Abstract
Nicotine enhances the value of environmental stimuli and rewards, and reward-enhancement can maintain nicotine consumption. Stimulants such as d-amphetamine are misused more by women and are commonly co-used with nicotine. d-Amphetamine potentiates nicotine’s effects in human and animal research. To date, there are no published studies examining this interaction in a reward-enhancement task. The current study sought to investigate the reward-enhancing effects of nicotine alongside and co-administered with d-amphetamine. Further, we evaluated the persistence of reward-enhancement across ratio and temporal schedules of reinforcement. We used 10 male and 10 female Sprague-Dawley rats. Enhancement was assessed within-subjects by examining active lever pressing for a visual stimulus reinforcer on Variable Ratio 3, Variable Interval 30s, and Variable Time 30s-Variable Ratio 3 schedules. Before one-hour sessions, rats received one injection of saline, 0.1, or 0.3 mg/kg d-amphetamine and one of saline or 0.4 mg/kg nicotine, making six possible drug combinations (saline + saline, saline + nicotine, 0.1 d-amphetamine + saline, 0.1 d-amphetamine + nicotine, 0.3 d-amphetamine + saline, and 0.3 d-amphetamine + nicotine) experienced in a randomized order by each rat. When d-amphetamine was co-administered with nicotine, we found an interaction effect on reward-enhancement that persisted across schedules of reinforcement. Males and females exhibited reward-enhancement by 0.3 d-amphetamine, while only females showed reward-enhancement by 0.1 d-amphetamine. Further, females responded more for the visual stimulus than males in all d-amphetamine conditions. Future studies should assess how reward-enhancement is involved in high nicotine-amphetamine comorbidity rates and enhanced amphetamine misuse in women.
Keywords: Adderall, ADHD, amphetamine, Dexedrine, nicotine, operant, prescription psychostimulants, reward-enhancement, sex differences, smoking
Introduction
There are 480,000 deaths every year from cigarette smoking in the United States, making smoking the leading cause of preventable death (U.S. Department of Health and Human Services [USDHHS], 2014). About 15% of the population smokes cigarettes, with most smokers expressing a desire to quit. However, only 7.5% of smokers maintained abstinence for at least six months (USDHSS, 2020). Notwithstanding the prevalence and persistence of nicotine consumption, nicotine is a weak primary reinforcer. This claim is evidenced by low and inconsistent rates of intravenous (IV) self-administration in humans and animals (Donny et al., 2003; for a review see Goodwin et al. [2015]). In fact, a wide variety of environmental stimuli are often required to attain robust nicotine self-administration (for reviews see Caggiula et al. [2001] and Peartree et al. [2012]). A large body of research suggests that nicotine dependency is largely maintained by processes other than primary reinforcement (Caggiula et al., 2009). These include learning processes such as conditioning involving the interoceptive stimulus effects of nicotine (Huynh et al., 2020), conditioned reinforcement of nicotine associated stimuli (Charntikov et al., in press; Palmatier et al., 2007a), and enhancement of the rewarding effects of other environmental stimuli by nicotine (Barrett and Bevins, 2012).
This reward-enhancing effect has been characterized in a growing body of research as increased responding for and consumption of non-nicotine reinforcers when nicotine is administered (cf. Caggiula et al., 2009). For example, IV self-administration studies have found up to eight-fold increases in responding maintained by nicotine infusions when infusions are delivered with a paired visual stimulus (Caggiula et al., 2002; Donny et al., 2003; Palmatier et al., 2006). Additionally, non-contingent IV or subcutaneously administered nicotine can elicit two-fold or higher increases in responding for a visual stimulus (Barrett and Bevins, 2013; Barrett et al., 2017, 2018; Barrett and Bevins, 2012; Cassidy and Dallery, 2014; Donny et al., 2003). Relative to nicotine-free controls, human participants responded (key-pressed) more in a computer task for video reinforcers when nicotine was delivered via cigarettes, nasal spray, patches, and e-cigarettes (Perkins et al., 2015, 2017, 2019; Perkins and Karelitz, 2014). Reward-enhancement by nicotine can contribute to the tenacity of nicotine dependency by having an important role in the acquisition and maintenance of nicotine consumption (Caggiula et al., 2002, 2009; Rupprecht et al., 2015).
In the present work, we first sought to investigate the reward-enhancing effects of nicotine in tandem with those of d-amphetamine in male and female rats. d-Amphetamine is the primary active ingredient of Dexedrine and Adderall, psychostimulant drugs commonly used in the treatment of attention deficit hyperactivity disorder (ADHD; Heal et al., 2013, Reeves and Schweitzer, 2005). Of the 16 million Americans that used prescription stimulants in 2015–2016, 5 million were cases of misuse (Compton et al., 2018). Like nicotine, d-amphetamine can enhance the reinforcing effects of a visual stimulus at doses ranging from 0.25 to 4 mg/kg (Winterbauer and Balleine, 2007; Wright et al., 2018).
A second goal of the present work was to examine sex differences in reward-enhancement by d-amphetamine. Of note, no studies thus far have examined female rats and reward-enhancement by d-amphetamine. This gap in the literature is surprising given that women have higher rates of amphetamine dependency, more severe dependency, and greater frequency and intensity of amphetamine use than men (Holdcraft and Iacono, 2004; Rungnirundorn et al., 2017). Consistent with this research in humans, female rats acquire d-amphetamine self-administration faster, earn more infusions, and have higher breakpoints on a Progressive Ratio schedule when responding for d-amphetamine infusions (Shahbazi et al., 2008). A full picture of the factors driving d-amphetamine abuse must characterize and better understand its effects in both males and females.
A third aim of this study was to examine the effects of concomitantly administered nicotine and d-amphetamine. Nicotine use is highly comorbid with the use of other psychostimulants (Compton et al., 2018; Kandel and Kandel, 2014; Silveira et al., 2018). Individuals with ADHD, who are commonly treated with psychostimulants, are 2 to 3 times more likely to smoke than those in the general population (Van Amsterdam et al., 2018) and smoking rates among stimulant users are exceptionally high (70–90%; Weinberger and Sofuoglu, 2009). Further, there are many anecdotal reports of nicotine and d-amphetamine co-use and interaction. A search of Reddit posts from the past year (www.reddit.com; Jan 2020 – Jan 2021) on the r/Adderall and r/Stims forums yielded 79 posts discussing prescription amphetamine and nicotine co-use, which garnered 907 comments (data gathered from redditsearch.io). Some examples of these posts include: “I have noticed I crave nicotine far more when I take my medicine [Adderall]” and “I can’t get enough of the intense nicotine rush I get on amphetamines [Dexedrine].” Research suggests this high comorbidity rate is not merely coincidental. In humans, acute doses of d-amphetamine have been shown to increase smoking (Cousins et al., 2001; Sigmon et al., 2003; Tidey et al., 2000), increase smoking satisfaction (Henningfield and Griffiths, 1981), and accelerate nicotine metabolism in smokers with ADHD (Gehricke et al., 2011). In rats, co-administered nicotine and d-amphetamine have been shown to produce additive or more than additive effects on locomotor behavior and dopaminergic transmission (Birrell and Balfour, 1998; Jutkiewicz et al., 2008a; Kim et al., 2011). The converging evidence suggests d-amphetamine can potentiate the effects of nicotine and vice versa. To date, we are not aware of any published studies examining how co-administered nicotine and d-amphetamine interact in a reward-enhancement task.
Finally, we sought to examine whether reward-enhancement persists within-subject on Variable Ratio (VR) 3, Variable Interval (VI) 30s, and compound Variable Time (VT) 30s-VR3 schedules of reinforcement. Reinforcement schedule has been largely excluded in the reward-enhancement literature (but see Chaudhri et al., 2007), with studies thus far relying on ratio schedules (e.g., Barrett and Bevins, 2012; Chaudhri et al., 2007; Donny et al., 2003; Palmatier et al., 2006). The choice of ratio schedules is consistent with the finding that higher reinforcer magnitude increases response rates on ratio schedules (Reed, 1991; Reed and Wright, 1988). In contrast, interval schedules appear less sensitive to changes in reinforcer magnitude. That is, response rate remains consistent when reinforcer magnitude is changed (Harzem et al., 1978; Leslie and Toal, 1994a; Reed and Wright, 1988; Zuriff, 1970). Further, nicotine and d-amphetamine have been shown to increase or decrease VI responding differentially depending on baseline response-rate (Gonzalez and Goldberg, 1977; McMillan, 1969). Additionally, stimulants can speed-up the perception of interval timing. That is, stimulant administration can produce a leftward shift in the typical post-reinforcement pause observed on Fixed Interval schedules (Body et al., 2009; Daniels et al., 2015; Taylor et al., 2007). Considering these factors, it is unknown whether reward-enhancement by nicotine and d-amphetamine would persist on temporal or compound schedules.
The present study extends previous work by characterizing the reward-enhancing effects of nicotine and d-amphetamine, alone and in combination, in male and female rats. We examined these reward-enhancing effects on a VR3, VI30s, and VT30s-VR3 schedule of reinforcement. Here, we seek to answer four research questions: (1) does d-amphetamine alone have reward-enhancement effects in male and female rats; (2) do reward-enhancement effects of d-amphetamine differ by sex; (3) do nicotine and d-amphetamine interact to potentiate reward-enhancement; and (4) does evidence of reward-enhancement persist within-subject on temporally-controlled schedules?
Methods
Subjects
20 Sprague-Dawley rats (10 male and 10 female; Envigo, Indianapolis, Indiana) arrived at 9 weeks old and were maintained in a temperature- and humidity-controlled colony room. Rats acclimated to the colony room for one day and were subsequently handled for seven days before beginning experimental procedures. Unless otherwise specified, water was available ad libitum and food was restricted to 12 g per day for females and 15 g per day for males. Experiments were conducted during the first four hours of the light phase of a 12-hour light/dark cycle. All procedures were approved by University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
Apparatus
Experiments were conducted using 10 conditioning chambers (ENV-008CT; Med-Associates, Inc., St. Albans, VT) which measured 30.5 × 24.1 × 21.0 cm (L × W × H). Chambers were enclosed within light- and sound-attenuating polyvinyl chloride cubicles. These were equipped with a fan for white noise and air circulation. Two sidewalls of the chamber were stainless steel. The front and rear walls were clear polycarbonate, and floors were constructed of stainless-steel rods. The right sidewall housed a dipper receptacle within a 5 × 4 × 5 cm (L × W × H) recessed opening where a dipper arm, when raised, provided access to 0.1 mL of 26% sucrose solution (weight/volume). Retractable metal levers were present 5 cm above the rod flooring; one on each side of the dipper receptacle. White 28-V DC (100-mA) cue-lights were located 3 cm above each lever. Two 28-V DC (100-mA) bulbs were located above the conditioning chamber but within the sound-attenuating cubicle; hereafter referred to as the house-light. The visual stimulus (VS) reinforcer was comprised of both cue-lights illuminating for five seconds and simultaneously turning off the main house-light for one minute. The MedPC program was utilized to record data and present programmed events in the chambers (MedPC for Windows V). All program code is available upon request.
Drugs
d-Amphetamine sulfate salt at 0.1 and 0.3 mg/mL (Sigma-Aldrich; St. Louis, MO) and (−)-nicotine tartrate at 0.4 mg/mL (MP Biomedicals; Irvine, CA) were dissolved in 0.9% saline and injected at a volume of 1 mL of solution/kg of body weight. d-Amphetamine was injected intraperitoneally (IP) using a 15-minute injection-to-placement interval (IPI) and nicotine was injected subcutaneously (SC) using a 5-minute IPI. The IPI is defined as the length of time between the injection and the start of the session. Rats were returned to the home cage after each injection. Before the session, rats were transported to the experimental room in a transport cart and placed in the conditioning chamber within 1-minute of the session starting. Nicotine was brought to a pH of 7.0 ± 0.2 with a dilute NaOH solution. d-Amphetamine doses are reported in salt form while nicotine doses are in base form — per field standards. All doses, IPIs, and routes of administration were based on published research (Huynh et al., 2020; Palmatier et al., 2005; Slezak et al., 2018).
Response Acquisition
All experimental sessions were one hour. For response acquisition training only, food was available ad libitum and water was restricted for 23-hours per day, with one hour of water access immediately following the session. Rats were placed on a series of ascending non-contingent VT schedules (VT30, 60, 120, and 180 seconds), where the schedule changed each day and in the same ascending order for each rat. Sessions began with the extension of both levers. Events were programmed such that rats received 4-seconds of access to sucrose reinforcement automatically at every interval on the VT schedule for the duration of the session. For the duration of the response acquisition phase, no cues were associated with sucrose delivery. This approach was taken to familiarize rats to the availability of sucrose in the dipper receptacle. To facilitate the lever pressing response, rats could concurrently lever press on a Fixed Ratio (FR) 1 schedule to earn additional sucrose reinforcers. FR1 performance had no impact on sucrose delivery on the VT schedule. Once the rat emitted a response on either lever, that lever retracted and the opposite lever extended. This forced lever alternation ensured equal sucrose experience with each lever for the duration of response acquisition training. After the four days, the VT schedule was removed and rats responded on the same FR1 schedule with forced lever alternation for seven days. Four rats that did not acquire the lever pressing response were hand-shaped by successive approximation before proceeding to VS training.
Visual Stimulus Training
Before the start of VS training, rats were assigned an active lever, counterbalanced by sex and conditioning chamber. Throughout the remainder of the study, rats only received the VS reinforcer for responding on their assigned active lever. At the start of the session, the house-lights were illuminated and both levers were extended. There were no programmed consequences for pressing the inactive lever. Although both levers remained extended, rats could not earn additional reinforcement for responding during the one-minute VS (cf. Barrett and Bevins, 2012). Rats were started on a FR1 schedule of reinforcement. Upon completion of the one-minute VS, the main house-lights were again illuminated and the FR1 was in force. Once behavior was stable on a FR1 schedule, rats were moved to a VR2, and subsequently maintained on a VR3 schedule for 12 sessions. For all VR schedules, programmed ratios were based on a symmetrical distribution (i.e., for VR3, the possible response requirements were from 1 to 5).
For the first 14 sessions of VS acquisition, rats received a SC injection of saline 5-minutes before the one-hour session and a SC injection of 0.4 mg/kg nicotine 15-minutes after the session. This approach habituated rats to injections before the session and ameliorated the initial locomotor suppressant effects associated with early nicotine exposure (Barrett and Odum, 2011; Palmatier et al., 2007a). For the next 16 sessions, rats were pseudo-randomly assigned an injection of either saline or 0.4 mg/kg nicotine 5-minutes before the session with the restriction that they could not receive the same injection more than two days in a row. This pre-session nicotine exposure was conducted due to observation of low overall response rates during initial training. Further, our lab and others have found that pre-session exposure of nicotine facilitates emergence of reward-enhancement by nicotine (Palmatier et al., 2007a; Wright et al., 2018). This pre-session protocol was not required for emergence of d-amphetamine reward-enhancement (cf. Wright et al., 2018).
Enhancement Assessments
The day following the completion of VS training, rats now received two injections before each session: one injection 15-minutes before the session of saline (SAL), 0.1, or 0.3 mg/kg d-amphetamine (AMP) and a second injection five-minutes before the session of SAL or 0.4 mg/kg nicotine (NIC). Thus, there were six possible drug combinations: SAL + SAL, SAL + NIC, 0.1 AMP + SAL, 0.1 AMP + NIC, 0.3 AMP + SAL, and 0.3 AMP + NIC. All six drug combinations were administered to each rat in a pseudo-randomized order, hereafter referred to as a block. Randomization was done with the restriction that the entire block had to be completed before the next block began. Additionally, no drug combination could be experienced twice successively. Rats completed four blocks (24 sessions) on each schedule of reinforcement (described below).
Rats began the enhancement assessment phase by responding on a VR3 schedule to attain a baseline measure of responding on a commonly used reinforcement schedule in our laboratory. Following testing on the VR3, rats were switched to a VI30s schedule to examine if enhancement of responding persisted on a temporal schedule. Programmed intervals were based on the Fleshler-Hoffman distribution (Fleshler and Hoffman, 1962). To examine enhancement on a compound schedule, rats were then moved to a VT30s-VR3 schedule. This schedule combined the VR3 and VI30s such that the rat could contact reinforcement for pressing 1 to 5 times on the active lever, but only after the programmed interval had elapsed. We chose this compound schedule due to observation of response rate increases when rats were transitioned to the VI-30s schedule. The VT30s-VR3 schedule was applied to examine whether the response rates would again increase, or rather decrease due to increased response effort for the weakly reinforcing VS.
Statistical Analysis
Prior to analyses, data from the first block for each schedule of reinforcement was removed to avoid examination of possible carry-over effects and ensure we are analyzing schedule-specific behavior (cf. Barrett et al., 2017). Our primary dependent variable to examine VS reward-enhancement was the number of active lever presses in the session. This included lever pressing during the VS presentation. Additional analyses were performed on inactive lever pressing as a measure of the non-specific effects of AMP and NIC on responding. For example, if inactive lever pressing increased in parallel and extent with active lever pressing, this data pattern could be interpreted as increasing overall locomotor behavior rather than enhancing the VS reward. All statistical analyses were conducted using R Statistical Program version 4.0.0 (R-Core Team, 2020). Because reinforcement schedule co-varies with time, each schedule of reinforcement was analyzed separately. Active and inactive lever pressing data were analyzed with a three-way mixed-groups analysis of variance (ANOVA) using the afex package (Singmann et al., 2020) with Sex as a between-groups factor and AMP dose and NIC dose as within-subjects factors. To provide the most direct answer to our research questions, post-hoc pairwise comparisons were conducted on the Sex * AMP * NIC interaction for active lever pressing where appropriate. All significant effects of inactive lever pressing were also explored. Post-hoc comparisons were corrected for multiple comparisons using Tukey’s test computed within the emmeans package (Lenth et al., 2019).
Results
Variable-Ratio 3
For the three-way mixed-groups ANOVA on active lever pressing maintained by a VR3 schedule of reinforcement, there were main effects of NIC dose [F(1,18) = 154.07, p < 0.001], AMP dose [F(2,36) = 103.95, p < 0.001], and Sex [F(1,18) = 6.39, p = 0.021] which are visualized in Figure 1a. Exploring the main effect of NIC, there was a significant increase in responding by NIC relative to SAL (p < 0.001; Figure 1a, left). For the main effect of AMP, there was an increase in active lever pressing by both 0.1 AMP and 0.3 AMP, and 0.3 AMP engendered significantly higher active lever pressing than 0.1 AMP (ps ≤ 0.003; Figure 1a, middle). Thus, the magnitude of these behavioral differences was dose-dependent. The main effect of Sex indicated that females responded more for the VS than males across drug conditions (p = 0.021; Figure 1a, right).
Figure 1.
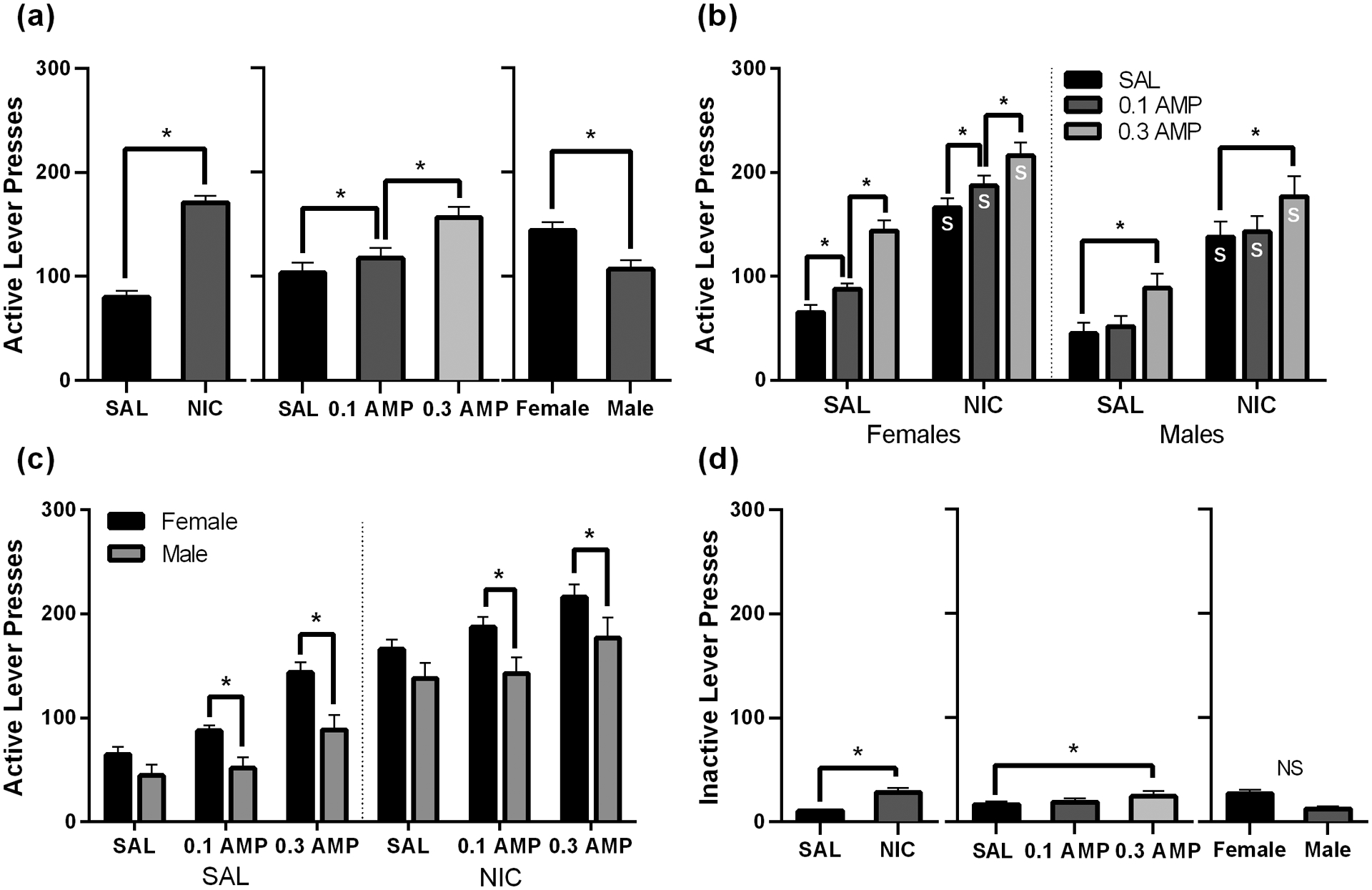
Active (a-c) and inactive (d) lever pressing maintained by a VR3 schedule of reinforcement under the six possible drug co-administration conditions: SAL + SAL, 0.1 AMP + SAL, 0.3 AMP + SAL, SAL + NIC, 0.1 AMP + NIC, and 0.3 AMP + NIC. Panel (a) presents marginal means from each main effect (NIC, AMP, and Sex) from analysis of active lever pressing. Panel (b) presents active lever pressing data within the AMP * NIC * Sex interaction organized to facilitate comparisons between drug conditions. The x-axis reflects NIC dose and bars indicate AMP dose. Data are separated by sex using the dotted line. An s indicates a significant difference (p < 0.05) from the respective saline condition. Panel (c) presents the same data from the three-way interaction but reorganized to facilitate visual comparisons between males and females. The x-axis now reflects AMP dose, separated by NIC dose via the dotted line; the bars represent Sex. Panel (d) shows marginal means from main effects of inactive lever pressing NS indicates the absence of a significant main effect. For all panels, asterisks indicate a significant difference at p < 0.o5. Data are presented as mean + 1 SEM.
There were also two-way interactions of Sex * AMP [F(2, 36) = 4.76, p = 0.015] and AMP * NIC [F(2,36) = 5.51, p = 0.008]. However, these were not explored because the three-way interaction of Sex * AMP * NIC approached conventional levels of significance [F(2,36) = 3.05, p = 0.060]. Based on our hypotheses that females will be more sensitive than males to the reward-enhancing effects of d-amphetamine, and therefore the effects of co-administered nicotine and d-amphetamine, the three-way interaction was explored. Figure 1b presents active lever pressing data from the VR3 schedule of reinforcement arranged to facilitate visual comparisons between drug conditions. Examining the effects of NIC for each Sex and AMP condition, all NIC conditions engendered higher active lever pressing than their respective SAL conditions (ps < 0.001). Also visualized in Figure 1b are the effects of AMP for each Sex and NIC condition. We found sex-specific effects of AMP. Females had an increase in active lever pressing relative to SAL + SAL by both 0.1 AMP + SAL (p = 0.004) and 0.3 AMP + SAL (p < 0.001). Additionally, there were dose-dependent increases in lever pressing relative to SAL + NIC by both 0.1 AMP + NIC and 0.3 AMP + NIC (ps < 0.001). For males, there was increased active lever pressing relative to SAL + SAL by 0.3 AMP + SAL (p < 0.001), but not 0.1 AMP + SAL (p = 0.563). Similarly, there was increased active lever pressing relative to SAL + NIC by 0.3 AMP + NIC (p < 0.001), but not 0.1 AMP + NIC (p = 0.737).
Figure 1c displays the same active lever pressing data rearranged to facilitate visual comparisons between males and females within the three-way interaction. Females pressed the active lever more than males only in the 0.1 and 0.3 AMP conditions for both AMP + SAL (ps = 0.046 and 0.003, respectively) and AMP + NIC conditions (ps = 0.015 and 0.030, respectively). There were no significant sex differences in SAL + SAL (p = 0.249) or SAL + NIC conditions (p = 0.113).
Figure 1d presents the main effects for inactive lever pressing. For the three-way ANOVA, there were main effects of only NIC [F(1,18) = 14.50, p = 0.001] and AMP [F(2,36) = 5.22, p = 0.010], with no significant interactions (Fs ≤ 3.14, ps ≥ 0.093) or main effect of Sex [F(1,18)= 3.00, p = 0.100]. The main effect of NIC on inactive lever pressing reflects the slight increase in inactive lever pressing by NIC relative to SAL (p = 0.001). Similarly, there was a small increase in inactive lever pressing by 0.3 AMP (p = 0.009); this was not the case for 0.1 AMP (p = 0.659). There was no effect of Sex on inactive lever pressing (p = 0.10).
Variable-Interval 30s
Using a three-way mixed groups ANOVA for active lever pressing on a VI30s schedule of reinforcement, there were main effects of NIC [F(1,18) = 197.88, p < 0.001], AMP [F(2,36) = 87.81, p < 0.001], and Sex [F(1,18) = 6.28, p = 0.022] which are visualized in Figure 2a. The patterns of the main effects were consistent with the analyses of the VR3 schedule. For the main effect of NIC, there were increases in responding by NIC relative to SAL conditions (p < 0.001; Figure 2a, left). The main effect of AMP reflects a dose-dependent (p < 0.001) increase in active lever pressing by 0.1 AMP and 0.3 AMP (ps ≤ 0.005; Figure 2a, middle). For the main effect of Sex, females responded more for the VS than males (p = 0.022; Figure 2a, right).
Figure 2.
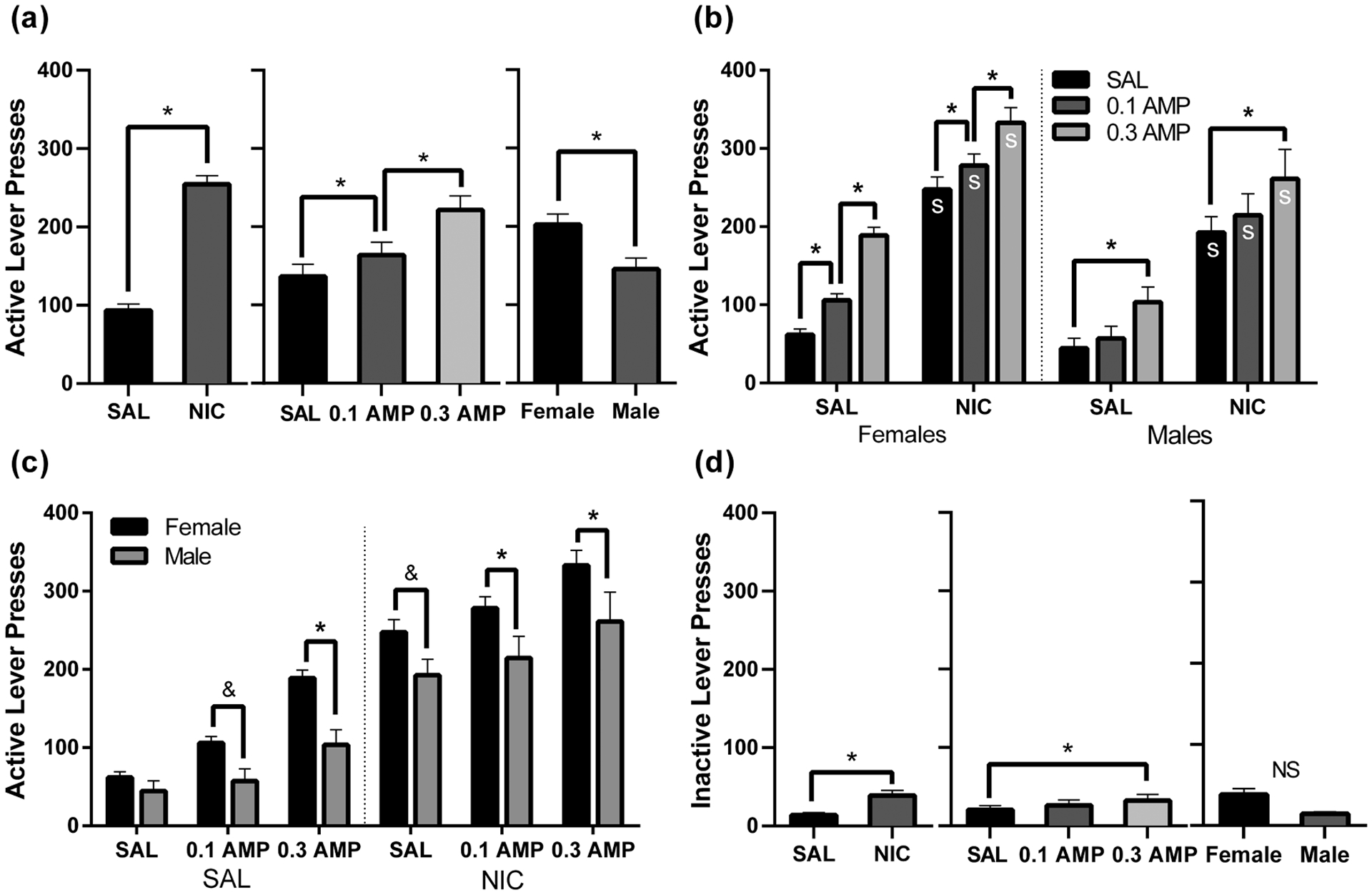
Active (a-c) and inactive (d) lever pressing maintained by a VI30s schedule of reinforcement, under the six possible drug co-administration conditions: SAL + SAL, 0.1 AMP + SAL, 0.3 AMP + SAL, SAL + NIC, 0.1 AMP + NIC, and 0.3 AMP + NIC. Panel (a) presents marginal means from each main effect (NIC, AMP, and Sex) from analysis of active lever pressing. Panel (b) presents active lever pressing data within the AMP * NIC * Sex interaction, organized to facilitate comparisons between drug conditions. The x-axis reflects NIC dose and bars indicate AMP dose. Data are separated by sex using the dotted line. An s indicates a significant difference (p < 0.05) from the respective saline condition. Panel (c) presents the same data from the three-way interaction but reorganized to facilitate visual comparisons between males and females. The x-axis now reflects AMP dose, separated by NIC dose via the dotted line; the bars represent Sex. Panel (d) shows marginal means from main effects of inactive lever pressing NS indicates the absence of a significant main effect. For all panels, asterisks indicate a significant difference at p < 0.o5, and ampersands indicate a trend towards significance at p < 0.10. Data are presented as mean + 1 SEM. Note that the y-axis value has increased from Figure 1.
There was a two-way interaction of Sex * AMP [F(2, 36) = 5.28, p = 0.010], but not AMP * NIC [F(2,36) = 1.39, p = 0.262]. The three-way interaction of Sex * AMP * NIC again approached significance [F(2,36) = 3.04, p = 0.060] and was therefore explored in lieu of the two-way interactions. Figure 2b illustrates active lever pressing on a VI30s schedule arranged to facilitate visual comparisons between drug conditions. Examining the effects of NIC for each Sex and AMP dose, there was higher active lever pressing in all NIC conditions versus their respective SAL conditions regardless of AMP dose or Sex (ps < 0.001). Examining the effects of AMP for each Sex and NIC condition, there were again sex-specific effects of AMP. For females, there were dose-dependent increases in active lever pressing relative to SAL + SAL by 0.1 AMP + SAL and 0.3 AMP + SAL (ps ≤ 0.002), and relative to SAL + NIC by 0.1 AMP + NIC and 0.3 AMP + NIC (ps ≤ 0.030). For males, there was increased responding relative to SAL + SAL by 0.3 AMP + SAL (p < 0.001), but not 0.1 AMP + SAL (p = 0.539), and increased responding relative to SAL + NIC by 0.3 AMP + NIC (p < 0.001), but not 0.1 AMP + NIC (p = 0.158).
Figure 2c presents the same active lever pressing data for the three-way interaction rearranged to enable visual comparisons between males and females for each drug condition. Comparing the sexes for each AMP and NIC condition, females responded more than males in the 0.3 AMP + SAL condition (p = 0.004), as well as in 0.1 AMP + NIC and 0.3 AMP + NIC conditions (ps < 0.026), but not in the SAL + SAL condition (p = 0.533). Unlike the VR3 schedule, females did not respond more than males in the 0.1 AMP + SAL condition (p = 0.083). Of note, increased lever pressing by females during the SAL + NIC condition approached significance (p = 0.051).
The main effects for the three-way ANOVA for inactive lever pressing are visualized in Figure 2d. There were main effects of AMP [F(2,36) = 5.85, p = 0.006] and NIC [F(1,18) = 15.40, p < 0.001], but not Sex [F(1,18) = 2.07, p = 0.167]. There were no significant interactions [Fs ≤ 1.84, ps ≥ 0.173]. Similarly to VR3 inactive lever pressing analyses, the main effect of NIC indicated a small increase in inactive responding by NIC versus SAL conditions (p < 0.001). The main effect of AMP was reflective of a small increase in inactive responding by 0.3 AMP (p = 0.004), but not by o.1 AMP (p = 0.259).
Variable-Time 30s/Variable-Ratio 3
For the three-way ANOVA for active lever pressing maintained by a VT30s-VR3 schedule of reinforcement, there were main effects of NIC [F(1,18) = 143.74, p < 0.001] and AMP [F(2,36) = 87.67, p < 0.001] with the behavioral patterns consistent with the analyses of the VR3 and VI30s schedules. These main effects are displayed in Figure 3a. Exploring the main effects, there were dose-dependent increases in active lever pressing by NIC relative to SAL conditions and increases relative to SAL by 0.1 and 0.3 AMP (Figure 3a, left and middle; ps < 0.001). However, the main effect of Sex [F(1,18) = 3.72, p = 0.070] only approached significance, with females tending to respond more than males (Figure 3a, right).
Figure 3.
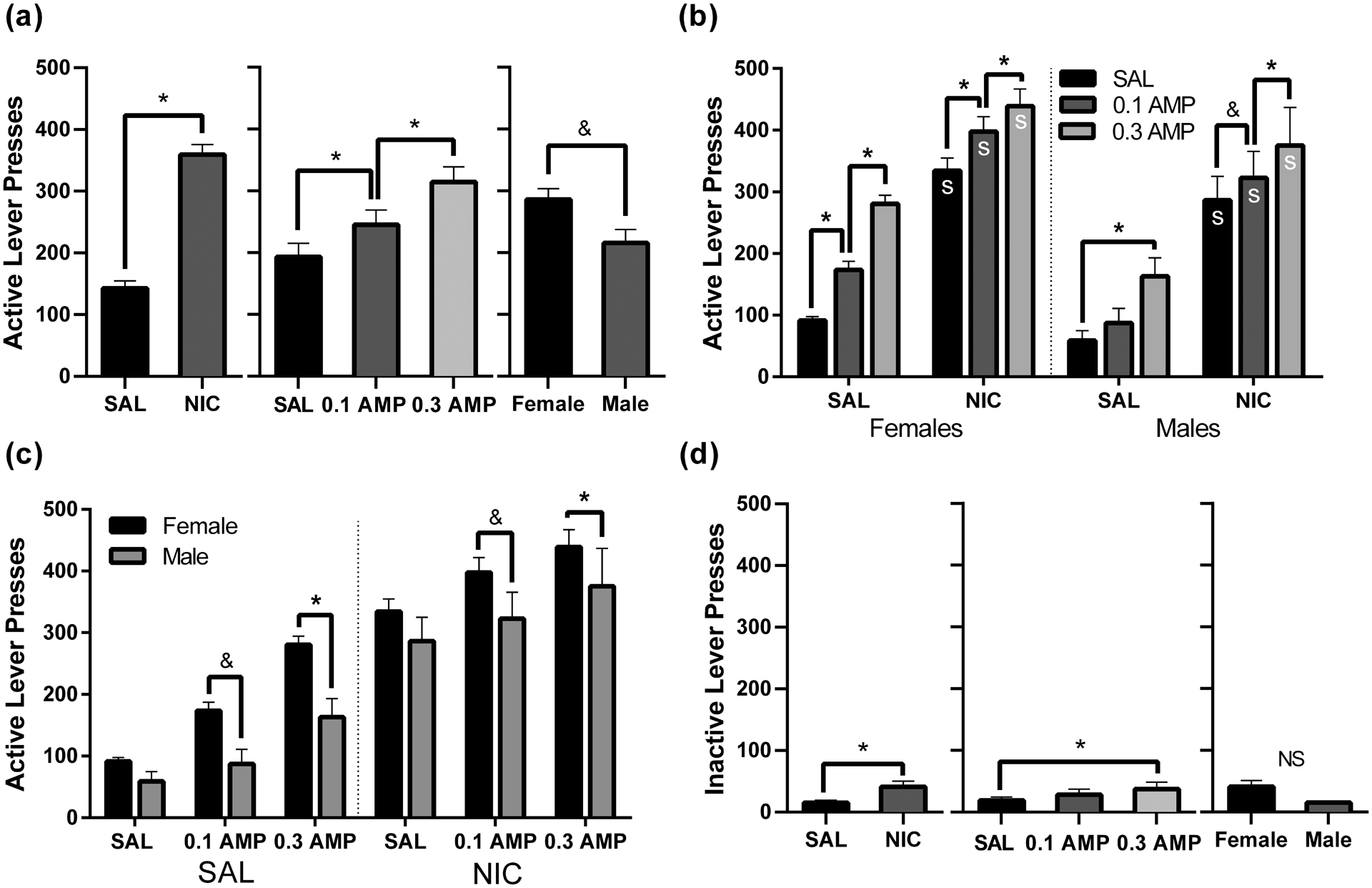
Active (a-c) and inactive (d) lever pressing maintained by a VT30s-VR3 schedule of reinforcement, under the six possible drug co-administration conditions: SAL + SAL, 0.1 AMP + SAL, 0.3 AMP + SAL, SAL + NIC, 0.1 AMP + NIC, and 0.3 AMP + NIC. Panel (a) presents marginal means from each main effect (NIC, AMP, and Sex) from analysis of active lever pressing. Panel (b) presents active lever pressing data within the AMP * NIC * Sex interaction, organized to facilitate comparisons between drug conditions. The x-axis reflects NIC dose and bars indicate AMP dose. Data are separated by sex using the dotted line. An s indicates a significant difference (p < 0.05) from the respective saline condition. Panel (c) presents the same data from the three-way interaction but reorganized to facilitate visual comparisons between males and females. The x-axis now reflects AMP dose, separated by NIC dose via the dotted line; the bars represent Sex. Panel (d) shows marginal means from main effects of inactive lever pressing NS indicates the absence of a significant main effect. For all panels, asterisks indicate a significant difference at p < 0.o5, and ampersands indicate a trend towards significance at p < 0.10. Data are presented as mean + 1 SEM. Note that the y-axis value has increased from Figures 1 & 2.
Additionally, there were two-way interactions of Sex * AMP [F(2,36) = 4.18, p = 0.023] and AMP * NIC [F(2,36) = 9.05, p < 0.001], which were not explored given the three-way interaction of Sex * AMP * NIC [F(2,36) = 3.71, p = 0.034]. Figure 3b shows active lever pressing data on a VT30s-VR3 schedule arranged to ease visual comparisons between drug conditions. We found increased responding in all NIC conditions versus their respective SAL conditions when examining the effects of NIC for each Sex and AMP conditions (ps < 0.001). Examining the effects of AMP for each Sex and NIC condition, there were similar sex-specific effects of AMP. Females pressed more relative to SAL + SAL in both AMP + SAL conditions (ps < 0.001) and more relative to SAL + NIC in both AMP + NIC conditions (ps < 0.001). The magnitude of these differences was dose-dependent (p < 0.001). Males pressed more relative to SAL + SAL in the 0.3 AMP + SAL condition, but not the 0.1 AMP + SAL condition (p = 0.182). Similarly, males responded more relative to SAL + NIC in only the 0.3 AMP + NIC condition (ps < 0.001); however, differing from VR3 and VI30s schedules, males responding in the 0.1 AMP + NIC condition approached a significant increase relative to SAL + NIC (p = 0.068).
Figure 3c shows the same active lever pressing data arranged to facilitate visual comparisons between males and females for each drug condition. Comparing males and females within the three-way interaction, females pressed more than males only in the 0.3 AMP + SAL condition (p = 0.010), while only approaching significantly higher active lever pressing than males in the 0.1 AMP + SAL (p = 0.053) and 0.1 AMP + NIC (p = 0.088) conditions.
Using a three-way ANOVA for inactive lever pressing on the VT30s-VR3 schedule of reinforcement, there were main effects of AMP [F(2,36) = 4.24, p = 0.022] and NIC [F(1,18) = 8.85, p = 0.008], but not Sex [F(1,18) = 1.41, p = 0.251], which are visualized in Figure 3d. The main effect of NIC indicated a slight increase in inactive lever pressing by NIC versus SAL (p = 0.008). The main effect of AMP reflects the small increase in inactive lever pressing by 0.3 AMP (p = 0.016); o.1 AMP did not increase inactive lever pressing (p = 0.330).
Discussion
Nicotine and d-amphetamine, alone and in combination, enhanced responding for the VS in males and females across a VR3, VI-30s, and VT30s-VR3 schedule of reinforcement. We replicated previous work by showing increased responding for a VS by nicotine in the absence of sex differences (e.g., Barrett and Bevins, 2013; Barrett et al., 2017, 2018; Barrett and Bevins, 2012). Nicotine evoked a roughly two-fold increase in responding for the VS. While inactive lever pressing did increase when nicotine was administered, this increase was slight. This finding suggests that reward-enhancement by nicotine cannot be solely attributed to general locomotor activating effects; a conclusion consistent with previous work (e.g., Barrett et al., 2017; Barrett and Bevins, 2012; cf. Donny et al., 2004).
Although the lack of sex differences in responding for the VS by nicotine is consistent with our previous studies (e.g., Barrett et al., 2017, 2018), there are some reports that females are more sensitive to some effects of nicotine (i.e., smoking associated cues and stimuli; Chaudhri et al., 2005; Perkins, 2009). One possible explanation for this difference is that the dose of nicotine (0.4 mg/kg) used in the current study produced a ceiling effect, masking possible sex differences. In support of this interpretation, we recently found sex differences in a nicotine drug discrimination task utilizing 0.1 mg/kg as the training dose (Huynh et al., 2020). In that study by Huynh et al. and in an earlier study (Charntikov et al., 2017), sex differences were not seen when 0.4 mg/kg of nicotine served as the training dose. Further, a recent study in our lab examined the effects of pre-session nicotine on enhancement of ethanol reinforcement. We found that while only 0.4 mg/kg nicotine enhanced the reinforcing effects of alcohol in male rats, doses as low as 0.05 mg/kg nicotine increased the reinforcing value of alcohol in females (Barrett et al., 2020). Published studies that have examined the sensory reward-enhancing effects of lower doses of nicotine have only utilized male rats (e.g., Barrett and Odum, 2011; Cassidy and Dallery, 2014; Swalve et al., 2015; Wright et al., 2018), leaving a gap in our understanding of sex differences in nicotine-evoked reward-enhancement. Further research in our lab is characterizing the reward-enhancing effects of a wide-range of nicotine doses using both male and female rats to better understand mechanisms other than the primary reinforcing effects of nicotine that impact the use liability of nicotine-containing products in both sexes.
Despite the lack of sex differences in reward-enhancement by nicotine, we found that females displayed greater sensitivity to the reward-enhancing effects of d-amphetamine. While males and females show increased responding in the 0.3 mg/kg d-amphetamine conditions (0.3 AMP + SAL and 0.3 AMP + NIC) relative to respective control conditions (SAL + SAL and SAL + NIC), only females exhibited heightened responding in the 0.1 mg/kg d-amphetamine conditions (0.1 AMP + SAL and 0.1 AMP + NIC) relative to control conditions. Additionally, largely consistent across schedules, females responded more than males in d-amphetamine conditions (0.1 AMP + SAL, 0.1 AMP + NIC, 0.3 AMP + SAL, and 0.3 AMP + NIC), despite not showing significantly higher responding relative to control conditions (SAL + SAL and SAL + NIC). Levels of reward-enhancement by 0.3 AMP + SAL approached that of SAL + NIC in females, whereas 0.3 AMP + SAL evoked increases in active lever pressing roughly half the size of NIC + SAL in males.
Our study adds to a body of published research showing that females are more sensitive to the behavioral effects of amphetamines. In humans, low doses of d-amphetamine (8 to 10 mg, orally administered) act as a reinforcer in women but not in men (Vansickel et al., 2010). Additionally, a low dose of methamphetamine (20 mg, orally administered) decreased reaction times for trials signaling monetary reward in women but not men (Mayo et al., 2019). In female but not male rats, d-amphetamine disrupted performance on a delay-discounting task (Eubig et al., 2014). Further, female rats exhibited heightened effects of d-amphetamine on locomotor activity (Mathews and McCormick, 2007; Milesi-Hallé et al., 2007) and showed faster behavioral sensitization in response to repeated d-amphetamine treatment (Becker et al., 2001).
Increased sensitivity to the locomotor activating effects of d-amphetamine in females may have contributed to the sex differences we observed; however, doses in the range of 0.1 to 0.3 mg/kg have been shown to produce only modest to no increases in locomotor activity in males or females (Jutkiewicz et al., 2008; Turner et al., 2017). Additionally, while active lever responding was enhanced more so in female rats, there was no effect of sex in our analysis of inactive lever pressing on any schedule and we did not see an increase in amphetamine-evoked inactive lever pressing by 0.1 mg/kg d-amphetamine. Prompted by a critique of an anonymous reviewer, we went back and conducted and analyses of covariance on active lever pressing data for each schedule of reinforcement. To identify the extent that our effects could be explained by inactive responding, inactive lever pressing was added as a time-varying covariate to our original analysis. We did identify a significant effect of inactive lever pressing only in the VI analysis. However, the addition of inactive lever pressing as a covariate did not alter the effects of any variable on active lever pressing for any schedule (i.e., did not make any effects nonsignificant that were significant in the original ANOVAs). The fact that we did not see an increase in inactive lever pressing that paralleled active lever pressing, nor any changes in results from the addition of our covariate, suggests that our observed effects on reward-enhancement by d-amphetamine cannot be solely attributed to non-specific behavioral activation; a suggestion consistent with previous reward-enhancement work on non-nicotine psychostimulants (Barrett et al., 2017; Wright et al., 2018).
Estradiol, the primary female sex-hormone, has been implicated in sex differences in response to d-amphetamine (Becker et al., 2001; Peris et al., 1991; Walker et al., 2012; White et al., 2002; Zovkic and McCormick, 2019). Amphetamine’s primary mechanism of action is via the dopaminergic system (Easton et al., 2007; reviewed by Heal et al. [2013]), and higher estrogen has been shown to increase dopamine receptor density (Lévesque et al., 1989) and sensitivity (Hruska and Silbergeld, 1980). Further, testosterone, the primary male sex-hormone, has been shown to accelerate amphetamine metabolism, generally resulting in lower brain levels of amphetamine and thus smaller behavioral effects in males (Meyer and Lytle, 1978). Contrary to this interpretation, numerous studies have shown that sex differences in the behavioral effects of amphetamine persist even when brain amphetamine levels are matched between males and females (Becker et al., 2001; Camp and Robinson, 1988a, 1988b; Robinson, 1984). Nevertheless, the role of estradiol specifically, or gonadal hormones more generally, in reward-enhancement by psychostimulants is, to our knowledge, an unexplored area of research. To parse out the role gonadal hormones play in sex differences in d-amphetamine-evoked reward-enhancement, monitoring of rat estrous cycle or exogenously manipulating estradiol or testosterone in studies of reward-enhancement would be of interest.
We also found that co-administered nicotine and d-amphetamine interact to potentiate VS reward-enhancement. Increases in active lever pressing by d-amphetamine were similar whether d-amphetamine was administered with saline or administered with nicotine. This effect is consistent with other animal studies examining acutely co-administered nicotine and d-amphetamine. Two studies with rats found that when nicotine and d-amphetamine were administered simultaneously, additive effects were observed (Jutkiewicz et al., 2008; Kim et al., 2011). Our finding that d-amphetamine increases nicotine’s reward-enhancing effects supports reward-enhancement as a plausible mechanism contributing to increased smoking behavior with acute d-amphetamine treatment (Cousins et al., 2001; Sigmon et al., 2003; Tidey et al., 2000), high nicotine-amphetamine comorbidity rates (Weinberger and Sofuoglu, 2009), and reports of d-amphetamine increasing smoking satisfaction (Henningfield and Griffiths, 1981). Future studies should determine the extent to which reward-enhancement contributes to this increased comorbidity.
Considering that there has been limited research examining how acute d-amphetamine impacts nicotine’s behavioral effects, studying a wider range of doses may be required to fully assess the nature of this interaction. The two aforementioned studies (Jutkiewicz et al., 2008; Kim et al., 2011) used low doses of d-amphetamine (0.32 mg/kg) and nicotine (0.32 or 0.1 mg/kg); perhaps higher doses will produce supra-additive effects. For example, Gerasimov et al. (2000) found that while 0.4 mg/kg nicotine co-administered with 10 mg/kg cocaine or 5 mg/kg methylphenidate had additive effects on dopamine transmission, the same dose of nicotine co-administered with higher doses of cocaine (20 mg/kg) or methylphenidate (10 mg/kg) had supra-additive effects. Further research should consider examining higher doses of d-amphetamine concomitantly administered with nicotine to assess if similar effects occur in behavioral tasks.
We found that reward-enhancement by nicotine and d-amphetamine persists within-subject across VR3, VI-30s, and VT30s-VR3 schedules of reinforcement. Our findings are notable considering previous findings that indicate VI response rates do not increase with higher magnitude of reinforcement (Harzem et al., 1978; Leslie and Toal, 1994b; Reed, 1991). Using reinforcer demand modeling, we have previously shown nicotine to increase the value of the VS (cf. Barrett and Bevins, 2012). Knowing this, we might expect our results to reflect changes in magnitude of reinforcement. However, perceived reinforcer magnitude may not be the sole factor involved in enhancement of the VS reward. Alternatively, nicotine and d-amphetamine could be increasing different facets of reinforcer value such as overall hedonic value or quality, which have both been shown to increase response rates (Clarkson et al., 2018; Covarrubias and Aparicio, 2008). Another possibility is that the relative temporal density of reinforcement in our VI30s schedule may be too high to represent the effects typically seen on VI schedules. For example, examination of a VI-40s schedule did show reinforcer magnitude effects (Grace and Bragason, 2005). In contrast, research identifying VI schedules as insensitive to changes in reinforcer magnitude has typically used VI-60s schedules or greater (Harzem et al., 1978; Leslie and Toal, 1994). Of note, magnitude of reinforcement can be manipulated by increasing or decreasing reinforcer duration, intensity, or quantity. While Grace and Bragason (2005) manipulated reinforcer magnitude by increasing reinforcer duration, Harzem and colleagues (1978) and Leslie and Toal (1994) manipulated reinforcer intensity and quantity, respectively. Thus, it is possible that our long duration reinforcer allowed for observation of magnitude effects, while we may have observed a different pattern of effects had we used a shorter duration reinforcer.
Another important consideration is that in addition to the VI intervals, the one-minute duration of our VS limited the number of reinforcers available during the session. That is, on the VR3 schedule, rats could earn 60 reinforcers if their responding was optimally matched to the schedule. However, because both the interval and reinforcer duration limited available reinforcers on the VI30s and VT30s-VR3 schedules, rats could only earn a maximum of 40 reinforcers (e.g., one reinforcer every 90s). On the temporal schedules, several rats in the present study approached this maximum when nicotine was co-administered with d-amphetamine. Perhaps examining enhancement of a shorter duration reinforcer would have ameliorated this ceiling effect and thus produced a different outcome. Further exploration of facets of reinforcer value responsible for the reward-enhancing effects of nicotine and the specific temporal distributions of reinforcement under which reward-enhancement persists will be of much interest to further understand the mechanisms of nicotine increasing reinforcer value.
Perhaps some portion of the persistence of reward-enhancement on temporal schedules could be attributed to the rate-dependent effects that have been described for nicotine and d-amphetamine (for a review see Bickel et al. [2016]). That is, nicotine and d-amphetamine have effects on schedule-controlled responding that are dependent on baseline response rates. The typical pattern of results is that low baseline response rates are increased by nicotine and d-amphetamine, whereas high baseline response rates are unchanged or decreased (Branch, 1984; Heffner et al., 1974; Morrison and Armitage, 1967; Raiff and Dallery, 2008). Accordingly, since the VS is a low value reinforcer that typically generates low baseline response rates, we would expect to see increased responding by nicotine and d-amphetamine (cf. Krebs et al., 2016). Notably, not all effects of nicotine and d-amphetamine on reward-enhancement can be attributed to rate-dependency (cf. Raiff and Dallery, 2008). If nicotine solely increased low response rates, we would expect nicotine to increase response rates for minimally or non-reinforcing stimuli. However, a previous study found that the degree to which nicotine increased response rates was dependent on the initial value of the reinforcer, insofar that responding for non-reinforcing stimuli was not enhanced by nicotine (Palmatier et al., 2007b). Our findings demonstrate the persistence of reward-enhancement across reinforcement schedules. However, the present study was not designed to directly compare the schedules nor examine whether evidence of reward-enhancement would be present on temporal schedules in the absence of previous schedule history. Since the schedule manipulations co-varied with time, we cannot determine whether potential schedule differences would reflect reinforcement contingencies or some combination of reinforcement history, repeated drug exposure, or other time-related factors. Persistence across schedules could have also been altered by these same factors. This point is consistent with evidence that behavioral history can alter the effects drugs (for a review see Tatham and Wanchisen [1998]). Thus, a different pattern of effects could have emerged if we had started with a different schedule or counterbalanced the order in which each schedule was experienced. Further investigation using reversal designs or experimental designs that would allow for direct comparison between schedules are required to fully understand the impact of schedule of reinforcement on reward-enhancement.
In conclusion, this study adds to a mounting body of literature describing the reward-enhancing effects of nicotine and d-amphetamine (Barrett et al., 2017; Barrett and Bevins, 2012; Cassidy and Dallery, 2014; Wright et al., 2018). We extended this previous work by identifying additive effects of concomitantly administered nicotine and d-amphetamine and adding reward-enhancement to the list of behavioral effects of d-amphetamine that are more prominent in female than male rats. Finally, we found that the reward-enhancing effects of nicotine and d-amphetamine persist on a VR3, VI30s, and VT30s-VR3 schedule of reinforcement. Future research should consider examining the degree to which increased reward-enhancement by d-amphetamine is involved in the high rates of amphetamine misuse in females (Holdcraft and Iacono, 2004; Rungnirundorn et al., 2017) and parse out the underlying neurobiological mechanisms along with the import of gonadal hormones such as estradiol (Becker et al., 2001; Zovkic and McCormick, 2019). Additionally, there is a growing body of research suggesting that polysubstance use is an important factor in drug addiction treatment outcomes (Crummy et al., 2020; Wang et al., 2017). Knowing this, we suggest that careful consideration of existing nicotine use in the prescription of amphetamines, in addition to nicotine-amphetamine comorbidities in smoking cessation treatment, is warranted. This suggestion also takes into consideration that reward-enhancement can greatly contribute to smoking acquisition and maintenance (cf. Caggiula et al., 2009), so anything that potentiates these reward-enhancing effects could further enhance abuse liability and impair treatment outcomes. Future studies may examine the degree to which d-amphetamine potentiating reward-enhancement by nicotine is involved in high rates of amphetamine-nicotine comorbidity (Compton et al., 2018; Silveira et al., 2018; Weinberger and Sofuoglu, 2009). Additionally, the anecdotal reports of d-amphetamine enhancing the effects of nicotine are numerous and not limited to those described earlier in this paper. Users on Reddit (www.reddit.com) in r/Adderall and r/Stims forums continually report anecdotes such as: “I’ve been in a 5 year relationship with the glorious combo of cigs and Adderall” and “a handy Adderall prescription made me addicted to cigarettes.” Further research is warranted to investigate this comorbidity considering the converging evidence that d-amphetamine enhances the abuse liability of nicotine-containing products.
ACKNOWLEDGEMENTS
The authors would like to thank Allissa Flynn, Andrew Finkner, Bailey Newsome, and Alex Johnson for their assistance in running experimental sessions.
Conflicts of Interest and Source of Funding
This research was supported by grant DA046109 from the National Institutes of Health. RAB was partially supported by GM130461 while preparing this manuscript for publication. No potential conflicts of interest were declared by the authors.
References
- Barrett ST, Bevins RA (2013). Nicotine enhances operant responding for qualitatively distinct reinforcers under maintenance and extinction conditions. Pharmacol Biochem Behav 114–115:9–15. [PMC free article] [PubMed] [Google Scholar]
- Barrett ST, Bevins RA (2012). A quantitative analysis of the reward-enhancing effects of nicotine using reinforcer demand: Behav Pharmacol 23:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ST, Geary TN, Steiner AN, Bevins RA (2017). Sex differences and the role of dopamine receptors in the reward-enhancing effects of nicotine and bupropion. Psychopharmacology (Berl) 234:187–198. [DOI] [PubMed] [Google Scholar]
- Barrett ST, Geary TN, Steiner AN, Bevins RA (2018). A behavioral economic analysis of the value-enhancing effects of nicotine and varenicline and the role of nicotinic acetylcholine receptors in male and female rats. Behav Pharmacol 29:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ST, Odum AL (2011). The effects of repeated exposure on the reward-enhancing effects of nicotine: Behav Pharmacol 22:283–290. [DOI] [PubMed] [Google Scholar]
- Barrett ST, Thompson BM, Emory JR, Larsen CE, Pittenger ST, Harris EN, et al. (2020). Sex differences in the reward-enhancing effects of nicotine on ethanol reinforcement: A reinforcer demand analysis. Nicotine Tob Res Off J Soc Res Nicotine Tob 22:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL (2001). Gender differences in the behavioral responses to cocaine and amphetamine: Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci 937:172–187. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Quisenberry AJ, Snider SE (2016). Does impulsivity change rate dependently following stimulant administration? A translational selective review and re-analysis. Psychopharmacology (Berl) 233:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell CE, Balfour DJK (1998). The influence of nicotine pretreatment on mesoaccumbens dopamine overflow and locomotor responses to D-amphetamine. Psychopharmacology (Berl) 140:142–149. [DOI] [PubMed] [Google Scholar]
- Body S, Cheung THC, Hampson CL, Boon FS den, Bezzina G, Fone KCF, et al. (2009). Attenuation of the effects of d-amphetamine on interval timing behavior by central 5-hydroxytryptamine depletion. Psychopharmacology (Berl) 203:547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch MN (1984). Rate dependency, behavioral mechanisms, and behavioral pharmacology. J Exp Anal Behav 42:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF (2009). Chapter 6: The role of nicotine in smoking: A dual-reinforcement model. Neb Symp Motiv Neb Symp Motiv 55:91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. (2001). Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav 70:515–530. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. (2002). Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 163:230–237. [DOI] [PubMed] [Google Scholar]
- Camp DM, Robinson TE (1988a). Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic d-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behav Brain Res 30:55–68. [DOI] [PubMed] [Google Scholar]
- Camp DM, Robinson TE (1988b). Susceptibility to sensitization. II. The influence of gonadal hormones on enduring changes in brain monoamines and behavior produced by the repeated administration of d-amphetamine or restraint stress. Behav Brain Res 30:69–88. [DOI] [PubMed] [Google Scholar]
- Cassidy RN, Dallery J (2014). Quantifying nicotine’s value-enhancement effect using a behavioral economic approach. J Exp Anal Behav 102:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Falco AM, Fink K, Dwoskin LP, Bevins RA (2017). The effect of sazetidine-A and other nicotinic ligands on nicotine controlled goal-tracking in female and male rats. Neuropharmacology 113:354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Pittenger ST, Swalve N, Barrett ST, Bevins RA (2020). Conditioned enhancement of the nicotine reinforcer. Exp Clin Psychopharmacol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, et al. (2007). Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology (Berl) 190:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, et al. (2005). Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 180:258–266. [DOI] [PubMed] [Google Scholar]
- Clarkson JM, Dwyer DM, Flecknell PA, Leach MC, Rowe C (2018). Handling method alters the hedonic value of reward in laboratory mice. Sci Rep 8:2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Han B, Blanco C, Johnson K, Jones CM (2018). Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry 175:741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, Wit H de (2001). Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology (Berl) 157:243–253. [DOI] [PubMed] [Google Scholar]
- Covarrubias P, Aparicio CF (2008). Effects of reinforcer quality and step size on rats’ performance under progressive ratio schedules. Behav Processes 78:246–252. [DOI] [PubMed] [Google Scholar]
- Crummy EA, O’Neal TJ, Baskin BM, Ferguson SM (2020). One is not enough: Understanding and modeling polysubstance use. Front Neurosci 14:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels CW, Watterson E, Garcia R, Mazur GJ, Brackney RJ, Sanabria F (2015). Revisiting the effect of nicotine on interval timing. Behav Brain Res 283:238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, et al. (2003). Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 169:68–76. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Sved AF (2004). Multiple effects of nicotine on behavior: a reply to Frenk and Dar (2003). Psychopharmacology (Berl) 171:474–476. [Google Scholar]
- Easton N, Steward C, Marshall F, Fone K, Marsden C (2007). Effects of amphetamine isomers, methylphenidate and atomoxetine on synaptosomal and synaptic vesicle accumulation and release of dopamine and noradrenaline in vitro in the rat brain. Neuropharmacology 52:405–414. [DOI] [PubMed] [Google Scholar]
- Eubig PA, Noe TE, Floresco SB, Sable JJ, Schantz SL (2014). Sex differences in response to amphetamine in adult Long-Evans rats performing a delay-discounting task. Pharmacol Biochem Behav 118:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshler M, Hoffman HS (1962). A progression for generating variable-interval schedules. J Exp Anal Behav 5:529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehricke JG, Hong N, Wigal TL, Chan V, Doan A (2011). ADHD medication reduces cotinine levels and withdrawal in smokers with ADHD. Pharmacol Biochem Behav 98:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Rice O, Schiffer WK, Dewey SL (2000). Synergistic interactions between nicotine and cocaine or methylphenidate depend on the dose of dopamine transporter inhibitor. Synap 38:432–437. [DOI] [PubMed] [Google Scholar]
- Gonzalez FA, Goldberg SR (1977). Effects of cocaine and d-amphetamine on behavior maintained under various schedules of food presentation in squirrel monkeys. J Pharmacol Exp Ther 201:33–43. [PubMed] [Google Scholar]
- Goodwin AK, Hiranita T, Paule MG (2015). The reinforcing effects of nicotine in humans and nonhuman primates: A review of intravenous self-administration evidence and future directions for research. Nicotine Tob Res 17:1297–1310. Oxford Academic. [DOI] [PubMed] [Google Scholar]
- Grace RC, Bragason O (2005). Does sensitivity to magnitude depend on the temporal distribution of reinforcement? J Exp Anal Behav 83:169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Starosciak AK, Chwa A, Grunberg NE (2012). Nicotine behavioral sensitization in Lewis and Fischer male rats. Exp Clin Psychopharmacol 20:345–351. [DOI] [PubMed] [Google Scholar]
- Harzem P, Lowe CF, Priddle-Higson PJ (1978). Inhibiting function of reinforcement: Magnitude effects on variable-interval schedules. J Exp Anal Behav 30:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Gosden J, Nutt DJ (2013). Amphetamine, past and present – a pharmacological and clinical perspective. J Psychopharmacol (Oxf) 27:479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner TG, Drawbaugh RB, Zigmond MJ (1974). Amphetamine and operant behavior in rats: Relationship between drug effect and control response rate. J Comp Physiol Psychol 86:1031–1043. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Griffiths RR (1981). Cigarette smoking and subjective response: effects of d-amphetamine. Clin Pharmacol Ther 30:497–505. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG (2004). Cross-generational effects on gender differences in psychoactive drug abuse and dependence. Drug Alcohol Depend 74:147–158. [DOI] [PubMed] [Google Scholar]
- Hruska RE, Silbergeld EK (1980). Increased dopamine receptor sensitivity after estrogen treatment using the rat rotation model. Science 208:1466–1468. [DOI] [PubMed] [Google Scholar]
- Huynh YW, Raimondi A, Finkner A, Kuck JD, Selleck C, Bevins RA (2020a). Menthol blunts the interoceptive discriminative stimulus effects of nicotine in female but not male rats. Psychopharmacology (Berl) 237:2395–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Nicolazzo DM, Kim MN, Gnegy ME (2008). Nicotine and amphetamine acutely cross-potentiate their behavioral and neurochemical responses in female Holtzman rats. Psychopharmacology (Berl) 200:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Kandel DB (2014). A molecular basis for nicotine as a gateway drug. N Engl J Med 371:932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MN, Jutkiewicz EM, Zhang M, Gnegy ME (2011). The sensitizing effect of acute nicotine on amphetamine-stimulated behavior and dopamine efflux requires activation of β2 subunit-containing nicotinic acetylcholine receptors and glutamate N-methyl-D-aspartate receptors. Neuropharmacology 60:1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs CA, Reilly WJ, Anderson KG (2016). Reinforcer magnitude affects delay discounting and influences effects of d-amphetamine in rats. Behav Processes 130:39–45. [DOI] [PubMed] [Google Scholar]
- Lenth R (2020). emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.4.7. https://CRAN.R-project.org/package=emmeans
- Leslie JC, Toal L (1994). Varying reinforcement magnitude on interval schedules. Q J Exp Psychol Sect B 47:105–122. [Google Scholar]
- Lévesque D, Gagnon S, Di Paolo T (1989). Striatal D1 dopamine receptor density fluctuates during the rat estrous cycle. Neurosci Lett 98:345–350. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, McCormick CM (2007). Female and male rats in late adolescence differ from adults in amphetamine-induced locomotor activity, but not in conditioned place preference for amphetamine. Behav Pharmacol 18:641–650. [DOI] [PubMed] [Google Scholar]
- Mayo LM, Paul E, DeArcangelis J, Van Hedger K, Wit H de (2019). Gender differences in the behavioral and subjective effects of methamphetamine in healthy humans. Psychopharmacology (Berl) 236:2413–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DE (1969). Effects of d-amphetamine on performance under several parameters of multiple fixed-ratio, fixed-interval schedules. J Pharmacol Exp Ther 167:26–33. [PubMed] [Google Scholar]
- Meyer EM, Lytle LD (1978). Sex related differences in the physiological disposition of amphetamine and its metabolites in the rat. Proc West Pharmacol Soc 21:313–316. [PubMed] [Google Scholar]
- Milesi-Hallé A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM (2007). Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav 86:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CF, Armitage AK (1967). Effects of nicotine upon the free operant behavior of rats and spontaneous motor activity of mice. Ann N Y Acad Sci 142:268–276. [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, et al. (2006). Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 184:391–400. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Matteson GL, Donny EC, Caggiula AR, Sved AF (2007a). Conditioned reinforcement in rats established with self-administered nicotine and enhanced by noncontingent nicotine. Psychopharmacology (Berl) 195:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, et al. (2007b). The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend 89:52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Wilkinson JL, Metschke DM, Bevins RA (2005). Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a Pavlovian appetitive discrimination task in rats. Neuropsychopharmacology 30:731–741. [DOI] [PubMed] [Google Scholar]
- Peartree NA, Sanabria F, Thiel KJ, Weber SM, Cheung THC, Neisewander JL (2012). A new criterion for acquisition of nicotine self-administration in rats. Drug Alcohol Depend 124:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, Decambre N, Coleman-Hardee ML, Simpkins JW (1991). Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated striatal [3H]dopamine release. Brain Res 566:255–264. [DOI] [PubMed] [Google Scholar]
- Perkins KA (2009). Sex differences in nicotine reinforcement and reward: Influences on the persistence of tobacco smoking. In Caggiula AR, Bevins RA, editors. Motiv Impact Nicotine Its Role Tob Use. New York, NY: Springer (U.S.). [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL (2014). Sensory reinforcement-enhancing effects of nicotine via smoking. Exp Clin Psychopharmacol 22:511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Boldry MC (2017). Nicotine acutely enhances reinforcement from non-drug rewards in humans. Front Psychiatry 8:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Boldry MC (2019). Reinforcement enhancing effects of nicotine via patch and nasal spray. Nicotine Tob Res Off J Soc Res Nicotine Tob 21:778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Michael VC (2015). Reinforcement enhancing effects of acute nicotine via electronic cigarettes. Drug Alcohol Depend 153:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- Raiff BR, Dallery J (2008). The generality of nicotine as a reinforcer enhancer in rats: effects on responding maintained by primary and conditioned reinforcers and resistance to extinction. Psychopharmacology (Berl) 201:305–314. [DOI] [PubMed] [Google Scholar]
- Reed P (1991). Multiple determinants of the effects of reinforcement magnitude on free-operant response rates. J Exp Anal Behav 55:109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P, Wright JE (1988). Effects of magnitude of food reinforcement on free-operant response rates. J Exp Anal Behav 49:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves G, Schweitzer J (2005). Pharmacological management of attention-deficit hyperactivity disorder. Expert Opin Pharmacother 5:1313–1320. [DOI] [PubMed] [Google Scholar]
- Robinson TE (1984). Behavioral sensitization: Characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacology (Berl) 84:466–475. [DOI] [PubMed] [Google Scholar]
- Rungnirundorn T, Verachai V, Gelernter J, Malison RT, Kalayasiri R (2017). Sex dfferences in methamphetamine use and dependence in a Thai treatment center. J Addict Med 11:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht LE, Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC (2015). Behavioral mechanisms underlying nicotine reinforcement. Curr Top Behav Neurosci 24:19–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi M, Moffett AM, Williams BF, Frantz KJ (2008). Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology (Berl) 196:71–81. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Tidey JW, Badger GJ, Higgins ST (2003). Acute effects of D-amphetamine on progressive-ratio performance maintained by cigarette smoking and money. Psychopharmacology (Berl) 167:393–402. [DOI] [PubMed] [Google Scholar]
- Silveira ML, Conway KP, Green VR, Kasza KA, Sargent JD, Borek N, et al. (2018). Longitudinal associations between youth tobacco and substance use in waves 1 and 2 of the Population Assessment of Tobacco and Health (PATH) Study. Drug Alcohol Depend 191:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singmann H, Bolker B, Westfall J, Aust F, Ben-Shachar MS, Højsgaard S, et al. (2020). afex: Analysis of Factorial Experiments [Internet]. Available from: https://CRAN.R-project.org/package=afex.
- Slezak JM, Mueller M, Ricaurte GA, Katz JL (2018). Pharmacokinetic and pharmacodynamic analysis of d-amphetamine in an attention task in rodents: Behav Pharmacol 29:551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalve N, Barrett ST, Bevins RA, Li M (2015). Examining the reinforcement-enhancement effects of phencyclidine and its interactions with nicotine on lever-pressing for a visual stimulus. Behav Brain Res 291:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatham TA, Wanchisen BA (1998). Behavioral history: A definition and some common findings from two areas of research. Behav Anal 21:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KM, Horvitz JC, Balsam PD (2007). Amphetamine affects the start of responding in the peak interval timing task. Behav Processes 74:168–175. [DOI] [PubMed] [Google Scholar]
- Tidey JW, O’Neill SC, Higgins ST (2000). d-Amphetamine increases choice of cigarette smoking over monetary reinforcement. Psychopharmacology (Berl) 153:85–92. [DOI] [PubMed] [Google Scholar]
- Turner KM, Peak J, Burne THJ (2017). Baseline-dependent effects of amphetamine on attention are associated with striatal dopamine metabolism. Sci Rep 7:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2014). The Health Consequences of Smoking -- 50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention (U.S.). [Google Scholar]
- U.S. Department of Health and Human Services (2020). Smoking Cessation: A Report of the Surgeon General. Washington D.C.: U.S. Department of Health and Human Services. [Google Scholar]
- Van Amsterdam J, Van Der Velde B, Schulte M, Van Den Brink W (2018). Causal factors of increased smoking in ADHD: A systematic review. Subst Use Misuse 53:432–445. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Rush CR (2010). Human sex differences in d-amphetamine self-administration. Addict Abingdon Engl 105:727–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Johnson ML, Van Swearingen AED, Arrant AE, Caster JM, Kuhn CM (2012). Individual differences in psychostimulant responses of female rats are associated with ovarian hormones and dopamine neuroanatomy. Neuropharmacology 62:2267–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Min JE, Krebs E, Evans E, Huang D, Liu L, et al. (2017). Polydrug use and its association with drug treatment outcomes among primary heroin, methamphetamine, and cocaine users. Int J Drug Policy 49:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Sofuoglu M (2009). The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse 35:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TL, Justice AJH, Wit H de (2002). Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav 73:729–741. [DOI] [PubMed] [Google Scholar]
- Winterbauer NE, Balleine BW (2007). The influence of amphetamine on sensory and conditioned reinforcement: Evidence for the re-selection hypothesis of dopamine function. Front Integr Neurosci [Internet] 1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JM, Ren S, Constantin A, Clarke PBS (2018). Enhancement of a visual reinforcer by d-amphetamine and nicotine in adult rats: relation to habituation and food restriction. Psychopharmacology (Berl) 235:803–814. [DOI] [PubMed] [Google Scholar]
- Zovkic IB, McCormick CM (2019). A rapid enhancement of locomotor sensitization to amphetamine by estradiol in female rats. Physiol Behav 203:51–59. [DOI] [PubMed] [Google Scholar]
- Zuriff GE (1970). A comparison of variable-ratio and variable-interval schedules of reinforcement. J Exp Anal Behav 13:369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]


