Abstract
Objectives:
Patients treated with cancer chemotherapeutics frequently report chemotherapy-induced peripheral neuropathy (CIPN), changes in mood (depression, anxiety), and functional impairments. Rodent models of CIPN elicit limited alterations in functional behaviors, which pose challenges in developing preclinical models of chemotherapy-induced behavioral depression.
Methods:
The present study examined the consequences of chemotherapy-induced mechanical hypersensitivity (paclitaxel: 32 mg/kg or 64 mg/kg, cumulative; oxaliplatin: 30 mg/kg, cumulative) on behavioral depression, as measured with operant responding for palatable food during periods of food restriction and ad libitum chow, consumption of non-contingently available palatable food in the presence of ad libitum chow, and voluntary wheel running. These studies employed two inbred mouse strains (C57BL/6J and Balb/cJ) and also examined potential sex differences.
Results:
All chemotherapeutic regimens caused profound mechanical hypersensitivity for the duration of the observation periods of the various studies (a minimum of 1 month, and up to 7 months), but none of the treatments changed voluntary wheel running or consumption of non-contingent palatable food. The high dose of paclitaxel temporarily reduced operant responding for palatable food in male C57BL/6J mice undergoing food restriction or maintained on ad libitum chow. However, paclitaxel failed to decrease operant responding for palatable food in free-feeding female C57BL/6J mice or Balb/cJ mice of either sex. Moreover, oxaliplatin did not significantly alter operant responding for palatable food in male or female C57BL/6J mice maintained on ad libitum chow.
Conclusions:
These findings demonstrate a dissociation between chemotherapy-induced mechanical hypersensitivity and behavioral depression in the assays tested. The transient effects of paclitaxel on operant responding in male C57BL/6J mice may represent a fleeting behavioral correlate of chemotherapy-associated pain-like behaviors in this strain.
Keywords: Paclitaxel, Oxaliplatin, Chemotherapy-Induced Peripheral Neuropathy, Mechanical Hypersensitivity, Operant Responding, Palatable Food, Balb/cJ Mice, C57BL/6J Mice, Motivation, Depression
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect associated with taxane-based compounds (paclitaxel, tradename Taxol ®) and platinum-based compounds (oxaliplatin, tradename Eloxatin ®). CIPN is a dose-limiting side effect that produces spontaneous and stimulus-evoked pain that can last for years after completion of treatment in some cancer survivors (Reeves et al. 2012; Seretny et al. 2014; Beijers et al. 2017). The prevalence of CIPN varies widely; approximately 15% to 90% of multi-agent chemotherapy-treated cancer patients develop CIPN (Pachman et al. 2015; Beijers et al. 2017; Kaplan et al. 2019; Nyrop et al. 2019), with CIPN rates skewing higher in patients receiving adjuvant paclitaxel or oxaliplatin. The large variance in the prevalence of CIPN is due to heterogeneity in chemotherapy regimens, duration of exposure, risk factors, and method of assessment of CIPN. Paclitaxel and oxaliplatin improve the survival and prognosis of many cancer patients. However, CIPN and the associated functional impairment, as well as affective disorders (depression and anxiety) induced by these chemotherapeutics, reduce quality of life (Reeves et al. 2012; Pachman et al. 2016; Avan et al. 2018; Oh and Cho 2020).
Similar to humans, paclitaxel and oxaliplatin induce dose-dependent, long-term mechanical and cold hypersensitivity in mice (for a recent review see (Bruna et al. 2020)). In addition to sensory changes, these chemo agents cause changes in behavioral responses in mouse assays used to screen anxiolytics and antidepressants, such as immobility in the forced swim test, avoidance of the light side of the light-dark box, and sucrose preference deficit (Toma et al. 2017), as well as increased immobility in the tail suspension test (Hache et al. 2015). Examining discrepancies in the relationship between chemotherapy-induced nociception and performance in rodent behavioral assays may provide some mechanistic insight into the complex relationship between CIPN and mood reported in cancer survivors. Further, improved animal models of CIPN-induced behavioral changes create better opportunities for discovering novel treatments for chemotherapy-induced depression.
The goal of this study was to examine the impact of chemotherapy on mechanical hypersensitivity and on voluntary behaviors, such as operant responding for palatable food, consumption of palatable food available noncontingently, and wheel running. To that end, we compared the effects of paclitaxel and oxaliplatin on male and female C57BL/6J mice. We hypothesize that mechanical hypersensitivity induced by paclitaxel and oxaliplatin will be associated with behavioral depression in C57BL/6J mice of both sexes.
We also examined the effects of paclitaxel on male and female Balb/cJ mice. Balb/c mice are an inbred strain widely used in cancer research. They are reported to show prolonged accumulation of paclitaxel in nervous tissue upon systemic administration (Wozniak et al. 2016). Further, Balb/c mice show greater sensitivity to the neurotoxic effects of chemotherapy (mechanical hypersensitivity, cold hypersensitivity, degradation of caudal nerve conduction velocity, dorsal root ganglia morphological changes, and intra-epidermal nerve fiber degeneration) than C57BL/6 mice (Marmiroli et al. 2017). Therefore, we hypothesize that Balb/cJ mice will have greater mechanical hypersensitivity and behavioral depression than C57BL/6J mice following treatment with paclitaxel.
Methods
Animals
Adult male and female C57BL/6J and Balb/cJ mice purchased from Jackson Laboratory (Bar Harbor, ME) were used in these studies. Cohort ages at the start of the experiments ranged from 3 to 6 months, with 8 – 12 subjects per sex/treatment/cohort. Animals were individually housed in a temperature- and humidity-controlled vivarium room with a 12-hr light/dark schedule (lights on at 7:00 A.M.). Cages contained enrichment items (corncob bedding, compressed cotton nestlet bedding material, in addition to cardboard mouse tunnels and/or pulp mouse huts). Mice were sacrificed by carbon dioxide asphyxiation and cervical dislocation following completion of experiments. All procedures were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University, in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Paclitaxel Injections
Paclitaxel (National Drug Codes List 70860-200-50, Athenex, Richmond, VA, USA) was dissolved in a mixture of 1:1:18 (1 volume ethanol/1 volume kolliphor [Sigma-Aldrich]/18 volumes distilled water). One complete regimen of paclitaxel consisted of four every-other-day intraperitoneal injections of 8 mg/kg solution. C57BL/6J and Balb/cJ mice of both sexes were treated with paclitaxel injections on Days 0, 2, 4, and 6, for a cumulative dose of 32 mg/kg paclitaxel, as per our previous reports (Toma et al. 2017, 2019; Curry et al. 2018; Kyte et al. 2018; Meade et al. 2020). Select cohorts received a second regimen of paclitaxel injections on Days 10, 12, 14, and 16, for a cumulative dose of 64 mg/kg paclitaxel. Control mice received 1:1:18 at a volume of 10 ml/kg, i.p., on the same injection regimen.
Oxaliplatin Injections
Oxaliplatin (National Drug Codes List 16729-332-05, Accord Healthcare Limited, London, ENG) was diluted in sterile 5% dextrose solution (Hospira, Lake Forest, IL), for a final dose of 3 mg/kg. Mice were injected i.p. with vehicle (5% dextrose) or oxaliplatin on Day 0, 1, 2, 3, 4, 10, 11, 12, 13, and 14, for a cumulative oxaliplatin dose of 30 mg/kg, as previously described (Ta et al. 2009).
von Frey Test and Acetone Test
Mice were habituated in plexiglass cups (approximately 10 cm diameter x 10 cm high) on an elevated mesh platform for 1 hr. Cups were draped with dark, opaque fabric. Stimuli were applied to the ventral surface of the hind paws. Nociception-like behaviors were recorded in both hind paws per subject and averaged together.
For the von Frey test, von Frey filaments were applied to the ventral surface of the hind paw as described in our previous publication (Meade et al. 2020). The mechanical thresholds are represented as the amount of force (g) applied by the von Frey filament to cause paw withdrawal, expressed as the mean force ± SEM.
For the acetone test, approximately 20 μL of acetone was briskly squirted onto the ventral surface of the hind paw. The duration of acetone-elicited behaviors (paw licking, flinching, paw elevation), irrespective of disruptions or pauses, was measured during the 60 seconds immediately after application of acetone. Target behaviors wear timed by stopwatch. Acetone test data are expressed as the mean time ± SEM.
Food Restriction and Target Weight Calculation
Mice were individually housed for one week on ad libitum Teklad rodent diet to determine free-feeding body weights. Target weights were calculated as 85-90% of free-feed body weight (in grams). Mice were fed 4 g of food daily in the home cage to gradually reduce their body weight. Food amounts were further reduced by half-gram increments when needed, to facilitate weight loss and maintenance of the target weight.
Mice were maintained at their target weights during operant training periods. Mice subjected to the fixed ratio studies continued food restriction for the duration of the experiment, while animals used for progressive ratio studies were given food ad libitum following completion of behavior acquisition training.
Operant Apparatus
Sound- and light-attenuated operant conditioning boxes (18 × 18 × 18 cm) containing two nose-poke apertures (left and right) were used (MED Associates, St. Albans, VT). Nose-poking at the active nose-poke led to delivery of Bio-Serv™ Dustless Precision Pellets™ Purified Rodent Diet (18.4% protein, 5.5% fat, 4.6% fiber, 6.5% ash, <10% moisture, 59.1% carbohydrate, 14 mg size; Bio-Serv, Flemington, NJ) to a recessed receptacle located between the two nose-poke apertures. The inactive nose-poke was plugged with a rubber stopper for all experiments. MED-PC IV software (MED Associates, St. Albans, VT) was used to run operant programs, record nose-pokes, and control pellet deliveries.
Operant Responding Behavior Acquisition Training
Upon reaching target weight, mice were placed in operant boxes during the dark cycle. The inactive left nose-poke aperture was plugged. The light above both apertures was turned on, and the inside compartment of the nose-poke was illuminated. One noncontingent pellet was provided in the recessed receptacle. Inserting the nose into the active right nose-poke aperture was recorded as one response. One nose-poke response triggered the release of one pellet into the recessed receptacle. Following 1 hr of nose-poking for pellets on this fixed ratio 1 (FR1) schedule (one nose-poke earns one pellet), mice that successfully earned a minimum of 50 pellets were increased to an FR2 schedule directly thereafter. Mice stayed in the chamber overnight (up to 12 hours), to have an extended training session on the FR2 schedule. While mice often earned upwards of 300 pellets during the initial overnight training session, mice did not consume all pellets earned; the majority of pellets remained in the recessed receptacle or on the operant chamber floor. Mice were fed rodent chow in the home cage following the overnight session.
Over the course of the following week, food-restricted mice continued fixed ratio training daily during the light cycle for 15-minute sessions. The left nose-poke was plugged for the duration of these behavior acquisition sessions, and one noncontingent pellet was placed in the recessed receptacle at the start of all sessions to serve as a cue for the mice. Upon earning 10 reinforcers at the FR2 level, the demand was increased to FR3. Earning 5 reinforcers at FR levels greater than FR2 led to promotion to the next higher FR level, with FR10 as the highest level. If the mice completed their current FR level during the 15-minute training session, they were promoted to the next highest FR level within that session. The FR level the individual mouse ended on for that training day was the FR level the mouse would begin on for the next training day. Mice were not restricted in the number of training levels they could be promoted to within a 15-minute training session. Mice that reached FR10 were tested daily at FR10 for ten days to establish stable rates of behavior. Unlike the prolonged overnight training session, mice consumed all pellets earned during the 15-minute daytime training sessions. Mice were fed in the home cage following completion of daily behavior acquisition training.
Subjects that failed to reach FR10 within 10 training days, or that completed FR10 training but had rates lower than 10 nose-pokes per minute following 10 additional FR10 training days, were excluded from the study. Between 0 and 2 subjects were excluded per cohort due to low baseline FR10 rates.
Fixed Ratio Operant Responding
Two cohorts of food-restricted male C57BL/6J mice with rates of FR10 nose-poking > 10 responses per minute received either vehicle (1:1:18, i.p.) or paclitaxel (8 mg/kg, i.p.) injections. Cohort 1 received one regimen of four injections and Cohort 2 received two regimens, for a total of eight injections. Food-restricted mice performed 15-minute FR10 sessions in the morning, were fed in the home cage, and received injections in the afternoon. Cohort 1 received testing daily for one month (with occasional breaks for weekends). Cohort 2 received daily testing for two months (with occasional breaks for weekends). The inactive left nose-poke was plugged for FR10 experiments.
FR10 data are represented as the number of nose-pokes per session ± SEM, relative to the performance of vehicle control-matched subjects on the same test day.
Progressive Ratio Operant Responding
Additional cohorts of food-restricted male and female C57BL/6J and Balb/cJ mice that successfully met the training criteria for FR10 performance were then trained to nose-poke on a progressive ratio of reinforcement for palatable pellets during the light cycle. The inactive right nose-poke was plugged for progressive ratio experiments. One-hour progressive ratio sessions began by presentation of one noncontingent pellet in the recessed receptacle. Response requirements increased exponentially upon earning each reinforcer. The nose-poke requirement for progressive ratio sessions was 2x-1, where x = number of pellets earned. Following this equation, the operant response requirement for pellets was as follows: [noncontingent], 1, 2, 4, 8, 16, 32, 64, 128, 256, 512, 1,024, 2,048, 4,096. The progressive ratio computer coding was modified from the program available on the MEDState Notation Repository (ProgressiveRatio_v2, http://www.mednr.com/programs/progressiveratio2.htm, originally designed by (Richardson et al. 1996) and updated by (Bamberger 2013)). Failure to nose-poke for 10 minutes led to termination of the session and establishment of the “breakpoint”: the maximum number of responses the subject is willing to perform on the manipulandum to earn a reinforcement, before displaying inactivity on the manipulandum for a specified amount of time after an increase in the amount of responses required to earn the reinforcer (Richardson et al. 1996). However, food-restricted mice and free-feeding mice in the present study seldom displayed 10 minutes of inactivity on the nose-pokes during the 1-hr progressive ratio session, or in pilot 4-hr progressive ratio sessions. Because we were not able to consistently determine the breakpoint in the progressive ratio experiments, the amount of effort subjects exerted in this task is represented as the total number of nose-poke responses ± SEM, or as a normalization of the total number of nose-pokes in chemotherapy-treated subjects relative to vehicle controls in the same cohort, tested during the same day, ± SEM. Food-restricted mice were fed in their home cages following completion of training sessions.
Food-restricted mice that earned < 5 pellets during any of three 1-hr progressive ratio training sessions were excluded from the study. Food-restricted mice that successfully met the training criteria for progressive ratio training were put on free-feed for one week, then their baseline performance while on free-feed was measured. Free-feeding mice that earned < 5 pellets during 1-hr progressive ratio sessions were excluded from the study.
Mice were assigned to treatment groups (vehicle for paclitaxel [1:1:18], vehicle for oxaliplatin [5% dextrose], 64 mg/kg paclitaxel cumulative, or 30 mg/kg oxaliplatin cumulative) based on their free-feeding operant performance, operant machine number, order of testing, and baseline mechanical thresholds as determined by the von Frey test (when available). Mice in all groups repeated progressive ratio testing after completing their first regimen of injections, after completing their second regimen of injections, and weekly thereafter for a minimum of 8 sessions.
Palatable Food Consumption
To determine if chemotherapy treatment reduces consumption of palatable food, mice maintained on ad libitum chow were placed in a clean, empty cage lacking bedding and enrichment items. Cages were equipped with a hopper containing water and standard rodent chow. Fifty 14 mg Bio-Serv™ Dustless Precision Pellets™ Purified Rodent Diet, which are the same pellets earned during operant experiments, were placed on the floor of the empty cage. The number of pellets remaining after 1 hr was counted. Palatable food consumption was measured after the second regimen of injections. Data are expressed as the mean number of pellets eaten ± SEM. Palatable food consumption sessions occurred during the light cycle, with overhead ambient lighting.
Voluntary Wheel Running
Voluntary wheel running is a behavior that rodents and other animals spontaneously perform in the wild and laboratory settings (Meijer and Robbers 2014). Mice maintained on ad libitum chow were placed in free-standing, sealed running wheels (0.68 m circumference) with the ability to spin in either direction. The number of rotations completed by each mouse was recorded after 2 hr. Wheels were not located in the home cage, so mice did not have access to chow or water during this period. Rotations were converted to distance traveled by multiplying the number of rotations by 0.68 meters. Data are expressed as distance traveled (m) ± SEM. Voluntary wheel running sessions occurred during the light cycle, with overhead ambient lighting.
Statistical Analysis
GraphPad Prism software, version 8.0 (GraphPad Software, Inc., La Jolla, CA), was used to perform statistical analyses on all data sets. T-tests and two-way repeated measures ANOVA followed by post-hoc Sidak’s multiple comparisons tests were used where appropriate to assess differences in treatments, sex, time, strain, repeated testing, or feeding condition.
von Frey thresholds (g), acetone-elicited behavior duration (s), voluntary wheel running (m), and pellet consumption (#) are represented as the mean ± SEM. For operant experiments, the data are expressed as the % nose-pokes relative to vehicle control, which were calculated by normalizing the average number of nose-pokes performed by vehicle-treated mice on a given test day to 100%, and multiplying the average number of nose-pokes performed by the corresponding cohort of chemotherapy-treated mice on the same test day by the same calculated normalization factor. This method accounts for intrasession variation in performance. Schwartz et al. (2014) employed a similar normalization calculation to highlight deficit in operant responding for palatable food in two mouse models of pain (Schwartz et al. 2014a), though did not account for intrasession variation in performance.
Results
All Chemotherapy Regimens Induced Mechanical Hypersensitivity in the Hind Paw
Upon their first exposure to the elevated mesh platform, C57BL/6J mice were able to adapt to the platform within the 1 hour habituation period (stand still, sleep). Food-restricted male C57BL/6J mice had baseline mechanical thresholds comparable to free-feeding male C57BL/6J mice (Toma et al. 2017; Meade et al. 2020) and developed mechanical hypersensitivity following treatment with paclitaxel. Upon completion of paclitaxel injections in Cohort 1 (32 mg/kg paclitaxel, cumulative), mice showed a significant reduction in mechanical thresholds when assessed at the 1 week time point (Veh = 7, PAC = 9; two-way ANOVA, F 32 mg/kg Paclitaxel (1, 14) = 27.55, P = 0.0001) (Fig. 1A). For Cohort 2, 32 mg/kg significantly reduced mechanical thresholds at 1 week (Veh = 12, PAC = 11, Sidak’s multiple comparisons test, t = 6.719, df = 66, P < 0.0001). Mechanical hypersensitivity was present when assessed at week 3 in this cohort, following the second regimen of injections (Veh = 12, PAC = 11, Sidak’s multiple comparisons test, t = 7.259, df = 66, P < 0.0001). The additional regimen of paclitaxel injections (64 mg/kg, cumulative) did not enhance mechanical hypersensitivity (Day 8 vs. Day 17, t-test, P > 0.05) (Fig. 1B).
Fig. 1: All Chemotherapy Regimens Induced Hypersensitivity in the Hind Paw.

Mechanical hypersensitivity was present in all subjects following treatment with chemotherapy: A) Food-restricted male C57BL/6J mice treated with vehicle (Veh, 1:1:18, n = 7) or a cumulative dose of 32 mg/kg paclitaxel (PAC, n = 8); B) Food-restricted male C57BL/6J mice treated with vehicle (1:1:18, n = 12) or a cumulative dose of 64 mg/kg paclitaxel (n = 12); C) Free-feeding male and female C57BL/6J mice treated with vehicle (1:1:18, male = 8, female = 10) or a cumulative dose of 64 mg/kg paclitaxel (male = 7, female = 10); D) Free-feeding male and female Balb/cJ mice treated with vehicle (1:1:18, male = 9, female = 8) or a cumulative dose of 64 mg/kg paclitaxel (male = 10, female = 8); E) Free-feeding male and female C57BL/6J mice treated with vehicle (5% dextrose solution, male = 10, female = 10) or a cumulative dose of 30 mg/kg oxaliplatin (male = 10, female = 10). F) Cold hypersensitivity was assessed with the acetone test in the hind paws of male and female C57BL/6J mice from the oxaliplatin cohort. Data are represented as mean values ± SEM. Arrows indicate one regimen of injections (4 every-other-day injections for paclitaxel cohorts, 5 every-day injections for oxaliplatin cohorts). Filled points are statistically significant from vehicle control (two-way ANOVA with Sidak’s multiple comparisons test, P < 0.05).
Paclitaxel treatment induced long-lasting mechanical hypersensitivity in the hind paws of free-feeding C57BL/6J mice, irrespective of sex (Two-way ANOVA F Paclitaxel (1, 196) = 465.9, P < 0.0001). The first regimen of injections (32 mg/kg paclitaxel, cumulative) induced mechanical hypersensitivity in free-feeding male C57BL/6J mice as early as Week 1 (Veh = 8, PAC = 7; two-way ANOVA with Sidak’s multiple comparisons test, t = 6.825, df =7, P < 0.0001). An additional regimen of injections (for 64 mg/kg paclitaxel, cumulative) did not enhance the previous mechanical hypersensitivity in male or female C57BL/6J, though robust mechanical hypersensitivity was present (Week 3, male: Veh = 8, PAC = 7; female: n = 10/group; two-way ANOVA with Sidak’s multiple comparisons test, t = 13.41, df = 196, P < 0.0001), perhaps representing a floor effect. Mechanical hypersensitivity remained when assessed in the von Frey test at the time point 7 months after the start of injections (week 27, male: Veh = 8, PAC = 7; female: n = 10/group; two-way ANOVA with Sidak’s multiple comparisons test, t = 10.09, df = 196, P < 0.0001) (Fig. 1C).
Unlike C57BL/6J mice, Balb/cJ mice were unable to fully habituate to the elevated mesh platform, despite repeated exposure to the platform on different days. When placed in plexiglass cups on the platform, Balb/cJ mice exhibited continuous locomotor activity (walking, climbing, digging, grooming, jumping, chewing the platform) for the duration of the habituation period. This excess locomotor activity made it impossible to stealthily apply the von Frey filament to the ventral surface of the hind paws on the majority of test days; Balb/cJ mice ran away from filaments or attacked them. Therefore, mechanical thresholds in Balb/cJ mice were only able to be measured at one time point, 1 month after initiation of vehicle or paclitaxel injections (Fig. 1D), which was a test day when their locomotor activity was sufficiently low enough to perform the test. Mechanical thresholds in vehicle-treated Balb/cJ mice (1.51 ± 0.13 grams) were significantly lower than mechanical thresholds in time course-matched vehicle-treated C57BL/6J mice (2.53 ± 0.20 grams) (two-way ANOVA, F Genotype (1, 31) = 16.48, P = 0.0003). Sidak’s multiple comparisons test revealed an effect of genotype in male mice (t = 3.298, df = 31, P = 0.0049) and female mice (t = 2.433, df = 31, P = 0.0414). Paclitaxel-treated Balb/cJ mice displayed lower mechanical thresholds than vehicle-matched Balb/cJ controls at the 1 month time point (two-way ANOVA, F Paclitaxel (1, 31) = 108.8, P < 0.0001). There were no sex differences in mechanical thresholds in Balb/cJ mice (two-way ANOVA, F Sex (1, 31) = 0.8639, P = 0.12) or interactions between sex and paclitaxel (two-way ANOVA, F Sex x Paclitaxel (1, 31) = 1.524, P = 0.23).
Fig. 1E shows that oxaliplatin induces long-lasting sensory changes in the C57BL/6J hind paw. The first regimen of oxaliplatin injections (15 mg/kg, cumulative) was sufficient to induce mechanical hypersensitivity in both sexes at week 1 (two-way ANOVA with Sidak’s multiple comparisons test, t = 11.68, df = 19.22, P < 0.0001). Over the course of the experimental period, three-way ANOVA identified an effect of oxaliplatin (F Oxaliplatin (1,18) = 238.7, P < 0.0001) and an effect of time (F Time (3,54) = 75.92, P < 0.0001) in the von Frey test, with a significant interaction between oxaliplatin and time (F Oxalipatin x Time (3,54) = 53.30, P < 0.0001). There was no effect of sex on mechanical thresholds in oxaliplatin-treated C57BL/6J mice (F Sex (0.6601, 11.88) = 0.8897, P = 0.32), and no interaction between sex, oxaliplatin, and time on mechanical thresholds (F Sex x Oxaliplatin x Time (3, 54) = 0.2610, P = 0.85). Mice of both sexes treated with oxaliplatin continued to show mechanical hypersensitivity when assessed at week 8 (two-way ANOVA with Sidak’s multiple comparisons test, t = 17.32, df = 19.5, P < 0.0001).
Mice in the oxaliplatin cohort were also subjected to the acetone test, as a secondary measure of evoked hypersensitivity. The cumulative dose of 30 mg/kg oxaliplatin increased the amount of time attending to the paw at week 4, and this elevated attention to the paw was present when measured at week 10 (Fig. 1F). Three-way ANOVA identified an effect of oxaliplatin (F Oxaliplatin (1,36) = 92.53, P < 0.0001) and an effect of time (F Time (2,72) = 44.86, P < 0.0001) in the acetone test, with a significant interaction between oxaliplatin and time (F Oxaliplatin x Time (2,72) = 38.15, P < 0.0001). Further, two-way ANOVA identified a significant increase in time attending to the paw in oxaliplatin-treated mice of both sexes between week 4 and week 10 (F Oxaliplatin x Time (1,18) = 14.19, P = 0.0014). There were no sex differences in time attending to the paw in any acetone test analyses.
High-Dose Paclitaxel Decreases Operant Responding in Food-Restricted C57BL/CJ Male Mice with Mechanical Hypersensitivity.
The consequences of fixed ratio operant responding for palatable food in male C57BL/6J mice administered 32 mg/kg paclitaxel are shown in Fig. 2A. The average number of nose-pokes performed by these subjects at baseline during a 15 min FR10 session was 365.0 ± 46.7 (Veh) and 389.2 ± 52.0 (PAC). Subjects in both treatment groups (veh = 7, PAC = 9) showed an increase in the number of responses across sessions regardless of drug, as reflected by a significant main effect of repeated testing (F Time (25, 362) = 3.528, P < 0.0001). There was not a main effect of 32 mg/kg paclitaxel (F Paclitaxel (1, 362) = 0.2412, P = 0.62) or an interaction between repeated testing and 32 mg/kg paclitaxel (F Time x Paclitaxel (25, 362) = 0.1678, P > 0.99). All subjects in this cohort successfully met the training criteria.
Fig. 2: Fixed Ratio 10 Performance in Food-Restricted C57BL/CJ Male Mice Treated with Low-Dose and High-Dose Paclitaxel.

Daily operant responding on a FR10 schedule of reinforcement in food-restricted mice treated with vehicle or paclitaxel. A) Vehicle treatment (1:1:18, n = 7) or a cumulative dose of 32 mg/kg paclitaxel (n = 8). B) Vehicle treatment (1:1:18, n = 12) or a cumulative dose of 64 mg/kg paclitaxel (n = 12). Total nose-poke numbers are normalized relative to vehicle controls in the same cohort, on the same test day, ± SEM. Arrows indicate one injection of vehicle or 8 mg/kg paclitaxel. On days with both operant sessions and injections, operant sessions preceded injections. Filled points are significantly different from vehicle control, t-test P < 0.05. $ = significant overall effect of treatment (two-way ANOVA, F Paclitaxel (1, 1113) = 56.66, P < 0.0001).
The average number of nose-pokes performed by male C57BL/6J mice in the 64 mg/kg paclitaxel experiment prior to drug administration was 229.3 ± 31.0 (Veh) and 240.4 ± 38.4 (PAC). Similar to the results in the 32 mg/kg paclitaxel experiment, subjects in this experiment (Veh = 12, PAC = 11) performed more responses with repeated testing (F Time (52, 1113) = 12.89, P < 0.0001). However, analysis of the raw data shows that the rate of increase in operant responding over time is stunted in mice treated with a cumulative dose of 64 mg/kg paclitaxel, as compared to vehicle controls in that cohort (two-way ANOVA, FPaclitaxel (1, 1113) = 56.66, P < 0.0001). The normalized data for this experiment are presented in Fig. 2B. All subjects in this cohort successfully met the training criteria, though one subject in the paclitaxel cohort died during the first regimen of injections.
Effects of Feeding on Operant Responding
Mice were food-restricted to facilitate operant responding for palatable food. Because food restriction can act as a form of chronic mild stress (Beck and Luine 1999), in the interest of mouse welfare, we tested if mice would continue to perform the operant task when the stressor of food restriction is removed. Ad libitum chow decreased FR10 responding for palatable food to below 5 reinforcers earned, which is below the experimental cutoff. Supplemental Digital Content 1 shows that food restriction produces robust operant responding for palatable food on a progressive ratio of reinforcement, and that while ad libitum feeding decreases operant performance, the total number of nose-pokes performed was sufficient to earn ≥ 5 reinforcers. Therefore, the remainder of the operant studies were performed under the parameters of ad libitum chow and a progressive ratio of reinforcement.
Paclitaxel has Sex-, Time-, and Strain-Dependent Effects on Operant Responding in Free-Feeding Mice with Mechanical Hypersensitivity
Because 64 mg/kg, but not 32 mg/kg, paclitaxel induced a deficit in fixed ratio operant responding in food-restricted male C57BL/6J mice, the cumulative dose of 64 mg/kg paclitaxel was selected for progressive ratio experiments in three new cohorts of free-feeding male C57BL/6J mice. Two subjects out of forty-eight did not successfully meet the training criteria and were excluded from further operant responding experiments. For subjects that met the training criteria, progressive ratio performance was measured at baseline, between the first and second regimen of injections (week 1), after completion of the second regimen of injections (week 3), and weekly thereafter. The average number of nose-pokes performed by free-feeding male C57BL/6J mice during 1-hr progressive sessions at baseline was 114.5 ± 11.2 (Veh = 24) and 115.5 ± 14.6 (PAC = 22). As was the case for the FR10 food-restriction experiments, male C57BL/6J mice in the progressive ratio ad libitum chow experiments also showed an increase in operant responding over time (mixed effects analysis, F Time (3.612, 147.2) = 3.607, P = 0.0100, n = 14 – 24/group/time point), suggesting that free-feeding mice continue to improve in the progressive ratio task over time. Hardware malfunctions on select test were responsible for variability in the number of subjects used in the operant task.
The cumulative dose of 64 mg/kg paclitaxel significantly decreased the total number of responses in the progressive ratio task in free-feeding male C57BL/6J mice, as compared to vehicle controls (Two-way ANOVA F Paclitaxel (1, 777) = 23.60, P < 0.0001). Three independent cohorts of male C57BL/6J mice exhibited the same pattern of decreased operant responding following completion of two regimens of injections, which remained for 2 - 4 weeks, depending on the cohort, then returned to baseline. Each cohort consisted of age-matched peers, with cohort ages ranging from 4 - 7 months at the start of injections. A statistically significant decrease in progressive ratio task performance emerged at week 6 (t-test, t = 2.724, df = 43, P = 0.0093) (Fig. 3A).
Fig. 3: Effects of Chemotherapy on Operant Responding for Palatable Food in Free-Feeding Mice.
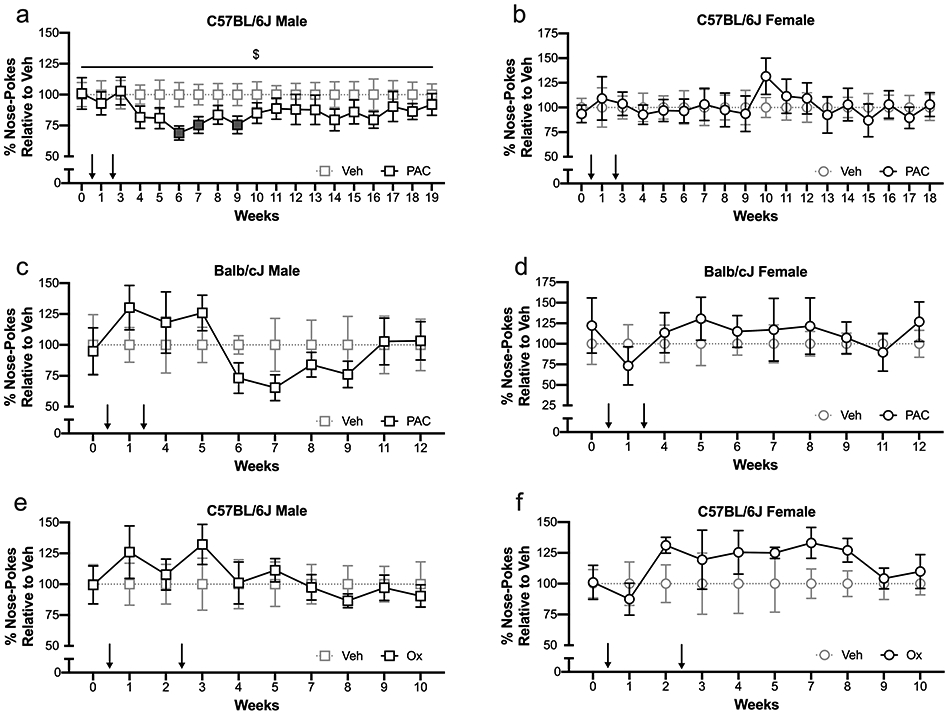
Weekly operant responding on a progressive ratio of reinforcement in free-feeding mice treated with vehicle or chemotherapy. A) Free-feeding male C57BL/6J mice treated with vehicle (1:1:18, n = 15 – 24) or a cumulative dose of 64 mg/kg paclitaxel (n = 14 – 22). B) Free-feeding female C57BL/6J mice treated with vehicle (1:1:18, n = 10) or a cumulative dose of 64 mg/kg paclitaxel (n = 10). C) Free-feeding male Balb/cJ mice treated with vehicle (1:1:18, n = 8 – 9) or a cumulative dose of 64 mg/kg paclitaxel (n = 10). D) Free-feeding female Balb/cJ mice treated with vehicle (1:1:18, n = 8) or a cumulative dose of 64 mg/kg paclitaxel (n = 7 – 8). E) Free-feeding male C57BL/6J mice treated with vehicle (5% dextrose solution, n = 10) or a cumulative dose of 30 mg/kg oxaliplatin (n = 10). F) Free-feeding female C57BL/6J mice treated with vehicle (5% dextrose solution, n = 10) or a cumulative dose of 30 mg/kg oxaliplatin (n = 10). Total nose-poke numbers are normalized relative to vehicle controls in the same cohort, on the same test day, ± SEM. Arrows indicate one regimen of injections (4 every-other-day injections for paclitaxel cohorts, 5 every-day injections for oxaliplatin cohorts). Filled points are significantly different from vehicle control, t-test P < 0.05. $ = significant overall effect of treatment (Two-way ANOVA, F Paclitaxel (1, 777) = 23.60, P < 0.0001).
To determine if there were sex differences in paclitaxel-mediated decreases in operant responding, female C57BL/6J mice were subjected to the same training schedule and dosing regimen as the males. All female C57BL/6J mice in the paclitaxel arm of the study successfully met the training criteria. The average number of nose-pokes performed by free-feeding female C57BL/6J mice at baseline was 127.7 ± 11.8 (Veh = 10) and 119.5 ± 11.3 (PAC = 10). Similar to male subjects, female C57BL/6J mice increased operant responding on a progressive ratio over time (mixed-effects analysis, F Time (17, 306) = 4.162, P < 0.0001, n = 9-10/group). However, unlike male subjects receiving a cumulative dose of 64 mg/kg paclitaxel, female C57BL/6J mice did not show a paclitaxel-mediated suppression of operant responding (mixed effects analysis, F Paclitaxel (1, 38) = 0.0005, P = 0.98, n = 9-10/group) (Fig. 3B).
To determine the susceptibility of the Balb/cJ mouse strain to paclitaxel-mediated changes in operant responding, Balb/cJ mice that successfully met the training criteria were subjected to the same injection and testing regimen as C57BL/6J mice. One Balb/cJ male mouse and four Balb/cJ female mice did not successfully meet the training criteria and were excluded from further operant responding experiments. Of mice that met the training criteria, the average number of nose-pokes performed by free-feeding Balb/cJ male mice on the progressive ratio task at baseline was 168.6 ± 41.3 (Veh = 9) and 160 ± 31.9 (PAC = 10). The average number of nose-pokes performed by Balb/cJ female mice at baseline was 64.6 ± 16.2 (Veh = 8) and 79.0 ± 21.7 (PAC = 8). Unlike C57BL/6J mice, free-feeding Balb/cJ mice of either sex did not increase their progressive ratio performance with repeated testing. Balb/cJ mice exposed to a cumulative dose of 64 mg/kg paclitaxel did not show decreased progressive ratio performance (male: mixed-effects analysis, F Paclitaxel (1, 17) = 0.1371, P = 0.72, n = 8-10/group (Fig. 3C); female: mixed-effects analysis, F Paclitaxel (1, 14) = 0.2654, P = 0.61, n = 7-8/group) (Fig. 3D). Due to lack of deficit in progressive ratio performance in paclitaxel-treated Balb/cJ mice of either sex, the experiment was terminated at 12 weeks.
To determine if a chemotherapeutic with a different mechanism of action than paclitaxel could cause operant responding deficit in C57BL/6J mice, free-feeding C57BL/6J mice of both sexes that successfully met the training criteria for operant task performance were subjected to oxaliplatin treatment, then underwent progressive ratio testing. The average number of nose-pokes performed by drug-naive male C57BL/6J mice in this cohort at baseline was 166.4 ± 26.5 (Veh = 10) and 165.4 ± 25.6 (Ox = 10). The average number of nose-pokes performed by drug-naïve female mice at baseline was 105.3 ± 12.0 (Veh = 10) and 106.4 ± 14.6 (Ox = 10). All forty C57BL/6J subjects in the oxaliplatin arm of the study met the training criteria and received injections.
Overall, female C57BL/6J mice had an increase in operant behavior over time (mixed-effects analysis, ANOVA, F Time (2.635, 47.43) = 4.269, P = 0.012), whereas male mice in this cohort did not. A cumulative dose of 30 mg/kg oxaliplatin did not decrease operant responding in either sex (male: two-way ANOVA, F Oxaliplatin (1, 18) = 0.09209, P = 0.77, n = 10/group (Fig. 3E); female: two-way ANOVA, F Oxaliplatin (1, 18) = 1.322, P = 0.2653, n = 10/group) (Fig. 3F). Oxaliplatin did not stunt increased responding over time in female C57BL/6J mice.
Chemotherapy Did Not Change Consumption of Noncontingently Available Palatable Food in Free-Feeding Mice
One-hour palatable food eating tests were performed in mice. Male C57BL/6J mice that were food-restricted or on ad libitum rodent chow ate equivalent numbers of palatable food pellets (t-test, P > 0.05) (Fig. 4A), supporting the protocol change from food-restriction to ad libitum chow. In free-feeding C57BL/6J mice from the 64 mg/kg paclitaxel-treated cohorts (Fig. 4B), there was no effect of sex (Two-way ANOVA, F Sex (1, 48) = 2.618, P = 0.11) or paclitaxel (Two-way ANOVA, F Paclitaxel (1, 48) = 1.656, P = 0.20) or interaction between sex and paclitaxel (Two-way ANOVA, F Sex x Paclitaxel (1, 48) = 1.732, P = 0.19) on the number of pellets eaten during the 1-hr session.
Fig. 4: Consumption of Non-Contingently Available Palatable Food.

Mice were allowed 1 hr to consume up to 50 non-contingently available palatable food pellets. A) Pellet eating in a cohort of drug-naïve male C57BL/6J mice that was food-restricted (~85 – 90% of free-feeding body weight, n = 15) and a second cohort of drug-naïve male C57BL/6J mice that was maintained on ad libitum chow (n = 12) (t-test, P > 0.05). B) Pellet consumption in ad libitum C57BL/6J mice 2 months after initiation of vehicle (1:1:18, male = 16, female = 10) or paclitaxel injections (64 mg/kg, cumulative, male = 16, female = 10). C) Pellet consumption in free-feeding Balb/cJ mice 1 month after initiation of vehicle (1:1:18, male = 9, female = 8) or paclitaxel injections (64 mg/kg, cumulative, male = 10, female = 8). D) Pellet consumption in free-feeding C57BL/6J mice 1 month after initiation of vehicle (5% dextrose solution, male = 10, female = 10) or oxaliplatin injections (30 mg/kg, cumulative, male = 10, female = 10).
At baseline, naïve free-feeding male Balb/cJ mice ate 41.7 ± 1.6 pellets (span: 28 – 50 pellets), and female Balb/cJ mice ate 36.8 ± 1.8 pellets (span: 20 – 50 pellets). Three-way ANOVA did not identify effects of repeated palatable food eating sessions (F Time (2, 62) = 3.065, P = 0.054), sex (F Sex (1, 31) = 3.091, P = 0.089), or paclitaxel (F Paclitaxel (1, 32) = 0.0513, P = 0.82) in Balb/cJ mice. Further, there was no interaction between these three factors in Balb/cJ mice (three-way ANOVA, F Time x Sex x Paclitaxel (2, 62) = 0.6622, P = 0.52) (Fig. 4C).
Similarly, there was no effect of oxaliplatin (F Oxaliplatin (1, 36) = 2.170, P = 0.15) or sex (FSex (1, 36) = 0.4483, P = 0.51) on the number of pellets eaten by C57BL/6J mice in the oxaliplatin cohort (Fig. 4D). It is important to note that all mice ate greater than 10 pellets during the palatable food eating session, but no subject earned more than 10 pellets during the progressive ratio task.
Chemotherapy Did Not Change Voluntary Wheel Running in Free-Feeding Mice
To determine if the chemotherapy regimens used in these studies decrease locomotion or wheel running performance, 2-hr voluntary wheel running sessions were performed in mice following completion of chemotherapy treatment. There was no effect of prior treatment with 64 mg/kg paclitaxel on total distance traveled by male C57BL/6J mice at the 2-month time point (Veh vs. PAC, t-test, t = 1.581, df = 30, P = 0.12) (Fig. 5A). Voluntary wheel running was not assessed in female C57BL/6J mice treated with 64 mg/kg paclitaxel. Female Balb/cJ mice ran further than male Balb/cJ mice at the 1-month time point (Two-way ANOVA F Sex (1, 31) = 9.226, P = 0.0048). However, paclitaxel did not change voluntary wheel running in Balb/cJ mice of either sex at this time point (Two-way ANOVA F Paclitaxel (1, 31) = 0.9618, P = 0.33) (Fig. 5B). There was no effect of oxaliplatin treatment on distance traveled in C57BL/6J mice at the 1-month time point (F Oxaliplatin (1, 36) = 0.05051, P = 0.8234). Also, there were no sex differences in total distance traveled in the oxaliplatin-treated cohort of C57BL/6J subjects (F Sex (1, 36) = 3.113, P = 0.086) (Fig. 5C).
Fig. 5: Voluntary Wheel Running in Free-Feeding Mice Treated with Chemotherapy.

Voluntary wheel running was assessed during 2-hr sessions following completion of chemotherapy. A) Male C57BL/6J mice 2 months after initiation of vehicle (1:1:18, n = 16) or a cumulative dose of 64 mg/kg paclitaxel (n = 16). B) Balb/cJ mice 1 month after initiation of vehicle (1:1:18, male = 9, female = 8) or a cumulative dose of 64 mg/kg paclitaxel (male = 10, female = 8). C) C57BL/6J mice 1 month after initiation of vehicle (5% dextrose solution, male = 10, female = 10) or a cumulative dose of 30 mg/kg oxaliplatin (male = 10, female = 10).
Discussion
Chronic pain in humans is a nuanced and complex phenomenon. Chronic pain can cause functional impairments, such as mobility issues, or more subtle forms as impairment, such as anhedonia or decreased motivation. Mimicking the various facets of chronic pain in animal models presents challenges, and any behavioral outcome will be an oversimplification of the human experience. Therefore, assessment of multiple behavioral endpoints are needed in order to reflect the pain experience. To this end, animal models of chronic pain should account for the effects of the chronic pain experience on mobility, reward, and motivation-related behavior, among others. Previously, we characterized the effects of repeated paclitaxel administration on mechanical hypersensitivity and behaviors related to mobility, reward, novelty, exploration, anxiety, depression, fatigue, and innate behaviors; we demonstrated that this chemotherapeutic alters some of these measures (Toma et al. 2017, 2019). These changes are consistent with changes in affective-like behaviors and cognitive flexibility reported with other rodent models of chronic pain (Descalzi et al. 2017; Cowen et al. 2018; Sieberg et al. 2018). Our results suggest that paclitaxel induced time-dependent changes in select measures, reinforcing the idea that chemotherapy-induced chronic pain states can have intricate effects on behavioral outcomes.
The present study builds on our previous reports and examines the effects of two mechanistically distinct and widely used chemotherapy drugs on mechanical hypersensitivity, activity, and affect-related behaviors in two inbred mouse strains. First, we observed that all chemotherapy regimens induced mechanical hypersensitivity. Second, we demonstrated that a high dose of paclitaxel temporarily decreased operant task performance in food-restricted and free-feeding male C57BL/6J mice, but not female C57BL/6J mice. Third, we observed no change in performance in the operant task in Balb/cJ mice of either sex when treated with the same dose of paclitaxel that decreased operant responding in C57BL/6J mice. Fourth, a dose of 30 mg/kg oxaliplatin did not change performance in the operant task in C57BL/6J mice of either sex. Fifth, none of the chemotherapy regimens changed voluntary wheel running, an example of activity, or consumption of noncontingently available palatable food pellets, an example of hedonic and motivated-like behaviors.
The deficit in the progressive ratio task performance in male C57BL/6J mice occurred 6-9 weeks following initiation of paclitaxel injections (64 mg/kg, cumulative). In contrast, paclitaxel-induced mechanical hypersensitivity first appeared 1 week after initiation of injections (32 mg/kg, cumulative) and persisted for up to 7 months. Therefore, mechanical hypersensitivity did not fully correspond with deficits in operant responding.
To determine whether temporary chemotherapy-induced changes in preference for the reinforcer were driving time-dependent deficits in operant responding in male C57BL/6J mice, mice were subjected to the palatable food eating test, where the same pellets earned during operant tasks were available non-contingently. None of the chemotherapy regimens changed consumption of palatable pellets while standard rodent chow was available, arguing against loss of preference for the pellets. Schwartz et al. (2014b, supplemental material) had a complementary finding: two mouse models of nerve injury did not induce changes in searching behavior for palatable food or consumption of standard rodent chow (Schwartz et al. 2014b).
We also investigated whether paclitaxel-induced deficits in operant responding were due to paclitaxel-induced changes in activity. In rodents, voluntary wheel running is a behavioral measure of activity and motivation that is affected by chronic pain (Cobos et al. 2012; Contreras et al. 2021). To that end, mice in the present study were subjected to voluntary wheel running. The results of the voluntary wheel running experiment suggest that none of the chemotherapy regimens reduced voluntary activity in mice, despite presence of mechanical hypersensitivity. Together, these findings suggest that the delayed and temporary depression of operant responding for palatable food in C57BL/6J male mice treated with 64 mg/kg paclitaxel occurs without global behavioral impairment.
Lack of motivation is an understudied side effect in patients with CIPN that directly and indirectly impairs many domains of quality of life. Because roughly half of breast cancer survivors report moderate to severe lack of motivation, peaking during the first six months of treatment (Person et al. 2020), we selected operant responding for food in the present study to model some behavioral aspects related to this phenomenon. Various rodent models of pain show a reduction in operant responding. Some of these models include: i.p. lipopolysaccharide (Grossberg et al. 2018; Vichaya et al. 2019), intraplantar complete Freund’s adjuvant (Schwartz et al. 2014a; Okun et al. 2016), lactic acid (Cone et al. 2018), and plantar incision (Warner et al. 2015).
There is limited research examining models of neuropathy, tonic pain, and chronic pain on operant responding in mice, though C57BL/6 mice with spared nerve injury earn fewer food pellets than controls (Schwartz et al. 2014a). In rats, paclitaxel decreases fixed ratio performance when the reinforcer is standard rodent chow (Legakis et al. 2019) but not intracranial self-stimulation (Legakis et al. 2018), even though paclitaxel induced mechanical hypersensitivity in both reports. In contrast, rats with spinal nerve ligation do not show changes in operant responding for sucrose pellets when tested on a fixed ratio or progressive ratio, despite presence of mechanical hypersensitivity (Okun et al. 2016). Similarly, rats with bilateral intraplantar formalin treatment do not show changes in intracranial self-stimulation (Selley et al. 2020). These studies show that different models of pain can produce overlapping as well as divergent effects on operant behavior. In the present study, we demonstrate that chemotherapy-induced mechanical hypersensitivity produced divergent effects on operant responding for palatable food in mice, which only occurred in male C57BL/6J mice treated with the higher dose of paclitaxel, with a delayed onset.
Following paclitaxel treatment, female C57BL/6 mice are reported to develop mechanical hypersensitivity equivalent to the mechanical hypersensitivity developed by male C57BL/6 mice (Neelakantan et al. 2016; Liang et al. 2020). Consistent with these reports, we observed that a cumulative dose of 64 mg/kg paclitaxel induced mechanical hypersensitivity in all C57BL/6J mice. Further, we show that mechanical hypersensitivity was present in both male and female C57BL/6J mice for up to 7 months.
While paclitaxel appears to induce mechanical hypersensitivity equally in both sexes of mice, the role of sex on paclitaxel-induced behavioral changes remains to be further investigated. One study found that paclitaxel elicits anxiogenic-like effects in male but not female C57BL/6 mice in the elevated plus maze, but that same study also reports no sex differences on paclitaxel-treated mice in the forced swim test (Liang et al. 2020). In the present study, a cumulative dose of 64 mg/kg paclitaxel had time-dependent decreases on operant responding in male C57BL/6J mice; however, this dose of paclitaxel did not change progressive ratio task performance in female C57BL/6J mice at any time point tested. These findings suggest that some of paclitaxel’s effects on behavior may be sex-dependent.
In order to determine if there were strain differences in paclitaxel-induced deficit in progressive ratio performance, free-feeding Balb/cJ mice that successfully met the training criteria for operant training were subjected to the same injection time course and testing schedule as the C57BL/6J mice. However, our Balb/cJ mice did not exhibit 64 mg/kg paclitaxel-induced deficits in the progressive ratio task at any time point tested. These strain-dependent effects of paclitaxel on operant responding for palatable food suggest the existence of possible genetic differences. Strain differences in pharmacodynamics of enzymes involved in paclitaxel metabolism (Rudeck et al. 2018), inflammation (Gant et al. 2003), and nociception (Wilson et al. 2003; Mazur-Bialy et al. 2011; Hanstein et al. 2014; Takahashi et al. 2016; Marmiroli et al. 2017) could explain these discrepancies.
Patients treated with paclitaxel (Thornton et al. 2008; Bao et al. 2016) or oxaliplatin (Mols et al. 2013; Bonhof et al. 2019; Oh and Cho 2020) report reduced quality of life. Two-thirds of patients treated with oxaliplatin in combination with fluorouracil show signs of depression (Oh 2017). Like paclitaxel, oxaliplatin induces peripheral neuropathy and long-term changes in mood, which may be interrelated: oxaliplatin-induced CIPN is associated with anxiety and depression in colorectal cancer survivors (Bonhof et al. 2019). Further, oxaliplatin-induced peripheral neuropathy significantly impairs activities of daily living, with a significant correlation between impairment of activities of daily living and depression (Oh et al. 2020). Therefore, the sensory and emotion-related side effects of oxaliplatin appear to be interdependent in patients. In contrast, Hsu et al. (2020) noted that patients treated with oxaliplatin who do not develop CIPN still have reduced quality of life (emotional distress: anxiety, depression, fatigue) (Hsu et al. 2020), suggesting that sensory disturbances can be independent of mood-related changes. In the present study, we observed that oxaliplatin induced mechanical hypersensitivity but did not cause behavioral depression in operant responding, consumption of palatable food, or voluntary wheel running, further suggesting that sensory disturbances can be independent of behavioral outcomes in mice.
Conclusion
We demonstrated that operant responding for palatable food, consumption of noncontingently available palatable food, and voluntary wheel running were differentially affected by chemotherapy treatment. We also observed that the occurrence of chemotherapy-induced mechanical hypersensitivity was not broadly correlated with deficit in operant responding for palatable food. Examining this discrepancy may provide some mechanistic insight into the complex relationship between CIPN and mood reported in cancer survivors. Indeed, the transient nature of this observation suggests that mechanisms other than those governing mechanical hypersensitivity or CIPN contribute to paclitaxel-induced changes in motivation-like behaviors. Finally, our results highlight the importance of sex and genotype as factors that modulate chemotherapy-induced changes on affect-like behaviors. Overall, the assessment of multiple behavioral outcome measures and experimental factors helps in creating an improved, integrated animal model of chemotherapy-induced behavioral depression. In addition, this approach creates opportunities to explore the mechanisms that underly chemotherapy-induced behavioral changes, which may lead to the discovery of novel pharmacological treatment options for cancer survivors with affective disorders.
Supplementary Material
Supplemental Digital Content 1: Effect of Feeding Condition on Progressive Ratio Operant Responding for Palatable Food.
Acknowledgements
We would like to thank Dr. Patrick M. Beardsley for consultation on operant study design.
Funding Source: NIH grants 1F31CA224930-01A1 and 5F31CA224930-02 (to J.A.M.), grant R01-CA206028 and CA219637 (to M.I.D.).
Footnotes
Conflicts of Interest: None declared.
References
- Avan R, Janbabaei G, Hendouei N, et al. (2018) The effect of pregabalin and duloxetine treatment on quality of life of breast cancer patients with taxane-induced sensory neuropathy: A randomized clinical trial. J Res Med Sci 23:. doi: 10.4103/jrms.JRMS_1068_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger G (2013) Progressive Ratio. In: MEDState Not. Repos http://www.mednr.com/programs/progressiveratio2.htm [Google Scholar]
- Bao T, Basal C, Seluzicki C, et al. (2016) Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat 159:327–333. doi: 10.1007/s10549-016-3939-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Luine VN (1999) Food deprivation modulates chronic stress effects on object recognition in male rats: Role of monoamines and amino acids. Brain Res 830:56–71. doi: 10.1016/S0006-8993(99)01380-3 [DOI] [PubMed] [Google Scholar]
- Beijers AJM, Oerlemans S, Mols F, et al. (2017) The magnitude of neurotoxicity in patients with multiple myeloma and the impact of dose modifications: results from the population-based PROFILES registry. Ann Hematol 96:653–663. doi: 10.1007/s00277-017-2927-8 [DOI] [PubMed] [Google Scholar]
- Bonhof CS, Poll-Franse LV., Vissers PAJ, et al. (2019) Anxiety and depression mediate the association between chemotherapy-induced peripheral neuropathy and fatigue: Results from the population-based PROFILES registry. Psychooncology 28:1926–1933. doi: 10.1002/pon.5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruna J, Alberti P, Calls-Cobos A, et al. (2020) Methods for in vivo studies in rodents of chemotherapy induced peripheral neuropathy. Exp. Neurol 325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos EJ, Ghasemlou N, Araldi D, et al. (2012) Inflammation-induced decrease in voluntary wheel running in mice: A nonreflexive test for evaluating inflammatory pain and analgesia. Pain 153:876–884. doi: 10.1016/j.pain.2012.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone K, Lanpher J, Kinens A, et al. (2018) Delta/mu opioid receptor interactions in operant conditioning assays of pain-depressed responding and drug-induced rate suppression: assessment of therapeutic index in male Sprague Dawley rats. Psychopharmacology (Berl) 235:1609–1618. doi: 10.1007/s00213-018-4876-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras KM, Caillaud M, Neddenriep B, et al. (2021) Deficit in voluntary wheel running in chronic inflammatory and neuropathic pain models in mice: Impact of sex and genotype. Behav Brain Res 399:113009. doi: 10.1016/j.bbr.2020.113009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen SL, Phelps CE, Navratilova E, et al. (2018) Chronic pain impairs cognitive flexibility and engages novel learning strategies in rats. Pain 159:1403–1412. doi: 10.1097/j.pain.0000000000001226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry ZA, Wilkerson JL, Bagdas D, et al. (2018) Monoacylglycerol Lipase Inhibitors Reverse Paclitaxel-Induced Nociceptive Behavior and Proinflammatory Markers in a Mouse Model of Chemotherapy-Induced Neuropathy s. J Pharmacol Exp Ther J Pharmacol Exp Ther 366:169–183. doi: 10.1124/jpet.117.245704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descalzi G, Mitsi V, Purushothaman I, et al. (2017) Neuropathic pain promotes adaptive changes in gene expression in brain networks involved in stress and depression. Sci Signal 10:. doi: 10.1126/scisignal.aaj1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant TW, Baus PR, Clothier B, et al. (2003) Gene expression profiles associated with inflammation, fibrosis, and cholestasis in mouse liver after griseofulvin. Toxicogenomics 111:847–853. doi: 10.1289/txg.5849 [DOI] [PubMed] [Google Scholar]
- Grossberg AJ, Vichaya EG, Christian DL, et al. (2018) Tumor-associated fatigue in cancer patients develops independently of il1 signaling. Cancer Res 78:695–705. doi: 10.1158/0008-5472.CAN-17-2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hache G, Guiard BP, Nguyen TH, et al. (2015) Antinociceptive activity of the new triple reuptake inhibitor NS18283 in a mouse model of chemotherapy-induced neuropathic pain. Eur J Pain (United Kingdom) 19:322–333. doi: 10.1002/ejp.550 [DOI] [PubMed] [Google Scholar]
- Hanstein R, Zhao JB, Basak R, et al. (2014) Focal Inflammation Causes Carbenoxolone-Sensitive Tactile Hypersensitivity in Mice. Open Pain J 3:123–133. doi: 10.2174/1876386301003010123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H-T, Wu L-M, Lin P-C, et al. (2020) Emotional distress and quality of life during folinic acid, fluorouracil, and oxaliplatin in colorectal cancer patients with and without chemotherapy-induced peripheral neuropathy A cross-sectional study. doi: 10.1097/MD.0000000000019029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MA, Oruc Z, Gumus M, et al. (2019) The efficacy and reliability of sequential adjuvant anthracycline-based chemotherapy and weekly paclitaxel regimen in human epidermal growth factor receptor 2 negative breast cancer: A retrospective analysis of a multicentre study. J BUON 24:1081–1086 [PubMed] [Google Scholar]
- Kyte SL, Toma W, Bagdas D, et al. (2018) Nicotine prevents and reverses paclitaxel-induced mechanical allodynia in a mouse model of CIPN. J Pharmacol Exp Ther 364:110–119. doi: 10.1124/jpet.117.243972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis LP, Bigbee JW, Negus SS (2018) Lack of paclitaxel effects on intracranial Self-Stimulation in male and female rats: Comparison to mechanical sensitivity. Behav Pharmacol 29:290–298. doi: 10.1097/FBP.0000000000000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis LP, Diester CM, Townsend EA, et al. (2019) Comparison of chemotherapy effects on mechanical sensitivity and food-maintained operant responding in male and female rats. Behav Pharmacol 1. doi: 10.1097/fbp.0000000000000527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Wei J, Tian L, et al. (2020) Paclitaxel Induces Sex-biased Behavioral Deficits and Changes in Gene Expression in Mouse Prefrontal Cortex. Neuroscience 426:168–178. doi: 10.1016/j.neuroscience.2019.11.031 [DOI] [PubMed] [Google Scholar]
- Marmiroli P, Riva B, Pozzi E, et al. (2017) Susceptibility of different mouse strains to oxaliplatin peripheral neurotoxicity: Phenotypic and genotypic insights. PLoS One 12:1–25. doi: 10.1371/journal.pone.0186250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur-Bialy AI, Majka A, Wojtas L, et al. (2011) Strain-specific effects of riboflavin supplementation on zymosan-induced peritonitis in C57BL/6J, BALB/c and CBA mice. Life Sci 88:265–271. doi: 10.1016/j.lfs.2010.11.016 [DOI] [PubMed] [Google Scholar]
- Meade JA, Alkhlaif Y, Contreras KM, et al. (2020) Kappa opioid receptors mediate an initial aversive component of paclitaxel-induced neuropathy. Psychopharmacology (Berl). doi: 10.1007/s00213-020-05572-2 [DOI] [PubMed] [Google Scholar]
- Meijer JH, Robbers Y (2014) Wheel running in the wild. Proc R Soc B Biol Sci 281:. doi: 10.1098/rspb.2014.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mols F, Beijers T, Lemmens V, et al. (2013) Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: Results from the population-based PROFILES registry. J Clin Oncol 31:2699–2707. doi: 10.1200/JCO.2013.49.1514 [DOI] [PubMed] [Google Scholar]
- Neelakantan H, Ward SJ, Walker EA (2016) Effects of paclitaxel on mechanical sensitivity and morphine reward in male and female C57Bl6 mice. Exp Clin Psychopharmacol 24:485–495. doi: 10.1037/pha0000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyrop KA, Deal AM, Reeder-Hayes KE, et al. (2019) Patient-reported and clinician-reported chemotherapy-induced peripheral neuropathy in patients with early breast cancer: Current clinical practice. Cancer 125:2945–2954. doi: 10.1002/cncr.32175 [DOI] [PubMed] [Google Scholar]
- Oh PJ (2017) Predictors of cognitive decline in people with cancer undergoing chemotherapy. Eur J Oncol Nurs 27:53–59. doi: 10.1016/j.ejon.2016.12.007 [DOI] [PubMed] [Google Scholar]
- Oh PJ, Cho JR (2020) Changes in Fatigue, Psychological Distress, and Quality of Life After Chemotherapy in Women with Breast Cancer: A Prospective Study. Cancer Nurs 43:E54–E60. doi: 10.1097/NCC.0000000000000689 [DOI] [PubMed] [Google Scholar]
- Oh PJ, Lee JR, Kim SK, Kim JH (2020) Changes in chemotherapy-induced peripheral neuropathy, disturbance in activities of daily living, and depression following chemotherapy in patients with colorectal cancer: A prospective study. Eur J Oncol Nurs 44:101676. doi: 10.1016/j.ejon.2019.101676 [DOI] [PubMed] [Google Scholar]
- Okun A, Mckinzie DL, Witkin JM, et al. (2016) Hedonic and motivational responses to food reward are unchanged in rats with neuropathic pain. Pain 157:2731–2738. doi: 10.1097/j.pain.0000000000000695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachman DR, Qin R, Seisler D, et al. (2016) Comparison of oxaliplatin and paclitaxel-induced neuropathy (Alliance A151505). Support Care Cancer 24:5059–5068. doi: 10.1007/s00520-016-3373-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachman DR, Qin R, Seisler DK, et al. (2015) Clinical course of oxaliplatin-induced neuropathy: Results from the randomized phase III trial N08CB (Alliance). J Clin Oncol 33:3416–3422. doi: 10.1200/JCO.2014.58.8533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person H, Guillemin F, Conroy T, et al. (2020) Factors of the evolution of fatigue dimensions in patients with breast cancer during the 2 years after surgery. Int J Cancer 146:1827–1835. doi: 10.1002/ijc.32527 [DOI] [PubMed] [Google Scholar]
- Reeves BN, Dakhil SR, Sloan JA, et al. (2012) Further data supporting that paclitaxel-associated acute pain syndrome is associated with development of peripheral neuropathy: North Central Cancer Treatment Group trial N08C1. Cancer 118:5171–5178. doi: 10.1002/cncr.27489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS, Richardson NR, Roberts DCS (1996) Progressive ratio schedules in drug self-administration studies in rats : a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11 [DOI] [PubMed] [Google Scholar]
- Rudeck J, Bert B, Marx-Stoelting P, et al. (2018) Liver lobe and strain differences in the activity of murine cytochrome P450 enzymes. Toxicology 404–405:76–85. doi: 10.1016/j.tox.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Schwartz N, Temkin P, Jurado S, et al. (2014a) Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Sci (New York, NY) 345:535–542. doi: 10.1126/science.1253994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N, Temkin P, Jurado S, et al. (2014b) Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science (80- ) 345:535–542. doi: 10.1126/science.1253994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Lazenka MF, Sim-Selley LJ, et al. (2020) Attenuated dopamine receptor signaling in nucleus accumbens core in a rat model of chemically-induced neuropathy. Neuropharmacology 166:107935. doi: 10.1016/j.neuropharm.2020.107935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seretny M, Currie GL, Sena ES, et al. (2014) Comprehensive review Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 155:2461–2470. doi: 10.1016/j.pain.2014.09.020 [DOI] [PubMed] [Google Scholar]
- Sieberg CB, Taras C, Gomaa A, et al. (2018) Neuropathic Neuropathic pain drives anxiety behavior in mice, results consistent with anxiety levels in diabetic neuropathy patients. doi: 10.1097/PR9.0000000000000651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta LE, Low PA, Windebank AJ (2009) Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Mol Pain 5:. doi: 10.1186/1744-8069-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Shima K, Tsuchiya M, et al. (2016) Analgesic Effects of 1st Generation Anti-histamines in Mice [DOI] [PubMed] [Google Scholar]
- Thornton LM, Carson WE, Shapiro CL, et al. (2008) Delayed emotional recovery after taxane-based chemotherapy. Cancer 113:638–647. doi: 10.1002/cncr.23589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma W, Kyte SL, Bagdas D, et al. (2017) Effects of paclitaxel on the development of neuropathy and affective behaviors in the mouse. Neuropharmacology 117:305–315. doi: 10.1016/j.neuropharm.2017.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma W, Kyte SL, Bagdas D, et al. (2019) The α7 nicotinic receptor silent agonist R-47 prevents and reverses paclitaxel-induced peripheral neuropathy in mice without tolerance or altering nicotine reward and withdrawal. Exp Neurol 320:. doi: 10.1016/j.expneurol.2019.113010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichaya EG, Laumet G, Christian DL, et al. (2019) Motivational changes that develop in a mouse model of inflammation-induced depression are independent of indoleamine 2,3 dioxygenase. Neuropsychopharmacology 44:364–371. doi: 10.1038/s41386-018-0075-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner E, Krivitsky R, Cone K, et al. (2015) Evaluation of a Postoperative Pain-Like State on Motivated Behavior in Rats: Effects of Plantar Incision on Progressive-Ratio Food-Maintained Responding. Drug Dev Res 76:432–441. doi: 10.1002/ddr.21284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SG, Smith SB, Chesler EJ, et al. (2003) The heritability of antinociception: Common pharmacogenetic mediation of five neurochemically distinct analgesics. J Pharmacol Exp Ther 304:547–559. doi: 10.1124/jpet.102.041889 [DOI] [PubMed] [Google Scholar]
- Wozniak KM, Vornov JJ, Wu Y, et al. (2016) Sustained accumulation of microtubule-binding chemotherapy drugs in the peripheral nervous system: Correlations with time course and neurotoxic severity. Cancer Res 76:3332–3339. doi: 10.1158/0008-5472.CAN-15-2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1: Effect of Feeding Condition on Progressive Ratio Operant Responding for Palatable Food.


