Abstract
Introduction:
Patients with post-stroke cognitive impairment appear to be at higher risk of recurrent stroke and death. However, whether cognitive impairment after lacunar stroke is associated with recurrent stroke and death remains unclear. We assessed whether global or domain-specific cognitive impairment after lacunar stroke is associated with recurrent stroke and death.
Methods:
We considered patients from the Secondary Prevention of Small Subcortical Strokes (SPS3) trial with a baseline cognitive exam administered in English by certified SPS3 personnel, 14 to 180 days after qualifying lacunar stroke. We considered a baseline score of ≤ 86 on the Cognitive Assessment Screening Instrument to indicate global cognitive impairment, <10 on the Clock Drawing on Command test to indicate executive function impairment, and domain-specific summary scores in the lowest quartile to indicate memory and non-memory impairment. We used Cox proportional hazards models to estimate the association between post-stroke cognitive impairment and subsequent risk of recurrent stroke and death.
Results:
The study included 1,528 participants with a median enrollment time 62 days after qualifying stroke. During a mean follow-up of 3.9 years, 11.4% of participants had recurrent stroke and 8.2% died. In the fully adjusted models, memory impairment was independently associated with an increased risk of recurrent stroke (hazard ratio, 1.48; 95% confidence interval [95% CI], 1.04 – 2.09) and death (hazard ratio, 1.87; 95% CI, 1.25 – 2.79). Global impairment (hazard ratio, 1.66; 95% CI, 1.06 – 2.59) and non-memory impairment (hazard ratio, 1.74; 95% CI, 1.14 – 2.67) were associated with an increased risk of death.
Discussion/Conclusion:
After lacunar stroke, memory impairment was an independent predictor of recurrent stroke and death, while global and non-memory impairment were associated with death. Cognitive screening in lacunar stroke may help identify populations at higher risk of recurrent stroke and death.
Keywords: Lacunar Stroke, Cognitive Impairment, Cognitive Testing, Cohort Study
Introduction
Small vessel-related ischemic or lacunar stroke is a common stroke subtype [1, 2]. Although patients with lacunar stroke have smaller infarct size compared to other stroke subtypes, the risk of developing cognitive impairment or dementia is similar [3]. In patients with ischemic stroke, those with post-stroke dementia or cognitive impairment appear to be at higher risk of recurrent stroke and death [4–11]. However, whether cognitive impairment without dementia after lacunar stroke is associated with recurrent stroke and death, and whether any such relationship is specific to impairment in specific cognitive domains, remains unclear.
The Secondary Prevention of Small Subcortical Stroke (SPS3) trial provides a unique opportunity to investigate the effects of cognitive status after lacunar stroke on recurrent stroke and death [12]. The goal of this study was to assess whether global and domain-specific cognitive impairment after lacunar stroke is associated with recurrent stroke and death in the English-speaking participants of the SPS3 trial.
Materials/Methods
Here, we briefly describe our methods. Additional details are available in Appendix A.
Study Population
The SPS3 trial-design, eligibility, and results were reported elsewhere [12, 13]. We included all SPS3 participants who were administered their baseline cognitive exam at the time of randomization in English by a certified SPS3 personnel 14 to 180 days after their qualifying lacunar stroke (n=1,559). We then excluded 31(2%) participants with missing covariate data for a final sample size of 1528 participants (Figure 1).
Figure 1.
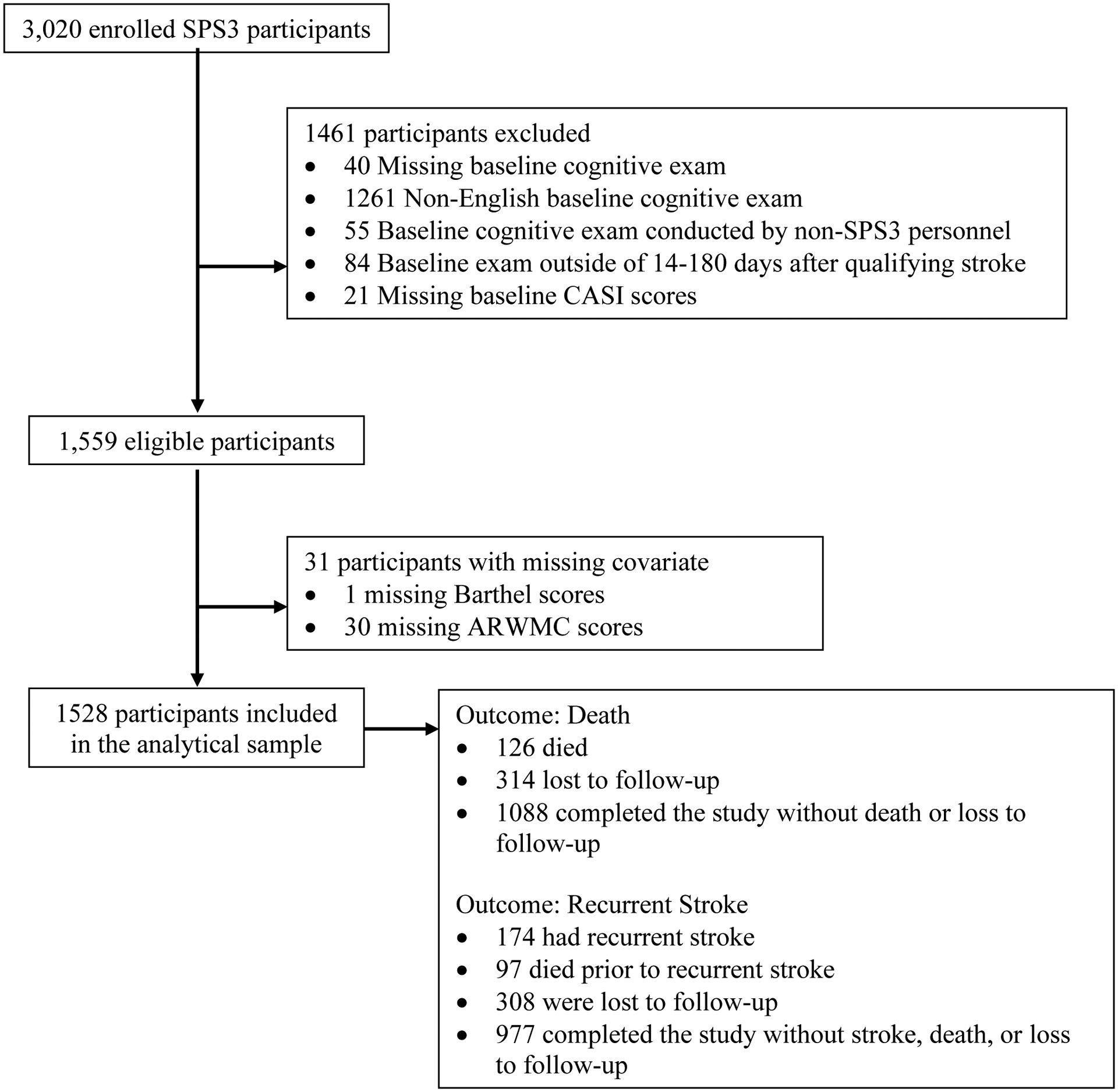
Flow Chart for Sample Derivation and Analysis
Assessment of Cognitive Function
The SPS3 cognitive battery included the Cognitive Assessment Screening Instrument (CASI) [13,14,16], immediate and delayed recall from the California Verbal Learning Test (CVLT), the Controlled Oral Word Association (COWA) test, and three tests from the Wechsler Adult Intelligence Scale III (WAIS-III) (block design, symbol search, and digit span), and the Clock Drawing on Command (CLOX) test.
Global cognitive impairment was defined by a cutoff score of ≤ 86 on the CASI [14]. Executive function impairment was determined by a cutoff score of < 10 on the CLOX test [15]. For the remaining tests, we created z-scores by subtracting the sample mean and dividing by the standard deviation, inverting sores where appropriate so higher z-scores indicate better performance. We then calculated a summary scores by averaging test-specific z-scores. The memory domain score was the average of z-scores from the two CVLT tests, while the non-memory domain included COWA and the three subtests from the WAIS-III [16, 17]. Memory impairment and non-memory impairment was defined as scoring below the 25th percentile.
Outcome Assessment
The primary endpoint was all stroke recurrence (first ischemic stroke or intracranial hemorrhage), and secondary endpoint was all-cause mortality [12].
Covariates
Demographics including age, sex, race, and education were self-reported at baseline prior to randomization [12]. Medical history, stroke location, Modified Rankin score (mRs), Barthel activities of daily living, and white matter hyperintensities were obtained after qualifying stroke and categorized based on previously published articles [18, 19].
Statistical Methods
Participants accumulated follow-up time from their baseline examination until either the primary outcome of interest or censorship due to death, loss to follow up, or the conclusion of the study, whichever came first (Figure 1). We used Cox proportional hazards models to quantify the impact of overall and domain-specific cognitive impairment on risk of recurrent stroke and death. Sequential models assessed the influence of potential confounders. Sensitivity analyses, including analyses to understand the influence of variable time between qualifying stroke and baseline cognitive assessment, are described in Appendix A. Statistical analyses were performed using SAS 9.4. We reported 95% confidence intervals and considered two-sided p-values of less than 0.05 to be statistically significant.
Results
Characteristics of the sample are provided in Table 1. The median time from qualifying stroke to baseline cognitive exam was 62 days. During mean follow-up of 3.9 ± 2.3 years, there were 174 (11.4%) recurrent stroke episodes and 126 (8.2%) deaths. The majority of recurrent strokes were ischemic stroke (n=159, 91%) and roughly half (n=81, 47%) were lacunar stroke. Cause of death was vascular in 45 decedents (36%), non-vascular in 52 decedents (41%), and uncertain in 29 decedents (23%). There were 403 (26.4%) participants with global cognitive impairment (CASI ≤ 86) and 1,125 (73.6%) without it (CASI > 86). Comparison of the eligible sample to the full SPS3 sample is available in Appendix B and Appendix Table A1.
Table 1.
Baseline characteristics of SPS3 participants according to global cognitive impairment
| Parameters | Overall (n = 1528) | Global cognitive impairment (n = 403) | No global cognitive impairment (n = 1125) | p-value* |
|---|---|---|---|---|
| Age, mean (SD) | 61.6 (10.7) | 62.6 (11.2) | 61.3 (10.4) | 0.04 |
| Male, n (%) | 893 (58.4%) | 217 (53.8%) | 676 (60.1%) | 0.03 |
| Race/Ethnicity, n (%) | <0.001 | |||
| White | 942 (61.6%) | 138 (34.2%) | 804 (71.5%) | |
| Black | 406 (26.6%) | 187 (46.4%) | 219 (19.5%) | |
| Hispanic | 116 (7.6%) | 50 (12.4%) | 66 (5.9%) | |
| Other Races | 64 (4.2%) | 28 (6.9%) | 36 (3.2%) | |
| Education, n (%) | <0.001 | |||
| 0–8 Years | 113 (7.4%) | 71 (17.6%) | 42 (3.7%) | |
| 9–12 Years | 604 (39.5%) | 225 (55.8%) | 379 (33.7%) | |
| >12 Years | 811 (53.1%) | 107 (26.6%) | 704 (62.6%) | |
| Aspirin + Clopidogrel Group, n (%) | 756 (49.5%) | 178 (44.2%) | 578 (51.4%) | 0.01 |
| 130–149 mmHg SBP Group, n (%) | 763 (49.9%) | 211 (52.4%) | 552 (49.1%) | 0.26 |
| Stroke History, n (%) | 189 (12.4%) | 63 (15.6%) | 126 (11.2%) | 0.02 |
| Family Stroke History, n (%) | 618 (40.4%) | 168 (41.7%) | 450 (40%) | 0.55 |
| Hypertension, n (%) | 1207 (79%) | 339 (84.1%) | 868 (77.2%) | 0.003 |
| Hyperlipidemia, n (%) | 889 (58.2%) | 230 (57.1%) | 659 (58.6%) | 0.6 |
| Diabetes Mellitus, n (%) | 502 (32.9%) | 167 (41.4%) | 335 (29.8%) | <0.001 |
| CVD History†, n (%) | 271 (17.7%) | 83 (20.6%) | 188 (16.7%) | 0.08 |
| Smoking Status, n (%) | 0.48 | |||
| Current | 351 (23%) | 101 (25.1%) | 250 (22.2%) | |
| Former | 629 (41.2%) | 159 (39.5%) | 470 (41.8%) | |
| Never | 548 (35.9%) | 143 (35.5%) | 405 (36%) | |
| Weekly Regular Alcohol Use, n (%) | 482 (31.5%) | 91 (22.6%) | 391 (34.8%) | <0.001 |
| Days of Exercise Weekly, n (%) | <0.001 | |||
| 0 Days | 610 (39.9%) | 193 (47.9%) | 417 (37.1%) | |
| 1–6 Days | 544 (35.6%) | 123 (30.5%) | 421 (37.4%) | |
| 7 Days | 374 (24.5%) | 87 (21.6%) | 287 (25.5%) | |
| Lacunar Stroke Location, n (%) | 0.05 | |||
| Thalamus | 395 (25.9%) | 93 (23.1%) | 302 (26.8%) | |
| Basal Ganglia/Internal Capsule | 437 (28.6%) | 116 (28.8%) | 321 (28.5%) | |
| Centrum Semiovale/Corona Radiata | 321 (21%) | 76 (18.9%) | 245 (21.8%) | |
| Medulla/Midbrain/Pons/Cerebellum | 375 (24.5%) | 118 (29.3%) | 257 (22.8%) | |
| Modified Rankin Score > 1, n (%) | 507 (33.2%) | 191 (47.4%) | 316 (28.1%) | <0.001 |
| Barthel ADL Score, n (%) | <0.001 | |||
| Score 100 | 1133 (74.1%) | 250 (62%) | 883 (78.5%) | |
| Score 90–99 | 154 (10.1%) | 57 (14.1%) | 97 (8.6%) | |
| Score <90 | 241 (15.8%) | 96 (23.8%) | 145 (12.9%) | |
| WMHs ARWMC Score, n (%) | <0.001 | |||
| 0–4 | 777 (50.9%) | 173 (42.9%) | 604 (53.7%) | |
| 5–8 | 419 (27.4%) | 114 (28.3%) | 305 (27.1%) | |
| >9 | 332 (21.7%) | 116 (28.8%) | 216 (19.2%) |
Abbreviations: SPS3, Secondary Prevention of Small Subcortical Stroke; global cognitive impairment, global cognitive impairment; SBP, systolic blood pressure; CVD, Cardiovascular Disease; ADL, Activities of Daily Living; WMHs, White Matter Hyperintensities; ARWMC, Age-related White Matter Changes Scale.
p-values will be obtained from Chi-Square Tests for categorical variables & T-test for continuous variables; α = 0.05.
Cardiovascular diseases history includes any previous myocardial infarction, angina, congestive heart failure, coronary artery bypass graft, coronary angioplasty, coronary stent, contralateral carotid endarterectomy, carotid stenosis, pacemaker, peripheral vascular disease, or intermittent claudication.
In the crude analysis, global cognitive impairment, memory impairment, and non-memory impairment were associated with a higher risk of recurrent stroke (Table 2). In the fully-adjusted model, memory impairment remained associated with a higher risk of recurrent stroke (hazard ratio [HR], 1.48; 95% confidence interval [CI], 1.04 – 2.09).
Table 2.
Unadjusted and adjusted hazards ratio (95% CI) of recurrent stroke and death according to global and domain specific cognitive impairment.
| Outcomes | Model 1* | Model 2† | Model 3‡ | Model 4§ | ||||
|---|---|---|---|---|---|---|---|---|
| Cognitive Domain | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value |
| Recurrent Stroke | ||||||||
| Global Impairment (CASI ≤86) | 1.75 (1.28, 2.39) | <0.001 | 1.50 (1.05, 2.14) | 0.03 | 1.30 (0.91, 1.87) | 0.15 | 1.24 (0.86, 1.79) | 0.24 |
| CLOX Impairment (<10) | 1.29 (0.89, 1.86) | 0.18 | 1.10 (0.75, 1.61) | 0.64 | 0.98 (0.66, 1.45) | 0.92 | 0.94 (0.63, 1.39) | 0.74 |
| Memory Impairment (<Q1) | 1.86 (1.37, 2.53) | <0.001 | 1.58 (1.13, 2.21) | 0.008 | 1.62 (1.15, 2.27) | 0.005 | 1.48 (1.04, 2.09) | 0.03 |
| Non-Memory Impairment (<Q1) | 1.80 (1.32, 2.46) | <0.001 | 1.53 (1.06, 2.19) | 0.02 | 1.43 (1.00, 2.05) | 0.05 | 1.32 (0.92, 1.90) | 0.13 |
| All-Cause Mortality | ||||||||
| Global Impairment (CASI <86) | 1.77 (1.23, 2.54) | 0.002 | 1.88 (1.24, 2.83) | 0.003 | 1.61 (1.05, 2.46) | 0.03 | 1.66 (1.06, 2.59) | 0.03 |
| CLOX Impairment (<10) | 1.43 (0.94, 2.17) | 0.1 | 1.04 (0.67, 1.63) | 0.85 | 0.95 (0.60, 1.49) | 0.81 | 0.92 (0.58, 1.44) | 0.71 |
| Memory Impairment (<Q1) | 2.33 (1.64, 3.32) | <0.001 | 2.00 (1.36, 2.94) | <0.001 | 1.87 (1.27, 2.76) | 0.002 | 1.87 (1.25, 2.79) | 0.002 |
| Non-Memory Impairment (<Q1) | 1.93 (1.35, 2.76) | <0.001 | 1.87 (1.24, 2.82) | 0.003 | 1.79 (1.19, 2.70) | 0.006 | 1.74 (1.14, 2.67) | 0.01 |
Abbreviations: CASI, Cognitive Assessment Screening Instrument; CLOX, Clock Drawing to Command; Q1, First quartile (<25th percentile) on sample z score.
Model 1: Unadjusted.
Model 2: Adjusted for age, sex, race/ethnicity, education, blood pressure control group, & dual antiplatelet treatment.
Model 3: Adjusted for Model 2 + stroke history, family stroke history, hypertension, hyperlipidemia, diabetes mellitus, cardiovascular disease history, smoking status, weekly alcohol use, & days of exercise weekly.
Model 4: Adjusted for Model 3 + lacunar stroke location, modified Rankin score, Barthel activities of daily living, and age-related white matter changes scale score.
The risk of all-cause mortality was similarly higher for patients with global cognitive impairment, memory impairment, and non-memory impairment in the crude analysis. In the fully-adjusted model, the risk of all-cause mortality remained higher among participants with global cognitive impairment (HR, 1.66; 95% CI, 1.06 – 2.59), memory impairment (HR, 1.87; 95% CI, 1.25 – 2.79), and non-memory impairment (HR, 1.74; 95% CI, 1.14 – 2.67; Table 2).
The unadjusted and adjusted hazard ratios for various outcomes based on the continuous memory and non-memory domain scores as predictors are presented in Table 3. The association between memory domain performance and recurrent stroke was positive, but only marginally significant in fully adjusted models (HR, 1.18; 95% CI, 0.99 – 1.42). However, worse performance in the non-memory domain tests (HR, 1.35; 95% CI, 1.05 – 1.74) was associated with an increased risk of recurrent stroke. The risk of all-cause mortality was higher in those with worse performance in both the memory domain (HR, 1.40; 95% CI, 1.11 – 1.76) and non-memory domain tests (HR, 1.71; 95% CI, 1.24 – 2.35; Table 3). Findings of sensitivity analyses were consistent with those of primary analyses (Appendix C, Appendix Table A2, Appendix Table A3).
Table 3:
Unadjusted and adjusted hazards ratio (95% CI) of recurrent stroke and death for every one-unit change in continuous composite memory and non-memory domain scores.
| Outcomes | Model 1* | Model 2† | Model 3‡ | Model 4§ | ||||
|---|---|---|---|---|---|---|---|---|
| Cognitive Domain | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value |
| Recurrent Stroke | ||||||||
| Memory Domain | 1.37 (1.17, 1.61) | <0.001 | 1.26 (1.05, 1.51) | 0.01 | 1.25 (1.04, 1.49) | 0.02 | 1.18 (0.99, 1.42) | 0.07 |
| Non-Memory Domain | 1.69 (1.37, 2.07) | <0.001 | 1.64 (1.27, 2.11) | <0.001 | 1.47 (1.15, 1.89) | 0.002 | 1.35 (1.05, 1.74) | 0.02 |
| All-Cause Mortality | ||||||||
| Memory Domain | 1.60 (1.32, 1.93) | <0.001 | 1.45 (1.17, 1.79) | <0.001 | 1.42 (1.14, 1.78) | 0.002 | 1.40 (1.11, 1.76) | 0.004 |
| Non-Memory Domain | 1.86 (1.45, 2.37) | <0.001 | 1.98 (1.47, 2.68) | <0.001 | 1.76 (1.29, 2.38) | <0.001 | 1.71 (1.24, 2.35) | <0.001 |
Model 1: Unadjusted.
Model 2: Adjusted for age, sex, race/ethnicity, education, blood pressure control group, & dual antiplatelet treatment.
Model 3: Adjusted for Model 2 + stroke history, family stroke history, hypertension, hyperlipidemia, diabetes mellitus, cardiovascular disease history, smoking status, weekly alcohol use, & days of exercise weekly.
Model 4: Adjusted for Model 3 + lacunar stroke location, modified Rankin score, Barthel activities of daily living, and age-related white matter changes scale score.
Discussion
In this large, well-characterized cohort of nondisabled and nondemented patients with lacunar stroke, post-stroke global cognitive impairment was associated with an increased risk of all-cause mortality. In addition, memory impairment was associated with an increased risk of recurrent stroke and death, while impairment in the non-memory domain was associated with an increased risk of death.
This study is unique as it reported on the effects of global and domain-specific cognitive impairment on the risk of recurrent stroke and death after lacunar stroke, rather than all ischemic stroke. In a study conducted in Singapore, cognitive impairment without dementia 3 months after ischemic stroke was predictive of dependency and death [6]. In a Helsinki study of 486 ischemic stroke patients, post-stroke dementia predicted recurrent stroke, but cognitive impairment without dementia did not [7]. Although our study enrolled only lacunar stroke patients versus all ischemic strokes in these studies, it follows a similar pattern, as global cognitive impairment is associated with mortality but not recurrent stroke. Lacunar stroke is caused by small vessel disease, which is a systemic pathology. The presence of global cognitive impairment after lacunar stroke probably is indicative of advanced microangiopathy. Therefore, higher risk of all-cause mortality in this cohort might be related to the small vessel disease progression to other beds including kidney and heart [20, 21].
In this study, memory impairment was associated with increased risk of death, which is consistent with prior work elsewhere considering all ischemic stroke [9]. While memory impairment was also associated with a higher risk of recurrent stroke, worse memory performance trended towards an increased risk of recurrent stroke, but the association was not statistically significant. Since memory impairment and memory performance were derived from the same composite score, this suggests that the relationship may not be linear, and decline to impairment, rather than simple decline in cognition, may drive this association. In the large meta-analysis of non-stroke patients, memory-predominant cognitive impairment was associated with an increased risk of stroke [22]. Memory impairment after lacunar stroke may indicate the severity of small vessel disease [22]. In SPS3, patients with cerebral microbleeds on their MRI were at high risk of stroke recurrence and death indicating that this subgroup of patients likely harbors a more advanced form of cerebral small vessel disease [23]. In vascular cognitive impairment there is substantial overlap between neurodegenerative and cerebrovascular pathologies including stroke [24]. Although SPS3 excluded patients with dementia (defined as MMSE < 24), some of enrolled patients may have had amnestic mild cognitive impairment consistent with Alzheimer’s pathology. How Alzheimer’s pathology affects small vessel function and how vascular dysfunction contributes to the molecular pathology of Alzheimer’s are areas of intense research [24]. Lastly, it is also possible that patients with memory-predominant cognitive impairment after lacunar stroke were less compliant with secondary stroke prevention medications due to their memory problems, leading to increased risk of recurrent stroke.
This study has clinical implications. First, it highlights the potential for cognitive testing in patients with recent lacunar stroke to identify those at higher risk of recurrent stroke and death. Given cognitive impairment is associated with increased risk of recurrent stroke and death, persons with cognitive impairment may benefit from more frequent follow-up and more aggressive secondary stroke prevention efforts. Secondly, these patients appear at higher risk of both vascular and nonvascular mortality. Therefore, increased communication with primary care practitioners may be warranted to ensure all primary preventive diagnostic strategies are up to date.
The strengths of this study include the large number of well-defined, MRI-confirmed lacunar stroke cases, the standardized measurement of global and domain-specific cognitive status, and the standardized assessment of recurrent stroke and death. We acknowledge that this study also has limitations. We were not able to adjust for the presence of brain microbleeds, as only a fraction of participants had an interpretable axial T2*-weighted gradient echo sequence during their baseline MRI [23]. We do not know the participants’ pre-stroke cognitive status; however, as the SPS3 trial excluded persons with severe cognitive impairment or dementia, we can safely assume our participants did not have severe cognitive impairment or dementia prior to their qualifying stroke. The SPS3 trial also excludes potential participants if they had a disabling stroke (mRs > 4), so the results of this study could underestimate the true effect among patients that had a lacunar stroke. Future work is needed to confirm these findings in other populations and to identify potential mediators of this association. For example, cognitive impairment could lead to increased risk of cardiovascular disease or greater risk factor burden, which in turn could increase risk of recurrent stroke or death.
In conclusion, memory impairment was independently associated with recurrent stroke and death, while global cognitive impairment and non-memory impairment was associated with death. Cognitive screening after lacunar stroke may help to identify populations at higher risk of recurrent stroke and death.
Acknowledgements
We would like to acknowledge the SPS3 Investigators and the National Institute of Health - National Institute of Neurological Disorders and Stroke (NINDS) for providing the data set.
Funding Sources
SPS3 was supported by NINDS/NIH grant #U01NS091951
Appendix A. Additional Details of Study Methods
We provide additional details of our methods below. However, we refer the reader to the manuscript text for an overview of our approach.
Study Population
The SPS3 trial was a 2 × 2 factorial randomized control trial that looked into the effects of dual antiplatelet therapy and blood pressure control on individuals who had a symptomatic, MRI-confirmed lacunar stroke [1–4]. MRI criteria for a clinical lacunar syndrome included a lesion measuring 2.0 cm or less in diameter on diffusion-weighted imaging that corresponded to a positive apparent-diffusion-coefficient image or a lesion with a well-delineated area of focal hyperintensity that was 2.0 cm or less in diameter on fluid-attenuated inversion recovery imaging or T2-weighted imaging that corresponded to the clinical syndrome [1]. Hypointense lesions at the level of the anterior commissure, convexity, or midbrain, were considered enlarged peri-vascular spaces and not classified as infarcts, unless the lesion was surrounded by a hyperintense halo on FLAIR [5].
At study entry, between 14 and 180 days after qualifying stroke, neuropsychological testing, Mini Mental State Examination (MMSE), Barthel Activities of Daily Living Index, and modified Rankin Scale (m-Rankin) were administered. Participants with a disabling stroke (m-Rankin ≥ 4), significant cognitive impairment (MMSE > 2 SD below the mean for age and education, i.e., an adjusted score < 24, generally accepted as the cutoff for mild dementia) cortical ischemia, carotid stenosis, or any major-risk cardioembolic source were excluded from the trial [6].
Cognitive assessments were performed at baseline and at every annual visit. All participants, regardless of compliance with the treatment arms, were followed up to the end of the study on April 30, 2012 [1]. The primary outcome of the trial was recurrent stroke, and the secondary outcomes included cognitive status, major vascular events, and death [1].
Administration of Cognitive Function
A neuropsychological test battery was administered in the participant’s preferred language (English, Spanish, or French) by certified SPS3 personnel at the time of randomization. Participants with uncorrected visual impairment were not administered any vision-related tests [1, 6, 7].
Outcome Assessment
Recurrent stroke was clinically defined as the presence of focal neurological deficit for more than 24 hours with supplemental non-contrast CT or brain MRI [1]. Death was determined via medical records or autopsy reports, verified by an SPS3 study physician [1].
Covariates
Demographic information and the participants’ medical history prior to qualifying lacunar stroke were also collected at baseline prior to randomization [1]. Education was defined as years of formal education and grouped into three categories: 0–8 years, 9–12 years, and > 12 years. Stroke location was divided into four groups: 1) thalamus, 2) basal ganglia and internal capsule, 3) centrum semiovale and corona radiata, and 4) medulla, midbrain, pons, cerebellum [8]. Modified Rankin scores and Barthel activities of daily living scores were collected at baseline after qualifying stroke. Modified Rankin scores were dichotomized into grades of ≤ 1 or > 1 where a score of ≤ 1 signifies no significant neurologic disability. Barthel activities of daily living scores were categorized into a score of 100, 90–99, and < 90, and a score of 100 means complete functional independence. White matter hyperintensities were defined using the age-related white matter changes (ARWMC) rating scale and were evaluated visually on FLAIR images using the age-related white matter change (range 0–16) [5]. The average ARWMC scores were categorized a priori into scores of 0–4 for none or mild disease, 5–8 for moderate, and > 9 for severe [5]. Interrater agreement was good-excellent on a sample of 40 MRIs (range: κ = 0·64, 77% agreement to κ = 0·89, 95% agreement) [8].
Statistical Methods
We compared the baseline characteristics between those with and without global cognitive impairment (CASI ≤ 86 and a CASI > 86) using the student t-test for continuous variables or chi-square test for categorical variables. Model 1 of Cox proportional hazards model was unadjusted. Model 2 was adjusted for demographics including age, sex, race, and education as well as the participant’s dual antiplatelet and blood pressure control group. Model 3 was further adjusted for the participant’s medical history including stroke history, family stroke history, hypertension, hyperlipidemia, diabetes mellitus, cardiovascular disease history, smoking status, alcohol use, and days of exercise weekly. Model 4 was further adjusted for qualifying stroke characteristics including lacunar stroke location, modified Rankin score, Barthel activities for daily living score, and age-related white matter changes scale score.
We chose variables likely to meet the definition of confounders for inclusion in our models, and provide multiple levels of adjustment to illustrate the influence of adjusting for different types of confounders. All covariates, and specifically those for model 3 (cardiovascular risk factors, stroke history, and history of cardiovascular disease) and 4 (markers of lacunar stroke subtype and severity) were selected based on author knowledge of predictors of recurrent stroke and death, as well as covariates considered in prior studies that looked at the association between cognitive impairment and recurrent stroke or death [9–14]. For example, previous stroke history, diabetes mellitus, hypertension, smoking, and cardiovascular disease history were predictors of recurrent stroke in SPS3 trial [15]. Hyperlipidemia was added based on another SPS3 study showing increased risk of recurrent stroke in patients with hyperlipidemia [16]. Furthermore, family stroke history, alcohol use, and exercise are known correlates of recurrent stroke and post-stroke cognitive impairment [17–19]. The covariates related to qualifying stroke severity (lacunar stroke location, modified Rankin score, Barthel activity of daily living, and age-related white matter hyperintensity changes) were grouped and further adjusted for in model 4. In Jacova et al., these covariates were all factors associated with cognitive impairment in the univariate analysis [6].
In secondary analyses, we estimated the association between a one-unit change in the continuous memory/non-memory domain scores and the hazards of recurrent stroke and death.
We assessed the proportional hazards assumption using Schoenfeld’s residual plots and interactions between each covariate and time. These diagnostics suggested violations of the proportional hazards assumption for stroke history and Barthel activities to daily living score; therefore, we stratified on these covariates within all reported models. These diagnostics also suggested mild violations of the proportional hazards assumption towards the end of the follow-up time. Therefore, we conducted a sensitivity analysis censoring all participants at year 5.
We conducted several other sensitivity analyses. We excluded participants with a history of stroke in order to quantify the impact of cognitive impairment after first stroke. Since the time from qualifying stroke to baseline examination varied between one to three months, we conducted sensitivity analyses adding the time between qualifying stroke and baseline examination to each participant’s follow-up time and assessed whether there was an interaction between the time from qualifying stroke to baseline examination and their cognitive test score. Finally, we conducted analyses adding adjustment for imaging evidence of stenosis of the intracranial arteries among those with necessary imaging data.
Appendix B. Comparison of the analytical sample to the SPS3 cohort
Compared to the full SPS3 cohort, which included both English and Spanish-speaking participants, participants in our analytical sample were generally younger, female, white, and had more years of education (Appendix Table A1). The studied SPS3 subgroup in this analysis was also more likely to have hypertension, hyperlipidemia, a history of CVD, and a family history of stroke, more likely to be current or former smokers, more likely to drink alcohol weekly, and more likely to exercise multiple times a week. Participants in this subgroup were also more likely to have a qualifying lacunar strokes in thalamus and no disability after qualifying lacunar stroke.
Appendix C. Results of Sensitivity Analyses
Findings of sensitivity analyses were consistent with those of primary analyses, although whether a specific result was statistically significant varied slightly from analysis to analysis (Table A2 and A3). After censoring participants at year 5, impairment in the memory domain remained associated with a higher risk of recurrent stroke (HR, 1.59; 95% CI, 1.11 – 2.30), and the association between non-memory impairment and recurrent stroke was stronger, and became statistically significant (HR, 1.48, 95% CI, 1.01, 2.17). With the exception of the association between non-memory impairment and higher risk of all-cause mortality (HR, 2.04; 95% CI, 1.26 – 3.30), associations between cognitive impairment and all-cause mortality were slightly weaker after censoring all participants after 5 years of follow-up. Worse performance in memory and non-memory domain tests, using test scores as a continuous variable, was associated with both recurrent stroke and death after censoring participants at year 5. Excluding participants with a history of stroke did not materially change the pattern of findings for recurrent stroke or death. Adding the time between qualifying stroke and baseline examination to each participant’s follow up time also produced similar findings. Furthermore, the interaction between the time from qualifying stroke to baseline examination and cognitive performance was not significant for all cognitive domains. Results of sensitivity analyses additionally adjusting for stenosis of the intracranial arteries were consistent with primary analyses (Appendix Table A4 and A5).
Appendix Tables
Table A1.
Comparison of those included in the analytical sample to the full SPS3 cohort and those excluded from the analytical sample
| Parameters | SPS3 Cohort | Included | Excluded | p-valuê |
|---|---|---|---|---|
| (n = 3020) | (n = 1528) | (n = 1492) | ||
| CASI <86, n (%) | 1290 (42.7%) | 403 (26.4%) | 887 (59.5%) | <0.001 |
| Age, mean (SD) | 62.8 (10.8) | 61.6 (10.7) | 64.0 (10.7) | <0.001 |
| Male, n (%) | 1902 (63%) | 893 (58.4%) | 1009 (67.6%) | <0.001 |
| Race/Ethnicity, n (%) | <0.001 | |||
| White | 1626 (53.8%) | 942 (61.6%) | 684 (45.8%) | |
| Black | 464 (15.4%) | 406 (26.6%) | 58 (3.9%) | |
| Hispanic | 662 (21.9%) | 116 (7.6%) | 546 (36.6%) | |
| Other Races | 268 (8.9%) | 64 (4.2%) | 204 (13.7%) | |
| Education, n (%) | <0.001 | |||
| 0–8 Years | 790 (26.2%) | 113 (7.4%) | 677 (45.4%) | |
| 9–12 Years | 1146 (37.9%) | 604 (39.5%) | 542 (36.3%) | |
| >12 Years | 1084 (35.9%) | 811 (53.1%) | 273 (18.3%) | |
| Aspirin + Clopidogrel Group, n (%) | 1517 (50.2%) | 756 (49.5%) | 761 (51%) | 0.4 |
| <130 mmHg SBP Group, n (%) | 1519 (50.3%) | 763 (49.9%) | 756 (50.7%) | 0.69 |
| Stroke History, n (%) | 400 (13.2%) | 189 (12.4%) | 211 (14.1%) | 0.15 |
| Family Stroke History, n (%) | 1060 (35.1%) | 618 (40.4%) | 442 (29.6%) | <0.001 |
| Hypertension, n (%) | 2264 (75%) | 1207 (79%) | 1057 (70.8%) | <0.001 |
| Hyperlipidemia, n (%) | 1471 (48.7%) | 889 (58.2%) | 582 (39%) | <0.001 |
| Diabetes Mellitus, n (%) | 1002 (33.2%) | 502 (32.9%) | 500 (33.5%) | 0.7 |
| CVD History*, n (%) | 409 (13.5%) | 271 (17.7%) | 138 (9.2%) | <0.001 |
| Smoking Status, n (%) | <0.001 | |||
| Current | 617 (20.4%) | 351 (23%) | 266 (17.8%) | |
| Former | 1207 (40%) | 629 (41.2%) | 578 (38.7%) | |
| Never | 1196 (39.6%) | 548 (35.9%) | 648 (43.4%) | |
| Weekly Regular Alcohol Use, n (%) | 848 (28.1%) | 482 (31.5%) | 366 (24.5%) | <0.001 |
| Days of Exercise Weekly, n (%) | <0.001 | |||
| 0 Days | 1195 (39.6%) | 610 (39.9%) | 585 (39.2%) | |
| 1–6 Days | 986 (32.7%) | 544 (35.6%) | 442 (29.6%) | |
| 7 Days | 838 (27.8%) | 374 (24.5%) | 464 (31.1%) | |
| Lacunar Stroke Location, n (%) | <0.001 | |||
| Thalamus | 677 (22.4%) | 395 (25.9%) | 282 (18.9%) | |
| Basal Ganglia/Internal Capsule | 841 (27.9%) | 437 (28.6%) | 404 (27.1%) | |
| Centrum Semiovale/Corona Radiata | 719 (23.8%) | 321 (21%) | 398 (26.7%) | |
| Medulla/Midbrain/Pons/Cerebellum | 781 (25.9%) | 375 (24.5%) | 406 (27.2%) | |
| Modified Rankin Score > 1, n (%) | 1009 (33.4%) | 507 (33.2%) | 502 (33.6%) | 0.79 |
| Barthel ADL Score, n (%) | <0.001 | |||
| Score 100 | 2112 (70%) | 1133 (74.1%) | 979 (65.7%) | |
| Score 90–99 | 298 (9.9%) | 154 (10.1%) | 144 (9.7%) | |
| Score <90 | 609 (20.2%) | 241 (15.8%) | 368 (24.7%) | |
| WMHs ARWMC Score, n (%) | 0.7 | |||
| 0–4 | 1491 (50.2%) | 777 (50.9%) | 714 (49.5%) | |
| 5–8 | 833 (28%) | 419 (27.4%) | 414 (28.7%) | |
| >9 | 646 (21.8%) | 332 (21.7%) | 314 (21.8%) |
Table A2:
Adjusted hazards ratio (95% CI) of recurrent stroke and death according to global and domain specific cognitive impairment across sensitivity analyses
| Outcomes | Censoring all participants after 5 years of follow-up* | Excluding participants with a history of stroke* | Adding time from qualifying stroke to baseline exam to follow-up* | |||
|---|---|---|---|---|---|---|
| Cognitive Domain | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value |
| Recurrent Stroke | ||||||
| Global Impairment (CASI ≤86) | 1.40 (0.95, 2.06) | 0.09 | 1.28 (0.85, 1.92) | 0.24 | 1.24 (0.86, 1.79) | 0.24 |
| CLOX Impairment (<10) | 1.08 (0.72, 1.63) | 0.7 | 1.10 (0.72, 1.67) | 0.67 | 0.93 (0.62, 1.38) | 0.71 |
| Memory Impairment (<Q1) | 1.59 (1.11, 2.30) | 0.01 | 1.57 (1.07, 2.31) | 0.02 | 1.47 (1.04, 2.08) | 0.03 |
| Non-Memory Impairment (<Q1) | 1.48 (1.01, 2.17) | 0.05 | 1.26 (0.84, 1.89) | 0.26 | 1.32 (0.92, 1.90) | 0.13 |
| All-Cause Mortality | ||||||
| Global Impairment (CASI <86) | 1.51 (0.90, 2.51) | 0.12 | 1.69 (1.03, 2.75) | 0.04 | 1.69 (1.08, 2.64) | 0.02 |
| CLOX Impairment (<10) | 0.92 (0.54, 1.55) | 0.75 | 1.00 (0.61, 1.62) | 0.98 | 0.91 (0.58, 1.44) | 0.69 |
| Memory Impairment (<Q1) | 1.54 (0.97, 2.45) | 0.07 | 1.90 (1.23, 2.94) | 0.004 | 1.89 (1.27, 2.82) | 0.002 |
| Non-Memory Impairment (<Q1) | 2.04 (1.26, 3.30) | 0.004 | 1.70 (1.08, 2.68) | 0.02 | 1.76 (1.15, 2.69) | 0.01 |
Abbreviations: CASI, Cognitive Assessment Screening Instrument; CLOX, Clock Drawing to Command; Q1, First quartile (<25th percentile) on sample z score.
All models are adjusted for Model 4 covariates: age, sex, race/ethnicity, education, blood pressure control group, dual antiplatelet treatment, stroke history, family stroke history, hypertension, hyperlipidemia, diabetes mellitus, cardiovascular disease history, smoking status, weekly alcohol use, days of exercise weekly, lacunar stroke location, modified Rankin score, Barthel activities of daily living, and age-related white matter changes scale score.
Table A3:
Adjusted hazards ratio (95% CI) of recurrent stroke and death for every one-unit change in continuous composite memory and non-memory domain scores performance across our sensitivity analyses
| Outcomes | Censoring all participants after 5 years of follow-up* | Excluding participants with a history of stroke* | Adding time from qualifying stroke to baseline exam to follow-up* | |||
|---|---|---|---|---|---|---|
| Cognitive Domain | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value |
| Recurrent Stroke | ||||||
| Memory Domain | 1.29 (1.06, 1.56) | 0.01 | 1.21 (0.98, 1.50) | 0.07 | 1.19 (0.99, 1.42) | 0.07 |
| Non-Memory Domain | 1.40 (1.07, 1.84) | 0.02 | 1.31 (0.98, 1.75) | 0.07 | 1.35 (1.05, 1.74) | 0.02 |
| All-Cause Mortality | ||||||
| Memory Domain | 1.31 (1.01, 1.70) | 0.04 | 1.43 (1.11, 1.85) | 0.005 | 1.41 (1.12, 1.78) | 0.003 |
| Non-Memory Domain | 1.95 (1.35, 2.81) | <0.001 | 1.76 (1.25, 2.50) | 0.001 | 1.73 (1.26, 2.38) | <0.001 |
All models are adjusted for Model 4 covariates: age, sex, race/ethnicity, education, blood pressure control group, dual antiplatelet treatment, stroke history, family stroke history, hypertension, hyperlipidemia, diabetes mellitus, cardiovascular disease history, smoking status, weekly alcohol use, days of exercise weekly, lacunar stroke location, modified Rankin score, Barthel activities of daily living, and age-related white matter changes scale score.
Table A4:
Adjusted hazards ratio (95% CI) of recurrent stroke and death according to global and domain specific cognitive impairment with additional adjustment for stenosis of the intracranial arteries
| Outcomes | HR (95% CI) | p value |
|---|---|---|
| Cognitive Domain | ||
| Recurrent Stroke | ||
| Global Impairment (CASI ≤86) | 1.17 (0.79, 1.73) | 0.44 |
| CLOX Impairment (<10) | 0.84 (0.55, 1.30) | 0.44 |
| Memory Impairment (<Q1) | 1.43 (0.99, 2.07) | 0.06 |
| Non-Memory Impairment (<Q1) | 1.28 (0.87, 1.88) | 0.22 |
| All-Cause Mortality | ||
| Global Impairment (CASI <86) | 1.48 (0.91, 2.40) | 0.12 |
| CLOX Impairment (<10) | 1.00 (0.62, 1.60) | 0.99 |
| Memory Impairment (<Q1) | 1.75 (1.14, 2.68) | 0.01 |
| Non-Memory Impairment (<Q1) | 1.65 (1.04, 2.63) | 0.03 |
Abbreviations: CASI, Cognitive Assessment Screening Instrument; CLOX, Clock Drawing to Command; Q1, First quartile (<25th percentile) on sample z score.
All models are adjusted for Model 4 covariates: age, sex, race/ethnicity, education, blood pressure control group, dual antiplatelet treatment, stroke history, family stroke history, hypertension, hyperlipidemia, diabetes mellitus, cardiovascular disease history, smoking status, weekly alcohol use, days of exercise weekly, lacunar stroke location, modified Rankin score, Barthel activities of daily living, and age-related white matter changes scale score.
Table A5:
Adjusted hazards ratio (95% CI) of recurrent stroke and death for every one-unit change in continuous composite memory and non-memory domain scores performance with additional adjustment for stenosis of the intracranial arteries
| Outcomes | HR (95% CI) | p value |
|---|---|---|
| Cognitive Domain | ||
| Recurrent Stroke | ||
| Memory Domain | 1.17 (0.97, 1.42) | 0.11 |
| Non-Memory Domain | 1.29 (0.99, 1.69) | 0.06 |
| All-Cause Mortality | ||
| Memory Domain | 1.38 (1.08, 1.77) | 0.01 |
| Non-Memory Domain | 1.67 (1.18, 2.37) | 0.004 |
All models are adjusted for Model 4 covariates: age, sex, race/ethnicity, education, blood pressure control group, dual antiplatelet treatment, stroke history, family stroke history, hypertension, hyperlipidemia, diabetes mellitus, cardiovascular disease history, smoking status, weekly alcohol use, days of exercise weekly, lacunar stroke location, modified Rankin score, Barthel activities of daily living, and age-related white matter changes scale score.
Footnotes
Statement of Ethics
All participants in the SPS3 trial provided written informed consent and the SPS3 trial was approved by the local institutional review boards of all participating centers. De-identified data from the study was acquired with approval from the NINDS, and the study was ruled not human subjects research by the George Washington University institutional review board.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ornello R, Degan D, Tiseo C, Di Carmine C, Perciballi L, Pistoia F, et al. Distribution and temporal trends from 1993 to 2015 of ischemic stroke subtypes: a systematic review and meta-analysis. Stroke. 2018;49(4):814–9. [DOI] [PubMed] [Google Scholar]
- 3.Makin SDJ, Turpin S, Dennis MS, Wardlaw JM. Cognitive impairment after lacunar stroke: systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J Neurol Neurosurg Psychiatry. 2013;84(8):893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon HS, Lee D, Lee MH, Yu S, Lim J-S, Yu K-H, et al. Post-stroke cognitive impairment as an independent predictor of ischemic stroke recurrence: PICASSO sub-study. Journal of neurology. 2019:1–6. [DOI] [PubMed] [Google Scholar]
- 5.Melkas S, Oksala NK, Jokinen H, Pohjasvaara T, Vataja R, Oksala A, et al. Poststroke dementia predicts poor survival in long-term follow-up: influence of prestroke cognitive decline and previous stroke. Journal of Neurology, Neurosurgery & Psychiatry. 2009;80(8):865–70. [DOI] [PubMed] [Google Scholar]
- 6.Narasimhalu K, Ang S, De Silva DA, Wong M-C, Chang H-M, Chia K-S, et al. The prognostic effects of poststroke cognitive impairment no dementia and domain-specific cognitive impairments in nondisabled ischemic stroke patients. Stroke. 2011;42(4):883–8. [DOI] [PubMed] [Google Scholar]
- 7.Sibolt G, Curtze S, Melkas S, Putaala J, Pohjasvaara T, Kaste M, et al. Poststroke dementia is associated with recurrent ischaemic stroke. J Neurol Neurosurg Psychiatry. 2013;84(7):722–6. [DOI] [PubMed] [Google Scholar]
- 8.Henon H, Vroylandt P, Durieu I, Pasquier F, Leys D. Leukoaraiosis more than dementia is a predictor of stroke recurrence. Stroke. 2003;34(12):2935–40. [DOI] [PubMed] [Google Scholar]
- 9.Oksala N, Jokinen H, Melkas S, Oksala A, Pohjasvaara T, Hietanen M, et al. Cognitive impairment predicts poststroke death in long-term follow-up. Journal of Neurology, Neurosurgery & Psychiatry. 2009;80(11):1230–5. [DOI] [PubMed] [Google Scholar]
- 10.Tatemichi T, Paik M, Bagiella E, Desmond D, Pirro M, Hanzawa L. Dementia after stroke is a predictor of long-term survival. Stroke. 1994;25(10):1915–9. [DOI] [PubMed] [Google Scholar]
- 11.Woo J, Kay R, Yuen Y, Nicholls M. Factors influencing long-term survival and disability among three-month stroke survivors. Neuroepidemiology. 1992;11(3):143–50. [DOI] [PubMed] [Google Scholar]
- 12.SPS3 Investigators, Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367(9):817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The SPS3 Study Group, Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCurry SM, Edland SD, Teri L, Kukull WA, Bowen JD, McCormick WC, et al. The cognitive abilities screening instrument (CASI): data from a cohort of 2524 cognitively intact elderly. International journal of geriatric psychiatry. 1999;14(10):882–8. [PubMed] [Google Scholar]
- 15.Royall DR, Cordes JA, Polk M. CLOX: an executive clock drawing task. Journal of Neurology, Neurosurgery & Psychiatry. 1998;64(5):588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacova C, Pearce LA, Costello R, McClure LA, Holliday SL, Hart RG, et al. Cognitive impairment in lacunar strokes: the SPS3 trial. Ann Neurol. 2012;72(3):351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacova C, Pearce LA, Roldan AM, Arauz A, Tapia J, Costello R, et al. Cognitive performance following lacunar stroke in Spanish-speaking patients: results from the SPS3 trial. Int J Stroke. 2015;10(4):519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhamoon MS, McClure LA, White CL, Lakshminarayan K, Benavente OR, Elkind MS, et al. Long-term disability after lacunar stroke: secondary prevention of small subcortical strokes. Neurology. 2015;84(10):1002–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benavente OR, Pearce LA, Bazan C, Roldan AM, Catanese L, Bhat Livezey VM, et al. Clinical-MRI correlations in a multiethnic cohort with recent lacunar stroke: the SPS3 trial. Int J Stroke. 2014;9(8):1057–64. [DOI] [PubMed] [Google Scholar]
- 20.Berry C, Sidik N, Pereira AC, Ford TJ, Touyz RM, Kaski JC, et al. Small-vessel disease in the heart and brain: current knowledge, unmet therapeutic need, and future directions. Journal of the American Heart Association. 2019;8(3):e011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toyoda K. Cerebral small vessel disease and chronic kidney disease. Journal of stroke. 2015;17(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rostamian S, Mahinrad S, Stijnen T, Sabayan B, de Craen AJ. Cognitive impairment and risk of stroke: a systematic review and meta-analysis of prospective cohort studies. Stroke. 2014;45(5):1342–8. [DOI] [PubMed] [Google Scholar]
- 23.Shoamanesh A, Pearce LA, Bazan C, Catanese L, McClure LA, Sharma M, et al. Microbleeds in the Secondary Prevention of Small Subcortical Strokes Trial: Stroke, mortality, and treatment interactions. Ann Neurol. 2017;82(2):196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, et al. The science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cellular and molecular neurobiology. 2016;36(2):281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Appendix References
- 1.SPS3 Investigators, Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367(9):817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClure LA, Szychowski JM, Benavente O, Coffey CS. Sample size re-estimation in an on-going NIH-sponsored clinical trial: the secondary prevention of small subcortical strokes experience. Contemp Clin Trials. 2012;33(5):1088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White CL, Szychowski JM, Roldan A, Benavente MF, Pretell EJ, Del Brutto OH, et al. Clinical features and racial/ethnic differences among the 3020 participants in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial. J Stroke Cerebrovasc Dis. 2013;22(6):764–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The SPS3 Study Group, Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benavente OR, Pearce LA, Bazan C, Roldan AM, Catanese L, Bhat Livezey VM, et al. Clinical-MRI correlations in a multiethnic cohort with recent lacunar stroke: the SPS3 trial. Int J Stroke. 2014;9(8):1057–64. [DOI] [PubMed] [Google Scholar]
- 6.Jacova C, Pearce LA, Costello R, McClure LA, Holliday SL, Hart RG, et al. Cognitive impairment in lacunar strokes: the SPS3 trial. Ann Neurol. 2012;72(3):351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacova C, Pearce LA, Roldan AM, Arauz A, Tapia J, Costello R, et al. Cognitive performance following lacunar stroke in Spanish-speaking patients: results from the SPS3 trial. Int J Stroke. 2015;10(4):519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhamoon MS, McClure LA, White CL, Lakshminarayan K, Benavente OR, Elkind MS, et al. Long-term disability after lacunar stroke: secondary prevention of small subcortical strokes. Neurology. 2015;84(10):1002–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon HS, Lee D, Lee MH, Yu S, Lim J-S, Yu K-H, et al. Post-stroke cognitive impairment as an independent predictor of ischemic stroke recurrence: PICASSO sub-study. Journal of neurology. 2019:1–6. [DOI] [PubMed] [Google Scholar]
- 10.Melkas S, Oksala NK, Jokinen H, Pohjasvaara T, Vataja R, Oksala A, et al. Poststroke dementia predicts poor survival in long-term follow-up: influence of prestroke cognitive decline and previous stroke. Journal of Neurology, Neurosurgery & Psychiatry. 2009;80(8):865–70. [DOI] [PubMed] [Google Scholar]
- 11.Narasimhalu K, Ang S, De Silva DA, Wong M-C, Chang H-M, Chia K-S, et al. The prognostic effects of poststroke cognitive impairment no dementia and domain-specific cognitive impairments in nondisabled ischemic stroke patients. Stroke. 2011;42(4):883–8. [DOI] [PubMed] [Google Scholar]
- 12.Sibolt G, Curtze S, Melkas S, Putaala J, Pohjasvaara T, Kaste M, et al. Poststroke dementia is associated with recurrent ischaemic stroke. J Neurol Neurosurg Psychiatry. 2013;84(7):722–6. [DOI] [PubMed] [Google Scholar]
- 13.Henon H, Vroylandt P, Durieu I, Pasquier F, Leys D. Leukoaraiosis more than dementia is a predictor of stroke recurrence. Stroke. 2003;34(12):2935–40. [DOI] [PubMed] [Google Scholar]
- 14.Oksala N, Jokinen H, Melkas S, Oksala A, Pohjasvaara T, Hietanen M, et al. Cognitive impairment predicts poststroke death in long-term follow-up. Journal of Neurology, Neurosurgery & Psychiatry. 2009;80(11):1230–5. [DOI] [PubMed] [Google Scholar]
- 15.Hart RG, Pearce LA, Bakheet MF, Benavente OR, Conwit RA, McClure LA, et al. Predictors of stroke recurrence in patients with recent lacunar stroke and response to interventions according to risk status: secondary prevention of small subcortical strokes trial. Journal of stroke and cerebrovascular diseases. 2014;23(4):618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arboix A, Font A, Garro C, Garcia-Eroles L, Comes E, Massons J. Recurrent lacunar infarction following a previous lacunar stroke: a clinical study of 122 patients. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78(12):1392–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung J-W, Kim BJ, Han M-K, Kang K, Park J-M, Park S-S, et al. Family history and risk of recurrent stroke. Stroke. 2016;47(8):1990–6. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds K, Lewis B, Nolen JDL, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: a meta-analysis. Jama. 2003;289(5):579–88. [DOI] [PubMed] [Google Scholar]
- 19.Oberlin LE, Waiwood AM, Cumming TB, Marsland AL, Bernhardt J, Erickson KI. Effects of physical activity on poststroke cognitive function: a meta-analysis of randomized controlled trials. Stroke. 2017;48(11):3093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]


