Abstract
Mastocytosis is a disorder characterized by the abnormal proliferation and/or accumulation of mast cells in different organs. More than 90% of patients with systemic mastocytosis have a gain-of-function mutation in codon 816 of the KIT receptor on mast cells (MCs). The symptoms of mastocytosis patients are related to the MC-derived mediators that exert local and distant effects. MCs produce angiogenic and lymphangiogenic factors, including vascular endothelial growth factors (VEGFs) and angiopoietins (ANGPTs). Serum concentrations of VEGF-A, VEGF-C, VEGF-D, ANGPT1 and ANGPT2 were determined in 64 mastocytosis patients and 64 healthy controls. Intracellular concentrations and spontaneous release of these mediators were evaluated in the mast cell lines ROSAKIT WT and ROSA KIT D816V and in human lung mast cells (HLMCs). VEGF-A, ANGPT1, ANGPT2 and VEGF-C concentrations were higher in mastocytosis patients compared to controls. The VEGF-A, ANGPT2 and VEGF-C concentrations were correlated with the symptom severity. ANGPT1 concentrations were increased in all patients compared to controls. ANGPT2 levels were correlated with severity of clinical variants and with tryptase levels. VEGF-A, ANGPT1 and VEGF-C did not differ between indolent and advanced mastocytosis. ROSAKIT WT, ROSAKIT D816V and HLMCs contained and spontaneously released VEGFs and ANGPTs. Serum concentrations of VEGFs and ANGPTs are altered in mastocytosis patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-021-00693-0.
Keywords: Angiopoietins, Mast cells, Mastocytosis, Stem cell factor, Tryptase, Vascular endothelial growth factors
Introduction
Mastocytosis is a rare clonal disorder characterized by uncontrolled proliferation, abnormal accumulation and survival of mast cells in several organs [1, 2]. This pathological condition is due to a somatic activating mutations of KIT gene that encodes for tyrosine kinase receptor KIT (CD117) largely expressed on mast cells [3, 4]. More than 90% of patients with systemic mastocytosis have a gain-of-function mutation in codon 816 of the receptor tyrosine kinase KIT, where a valine is substituted for an aspartate (KIT D816V) [5]. This mutation leads to an autophosphorylation of KIT receptor which results in MC proliferation also in the absence of the KIT ligand, stem cell factor (SCF) [5], which induces maturation, activation and proliferation of MCs [6, 7]. Mastocytosis is a heterogeneous group of neoplastic conditions [8] ranging from a skin-limited disorder [e.g., cutaneous mastocytosis (CM)] to severe forms involving multiple organs [e.g., systemic mastocytosis (SM)] [8, 9]. The symptoms of mastocytosis are the consequence of infiltration, activation and degranulation of MCs which exert local and systemic effects [10].
MCs are immune cells derived from bone marrow CD34+/CD117+ progenitors [11] which are localized in all tissues, including the skin [12], the mucosa of respiratory tract [13], the gastrointestinal system [14] and the heart [15]. Their number increases in several disorders such as bacterial, viral and parasitic infections [16, 17], allergic disorders [18, 19], arthritis [20], cardiovascular disorders [15, 21], cancer [22–24] and mastocytosis [10].
A plethora of stimuli (i.e., antigens, allergens, bacterial and viral superallergens, cytokines, chemokines) activates MCs to release two groups of inflammatory molecules: pre-formed mediators stored in their cytoplasmatic granules (e.g., histamine, α- and β-tryptase, chymase, and heparin) and de novo synthesized molecules (e.g., cytokines, chemokines, growth factors and lipid mediators) [25–27]. Immunological and non-immunological stimuli can induce the release of vascular endothelial growth factors (VEGFs) [28–34] and angiopoietins (ANGPTs) [35] from human MCs.
The VEGF family, in humans, consists of five separate gene products: VEGF-A, VEGF-B and placental growth factor (PlGF) are key regulators of physiological and pathological blood vessel growth [36, 37], whereas VEGF-C and VEGF–D modulate lymphangiogenesis [38]. VEGFs bind three tyrosine kinase receptors (VEGFR-1, -2, -3) expressed on blood (BEC) and lymphatic endothelial cells (LECs) [39]. VEGF-A, initially named vascular permeability factor (VPF), was discovered by Dvorak and collaborators for its permeabilizing activity [40]. It was found that VEGF-A is at least 50 times more potent than histamine in inducing vascular permeability [41, 42].
The ANGPT system is another pathway regulating vascular barrier functions [43]. In humans, ANGPT1 and ANGPT2 are the two primary angiopoietins: ANGPT1 is a vascular stabilizer acting on Tie2 receptor on BECs [44]. By contrast, ANGPT2 is an inhibitory ligand of the Tie2 receptor that disrupts the integrity of the blood vessel wall, thus counteracting vascular normalization [45].
Increased concentrations of circulating VEGFs and/or ANGPTs have been found in different human disorders characterized by increased vascular permeability or angiogenesis such as cardiovascular diseases [39, 46–48], cancer [49, 50], systemic capillary leak syndrome [51], angioedema [52, 53] and sepsis [54–56].
The role of VEGFs and ANGPTs in the pathophysiology of different forms of mastocytosis has not been thoroughly investigated. The aim of this paper was to evaluate the serum concentrations of VEGF-A, VEGF-C, VEGF-D, ANGPT1 and ANGPT2 in patients with different variants of mastocytosis and the expression of angiogenic and lymphangiogenic factors in human MC lines with or without D816V mutation.
Materials and methods
Patients selection
In a retrospective study, we evaluated 64 Caucasian patients with mastocytosis (31 males and 33 females; age range: 21–79 years; median age 46 years) followed at the University of Naples Federico II and at the University of Salerno whose clinical characteristics are summarized in Table 1 and Supplementary Table 1. None of patients was treated for mastocytosis with anti-inflammatory, immunosuppressant or immunomodulatory drugs at the time of blood sampling. A total of 64 healthy controls (HC) (31 males and 33 females; age range: 29–70 years; median age 43 years) were recruited as control. Inclusion criteria for this study were: the absence of any pathological conditions at the time of enrollment; expression of written informed consent. Exclusion criteria were: the presence of any condition that, in the opinion of the investigators, could interfere with the completion of the study such as pregnancy. Mediator-related symptoms were classified according to severity and frequency as follows: 13 patients had grade 0 (no symptoms), 13 had grade 1 (mild/ infrequent: prophylaxis and or as needed therapy), 26 had grade 2 (moderate: kept under control with anti-mediator type drugs daily), and 12 had grade 3 (severe and frequent: not sufficiently controlled with therapy). None of the patients had grade 4 characterized by severe adverse events which require immediate therapy and hospitalization. The diagnosis and classification of mastocytosis were made according to the recommendation of the World Health Organization (WHO) on the histological examination of a skin biopsy for cutaneous mastocytosis (CM) and of bone marrow biopsy for systemic mastocytosis (SM) [9, 57]. Patients were divided according to cutaneous and/or systemic involvement and the severity and frequency of symptoms. The first group (indolent) included maculopapular cutaneous mastocytosis (MPCM) (n = 3), mastocytosis in the skin (MIS) (n = 4), and indolent systemic mastocytosis (ISM) (n = 38). The second group (advanced) included patients with smoldering systemic mastocytosis (n = 9), aggressive systemic mastocytosis (n = 7), systemic mastocytosis associated with hematologic disease (SM-AHD) (n = 2) and mast cell leukemia (MCL) (n = 1) [2, 8, 9]. The most common mutation of KIT receptor in patients with indolent and aggressive SM is KIT D816V [2, 58]. The search of KIT D816V mutation was performed in 30 patients. In 21 of those patients, the presence of KIT mutation was found and in 9 patients was not found. Many patients with provisional diagnosis of isolated skin mastocytosis refused to undergo bone marrow biopsy. Circulating concentrations of angiogenic (VEGF-A), lymphangiogenic (VEGF-C and VEGF-D) factors, and angiopoietins (ANGPT1 and ANGPT2) were assessed in all patients and controls.
Table 1.
Characteristics of patients with mastocytosis compared to healthy donors
| Characteristics | Healthy (n = 64) | Patients (n = 64) |
|---|---|---|
| Age (years range) | 43 (29–70) | 46 (21–79) |
| Gender Male (%) | 31 (48%) | 31 (48%) |
| Tryptase (µg/L)a | 5.6 ± 3.3 | 99.5 ± 243.4b |
| Symptom Grading (%) | ||
| 0 | 13 (20%) | |
| 1 | NA | 13 (20%) |
| 2 | 26 (41%) | |
| 3 | 12 (19%) | |
| Indolent | NA | |
| MPCM | 3 (5%) | |
| MIS | 4 (6%) | |
| I SM | 38 (60%) | |
| Clinical variants(%) | ||
| Advanced | NA | |
| SSM | 9 (14%) | |
| ASM | 7 (11%) | |
| SM-AHD | 2 (3%) | |
| MCL | 1 (1%) | |
| Skin lesions (%) | NA | 30 (47%) |
| Anaphylaxis (%) | NA | 19 (30%) |
| Urticaria (%) | NA | 35 (55%) |
| Flushing (%) | NA | 46 (72%) |
| Pruritus (%) | NA | 51 (80%) |
ASM aggressive systemic mastocytosis; ISM: indolent systemic mastocytosis; MCL mast cell leukemia; MIS mastocytosis in skin; MPCM maculopapular cutaneous mastocytosis; SM-AHD systemic mastocytosis associated with hematologic disease; SSM smoldering systemic mastocytosis; NA not applicable; Data were analyzed by t-test
aData are expressed as median values ± SD
bp < 0.005
Serum collection
The Ethics Committee of Campania ASL Napoli 3 Sud (protocol number 68863) approved that serum, obtained during routine diagnostics, could be used for research investigating the pathophysiology of mastocytosis. Written informed consent was obtained from both mastocytosis patients and HC according to the principles expressed in the Declaration of Helsinki. The blood samples were collected by a clean venepuncture. After centrifugation (2000 × g, 22 °C, 20 min), the serum was divided into aliquots and stored at − 80 °C until tested. We collected the data about the clinical manifestations at onset of mastocytosis and 30% of patients have an history of anaphylaxis at the onset of disease. The collection of the samples for measurement of all metabolites was performed at least after 3 months from anaphylactic episodes.
Tryptase assay
Serum tryptase concentration was measured by fluoro-enzyme immune assay (FEIA) using Uni-CAP100 (Phadia Diagnostics AB, Uppsala, Sweden). This technique allows to measure both α-tryptase and β-tryptase.
Culture of human mast cells
The human mast cell lines ROSAKIT WT and ROSAKIT D816V were a generous gift from Michel Arock (Laboratoire de Biologie et de Pharmacologie Appliquee, Ecole Normale Supérieure de Cachan) [59]. ROSAKIT WT and ROSAKIT D816V mast cell lines were cultured at the density of 4 × 105 cells/mL with and without recombinant SCF (80 ng/mL) (Peprotech, London, UK), respectively, in IMDM (Microgem, Naples, Italy) supplemented with 0.3% bovine serum albumin (Microgem, Naples, Italy), 1% L-glutamine (Sigma-Aldrich St. Louis, MO, USA), 2% nonessential aminoacids (Microgem, Naples, Italy), 1% vitamins solution (Gibco-Thermo-Fisher Waltham, MA, USA), 1% insulin-transferrin-selenium (Thermo-Fisher Waltham, MA, USA), and 1% sodium pyruvate (Gibco-Thermo-Fisher Waltham, MA, USA), 1% antibiotic–antimycotic solution (Lonza, Basel, CH). ROSAKIT WT and ROSAKIT D816V cells were incubated in 25 cm2 flask at 37 °C and 5% CO2 and counted after 4 days of culture. Primary human mast cells were isolated from lung parenchyma of patients undergoing thoracic surgery for lung cancer and were purified (> 98%) by immunomagnetic selection, as previously described [21]. The study protocol involving the use of human blood cells was approved by the Ethics Committee of the University of Naples Federico II (Protocol number 301/12), and written informed consent was obtained from donors according to the principles expressed in the Declaration of Helsinki. For VEGFs and ANGPTs analysis, all types of mast cells were incubated (37 °C, 5% CO2, 24 h) in complete medium. At the end of the experiments, the cells were centrifuged (1000 × g, 4 °C, 5 min) and the supernatants were stored at − 80 °C for subsequent determination of mediators. The cellular pellets were lysated in Tryton X-100 0.1% (Sigma-Aldrich, Saint Louis, MO, USA) and stored at − 80 °C for subsequent determination of intracellular mediator content.
ELISA assay
VEGF-A, VEGF-C, VEGF-D, ANGPT1 and ANGPT2 concentrations in serum, in supernatant and in cellular lysates of ROSAKIT WT and ROSAKIT D816V were measured using commercially available ELISA KITs (R&D System, Minneapolis, MN, USA) according to the manufacturer's instructions. The serum concentrations of these mediators from mastocytosis patients and HC were expressed as pg/mL. Intracellular and released mediators from ROSAKIT WT and ROSAKIT D816V were expressed as pg/106 cells.
Statistical analysis
Data were analyzed with the GraphPad Prism 5 software package. Data were tested for normality using the D'Agostino–Pearson normality test. If normality was not rejected at 0.05 significance level, we used parametric tests. Otherwise, for not-normally distributed data we used nonparametric tests. Statistical analysis was performed by unpaired two-tailed t-test or two-tailed Mann–Whitney test as indicated in figure legends. Correlations between two variables were assessed by Spearman’s correlation analysis and reported as coefficient of correlation (r). A p value ≤ 0.05 was considered statistically significant. Serum levels of VEGF-A, VEGF-C, VEGF-D, ANGPT1 and ANGPT2 are shown as the median (horizontal black line), the 25th and 75th percentiles (boxes) and the 5th and 95th percentiles (whiskers) of 64 controls and 64 patients.
Results
VEGF and ANGPT serum concentrations in patients with mastocytosis
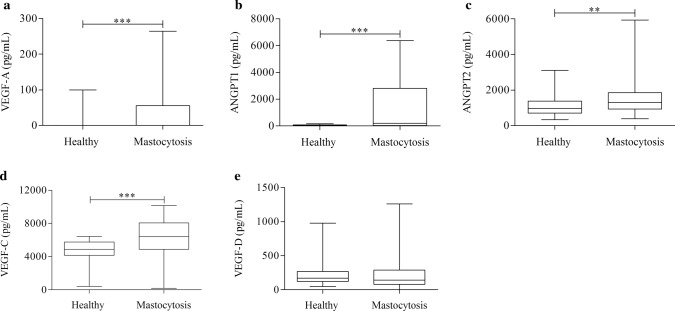
VEGF-A concentrations in mastocytosis patients have been studied only on small number of patients by Brockow et al. [60], whereas the ANGPTs levels have not yet been investigated. Therefore, in this study we evaluated the serum concentrations of VEGFs and ANGPTs in patients with mastocytosis (N = 64) compared to HC (N = 64). The characteristics of patients are reported in supplementary Table 1 (Supplementary Table 1). Figure 1a shows that VEGF-A serum levels of mastocytosis patients were higher than HC [VEGF-A: (34.63 ± 56.76) vs (9.21 ± 24.59) pg/mL]. Interestingly, both ANGPT2, which increases vascular permeability [61], and its antagonist ANGPT1 [62, 63] were increased in mastocytosis patients compared to HC [ANGPT1: (1,538 ± 2,136) vs (39.93 ± 45.60 pg/mL)] [ANGPT2: (1,492 ± 976) vs (1,085 ± 607) pg/mL] (Fig. 1b, c). In mastocytosis patients, the concentrations of different mediators did not correlate with each other (data not shown).
Fig. 1.
VEGF-A (a), ANGPT1 (b), ANGPT2 (c), VEGF-C (d) and VEGF-D (e) serum levels in healthy donors and in patients with mastocytosis. Data are shown as the median (horizontal black line), the 25th and 75th percentiles (boxes) and the 5th and 95th percentiles (whiskers) of 64 patients with mastocytosis and 64 healthy donors. **p < 0.01; ***p < 0.001
No data are available on the serum concentrations of lymphangiogenic factors in mastocytosis patients. Serum concentrations of VEGF-C were significantly higher in mastocytosis patients compared to the control group [VEGF-C: (6228 ± 2188) vs (4741 ± 1266) pg/mL] (Fig. 1d). Interestingly, the concentrations of VEGF-D, another lymphangiogenic factor, did not differ between the two groups [VEGF-D: (204.9 ± 213.1) vs (234.8 ± 184.5) pg/mL] (Fig. 1e). Surprisingly, VEGF-C and VEGF-D concentrations were positively correlated with each other (Supplementary Fig. 1).
There was no difference in VEGF and/or ANGPT concentrations between male and female values in both controls and patients (Supplementary Fig. 2). Moreover, the age of patients and the concentrations of the different mediators examined did not correlate (Supplementary Fig. 3, Additional File 4).
Effects of disease severity on serum concentrations of VEGF and ANGPT in patients with mastocytosis
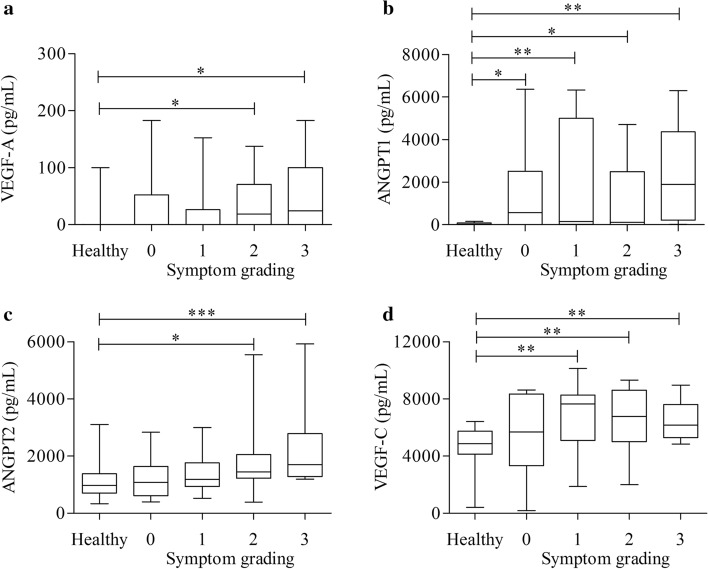
A triplex experimental analysis was used to verify whether enhanced levels of VEGF-A, ANGPT1, ANGPT2 and VEGF-C were correlated with mastocytosis severity. Mastocytosis patients were divided according to the severity of mediator-related symptoms, from grading 0 to grading 3 (see Materials and methods), and concentrations of VEGFs and ANGPTs were compared among groups. VEGF-A, ANGPT2 and VEGF-C levels were not increased in asymptomatic patients (grading 0) compared to controls (Fig. 2a, c, d). Symptomatic patients (grading 1 to 3) had elevated VEGF-C concentrations compared to HC (Fig. 2d); conversely, VEGF-A and ANGPT2 were altered only in symptomatic patients with grading 2 and 3 (Fig. 2a, c). Interestingly, ANGPT1 levels were increased in both asymptomatic and symptomatic patients compared to HC (Fig. 2b).
Fig. 2.
Effects of symptom grading on serum concentrations of VEGF-A (a), ANGPT1 (b), ANGPT2 (c) and VEGF-C (d). Serum levels of VEGFs and ANGPTs were measured in 13 patients with symptom grading 0, 13 patients with symptom grading 1, 20 patients with symptom grading 2 and 12 patients with symptom grading 3. *p < 0.05; **p < 0.01; ***p < 0.001
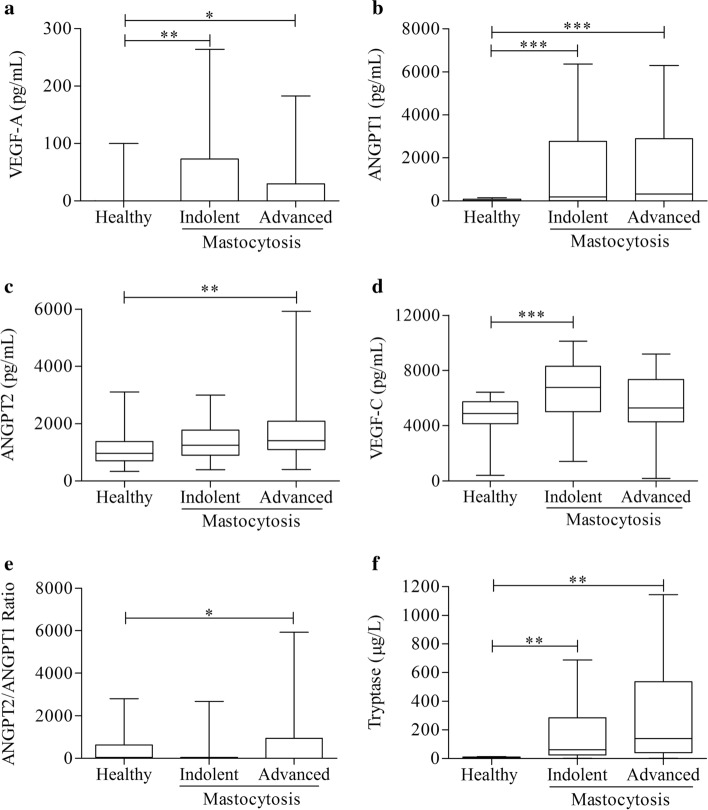
We also grouped patients according to their clinical variants in two groups (see Materials and methods): indolent (MPCM/MIS/ISM) and advanced (SSM/SM-AHD/ASM/MCL) mastocytosis. Figure 3 shows that VEGF-A (panel A) and ANGPT1 (panel B) concentrations did not differ between patients with indolent and advanced variants, but were altered in both groups when compared to controls. ANGPT2 levels, like tryptase (panel F), were higher in patients with advanced mastocytosis compared to indolent variants (panel C). Interestingly, VEGF-C was increased only in indolent mastocytosis compared to controls (panel D). The ANGPT2/ANGPT1 ratio (an index of vascular permeability) [53] was also increased in more severe patients (panel E).
Fig. 3.
Effects of clinical variants of mastocytosis on serum concentrations of VEGF-A, VEGF-C, ANGPT1, ANGPT2, ANGPT2/ANGPT1 ratio, tryptase. VEGF-A (a), ANGPT1 (b), ANGPT2 (c), VEGF-C (d), ANGPT2/ANGPT1 (e) and tryptase (f) serum levels were determined in 64 healthy controls, in 45 patients with indolent variants and in 19 patients with advanced variants. *p < 0.05; **p < 0.01; ***p < 0.001
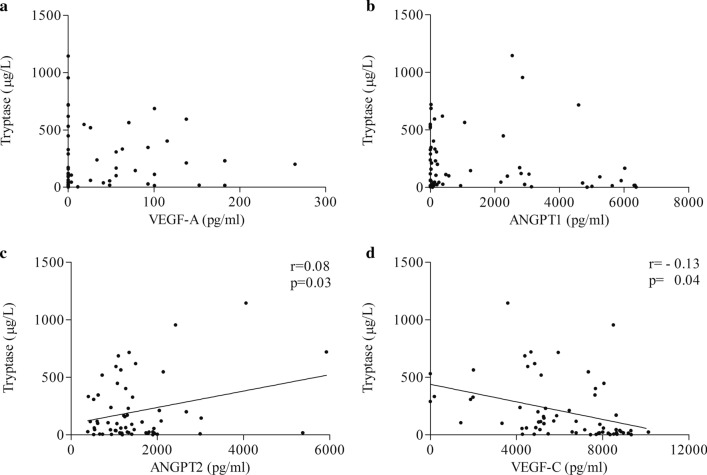
Patients with advanced forms of mastocytosis have elevated tryptase levels compared with those with indolent forms [64, 65]. Thus, we analyzed the correlations between VEGFs/ANGPTs and this important marker of mast cell activation/proliferation. The concentrations of VEGF-A (Fig. 4a) and ANGPT1 (Fig. 4b) were not correlated with tryptase levels. The concentrations of ANGPT2 positively correlated with tryptase concentrations (Fig. 4c). By contrast, the serum levels of VEGF-C negatively correlated with tryptase concentrations (Fig. 4d).
Fig. 4.
Correlations between VEGF-A, ANGPT1, ANGPT2, VEGF-C and tryptase concentrations. Correlations between two variables: VEGF-A and tryptase (a), ANGPT1 and tryptase (b), ANGPT2 and tryptase (c) and VEGF-C and tryptase (d) were assessed by Spearman’s correlation analysis and reported as coefficient of correlation (r). p < 0.05 was considered statistically significant
Angiogenic and lymphangiogenic factors in primary human mast cells and in human mast cell lines
We have previously reported that VEGF-A, VEGF-C, and VEGF-D can be detected by immunohistochemistry in human lung mast cells [66]. The ROSAKIT WT is a SCF-dependent human MC line expressing the high affinity receptor for IgE (FcεRI) [59]. The most frequent mutation affecting patients with mastocytosis is the D816V [5, 67]. The transfection with KIT D816V converted ROSAKIT WT cells into an SCF-independent clone, ROSAKIT D816V, which produced a mastocytosis-like disease in mice [59].
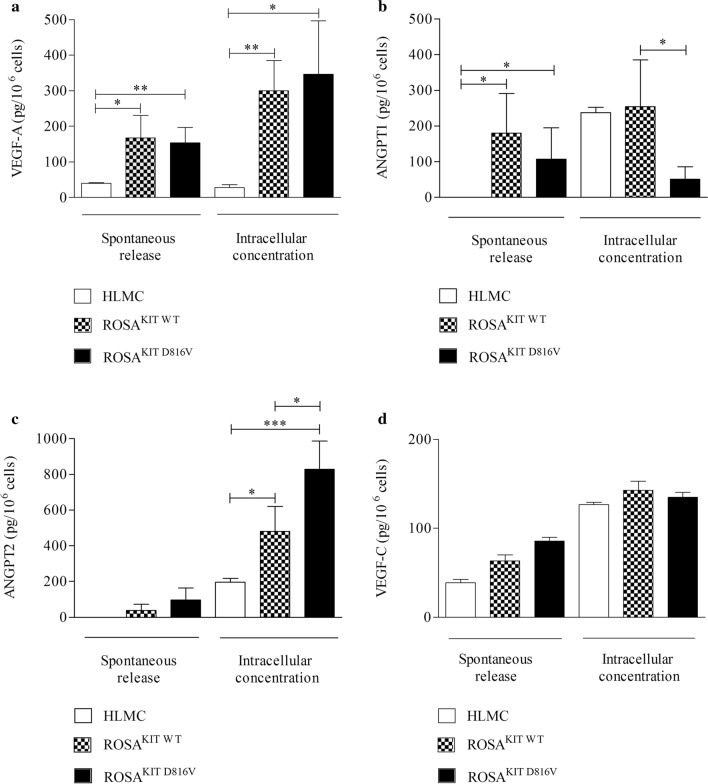
We analyzed the basal content of VEGFs and ANGPTs in ROSAKIT WT and ROSAKIT D816V and also primary human MC derived from lung tissue (HLMCs). Figure 5 shows that both ROSAKIT WT and ROSAKIT D816V spontaneously released a large amount of VEGF-A (panel A), ANGPT1 (panel B), ANGPT2 (panel C) and VEGF-C (panel D). The spontaneous release of these mediators did not differ between the two mast cell lines. HLMCs release lower concentration of VEGF-A and ANGPT1 compared ROSA cells; by contrast, the release of ANGPT2 and VEGF-C did not differ among the three different human MCs.
Fig. 5.
Spontaneous release and intracellular concentrations of VEGF-A (a), ANGPT1 (b), ANGPT2 (c), and VEGF-C (d) from human lung mast cells (HLMC), ROSAKIT WT and ROSAKIT D816. ROSAKIT WT and ROSAKIT D816V were incubated (5% CO2, 37 °C, 24 h) with and without SCF (80 ng/ml), respectively. Data are the mean ± SD of 5 independent experiments. *p < 0.05; *p < 0.01; ***p < 0.001
ROSAKIT WT and ROSAKIT D816V contained VEGF-A (Fig. 5a), ANGPT1 (Fig. 5b), ANGPT2 (Fig. 5c) and VEGF-C (Fig. 5d). The content of VEGF-A and of VEGF-C was similar between wild type and mutated MC lines (Fig. 5a, d). ANGPT1 in lysates of ROSAKIT WT was higher than in ROSAKIT D816V. By contrast, the intracellular content of ANGPT2 was higher in ROSAKIT D816V than in ROSAKIT WT (Fig. 5b, c). HLMCs contained lower levels of VEGF-A and ANGPT2 compared to ROSA cells.
Discussion
Serum concentrations of VEGF-A, VEGF-C, ANGPT1 and ANGPT2 are increased in patients with mastocytosis compared to healthy controls. Some of these mediators such as VEGF-A [21, 31–34], VEGF-C [21, 29] and ANGPT1 and ANGPT2 [35] are expressed by human primary and neoplastic (e.g., LAD2, HMC-1) mast cells. There is a clinical correlation between the severity of mastocytosis and the plasma levels of these mast cell-derived mediators. In fact, circulating levels of VEGF-A, ANGPT2 and VEGF-C are increased in symptomatic, but not asymptomatic mastocytosis patients. Interestingly, the serum concentration of ANGPT1, which is mainly produced by pericytes and inhibits endothelial cell permeability [63], is increased in all mastocytosis patients.
The angiopoietin (ANGPT) family is an important group of factors, specific for vascular endothelium, whose functions are mediated through two tyrosine kinase receptors, Tie1 and Tie2 [43, 63]. The ANGPT-Tie ligand–receptor system exerts a key role in regulating vascular integrity [68, 69]. Besides their roles in the regulation of angiogenesis [62, 70] and lymphangiogenesis [71, 72], ANGPTs also modulate inflammation in several disorders [39, 48, 69, 73]. ANGPT1, produced by peri-endothelial mural cells (pericytes) [74] and immune cells [35, 75], is a potent agonist of Tie2 receptor on endothelial cells [44, 70]. ANGPT1 is an anti-inflammatory molecule that maintains vascular integrity [68, 76, 77]. ANGPT2, stored in Weibel–Palade bodies in endothelial cells [78], is considered a pro-inflammatory molecule [61, 79]. ANGPT2 inhibits ANGPT1/Tie2 interaction [62, 63], resulting in vascular instability and leakage [61].
It has been demonstrated that ANGPT1 inhibits the in vitro activation of the mouse mastocytoma cell line P815 and experimental anaphylactic shock in mice [80]. Anaphylaxis and anaphylactoid reactions are more frequent in mastocytosis patients compared to the general population [81]. It is possible to speculate that the increase in circulating ANGPT1 in all patients with mastocytosis might represent a protective factor in counterbalancing the vasopermeability effect of VEGF-A [41, 42, 82] and ANGPT2 [61].
Tryptase is a serine protease highly expressed by human mast cells and to a minor extent by basophils [83, 84]. Measurements of tryptase levels in serum have been used to assess mast cell load in systemic mastocytosis [64, 85–87]. In this study, we found that serum concentrations of tryptase are increased in indolent and advanced mastocytosis. Tryptase concentrations are positively correlated with the circulating levels of ANGPT2 and negatively correlated with VEGF-C. The latter observation is difficult to reconcile because there is evidence that activated human mast cells release both tryptase and VEGF-C [21, 39]. Moreover, tryptase serum concentrations are not correlated with the levels of VEGF-A and ANGPT1. Again, these results are rather unexpected because VEGF-A [28–34] and ANGPT1 [35] are expressed by human mast cells. However, many other immune and non-immune cells can produce and release VEGF-A [88–90] and ANGPT1 [63].
This study also examined the differential expression of several mediators in indolent and advanced mastocytosis. There is compelling evidence that mastocytosis is a heterogeneous condition with strikingly different prognostic profiles [2, 91]. Serum concentrations of tryptase, VEGF-A and ANGPT1 are increased in indolent and advanced mastocytosis compared to healthy controls. However, the lack of correlation between tryptase and both VEGF-A and ANGPT1 might indicate that alternative sources of the two latter mediators are involved in mastocytosis. This observation suggests that there are complex cellular and biochemical alterations in mastocytosis, in addition to the proliferation of mast cells.
ANGPT2, which is released mainly by endothelial cells [78], and ANGPT1/ANGPT2 ratio, an index of vascular permeability [53], are increased only in advanced mastocytosis. We found that serum concentrations of ANGPT2 are correlated with those of tryptase. The latter correlation might indicate that in patients with advanced mastocytosis these mediators are mainly derived from activated mast cells. These results are in line with those of our previous work in which we demonstrated that an endothelial dysfunction is detectable in patients with mastocytosis and is more severe in patients with high tryptase levels and advanced disease. Endothelial function appears to be negatively influenced by MC proliferation rather than by the severity of mediator-related symptoms [92].
VEGF-C and VEGF-D are the most important modulators of inflammatory and tumor lymphangiogenesis [93, 94] acting on VEGF receptor 3 (VEGFR-3) on LECs [38, 95]. These factors can be detected [66] and can be produced by activated human mast cells [21, 39]. Our results indicate that the serum concentrations of VEGF-C but not VEGF-D are markedly increased in patients with mastocytosis compared to healthy controls. The differential alterations of VEGF-C and VEGF-D in these patients are intriguing but not surprising. Recent evidence indicates that VEGF-C and VEGF-D can differently modulate the immune system [93]. The possible role of VEGF-C in mastocytosis deserves further investigations.
Human mast cells constitutively express VEGF receptors and Tie receptors for ANGPTs [28, 29, 35]. These receptors are functionally active because VEGFs [29] and ANGPT1 exert a chemotactic effect on human mast cells [35]. In this scenario, one may envisage a novel autocrine-loop involving angiogenic factors (i.e., VEGFs, ANGPTs) and their receptors on mast cells. In fact, VEGFs and ANGPT1 released by activated mast cells might attract progenitors of these cells to sites of neoplastic growth through the engagement of VEGFs and Tie2 receptors, respectively.
There is compelling evidence that human mast cells are a major source of several canonical (VEGF-A, ANGPTs) [21, 31–35] and non-canonical angiogenic factors (LTC4, LTD4, tryptase) [96, 97]. Valent and collaborators demonstrated that bone marrow microvessel density (MVD) is increased in patients with mastocytosis [98]. Moreover, BM MVD was significantly higher in systemic mastocytosis compared to cutaneous mastocytosis and healthy controls. Immunohistochemical staining revealed expression of VEGF-A in mast cell infiltrates. The same group of investigators extended the previous observation to canine mastocytosis by demonstrating the presence of VEGF in primary dog mastocytomas by immunohistochemistry and VEGF mRNA by PCR [99].
We have previously shown by immunohistochemistry that HLMCs contain VEGF-A, VEGF-C, and VEGF-D [66]. In this study, we confirm that immunoreactive VEGF-A is present in HLMCs and can be spontaneously released. We also examined the content and spontaneous release of several angiogenic factors in ROSA mast cell lines with (ROSAKIT D816V) and without KIT mutation (ROSAKIT WT) [59]. Both ROSAKIT WT and ROSAKIT D816V contained and spontaneously released VEGF-A, VEGF-C and ANGPT1. These findings agree with previous observations that the histamine content and FcεRI expression did not differ between both ROSAKIT WT and ROSAKIT D816V cell lines [59].
MicroRNA (miRNAs) are a large class of single-stranded RNA molecules that regulate a wide spectrum of cellular functions [100, 101]. Several miRNAs modulate different genes during mast cell activation [100, 102, 103] and the expression of pro- and anti-angiogenic factors [101, 104]. In particular, miR-221 and miR-222, highly expressed in endothelial cells, are upregulated during mast cell activation [102] and modulate the secretion of cytokines [105] and angiogenesis [104]. Therefore, it would be of interest to evaluate the expression of miR-221/222 in patients with different variants of mastocytosis. We cannot exclude the possibility that complex interplay between miR-221/222 and angiogenic/lymphangiogenic factors could contribute to the neoplastic progression of mastocytosis.
This study has a limitation that should be pointed out. Although more than 90% of patients with systemic mastocytosis have a mutation in codon 816 of KIT (KIT D816V) [5, 58], alternative KIT mutation in codon 816 (e.g., D816A/F/H/I/N/T/Y) has been described. In addition, to the tyrosine kinase domain (exons 17 and 18; e.g., D820G or N8221/K), at least 30 different KIT mutations have been identified in the extracellular (exon 8–9), transmembrane (exon 19; e.g., F522C) and juxtamembrane domains (exon 11; e.g., V560 G/I) in a small percentage of mastocytosis patients [106–109]. In this study, the identification of KIT D816V was not performed in all patients examined. In addition, other less common mutations were not investigated.
Mastocytosis is a heterogeneous group of neoplastic disorders characterized by complex pathology, distinct subtypes, and highly variable clinical courses [2, 8, 9]. Our findings indicate that VEGF and ANGPT concentrations are increased in patients with mastocytosis compared to controls. Several studies have shown that in addition to activating KIT mutations, additional mutations in other genes may occur in mastocytosis [106–108]. The contribution of KIT and other mutations to the altered production of VEGFs and ANGPTs in patients with different forms of mastocytosis remains to be investigated. In addition, further studies on larger cohorts of patients with different variants of mastocytosis could highlight the theragnostic significance of VEGF and ANGPT assays in these patients. Finally, classical and novel inhibitors of angiogenesis and/or lymphangiogenesis alone or in combination with other anti-neoplastic drugs are used in the treatment of cancer [63]. The current treatment options for patients with advanced mastocytosis need to be improved [2, 91]. Perhaps, the use of angiogenic/lymphangiogenic inhibitors could be considered for the treatment of selected patients with severe mastocytosis and high levels of circulating angiogenic factors.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Dr. Gjada Criscuolo for critical reading of the manuscript and the administrative staff (Dr. Roberto Bifulco and Dr. Anna Ferraro), without whom it would not be possible to work as a team.
Abbreviations
- ASM
Aggressive systemic mastocytosis
- ANGPT
Angiopoietin
- BEC
Blood endothelial cell
- BM
Bone marrow
- BSA
Bovine serum albumin
- CM
Cutaneous mastocytosis
- DCM
Diffuse cutaneous mastocytosis
- FEIA
Fluoro-enzyme immune assay
- HC
Healthy control
- HLMC
Human lung mast cell
- ISM
Indolent systemic mastocytosis
- LEC
Lymphatic endothelial cell
- MC
Mast cell
- MCL
Mast cell leukemia
- MCS
Mast cell sarcoma
- MIS
Mastocytosis in skin
- MPCM
Maculopapular cutaneous mastocytosis
- MVD
Microvessel density
- PlGF
Placental growth factor
- SM-AHD
Systemic mastocytosis associated to hematologic disease
- SSM
Smoldering systemic mastocytosis
- SCF
Stem cell factor
- SM
Systemic mastocytosis
- Tie
Tyrosine kinase with immunoglobulin-like and EGF-like domains
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
- VPF
Vascular permeability factor
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. This work was supported in part by grants from the CISI-Lab Project (University of Naples Federico II), TIMING Project and Campania Bioscience (Regione Campania), and MIUR PRIN 2017 M8Y MR8_005.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interest.
Ethical approval
This study has received ethical, regulatory and institutional approvals at Ethics Committee Campania ASL Napoli 3 Sud (Protocol Number 68863) and by the Ethics Committee of the University of Naples Federico II (Protocol Number 301/12) and was run in accordance with the recommendations from the Declaration of Helsinki. All participants or their legal representative provided informed consent before their enrolment in the registry.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gilda Varricchi, Email: gildanet@gmail.com.
Stefania Loffredo, Email: stefanialoffredo@hotmail.com.
References
- 1.Valent P, Akin C, Gleixner KV, et al. Multidisciplinary challenges in mastocytosis and how to address with personalized medicine approaches. Int J Mol Sci. 2019;20(12):2976. doi: 10.3390/ijms20122976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiter A, George TI, Gotlib J. New developments in diagnosis, prognostication, and treatment of advanced systemic mastocytosis. Blood. 2020;135(16):1365–1376. doi: 10.1182/blood.2019000932. [DOI] [PubMed] [Google Scholar]
- 3.Arock M. Mastocytosis, classification, biological diagnosis and therapy. Ann Biol Clin (Paris) 2004;62(6):657–669. [PubMed] [Google Scholar]
- 4.Shomali W, Gotlib J. The new tool "KIT" in advanced systemic mastocytosis. Hematol Am Soc Hematol Educ Program. 2018;2018(1):127–136. doi: 10.1182/asheducation-2018.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komi DEA, Rambasek T, Wohrl S. Mastocytosis: from a molecular point of view. Clin Rev Allergy Immunol. 2018;54(3):397–411. doi: 10.1007/s12016-017-8619-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuch A, Brockow K. Mastocytosis and anaphylaxis. Immunol Allergy Clin North Am. 2017;37(1):153–164. doi: 10.1016/j.iac.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 7.de Paulis A, Minopoli G, Arbustini E, et al. Stem cell factor is localized in, released from, and cleaved by human mast cells. J Immunol. 1999;163(5):2799–2808. [PubMed] [Google Scholar]
- 8.Valent P, Akin C, Hartmann K, et al. Advances in the classification and treatment of mastocytosis: current status and outlook toward the future. Cancer Res. 2017;77(6):1261–1270. doi: 10.1158/0008-5472.CAN-16-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardanani A. Systemic mastocytosis in adults: 2019 update on diagnosis, risk stratification and management. Am J Hematol. 2019;94(3):363–377. doi: 10.1002/ajh.25371. [DOI] [PubMed] [Google Scholar]
- 10.Akin C. Mast cell activation syndromes. J Allergy Clin Immunol. 2017;140(2):349–355. doi: 10.1016/j.jaci.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Varricchi G, Rossi FW, Galdiero MR, et al. Physiological roles of mast cells: collegium internationale allergologicum update 2019. Int Arch Allergy Immunol. 2019;179(4):247–261. doi: 10.1159/000500088. [DOI] [PubMed] [Google Scholar]
- 12.Varricchi G, Pecoraro A, Loffredo S, et al. Heterogeneity of human mast cells with respect to MRGPRX2 receptor expression and function. Front Cell Neurosci. 2019;13:299. doi: 10.3389/fncel.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang YL, Wang Z, Igawa S, et al. Lipocalin 2: a new antimicrobial in mast cells. Int J Mol Sci. 2019;20(10):2380. doi: 10.3390/ijms20102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frossi B, De Carli M, Calabro A. Coeliac disease and mast cells. Int J Mol Sci. 2019;20(14):3400. doi: 10.3390/ijms20143400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varricchi G, Marone G, Kovanen PT. Cardiac mast cells: underappreciated immune cells in cardiovascular homeostasis and disease. Trends Immunol. 2020;41(8):734–746. doi: 10.1016/j.it.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Marshall JS, Portales-Cervantes L, Leong E. Mast cell responses to viruses and pathogen products. Int J Mol Sci. 2019;20(17):4241. doi: 10.3390/ijms20174241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piliponsky AM, Acharya M, Shubin NJ. Mast cells in viral, bacterial, and fungal infection immunity. Int J Mol Sci. 2019;20(12):2851. doi: 10.3390/ijms20122851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borriello F, Granata F, Varricchi G, et al. Immunopharmacological modulation of mast cells. Curr Opin Pharmacol. 2014;17:45–57. doi: 10.1016/j.coph.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Varricchi G, de Paulis A, Marone G, Galli SJ. Future needs in mast cell biology. Int J Mol Sci. 2019;20(18):4397. doi: 10.3390/ijms20184397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivellese F, Mauro D, Nerviani A, et al. Mast cells in early rheumatoid arthritis associate with disease severity and support B cell autoantibody production. Ann Rheum Dis. 2018;77(12):1773–1781. doi: 10.1136/annrheumdis-2018-213418. [DOI] [PubMed] [Google Scholar]
- 21.Varricchi G, Loffredo S, Borriello F, et al. Superantigenic activation of human cardiac mast cells. Int J Mol Sci. 2019;20(8):1828. doi: 10.3390/ijms20081828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sammarco G, Varricchi G, Ferraro V, et al. Mast cells, angiogenesis and lymphangiogenesis in human gastric cancer. Int J Mol Sci. 2019;20(9):2106. doi: 10.3390/ijms20092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrari SM, Fallahi P, Galdiero MR, et al. Immune and inflammatory cells in thyroid cancer microenvironment. Int J Mol Sci. 2019;20(18):4413. doi: 10.3390/ijms20184413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varricchi G, Galdiero MR, Loffredo S, et al. Are mast cells MASTers in cancer? Front Immunol. 2017;8:424. doi: 10.3389/fimmu.2017.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 2018;282(1):121–150. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon TC, Befus AD, Kulka M. Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol. 2014;5:569. doi: 10.3389/fimmu.2014.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afferni C, Buccione C, Andreone S, et al. The pleiotropic immunomodulatory functions of IL-33 and its implications in tumor immunity. Front Immunol. 2018;9:2601. doi: 10.3389/fimmu.2018.02601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loffredo S, Staiano RI, Granata F, Genovese A, Marone G. Immune cells as a source and target of angiogenic and lymphangiogenic factors. Chem Immunol Allergy. 2014;99:15–36. doi: 10.1159/000353316. [DOI] [PubMed] [Google Scholar]
- 29.Detoraki A, Staiano RI, Granata F, et al. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J Allergy Clin Immunol. 2009;123(5):1142–1149. doi: 10.1016/j.jaci.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 30.Marone G, Varricchi G, Loffredo S, Granata F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur J Pharmacol. 2016;778:146–151. doi: 10.1016/j.ejphar.2015.03.088. [DOI] [PubMed] [Google Scholar]
- 31.Boesiger J, Tsai M, Maurer M, et al. Mast cells can secrete vascular permeability factor/ vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of fc epsilon receptor I expression. J Exp Med. 1998;188(6):1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdel-Majid RM, Marshall JS. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J Immunol. 2004;172(2):1227–1236. doi: 10.4049/jimmunol.172.2.1227. [DOI] [PubMed] [Google Scholar]
- 33.Grutzkau A, Kruger-Krasagakes S, Baumeister H, et al. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol Biol Cell. 1998;9(4):875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theoharides TC, Zhang B, Kempuraj D, et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010;107(9):4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prevete N, Staiano RI, Granata F, et al. Expression and function of Angiopoietins and their tie receptors in human basophils and mast cells. J Biol Regul Homeost Agents. 2013;27(3):827–839. [PubMed] [Google Scholar]
- 36.Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. 2013;123(8):3190–3200. doi: 10.1172/JCI70212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 38.Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest. 2014;124(3):878–887. doi: 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marone G, Rossi FW, Pecoraro A, et al. HIV gp120 induces the release of proinflammatory, angiogenic, and lymphangiogenic factors from human lung mast cells. Vaccines (Basel) 2020;8(2):208. doi: 10.3390/vaccines8020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 41.Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274(3):H1054–H1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- 42.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 43.Saharinen P, Leppanen VM, Alitalo K. SnapShot: angiopoietins and their functions. Cell. 2017;171(3):724–724. doi: 10.1016/j.cell.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87(7):1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 45.Moss A. The angiopoietin: Tie 2 interaction: a potential target for future therapies in human vascular disease. Cytokine Growth Factor Rev. 2013;24(6):579–592. doi: 10.1016/j.cytogfr.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Tao Z, Chen B, Tan X, et al. Coexpression of VEGF and angiopoietin-1 promotes angiogenesis and cardiomyocyte proliferation reduces apoptosis in porcine myocardial infarction (MI) heart. Proc Natl Acad Sci U S A. 2011;108(5):2064–2069. doi: 10.1073/pnas.1018925108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsunaga T, Warltier DC, Tessmer J, et al. Expression of VEGF and angiopoietins-1 and -2 during ischemia-induced coronary angiogenesis. Am J Physiol Heart Circ Physiol. 2003;285(1):H352–H358. doi: 10.1152/ajpheart.00621.2002. [DOI] [PubMed] [Google Scholar]
- 48.Lee SJ, Lee CK, Kang S, et al. Angiopoietin-2 exacerbates cardiac hypoxia and inflammation after myocardial infarction. J Clin Invest. 2018;128(11):5018–5033. doi: 10.1172/JCI99659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu C, Wang W, Wang Y, et al. Serum angiopoietin-2 as a clinical marker for lung cancer in patients with solitary pulmonary nodules. Ann Clin Lab Sci. 2016;46(1):60–64. [PubMed] [Google Scholar]
- 50.Nowacka A, Smuczynski W, Rosc D, Wozniak-Dabrowska K, Sniegocki M. Serum VEGF-A concentrations in patients with central nervous system (CNS) tumors. PLoS ONE. 2018;13(3):e0192395. doi: 10.1371/journal.pone.0192395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie Z, Ghosh CC, Patel R, et al. Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome) Blood. 2012;119(18):4321–4332. doi: 10.1182/blood-2011-08-375816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrara AL, Bova M, Petraroli A, et al. Hereditary angioedema attack: what happens to vasoactive mediators? Int Immunopharmacol. 2020;78:106079. doi: 10.1016/j.intimp.2019.106079. [DOI] [PubMed] [Google Scholar]
- 53.Loffredo S, Bova M, Suffritti C, et al. Elevated plasma levels of vascular permeability factors in C1 inhibitor-deficient hereditary angioedema. Allergy. 2016;71(7):989–996. doi: 10.1111/all.12862. [DOI] [PubMed] [Google Scholar]
- 54.van der Flier M, van Leeuwen HJ, van Kessel KP, et al. Plasma vascular endothelial growth factor in severe sepsis. Shock. 2005;23(1):35–38. doi: 10.1097/01.shk.0000150728.91155.41. [DOI] [PubMed] [Google Scholar]
- 55.Lin SM, Chung FT, Kuo CH, et al. Circulating angiopopietin-1 correlates with the clinical course of multiple organ dysfunction syndrome and mortality in patients with severe sepsis. Medicine (Baltimore) 2015;94(20):e878. doi: 10.1097/MD.0000000000000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lymperopoulou K, Velissaris D, Kotsaki A, et al. Angiopoietin-2 associations with the underlying infection and sepsis severity. Cytokine. 2015;73(1):163–168. doi: 10.1016/j.cyto.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 57.Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129(11):1420–1427. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagata H, Worobec AS, Oh CK, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A. 1995;92(23):10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saleh R, Wedeh G, Herrmann H, et al. A new human mast cell line expressing a functional IgE receptor converts to tumorigenic growth by KIT D816V transfection. Blood. 2014;124(1):111–120. doi: 10.1182/blood-2013-10-534685. [DOI] [PubMed] [Google Scholar]
- 60.Brockow K, Akin C, Huber M, et al. Levels of mast-cell growth factors in plasma and in suction skin blister fluid in adults with mastocytosis: correlation with dermal mast-cell numbers and mast-cell tryptase. J Allergy Clin Immunol. 2002;109(1):82–88. doi: 10.1067/mai.2002.120524. [DOI] [PubMed] [Google Scholar]
- 61.Roviezzo F, Tsigkos S, Kotanidou A, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther. 2005;314(2):738–744. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- 62.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 63.Saharinen P, Eklund L, Alitalo K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug Discov. 2017;16(9):635–661. doi: 10.1038/nrd.2016.278. [DOI] [PubMed] [Google Scholar]
- 64.Lim KH, Tefferi A, Lasho TL, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113(23):5727–5736. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 65.Valent P, Bonadonna P, Hartmann K, et al. Why the 20% + 2 tryptase formula is a diagnostic gold standard for severe systemic mast cell activation and mast cell activation syndrome. Int Arch Allergy Immunol. 2019;180(1):44–51. doi: 10.1159/000501079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Detoraki A, Granata F, Staibano S, et al. Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy. 2010;65(8):946–958. doi: 10.1111/j.1398-9995.2010.02372.x. [DOI] [PubMed] [Google Scholar]
- 67.Chatterjee A, Ghosh J, Kapur R. Mastocytosis: a mutated KIT receptor induced myeloproliferative disorder. Oncotarget. 2015;6(21):18250–18264. doi: 10.18632/oncotarget.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27(12):552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019;8(5):471. doi: 10.3390/cells8050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87(7):1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 71.Fagiani E, Lorentz P, Kopfstein L, Christofori G. Angiopoietin-1 and -2 exert antagonistic functions in tumor angiogenesis, yet both induce lymphangiogenesis. Cancer Res. 2011;71(17):5717–5727. doi: 10.1158/0008-5472.CAN-10-4635. [DOI] [PubMed] [Google Scholar]
- 72.Schulz P, Fischer C, Detjen KM, et al. Angiopoietin-2 drives lymphatic metastasis of pancreatic cancer. FASEB J. 2011;25(10):3325–3335. doi: 10.1096/fj.11-182287. [DOI] [PubMed] [Google Scholar]
- 73.Eklund L, Kangas J, Saharinen P. Angiopoietin-Tie signalling in the cardiovascular and lymphatic systems. Clin Sci (Lond) 2017;131(1):87–103. doi: 10.1042/CS20160129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fang HY, Hughes R, Murdoch C, et al. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114(4):844–859. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jeansson M, Gawlik A, Anderson G, et al. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest. 2011;121(6):2278–2289. doi: 10.1172/JCI46322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6(4):460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 78.Fiedler U, Scharpfenecker M, Koidl S, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103(11):4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 79.Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12(2):235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 80.Yao JH, Cui M, Li MT, et al. Angiopoietin1 inhibits mast cell activation and protects against anaphylaxis. PLoS ONE. 2014;9(2):e89148. doi: 10.1371/journal.pone.0089148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muller UR, Haeberli G. The problem of anaphylaxis and mastocytosis. Curr Allergy Asthma Rep. 2009;9(1):64–70. doi: 10.1007/s11882-009-0010-9. [DOI] [PubMed] [Google Scholar]
- 82.Grover TR, Zenge JP, Parker TA, Abman SH. Vascular endothelial growth factor causes pulmonary vasodilation through activation of the phosphatidylinositol-3-kinase-nitric oxide pathway in the late-gestation ovine fetus. Pediatr Res. 2002;52(6):907–912. doi: 10.1203/00006450-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 83.Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014;14(7):478–494. doi: 10.1038/nri3690. [DOI] [PubMed] [Google Scholar]
- 84.Jogie-Brahim S, Min HK, Fukuoka Y, Xia HZ, Schwartz LB. Expression of alpha-tryptase and beta-tryptase by human basophils. J Allergy Clin Immunol. 2004;113(6):1086–1092. doi: 10.1016/j.jaci.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 85.Schwartz LB, Sakai K, Bradford TR, et al. The alpha form of human tryptase is the predominant type present in blood at baseline in normal subjects and is elevated in those with systemic mastocytosis. J Clin Invest. 1995;96(6):2702–2710. doi: 10.1172/JCI118337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316(26):1622–1626. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- 87.Sperr WR, El-Samahi A, Kundi M, et al. Elevated tryptase levels selectively cluster in myeloid neoplasms: a novel diagnostic approach and screen marker in clinical haematology. Eur J Clin Invest. 2009;39(10):914–923. doi: 10.1111/j.1365-2362.2009.02184.x. [DOI] [PubMed] [Google Scholar]
- 88.Staiano RI, Loffredo S, Borriello F, et al. Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J Leukoc Biol. 2016;99(4):531–540. doi: 10.1189/jlb.3HI1214-584R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Granata F, Frattini A, Loffredo S, et al. Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. J Immunol. 2010;184(9):5232–5241. doi: 10.4049/jimmunol.0902501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Loffredo S, Borriello F, Iannone R, et al. Group V secreted phospholipase A2 induces the release of proangiogenic and antiangiogenic factors by human neutrophils. Front Immunol. 2017;8:443. doi: 10.3389/fimmu.2017.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Radia DH, Green A, Oni C, Moonim M. The clinical and pathological panoply of systemic mastocytosis. Br J Haematol. 2020;188(5):623–640. doi: 10.1111/bjh.16288. [DOI] [PubMed] [Google Scholar]
- 92.Bucci T, Parente R, De Feo G, Cardamone C, Triggiani M. Flow-mediated dilation shows impaired endothelial function in patients with mastocytosis. J Allergy Clin Immunol. 2019;144(4):1106–1111. doi: 10.1016/j.jaci.2019.05.037. [DOI] [PubMed] [Google Scholar]
- 93.Fankhauser M, Broggi MAS, Potin L, et al. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci Transl Med. 2017;9:407. doi: 10.1126/scitranslmed.aal4712. [DOI] [PubMed] [Google Scholar]
- 94.Stacker SA, Williams SP, Karnezis T, et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14(3):159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 95.Randolph GJ, Ivanov S, Zinselmeyer BH, Scallan JP. The lymphatic system: integral roles in immunity. Annu Rev Immunol. 2017;35:31–52. doi: 10.1146/annurev-immunol-041015-055354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gruber BL, Marchese MJ, Kew R. Angiogenic factors stimulate mast-cell migration. Blood. 1995;86(7):2488–2493. [PubMed] [Google Scholar]
- 97.Duah E, Teegala LR, Kondeti V, et al. Cysteinyl leukotriene 2 receptor promotes endothelial permeability, tumor angiogenesis, and metastasis. Proc Natl Acad Sci U S A. 2019;116(1):199–204. doi: 10.1073/pnas.1817325115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wimazal F, Jordan JH, Sperr WR, et al. Increased angiogenesis in the bone marrow of patients with systemic mastocytosis. Am J Pathol. 2002;160(5):1639–1645. doi: 10.1016/S0002-9440(10)61111-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rebuzzi L, Willmann M, Sonneck K, et al. Detection of vascular endothelial growth factor (VEGF) and VEGF receptors Flt-1 and KDR in canine mastocytoma cells. Vet Immunol Immunopathol. 2007;115(3–4):320–333. doi: 10.1016/j.vetimm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 100.Shefler I, Salamon P, Mekori YA. MicroRNA involvement in allergic and non-allergic mast cell activation. Int J Mol Sci. 2019;20(9):2145. doi: 10.3390/ijms20092145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leone P, Buonavoglia A, Fasano R, et al. Insights into the regulation of tumor angiogenesis by micro-RNAs. J Clin Med. 2019;8(12):2030. doi: 10.3390/jcm8122030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mayoral RJ, Pipkin ME, Pachkov M, et al. MicroRNA-221-222 regulate the cell cycle in mast cells. J Immunol. 2009;182(1):433–445. doi: 10.4049/jimmunol.182.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mayoral RJ, Deho L, Rusca N, et al. MiR-221 influences effector functions and actin cytoskeleton in mast cells. PLoS ONE. 2011;6(10):e26133. doi: 10.1371/journal.pone.0026133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dentelli P, Rosso A, Orso F, et al. microRNA-222 controls neovascularization by regulating signal transducer and activator of transcription 5A expression. Arterioscler Thromb Vasc Biol. 2010;30(8):1562–1568. doi: 10.1161/ATVBAHA.110.206201. [DOI] [PubMed] [Google Scholar]
- 105.Zhou Y, Yang Q, Xu H, et al. miRNA-221-3p enhances the secretion of interleukin-4 in mast cells through the phosphatase and tensin homolog/p38/nuclear factor-kappaB pathway. PLoS ONE. 2016;11(2):e0148821. doi: 10.1371/journal.pone.0148821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Traina F, Visconte V, Jankowska AM, et al. Single nucleotide polymorphism array lesions, TET2, DNMT3A, ASXL1 and CBL mutations are present in systemic mastocytosis. PLoS ONE. 2012;7(8):e43090. doi: 10.1371/journal.pone.0043090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schwaab J, Schnittger S, Sotlar K, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122(14):2460–2466. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
- 108.Jawhar M, Schwaab J, Schnittger S, et al. Molecular profiling of myeloid progenitor cells in multi-mutated advanced systemic mastocytosis identifies KIT D816V as a distinct and late event. Leukemia. 2015;29(5):1115–1122. doi: 10.1038/leu.2015.4. [DOI] [PubMed] [Google Scholar]
- 109.Arock M, Sotlar K, Akin C, et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia. 2015;29(6):1223–1232. doi: 10.1038/leu.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.