Abstract
Recent theories in computational psychiatry propose that unusual perceptual experiences and delusional beliefs may emerge as a consequence of aberrant inference and disruptions in sensory learning. The current study investigates these theories and examines the alterations that are specific to schizophrenia spectrum disorders vs those that occur as psychotic phenomena intensify, regardless of diagnosis. We recruited 66 participants: 22 schizophrenia spectrum inpatients, 22 nonpsychotic inpatients, and 22 nonclinical controls. Participants completed the reversal oddball task with volatility manipulated. We recorded neural responses with electroencephalography and measured behavioral errors to inferences on sound probabilities. Furthermore, we explored neural dynamics using dynamic causal modeling (DCM). Attenuated prediction errors (PEs) were specifically observed in the schizophrenia spectrum, with reductions in mismatch negativity in stable, and P300 in volatile, contexts. Conversely, aberrations in connectivity were observed across all participants as psychotic phenomena increased. DCM revealed that impaired sensory learning behavior was associated with decreased intrinsic connectivity in the left primary auditory cortex and right inferior frontal gyrus (IFG); connectivity in the latter was also reduced with greater severity of psychotic experiences. Moreover, people who experienced more hallucinations and psychotic-like symptoms had decreased bottom-up and increased top-down frontotemporal connectivity, respectively. The findings provide evidence that reduced PEs are specific to the schizophrenia spectrum, but deficits in brain connectivity are aligned on the psychosis continuum. Along the continuum, psychotic experiences were related to an aberrant interplay between top-down, bottom-up, and intrinsic connectivity in the IFG during sensory uncertainty. These findings provide novel insights into psychosis neurocomputational pathophysiology.
Keywords: schizophrenia spectrum, psychosis continuum, predictive coding, effective connectivity, sensory inference
Introduction
Individuals with a schizophrenia spectrum disorder (SZS) form beliefs and percepts that are, at times, disconnected from reality. From a predictive coding perspective, disrupted belief formation and perceptual abnormalities in SZS arise due to an impairment in the brain’s ability to make inferences about the world.1–3 Individuals experiencing psychosis may have a distorted internal representation of the sensory world, due to difficulties in “regularity learning”—the ability to learn patterns that emerge from statistical dependencies of events in the sensory environment. This may lead to a perceived chaotic sensory environment, with misattribution of salience to irrelevant sensory information that may trigger unusual beliefs and percepts.2,4 These aforementioned theories need further empirical examination. Here, we elucidate whether difficulties in regularity learning do indeed underlie psychotic phenomena.
Our internal representation or model of the world depends not only on our regularity learning ability but also on how we estimate the volatility in our environment. In stable conditions, the source of uncertainty is in the estimation of sensory information (ie, local regularities). In contrast, in volatile conditions, a second level of uncertainty exists, reflecting the possibility that the local regularities will change with time (ie, global regularities). Individuals with SZS show overestimation of environmental volatility.5 However, it is unknown whether this leads to impaired learning of local regularities in stable contexts.
An attenuated prediction error (PE) response evoked in auditory oddball paradigms is arguably the most robust biomarker of impaired sensory learning in SZS.6–9 Measured using electroencephalography (EEG), PEs occur after a surprising “deviant” sound is played in a sequence of repetitive “standard” sounds. PEs, notably the mismatch negativity (MMN) and the P300 components, typically occur at 150–250 ms10,11 and 250—500 ms,12 respectively. In SZS, deficits in PEs are thought to result from a failure to learn about regularities (ie, the relative probability of standard and deviant sounds).1,13 These findings offer neurophysiological support for aberrant sensory learning in SZS. Previous work has found that alterations in a frontotemporal network underlie reduced PE response in SZS, in particular, top-down and intrinsic (within region) connections in this network.14–16 However, it is currently unknown if similar brain dysconnectivity in SZS underlies difficulty in regularity learning behavior.
A number of studies have demonstrated decreasing PE response with increasing severity and chronicity of SZS.17 In addition, impairments in regularity learning behavior and complex PEs have been shown to increase as psychotic-like experiences increase in nonclinical populations.18,19 These findings provide some evidence that aberrations in the brain’s prediction model align on a continuum of psychosis.20 The “psychosis continuum” comprises the full range of psychotic experiences: from healthy individuals with psychotic-like experiences to individuals experiencing florid psychosis at the severe end of the schizophrenia spectrum.21,22 The importance of studying psychotic phenomena in a broader dimensional way has recently been highlighted, as it has the potential to uncover risk and resilience factors before the onset of frank symptoms.23 Here, we explore if the anomalies in neurophysiology and brain networks, previously discovered in individuals diagnosed with SZS during sensory learning, also align on the full psychosis continuum, independent of diagnosis.
Thus, the aims of this study were to investigate if anomalies in regularity learning behavior, PEs, and brain connectivity are: (1) specific to the schizophrenia spectrum or (2) generally related to hallucinations/delusions as well as psychotic-like experiences (independent of diagnosis). We hypothesized that the SZS group would have difficulties in local learning of regularities in stable contexts, which would be reflected in a reduced PE response and increased learning errors, and that impaired regularity learning would relate to disruptions in frontotemporal connectivity as psychotic phenomena intensify, across all 3 groups.
Methods and Materials
Participants
This study included 66 participants, of which 22 were inpatients with SZS, 22 were nonpsychotic (NP) inpatients, and 22 were nonclinical controls (NC). The inpatients were recruited from the Monash Medical Centre, Adult Inpatient Psychiatric Unit. The NP inpatient group was selected from those patients without any clinically relevant or diagnosed psychotic symptoms. The NCs were recruited through the university Research Participation Scheme, flyers, and community websites. Prescreening confirmed that all NC participants did not have a history of psychiatric or neurological disorders. All participants were assessed using the Community Assessment of Psychic Experience (CAPE)24 questionnaire, which measures psychotic-like experiences, and additionally patients were assessed using the Positive and Negative Syndrome Scale (PANSS)25 to measure clinical psychotic symptoms. Details on the clinico-demographics of the sample are summarized in the supplementary materials and supplementary table S1 and figure S1.
Materials and Procedure
Reversal Oddball Task
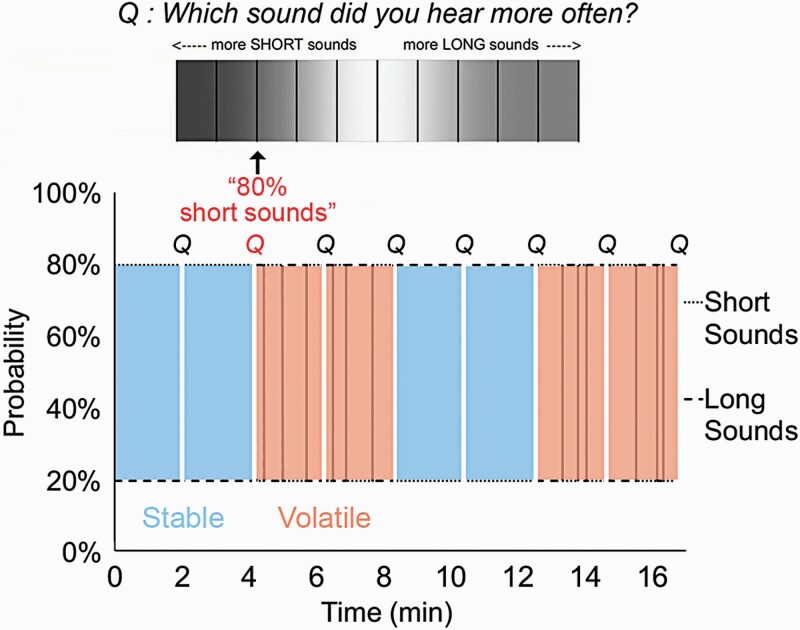
We used an auditory “duration” oddball paradigm, which included stable and volatile blocks (adapted from Weber and colleagues26,27 and first introduced by Dzafic and colleagues19) (see figure 1). In a stable block, a particular sound was always more likely than another sound (eg, short sounds presented with 80% probability). In volatile blocks, a particular sound, which was more likely at first, was then less likely, with 3 reversals of probability in each volatile block. Each block for the stable and volatile conditions was 125 seconds in duration. Reversals within the volatile condition randomly occurred at different time-points, with a mean duration of 31.25 seconds before each reversal. The order of stable and volatile blocks was counterbalanced across participants (further methodological details provided in the supplementary materials).
Fig. 1.
Reversal oddball task. A schematic diagram showing 4 stable and 4 volatile blocks (16.67 min total duration), each separated by a break and questions (approximately 3 min duration). The total duration of the experiment was approximately 20 min. The order of the blocks was counterbalanced between participants. In the first stable block depicted here, the short sound (50 ms) was more probable (80%) throughout, whereas in the first volatile block, the long sound (100 ms) was more probable at first, and then the short sound was more probable, with 3 reversals (vertical lines) of probability in total in each volatile block. Participants were asked to listen to the sounds and estimate the probability (top Q) of the most frequent sound and also rate their confidence on this judgment, every 2:08 min. The colored Q in shows an example of a correct response for the second stable block.
EEG Recording
A Biosemi Active Two system recorded continuous EEG data from 64 scalp electrodes arranged according to the international 10–20 system28 and sampling at 1024 Hz. Standard preprocessing and data analyses were performed with SPM12 (http://www.fil.ion.ucl.ac.uk/spm/); for further details, please refer to supplementary materials.
Dynamic Causal Modeling
Dynamic causal modeling (DCM) was employed in order to investigate how effective connectivity is related to both regularity learning and psychotic symptoms/experiences. DCM has an embedded source reconstruction algorithm based on a spatial forward model. However, in addition to this, DCM incorporates a biologically informed model, the neural mass model, which defines responses of neuronal subpopulations29,30 and places empirically derived constraints on the inversion, allowing inferences to be made about the source connectivity.31
In the model specification, we defined a brain network based on previous robust findings.32,33 This model included: bilateral A1 (left [−42, −22, 7] and right [46, −14, 8]; chosen as the cortical input sources), bilateral superior temporal gyrus (STG; left [−61, −32, 8] and right [59, −25, 8]), and bilateral inferior frontal gyrus (IFG) (left [−46, 20, 8] and right [46, 20, 8]). In this brain model, we examined forward and backward connections at all levels. We explored intrinsic connections within bilateral A1, given that modulations in intrinsic connectivity within A1 have shown a consistent and substantial explanatory contribution to the model.34,35 In addition, we investigated intrinsic connections within the bilateral IFG, as they are found to be altered in those with a psychotic disorder15 and after ketamine administration.36
Under simplifying assumptions, forward connections model PEs (encoded by superficial pyramidal cells), backward connections model predictions (encoded by deep pyramidal cells),37 and intrinsic connections model the local adaptation of neurons within a particular region.38,39 It is important to note that the equations underlying DCM are agnostic about the computational quantities that the connections confer (such as predictions and PEs). Here, we use the notion of intrinsic connectivity as defined in Kiebel and colleagues,40 which models the maximum postsynaptic potential. For further details regarding the DCM, please refer to supplementary materials.
Data Analysis
Behavioral analyses were conducted using the SPSS package (IBM Corp, 2012), and multiple correlations were corrected using the Bonferroni method. We used SPM12 for the neuroimaging analyses. One SZS and one NP participant were removed from all EEG analyses due to inadequate signal (low signal-to-noise ratio) after preprocessing. We conducted between-group analyses to test specificity to the schizophrenia spectrum. In addition, we conducted within-group analyses (by pooling the 3 groups of NC, NP, and SZS) in order to test the psychosis continuum. In the analyses for the pooled groups, we controlled for the effect of group in order to show that our findings on the psychosis continuum were not merely driven by group differences. This was done by adding group membership as a covariate of no interest. In addition to this, we have conducted slope tests to ensure that none of our findings were spurious (which they were not), hence enabling merging the data across groups (please see supplementary materials and supplementary table S2).
For the behavioral analysis, we assessed the effect of group and volatility on regularity learning, using a mixed-design ANOVA. For the event-related potential (ERP) analysis, we report results only from the Fz channel and assess the effect of group and volatility on brain PEs (MMN and P300), using a mixed-design ANOVA. We also investigated the association between psychotic-like experiences (CAPE positive scores), MMN, P300, and regularity learning errors with Pearson’s correlations. For the DCM, we applied parametric empirical Bayes (PEB) to determine the strength of effective connectivity at each connection, across all participants, and to assess group, regularity learning, and psychotic trait effects. From the PEB, we discuss the results that exceeded 95% posterior probability, indicating strong evidence that these connections are being modulated. For further details on the data analyses, please refer to supplementary materials.
Results
Regularity Learning Errors
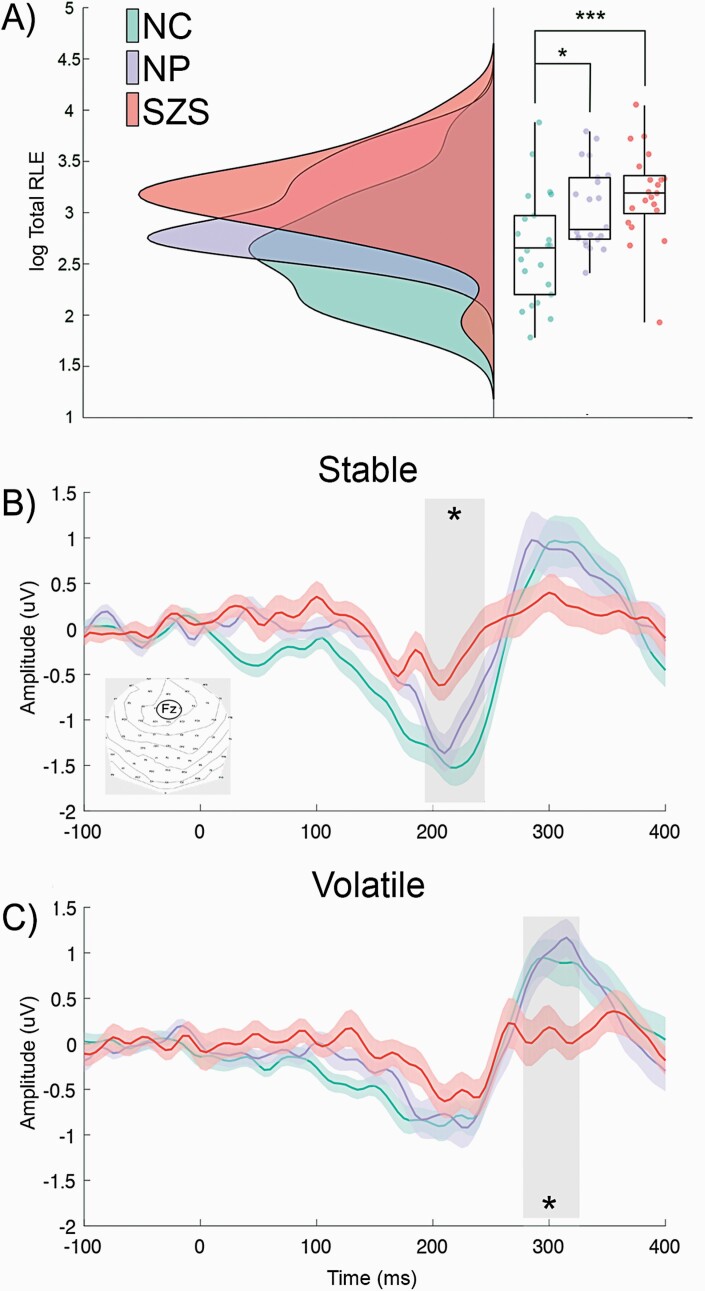
First, we explored regularity learning between the 3 groups. A main effect of volatility, F(1, 62) = 11.5, P = .001, η 2 = 0.16, was found with more errors made in volatile compared with stable environments. We also found a main effect of group, F(2, 62) = 7.36, P = .001, η 2 = 0.19, due to SZS (M = 3.82, SD = 0.42) and NP (M = 3.57, SD = 0.40) having increased errors in regularity learning compared with NC (M = 3.24, SD = 0.54) (SZS vs NC: P = .001, d = 1.20; NP vs NC: P = .027, d = 0.70). Interestingly, regularity learning errors did not significantly differ between SZS and NP (P = .66) (figure 2a). No significant interaction was seen between group and volatility, F(2, 62) = 0.35, P = .71; henceforth, regularity learning errors are averaged across stable and volatile conditions.
Fig. 2.
Regularity learning errors and attenuated prediction errors in the schizophrenia spectrum. (A) Regularity learning errors are significantly increased in both clinical groups compared with nonclinical controls. (B) The SZS group has significantly attenuated MMN in stable conditions and (C) P300 in volatile conditions, compared with both control groups. Shaded areas correspond to the time window with the maximum amplitude across the 3 groups. Note: SZS, schizophrenia spectrum disorder; MMN, mismatch negativity; *P < .05; ***P < .001;. Regularity learning errors were log transformed.
We explored if regularity learning was associated with psychotic phenomena regardless of diagnosis by correlating it with CAPE positive scores across our entire sample. Pearson’s correlations revealed a relationship between regularity learning errors and CAPE positive scores (r = .35, P = .004, Pcorr = .016); however, this was not significant after controlling for the effect of patient group (r = .22, P = .08).
PEs and Specificity to Schizophrenia Spectrum
We investigated PE updating between the 3 groups in stable and volatile contexts and their relationship with impaired regularity learning. We found a main effect of MMN, F(1, 61) = 80.47, P < .001, η 2 = 0.57, a 2-way interaction between group and MMN, F(2, 61) = 3.76, P = .029, η 2 = 0.11, and a 3-way interaction between volatility, group, and MMN, F(2, 61) = 3.67, P = .03, η 2 = 0.11. Follow-up tests revealed that MMN was different between groups during stable but not volatile conditions (stable MMN: F(2, 61) = 8.67, P = .03, η 2 = 0.22; volatile MMN: F(2, 61) = 1.00, P = .37). Further follow-up tests determined that stable MMN was specifically attenuated in SZS (M = −0.38, SD = 0.77) compared with NC (M = −1.39, SD = 0.84), P < .0001, d = 1.25, and NP (M = −1.08, SD = 0.84), P = .02, d = 0.87, but not significantly different between NP and NC, P = .52 (figure 2b).
We examined P300 with the nonparametric independent-samples Kruskal-Wallis test and found that P300 was different between groups during volatile but not stable conditions (stable P300: P = .269; volatile P300: P = .014). Follow-up tests revealed that volatile P300 was specifically attenuated in SZS (M = 0.077, SD = 0.91) compared with NC (M = 0.88, SD = 1.03), P = .009, d = 0.83, and NP (M = 0.86, SD = 0.83), P = .015, d = 0.90, but not significantly different between NP and NC (P = .88) figure 2c).
Furthermore, we asked whether behavioral regularity learning errors were related to learning at the neurophysiological level irrespective of diagnosis. Correlations revealed that regularity learning errors were related to attenuation in both stable MMN and volatile P300 (stable MMN: r = .29, P = .02, Pcorr = .04; volatile P300: r = −.30, P = .02, Pcorr = .03). However, these relationships were no longer significant after controlling for the effect of group (stable MMN: r = .05, P = .72; volatile P300: r = −.19, P = .13).
Effective Connectivity Underpinning Aberrant Inference and Psychotic Experiences
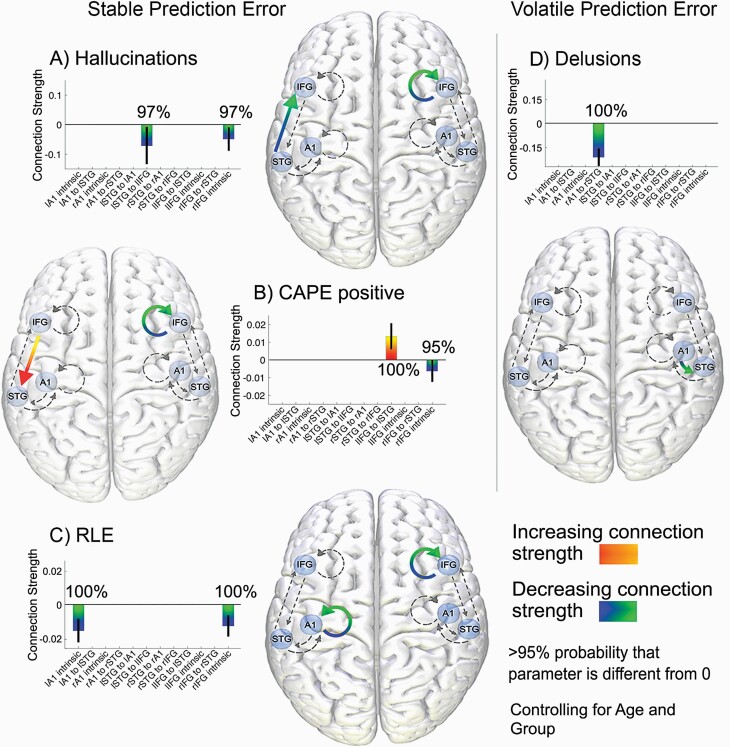
We examined brain connectivity across the psychosis continuum; we investigated regularity learning ability and CAPE positive scores pooled over the 3 groups, and PANSS hallucinations and delusions pooled over SZS and NP inpatients. People with greater regularity learning errors showed less intrinsic connectivity within the left A1 and right IFG in stable conditions (see figure 3c). Interestingly, people with greater CAPE positive scores and patients with more severe hallucinations also had decreased intrinsic connectivity within the right IFG. We also found that CAPE positive scores increased with greater top-down left IFG to STG connectivity, and those with more severe hallucinations had weaker bottom-up left STG to IFG connectivity (figures 3a and 3b). People with more severe delusions also showed weaker bottom-up connections from the right A1 to STG during volatile conditions (figure 3d). All the effective connectivity findings remain even after controlling for the effect of group, suggesting that these relationships exist on the continuum of psychosis, regardless of diagnosis. We did not find any connectivity differences between groups that survived the >95% threshold.
Fig. 3.
Increased top-down and decreased bottom-up connectivity across the psychosis continuum and decreased intrinsic connectivity in poorer regularity learning. During stable PEs: (A) Bottom-up connections from lSTG to lIFG and intrinsic connections in the rIFG are modulated by PANSS hallucinations. (B) Top-down connections from lIFG to lSTG and intrinsic connections in the rIFG are modulated by CAPE positive scores. (C) lA1 and rIFG intrinsic connections are modulated by regularity learning errors. During volatile PEs, (D) bottom-up connections from rA1 to rSTG are modulated by PANSS delusions. Note: PEs, prediction errors; STG, superior temporal gyrus; IFG, inferior frontal gyrus; r, right; l, left; PANSS, Positive and Negative Syndrome Scale; CAPE, Community Assessment of Psychic Experience. All connections are >95% probability that parameter is different from 0. All analyses control for the effect of age and group.
The Effect of Medication
Chlorpromazine equivalents were not associated with regularity learning ability (r = .12, P = .64), stable MMN (r = −.081, P = .76), volatile MMN (r = −.15, P = .56), stable P300 (r = −.08, P = .75), volatile P300 (r = −0.18, P = .49), or effective connectivity (across all connectivity parameters: r < .33, P > .19).
Confidence
Confidence ratings were included to monitor the participants’ understanding and attention of the task throughout. We did not find a main effect of group on confidence ratings, suggesting that the SZS, NP, and NC were equally confident in their regularity learning estimates, F(2, 65) = 0.078, P = .925.
Discussion
The aims of the current study were to investigate sensory learning and inference, in the schizophrenia spectrum, and as psychotic phenomena intensify across the psychosis continuum. Our results revealed specific attenuations for the SZS group in stable MMN and volatile P300, which suggest difficulties in the extraction of both local and global learnt regularities at the neurophysiological level. However, along the psychosis continuum, impaired behavioral regularity learning in stable contexts was related to decreases in the left A1 and the right IFG intrinsic connectivity, the latter was also related to increases in psychotic-like experiences and hallucinations. Moreover, individuals with more psychotic-like experiences showed increases in top-down left IFG-STG, and those with more severe hallucinations had decreases in bottom-up left STG-IFG connectivity. Therefore, there may be an interplay between strong top-down and weak bottom-up frontotemporal connectivity during sensory learning and decreased right IFG intrinsic connectivity during sensory inference, which underlies psychosis pathophysiology.
Impaired Neurophysiological Extraction of Regularities Specific to the Schizophrenia Spectrum
Attenuated PEs, namely the MMN and P300, were specifically observed in the schizophrenia spectrum group. Previous studies have also found that MMN attenuation is specific for patients with schizophrenia, when compared with people with bipolar disorder, Alzheimer’s disease,41 major depression,42 and obsessive-compulsive disorder.43 Our results go further and indicate that SZS has deficits in extracting local and global regularities reflected in stable MMN and volatile P300 attenuation, respectively. MMN is thought to be an automatic response to local errors in prediction, signaling short-term deviance detection, whereas long-term (global), complex errors are captured to a greater extent by the P300, which is contingent on attention.44–46 Therefore, MMN may be more crucial in stable conditions, in which there is only uncertainty in what sound will play next. In the volatile conditions, on the other hand, there is a second layer of uncertainty that pertains to knowing when the more probable sound becomes the less probable sound. This deficit in neurophysiological local and global regularity extraction seems to be specific to the SZS group as the NP inpatients did not significantly differ from the NC in their PEs.
Decreased Intrinsic Connectivity Is Related to Poorer Regularity Learning Behavior Along the Psychosis Continuum
Individuals with greater errors in regularity learning showed decreased intrinsic connectivity within the left A1. Intrinsic connectivity in DCM is intended to model local adaptation of neurons,40 which occurs to repeated stimuli (such as standard sounds).38 Previous work found that the left A1 may be involved in the encoding of temporal (rather than spectral) information of sounds47 as well as in processing very rapid sound changes.48,49 Less adaptation within the left A1 may hinder the ability to extract the temporal information from sounds, such as sound duration, which critically defined the regularity in our experiment.
The reduced intrinsic connectivity in the A1 may also suggest that participants who paid less attention had poorer regularity learning, as attention has previously been linked to intrinsic connections in the A1 during an auditory oddball task.50 Attentional deficits are inherent in individuals with psychiatric illness and may also arise due to the sedative effects of medication. Having said that, we report no association between regularity learning errors and chlorpromazine equivalents nor any group differences in participants’ subjective confidence ratings. This diminishes but does not eliminate the possible contribution of attention to these findings.
We also found that people with greater regularity learning errors and those who reported more psychotic-like experiences and hallucinations had decreased intrinsic connectivity within the right IFG. Decreased intrinsic connectivity may result in disinhibition, or increased firing, due to decreased neuronal adaptation within that region. In a recent DCM study, ketamine-induced N-Methyl-D-aspartate (NMDA) receptor change during an auditory oddball task resulted in attenuated PE responses and disinhibition in the IFG.36 The administration of ketamine is known to produce psychotic-like experiences in nonclinical individuals.51 Future work is needed to causally test whether improved NMDA function recovers regularity learning in individuals diagnosed with a psychotic disorder.
Increased Top-Down and Decreased Bottom-Up Connectivity During Sensory Learning Along the Psychosis Continuum
We found that people with more psychotic-like experiences had increased top-down connectivity from the left IFG to STG. In addition, we found that people with more severe hallucinations and delusions had decreased bottom-up connectivity from the left STG to IFG and the right A1 to STG, respectively. This may indicate that maladaptive strong top-down connections may only be associated with milder psychotic-like symptoms, whereas weak bottom-up connections may be associated with more severe clinical psychosis. It is worth pointing out that stronger top-down connections are entirely consistent with weaker bottom-up connections. We speculate that this increase in top-down connectivity, relating to maladaptive strong priors, may suppress sensory information in the reverse bottom-up direction (in order to maintain cogent perception). In contrast, this plausibly compensatory process may be diminished or absent in psychotic disorders and instead only weakened sensory updating present. Of course, future research is needed to elucidate whether these speculations indeed hold by employing paradigms that tap directly into priors and sensory information as well as future longitudinal research that includes ultra-high risk/first-episode psychosis participants where these changes may be the first emergent.
Limitations
We attempted to control for the effect of medication by (1) including a medicated inpatient control group and (2) running post hoc analyses to evaluate any associations with antipsychotic drugs within the SZS group. We did not find associations between chlorpromazine equivalents with any behavioral, electrophysiological, or effective connectivity findings. However, we cannot completely rule out a possible contribution of medication given that the medicated NP inpatient group was on significantly lower doses than the schizophrenia spectrum group.
We only found increased top-down frontotemporal connectivity related to CAPE scores and not to the more severe hallucinations and delusions, or schizophrenia spectrum patient status. It is possible that the effect did not reach significance in the relatively smaller sample of patients for whom we had a measure of hallucinations and delusions (two-third of the sample; 44 participants) and schizophrenia spectrum patients (one-third of the sample; 22 participants). In contrast, we had CAPE scores for the full group of participants (66 participants). Future studies with a larger sample size are needed to determine whether top-down alterations in frontotemporal connectivity are specifically related to milder psychotic-like traits, or whether it is indeed related to clinical psychosis.
Conclusions
In this study, we examined sensory learning impairments specific to the schizophrenia spectrum vs those that align on the continuum of psychosis. Our findings demonstrate that sensory PEs are specifically impaired in SZS, whereas frontotemporal dysconnectivity occurs on the psychosis continuum, regardless of diagnosis. We show that reduced adaptation in the right IFG relates to impairments in sensory inference and more psychotic experiences. Moreover, we reveal the neural interplay, ie, increasing top-down and decreasing bottom-up frontotemporal connectivity, as the severity of psychotic phenomena increases. These findings provide novel insights into the neurocomputational pathophysiology underlying psychotic experiences.
Supplementary Material
Acknowledgments
We thank the participants for their time and the nurses in the psychiatric ward for their assistance. All the authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
M.I.G. is supported by the University of Queensland Fellowship (2016000071) and Women’s Academic Fund from Queensland Government and O.C. is supported by Australian Research Council Future Fellowship (FT140100807).
Data availability
We have made the behavioral, questionnaire, and EEG data, and PEB script publicly available here: https://doi.org/10.26188/12752399
References
- 1. Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ. The computational anatomy of psychosis. Front Psychiatry. 2013;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams RA, Huys QJ, Roiser JP. Computational psychiatry: towards a mathematically informed understanding of mental illness. J Neurol Neurosurg Psychiatry. 2016;87(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Powers AR, Mathys C, Corlett PR. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science. 2017;357(6351):596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sterzer P, Adams RA, Fletcher P, et al. The predictive coding account of psychosis. Biol Psychiatry. 2018;84(9):634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deserno L, Boehme R, Mathys C, et al. Volatility estimates increase choice switching and relate to prefrontal activity in Schizophrenia. In: Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2020;5(2):173–183. doi: 10.1016/j.bpsc.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 6. Bodatsch M, Ruhrmann S, Wagner M, et al. Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011;69(10):959–966. [DOI] [PubMed] [Google Scholar]
- 7. Näätänen R, Shiga T, Asano S, Yabe H. Mismatch negativity (MMN) deficiency: a break-through biomarker in predicting psychosis onset. Int J Psychophysiol. 2015;95(3):338–344. [DOI] [PubMed] [Google Scholar]
- 8. Michie PT. What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiol. 2001;42(2):177–194. [DOI] [PubMed] [Google Scholar]
- 9. Todd J, Harms L, Schall U, Michie PT. Mismatch negativity: translating the potential. Front Psychiatry. 2013;4:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol. 2007;118(12):2544–2590. [DOI] [PubMed] [Google Scholar]
- 11. Earls HA, Curran T, Mittal V. A meta-analytic review of auditory event-related potential components as endophenotypes for schizophrenia: perspectives from first-degree relatives. Schizophr Bull. 2016;42(6):1504–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol. 1975;38(4):387–401. [DOI] [PubMed] [Google Scholar]
- 13. Lecaignard F, Bertrand O, Gimenez G, Mattout J, Caclin A. Implicit learning of predictable sound sequences modulates human brain responses at different levels of the auditory hierarchy. Front Hum Neurosci. 2015;9:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dima D, Frangou S, Burge L, Braeutigam S, James AC. Abnormal intrinsic and extrinsic connectivity within the magnetic mismatch negativity brain network in schizophrenia: a preliminary study. Schizophr Res. 2012;135(1-3):23–27. [DOI] [PubMed] [Google Scholar]
- 15. Ranlund S, Adams RA, Díez Á, et al. Impaired prefrontal synaptic gain in people with psychosis and their relatives during the mismatch negativity. Hum Brain Mapp. 2016;37(1):351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larsen KM, Mørup M, Birknow MR, et al. Altered auditory processing and effective connectivity in 22q11.2 deletion syndrome. Schizophr Res. 2018;197:328–336. [DOI] [PubMed] [Google Scholar]
- 17. Randeniya R, Oestreich LKL, Garrido MI. Sensory prediction errors in the continuum of psychosis. Schizophr Res. 2017; 191:109–122. [DOI] [PubMed] [Google Scholar]
- 18. Oestreich LKL, Randeniya R, Garrido MI. Auditory prediction errors and auditory white matter microstructure associated with psychotic-like experiences in healthy individuals. Brain Struct Funct. 2019;224(9):3277–3289. [DOI] [PubMed] [Google Scholar]
- 19. Dzafic I, Randeniya R, Harris CD, Bammel M, Garrido MI. Statistical learning and inference is impaired in the non-clinical continuum of psychosis. J Neurosci. 2020;40(35):6759–6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verdoux H, van Os J. Psychotic symptoms in non-clinical populations and the continuum of psychosis. Schizophr Res. 2002;54(1-2):59–65. [DOI] [PubMed] [Google Scholar]
- 21. DeRosse P, Karlsgodt KH. Examining the psychosis continuum. Curr Behav Neurosci Rep. 2015;2(2):80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Os J, Reininghaus U. Psychosis as a transdiagnostic and extended phenotype in the general population. World Psychiatry. 2016;15(2):118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor JH, Calkins ME, Gur RE. Markers of psychosis risk in the general population. Biol Psychiatry. 2020;88(4):337–348. [DOI] [PubMed] [Google Scholar]
- 24. Konings M, Bak M, Hanssen M, van Os J, Krabbendam L. Validity and reliability of the CAPE: a self-report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatr Scand. 2006;114(1):55–61. [DOI] [PubMed] [Google Scholar]
- 25. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 26. Weber LA, Diaconescu AO, Mathys C, et al. Ketamine affects prediction errors about statistical regularities: a computational single-trial analysis of the mismatch negativity. J Neurosci. 2020;40(29):5658–5668. doi: 10.1523/JNEUROSCI.3069-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weber L, Diaconescu A, Tomiello S, et al. F157. Hierarchical prediction errors during auditory mismatch under pharmacological manipulations: a computational single-trial EEG analysis. Schizophr Bull. 2018;44(Suppl 1):S281–S282. [Google Scholar]
- 28. Oostenveld R, Praamstra P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol. 2001;112(4):713–719. [DOI] [PubMed] [Google Scholar]
- 29. Jansen BH, Rit VG. Electroencephalogram and visual evoked potential generation in a mathematical model of coupled cortical columns. Biol Cybern. 1995;73(4):357–366. [DOI] [PubMed] [Google Scholar]
- 30. David O, Friston KJ. A neural mass model for MEG/EEG: coupling and neuronal dynamics. Neuroimage. 2003;20(3):1743–1755. [DOI] [PubMed] [Google Scholar]
- 31. Kiebel SJ, Garrido MI, Moran R, Chen CC, Friston KJ. Dynamic causal modeling for EEG and MEG. Hum Brain Mapp. 2009;30(6):1866–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Opitz B, Rinne T, Mecklinger A, von Cramon DY, Schröger E. Differential contribution of frontal and temporal cortices to auditory change detection: fMRI and ERP results. Neuroimage. 2002;15(1):167–174. [DOI] [PubMed] [Google Scholar]
- 33. Garrido MI, Kilner JM, Kiebel SJ, Stephan KE, Friston KJ. Dynamic causal modelling of evoked potentials: a reproducibility study. Neuroimage. 2007;36(3):571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garrido MI, Friston KJ, Kiebel SJ, Stephan KE, Baldeweg T, Kilner JM. The functional anatomy of the MMN: a DCM study of the roving paradigm. Neuroimage. 2008;42(2):936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garrido MI, Kilner JM, Kiebel SJ, Friston KJ. Dynamic causal modeling of the response to frequency deviants. J Neurophysiol. 2009;101(5):2620–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosch RE, Auksztulewicz R, Leung PD, Friston KJ, Baldeweg T. Selective prefrontal disinhibition in a roving auditory oddball paradigm under N-methyl-d-aspartate receptor blockade. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(2):140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kiebel SJ, Garrido MI, Moran RJ, Friston KJ. Dynamic causal modelling for EEG and MEG. Cogn Neurodyn. 2008;2(2):121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garrido MI, Kilner JM, Kiebel SJ, Stephan KE, Baldeweg T, Friston KJ. Repetition suppression and plasticity in the human brain. Neuroimage. 2009;48(1):269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garrido MI, Friston KJ, Kiebel SJ, Stephan KE, Baldeweg T, Kilner JM. The functional anatomy of the MMN: a DCM study of the roving paradigm. Neuroimage. 2008;42(2):936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kiebel SJ, Garrido MI, Friston KJ. Dynamic causal modelling of evoked responses: the role of intrinsic connections. Neuroimage. 2007;36(2):332–345. [DOI] [PubMed] [Google Scholar]
- 41. Baldeweg T, Hirsch SR. Mismatch negativity indexes illness-specific impairments of cortical plasticity in schizophrenia: a comparison with bipolar disorder and Alzheimer’s disease. Int J Psychophysiol. 2015;95(2):145–155. [DOI] [PubMed] [Google Scholar]
- 42. Umbricht D, Koller R, Schmid L, et al. How specific are deficits in mismatch negativity generation to schizophrenia? Biol Psychiatry. 2003;53(12):1120–1131. [DOI] [PubMed] [Google Scholar]
- 43. Oades RD, Dittmann-Balcar A, Zerbin D, Grzella I. Impaired attention-dependent augmentation of MMN in nonparanoid vs paranoid schizophrenic patients: a comparison with obsessive-compulsive disorder and healthy subjects. Biol Psychiatry. 1997;41(12):1196–1210. [DOI] [PubMed] [Google Scholar]
- 44. Chennu S, Noreika V, Gueorguiev D, et al. Expectation and attention in hierarchical auditory prediction. J Neurosci. 2013;33(27):11194–11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marti S, Thibault L, Dehaene S. How does the extraction of local and global auditory regularities vary with context? PLoS One. 2014;9(9):e107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wacongne C, Labyt E, van Wassenhove V, Bekinschtein T, Naccache L, Dehaene S. Evidence for a hierarchy of predictions and prediction errors in human cortex. Proc Natl Acad Sci U S A. 2011;108(51):20754–20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends Cogn Sci. 2002;6(1):37–46. [DOI] [PubMed] [Google Scholar]
- 48. Näätänen R, Lehtokoski A, Lennes M, et al. Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature. 1997;385(6615):432–434. [DOI] [PubMed] [Google Scholar]
- 49. Tervaniemi M, Hugdahl K. Lateralization of auditory-cortex functions. Brain Res Brain Res Rev. 2003;43(3):231–246. [DOI] [PubMed] [Google Scholar]
- 50. Auksztulewicz R, Friston K. Attentional enhancement of auditory mismatch responses: a DCM/MEG study. Cereb Cortex. 2015;25(11):4273–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We have made the behavioral, questionnaire, and EEG data, and PEB script publicly available here: https://doi.org/10.26188/12752399