which may not be tolerated by patients with underlying conditions, especially cirrhosis and hypersplenism [32]. Thus, the optimal antifungal therapy for CM in patients with cirrhosis remains unclear. Three kinds of initial antifungal regimens were administered in our patients (amphotericin B with or without flucytosine in 7, amphotericin B plus fluconazole with or without flucytosine in 5, and fluconazole with or without flucytosine in 12), achieving comparable efficacies at the end of initial therapy. However, amphotericin B–related ADRs occurred in 69.2% of patients while fluconazole with or without flucytosine appeared to be much safer, with only 1 patient developing a mild abnormity of serum creatinine. It is noteworthy that 4 patients had no response to fluconazole with or without flucytosine therapy because of the lower doses (<400 mg/day) they received, which could not efficiently treat CM according to a previous study [33]. Moreover, 1-year mortality was 0% in the fluconazole with or without flucytosine regimen, and 14.3%–20.0% in the other 2 groups. Taken together, high-dose fluconazole (>400 mg/day) alone or combined with flucytosine is a safe alternative of initial therapy for cirrhosis-associated cryptococcosis.
Cirrhosis has been demonstrated as a strong predictor for mortality among cryptococcosis. For HIV-uninfected patients, mortality rates in cirrhosis-associated cryptococcosis have ranged between 57% and 100% [21, 34, 35]. In our study, we found that survival of CM during 1-year follow-up showed significant difference among patients with Child-Pugh class A, B, and C cirrhosis, as demonstrated before. Additionally, Singh et al conducted a 14-year multicenter study comprising 112 HIV-infected patients who had concurrent cirrhosis and cryptococcosis, and observed higher 90-day mortality in patients with more advanced cirrhosis. In detail, Child-Pugh class C cirrhosis increased the risk of death by 20.12-fold compared with Child-Pugh class A [21]. In our study, we found an all-cause mortality of 23.7% and Child-Pugh class C cirrhosis as an independent factor for 1-year mortality in CM (HR, 7.555 [95% CI, 1.393–40.971]; P = .019), demonstrating the associations between severity of cirrhosis and outcome of cryptococcosis.
The limitations of this study should be acknowledged. First, it was a single center–based study and the number of cases included were limited. Meanwhile, due to the retrospective nature of our study, some clinical data are incomplete, which may restrict statistical power. For instance, CrAg tests were not available in a number of patients who were diagnosed with cryptococcosis before being transferred to our hospital. Larger-scale multicenter randomized controlled trials are required to provide suggestions to compare the different antifungal regimens for cryptococcosis in patients with cirrhosis.
In summary, our retrospective study presented a characteristic profile of cryptococcosis in HIV-uninfected patients with cirrhosis. Patients with cirrhosis, regardless of its etiology, were predisposed to Cryptococcus. Severity of cirrhosis was associated with developing extrapulmonary cryptococcosis and survival in CM. Child-Pugh C cirrhosis and delayed diagnosis of CM (>120 days) were independent predictive factors of outcome. High-dose fluconazole could be a safe alternative for such patients. Our study not only provided clinically significant implications for the management of cryptococcosis in patients with cirrhosis, but also suggested that a high level of vigilance for cryptococcal infection should be kept in patients with cirrhosis. Early recognition and timely intervention are key for outcome.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank all the patients and healthcare workers for their contribution and participation in this study.
Author contributions. L.-P. Z. designed this study. C.-W. Y., J.-H. C., C.-X. Q., and Y. L. collected the data from medical records. J.-H. C., H.-Z. Z., L.-H. Z., and X. W. analyzed and interpreted the data. J.-H. C., C.-W. Y., and Y.-K. J. were equal contributors in writing the manuscript. All authors read and approved the final manuscript.
Financial support. This work was supported by the National Natural Science Foundation of China (grant number 81971911 to L. P. Z.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol 2019; 70:151–71. [DOI] [PubMed] [Google Scholar]
- 2. Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010; 139:1246–56, 1256.e1-–5. [DOI] [PubMed] [Google Scholar]
- 3. Piano S, Singh V, Caraceni P, et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology 2019; 156:1368–80.e1310. [DOI] [PubMed] [Google Scholar]
- 4. Alexopoulou A, Vasilieva L, Agiasotelli D, Dourakis SP. Fungal infections in patients with cirrhosis. J Hepatol 2015; 63:1043–5. [DOI] [PubMed] [Google Scholar]
- 5. Hassan EA, Abd El-Rehim AS, Hassany SM, et al. Fungal infection in patients with end-stage liver disease: low frequency or low index of suspicion. Int J Infect Dis 2014; 23:69–74. [DOI] [PubMed] [Google Scholar]
- 6. Spec A, Raval K, Powderly WG. End-stage liver disease is a strong predictor of early mortality in cryptococcosis. Open Forum Infect Dis 2016; 3:ofv197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pappas PG. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc 2013; 124:61–79. [PMC free article] [PubMed] [Google Scholar]
- 8. Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am 2016; 30:179–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu LP, Wu JQ, Xu B, et al. Cryptococcal meningitis in non-HIV-infected patients in a Chinese tertiary care hospital, 1997-2007. Med Mycol 2010; 48:570–9. [DOI] [PubMed] [Google Scholar]
- 11. Zhou LH, Jiang YK, Li RY, et al. Risk-based estimate of human fungal disease burden, China. Emerg Infect Dis 2020; 26:2137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, et al. STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370:1453–7. [DOI] [PubMed] [Google Scholar]
- 13. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020; 71:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang RY, Chen YQ, Wu JQ, et al. Cryptococcosis in patients with hematological diseases: a 14-year retrospective clinical analysis in a Chinese tertiary hospital. BMC Infect Dis 2017; 17:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bassegoda O, Huelin P, Ariza X, et al. Development of chronic kidney disease after acute kidney injury in patients with cirrhosis is common and impairs clinical outcomes. J Hepatol 2020; 72:1132–9. [DOI] [PubMed] [Google Scholar]
- 16. Gambato M, Lens S, Navasa M, Forns X. Treatment options in patients with decompensated cirrhosis, pre- and post-transplantation. J Hepatol 2014; 61:S120–31. [DOI] [PubMed] [Google Scholar]
- 17. Segal BH, Herbrecht R, Stevens DA, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis 2008; 47:674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Cancer Institute. Common terminology criteria for adverse events (CTCAE, version 5.0). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed 21 February 2021.
- 19. World Health Organization. The use of the WHO–UMC system for standardised case causality assessment. https://www.who-umc.org/media/164200/who-umc-causality-assessment_new-logo.pdf. Accessed 21 February 2021.
- 20. Baddley JW, Perfect JR, Oster RA, et al. Pulmonary cryptococcosis in patients without HIV infection: factors associated with disseminated disease. Eur J Clin Microbiol Infect Dis 2008; 27:937–43. [DOI] [PubMed] [Google Scholar]
- 21. Singh N, Sifri CD, Silveira FP, et al. Cryptococcosis in patients with cirrhosis of the liver and posttransplant outcomes. Transplantation 2015; 99:2132–41. [DOI] [PubMed] [Google Scholar]
- 22. Liu K, Ding H, Xu B, et al. Clinical analysis of non-AIDS patients pathologically diagnosed with pulmonary cryptococcosis. J Thorac Dis 2016; 8:2813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang X, Lin SX, Tao J, et al. Study of liver cirrhosis over ten consecutive years in southern China. World J Gastroenterol 2014; 20:13546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu L, Hu C, Hu H, et al. Importance of fibrosis 4 index score and mode of anti-fungal treatment to the outcome of cryptococcal meningitis in hepatitis B virus-infected patients. Infect Dis (Lond) 2019; 51:113–21. [DOI] [PubMed] [Google Scholar]
- 25. Zhong YH, Tan F, Li M, et al. Comparisons of presentations and outcomes of cryptococcal meningitis between patients with and without hepatitis B virus infection. Int J Infect Dis 2014; 20:31–6. [DOI] [PubMed] [Google Scholar]
- 26. Spies FS, de Oliveira MB, Krug MS, et al. Cryptococcosis in patients living with hepatitis C and B viruses. Mycopathologia 2015; 179:307–12. [DOI] [PubMed] [Google Scholar]
- 27. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 2014; 61:1385–96. [DOI] [PubMed] [Google Scholar]
- 28. Lin YY, Shiau S, Fang CT. Risk factors for invasive Cryptococcus neoformans diseases: a case-control study. PLoS One 2015; 10:e0119090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun D, Sun P, Li H, et al. Fungal dissemination is limited by liver macrophage filtration of the blood. Nat Commun 2019; 10:4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang X, Cheng JH, Zhou LH, et al. Evaluation of low cryptococcal antigen titer as determined by the lateral flow assay in serum and cerebrospinal fluid among HIV-negative patients: a retrospective diagnostic accuracy study. IMA Fungus 2020; 11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Molloy SF, Kanyama C, Heyderman RS, et al. ACTA Trial Study Team . Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med 2018; 378:1004–17. [DOI] [PubMed] [Google Scholar]
- 33. Zhao HZ, Wang RY, Wang X, et al. High dose fluconazole in salvage therapy for HIV-uninfected cryptococcal meningitis. BMC Infect Dis 2018; 18:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jean SS, Fang CT, Shau WY, et al. Cryptococcaemia: clinical features and prognostic factors. QJM 2002; 95:511–8. [DOI] [PubMed] [Google Scholar]
- 35. Chuang YM, Ho YC, Chang HT, et al. Disseminated cryptococcosis in HIV-uninfected patients. Eur J Clin Microbiol Infect Dis 2008; 27: 307–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.