Abstract
Gestating sows may be more susceptible to increasing dry bulb temperatures (TDB) due to greater metabolic heat production and increased body mass, especially as gestation advances. However, there are few studies on the thermoregulatory and physiological responses of sows at differing gestation stages exposed to gradually increasing temperatures. The study objective was to determine the thermoregulatory and physiological responses of nonpregnant (n = 12; parity 3.27 ± 0.86), mid-gestation (59.7 ± 9.6 d pregnant, n = 12; parity 3.25 ± 0.83), and late-gestation (99.0 ± 4.8 d pregnant, n = 12; parity 3.33 ± 0.75) sows exposed to increasing TDB. Prior to the experiment (5.0 ± 0.7 d), jugular catheters were placed in all sows. During the experiment, the TDB was increased incrementally by 2.45 ± 0.43 °C every 60 min from 19.84 ± 2.15 to 35.54 ± 0.43 °C over 400 min, and relative humidity was recorded at 40.49 ± 18.57%. Respiration rate (RR), heart rate (HR), skin temperature, and vaginal temperature were measured, and blood samples were obtained via the jugular catheter every 20 min. Data were analyzed using PROC MIXED in SAS 9.4. RR increased at a lower TDB (P < 0.01) in late-gestation sows compared with mid-gestation and nonpregnant sows, but no differences were detected between mid-gestation and nonpregnant sows. Overall, late-gestation sows had greater RR (P < 0.01; 23 ± 2 breaths per min [brpm]) compared with mid-gestation (16 ± 2 brpm) and nonpregnant (15 ± 2 brpm) sows. Late-gestation sows had an overall greater HR (P < 0.01; 84 ± 5 beats per min [bpm]) than mid-gestation (76 ± 5 bpm) and nonpregnant (69 ± 5 bpm) sows. Late-gestation sows had overall reduced bicarbonate and total carbon dioxide levels (P = 0.02; 23.89 ± 1.97 and 25.41 ± 2.07 mmol/L, respectively) compared with mid-gestation (27.03 ± 1.97 and 28.58 ± 2.07 mmol/L, respectively) and nonpregnant (26.08 ± 1.97 and 27.58 ± 2.07 mmol/L, respectively) sows. Moreover, late-gestation sows had overall greater nitric oxide levels (P < 0.01; 248.82 ± 34.54 µM) compared with mid-gestation (110.47 ± 34.54 µM) and nonpregnant (41.55 ± 34.54 µM) sows. In summary, late-gestation sows appear to be more sensitive to increasing TDB as indicated by thermoregulatory and physiological responses when compared with mid-gestation or nonpregnant sows. The results from this study provide valuable information regarding thermoregulatory thresholds of sows at differing gestation stages.
Keywords: gestation, heat stress, physiology, sow, thermoregulation
Introduction
Physiological heat stress (HS) in sows may occur when the environmental heat load rises above the sows’ upper critical temperature limit (Silanikove, 2000). Acclimation to HS can occur via reduced heat production and increased heat loss to maintain euthermia (Nichols et al., 1982). Heat loss mechanisms may include behavioral thermoregulation (e.g., postural changes), blood redistribution to the skin (vasodilation), and increased evaporative heat loss through greater respiration rate (RR; Silanikove, 2000, Parois et al., 2018). Unfortunately, these mechanisms may not be sufficient under high heat loads or if sows are under altered physiological states such as gestation (Heitman et al., 1951; Omtvedt et al., 1971), which can decrease farrowing rate, fertility, and litter size and negatively impact sow welfare (Renaudeau et al., 2003; Tummaruk et al., 2004; Almond and Bilkei, 2005; Ross et al., 2015). Therefore, it is important to understand how physiological changes impact a sow’s response to increasing heat loads, especially in sows selected for greater lean gain and litter size and overall higher heat production than genetics from >50 yr ago with which current environmental management recommendations are often based upon (Stinn and Xin, 2014).
HS susceptibility in sows is influenced by physiological changes that occur during gestation. For example, previous research indicates that RR and rectal temperature increase at a greater rate in late-gestation vs. nonpregnant sows exposed to HS (Heitman et al., 1951; Omtvedt et al., 1971). In addition, gestation stage can influence HS susceptibility and reproductive efficiency because sows that are exposed to HS during late-gestation (102 to 110 d pregnant) have reduced numbers of live piglets born and birth weights of live piglets relative to those exposed to HS during mid-gestation (53 to 61 d pregnant; Omtvedt et al., 1971). While it is known that pregnancy can influence the HS response in sows, there is a lack of current pertinent literature on how reproductive stage (nonpregnant vs. mid-gestation vs. late-gestation) influences a sow’s thermoregulatory and physiological response to increasing environmental heat load, particularly in sows with current genetics. Therefore, the study objective was to determine the thermoregulatory and physiological responses of sows with current genetics exposed to increasing environmental heat load at three reproductive stages: nonpregnant, mid-gestation (59.7 ± 9.6 d pregnant), and late-gestation (99.0 ± 4.8 d pregnant). Our alternative hypothesis was that late-gestation sows would have a more sensitive thermoregulatory and physiological response to increasing environmental heat load relative to mid-gestation and nonpregnant sows.
Materials and Methods
Animals
All procedures involving pigs were approved by the Purdue University Animal Care and Use Committee (protocol # 1811001823). Animal care and use standards were based upon the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Federation of Animal Sciences Societies, 2010). In 4 repetitions, 12 nonpregnant multiparous sows (n = 3/repetition; parity 3.27 ± 0.86; 129.9 ± 52.8 d postweaning), 12 mid-gestation multiparous sows (n = 3/repetition; parity 3.25 ± 0.83; 65.7 ± 17.2 d postweaning), and 12 late-gestation multiparous sows (n = 3/repetition; parity 3.33 ± 0.75; 102.8 ± 4.6 d postweaning) were tested. The sample size was selected based on the Mead Resource Equation (Mead, 1988), and sows were selected based on reproductive stage and parity. Body weight was recorded before and after the experiment and change in body weight was calculated. Following the experiment, it was discovered that one nonpregnant sow was pregnant during the time of testing and was placed into the mid-gestation category, post hoc, yielding 11 nonpregnant, 13 mid-gestation, and 12 late-gestation sows. All sows were Yorkshire × Landrace and pregnant sows were bred to Duroc sires. Sows were limit-fed (2.27 kg/d) a diet containing primarily corn (62.0%), soybean meal (12.9%), and distillers dried grains with solubles (20.0%) formulated to meet or exceed nutrient requirements for sows throughout the experiment per normal production practices (NRC, 2012).
Jugular catheter placement
Jugular catheters were placed in all sows following the procedures outlined by Marchant-Forde et al. (2012). Briefly, daily feed allotments were withheld on the day of the surgery, but water was available ad libitum. Anesthesia was induced by an intramuscular (IM) injection of a drug cocktail (0.02 mL/kg BW dose) that included tiletamine/zolazepam (100 mg/mL; Telazol C IIIN; Zoetis, Parsippany, NJ, USA), ketamine (50 mg/mL; KetaVed C III; Vedco Inc., Saint Joseph, MO), and xylazine (50 mg/mL; Anased; Lloyd Inc., Shenandoah, IA), and then anesthesia was maintained using 1% to 4% isoflurane with oxygen. An incision was made at the jugular fossa, the jugular vein was located, and tubing (Saint-Gobain Tygon ND 100-80 tubing; 1.27 mm inside diameter and 2.286 mm outside diameter; Saint-Gobain North America, Malvern, PA, USA) was inserted toward the heart and secured inside the jugular vein using suture (2-0 absorbable suture; Model #Z317H; Ethicon Inc, Somerville, NJ, USA). The catheter tubing was subsequently routed around the neck under the skin to an exit point just below the dorsal base of the neck using a trocar. A modified 16-gauge needle with the tip removed (BD PrecisionGlide Needle; Model # BD 305197; Fisher Scientific; Waltham, MA, USA) was inserted into the catheter tubing that exited the neck, the catheter was blocked with taurolidine-citrate (approximately 2 mL/catheter; Access Technologies; #TCS-04; Skokie, Illinois, USA), and a cap (Luer-Lok Caps; Model # 408530; Becton, Dickinson and Company; Franklin Lakes, NJ) was used to seal the catheter. Sows were given analgesia (2.2 mg/kg IM flunixin meglumine; Merck Animal Health, Madison, NJ, USA) both during and 24 hr post-surgery as well as an antibiotic (ceftiofur; 5 mg/kg IM; Zoetis, Parsippany, NJ, USA) during surgery. Following surgery and recovery, sows were moved into individual pens (1.22 × 2.01 m) in a thermoneutral (TN; 21.1 ± 2.0 °C and 29.4 ± 1.6% RH) room for 5.0 ± 0.7 d prior to the experiment.
Environmental exposure
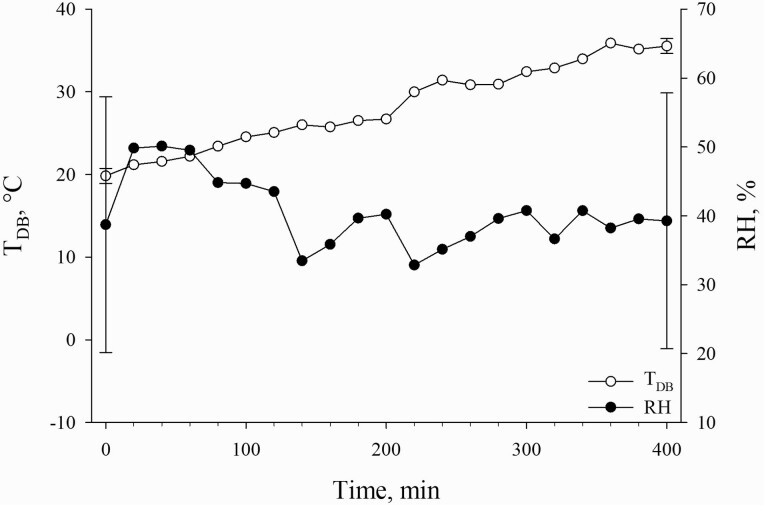
Sows were moved (approximately, 3 m walking distance as described by Kpodo et al., 2020) into individual pens (1.22 × 2.01 m) within an environmentally controlled room where the dry bulb temperature (TDB) was recorded at 15.1 ± 1.9 °C and the relative humidity (RH) was recorded at 50.7 ± 5.6%. Sows were allowed to acclimate to their new environment for 270 min, and then at the start of the experimental period, the TDB was recorded at 19.84 ± 2.15 °C. During the experiment, the TDB was increased incrementally by 2.45 ± 0.43 °C every 60 min from 19.84 ± 2.15 to 35.54 ± 0.43 °C over 400 min, and RH was measured at 40.49 ± 18.57% (Figure 1). The temperature load and pattern imposed were not designed to elicit a substantial HS response (i.e., a large increase in body temperature measures) but rather to characterize the sow’s response to increasing TDB and to determine thermoregulatory thresholds. The environmental room contained two data loggers (Hobo; data logger temperature/RH; accuracy ± 0.20 °C; Onset; Bourne, MA) mounted above the floor at sow standing level (approximately 1 m) on opposite ends of the room to record TDB and RH in 5-min intervals. Water disappearance was measured (1.9 cm NPT Water Meter; 1,033.5 kPa; 93.3 °C; ISTEC 1700 Series; Sparta, NJ) every 20 min during the entire experiment.
Figure 1.
Dry bulb temperature (TDB) and RH for the duration of the 400-min experimental period.
Physiological measurements
During the experiment, vaginal temperature (TV), skin temperature (TS), heart rate (HR), and RR were measured for all sows in 20-min intervals. A calibrated thermochron temperature recorder (iButton, calibrated accuracy ± 0.11 °C; resolution = 0.125 °C; Dallas Semi-conductor, Maxim, Irving, TX) was used to collect TV as previously described by Johnson and Shade (2017). Skin temperature was measured by taking a broadside photo of individual sows using an infrared camera (FLIR Model T440, accuracy ± 2%; emissivity = 0.98; resolution = 0.04 °C; FLIR Systems Inc.; Wilsonville, OR). Infrared photos were analyzed with the FLIR Tools Software (version 5.13). For image analysis, the minimum, maximum, and mean temperatures of the trunk region of the sow (i.e., all skin caudal to the neck and dorsal to the elbow and stifle) were recorded as previously described in Kpodo et al. (2019). HR (beats per min [bpm]) was measured using telemetric HR monitors (Polar S810i, Polar Electro Öy, Kempele, Finland), which have been previously validated for use in pigs (Marchant-Forde et al., 2004). RR (breaths per min [brpm]) was determined by counting flank movements for 15 s through visual observation and then multiplying by 4 as previously described (Kpodo et al., 2020). Post-experiment, sows were followed through gestation to record subsequent litter production data. Litter production data included: born, born alive, stillborn, dead (crushed), dead (other), birth weight (kg), and weaning weight (kg) on a per litter basis.
Blood collection and analyses
Whole blood samples (5 mL) were collected from jugular catheters every 20 min using 6 mL syringes (Monoject, Minneapolis, MN) and then placed into 6 mL serum tubes (BD vacutainers; Franklin Lakes, NJ). Additionally, at 1 h intervals, the whole blood samples (5 mL) were split into a 6-mL serum tube (3 mL of whole blood), a 2-mL K2 ethylenediaminetetraacetic acid tube (1 mL of whole blood; BD vacutainers; Franklin Lakes, NJ), and a 2-mL lithium heparin tube (1 mL whole blood; BD vacutainers; Franklin Lakes, NJ). Serum tube samples were centrifuged at 4 °C and 1,900 × g for 15 min, aliquoted, and stored at −80 °C for nitric oxide analysis. Blood samples collected in K2 ethylenediaminetetraacetic acid tubes were used to determine neutrophil and lymphocyte concentrations using a hematology analyzer (Genesis, Oxford Science Inc., Oxford, CT) within an hour post-collection. Lithium heparin samples were used to measure blood pH, carbon dioxide (CO2), oxygen (O2), base excess in the extracellular fluid compartment (BEECF), bicarbonate, and concentrations of sodium, potassium, glucose, hematocrit, and hemoglobin immediately post-collection using an on-site iStat Portable Clinical Analyzer (VetScan i-STAT, Abaxis Inc., Union City, CA) with a CG8+ cartridge (VetScan i-STAT1 CG8+ Cartridges; Model# 600-9001-25; Abaxis Inc., Union City, CA).
Prior to performing the nitric oxide analysis, serum was filtered using 30,000 MW cutoff filters (Millipore, Bedford, MA) to minimize peptide interference with the spectrophotometric reading. Samples were then diluted 1:5 and analyzed using a commercially available enzyme-linked immunosorbent assay kit (minimum detectable level: 2.5 µM; Nitrate/Nitrite Colorimetric Assay Kit, Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions. The nitric oxide intra- and inter-assay coefficients of variation were 4.57% and 11.43%, respectively.
Statistics
Data were analyzed via PROC MIXED in SAS 9.4 (SAS Institute Inc., Cary, NC). Reproductive stage was considered a fixed effect, and repetition, parity, and sow were considered random effects. Data collected over time were analyzed with repeated measures with the time as a fixed effect, the subject as sow(reproductive stage), and using the optimal covariance structure for each response variable as determined by the Bayesian information criterion goodness-of-fit criteria (Littell et al., 1998). A Kenward–Rogers degrees of freedom correction was applied to all repeated measures analysis via the ddfm = kr option of the model statement (Kenward and Roger, 1997). For all analyses, a preplanned statistical comparison was conducted for late-gestation vs. nonpregnant and mid-gestation sows using the CONTRAST statement in SAS. This contrast is a main effect across all time points. Time data will only be discussed when interacting with reproductive stage treatment, as only reproductive stage effects were of interest in the present study because it was expected that thermoregulatory and physiological differences would occur as time progressed (Liu et al., 2021). RR and offspring weaning weight data were log transformed, whereas stillborn numbers, dead piglet numbers, and neutrophil:lymphocyte were square root transformed to meet normality assumptions. However, back-transformed least squares means (LSM) and SEs are reported for ease of interpretation. Results are presented as LSM ± SEM unless otherwise stated. Statistical significance was defined as P ≤ 0.05 and a tendency was defined as 0.05 < P ≤ 0.10.
Results
Thermoregulation
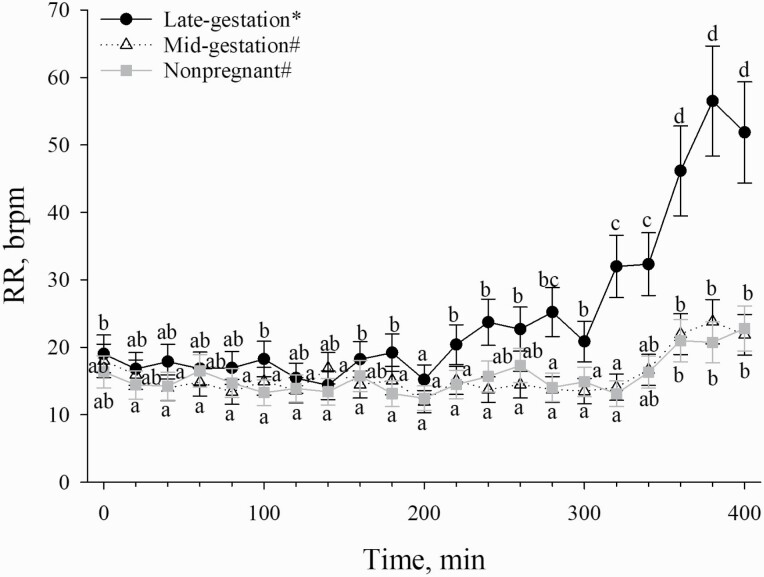
RR was greater (P < 0.01) in late-gestation sows when compared with mid-gestation sows at 100 min, from 160 to 180 min, and from 220 to 400 min (Figure 2). Additionally, RR was greater in late-gestation sows (P < 0.01) when compared with nonpregnant sows at 100, 180, and 220 min and from 280 to 400 min (Figure 2). The aforementioned increase in RR began at a lower TDB for late-gestation sows when compared with mid-gestation and nonpregnant sows (Figures 1 and 2). However, no RR differences were detected between nonpregnant sows and mid-gestation sows (Table 1; Figure 2). Overall, late-gestation sows had greater RR (P < 0.01; 23 ± 2 brpm) compared with mid-gestation (16 ± 2 brpm) and nonpregnant (15 ± 2 brpm) sows (Table 1; Figure 2). No other differences (P > 0.05) or tendencies (P > 0.10) in thermoregulation characteristics related to reproductive stage were detected (Table 1; Figures 2–4).
Figure 2.
Effects of reproductive stage (late-gestation, mid-gestation, and nonpregnant) on RR in sows exposed to incrementally increasing TDB over a 400-min period. Data are shown as LSM ± SEM. Lettersa–d indicate reproductive stage by time differences (P ≤ 0.05). Symbols*,# on the legend indicate the overall reproductive stage treatment differences (P ≤ 0.05).
Table 1.
Effect of reproductive stage on thermoregulatory characteristics of sows exposed to incrementally increasing TDB
| Characteristics | Reproductive stage | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Nonpregnant | Mid-gestation | Late-gestation | R1 | T2 | R × T3 | Contrast4 | ||
| TS, °C | 33.16 | 33.28 | 33.18 | 0.44 | 0.88 | <0.01 | 0.95 | 0.86 |
| TV, °C | 38.25 | 38.23 | 38.27 | 0.14 | 0.97 | <0.01 | 0.80 | 0.84 |
| RR, brpm | 15a | 16a | 23b | 2 | <0.01 | <0.01 | <0.01 | <0.01 |
| HR, bpm | 69a | 76b | 84c | 5 | <0.01 | <0.01 | 0.32 | <0.01 |
1Reproductive stage.
2Time.
3Reproductive stage × time interaction.
4Late-gestation vs. mid-gestation and nonpregnant.
a–cLetters indicate differences (P ≤ 0.05) between LSM within a row for reproductive stage differences.
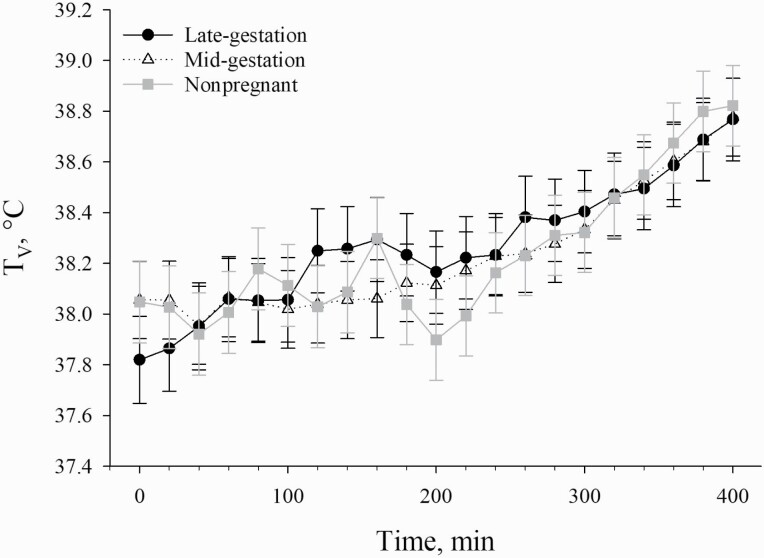
Figure 4.
Effects of reproductive stage (late-gestation, mid-gestation, and nonpregnant) on TV in sows exposed to incrementally increasing TDB over a 400-min period. Data are shown as LSM ± SEM.
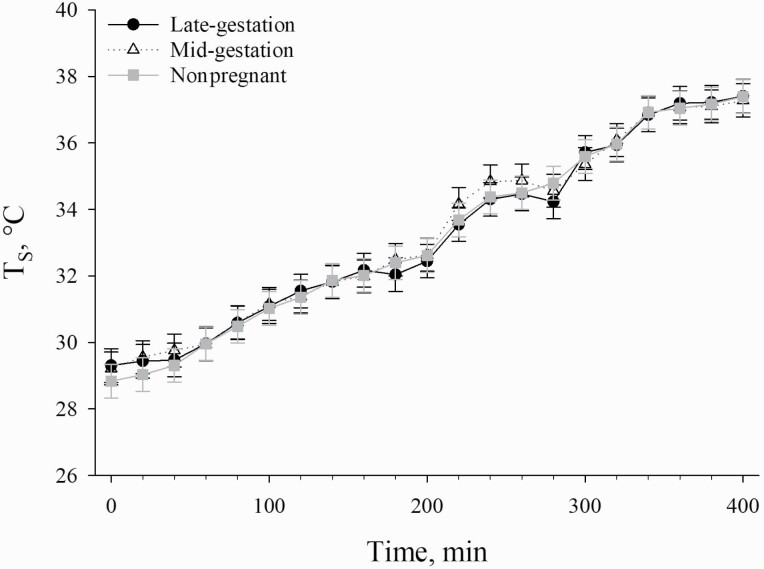
Figure 3.
Effects of reproductive stage (late-gestation, mid-gestation, and nonpregnant) on TS in sows exposed to incrementally increasing TDB over a 400-min period. Data are shown as LSM ± SEM.
Heart rate
Overall, late-gestation sows had increased HR (P < 0.01; 15.9%) when compared with mid-gestation and nonpregnant sows (Table 1). Mid-gestation sows had overall greater (P < 0.01; 10.1%) HR when compared with nonpregnant sows (Table 1). No other differences (P > 0.05) or tendencies (P > 0.10) in HR related to reproductive stage were detected (Table 1).
Blood characteristics
Overall, late-gestation sows tended to have reduced BEECF (P = 0.06; 344.0%) when compared with mid-gestation and nonpregnant sows, but no differences were detected between mid-gestation and nonpregnant sows (Table 2). Late-gestation sows had overall reduced bicarbonate levels (P = 0.02; 10.0%) compared with mid-gestation and nonpregnant sows, but no differences were detected between mid-gestation and nonpregnant sows (Table 2). Total CO2 levels were reduced overall for late-gestation sows (P = 0.02; 9.5%) when compared with mid-gestation and nonpregnant sows, but no differences were detected between mid-gestation and nonpregnant sows (Table 2). Sodium levels tended to be greater overall in late-gestation sows (P = 0.09; 1.7%) when compared with mid-gestation and nonpregnant sows, but no differences were detected between mid-gestation and nonpregnant sows (Table 2). Nitric oxide levels were greater for late-gestation sows (P < 0.01; 312.0%) when compared with mid-gestation and nonpregnant sows (Table 2). Mid-gestation sows had greater nitric oxide levels (P < 0.01; 166.0%) when compared with nonpregnant sows (Table 2). Contrasts indicated that late-gestation sows tended to have reduced (P = 0.09) hematocrit (6.6%) and hemoglobin levels (6.2%) compared with mid-gestation and nonpregnant sows (Table 2). No other differences (P > 0.05) or tendencies (P > 0.10) in blood characteristics related to reproductive stage were detected (Table 2).
Table 2.
Effect of reproductive stage on blood characteristics of sows exposed to incrementally increasing TDB
| Characteristics | Reproductive stage | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Nonpregnant | Mid-gestation | Late-gestation | R1 | T2 | R × T3 | Contrast4 | ||
| Neutrophil:lymphocyte | 0.98 | 0.98 | 1.00 | 0.24 | 0.99 | 0.54 | 0.39 | 0.91 |
| pH | 7.36 | 7.39 | 7.33 | 0.06 | 0.43 | 0.20 | 0.80 | 0.26 |
| Partial pressure CO2, mmHg | 46.30 | 45.20 | 46.75 | 4.27 | 0.89 | 0.08 | 0.71 | 0.71 |
| Partial pressure O2, mmHg | 32.15 | 32.41 | 37.48 | 18.30 | 0.60 | 0.04 | 0.72 | 0.32 |
| BEECF, mmol/L | 0.60 | 1.76 | −2.18 | 2.45 | 0.06 | 0.04 | 0.57 | 0.02 |
| Bicarbonate, mmol/L | 26.08a | 27.03a | 23.89b | 1.97 | 0.02 | 0.02 | 0.42 | <0.01 |
| Total CO2, mmol/L | 27.58a | 28.58a | 25.41b | 2.07 | 0.02 | <0.01 | 0.34 | <0.01 |
| Saturated O2, % | 73.79 | 71.49 | 70.50 | 5.27 | 0.69 | <0.01 | 0.75 | 0.42 |
| Sodium, mmol/L | 146.23 | 145.66 | 148.43 | 1.87 | 0.09 | 0.03 | 0.44 | 0.03 |
| Potassium, mmol/L | 3.51 | 3.68 | 3.60 | 0.32 | 0.35 | 0.18 | 0.81 | 0.93 |
| Glucose, mg/dL | 79.37 | 76.22 | 75.21 | 5.02 | 0.44 | 0.02 | 0.12 | 0.36 |
| Hematocrit, percent packed cell volume | 28.91 | 27.93 | 26.65 | 0.88 | 0.18 | <0.01 | 0.49 | 0.09 |
| Hemoglobin, g/dL | 9.83 | 9.50 | 9.06 | 0.30 | 0.18 | <0.01 | 0.51 | 0.09 |
| Nitric Oxide, µM | 41.55a | 110.47b | 248.82c | 34.54 | <0.01 | 0.33 | 0.33 | <0.01 |
1Reproductive stage.
2Time.
3Reproductive stage × time interaction.
4Late-gestation vs. mid-gestation and nonpregnant.
a–cLetters indicate differences (P ≤ 0.05) between LSM within a row for reproductive stage differences.
Body weight and water disappearance
No differences (P > 0.05) or tendencies (P > 0.10) in body weight or water disappearance were observed (Table 3).
Table 3.
Effect of reproductive stage on body weight and water disappearance of sows exposed to incrementally increasing TDB
| Characteristics | Reproductive stage | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Nonpregnant | Mid-gestation | Late-gestation | R1 | T2 | R × T3 | Contrast4 | ||
| Initial body weight, kg | 244.2 | 218.9 | 251.2 | 25.52 | 0.19 | — | — | 0.15 |
| Final body weight, kg | 241.9 | 216.7 | 249.2 | 26.51 | 0.20 | — | — | 0.14 |
| Δ body weight5, kg | −2.3 | −2.2 | −2.0 | 0.99 | 0.22 | — | — | 0.18 |
| Water disappearance, L | 0.19 | 0.23 | 0.26 | 0.16 | 0.84 | 0.17 | 0.39 | 0.39 |
1Reproductive stage.
2Time.
3Reproductive stage × time interaction.
4Late-gestation sows vs. mid-gestation and nonpregnant sows.
5Final body weight minus initial body weight.
Reproductive performance
No differences (P > 0.05) or tendencies (P > 0.10) in reproductive performance were observed (Table 4).
Table 4.
Effects of reproductive stage on reproductive performance of sows exposed to incrementally increasing TDB
| Characteristics1 | Reproductive stage | SEM | P-value | |
|---|---|---|---|---|
| Late-gestation2 | Mid-gestation3 | R4 | ||
| Born | 17.06 | 15.78 | 7.41 | 0.48 |
| Born alive | 15.55 | 15.04 | 6.76 | 0.76 |
| Stillborn | 0.53 | 0.18 | 0.25 | 0.31 |
| Dead (crushed) | 0.77 | 0.26 | 0.27 | 0.19 |
| Dead (other) | 0.27 | 0.25 | 0.22 | 0.94 |
| Birth weight, kg | 2.19 | 2.13 | 0.03 | 0.46 |
| Weaning weight, kg | 5.88 | 4.90 | 1.01 | 0.25 |
1Litter basis.
2 n = 12 sows.
3 n = 13 sows.
4Reproductive stage.
Discussion
Advancing gestation causes an increase in metabolic heat production due to greater fetal growth and development (He et al., 2019). Unfortunately, this also increases heat gain by the sow, which may lead to an increased sensitivity to greater environmental heat loads (Zumbach et al., 2008). As a result of increased heat gain, it is likely that advancing gestation stage would require greater heat loss efforts by sows through vasodilation of the skin (i.e., radiation, convection, and conduction) and increasing RR (Omtvedt et al., 1971) in order to maintain euthermia. In the present study, although no reproductive stage-related TS differences were detected, RR began to increase at a lower TDB for late-gestation sows when compared with mid-gestation and nonpregnant sows. RR is a vital heat loss mechanism that relies on evaporation and is considered one of the most sensitive indicators of HS in pigs (Patience et al., 2005). Therefore, an earlier increase in RR for late-gestation sows at a lower TDB may suggest that they were more sensitive to the increasing environmental heat load compared with mid-gestation and nonpregnant sows.
Despite the earlier increase in RR at a lower TDB for late-gestation sows compared with mid-gestation and nonpregnant sows, there were no reproductive stage differences detected for TV. However, given the relatively short time frame, sows were exposed to TDB that exceeded their upper critical temperature limit, the incremental nature of the TDB increase, the fact that this study was not designed to elicit a substantial HS response, and the greater RR of late-gestation sows as a heat loss mechanism, this result was expected. Rather, these data likely indicate that the rapid increase in RR for late-gestation sows was effective in dissipating the additional heat generated by the developing fetuses when compared with mid-gestation fetuses, thereby allowing the late-gestation sows to maintain euthermia during the course of the experiment. Because all sows were limit fed (i.e., similar heat of nutrient processing), had a similar body weight, and had similar fetus numbers (as indicated by reproductive data), the need to increase RR to maintain TV at euthermia was likely a result of a rapid increase in fetal growth that begins to occur at approximately 40 to 60 d of gestation leading to an increase in heat production and subsequently heat gain by the sow (Zhao et al., 2020). Therefore, these data indicate that sow HS sensitivity differences exist based upon reproductive stage and are likely related to late-term fetal growth.
The greater and more rapid increase in RR for late gestation sows due to fetal metabolic heat production resulted in altered blood characteristics associated with greater HS sensitivity. When RR increases due to HS exposure, blood CO2 and bicarbonate are reduced, which may lead to respiratory alkalosis (West, 2003; Renaudeau et al., 2011; Cottrell et al., 2015). In the present study, blood CO2 and bicarbonate levels were reduced for late-gestation sows when compared with mid-gestation and nonpregnant sows, and this was a likely result of the aforementioned greater and more rapid increase in RR to dissipate excess body heat. Because previous research has demonstrated that reduced blood CO2 and bicarbonate are associated with greater HS sensitivity in pigs (Boddicker et al., 2014), this response likely indicates that late-gestation sows were more sensitive to increasing TDB when compared with mid-gestation and nonpregnant sows.
To support the increase of blood flow to the skin due to vasodilation, HR is increased, and previous studies have used HR as a measure of physiological strain in pigs exposed to greater heat loads (Moran et al., 1998). Despite the fact that late-gestation sows appeared to be more HS sensitive as indicated by a greater and more rapid increase in RR and altered blood CO2 and bicarbonate, no interactions between reproductive stage and increasing TDB were detected for HR. While this is somewhat unexpected given the apparent increase in HS sensitivity of late-gestation sows, there are conflicting reports regarding the use of HR to predict HS sensitivity in pigs. For example, while one study reported an increase in HR for pigs exposed to a diurnal HS pattern (Patience et al., 2005), others report a decrease in HR for HS-exposed sows (Fraser, 1970; Laspiur and Trottier, 2001). Nevertheless, an overall increase in HR was detected for late-gestation sows when compared with mid-gestation and nonpregnant sows, regardless of TDB exposure. Although literature on HR differences related to reproductive stage in sows is limited, previous reports indicate that a greater HR may be a result of greater sympathetic activity (Parois et al., 2018). Because pregnancy (especially during late-gestation; Mizuno et al., 2017) increases sympathetic activity (and subsequently HR) in humans, this may explain the overall increase in HR for late-gestation sows when compared with mid-gestation and nonpregnant sows in the present study. Future research should focus on the cardiac output increases that occur in gestating sows.
Nitric oxide may be an indirect measure of vasodilation (Kellogg et al., 1999) that is often associated with increased TS resulting from exposure to increasing TDB (Kellogg et al., 1998). Vasodilation increases in response to elevated TDB to support greater heat dissipation through the skin by increasing blood flow to the periphery to dissipate body heat (Farrell and Bishop, 1995; Charkoudian, 2003; Ogoh et al., 2013), which may be measured by increases in TS (Silanikove, 2000). Because there were no reproductive stage by TDB exposure-related differences in TS, it was expected that there would be no nitric oxide differences when comparing the interaction between reproductive stage and TDB exposure. Despite this, an overall effect of reproductive stage on nitric oxide levels was detected. Late-gestation sows had an approximately a 2-fold and 6-fold increase in circulating nitric oxide when compared with mid-gestation and nonpregnant sows, respectively. Although literature on nitric oxide levels in pigs is limited, especially when comparing different reproductive stages, previous research in sheep indicates that circulating nitric oxide levels are elevated during the last third of gestation, and this may be due to the regulation of glucose or blood flow (Massmann et al., 1999). Therefore, future research should elucidate the physiological role of nitric oxide in sows at different reproductive stages.
Conclusions
Advancing gestation increases metabolic heat production in sows and may result in greater susceptibility to increasing environmental heat load. However, knowledge about the thermoregulatory and physiological responses of gestating sows with current genetics is limited, which may result in inaccurate or imprecise barn environmental management under HS conditions. Based on previous research, we hypothesized that late-gestation sows would have a more sensitive thermoregulatory and physiological response to increasing environmental heat load relative to mid-gestation and nonpregnant sows. When compared with mid-gestation and nonpregnant sows, late-gestation sows had increased RR and altered blood characteristics in response to increasing TDB, which are indicative of greater HS sensitivity. However, minimal differences between mid-gestation and nonpregnant sows exist in thermoregulatory and physiological responses to increasing TDB. These data provide evidence that late-gestation sows should be managed differently during times of HS as they may succumb to HS at lower TDB when compared with mid-gestation and nonpregnant sows. Future studies should investigate interventions to reduce the negative impact of HS during gestation as well as to elucidate the sow’s response to long-term changes in TDB.
Acknowledgments
This research was supported by the USDA National Institute of Food and Agriculture—Agriculture and Food Research Initiative grant (2018-67015-28130). In addition, we would like to thank the Purdue University Swine Farm staff and students as well as the USDA-ARS Livestock Behavior Research Unit employees for their help with data collection, animal care, and lab analyses.
Glossary
Abbreviations
- BEECF
base excess in the extracellular fluid compartment
- bpm
beats per min
- brpm
breaths per min
- HR
heart rate
- HS
heat stress
- IM
intramuscular
- RH
relative humidity
- RR
respiration rate
- TDB
dry bulb temperature
- TN
thermoneutral
- TS
skin temperature
- TV
vaginal temperature
Conflict of interest statement
The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. In addition, this research was supported by an appointment to the Agricultural Research Service (ARS) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Agriculture (USDA). ORISE is managed by (Oak Ridge Associated Universities) ORAU under DOE contract number DE-SC0014664. All opinions expressed in this paper are the author’s and do not necessarily reflect the policies and views of USDA, ARS, DOE, or ORAU/ORISE. No conflict of interest, financial, or otherwise are declared by the authors.
Literature Cited
- Almond, P. K., and Bilkei G.. . 2005. Seasonal infertility in large pig production units in an Eastern-European climate. Aust. Vet. J. 83:344–346. doi: 10.1111/j.1751-0813.2005.tb15627.x [DOI] [PubMed] [Google Scholar]
- Boddicker, R. L., Seibert J. T., Johnson J. S., Pearce S. C., Selsby J. T., Gabler N. K., Lucy M. C., Safranski T. J., Rhoads R. P., Baumgard L. H., . et al. 2014. Gestational heat stress alters postnatal offspring body composition indices and metabolic parameters in pigs. PLoS One. 9:e110859. doi: 10.1371/journal.pone.0110859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian, N. 2003. Skin blood flow in adult human thermo-regulation: how it works, when it does not, and why. Mayo Clinic. Proceed. 78:603–612. doi: 10.4065/78.5.603 [DOI] [PubMed] [Google Scholar]
- Cottrell, J. J., Liu F., Hung A. T., DiGiacomo K., Chauhan S. S., Leury B. J., Furness J. B., Celi P., and Dunshea F. R.. . 2015. Nutritional strategies to alleviate heat stress in pigs. Anim. Prod. Sci. 55:1391–1402. doi: 10.1071/AN15255 [DOI] [Google Scholar]
- Farrell, D. M., and Bishop V. S.. . 1995. Permissive role for nitric oxide in active thermoregulatory vasodilation in rabbit ear. Am. J. Physiol. 269(5 Pt 2):H1613–H1618. doi: 10.1152/ajpheart.1995.269.5.H1613 [DOI] [PubMed] [Google Scholar]
- Federation of Animal Sciences Societies. 2010. Guide for the care and use of agricultural animals in research and teaching. 3rd ed. Champaign (IL):Federation of Animal Sciences Societies. Chap. 11. [Google Scholar]
- Fraser, A. F. 1970. Studies on heat stress in pigs in a tropical environment. Trop. Anim. Health Prod. 2:76–86. doi: 10.1007/BF02359573 [DOI] [Google Scholar]
- He, J., Guo H., Zheng W., Xue Y., Zhao R., and Yao W.. . 2019. Heat stress affects fecal microbial and metabolic alterations of primiparous sows during late gestation. J. Anim. Sci. Biotechnol. 10:1–12. doi: 10.1186/s40104-019-0391-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman, H., Hughes E. H., and Kelly C. F.. . 1951. Effects of elevated ambient temperature on pregnant sows. J. Anim. Sci. 10:907–915. doi: 10.2527/jas1951.104907x [DOI] [Google Scholar]
- Johnson, J. S., and Shade K. A.. . 2017. Characterizing body temperature and activity changes at the onset of estrus in replacement gilts. Livest. Sci. 199:22–24. doi: 10.1016/j.livsci.2017.03.004 [DOI] [Google Scholar]
- Kellogg, D. L.Jr, Crandall C. G., Liu Y., Charkoudian N., and Johnson J. M.. . 1998. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J. Appl. Physiol. (1985). 85:824–829. doi: 10.1152/jappl.1998.85.3.824 [DOI] [PubMed] [Google Scholar]
- Kellogg, D. L.Jr, Liu Y., Kosiba I. F., and O′Donnell D.. . 1999. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J. Appl. Physiol. (1985). 86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185 [DOI] [PubMed] [Google Scholar]
- Kenward, M. G., and Roger J. H.. . 1997. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997. doi: 10.2307/2533558 [DOI] [PubMed] [Google Scholar]
- Kpodo, K. R., Duttlinger A. W., and Johnson J. S.. . 2019. Effects of pen location on thermoregulation and growth performance in grow-finish pigs during late summer. Transl. Anim. Sci. 3:1375–1382. doi: 10.1093/tas/txz033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kpodo, K. R., Duttlinger A. W., Maskal J. M., and Johnson J. S.. . 2020. Effects of feed removal on thermoregulation and intestinal morphology in pigs recovering from acute hyperthermia. J. Anim. Sci. 98:1–10. doi: 10.1093/jas/skaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laspiur, J. P., and Trottier N. L.. . 2001. Effect of dietary arginine supplementation and environmental temperature on sow lactation performance. Livest. Prod. Sci. 70:159–165. doi: 10.1016/S0301-6226(01)00209-3 [DOI] [Google Scholar]
- Littell, R. C., Henry P. R., and Ammerman C. B.. . 1998. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 76:1216–1231. doi: 10.2527/1998.7641216x [DOI] [PubMed] [Google Scholar]
- Liu, L., Tai M., Yao W., Zhao R., and Shen M.. . 2021. Effects of heat stress on posture transitions and reproductive performance of primiparous sows during late gestation. J. Therm. Biol. 96:102828. doi: 10.1016/j.jtherbio.2020.102828 [DOI] [PubMed] [Google Scholar]
- Marchant-Forde, R. M., Marlin D. J., and Marchant-Forde J. N.. . 2004. Validation of a cardiac monitor for measuring heart rate variability in adult female pigs: accuracy, artefacts and editing. Physiol. Behav. 80:449–458. doi: 10.1016/j.physbeh.2003.09.007 [DOI] [PubMed] [Google Scholar]
- Marchant-Forde, J. N., Matthews D. L., Poletto R., McCain R. R., Mann D. D., DeGraw R. T., Hampsch J. M., Peters S., Knipp G. T., and Kissinger C. B.. . 2012. Plasma cortisol and noradrenalin concentrations in pigs: automated sampling of freely moving pigs housed in the PigTurn® versus manually sampled and restrained pigs. Anim. Welf. 21:197. doi: 10.7120/09627286.21.2.197 [DOI] [Google Scholar]
- Massmann, G. A., Zhang J., and Figueroa J. P.. . 1999. Functional and molecular characterization of nitric oxide synthase in the endometrium and myometrium of pregnant sheep during the last third of gestation. Am. J. Obstet. Gynecol. 181:116–125. doi: 10.1016/S0002-9378(99)70446-1 [DOI] [PubMed] [Google Scholar]
- Mead, R. 1988. The design of experiments. Cambridge/New York (NY): Cambridge University Press; 620 p. [Google Scholar]
- Mizuno, T., Tamakoshi K., and Tanabe K.. . 2017. Anxiety during pregnancy and autonomic nervous system activity: a longitudinal observational and cross-sectional study. J. Psychosom. Res. 99:105–111. doi: 10.1016/j.jpsychores.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Moran, D. S., Shitzer A., and Pandolf K. B.. . 1998. A physiological strain index to evaluate heat stress. Am. J. Physiol. 275:R129–R134. doi: 10.1152/ajpregu.1998.275.1.R129 [DOI] [PubMed] [Google Scholar]
- Nichols, D. A., Ames D. R., and Hines R. H.. . 1982. Effect of temperature on performance and efficiency of finishing swine. In: Proceedings of the 2nd International Livestock Environment Symposium, Ames, lA. ASAE Publ. No. 3-82. St. Joseph (MI): ASAE; p. 376–379. [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th ed. Washington (DC):National Academy Press. [Google Scholar]
- Ogoh, S., Sato K., Okazaki K., Miyamoto T., Hirasawa A., Morimoto K., and Shibasaki M.. . 2013. Blood flow distribution during heat stress: cerebral and systemic blood flow. J. Cereb. Blood Flow Metab. 33:1915–1920. doi: 10.1038/jcbfm.2013.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omtvedt, I. T., Nelson R. E., Edwards R. L., Stephens D. F., and Turman E. J.. . 1971. Influence of heat stress during early, mid and late pregnancy of gilts. J. Anim. Sci. 32:312–317. doi: 10.2527/jas1971.322312x [DOI] [PubMed] [Google Scholar]
- Parois, S. P., Cabezón F. A., Schinckel A. P., Johnson J. S., Stwalley R. M., and Marchant-Forde J. N.. . 2018. Effect of floor cooling on behavior and heart rate of late lactation sows under acute heat stress. Front. Vet. Sci. 5:223. doi: 10.3389/fvets.2018.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patience, J. F., Umboh J. F., Chaplin R. K., and Nyachoti C. M.. . 2005. Nutritional and physiological responses of growing pigs exposed to a diurnal pattern of heat stress. Livest. Prod. Sci. 96:205–214. doi: 10.1016/j.livprodsci.2005.01.012 [DOI] [Google Scholar]
- Renaudeau, D., Gourdine J. L., and St-Pierre N. R.. . 2011. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J. Anim. Sci. 89:2220–2230. doi: 10.2527/jas.2010-3329 [DOI] [PubMed] [Google Scholar]
- Renaudeau, D., Noblet J., and Dourmad J. Y.. . 2003. Effect of ambient temperature on mammary gland metabolism in lactating sows. J. Anim. Sci. 81:217–231. doi: 10.2527/2003.811217x [DOI] [PubMed] [Google Scholar]
- Ross, J. W., Hale B. J., Gabler N. K., Rhoads R. P., Keating A. F., and Baumgard L. H.. . 2015. Physiological consequences of heat stress in pigs. Anim. Prod. Sci. 55:1381–1390. doi: 10.1071/AN15267 [DOI] [Google Scholar]
- Silanikove, N. 2000. Effects of heat stress on the welfare of extensively managed domestic ruminants. Livest. Prod. Sci. 67:1–18. doi: 10.1016/S0301-6226(00)00162-7 [DOI] [Google Scholar]
- Stinn, J. P., and Xin H.. . 2014. Heat and moisture production rates of a modern U.S. swine breeding-gestation farrowing facility. Trans. ASABE. 57:1517–1528. doi: 10.13031/trans.57.10711 [DOI] [Google Scholar]
- Tummaruk, P., Tantasuparuk W., Techakumphu M., and Kunavongkrit A.. . 2004. Effect of season and outdoor climate on litter size at birth in purebred Landrace and Yorkshire sows in Thailand. J. Vet. Med. Sci. 66:477–482. doi: 10.1292/jvms.66.477 [DOI] [PubMed] [Google Scholar]
- West, J. W. 2003. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 86:2131–2144. doi: 10.3168/jds.S0022-0302(03)73803-X [DOI] [PubMed] [Google Scholar]
- Zhao, W., Liu F., Bell A. W., Le H. H., Cottrell J. J., Leury B. J., Green M. P., and Dunshea F. R.. . 2020. Controlled elevated temperatures during early-mid gestation cause placental insufficiency and implications for fetal growth in pregnant pigs. Sci. Rep. 10:20677. doi: 10.1038/s41598-020-77647-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbach, B., Misztal I., Tsuruta S., Sanchez J. P., Azain M., Herring W., Holl J., Long T., and Culbertson M.. . 2008. Genetic components of heat stress in finishing pigs: development of a heat load function. J. Anim. Sci. 86:2082–2088. doi: 10.2527/jas.2007-0523 [DOI] [PubMed] [Google Scholar]