Abstract
Purpose:
To generate diagnostic 18F-FDG PET images of pediatric cancer patients from ultra-low dose 18F-FDG PET input images, using a novel artificial intelligence (AI) algorithm.
Methods:
We used whole body 18F-FDG-PET/MRI scans of 33 children and young adults with lymphoma (3–30 years) to developed a convolutional neural network (CNN), which combines inputs from simulated 6.25% ultra-low-dose 18F-FDG PET scans and simultaneously acquired MRI scans to produce a standard dose 18F-FDG PET scan. The image quality of ultra-low-dose PET scans, AI-augmented PET scans and clinical standard PET scans was evaluated by traditional metrics in computer vision, and by expert radiologists and nuclear medicine physicians, using Wilcoxon signed rank tests and weighted kappa statistics.
Results:
The peak signal-to-noise ratio and structural similarity index were significantly higher, and the normalized root-mean-square error significantly lower on the AI-reconstructed PET images compared to simulated 6.25% dose images (p<0.001). Compared to the ground-truth standard-dose PET, SUVmax values of tumors and reference tissues were significantly higher on the simulated 6.25% ultra-low-dose PET scans as a result of image noise. After the CNN-augmentation, the SUVmax values were recovered to values similar to the standard-dose PET. Quantitative measures of the readers’ diagnostic confidence demonstrated significantly higher agreement between standard clinical scans and AI-reconstructed PET scans (kappa = 0.942) than 6.25% dose scans (kappa = 0.650).
Conclusions:
Our CNN model could generate simulated clinical standard 18F-FDG PET images from ultra-low dose inputs, while maintaining clinically relevant information in terms of diagnostic accuracy and quantitative SUV measurements.
Keywords: Pediatric cancer imaging, PET/MRI, whole-body PET reconstruction, PET denoising, deep-learning
Introduction
In many patients with cancer, the metabolic information from 18F-FDG PET/CT scans is required to provide accurate tumor diagnoses and to monitor response to treatment (1–3). However, diagnostic 18F-FDG PET/CT scans involve considerable radiation exposure (4, 5). Several groups independently reported that the radiation exposure from diagnostic CT scans is associated with an increased risk of developing secondary cancers later in life (6–8). This is particularly concerning for children, as they are more sensitive to radiation effects than adults (9). For example, a patient with lymphoma who undergoes five PET/CT scans will be exposed to 10–15 mSv of ionizing radiation per scan and 50–75 mSv total (10). Direct evidence from human population studies showed that doses of 50–100 mSv (protracted exposure) or 10–50 mSv (acute exposure) increase the risk of developing secondary cancers later in life (9). While advances in cancer therapy have increased the number of pediatric cancer survivors, these patients now live long enough to encounter secondary cancers (9, 11–13). Therefore, the image gently campaign advocates for practitioners to provide the least possible radiation exposure when examining pediatric patients (14). Integrated 18F-FDG PET/MRI saves radiation by replacing CT with radiation free MRI scans (1). This addresses the radiation exposure from CT scans. A clinical standard 18F-FDG PET/CT scan is associated with about 6–7 mSv of radiation exposure for a clinical CT scan and 6–7 mSv for the 18F-FDG PET (4, 15). While many studies have focused on replacing CT with MRI for anatomical co-registration of 18F-FDG data, the reduction of the injected radiotracer dose has received less attention thus far. A major bottleneck to reducing radiotracer doses for 18F-FDG PET scans is increased image noise (16) and resultant decreased diagnostic accuracy of ultra-low dose scans (17). We hypothesized that this problem could be solved by training a deep convolutional neural network (CNN) to integrate information from ultra-low-dose PET images with anatomical information from simultaenously acquired MRI images to generate simulated standard-dose PET images.
The main innovation of our work is fourfold: (1). We are the first to explore ultra-low-dose 18F-FDG PET imaging of the whole-body using CNN-based image data augmentation. (2). This is the first study that utilizes information from simultaneously acquired MRI scans to reconstruct ultra-low-dose whole-body 18F-FDG PET scans. The inclusion of MRI data into the CNN improves the depiction of anatomical detail that could be missed if only the low-dose 18F-FDG PET was used as input. (3). We incorporate an attention-weighted loss function to enhance sensitivity of our model to reconstruct the significant regions where lesions occur; this approach contributes more to the loss function during training in regions where lesions occur, such as perivascular areas where lymph nodes are common. Making the model pay more attention to the anatomical regions with high frequency of pathology could protect the loss and computed gradients from overwhelming by relatively irrelevant pixels in whole-body scans. In this manner, less training data are required, which is critical in the domain of pediatric cancer imaging where imaging data are relatively sparce. (4) We conducted a task-specific region-based clinical evaluation. The reconstructed PET images were not only evaluated by traditional metrics in computer vision, but also assessed by expert radiologists and nuclear medicine physicians in terms of the overall image quality, diagnostic accuracy and diagnostic confidence. To date, no comprehensive region-based clinical evaluation was conducted in such whole-body PET image enhancement studies. Thus, the purpose of our study was to generate diagnostic 18F-FDG PET images of pediatric cancer patients from ultra-low dose 18F-FDG PET input images, using a novel CNN algorithm.
Materials and Methods
Patients and image acquisition
This Health Insurance Portability and Accountability Act-compliant clinical study was approved by our respective institutional review boards and was performed as a secondary analysis of prospectively acquired data. Written informed consent was obtained from all adult patients and all parents of pediatric patients. In addition, children were asked to give their assent. Between July 2015 and June 2019, we enrolled 33 children and young adults (14 female, 9 male) with lymphoma at two centers (University of Tübingen, Germany and Stanford University, CA, USA). 23 patients enrolled at Stanford had a mean age of 17 ± 7years (range: 6–30 years) and 11 patients enrolled at Tübingen had a mean age of 14 ± 5 years (range: 3–18 years). The patients at Stanford underwent a whole body integrated 18F-FDG PET/MRI scan on a 3T Signa PET/MRI scanner (GE Healthcare, Milwaukee, WI, USA) at 1 hour after intravenous injection of 18F-FDG at a dose of 3 MBq/kg, using a 32-channel torso phased array coil and an eight-channel, receive-only head coil. PET data were acquired simultaneously with contrast-enhanced T1-weighted gradient echo scans, using a 25 cm transaxial FOV and 3:30 minute acquisitions per PET bed. Tübingen patients underwent a whole body integrated 18F-FDG PET/MRI scan on a 3T Signa PET/MRI scanner (Siemens Healthineers, Erlangen, Germany), using a 16-channel torso phased array coil and a 16-channel head coil. PET data were acquired simultaneously with contrast-enhanced T1-weighted gradient echo MRI scans, using a 25 cm transaxial FOV and 4 minute acquisitions per PET bed. Radiotracer input data were used to generate 100% dose 18F-FDG PET images. 6.25% (0.18 mBq/kg) low-dose 18F-FDG PET images were simulated by unlisting the PET list-mode data and reconstructing them based on the percentage of used counts (28).
CNN architecture
We trained and cross-validated a CNN reconstruction model to augment whole body 18F-FDG PET/MRI scans of 23 subjects with lymphoma. The inputs for the model are axial simulated ultra-low-dose 18F-FDG PET images and simultaneously acquired axial contrast-enhanced T1-weighted MRI image. The outputs are AI-reconstructed 18F-FDG PET images, which should resemble a standard-dose 18F-FDG PET scan (Figure 1).
FIGURE 1. The pipeline of the CNN reconstruction net.
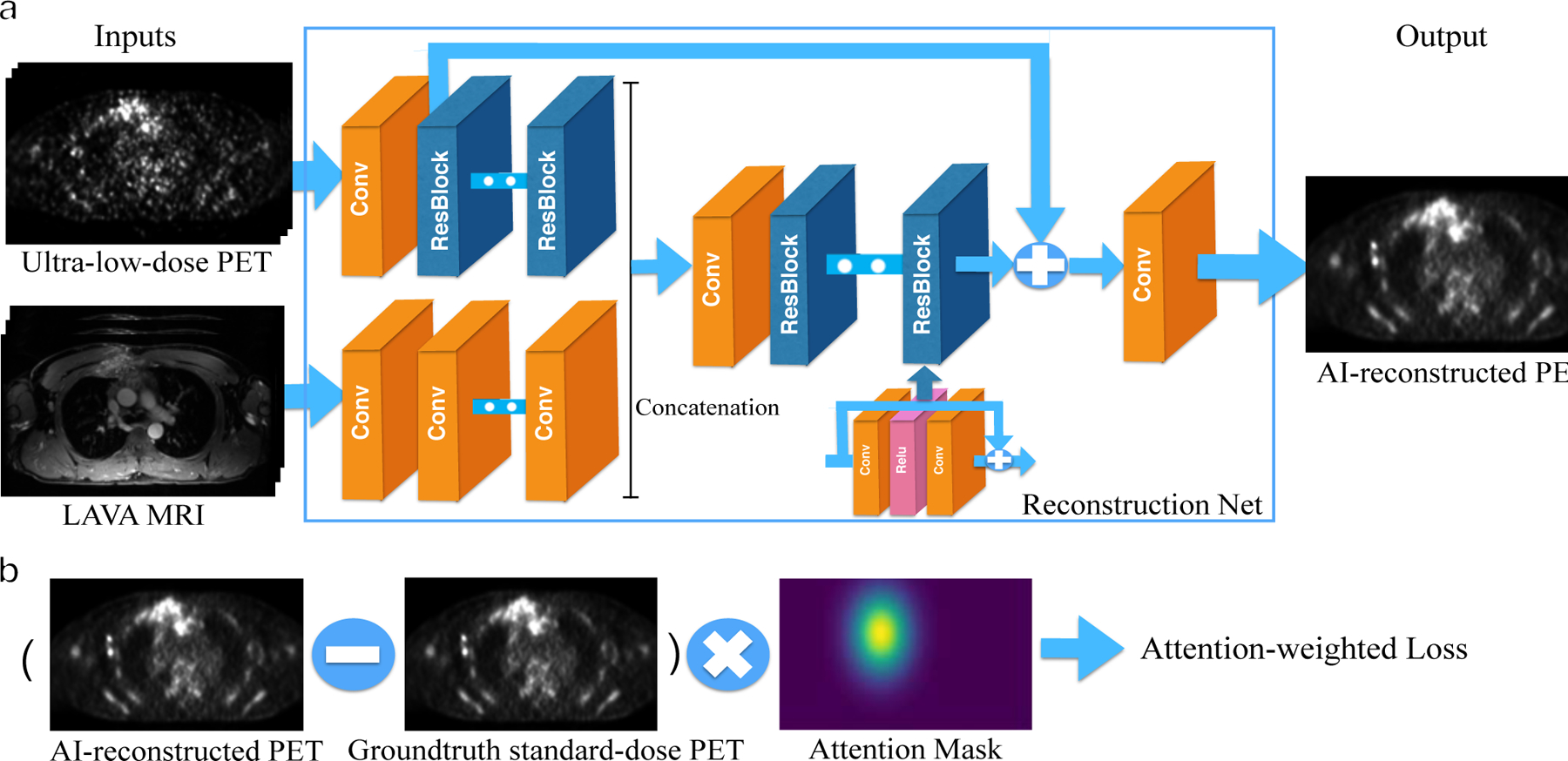
a). The evaluation framework of the PET reconstruction CNN. It inputs the simulated ultra-low-dose 18F-FDG PET and contrast-enhanced T1-weighted MRI images and outputs the synthetized standard-dose 18F-FDG PET images. The PET and MRI images are integrated at the mid-level after feature extraction; A skip-connection is added to the CNN in order to connect the ultra-low-dose 18F-FDG PET images with the final reconstruction. b). The calculation process of the attention-weighted loss. The attention mask highlights the tumor area.
We designed the reconstruction network based on an enhanced deep super-resolution network (EDSR) (29) - the state-of-the-art image reconstruction network. However, our model is significantly different from EDSR and particularly tailored for the whole-body 18F-FDG PET reconstruction. Our network is different in four key ways:
We utilized information from simultaneously acquired MRI scans. We hypothesized that using only a single simulated ultra-low-dose 18F-FDG PET image as input may not provide enough information to reconstruct detailed anatomical structures and may generate hallucination image artifacts. The information from simultaneously acquired MRI scans can be used to provide anatomical information.
We applied middle fusion to integrate MRI and 18F-FDG PET images. Rather than concatenating the MRI and 18F-FDG PET at the input level, we combine them in the feature-space. We assume that early fusion might lose information as the characteristics of 18F-FDG PET and MRI modalities are quite different. The benefit of mid-fusion as opposed to early fusion was observed in the initial experiment and relevant study (30). The two modalities are integrated after the fourth main residual block. Note that the PET branch is built upon a residual block, while the MRI branch is built upon pure convolutional layers.
A skip-connection between the ultra-low-dose 18F-FDG PET input and the final prediction layer is added to alleviate the burden of carrying identity information in the reconstruction network.
The reconstruction network is a slice-wise model which considers multi-slice inputs. The input consists of 5-slice LAVA MRI and 5-slice ultra-low-dose 18F-FDG PET images, and the output is a synthetic standard-dose 18F-FDG PET slice. Such input scheme provides the network with 2.5D information, reduces image noise and ensures vertical spatial consistency. The proposed network consists of 44 convolution layers in total.
Attention-weighted Loss
Weighted loss function was initially proposed to tackle the common issue of imbalanced data in background/foreground classification. By weighing underrepresented categories, a weighted loss function compensates the bias of training loss for the minority categories (31). In our work, we designed the attention-weighted loss by augmenting the loss function with a weight value corresponding to the significant regions of whole-body scans, specifically visceral organs and lymph node regions that are common areas where tumors occur. While all 18F-FDG PET data are augmented by our algorithm, the CNN pays particular attention to these areas. This encoded prior knowledge in spatial anatomy could enable the network to converge quickly, simplify the training, and improve quality of enhancing image quality of images of cancer lesions. Figure 1(b) shows the calculation of the attention-weighted loss function in the training phase.
The Generation of Attention Mask
For the training dataset, we used ITK-Snap (32) to obtain the attention masks which highlight the high-clinical-value regions. Potential tumor areas along main vessels, the mediastinum, liver and spleen were segmented as attention areas with the MRI image as the reference. Then, the rigid attention mask was transformed to a soft attention mask via Gaussian distribution. The final attention mask to the loss function is a Gaussian heatmap produced by four variables: the center and standard deviations of the Gaussian distribution of the rough target region mask of the scan, as shown in Supplementary Figure 7.
Training Details
We trained our model using Stanford baseline 18F-FDG-PET/MR scans of 23 children and young adults with malignant lymphoma. The large data requirement for training CNN is a limitation for pediatric applications, as there are not many of these studies. Proof-of-concept studies of CNN for pediatric oncology applications are hampered by sparse data (33). To overcome the challenge, we adopted leave-one-out cross validation. The dataset was divided into 23 folds. During training 22 of the folds were used as training set whereas the remaining one-fold was used for testing. We iterated 23 times to go through all combinations and produce the final AI-reconstructed PET images.
For the comparison study which should show the superiority of adding the MRI input information and attention-weighted loss, we trained the comparison models on 14 subjects, validated on 3 subjects and then tested on the remaining 6 subjects (Supplementary Data).
Computational Assessment
For the evaluation part, we used three computational metrics in computer vision to evaluate the performance of our networks, including peak signal-to-noise ratio (PSNR), the structural similarity index (SSIM) and the normalized root-mean-square error (NRMSE) (Supplementary Data).
Clinical Assessment
To understand the impact of our CNN on tumor detection, three clinical imaging experts (one radiologist, one nuclear medicine physician and one dual trained radiologist/nuclear medicine physician) determined the presence or absence of tumor lesions in 20 anatomical regions (Supplementary Table 1) per patient on the ultra-low-dose 18F-FDG PET scan, the AI-augmented ultra-low-dose 18F-FDG PET scan and the standard 100% dose 18F-FDG PET scan (in total 20 regions x 23 patients = 460 anatomical regions analyzed by each reviewer). The reviewers were blinded to clinical data and the type of the exam and analyzed the three different scan types in a random order and with an interval of at least 2 weeks to minimize recall bias. The clinical experts rated the visibility of lesions in these regions according to a Likert scale (1 - tumor definitely not present, 2 - tumor probably not present, 3 - undecided, 4 - tumor probably present, 5 - tumor definitely present). The combination of all clinical imaging tests and biopsies on all available imaging studies were used to generate a standard of reference for these evaluations. The lesion diagnostic metrics on the ultra-low-dose 18F-FDG PET scan, the AI-augmented ultra-low-dose 18F-FDG PET scan and the standard 100% dose 18F-FDG PET scan were compared with the standard of reference using confusionMatrix() function from R package caret.
Measurements of SUV values from tumors in the PET images are important for tumor detection and for quantitative monitoring of tumor therapy response. Reducing the 18F-FDG radiotracer dose can lead to increased image noise and affect these measurements. To evaluate, whether flawed SUV measurements could be recovered by our CNN, one nuclear medicine physician measured the SUVmax and SUVmean (standardized uptake value) of the lesion with the highest SUV in the 20 refined regions per patient as well as the SUVmean, SUVstd , SUVmax of the liver (3cm ROI) and mediastinal blood pool (2cm ROI) across the simulated ultra-low-dose 18F-FDG PET scan, the AI-augmented ultra-low-dose 18F-FDG PET scan and the standard 100% dose 18F-FDG PET scan. The measurement was obtained using MIM 6.5 (MIM Software, Inc., Cleveland, OH, USA) as “SUVmax = (tissue tracer activity (mCi/g))/((injected dose (mCi)*patient body weight (kg))). All metrics were compared across ultra-low-dose 18F-FDG PET scan, the AI-augmented ultra-low-dose 18F-FDG PET scan and the standard 100% dose 18F-FDG PET images using a Wilcoxon signed rank test.
Statistical Analysis
To evaluate the ability of the different scan types to provide clinically relevant information, we compared imaging experts’ scan assessments with the ground truth of whether lesions were present in each scan/region or not. All experts’ lesion classification results were grouped by scan types and the classification accuracy of each scan type was calculated as the positive predictive value (PPV), negative predictive value (NPV), and the balanced accuracy (average of sensitivity and specificity). All PPV, NPV, and balanced accuracy estimates were calculated using the confusionMatrix() function from R package caret. To evaluate the degree of agreement in Likert scale assessments between the 100% standard-dose PET scan and the other 2 scan types, we calculated weighted kappa statistics (using both linear weights and quadratic weights). All kappa estimates were generated using the kappa2() function from R package irr. The Wilcoxon signed rank test was used to compare all image quality metrics and SUV values that are paired between scan types.
Results
CNN reduces image noise of simulated ultra-low-dose PET scans
Figure 2 and Table 1 show the qualitative and quantitative results of the reconstruction model. A major problem of reducing radiotracer doses for 18F-FDG PET scans was an increased image noise (16). The CNN-augmented ultra-low dose 18F-FDG PET images demonstrated significantly less noise and better image quality compared to simulated 6.25% dose 18F-FDG PET images, measured by PSNR, SSIM and NRMSE. PSNR and SSIM were significantly higher, and NRMSE was significantly lower on the CNN-augmented ultra-low dose 18F-FDG PET as opposed to simulated 6.25% dose 18F-FDG PET images (all pair-wise t tests p < 0.001; Table 1). In addition, the standard deviation of the mean standardized uptake value (SUV) measurements of the liver and mediastinal blood pool were significantly lower on the CNN-augmented ultra-low dose 18F-FDG PET images compared to simulated 6.25% dose 18F-FDG PET images (Table 3 and Supplementary Figure 4). These qualitative and quantitative results show that the proposed CNN model reduces image noise of the ultra-low-dose 18F-FDG PET scans and reaches an overall image quality on CNN-augmented 18F-FDG PET images, which are similar to 100% dose 18F-FDG PET scans.
FIGURE 2. Representative 18F-FDG PET/MRI scan of a 16-year old female patient with Hodgkin lymphoma (HL).
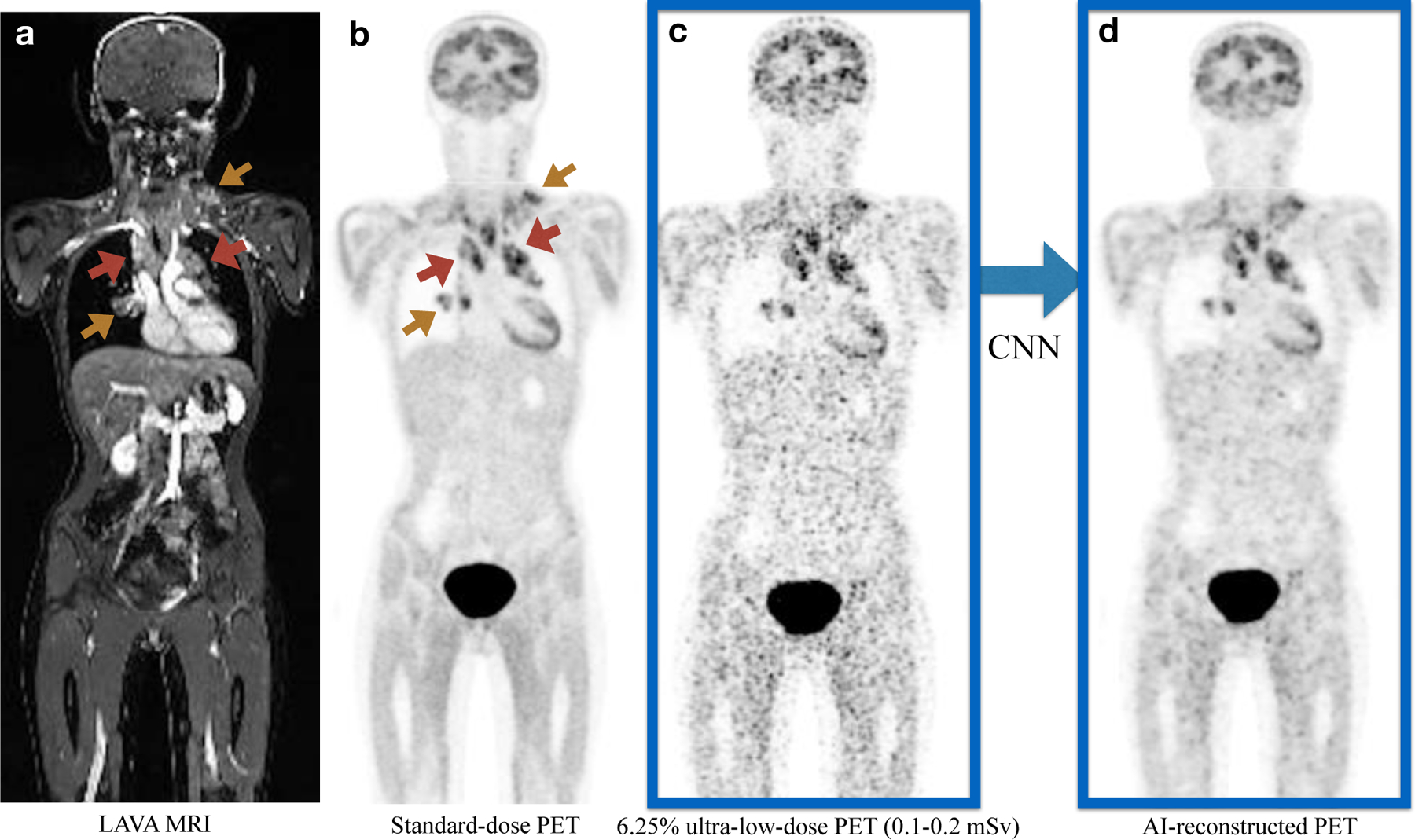
a). Coronal contrast-enhanced T1-weighted LAVA (liver acquisition and volume acquisition) MRI; b). Coronal view of a standard dose 18F-FDG dose PET scan (3 mBq/kg); c). Simulated ultra-low-dose PET scan at 6.25% 18F-FDG dose; d). The AI-reconstructed ultra-low-dose 18F-FDG PET image, reconstructed based on the 6.25% ultra-low dose PET and MRI scans as combined inputs. The red arrows point to the hypermetabolic tumors in the mediastinum. Additional hypermetabolic tumors are noted at the right hilum and left lower neck (yellow arrows). All lesions can be detected on all scans, but tumor-to-background contrast and confidence for lesion detection is improved on the AI-reconstructed 18F-FDG PET.
TABLE 1. Image quality metrics compare for simulated 6.25% ultra-low-dose18F-FDG PET and AI-reconstructed18F-FDG PET scans.
The AI-reconstructed 18F-FDG PET scan demonstrates improved image quality and significantly less noise for all three metrics compared to the ultra-low dose 18F-FDG PET scan: higher peak signal-to-noise ratio (PSNR), higher structural similarity index (SSIM) and lower normalized root-mean-square error (NRMSE), n=23 scans per group, Wilcoxon signed rank tests.
| 6.25% ultra-low-dose PET (N=23) |
Al-reconstructed PET (N=23) |
P-value* | |
|---|---|---|---|
| PSNR | |||
| Mean (SD) | 51.6 (8.50) | 55.6 (7.62) | <0.001 |
| Median [Q1, Q3] | 53.6 [43.9, 59.3] | 58.1 [48.8, 62.3] | |
| SSIM | |||
| Mean (SD) | 0.925 (0.0449) | 0.967 (0.0175) | <0.001 |
| Median [Q1, Q3] | 0.929 [0.916, 0.957] | 0.972 [0.961, 0.978] | |
| NRMSE | |||
| Mean (SD) | 0.257 (0.102) | 0.158 (0.0453) | <0.001 |
| Median [Q1, Q3] | 0.228 [0.182, 0.305] | 0.156 [0.127, 0.191] |
P-values were calculated using Wilcoxon Signed Rank Test.
TABLE 3. Standardized uptake values (SUV), as measured on 100% standard-dose18F-FDG PET, simulated 6.25% ultra-low-dose18F-FDG PET and AI-reconstructed18F-FDG PET scans.
Data represent mean and median SUVmax, SUVmean and SUVstd values of representative tumors, the liver and medastinal blood pool.
| 100% standard-dose PET | 6.25% ultra-low-dose PET | A1-reconstructed PET | |
|---|---|---|---|
| Liver | |||
| SUV_max | |||
| Mean (SD) | 2.65 (1.12) | 5.87 (2.44) | 2.92 (1.06) |
| Median [Q1, Q3] | 2.47 [2.19, 2.69] | 5.49 [4.79, 6.23] | 2.71 [2.46, 2.97] |
| SUV_mean | |||
| Mean (SD) | 1.89 (0.817) | 1.98 (0.866) | 1.90 (0.852) |
| Median [Q1, Q3] | 1.80 [1.54, 1.94] | 1.78 [1.56, 1.96] | 1.73 [1.48, 1.91] |
| SUV_std | |||
| Mean (SD) | 0.216 (0.0826) | 0.774 (0.277) | 0.270 (0.0894) |
| Median [Q1, Q3] | 0.200 [0.170, 0.240] | 0.710 [0.670, 0.845] | 0.250 [0.225, 0.300] |
| Mediastinal Blood Pool | |||
| SUV_max | |||
| Mean (SD) | 2.44 (1.13) | 5.11 (2.55) | 2.60 (121) |
| Median [Q1, Q3] | 2.22 [1.68, 2.68] | 4.22 [3.29, 6.40] | 2.44 [1.77, 2.97] |
| SUV_mean | |||
| Mean (SD) | 1.53 (0.863) | 1.61 (0.809) | 1.59 (0.794) |
| Median [Q1, Q3] | 1.25 [1.02, 1.67] | 1.38 [1.12, 1.82] | 1.47 [1.08, 1.70] |
| SUV_std | |||
| Mean (SD) | 0.237 (0.0951) | 0.690 (0.316) | 0.262 (0.119) |
| Median [Q1, Q3] | 0.220 [0.175, 0.275] | 0.580 [0.445, 0.905] | 0.240 [0.180, 0.330] |
| Tumor | |||
| SUV_max | |||
| Mean (SD) | 11.9 (6.44) | 15.0 (7.19) | 10.6 (5.95) |
| Median [Q1, Q3] | 11.6 [7.04, 15.4] | 14.2 [9.92, 18.8] | 9.77 [6.29, 13.8] |
| SUV_mean | |||
| Mean (SD) | 3.87 (2.33) | 3.79 (2.23) | 3.63 (2.24) |
| Median [Q1, Q3] | 3.17 [2.32, 5.39] | 3.24 [2.37, 4.94] | 2.90 [2.23, 4.96] |
Combining MRI and PET improves reconstruction quality
Supplementary Figure 1 shows the qualitative and quantitative performance of our CNN with and without additional MRI inputs. The CNN that predicts the standard-dose 18F-FDG PET with the least error is the CNN trained on both contrast-enhanced MRI and simulated ultra-low-dose 18F-FDG PET images, which demonstrates the benefit of including the MRI modality. The simultaneous 18F-FDG PET and MRI acquisition mode facilitates the integration of input data from the two modalities. The MRI images provided complementary anatomical information for depicting detailed high-resolution features that could be missed if only the simulated ultra-low-dose 18F-FDG PET images were used as input.
Attention-weighted loss boosts performance
Supplementary Figure 2 shows the performance comparison of two models - one with the attention-weighted loss function and one with a traditional mean-square-error (MSE) loss function. The attention-weighted loss function demonstrated a tendency to boost the performance of the CNN.
AI-reconstructed PET scans enable accurate tumor detection
Figure 3, Supplementary Figure 3, and Table 2 show the qualitative and quantitative results in terms of image diagnostic quality. Since pediatric tumors are highly metabolically active, there were only small differences in the clinical experts’ ability to detect tumor lesions on the different imaging scans: The clinical experts detected 225 of 249 tumors on the 100% dose 18F-FDG PET scan, 221 tumors on the simulated ultra-low-dose 18F-FDG PET scan and 223 tumors on the AI-augmented ultra-low-dose 18F-FDG PET scans. Most lesions were noted on all scans. Sensitivities, specificities and diagnostic accuracies were not significantly different for the three imaging modalities (Supplemental Table 2).
FIGURE 3. Illustration of image quality improvement in terms of tumor delineation on the AI-reconstructed 18F-FDG PET scan.
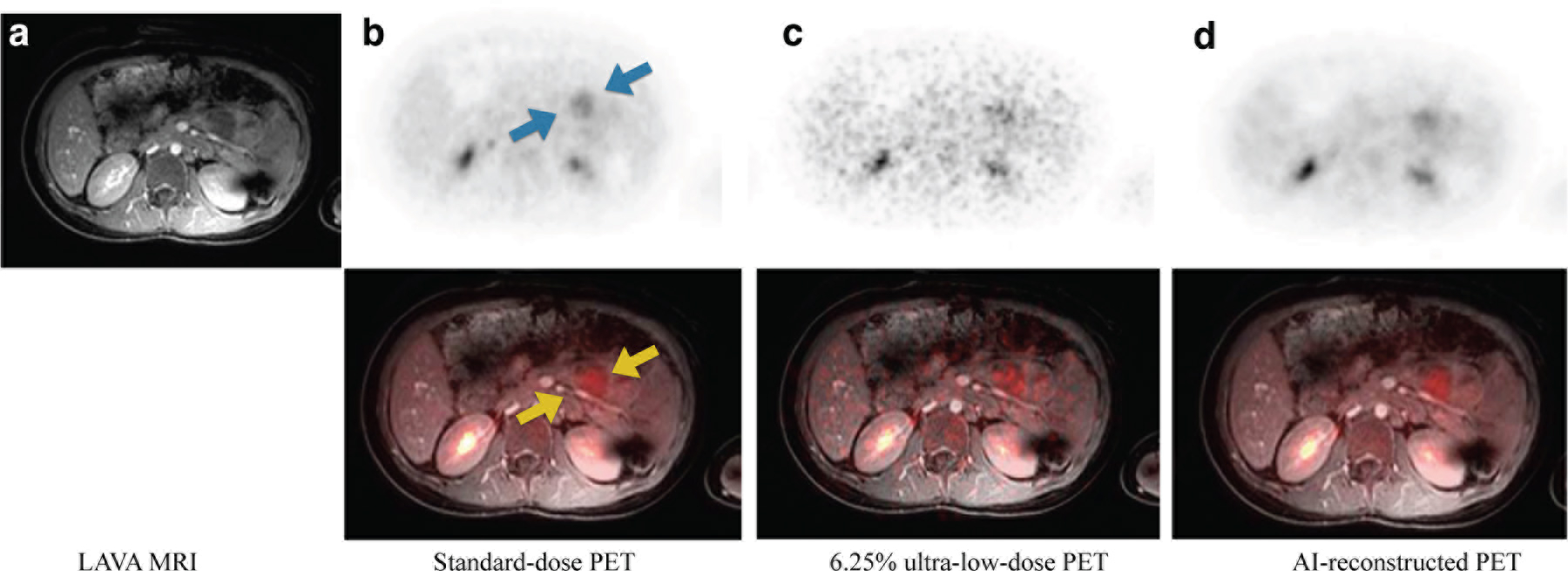
Representative 18F-FDG PET/MRI scan of a 10-year old male patient with diffuse large B-cell lymphoma (DLBCL). a). Axial T1-weighted contrast-enhanced LAVA MRI; b). Axial standard-dose 18F-FDG PET scan (upper panel), fused with T1-weighted MRI (lower panel); c). Axial simulated 6.25% ultra-low-dose 18F-FDG PET scan, fused with with T1-weighted MRI; d). Axial AI-reconstructed 18F-FDG PET image, fused with T1-weighted MRI. The arrows point to a tumor in the pancreas. The tumor can be well depicted in the original 100%-dose 18F-FDG PET scan and the AI-reconstructed 18F-FDG PET, but is nearly invisible on the 6.25% dose 18F-FDG PET scan.
TABLE 2. Agreement between expert reviewer tumor diagnoses on 100% standard-dose18F-FDG PET and simulated 6.25% ultra-low-dose18F-FDG PET and AI-reconstructed18F-FDG PET scan.
Three expert reviewers determined the presence of absence of tumor lesions in 20 anatomical regions per patient according to a Likert scale (1 - tumor definitely not present, 2 - tumor probably not present, 3 - undecided, 4 - tumor probably present, 5 - tumor definitely present). The agreement between 100% standard-dose PET images and 6.25% ultra-low-dose 18F-FDG PET and AI-reconstructed 18F-FDG PET scans was calculated with weighted Kappa estimates.
| Procedure | Weighted Kappa (Linear) |
Whighted Kappa (Quadratic) |
|---|---|---|
| Lymp Nodes | ||
| 625% ultra-low-dose | 0.702 | 0.859 |
| Al-reconstructed PET | 0.955 | 0.984 |
| Extralymphatic | ||
| 6.25% ultra-low-dose | 0.573 | 0.765 |
| AI-reconstructed PET | 0.910 | 0.965 |
| Bone Marrow | ||
| 6.25% ultra-low-dose | 0278 | 0.444 |
| AI-reconstructed PET | 0.828 | 0.916 |
| Whole Body | ||
| 6.25% ultra-low-dose | 0.650 | 0.820 |
| AI-reconstructed PET | 0942 | 0.977 |
However, the confidence of the readers regarding the presence or absence of tumor lesions in specific anatomical regions, as measured by a Likert scale, were significantly different between the different scans. The readers demonstrated a significantly lower confidence in the detection of lesions on 6.25% dose scans as opposed to on the AI-augmented ultra-low-dose 18F-FDG PET scans and the 100% dose 18F-FDG PET scans. The 100% dose 18F-FDG PET scans demonstrated significantly higher agreement with the AI-augmented ultra-low-dose 18F-FDG PET scans (kappa = 0.942) than the 6.25% dose 18F-FDG PET scans (kappa = 0.650). This also applied when considering tumors in different anatomical regions: The 100% dose 18F-FDG PET scans demonstrated significantly higher agreement with the AI-augmented ultra-low-dose 18F-FDG PET scans for tumor detection in lymph nodes (kappa = 0.955), visceral organs (kappa = 0.910) and the bone marrow (kappa = 0.828) compared to corresponding results of the simulated ultra-low-dose 18F-FDG PET scan for tumor detection in lymph nodes (kappa = 0.702), visceral organs (kappa = 0.573) and bone marrow (kappa = 0.278).
AI-reconstructed PET provides accurate quantitative tumor SUV measurements
Compared to the 100% dose 18F-FDG PET scans, SUVmax values of tumors, liver and mediastinal blood pool were significantly higher on the simulated 6.25% ultra-low-dose PET scans as a result of added image noise. On AI-augmented ultra-low-dose 18F-FDG PET scans, the SUVmax values were recovered to values which were similar to the standard-dose PET (Table 3 and Supplementary Figure 4).
Tumor SUV values are often compared to SUVmean values of liver and mediastinal blood pool as an internal standard of reference. Compared to 100% dose 18F-FDG PET scans, liver SUVmean and mediastinal blood pool SUVmean values were higher on 6.25% dose 18F-FDG PET scans (p=0.028 and 0.036, respectively), but were not significantly different on the AI-augmented ultra-low-dose 18F-FDG PET scans (p=0.523 and 0.316, respectively).
The CNN model generalizes in independent data
Next, we evaluate the model’s generalization by examining how the model performs when it augments reduced dose PET images from PET cases at another institution (Tübingen). We applied our CNN to PET scans of 11 additional subjects. The qualitative and quantitative reconstruction results on the 11 Tubingen scans are shown in Supplementary Table 3 and Supplementary Figure 5. The image quality is significantly improved on AI-augmented ultra-low-dose 18F-FDG PET scans as opposed to the original simulated reduced dose PET images, by 3.7 dB in PSNR, 2.8% in SSIM and 12.6% in NRMSE, which demonstrates good model generalization across data from different institutions. This is particularly noteworthy as the two institutions used different scanners from different vendors.
Discussion
Our data show that our CNN model could generate 18F-FDG PET images of the whole body from ultra low dose 18F-FDG PET inputs while maintaining clinically relevant information in terms of diagnostic accuracy and quantitative SUV measurements. Reducing the exposure to ionizing radiation from medical imaging procedures is important to minimize a potential risk of secondary cancer development later in life (10). Our CNN concept takes advantage of simultaneous 18F-FDG PET and MRI data acquisitions and could substantially advance the development of safer imaging tests for pediatric patients. We found that the inclusion of MRI data in addition to 18F-FDG PET images in the CNN model improves the depiction of anatomical detail in PET reconstruction. The rationale for this is that the MRI data provides detailed anatomic information that helps the CNN improve spatial detail in the reconstructed images. In addition, incorporation of attention-weighted loss into our model emphasizes the high-diagnostic-value regions on medical images, which further boosts the model performance. To our knowledge, this is a novel approach to enhancing low dose 18F-FDG PET image reconstruction.
Most prior PET low-dose reconstruction work focused on the brain. Previous authors (18, 19) showed that a low-dose PET scan of the brain can be obtained by combining 75% dose accelerated PET scans with T1-weighted MRI images as inputs to a CNN model to predict standard-dose brain PET images. Besides CNN, other frequently used neural network models in brain PET reconstruction are Generative Adversarial Networks (GANs) (20, 21), where the generator part of a GAN learns to create synthetic images with the goal of fooling the discriminator which is designed particularly to distinguish between real and synthetic images. Furthermore, several other attempts have focused on dose reduction below 10% (22–24). Chen et al (25) used ultra-low-dose brain PET images and MRI sequences as inputs to create AI augmented PET scans of the brain. However, brain image reconstruction is fundamentally different from whole-body image reconstruction, which is a much more challenging task due to its much more variable anatomical detail. Only two recent works (26, 27) focused on whole-body PET image reconstruction. A residual CNN was proposed to reconstruct full-dose PET images from 10% low dose counterparts (26). However, the research was conducted on only two whole-body scans which largely limits the generalizability of the developed model. In addition, the model was not tested with regards to its ability to render clinical diagnoses. The second proposed scheme (27) was built upon 50% low dose images which saves substantially less ionizing radiation than provided by our CNN model.
There are several limitations of our study. Some small lymph nodes can be less well delineated on the AI-reconstructed PET compared to the original standard-dose PET. In Supplementary Figure 6, we can see that sub-centimeter hypermetabolic lymph nodes are better delineated on the AI augmented scan than on the 6% dose scan. The AI augmented scan does not discriminate each individual lesion as well as the original 100% standard-dose scan. This technical limitation will be addressed with further improvements of our algorithm. The limitation of FDG-PET for the detection of sub-centimeter lesions is a well-described problem not only for our studies, but for the field in general. Another limitation is the need for simultaneously acquired MRI. There might be situations where only PET images are present or if the PET and MRI data are acquired separately on different scanners. It is worth noting that the proposed model could potentially be applied in PET/CT scenario where the CT provides the anatomical information for PET reconstruction. While this approach would not save irradiation, it could be used to save time by acquiring ultra-fast PET scans and augmenting them with CT data. Related problems due to sequential rather than simultaneous PET and CT data acquisition would have to be investigated. Meanwhile, hallucination signals could be introduced during reconstruction due to the lack of performance guarantee in deep learning models.
Conclusion
We have demonstrated that high quality 18F-FDG PET images can be reconstructed from ultra-low-dose imputs using a new CNN that includes the simultaneously acquired MRI in addition to PET data. The AI-augmented ultra-low dose 18F-FDG PET images maintain clinically relevant information in terms of diagnostic accuracy, diagnostic confidence and quantitative SUV measurements.
Supplementary Material
KEY POINTS.
QUESTION:
Can Artificial Intelligence augment whole body PET scans with minimal radiation exposure to the quality of standard-dose PET?
PERTINENT FINDINGS:
Using a cohort study of 23 clinical whole body 18F-FDG PET/MRI subjects, we demonstrated that the AI-reconstructed ultra-low-dose 18F-FDG PET images resemble high similarity with standard-dose 18F-FDG PET images, based on both quantitative and qualitative clinical evaluations. The proposed PET reconstruction model also generalizes in an independent cohort study of 11 clinical whole body 18F-FDG PET/MRI subjects.
IMPLICATIONS FOR PATIENT CARE:
We anticipate that our proposed model will enable a new generation of imaging exams for children that can be widely applied to interrogate health and disease without the risk of secondary cancer development later in life.
Acknowledgements
Funding:
This study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, R01 HD081123A) and the Andrew McDonough B+ Foundation. Statistical analysis for this work were also partially supported by the Biostatistics Shared Resource, which is funded by the Cancer Center Support Grant, P30CA124435.
Footnotes
Declarations:
Conflicts of interest/Competing interests:
There are no conflicts of interest or competing interests
Ethics approval and patient consent:
Research PET/MR imaging studies have been approved by the Institutional Review Board at Stanford University and the University of Tuebingen. All patients provided written informed consent to participate in a research PET/MR study and that results of this research will be published.
Availability of data and material: Data of this project will be made available
Code availability: Code details will be made available
CONFLICTS/COMPETING INTERESTS: NONE
References
- 1.Muehe AM, Theruvath AJ, Lai L, et al. How to provide gadolinium-free PET/MR cancer staging of children and young adults in less than 1 h: the Stanford approach. Molecular imaging and biology 2018;20:324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daldrup-Link HE. How PET/MR can add value for children with cancer. Current radiology reports 2017;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uslu L, Donig J, Link M, Rosenberg J, Quon A, Daldrup-Link HE. Value of 18F-FDG PET and PET/CT for evaluation of pediatric malignancies. Journal of Nuclear Medicine 2015;56:274–286. [DOI] [PubMed] [Google Scholar]
- 4.Huang B, Law MW-M, Khong P-L. Whole-body PET/CT scanning: estimation of radiation dose and cancer risk. Radiology 2009;251:166–174. [DOI] [PubMed] [Google Scholar]
- 5.Frush DP, Callahan M, Goske M, Kaste S, Hernanz-Schulman M. CT and radiation safety: content for community radiologists Image Gently website www.pedrad.org. 2009. [Google Scholar]
- 6.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. The Lancet 2012;380:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. Bmj 2013;346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meulepas JM, Ronckers CM, Smets AM, et al. Radiation exposure from pediatric CT scans and subsequent cancer risk in the Netherlands. JNCI: Journal of the National Cancer Institute 2019;111:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proceedings of the National Academy of Sciences 2003;100:13761–13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klenk C, Gawande R, Uslu L, et al. Ionising radiation-free whole-body MRI versus 18F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: a prospective, non-randomised, single-centre study. The lancet oncology 2014;15:275–285. [DOI] [PubMed] [Google Scholar]
- 11.Hall E, Brenner D. Cancer risks from diagnostic radiology. The British journal of radiology 2008;81:362–378. [DOI] [PubMed] [Google Scholar]
- 12.Robbins E. Radiation risks from imaging studies in children with cancer. Pediatric blood & cancer 2008;51:453–457. [DOI] [PubMed] [Google Scholar]
- 13.Council NR. Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2 Vol 7: National Academies Press; 2006. [PubMed] [Google Scholar]
- 14.Applegate KE, Frush DP. Image gently: a decade of international collaborations to promote appropriate imaging for children. Journal of the American College of Radiology 2017;14:956–957. [DOI] [PubMed] [Google Scholar]
- 15.Chawla SC, Federman N, Zhang D, et al. Estimated cumulative radiation dose from PET/CT in children with malignancies: a 5-year retrospective review. Pediatric radiology 2010;40:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karakatsanis NA, Fokou E, Tsoumpas C. Dosage optimization in positron emission tomography: state-of-the-art methods and future prospects. American journal of nuclear medicine and molecular imaging 2015;5:527. [PMC free article] [PubMed] [Google Scholar]
- 17.Niederkohr R, Hamill J, Jones J. Diagnostic accuracy of oncologic ultra-low-dose FDG-PET imaging: a pilot study. Journal of Nuclear Medicine 2020;61:1392–1392. [Google Scholar]
- 18.Xiang L, Qiao Y, Nie D, et al. Deep auto-context convolutional neural networks for standard-dose PET image estimation from low-dose PET/MRI. Neurocomputing 2017;267:406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaharchuk G. Next generation research applications for hybrid PET/MR and PET/CT imaging using deep learning. European Journal of Nuclear Medicine and Molecular Imaging 2019:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Zhou L, Yu B, et al. 3D auto-context-based locality adaptive multi-modality GANs for PET synthesis. IEEE transactions on medical imaging 2018;38:1328–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Yu B, Wang L, et al. 3D conditional generative adversarial networks for high-quality PET image estimation at low dose. Neuroimage 2018;174:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Gong E, Pauly J, Zaharchuk G. 200x low-dose PET reconstruction using deep learning. arXiv preprint arXiv:171204119 2017. [Google Scholar]
- 23.Ouyang J, Chen KT, Gong E, Pauly J, Zaharchuk G. Ultra‐low‐dose PET reconstruction using generative adversarial network with feature matching and task‐specific perceptual loss. Medical physics 2019;46:3555–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanaat A, Arabi H, Mainta I, Garibotto V, Zaidi H. Projection-space implementation of deep learning-guided low-dose brain PET imaging improves performance over implementation in image-space. Journal of Nuclear Medicine 2020:jnumed. 119.239327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen KT, Gong E, de Carvalho Macruz FB, et al. Ultra–low-dose 18F-florbetaben amyloid PET imaging using deep learning with multi-contrast MRI inputs. Radiology 2019;290:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan S, Zhu Y-M. Full-dose PET image estimation from low-dose PET image using deep learning: a pilot study. Journal of digital imaging 2019;32:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whiteley W, Luk WK, Gregor J. DirectPET: full-size neural network PET reconstruction from sinogram data. Journal of Medical Imaging 2020;7:032503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekine T, Delso G, Zeimpekis KG, et al. Reduction of 18F-FDG dose in clinical PET/MR imaging by using silicon photomultiplier detectors. Radiology 2018;286:249–259. [DOI] [PubMed] [Google Scholar]
- 29.Lim B, Son S, Kim H, Nah S, Mu Lee K. Enhanced deep residual networks for single image super-resolution. Paper presented at: Proceedings of the IEEE conference on computer vision and pattern recognition workshops, 2017. [Google Scholar]
- 30.Yi D, Grøvik E, Iv M, et al. MRI Pulse Sequence Integration for Deep-Learning Based Brain Metastasis Segmentation. arXiv preprint arXiv:191208775 2019. [Google Scholar]
- 31.Qin R, Qiao K, Wang L, Zeng L, Chen J, Yan B. Weighted focal loss: An effective loss function to overcome unbalance problem of chest X-Ray14. Paper presented at: Proc. IOP Conf. Ser., Mater. Sci. Eng, 2018. [Google Scholar]
- 32.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006;31:1116–1128. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee I, Crawley A, Bhethanabotla M, Daldrup-Link HE, Rubin DL. Transfer learning on fused multiparametric MR images for classifying histopathological subtypes of rhabdomyosarcoma. Computerized Medical Imaging and Graphics 2018;65:167–175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


