Abstract
Objective
Obesity and adverse metabolic outcomes in patients with severe mental illness are clinically significant but potentially preventable. Importantly, the evidence for switching to antipsychotics to reduce cardiometabolic burden is unclear.
Method
PubMED, Embase, PsycINFO, and Cochrane were searched from inception to March 8, 2020. Articles reporting weight and metabolic changes after antipsychotic switching vs staying on the previous antipsychotic were meta-analyzed both across and within group.
Results
Of 61 identified studies, 59 were meta-analyzed (40% rated high quality). In the switch-vs-stay pairwise meta-analyses, only aripiprazole significantly reduced weight (−5.52 kg, 95% CI −10.63, −0.42, P = .03), while olanzapine significantly increased weight (2.46 kg, 95% CI 0.34, 4.57, P = .02). Switching to aripiprazole also significantly improved fasting glucose (−3.99 mg/dl, 95% CI −7.34, −0.64, P = .02) and triglycerides (−31.03 mg/dl, 95% CI −48.73, −13.34, P = .0001). Dropout and psychosis ratings did not differ between switch and stay groups for aripiprazole and olanzapine. In before-to-after switch meta-analyses, aripiprazole (−1.96 kg, 95% CI −3.07, −0.85, P < .001) and ziprasidone (−2.22 kg, 95% CI −3.84, −0.60, P = .007) were associated with weight loss, whereas olanzapine (2.71 kg, 95% CI 1.87, 3.55, P < .001), and clozapine (2.80 kg, 95% CI 0.26, 5.34, P = .03) were associated with weight gain. No significant weight or other cardiometabolic changes were observed when switching to amisulpride, paliperidone/risperidone, quetiapine, or lurasidone.
Conclusions
Switching antipsychotics to agents with lower weight gain potential, notably to aripiprazole and ziprasidone, can improve weight profile and other cardiometabolic outcomes. When choosing switch agents, both the weight gain potential of the pre- and post-switch antipsychotic must be considered. Antipsychotic switching in psychiatrically stable patients must be weighed against the risk of psychiatric worsening.
Keywords: obesity, schizophrenia, bipolar disorder, antipsychotics, switching
Introduction
The prevalence of obesity in patients with severe mental illness (SMI) is up to 60%, twice the rate of the general population.1,2 In turn, obesity and dyslipidaemia, along with hypertension, and smoking, are major risk factors for cardiovascular disease, type 2 diabetes mellitus (T2DM) and cancer.3,4 These physical comorbidities contribute to the 3-fold increase in standardized mortality rate5 and a 15-year reduction in life expectancy6 compared to the general population. Being overweight or obese also increases the risk of all-cause mortality.7,8
Second-generation antipsychotics (SGA) are important contributors to weight gain.9,10 The resulting obesity can influence a patient’s perception of their treatment, resulting in poor adherence and subsequent exacerbation of symptoms, reduction in quality of life, and readmission to hospital.11–13
Strategies to counteract weight gain14 include lifestyle interventions15 and augmentation with metformin16 or glucagon-like peptide 1 receptor agonists (eg, exenatide, liraglutide).17 Given the challenges associated with people with SMI engaging in lifestyle interventions18 or the risks of polypharmacy,9 switching to an antipsychotic with a lower propensity to induce weight gain may be preferable.19
Although antipsychotic switching has been recommended as strategy to mitigate metabolic disorders among people with severe mental illness,2,9 the efficacy of switching and the most appropriate choice of agent are unclear. To our knowledge, only one preliminary meta-analysis (studies = 4, n = 636), published a decade ago, investigated the cardiometabolic effects of antipsychotic switching.20 We updated this review and included secondary outcomes, ie, other cardiometabolic measures, dropouts, and psychotic symptoms.
Method
Protocol
This systematic review followed a prespecified PROSPERO (International Prospective Register of Systematic Reviews) protocol (CRD42018114907) and we followed the PRISMA guidelines.21
Search Strategy
We searched PubMED, Embase, PsycINFO, and the Cochrane Library from inception to March 8, 2020. The basic search terms included “Switch* OR Substitut* OR Comparative AND Diabetes OR Obesity OR Weight OR BMI OR Obese OR Cholesterol OR Triglyceride* OR Blood Pressure OR “Metabolic Syndrome” AND (list of predefined antipsychotics) OR Antipsychotic*” (supplementary table 1.1). Two authors (W.M. and E.G.) screened at title abstract and full text levels. We hand searched included studies for relevant references and contacted experts regarding potential unpublished data.
Selection Criteria
We included randomized controlled trials or observational studies of people with SMI), such as schizophrenia and related psychoses, bipolar disorder, and major depressive disorder, that investigated a switch from any single or combination of antipsychotics to a different antipsychotic monotherapy for ≥4 weeks. Studies had to report endpoint and or mean change for cardiometabolic parameters, such as weight, body mass index (BMI), waist circumference, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, total cholesterol, triglycerides, systolic and or diastolic blood pressure, blood glucose levels, glycated hemoglobin (HbA1c), insulin, or presence of metabolic syndrome. We included comparisons of switching antipsychotics (“switch”) vs remaining on the same agent (“stay”), as well as studies without such controls. We excluded case reports, review articles, editorials, conference abstracts, articles using overlapping datasets to included studies, and studies solely switching from paliperidone to risperidone, or vice versa, as these SGAs were not considered sufficiently different. A switch to either paliperidone or risperidone was analyzed together.
Data Extraction
One author extracted data on study characteristics, participant details, clinical outcomes, efficacy measures, and metabolic parameters, which was validated by a second author (W.M. and E.G.). The primary outcome was change in weight when switching from one to another antipsychotic compared to staying in the same agent. We also examined before-to-after switch data on weight on commencing an antipsychotic agent. Secondary outcomes were pair-wise or before-to-after analyses of other cardiometabolic outcomes, including BMI, waist circumference, total, HDL and LDL cholesterol, triglycerides, fasting blood glucose, HbA1c, and insulin. Data were extracted on the antipsychotic used prior to the switch. Where switches from specific single antipsychotics were not available, we categorized prior antipsychotics as either mixed SGAs, mixed first-generation antipsychotics (FGAs), or mixed FGA/SGAs. Finally, we extracted data on drop-outs and changes in the scores of 2 total psychosis symptoms scales; the Brief Psychiatric Rating Scale (BPRS) and Positive and Negative Syndrome Scale (PANSS).
Risk of Bias Within Studies
We assessed studies, which compared switching to a new antipsychotic vs staying on a previous antipsychotic using the following criteria adapted from the Cochrane Collaboration guidelines22: (1) adequate generation of allocation sequence; (2) adequate random sequence generation; (3) blinding of allocation to conditions to participants; (4) blinding of allocation to conditions to assessor; (5) appropriate reporting on missing data; (6) reporting aligns with prespecified primary outcome measures; and (7) other sources of potential bias including pharmaceutical company funding. Studies were deemed to be of high quality if they had at least 4 domains with low risk of bias. To clarify selective outcome reporting, clinical trial registries were searched, and registered trials compared against the published studies.
For studies that only had data before-to-after switching, we used the modified Newcastle-Ottawa Quality Assessment Scale (supplementary table 4.3).23 A high-quality study has a score of 4 or more (maximum 5). We assessed 5 domains: representativeness of the sample; sample size (low bias if study size >200); loss to follow up; ascertainment of diagnosis of severe mental illness; and quality of descriptive statistics.
Statistical Analyses
We performed a pairwise meta-analyses using Comprehensive Meta-Analysis version 3.3 and Review Manager version 5.3.5 for comparisons of switching to a specific antipsychotic vs remaining on the previous agent. We also conducted a meta-analysis of before-to-after data of switching to a different antipsychotic. Meta-analyses were only included if at least 2 studies had useable data for the outcome of interest. The primary outcome of interest was the pairwise comparison of change in weight, with secondary outcomes of pairwise differences in other cardiometabolic symptoms, dropouts, and psychosis scores, and before-to-after changes in weight, plus other cardiometabolic outcomes, dropouts, and psychosis symptoms. Where possible, outcomes were presented as mean difference but we used the standardized mean difference (SMD) when primary data required conversion to effect size for combination in a meta-analysis, notably different psychosis scores. We used random effects models as we were unable to assume homogeneity among studies.
We conducted sensitivity analyses on prior antipsychotic, study setting (hospital or community), diagnosis, whether or not the study had weight loss as a primary outcome, age group of participants (child and youth or adult), and country of study. We undertook a meta-regression on dropouts, study duration, mean baseline BMI, mean age of participants, and mean duration of illness. We explored heterogeneity using the I2 statistic, with an I2 >50% indicating significant heterogeneity. We investigated publication bias, using Kendal’s Tau and Egger’s test,24 when meta-analyses included ≥10 studies.
Results
Included Studies
We identified 5,767 unique studies, of which 5,435 were excluded at title and abstract level. An additional 270 were excluded at full text review (supplementary table 3.1). We included 61 articles in the systematic review; 59 articles contributed to the meta-analyses (supplementary appendix 2). There were 8,554 participants with a mean age of 39.2 ± 9.9 years and 56.9% males. Mean study duration was 26.3 ± 19.7 weeks. Studies came from North America (n = 21), South America (n = 1), Europe (n = 8), Asia (n = 22), the Middle East (n = 2); and multiple countries (n = 7). The antipsychotics switched to included aripiprazole (n = 16), amisulpride (n = 2), blonanserin (n = 1), clozapine (n = 3), Iloperidone (n = 1), lurasidone (n = 2), olanzapine (n = 14), paliperidone/risperidone (n = 9), quetiapine (n = 8), and ziprasidone (n = 11). Five studies assigned patients to 2 or more different antipsychotics for comparison. Twenty-four studies provided data on a switch from a specific single antipsychotic, including risperidone (n = 5), clozapine (n = 1), amisulpride (n = 1), fluphenazine (n = 1), quetiapine (n = 1), olanzapine (n = 10), aripiprazole (n = 2), perphenazine (n = 1), and haloperidol (n = 2). Seven studies had data on switching from mixed FGAs, 10 from mixed SGAs, and 26 from mixed FGA/SGAs (supplementary table 7).
Thirty-three studies (54.1%) included people with schizophrenia, 17 studies (27.3%) people with schizophrenia or schizoaffective disorder, 2 studies (3.3%) people with bipolar disorder and 9 studies (14.8%) people with a mix of mental disorders. Most studies included community samples (n = 26, 43.3%) with the remainder from hospitals with or without community samples (supplementary table 7).
The overall study quality was low; only 4 of the 10 studies with data on switch-vs-stay were rated to be of high quality using the Cochrane tool, and only 20 of 51 before-to-after studies were rated to be of high quality using the Newcastle-Ottawa scale (supplementary appendix 4). Lack of blinding was the most common issue in the RCTs while in the before-to-after studies the main issues were small sample sizes and high attrition.
Ten studies (16.3%) had data on switching to a new antipsychotic vs staying on the previous agent.25–34 Of these, 4 were switches to aripiprazole,25,27,33,34 4 switched to olanzapine,29–32 1 switched to risperidone,26 and 1 switched to quetiapine.28 Fifty-one studies (83.6%) provided observational data on the outcomes of interest before-to-after a switch to a specific antipsychotic.35–84 Pairwise meta-analyses of switch-vs-stay were only possible for 2 antipsychotics, aripiprazole and olanzapine. By contrast, we conducted before-to-after meta-analyses for 9 antipsychotics: aripiprazole; amisulpride; clozapine; lurasidone; olanzapine; paliperidone/risperidone; quetiapine; and ziprasidone. Only qualitative results were available for blonanserin and iloperidone (supplementary table 7).
Pairwise Meta-Analyses of Switch-vs-Stay
Change in Body Weight
Aripiprazole
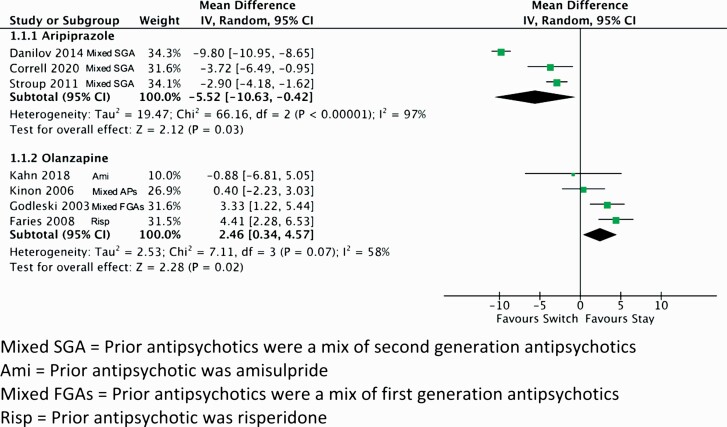
Four studies (2 high quality25,33 and 2 low quality27,34) contributed data for switching to aripiprazole (n = 182) vs staying on the previous antipsychotics (n = 214). Previous antipsychotics included a mix of SGAs. Three studies (2 high quality,25,33 1 low quality,27n = 333) contributed data for the primary outcome of body weight.25,27,33 Compared to staying on the previous antipsychotics, a switch to aripiprazole was associated with a 5.52 kg weight loss (figure 1 and supplementary table 5.1). The results remained significant when the low-quality study was omitted. It was not possible to disaggregate the results by prior antipsychotic.
Fig. 1.
Pairwise meta-analysis of switching to an antipsychotic for weight change. Mixed SGA = Prior antipsychotics were a mix of second generation antipsychotics; Ami = Prior antipsychotic was amisulpride; Mixed FGAs = Prior antipsychotics were a mix of first generation antipsychotics; Risp = Prior antipsychotic was risperidone.
Olanzapine
Four studies (1 high quality,31 3 low quality29,30,32) contributed data on switching to olanzapine compared to staying on previous agents. Previous antipsychotics included risperidone,29 amisulpride,31 long-acting injectable FGAs,30 and a mix of risperidone and FGAs.32 Compared to staying on previous antipsychotics, switching to olanzapine was associated with a 2.46 kg weight gain (figure 1 and supplementary table 5.2). The results were no longer significant when data were restricted to the one high-quality study or when the analysis was restricted to studies switching only from SGAs.
Other Cardiometabolic Outcomes
Aripiprazole
Two studies (1 high quality33 and 1 low quality,27n = 239) contributed data for other metabolic outcomes. Aripiprazole was associated with a reduction in fasting glucose triglycerides, but not HDL cholesterol (table 1 and supplementary table 5.1). When restricted to the one high-quality study, only triglycerides were significantly lower in the aripiprazole group. There was insufficient data to undertake meta-analyses of other cardiometabolic components.
Table 1.
Pairwise meta-analyses of metabolic and safety outcomes for switching to an antipsychotic
| Studies | Participants | Statistic | Mean | 95% CI | P value | I 2 | |
|---|---|---|---|---|---|---|---|
| Switch to Aripiprazole | |||||||
| Weight (kg) | 3 | 305 | MD | −5.52 kg | −10.63 kg to −0.42 kg | .03 | 97% |
| Fasting glucose (mg/dl) | 2 | 239 | MD | −3.99 | −7.34 to −0.64 | .02 | 0% |
| Triglycerides (mg/dl) | 2 | 239 | MD | −31.03 | −48.73 to −13.34 | .001 | 0% |
| HDL cholesterol (mg/dl) | 2 | 239 | MD | +2.90 | −2.11 to 7.92 | .26 | 70% |
| Psychotic symptoms | 3 | 301 | SMD | −0.44 | −1.20 to 0.32 | .26 | 88% |
| Drop outs | 3 | 334 | OR | +1.67 | 0.59 to 4.73 | .33 | 48% |
| Switch to Olanzapine | |||||||
| Weight (kg) | 4 | 353 | MD | +2.46 kg | 0.34 kg to 4.57 kg | .02 | 58% |
| Psychotic symptoms | 3 | 252 | SMD | +0.06 | −0.23 to 0.35 | .70 | 75% |
| Drop outs | 2 | 147 | OR | +0.86 | 0.19 to 3.95 | .85 | 69% |
Note: CI, confidence interval; HDL, high-density liposome; MD, mean difference; OR, odds ratio; SMD, standardized mean difference.
Olanzapine
Data were not available for meta-analyses of other cardiometabolic outcomes for a switch to olanzapine.
Safety
Aripiprazole
There was no significant difference between switching to aripiprazole or staying on previous antipsychotic for psychotic symptoms (1 high quality33 and 2 low quality,27,34n = 301) or dropouts (2 high quality,25,33 1 low quality,34n = 334) (table 1). The result remained nonsignificant with sensitivity analysis by study quality. There were insufficient data to explore other adverse drug reactions.
Olanzapine
There was no statistically significant difference between switching to olanzapine or staying on previous antipsychotic for psychotic symptoms (3 low-quality studies,29,30,32n = 252), or dropouts (1 high-quality,31 1 low-quality32 study, n = 147) (table 1). There were insufficient data to explore other adverse drug reactions.
Before-to-After Meta-analyses
Change in Body Weight
Aripiprazole
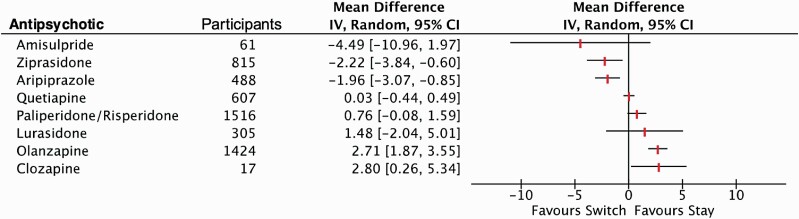
Fourteen studies (n = 488) contributed data for weight for aripiprazole.25,27,33,36,39,42,46,47,51,54,67,78,79,83 After switching to aripiprazole, participants had a mean weight loss of 1.96 kg (figure 2 and Supplementary table 6.1). When restricted to the 7 high-quality studies, there was no significant change in weight. Sensitivity analysis by recruitment site remained significant for mixed inpatient and community, but not for community alone. Weight loss remained statistically significant when prior antipsychotic was restricted to SGAs, but not for a mix of SGAs or FGAs. Weight loss remained significant irrespective of diagnosis, or whether weight loss was the primary outcome of the study. Sensitivity analysis of age group showed significant weight loss in the 13 adult studies but not in the one child and youth study. Studies from North America (n = 5) and Asia (n = 7) reported significant weight loss but not the 2 studies from Europe (supplementary table 6.2). Meta-regression by drop out proportion, study duration, baseline BMI, and mean sample age significantly impacted the overall result for weight change (supplementary table 6.3). There were insufficient data for meta-regression by duration of illness. There was no evidence of publication bias (Eggers regression = 8.48, SE = 4.92, 2-sided P = .11, Kendall’s Tau −0.20 P = .32; supplementary table 6.4).
Fig. 2.
Before-to-after meta-analysis of switching to an antipsychotic for weight change (in kg).
Amisulpride
One high-quality55 and one low-quality62 study contributed data on weight before-to-after switch to amisulpride from mixed SGAs (figure 2). There was no difference in weight after switching to amisulpride. There were insufficient data to undertake sensitivity analyses or meta-regression (supplementary table 6.5). There were no data for other metabolic outcomes.
Clozapine
One high-quality74 and one low-quality49 study contributed data on weight before-to-after switch to clozapine from a mix of FGA/SGAs. Patients switched to clozapine gained 2.8 kg (figure 2). There were insufficient data to undertake sensitivity analyses or meta-regression (supplementary table 6.6).
Lurasidone
Data from 2 high-quality studies of switch to lurasidone (1 from olanzapine75 and 1 from a mix of FGA/SGAs66) showed no difference in weight (figure 2). There were insufficient data to undertake sensitivity analyses or meta-regression (supplementary table 6.7).
Olanzapine
Thirteen studies (3 high quality59,64,74 and 10 low quality,29,30,32,35,38,49,70,77,80,85n = 1,426) contributed data on the effect of switching to olanzapine with a 2.7 kg weight gain (figure 2 and supplementary table 6.8). Sensitivity analyses by study quality, prior antipsychotic (mixed FGA/SGAs, mixed FGA, haloperidol, perphenazine, or risperidone), diagnosis, age group, or recruitment region had no impact on the weight gain. A hospital sample was not associated with weight gain whereas community or mixed setting samples were both associated with significant weight gain following a switch to olanzapine. Studies with weight as a primary outcome did not show any change in weight (supplementary table 6.9). Meta-regression by dropout proportion, study duration, mean sample age, and duration of illness showed these factors did not influence results (supplementary table 6.10). There was no evidence of publication bias with Kendall’s tau or Eggers regression (supplementary table 6.11).
Paliperidone/Risperidone
Four high-quality37,41,52,71 and 5 low-quality50,57,73,80,85 studies (n = 1,516) of paliperidone/risperidone showed no change in weight (figure 2 and supplementary table 6.12). When restricted to high-quality studies, there was significant weight gain. There was significantly greater weight gain with a change from mixed APs and mixed FGAs, but not olanzapine or perphenazine. One low-quality study of children switching from olanzapine reported >5 kg weight loss with switch to risperidone.73 This was the only study that reported weight loss with switch to paliperidone/risperidone. Studies in mixed settings reported significant weight gain, but not studies in community settings. Analyses restricted to studies from Europe or the United States reported significant weight gain (supplementary table 6.13). Meta-regression by increasing age was associated with significantly greater weight gain but this was no longer significant when the child and youth study was excluded. Study duration or dropout proportion did not influence weight change in meta-regressions (supplementary table 6.14).
Quetiapine
There were 1 high-quality44 and 6 low-quality41,43,58,65,68,85 studies of quetiapine (n = 607, all adults). There was no change in weight when switching to quetiapine (figure 2 and supplementary table 6.15). Sensitivity analyses by study quality, setting, diagnosis, and country did not affect the results. Two low-quality studies43,58 of switching from olanzapine reported significant weight loss, while one low-quality study58 of switching from haloperidol was associated with significant weight gain (supplementary table 6.16). There was no difference in weight when switching from risperidone, perphenazine, mixed FGA/SGAs, or mixed SGAs. Meta regression by dropout proportion, study duration, and mean sample age did not influence results (supplementary table 6.17).
Ziprasidone
Nine studies (3 high quality40,48,72 and 6 low quality,36,53,60,63,69,82 all adult, n = 815) of switching to ziprasidone showed a significant weight loss (figure 2 and supplementary table 6.18). This significant weight loss remained in sensitivity analyses for quality, setting, previous antipsychotics (aripiprazole, quetiapine, olanzapine, and mixed FGA/SGAs), diagnosis, weight as primary study outcome, and country (supplementary table 6.19). Meta-regression by dropout proportion, study duration, mean sample age, and duration of illness did not influence the results. Higher baseline BMI was associated with greater weight gain (supplementary table 6.20).
Other Metabolic Outcomes
Switching to aripiprazole, lurasidone, or ziprasidone was generally associated with improvements in most other cardiometabolic outcomes (supplementary appendix 5). By contrast, clozapine was associated with an increase in BMI. Olanzapine, quetiapine, and paliperidone/risperidone were neutral in terms of change in other cardiometabolic outcomes.
Safety
Switching to aripiprazole, paliperidone/risperidone, quetiapine, or ziprasidone was associated with a reduction in psychotic symptoms, while switching to olanzapine did not change psychotic symptoms (supplementary appendix 5).
Qualitative Analysis
Other Antipsychotics
One high-quality study (n = 52) examined switching to blonanserin from a mix of FGA and SGAs. They reported no change in weight or other metabolic outcome besides a small improvement in HbA1c.83 Another low-quality study (n = 500) investigated a switch to iloperidone from risperidone, olanzapine, or aripiprazole and reported significant weight gain (0.84 kg, 95% CI 0.50 kg, 1.19 kg, P < .001).81
Discussion
This systematic review is the most comprehensive synthesis of the impact of switching antipsychotics on metabolic outcomes; a previous meta-analysis only included 4 small trials.20 We included data from 61 studies and 8,554 participants on switching to 11 different antipsychotic medications and conducted meta-analyses for 9 antipsychotics.
Aripiprazole had the best evidence for weight loss upon antipsychotic switching; 5.5 kg in the pairwise and 2.0 kg in the before-to-after analysis. Switching to ziprasidone was also associated with weight loss but based on only before-to-after data. These findings are consistent with existing data on their lower propensity for weight gain.9 Switching to olanzapine was associated with 2.5–2.7 kg weight gain in switch-vs-stay and before-to-after meta-analyses. Switching to clozapine-induced weight gain in before-to-after studies. This finding reflects the adverse cardiometabolic profile of these agents.9 Switching to amisulpride, paliperidone/risperidone, quetiapine, or lurasidone had no effect on weight.
Switching from risperidone to olanzapine led to weight gain. Switching from olanzapine to lurasidone or quetiapine led to weight loss, but switching from olanzapine to paliperidone/risperidone had no effect. Switching from haloperidol to quetiapine or olanzapine led to weight gain but switching from aripiprazole or quetiapine to ziprasidone led to weight loss; there was no effect when switching from paliperidone/risperidone to quetiapine. Switching from perphenazine to olanzapine, was associated with weight gain while switching from perphenazine to quetiapine or paliperidone/risperidone was weight neutral.
Switching to aripiprazole improved triglycerides (both types of analyses) and total, LDL and HDL cholesterol (before-to-after analysis). Switching to ziprasidone improved metabolic outcomes (before-to-after analyses) but switching to clozapine or olanzapine (before-to-after analyses) worsened outcomes. Switching to aripiprazole, risperidone/paliperidone, quetiapine, or ziprasidone improved psychotic symptoms (before-to-after analysis).
This is the first study to comprehensively assess the effects of switching antipsychotics. We further explored the effects of potential confounders and effect modifiers, such as study duration, study quality, recruitment setting, prior antipsychotic, diagnoses, weight as a primary outcome, country of recruitment, dropouts, baseline BMI, mean sample age, and duration of illness. For the most part, these factors did not influence the overall results for weight changes with antipsychotic switching.
This study has several limitations. Although we were able to conduct pairwise switch-vs-stay meta-analyses for both aripiprazole and olanzapine, there were only usable data for before-to-after analyses for the other antipsychotics. These analyses are subject to regression to the mean and may therefore overstate changes in weight and other metabolic syndrome components associated with switching; results need to be interpreted with caution. By contrast, there might have been insufficient power to demonstrate a significant change with some comparisons, such as weight loss on switching to lurasidone, or weight gain when switching from risperidone to quetiapine, despite their known cardiometabolic profile.86,87 Thus, lack of demonstrated overall weight gain for the switch to quetiapine may have been due to lack of power and/or opposing/neutralizing results of significant weight gain when switching from haloperidol to quetiapine and significant weight loss when switching from olanzapine to quetiapine. This finding indicates the importance of considering the cardiometabolic profile of both the pre- and post-switch antipsychotic in the clinical decision-making process.
For the antipsychotics where both analyses types were conducted (ie, before-to-after and switch-vs-stay) there was consistency in weight changes across the analysis types, so observations of weight change from before-to-after studies may be broadly reflective of switch-vs-stay studies. While studies often reported whether participants as a group were on FGAs and/or SGAs, most studies did not specify the antipsychotic agent prior to switching. This lack of detail constrained our ability to conduct sensitivity analyses of switching from specific antipsychotics. The studies also lacked data on the duration of the previous antipsychotic and of the cardiometabolic impact of switching antipsychotics in specific disorders, such as bipolar disorder and depression. Most studies only considered people aged 18–64 years hence limiting generalizability to nonelderly adults. Overall study quality was low, with only 40% rated as being of high quality. Overall, heterogeneity was high in many of the meta-analyses, and as such, our results should be treated with caution. We explored heterogeneity in sensitivity analyses, but it remained high in most analyses.
Switching antipsychotics is only one strategy in managing obesity and metabolic syndrome among people with SMI.14,19 Switching to aripiprazole, with an SMD of −1.93 (95% CI −3.70 to −0.16, P = .03, supplementary table 5.2) in our pairwise meta-analyses of switch-vs-stay, compared favorably to other published weight loss interventions among people with schizophrenia. Lifestyle interventions including individual lifestyle counseling (SMD −0.98, 95% CI −1.15 to −0.81, P < .001) and physical activity (SMD −0.96, 95% CI −1.27 to −0.66, P < .001) can reduce weight and improve components of metabolic syndrome.14 Uptake remains a major challenge for their effectiveness.9 Moreover, the clinical relevance of lifestyle intervention associated weight loss has been challenged given that trials often recruit more motivated participants compared to observational studies with participants who may be more generalizable to real-world populations of people with SMI.18 Augmentation with pharmacological agents is also effective in reducing weight.14 For instance, augmentation with aripiprazole (SMD −0.73, 95% CI −0.97 to −0.48, P < .001) or topiramate (SMD −0.72, 95% CI −1.56 to −0.33, P < .001) can lead to weight loss.14 However, aripiprazole may only work when added to high-risk antipsychotics, such as olanzapine and clozapine.88 Topiramate augmentation has been associated with cognitive adverse drug reactions hence limiting its tolerability.14 The most promising nonpsychotropic agents include metformin (SMD −0.53, 95% CI −0.69 to −0.38, P < .001)14,16 and glucagon-like peptide receptor agonists (GLP1-RAs) (SMD −0.44, 95% CI −0.60 to −0.28, P < .001).14,17
Although switching to a different antipsychotic, notably aripiprazole and ziprasidone, may lead to weight loss, these benefits must be weighed against any potential risks of adverse drug reactions or deterioration in psychotic symptoms. Reassuringly, we found no change in psychotic symptoms in the switch to aripiprazole (switch-vs-stay). Indeed, aripiprazole and ziprasidone improved psychotic symptoms in the before-to-after analyses, but this finding may be confounded by regression to the mean. Nevertheless, switching antipsychotics in psychiatrically stable patients may risk relapse and detrimental consequences for at least some patients.89 Given clozapine is the most effective90 and efficacious91 medication for treatment refractory schizophrenia (TRS), it may not be feasible to switch from clozapine for patients with TRS. In these instances, consideration should be given to augmentation with metformin16 or a GLP1-RA.92 Despite clozapine’s association with poorer metabolic outcomes,93 it remains the most effective antipsychotic for reducing all-cause mortality.94
Managing obesity and metabolic syndrome therefore requires a combination of lifestyle and pharmacological strategies.19 The priority should be primary prevention of weight gain given the challenges of reversing weight gain and the potential for relapse in symptoms. This goal cannot be achieved solely by starting patients on an agent with a lower propensity for weight gain. All antipsychotics seem to induce weight gain in antipsychotic-naive patients95,96 and so patients will require early concomitant lifestyle treatments to manage this risk.19
In summary, switching to aripiprazole or ziprasidone is an evidence-based approach to improving cardiometabolic risk in people with SMI, while switching to olanzapine or clozapine can significantly worsen cardiometabolic status. These results need to be considered when weighing efficacy and safety of specific antipsychotics. However, there is a need for further, adequately powered, high-quality, randomized controlled trials, examining the effects of switching to antipsychotics with a lower propensity for weight gain, notably ziprasidone and aripiprazole and, possibly, lurasidone and the newly FDA-approved lumateperone that appears to have a low cardiometabolic risk profile,97 with data disaggregated for the pre-switch antipsychotic. Finally, further data on the impact of switching antipsychotics on other cardiometabolic parameters, including lipids, blood glucose levels, and blood pressure, as well as on psychopathology and patient-reported outcomes, like quality of life, treatment satisfaction, and adherence are needed.
Supplementary Material
Funding
D.S. was supported in part by an NHMRC ECF GNT1111136. W.M., E.G., K.W., C.C., S.K., and S.H. have no relevant funding to declare.
Conflict of Interest
D.S., W.M., E.G., K.W., and S.H. have no conflicts of interest to declare. C.C. has been a consultant and/or advisor to or have received honoraria from: Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Rovi, Supernus, and Teva. He has received grant support from Janssen and Takeda. He is also a stock option holder of LB Pharma. S.K. been a consultant and/or advisor to Lundbeck and Janssen
References
- 1. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Möller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry. 2009;24(6):412–424. [DOI] [PubMed] [Google Scholar]
- 3. Williams EP, Mesidor M, Winters K, Dubbert PM, Wyatt SB. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep. 2015;4(3):363–370. [DOI] [PubMed] [Google Scholar]
- 4. Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annu Rev Med. 2015;66:297–309. [DOI] [PubMed] [Google Scholar]
- 5. Oakley P, Kisely S, Baxter A, et al. Increased mortality among people with schizophrenia and other non-affective psychotic disorders in the community: a systematic review and meta-analysis. J Psychiatr Res. 2018;102:245–253. [DOI] [PubMed] [Google Scholar]
- 6. Lawrence D, Hancock KJ, Kisely S. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: retrospective analysis of population based registers. BMJ. 2013;346:f2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collaboration GBM. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Firth J, Siddiqi N, Koyanagi A, et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry. 2019;6(8):675–712. [DOI] [PubMed] [Google Scholar]
- 10. Mazereel V, Detraux J, Vancampfort D, van Winkel R, De Hert M. Impact of psychotropic medication effects on obesity and the metabolic syndrome in people with serious mental illness. Front Endocrinol (Lausanne). 2020;11:573479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kolotkin RL, Corey-Lisle PK, Crosby RD, et al. Impact of obesity on health-related quality of life in schizophrenia and bipolar disorder. Obesity (Silver Spring). 2008;16(4):749–754. [DOI] [PubMed] [Google Scholar]
- 12. Weiden PJ, Mackell JA, McDonnell DD. Obesity as a risk factor for antipsychotic noncompliance. Schizophr Res. 2004;66(1):51–57. [DOI] [PubMed] [Google Scholar]
- 13. Dibonaventura M, Gabriel S, Dupclay L, Gupta S, Kim E. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vancampfort D, Firth J, Correll CU, et al. The impact of pharmacological and non-pharmacological interventions to improve physical health outcomes in people with schizophrenia: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry. 2019;18(1):53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Firth J, Solmi M, Wootton RE, et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siskind DJ, Leung J, Russell AW, Wysoczanski D, Kisely S. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siskind D, Hahn M, Correll CU, et al. Glucagon-like peptide-1 receptor agonists for antipsychotic-associated cardio-metabolic risk factors: a systematic review and individual participant data meta-analysis. Diabetes Obes Metab. 2019;21(2):293–302. [DOI] [PubMed] [Google Scholar]
- 18. Speyer H, Jakobsen AS, Westergaard C, et al. Lifestyle interventions for weight management in people with serious mental illness: a systematic review with meta-analysis, trial sequential analysis, and meta-regression analysis exploring the mediators and moderators of treatment effects. Psychother Psychosom. 2019;88(6):350–362. [DOI] [PubMed] [Google Scholar]
- 19. Marteene W, Winckel K, Hollingworth S, et al. Strategies to counter antipsychotic-associated weight gain in patients with schizophrenia. Expert Opin Drug Saf. 2019;18(12): 1149–1160. [DOI] [PubMed] [Google Scholar]
- 20. Mukundan A, Faulkner G, Cohn T, Remington G. Antipsychotic switching for people with schizophrenia who have neuroleptic‐induced weight or metabolic problems. Cochrane Database Syst Rev. 2010;(12). doi: 10.1002/14651858.CD006629.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. [DOI] [PubMed] [Google Scholar]
- 24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Correll CU, Sikich L, Reeves G, et al. Metformin add-on vs. antipsychotic switch vs. continued antipsychotic treatment plus healthy lifestyle education in overweight or obese youth with severe mental illness: results from the IMPACT trial. World Psychiatry. 2020;19(1):69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Covell NH, McEvoy JP, Schooler NR, et al. ; Schizophrenia Trials Network . Effectiveness of switching from long-acting injectable fluphenazine or haloperidol decanoate to long-acting injectable risperidone microspheres: an open-label, randomized controlled trial. J Clin Psychiatry. 2012;73(5):669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Danilov DS. The use of aripiprazole in the treatment of obesity associated with the administration of neuroleptics of the second generation in patients with schizophrenia. Controlled Clinical Trial. 2014;2014(3):34–40. [PubMed] [Google Scholar]
- 28. Deberdt W, Lipkovich I, Heinloth AN, et al. Double-blind, randomized trial comparing efficacy and safety of continuing olanzapine versus switching to quetiapine in overweight or obese patients with schizophrenia or schizoaffective disorder. Ther Clin Risk Manag. 2008;4(4):713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Faries DE, Ascher-Svanum H, Nyhuis AW, Kinon BJ. Switching from risperidone to olanzapine in a one-year, randomized, open-label effectiveness study of schizophrenia. Curr Med Res Opin. 2008;24(5):1399–1405. [DOI] [PubMed] [Google Scholar]
- 30. Godleski LS, Goldsmith LJ, Vieweg WV, Zettwoch NC, Stikovac DM, Lewis SJ. Switching from depot antipsychotic drugs to olanzapine in patients with chronic schizophrenia. J Clin Psychiatry. 2003;64(2):119–122. [DOI] [PubMed] [Google Scholar]
- 31. Kahn RS, Winter van Rossum I, Leucht S, et al. ; OPTiMiSE study group . Amisulpride and olanzapine followed by open-label treatment with clozapine in first-episode schizophrenia and schizophreniform disorder (OPTiMiSE): a three-phase switching study. Lancet Psychiatry. 2018;5(10):797–807. [DOI] [PubMed] [Google Scholar]
- 32. Kinon BJ, Ahl J, Liu-Seifert H, Maguire GA. Improvement in hyperprolactinemia and reproductive comorbidities in patients with schizophrenia switched from conventional antipsychotics or risperidone to olanzapine. Psychoneuroendocrinology. 2006;31(5):577–588. [DOI] [PubMed] [Google Scholar]
- 33. Stroup TS, McEvoy JP, Ring KD, et al. ; Schizophrenia Trials Network . A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. 2011;168(9):947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wani RA, et al. “Effects of switching from olanzapine to aripiprazole on the metabolic profiles of patients with schizophrenia and metabolic syndrome: a randomized, open-label study”: corrigendum. Neuropsychiatric Disease and Treatment. 2015;11:685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baptista T, Dávila A, El Fakih Y, et al. Similar frequency of abnormal correlation between serum leptin levels and BMI before and after olanzapine treatment in schizophrenia. Int Clin Psychopharmacol. 2007;22(4):205–211. [DOI] [PubMed] [Google Scholar]
- 36. Chen Y, Bobo WV, Watts K, Jayathilake K, Tang T, Meltzer HY. Comparative effectiveness of switching antipsychotic drug treatment to aripiprazole or ziprasidone for improving metabolic profile and atherogenic dyslipidemia: a 12-month, prospective, open-label study. J Psychopharmacol. 2012;26(9):1201–1210. [DOI] [PubMed] [Google Scholar]
- 37. De Marinis T, et al. Switching to long-acting injectable risperidone is beneficial with regard to clinical outcomes, regardless of previous conventional medication in patients with schizophrenia. Pharmacopsychiatry. 2007;40(6):257–63. [DOI] [PubMed] [Google Scholar]
- 38. Dossenbach MRK, et al. Safety and efficacy of olanzapine in patients with severe haloperidol side effects. Research Article. 2000;12(3):243–52. [Google Scholar]
- 39. Dubuis J, et al. Switch of therapy to aripiprazole in swiss practices (TRACE: Treatment with Aripiprazole based on Case Experience). Article. 2007;158(3):115–22. [Google Scholar]
- 40. Fraile MG, et al. Switching to ziprasidone in the clinical practice setting: an open-label study. Int J Psychiat Med. 2013;45(2):125–42. [DOI] [PubMed] [Google Scholar]
- 41. Gaebel W, et al. Relapse prevention in schizophrenia and schizoaffective disorder with risperidone long-acting injectable vs quetiapine: results of a long-term, open-label, randomized clinical trial: corrigendum. Randomized Controlled Trial. 2011;36(2):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ganguli R, Brar JS, Garbut R, Chang CC, Basu R. Changes in weight and other metabolic indicators in persons with schizophrenia following a switch to aripiprazole. Clin Schizophr Relat Psychoses. 2011;5(2):75–79. [DOI] [PubMed] [Google Scholar]
- 43. Gupta S, Masand PS, Virk S, et al. Weight decline in patients switching from olanzapine to quetiapine. Schizophr Res. 2004;70(1):57–62. [DOI] [PubMed] [Google Scholar]
- 44. Hashimoto N, Toyomaki A, Honda M, et al. Long-term efficacy and tolerability of quetiapine in patients with schizophrenia who switched from other antipsychotics because of inadequate therapeutic response-a prospective open-label study. Ann Gen Psychiatry. 2015;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Howes OD, Smith S, Gaughran FP, Amiel SA, Murray RM, Pilowsky LS. The relationship between prolactin levels and glucose homeostasis in antipsychotic-treated schizophrenic patients. J Clin Psychopharmacol. 2006;26(6):629–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hsieh MH, Lin WW, Chen ST, et al. A 64-week, multicenter, open-label study of aripiprazole effectiveness in the management of patients with schizophrenia or schizoaffective disorder in a general psychiatric outpatient setting. Ann Gen Psychiatry. 2010;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hwang TJ, Lo WM, Chan HY, et al. Fast versus slow strategy of switching patients with schizophrenia to aripiprazole from other Antipsychotics. J Clin Psychopharmacol. 2015;35(6):635–644. [DOI] [PubMed] [Google Scholar]
- 48. Karayal ON, Glue P, Bachinsky M, et al. Switching from quetiapine to ziprasidone: a sixteen-week, open-label, multicenter study evaluating the effectiveness and safety of ziprasidone in outpatient subjects with schizophrenia or schizoaffective disorder. J Psychiatr Pract. 2011;17(2):100–109. [DOI] [PubMed] [Google Scholar]
- 49. Kelly DL, Conley RR, Richardson CM, Tamminga CA, Carpenter WT Jr. Adverse effects and laboratory parameters of high-dose olanzapine vs. clozapine in treatment-resistant schizophrenia. Ann Clin Psychiatry. 2003;15(3-4):181–186. [DOI] [PubMed] [Google Scholar]
- 50. Kim EY, Chang SM, Shim JC, et al. Long-term effectiveness of flexibly dosed paliperidone extended-release: comparison among patients with schizophrenia switching from risperidone and other antipsychotic agents. Curr Med Res Opin. 2013;29(10):1231–1240. [DOI] [PubMed] [Google Scholar]
- 51. Kim SH, Ivanova O, Abbasi FA, Lamendola CA, Reaven GM, Glick ID. Metabolic impact of switching antipsychotic therapy to aripiprazole after weight gain: a pilot study. J Clin Psychopharmacol. 2007;27(4):365–368. [DOI] [PubMed] [Google Scholar]
- 52. Kim SW, Chung YC, Lee YH, et al. Paliperidone ER versus risperidone for neurocognitive function in patients with schizophrenia: a randomized, open-label, controlled trial. Int Clin Psychopharmacol. 2012;27(5):267–274. [DOI] [PubMed] [Google Scholar]
- 53. Kim SW, Shin IS, Kim JM, Bae KY, Yang SJ, Yoon JS. Effectiveness of switching from aripiprazole to ziprasidone in patients with schizophrenia. Clin Neuropharmacol. 2010;33(3):121–125. [DOI] [PubMed] [Google Scholar]
- 54. Kim SW, Shin IS, Kim JM, et al. Effectiveness of switching to aripiprazole from atypical antipsychotics in patients with schizophrenia. Clin Neuropharmacol. 2009;32(5):243–249. [DOI] [PubMed] [Google Scholar]
- 55. Kim Y, Wang SM, Kwak KP, et al. Amisulpride switching in schizophrenic patients who showed suboptimal effect and/or tolerability to current antipsychotics in a naturalistic setting: an explorative study. Clin Psychopharmacol Neurosci. 2016;14(4):371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ko YH, Na KS, Kim CE, et al. The effectiveness of cross-tapering switching to ziprasidone in patients with schizophrenia or schizoaffective disorder. Psychiatry Investig. 2014;11(4):459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lai YC, Huang MC, Chen CH, Tsai CJ, Pan CH, Chiu CC. Pharmacokinetics and efficacy of a direct switch from conventional depot to risperidone long-acting injection in Chinese patients with schizophrenic and schizoaffective disorders. Psychiatry Clin Neurosci. 2009;63(4):440–448. [DOI] [PubMed] [Google Scholar]
- 58. Larmo I, de Nayer A, Windhager E, et al. ; Spectrum Study Group . Efficacy and tolerability of quetiapine in patients with schizophrenia who switched from haloperidol, olanzapine or risperidone. Hum Psychopharmacol. 2005;20(8):573–581. [DOI] [PubMed] [Google Scholar]
- 59. Lee CT, Conde BJ, Mazlan M, et al. Switching to olanzapine from previous antipsychotics: a regional collaborative multicenter trial assessing 2 switching techniques in Asia Pacific. J Clin Psychiatry. 2002;63(7):569–576. [DOI] [PubMed] [Google Scholar]
- 60. Lee HB, Yoon BH, Kwon YJ, et al. The efficacy and safety of switching to ziprasidone from olanzapine in patients with bipolar I disorder: an 8-week, multicenter, open-label study. Clin Drug Investig. 2013;33(10):743–753. [DOI] [PubMed] [Google Scholar]
- 61. Li CH, et al. A twenty-four-week, open-label study on Ziprasidones efficacy and influence on glucolipid metabolism in patients with schizophrenia and metabolic disorder. Comparative Study. 2013;17(16):2136–40. [PubMed] [Google Scholar]
- 62. Lin CC, Bai YM, Wang YC, et al. Improved body weight and metabolic outcomes in overweight or obese psychiatric patients switched to amisulpride from other atypical antipsychotics. J Clin Psychopharmacol. 2009;29(6): 529–536. [DOI] [PubMed] [Google Scholar]
- 63. Lindenmayer JP, Tedeschi F, Yusim A, et al. Ziprasidone’s effect on metabolic markers in patients with diabetes and chronic schizophrenia. Clin Schizophr Relat Psychoses. 2012;5(4):185–192. [DOI] [PubMed] [Google Scholar]
- 64. Lu Z, Hu J, Chen CK, et al. Effectiveness and safety of olanzapine in the treatment of schizophrenia among Asian patients switching from conventional antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):32–40. [DOI] [PubMed] [Google Scholar]
- 65. Mazeh D, Paleacu D, Barak Y. Quetiapine for elderly non-responsive schizophrenia patients. Psychiatry Res. 2008;157(1-3):265–267. [DOI] [PubMed] [Google Scholar]
- 66. McEvoy JP, Citrome L, Hernandez D, et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry. 2013;74(2):170–179. [DOI] [PubMed] [Google Scholar]
- 67. Pae CU, Serretti A, Chiesa A, et al. Immediate versus gradual suspension of previous treatments during switch to aripiprazole: results of a randomized, open label study. Eur Neuropsychopharmacol. 2009;19(8):562–570. [DOI] [PubMed] [Google Scholar]
- 68. Potvin S, Stip E, Lipp O, et al. Quetiapine in patients with comorbid schizophrenia-spectrum and substance use disorders: an open-label trial. Curr Med Res Opin. 2006;22(7):1277–1285. [DOI] [PubMed] [Google Scholar]
- 69. Ratner Y, Gibel A, Yorkov V, Ritsner MS. Effectiveness, safety, and tolerability of ziprasidone for treating schizophrenia patients undergoing usual care: a 12-month, open-label, flexible-dose, naturalistic observational trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1401–1409. [DOI] [PubMed] [Google Scholar]
- 70. Rodríguez-Pérez V, López A, Blanco C, et al. Olanzapine for the treatment of chronic refractory schizophrenia: a 12-month follow-up naturalistic study. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(6):1055–1062. [DOI] [PubMed] [Google Scholar]
- 71. Rosa F, Schreiner A, Thomas P, Sherif T. Switching patients with stable schizophrenia or schizoaffective disorder from olanzapine to risperidone long-acting injectable. Clin Drug Investig. 2012;32(4):267–279. [DOI] [PubMed] [Google Scholar]
- 72. Rossi A, Vita A, Tiradritti P, Romeo F. Assessment of clinical and metabolic status, and subjective well-being, in schizophrenic patients switched from typical and atypical antipsychotics to ziprasidone. Int Clin Psychopharmacol. 2008;23(4):216–222. [DOI] [PubMed] [Google Scholar]
- 73. Ruan LM, et al. Efficacy and safety of long-acting risperidone on early onset schizophrenia in adolescent patients. Afr J Pharm Pharmaco. 2010;4(5):184–92. [Google Scholar]
- 74. Shaw P, Sporn A, Gogtay N, et al. Childhood-onset schizophrenia: a double-blind, randomized clozapine-olanzapine comparison. Arch Gen Psychiatry. 2006;63(7):721–730. [DOI] [PubMed] [Google Scholar]
- 75. Stahl SM, Cucchiaro J, Simonelli D, Hsu J, Pikalov A, Loebel A. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry. 2013;74(5):507–515. [DOI] [PubMed] [Google Scholar]
- 76. Takaesu Y, Kishimoto T, Murakoshi A, Takahashi N, Inoue Y. Factors associated with discontinuation of aripiprazole treatment after switching from other antipsychotics in patients with chronic schizophrenia: a prospective observational study. Psychiatry Res. 2016;236:71–74. [DOI] [PubMed] [Google Scholar]
- 77. Takahashi H, Kamata M, Yoshida K, Ishigooka J, Higuchi H. Switching to olanzapine after unsuccessful treatment with risperidone during the first episode of schizophrenia: an open-label trial. J Clin Psychiatry. 2006;67(10):1577–1582. [DOI] [PubMed] [Google Scholar]
- 78. Takeuchi H, Suzuki T, Uchida H, et al. A randomized, open-label comparison of 2 switching strategies to aripiprazole treatment in patients with schizophrenia: add-on, wait, and tapering of previous antipsychotics versus add-on and simultaneous tapering. J Clin Psychopharmacol. 2008;28(5):540–543. [DOI] [PubMed] [Google Scholar]
- 79. Takeuchi H, Uchida H, Suzuki T, Watanabe K, Kashima H. Changes in metabolic parameters following a switch to aripiprazole in Japanese patients with schizophrenia: one-year follow-up study. Psychiatry Clin Neurosci. 2010;64(1):104–106. [DOI] [PubMed] [Google Scholar]
- 80. Wang X, Savage R, Borisov A, et al. Efficacy of risperidone versus olanzapine in patients with schizophrenia previously on chronic conventional antipsychotic therapy: a switch study. J Psychiatr Res. 2006;40(7):669–676. [DOI] [PubMed] [Google Scholar]
- 81. Weiden PJ, Citrome L, Alva G, et al. A trial evaluating gradual- or immediate-switch strategies from risperidone, olanzapine, or aripiprazole to iloperidone in patients with schizophrenia. Schizophr Res. 2014;153(1-3):160–168. [DOI] [PubMed] [Google Scholar]
- 82. Weiden PJ, Newcomer JW, Loebel AD, Yang R, Lebovitz HE. Long-term changes in weight and plasma lipids during maintenance treatment with ziprasidone. Neuropsychopharmacology. 2008;33(5):985–994. [DOI] [PubMed] [Google Scholar]
- 83. Woo YS, Bahk WM, Park YM, et al. Effects of switching to aripiprazole from current atypical antipsychotics on subsyndromal symptoms and tolerability in patients with bipolar disorder. Int Clin Psychopharmacol. 2016;31(5):275–286. [DOI] [PubMed] [Google Scholar]
- 84. Woo YS, Yoon BH, Jeon BH, et al. Switching antipsychotics to blonanserin in patients with schizophrenia: an open-label, prospective, multicenter study. Clin Psychopharmacol Neurosci. 2019;17(3):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Stroup TS, Lieberman JA, McEvoy JP, et al. ; CATIE Investigators . Effectiveness of olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia after discontinuing perphenazine: a CATIE study. Am J Psychiatry. 2007;164(3):415–427. [DOI] [PubMed] [Google Scholar]
- 86. Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kane JM, Correll CU, Goff DC, et al. A multicenter, randomized, double-blind, placebo-controlled, 16-week study of adjunctive aripiprazole for schizophrenia or schizoaffective disorder inadequately treated with quetiapine or risperidone monotherapy. J Clin Psychiatry. 2009;70(10):1348–1357. [DOI] [PubMed] [Google Scholar]
- 89. Olivares JM, Sermon J, Hemels M, Schreiner A. Definitions and drivers of relapse in patients with schizophrenia: a systematic literature review. Ann Gen Psychiatry. 2013;12(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Land R, Siskind D, McArdle P, Kisely S, Winckel K, Hollingworth SA. The impact of clozapine on hospital use: a systematic review and meta-analysis. Acta Psychiatr Scand. 2017;135(4):296–309. [DOI] [PubMed] [Google Scholar]
- 91. Siskind D, et al. Clozapine v. first-and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385–92. [DOI] [PubMed] [Google Scholar]
- 92. Siskind DJ, Russell AW, Gamble C, et al. Treatment of clozapine-associated obesity and diabetes with exenatide in adults with schizophrenia: a randomized controlled trial (CODEX). Diabetes Obes Metab. 2018;20(4):1050–1055. [DOI] [PubMed] [Google Scholar]
- 93. Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders–a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vermeulen JM, van Rooijen G, van de Kerkhof MPJ, Sutterland AL, Correll CU, de Haan L. Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1-12.5 Years. Schizophr Bull. 2019;45(2):315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One. 2014;9(4):e94112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(4):349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.