Abstract
3,4-Methylenedioxypyrovalerone (MDPV), and other structurally-related synthetic cathinones, are popular alternatives to prototypical illicit psychostimulants, such as cocaine and methamphetamine. These drugs are often referred to as “bath salts”, and function either as cocaine-like inhibitors of monoamine uptake, or amphetamine-like substrates for dopamine (DAT), norepinephrine (NET), and serotonin (SERT) transporters. These studies used male Sprague-Dawley rats trained to discriminate MDPV from saline to evaluate the substitution profiles of structurally-related synthetic cathinones, cocaine, and other direct acting dopamine and noradrenergic receptor agonists in order to characterize the relative contributions of dopamine, norepinephrine, and serotonin to the discriminative stimulus effects of MDPV. As expected, each of the cathinones and cocaine dose-dependently increased MDPV-appropriate responding, with a rank order potency that was positively correlated with their potency to inhibit DAT and NET, but not SERT, a relationship that is consistent with the rank order to maintain self-administration. The dopamine D2/3 receptor-preferring agonist quinpirole produced a modest increase in MDPV-appropriate responding whilst the dopamine D1/5 receptor agonist, SKF 82958, non-selective dopamine receptor agonist, apomorphine, as well as the α–1, and α–2 adrenergic receptor agonists, phenylephrine and clonidine, respectively, failed to increase MDPV-appropriate responding at doses smaller than those that suppressed responding altogether. Although these studies do not support a role for serotonergic or adrenergic systems in mediating/modulating the discriminative stimulus effects of MDPV, convergent evidence is provided to suggest that the discriminative stimulus effects of MDPV are primarily mediated by its capacity to inhibit DAT, and the subsequent activation of dopamine D2 and/or D3 receptors.
Keywords: 3,4-Methylenedioxypyrovalerone; Synthetic cathinones; Drug discrimination; Structure Activity Relationship
Introduction
Synthetic cathinones emerged on the illicit drug market in the early-2000s in the form of “bath salts” preparations and quickly became popular alternatives to psychostimulants, such as cocaine and methamphetamine. Indeed, synthetic cathinones function as either cocaine-like inhibitors of monoamine uptake, or amphetamine-like substrates for dopamine (DAT), norepinephrine (NET), and serotonin (SERT) transporters (Baumann et al. 2012, 2013; Simmler et al. 2013, 2014; Eshleman et al. 2013, 2017). They garnered attention in popular press because of their status as “legal highs” as well as the large number of adverse effects, emergency room visits and abnormal behaviors associated with the use of various “bath salts” preparations (Spiller et al. 2011; Kyle et al. 2011; Murray et al. 2012; Johnson and Johnson 2014). Those admitted to the emergency room were reported to exhibit both psychiatric (paranoia, hallucinations, agitation) and physiological (tachycardia, hyperthermia) symptoms that sometimes resulted in death. The United States Drug Enforcement Agency (US DEA) currently lists 13 synthetic cathinones as Schedule I compounds (Drug Enforcement Administration, Department of Justice 2011, 2014), including MDPV, α-pyrrolidinopentiophenone (α-PVP), and other pyrrolidine-containing cathinones. However, clandestine laboratories have skirted these regulations by introducing slight chemical modifications to these structures, resulting in a large number of “legal” synthetic cathinone derivatives. In addition to differences in their regulatory status, structurally-distinct synthetic cathinone derivatives have important functional differences in their mechanisms of action. The modest structural differences produced by chemical modifications convey both differences in potency to inhibit uptake at and selectivity for DAT, NET, and SERT. For instance, the monoamine uptake inhibitor, 3,4-methylenedioxypyrovalerone (MDPV), inhibits uptake at DAT and NET with ~750-fold more potency than at SERT but upon regulation by the US DEA, was quickly replaced in “bath salts” preparations with other pyrrolidine-containing cathinones, such as α-PVP (Baumann et al. 2012; Simmler et al. 2013; Baumann et al. 2013; Eshleman et al. 2013, 2017; Gannon et al. 2018a) (Figure 1). Functional studies have suggested that cathinones containing a pyrrolidine ring (e.g., MDPV) generally function as uptake inhibitors at monoamine transporters whereas those lacking a pyrrolidine ring or containing smaller substituents (e.g., methylone) function as substrates for monoamine transporters. Furthermore, potency to inhibit uptake at DAT has been demonstrated to be enhanced by the presence of a methylenedioxy moiety (e.g., MDPV versus α-PVP) or a longer α-alkyl side chain (e.g., MDPV versus 3,4-methylenedioxy-α-pyrrolidinobutyrophenone [MDPBP]) on pyrrolidine-containing cathinones whereas selectivity for DAT over SERT is greater for compounds lacking a methylenedioxy moiety and having a longer α-alkyl side chain (e.g., α-PVP > α-pyrrolidinopropiophenone (α-PPP) > MDPV). Potency to inhibit uptake at NET has also been demonstrated to be primarily influenced by the length of the α-alkyl side chain, but the absence of a methylenedioxy moiety also results in modest increases in potency to inhibit uptake at NET (Eshleman et al. 2017). Our laboratory has previously demonstrated that the rank order potencies of structurally-related synthetic cathinones to maintain responding under a fixed ratio schedule of reinforcement are consistent with their potencies to inhibit uptake at DAT (Gannon et al. 2018b), and that there is a significant positive correlation between reinforcing effectiveness, as assessed by either a progressive ratio schedule of reinforcement or demand curve analyses, and selectivity to inhibit uptake at DAT relative to SERT (Gannon et al. 2018a).
Figure 1.
Chemical structure of the synthetic cathinone MDPV (upper) and structurally-related synthetic cathinones MDPBP (middle left), MDPPP (lower left) α-PVP (middle right) and α-PPP (lower right).
Although the discriminative stimulus effects of MDPV have been described in numerous studies (Fantegrossi et al. 2013; Gatch et al. 2013; Collins et al. 2016; Harvey and Baker 2016; Gannon et al. 2016; Berquist and Baker 2017; DeLarge et al. 2017; Harvey et al. 2017; Berquist et al. 2017; Risca and Baker 2019; Gatch and Forster 2020), the vast majority of these studies have characterized these effects in subjects trained to discriminate other stimulants from saline. From these studies, it is clear that the discriminative stimulus effects of MDPV overlap with those produced by d-amphetamine (Harvey et al. 2017), cocaine (Gatch et al. 2013; Collins et al. 2016; Gannon et al. 2016), methamphetamine (Gatch et al. 2013), and MDMA (to a lesser extent) (Gatch and Forster 2020). Similarities between the discriminative stimulus effects of MDPV and other commonly abused stimulants led to further investigation of the mechanism(s) mediating the discriminative stimulus effects of MDPV. In those studies, rodents trained to discriminate MDPV from saline were used to demonstrate that the discriminative stimulus effects of cocaine (Fantegrossi et al. 2013; Risca and Baker 2019) and methamphetamine (Fantegrossi et al. 2013; Berquist and Baker 2017) overlap with those of MDPV, while substitution studies with MDMA, a drug with strong serotonin releasing properties, were mixed, with one study reporting MDMA producing high levels (Fantegrossi et al. 2013), and another reporting low levels (Berquist and Baker 2017) of MDPV-appropriate responding. Similarly mixed results have been reported for substitution studies with fenfluramine, another drug with strong serotonin releasing properties, in MDPV-trained subjects (Berquist and Baker 2017). Moreover, both dopamine D1-like (Sch 23390) and D2-like (haloperidol) receptor antagonists have been shown to dose-dependently blunt MDPV-appropriate responding, whereas 5-HT2 (pirenperone), 5-HT2A (MDL100907), and 5-HT1A (WAY 100635) receptor antagonists are without effect (Risca and Baker 2019).
While these findings support the notion that the discriminative stimulus properties of MDPV are primarily mediated by dopaminergic rather than serotonergic mechanisms, the noradrenergic contribution to the discriminative stimulus effects of MDPV remains largely unknown. Although the indirect-acting noradrenergic agonist desipramine failed to increase MDPV-appropriate responding when administered alone, it did increase the effectiveness of a small dose of MDPV when administered as a pretreatment (Risca and Baker 2019). Given the similarities in the discriminative stimulus effects of MDPV and cocaine, it is plausible that the noradrenergic system might modulate the discriminative stimulus effects of MDPV as has been reported for cocaine (Cunningham and Callahan 1991; Spealman 1995; Kleven and Koek 1997).
Despite a growing literature, much remains to be investigated regarding the relative contributions of dopamine, serotonin and norepinephrine to the discriminative stimulus effects of MDPV and related synthetic cathinones. To further elucidate the mechanism mediating the discriminative stimulus effects of MDPV, the current study utilized a structure-activity approach in rats trained to discriminate MDPV from saline to test the hypotheses that: (1) the potencies of five structurally related synthetic cathinones (MDPV, MDPBP, 3,4-methylenedioxy-α-pyrrolidinopropiophenone (MDPPP), α-PVP, α-PPP) and cocaine to produce MDPV-appropriate responding is positively correlated with their potencies to inhibit uptake at DAT (and NET), but not SERT; and (2) direct-acting dopamine receptor agonists will produce high levels of MDPV-appropriate responding, whereas noradrenergic receptor agonists are only expected to produce modest increases in MDPV-appropriate responding.
Methods
Subjects
Eight male Sprague-Dawley rats (275–300 g upon arrival) were purchased from Envigo (Indianapolis, IN, USA) and maintained in a temperature- and humidity-controlled room. Rats were singly housed and maintained on a 14/10-hour light/dark cycle. All experiments were conducted during the light cycle with sessions conducted at approximately the same time each day. Rats were provided ad libitum access to water and fed 15g of Purina rat chow per day. All studies were carried out in accordance with the Institutional Animal Care and Use Committees of the University of Texas Health Science Center at San Antonio and the eighth edition of the Guide for Care and Use of Laboratory Animals (National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals 2011).
Drugs
3,4-methylenedioxypyrovalerone (MDPV), 3,4-methylenedioxy-α-pyrrolidinobutyrophenone (MDPBP), 3,4-methylenedioxy-α-pyrrolidinopropiophenone (MDPPP), α-pyrrolidinopentiophenone (α-PVP), α-pyrrolidinopropiophenone (α-PPP), and 3,4-methylenedioxy-N-methylcathinone (methylone) were synthesized as racemic HCl salts by Agnieszka Sulima and Kenner Rice at the Intramural Research Program of the National Institute on Drug Abuse (Bethesda, MD, USA). Cocaine and fentanyl were provided by the National Institute on Drug Abuse Drug Supply Program. Caffeine, nicotine, SKF 82958, quinpirole, apomorphine, clonidine, phenylephrine, and desipramine were purchased from Sigma-Aldrich (St. Louis, MO, USA). All drugs were dissolved in physiological saline. Drugs were administered via intraperitoneal (IP) injection in a volume of 1.0 ml/kg with the exception of doses of caffeine larger than 10 mg/kg, which were administered in a volume of 1.78 ml/kg due to the solubility limit of caffeine (~14 mg/ml).
Apparatus
All experiments were conducted in standard operant conditioning chambers located within ventilated, sound-attenuating enclosures (Med Associates, Inc., St. Albans, VT). The right wall was equipped with two response levers and two stimulus lights (2.5 cm in diameter) above each lever that could be illuminated with a 100-mA white light. A pellet trough was located between the two levers and allowed for the delivery of 45 mg sucrose pellets. Experimental events were controlled, and data were collected using MED-PC IV software and a PC-compatible interface (Med Associates, Inc.).
Discrimination Training
Rats were trained to discriminate MDPV (1.0 mg/kg) from saline using a two-lever discrimination procedure as described previously (Collins et al. 2016). Briefly, following an IP injection of either MDPV or saline, rats were placed in a dark operant chamber for a 10-minute pretreatment period. The start of the session was signaled by the illumination of a white lights above the levers, and completion of the ratio requirement on the injection-appropriate lever resulted in the delivery of a 45 mg sucrose pellet. Responding on the alternate lever reset the response requirement on the injection-appropriate lever. At the beginning of training, a single response on the injection-appropriate lever resulted in delivery of a sucrose pellet. The response requirement was gradually increased to a fixed ratio (FR) of 10, based on performance. Sessions were terminated after 20 minutes, or 50 sucrose pellets were delivered, whichever occurred first. The position of the MDPV-appropriate lever (i.e., left or right) was counterbalanced across rats. MDPV and saline training sessions were conducted in a pseudorandom order, with the restriction that one training condition was not repeated on more than 3 consecutive days.
Discrimination Testing
Testing began once rats met the acquisition criteria, defined as four consecutive sessions (or five of six) in which ≥80% of responses occurred on the injection-appropriate lever, both before delivery of the first reinforcer and for the entire session. During test sessions, completion of 10 consecutive responses on either lever resulted in the delivery of a sucrose pellet; responding on the alternate lever reset the response requirement. Test sessions were terminated after 20 minutes, or 50 sucrose pellets were delivered, whichever occurred first. MDPV time course data were generated by administering 1 mg/kg MDPV either 10, 15, 30, 45, 60, 75, 90, or 120 minutes prior to the start of the session. For pretreatments longer than 10 minutes, rats were returned to their home cages and placed into the chamber for the final 10 minutes of the total pretreatment time. Pretreatment times were pseudorandomized for each rat. To generate dose-response curves, all test compounds were administered 10 minutes prior to the start of the session. Test drug order was pseudorandomized, with a full dose-response curve generated for MDPV (0.178–1 mg/kg), MDPBP (0.178–1.78 mg/kg), MDPPP (1–10 mg/kg), α-PVP (0.32–3.2 mg/kg), α-PPP (0.32–5.6 mg/kg), methylone (0.56–5.6 mg/kg), cocaine (1–17.8 mg/kg), nicotine (0.056–1 mg/kg), and fentanyl (0.01–0.178 mg/kg) first, with dose-response curves for quinpirole (0.01–0.178 mg/kg), SKF-82958 (0.1–1 mg/kg), apomorphine (0.0178–0.32 mg/kg), clonidine (0.01–0.1 mg/kg), phenylephrine (0.178–1 mg/kg), and desipramine (1–10 mg/kg) evaluated second. For individual subjects, drugs were tested up to doses that either resulted in >80% MDPV-appropriate responding, or decreased response rates to <20% of the average response rate observed in the previous four saline training sessions. Three training sessions (two saline, one MDPV; pseudorandom order) were conducted between tests to ensure stability criteria (≥80% of responses occurred on the injection-appropriate lever before delivery of the first reinforcer and for the entire session) continued to be met.
Statistical Analyses
Linear regression of the portion of the log dose-response curve for individual subjects spanning the 20% to 80% effect levels was used to estimate the dose required to produce 50% MDPV-appropriate responding (ED50). One dose with >80% effect and one dose with <20% effect were included in these analyses for each drug. Data for percent MDPV-appropriate responding are reported as the mean ± standard error of the mean (S.E.M.) and were included in analyses only for sessions in which rats received at least one reinforcer. ED50 values were reported as mean and 95% confidence intervals. The ED50 values for two drugs were considered statistically different if their 95% confidence intervals did not overlap. Response-rate and maximal effectiveness data are reported as the mean ±S.E.M. rate of responding in responses per second. Linear regression was performed to determine the relationship between the dose of drug required to produce 50% MDPV-appropriate responding, and the concentration of drug previously reported to inhibit dopamine, norepinephrine, or serotonin uptake by 50% in rat brain synaptosomes (Gannon et al. 2018a). Prism 8 software (GraphPad Software Inc., La Jolla, CA, USA) was used to conduct statistical analyses and plot figures.
Results
Discrimination training and time course
Drug discrimination performance (i.e., % MDPV-appropriate responding) across sequential MDPV and saline training sessions is shown in Figure 2 (upper left panel). As shown in Figure 2 (upper right panel), all 8 rats met acquisition criteria within 60 sessions, with the average number of training sessions being 39.5 ±3.7. Rates of responding for the five MDPV and five saline training sessions that immediately preceded the start of testing were 0.57 ±0.05 and 0.58 ±0.07 responses per second, respectively; rates during MDPV and saline training sessions remained stable throughout the course of the experiment. Responding on the injection-appropriate lever during training sessions remained above 95% correct for both the first reinforcer as well as through the session as a whole throughout the remainder of the experiment. When evaluated under testing conditions, MDPV dose-dependently increased MDPV-appropriate responding, with all rats responding almost exclusively on the MDPV-appropriate lever after being administered 1 mg/kg MDPV (Figure 2; lower left panel). The ED50 was determined to be 0.54 [0.41, 0.71] mg/kg and the maximum effect was 99% ±1. Figure 2 (lower right panel) depicts the time course for the discriminative stimulus effects of the training dose of 1 mg/kg MDPV. MDPV produced time-dependent increases in responding on the MDPV-paired lever and analysis via a one-way ANOVA with repeated measures revealed a main effect of pretreatment time [F(2.493, 17.5)=11.0; p<0.05]. Greater than 80% of responding occurred on the MDPV-appropriate lever in 5 of 8 rats at 60 minutes, in 6 of 8 rats at 75 minutes, in 5 of 8 rats at 90 minutes, and in 0 of 8 rats at 120 minutes.
Figure 2.
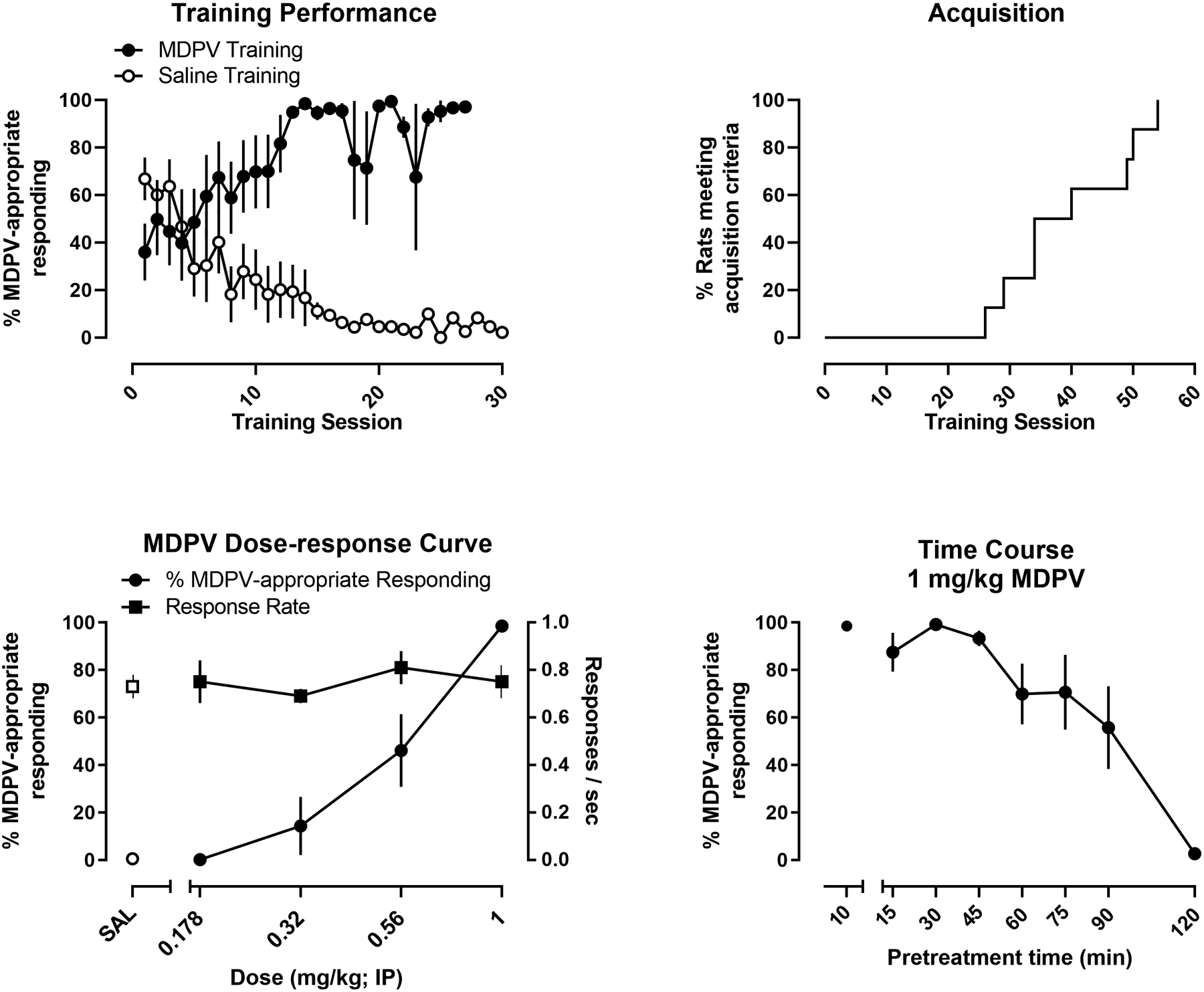
Upper left panel: Responding on the MDPV-appropriate lever during sequential training sessions preceded by MDPV (filled circles) or saline (open circles) injections. Upper right panel: Percentage of rats that met the acquisition criteria as a function of the number of training sessions Lower left panel: Percent MDPV-appropriate responding (filled circles) and rate (filled squares) as functions of MDPV dose. Lower right panel: Percent MDPV-appropriate responding following injection of 1 mg/kg MDPV as a function of pretreatment time. All data are represented as the mean ±S.E.M with the exception of the survival plot.
Substitution Tests
As shown in Figure 3 (left panels), MDPV as well as the other structurally related cathinones, α-PVP, α-PPP, MDPPP, MDPBP, and methylone all produced dose-dependent increases in MDPV-appropriate responding with a rank order potency of MDPV (ED50 = 0.54 [0.41, 0.71] mg/kg), MDPBP (ED50 = 0.77 [0.5, 1.2] mg/kg), α-PVP (ED50 = 1.0 [0.77, 1.4] mg/kg), α-PPP (ED50 = 1.4 [1.1, 1.7] mg/kg), MDPPP (ED50 = 2.46 [1.7, 3.7] mg/kg), and methylone (ED50 = 1.8 [1.2, 2.7] mg/kg). Regarding maximal effect, MDPV, α-PVP, α-PPP, MDPBP, and MDPPP all produced >95% MDPV-appropriate responding, whereas the maximum effect obtained with methylone was 88% ±18%. Although each pyrrolidine-containing cathinone produced high levels of MDPV-appropriate responding at doses smaller than those that suppressed responding, rate-decreasing effects limited the doses of methylone that could be tested, resulting in a maximum effect less than that produced by the other cathinones.
Figure 3.
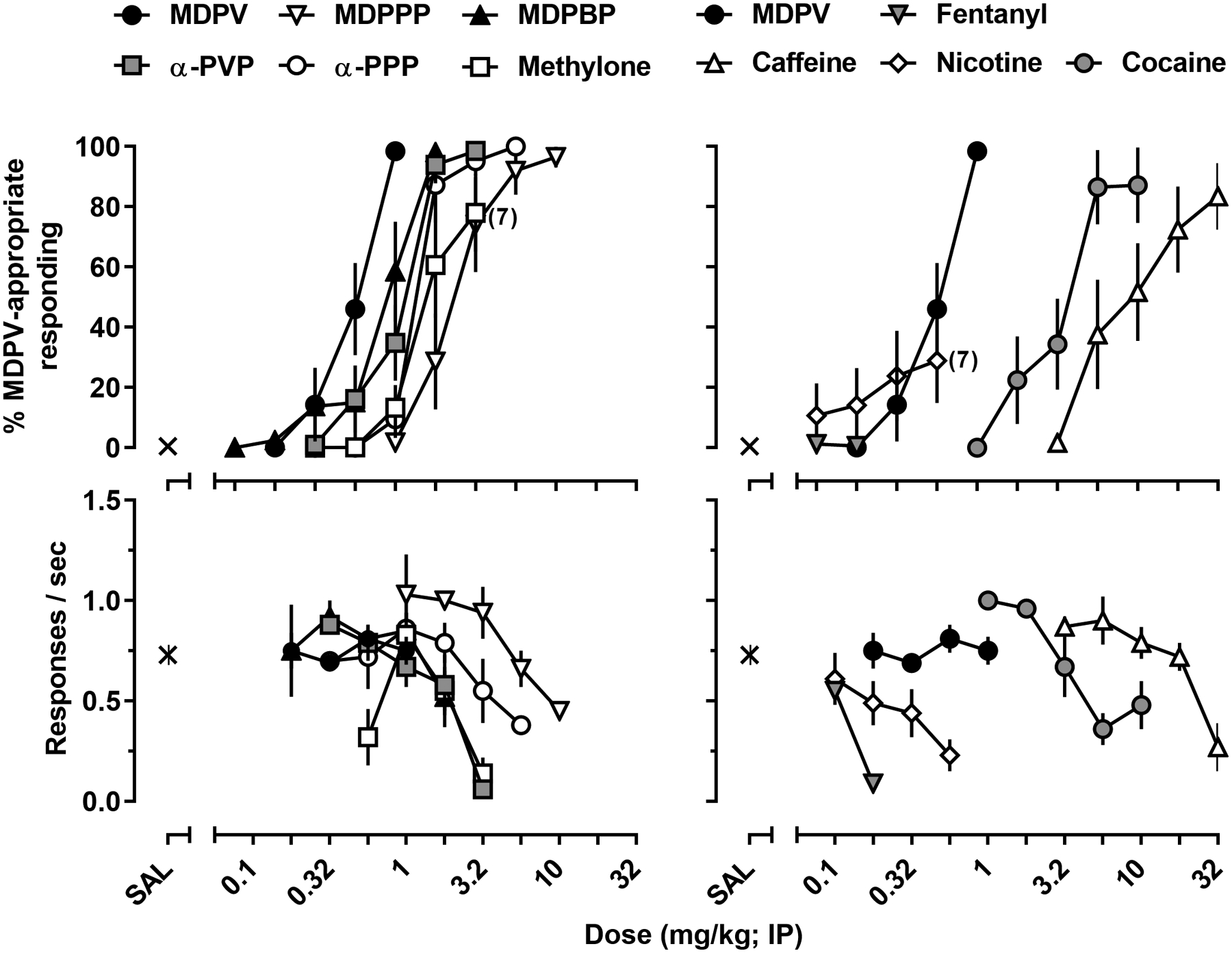
MDPV-like discriminative stimulus effects (upper left panel) and response rate (lower left panel) of MDPV, α-PVP, α-PPP, MDPPP, MDPBP, methylone (left panels) and MDPV, caffeine, fentanyl, nicotine and cocaine (right panels) in rats trained to discriminate MDPV (1 mg/kg) from saline. All data are represented as the mean ±S.E.M (n=8 unless otherwise shown).
The results of substitution tests with the non-cathinone drugs are shown in Figure 3 (right panels). Cocaine and caffeine both produced dose-dependent increases in MDPV-appropriate responding with ED50 values of 3.5 [2.1, 5.6] mg/kg, and 9.8 [5.4, 17.6] mg/kg, and maximal effects of 99% ±1, and 90% ±11, respectively. The mu-opioid receptor agonist, fentanyl, and nicotinic acetylcholine receptor agonist, nicotine, were also evaluated as negative controls. Although nicotine produced 100% MDPV-appropriate responding in one rat, nicotine and fentanyl failed to increase responding greater than 20% on the MDPV-appropriate lever in all other rats; nicotine and fentanyl were tested up to doses that suppressed response rate to ≤20% of that observed following saline administration.
Relationships between MDPV-like discriminative stimulus effects and potency to inhibit uptake at monoamine transporters
Linear regressions were performed between the potencies (ED50; mg/kg) of the pyrrolidine-containing cathinones and cocaine to increase MDPV-appropriate responding, and their potencies (IC50; μM) to inhibit uptake at DAT, NET, and SERT, as determined previously in rat brain synaptosomes (Gannon et al. 2018a). As shown in Figure 4, the potency of each drug to increase MDPV-appropriate responding showed a significant positive correlation with potency to inhibit uptake at DAT (R2=0.78; p=0.02), and no significant correlation with potency to inhibit uptake at NET (R2=0.51; p=0.11), or SERT (R2=0.09; p=0.57).
Figure 4.
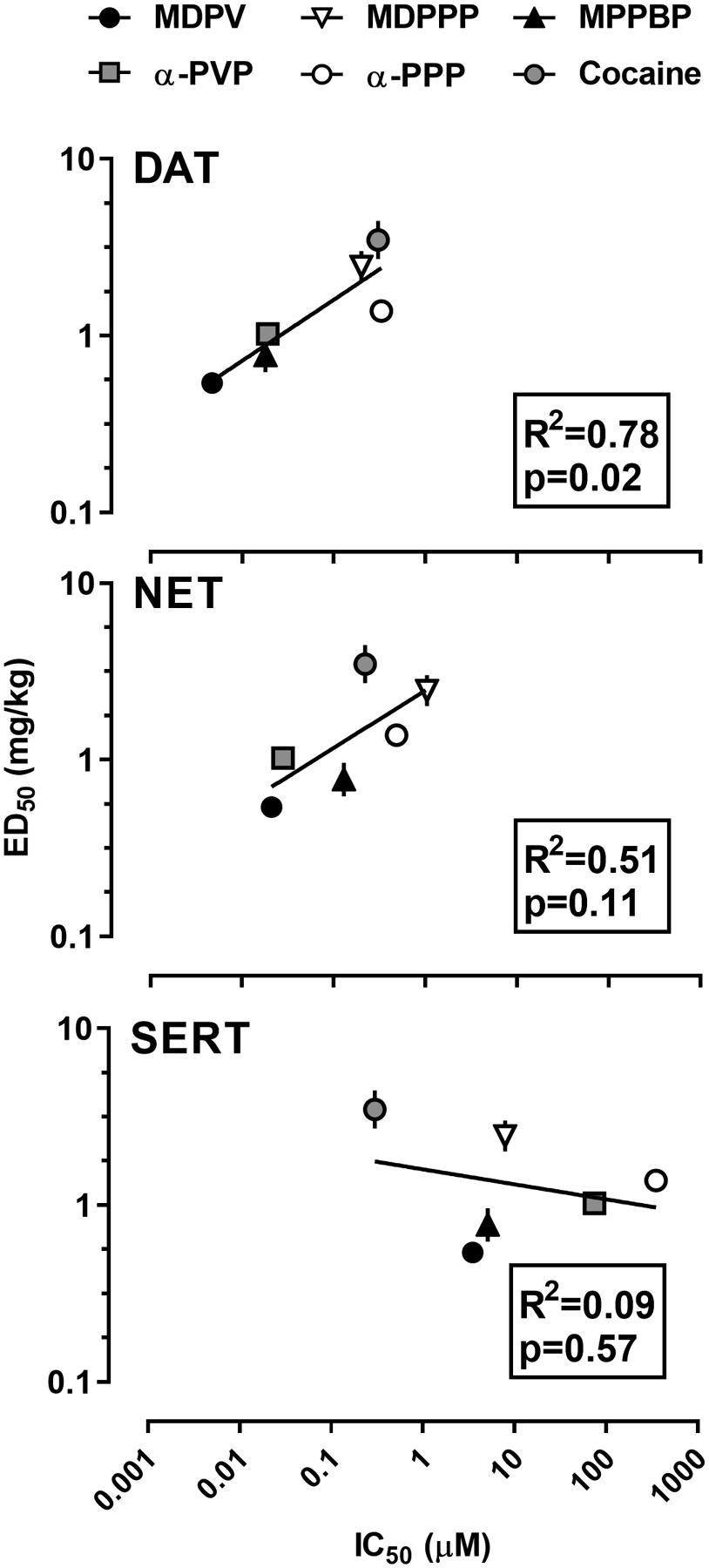
Correlational analyses of the relationships between potency to produce MDPV-appropriate responding and to inhibit uptake at monoamine transporters for MDPV (black circles), α-PVP (grey squares), α-PPP (open circles), MDPPP (downward triangles), MDPBP (upward triangles), and cocaine (grey circles). Abscissa: mean ±SEM potency (mg/kg) to produce MDPV-appropriate responding. Ordinate: Upper panel: Potency (μM) to inhibit [3H]dopamine uptake at DAT. Center panel: Potency (μM) to inhibit [3H]norepinephrine uptake at NET. Lower panel: Potency (μM) to inhibit [3H]serotonin uptake at SERT.
MDPV-like discriminative stimulus effects of dopaminergic and adrenergic receptor agonists
Given the MDPV-like discriminative stimulus effects of the cathinone derivatives and cocaine were significantly positively correlated with their potencies to inhibit monoamine uptake at DAT and the similar potencies of these compounds to inhibit monoamine uptake at DAT and NET, direct-acting agonists for dopaminergic and noradrenergic receptors were also evaluated for their ability to produce MDPV-appropriate responding (Figure 5). On the group level, dopamine receptor agonists produced only modest increases in MDPV-appropriate responding. The maximal effectiveness of the D2/D3 receptor-preferring agonist, quinpirole, was 47% ±27, while the D1/D5 receptor-preferring agonist, SKF 82958, and the relatively non-selective dopamine receptor agonist, apomorphine, produced maximal effects of 16% ±12 and 15% ±12, respectively (Figure 5; left panels). It should be noted that the doses of quinpirole that produced even low levels of MDPV-appropriate responding produced significant rate decreasing effects.
Figure 5.
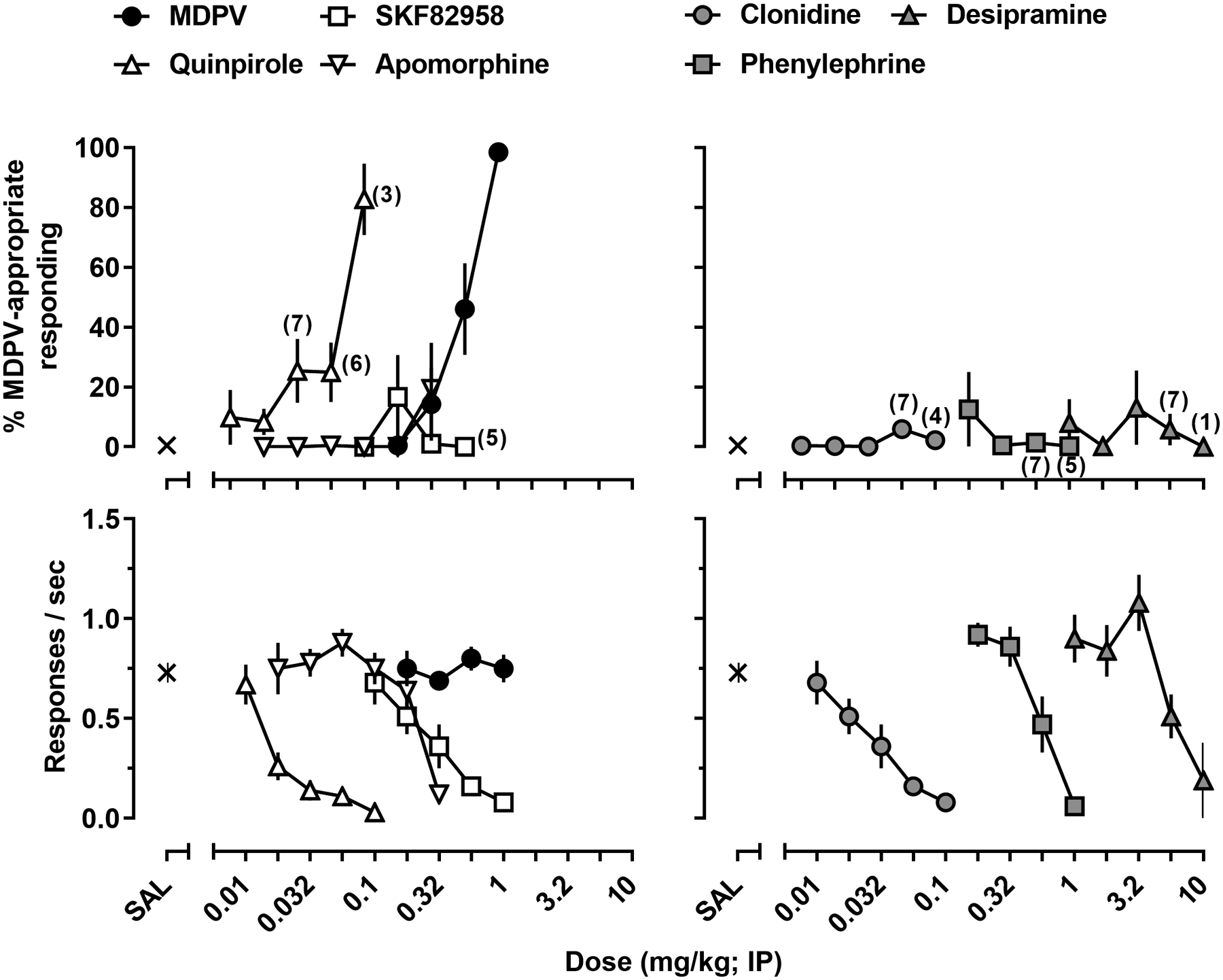
MDPV-like discriminative stimulus effects (upper panels) and response rate (lower panels) of MDPV, apomorphine, SKF82958, quinpirole (left panels) and clonidine, phenylephrine, and desipramine (right panels) in rats trained to discriminate MDPV (1 mg/kg) from saline. All data are represented as the mean ±S.E.M (n=8 unless otherwise shown).
Noradrenergic receptor agonists were not effective at producing MDPV-appropriate responding. The maximal effects of an indirect adrenergic receptor agonist, desipramine, an α−1 adrenergic receptor agonist, phenylephrine, and an α−2 adrenergic receptor agonist, clonidine, were 19% ±13, 2% ±1, and 6% ±3, respectively (Figure 5; right panels).
Discussion
Drug discrimination is a powerful tool to characterize the pharmacological properties of novel psychoactive stimulants (Fantegrossi et al. 2013; Gatch et al. 2013, 2015b, a, 2017, 2019, 2020; Harvey and Baker 2016; Gannon et al. 2016; Berquist and Baker 2017; Harvey et al. 2017; Berquist et al. 2017; Risca and Baker 2019; Gatch and Forster 2020). The current studies took advantage of the pharmacological specificity of the drug discrimination assay to characterize pharmacological differences in a series of structurally-related compounds in order to determine the relative roles of dopamine, norepinephrine, and serotonin in the discriminative stimulus properties of MDPV. There were four main findings: (1) all rats were trained to discriminate MDPV from saline, and MDPV increased responding on the MDPV-appropriate lever in a dose- and time-dependent manner; (2) every cathinone evaluated, in addition to cocaine and caffeine, produced high levels of MDPV-appropriate responding whereas fentanyl did not; (3) the dopamine D2/D3 receptor-preferring agonist quinpirole elicited responding on the MDPV-appropriate lever in the majority of subjects, whereas the other dopamine and noradrenergic receptor agonists produced little to no responding on the MDPV-appropriate lever in the majority of subjects; and (4) the potencies of pyrrolidine-containing cathinone and cocaine to produce MDPV-like discriminative stimulus effects were significantly positively correlated with their potencies to inhibit uptake at DAT but not at NET or SERT. Taken together, these data suggest that the discriminative stimulus effects of MDPV are primarily mediated by dopaminergic signaling.
The discriminative stimulus effects of 1 mg/kg MDPV served as a source of control over lever-responding and was demonstrated to be both dose and time-dependent. Stimulus control was established after an average of 39 training sessions, which is greater than the 15 training sessions reported by the only other study that has trained 1 mg/kg MDPV as a discriminative stimulus in rats (Berquist and Baker 2017). Curiously, in that study, 0.3 mg/kg MDPV produced high levels of MDPV-appropriate responding in contrast to the current study wherein administration of 0.32 mg/kg or 0.56 mg/kg MDPV produced ≤20% or ≤60% responding on the MDPV-appropriate lever, respectively. These differences may have arisen from procedural differences; in the current studies, rats were reinforced during test sessions as opposed to extinction conditions during test sessions in the previous study. This is the first study to characterize the time course of the discriminative stimulus effects of MDPV in rats. MDPV produced >80% of responding occurred on the MDPV-appropriate lever in the majority of subjects up to 90 minutes after administration. The prolonged stimulus control by MDPV observed in the current studies has also been demonstrated in mice (Fantegrossi et al. 2013) and is comparable to what has been observed with cocaine (Schechter 1997) and methamphetamine (Munzar et al. 1998).
Every pyrrolidine-containing cathinone tested (in addition to cocaine) dose-dependently increased MDPV-appropriate responding, each with a maximal effect of ≥95% responses being on the drug-paired lever. This finding is supported by previous literature demonstrating that that each of these drugs function as inhibitors of monoamine uptake (Baumann et al. 2012, 2013; Simmler et al. 2013, 2014; Eshleman et al. 2013, 2017; Gannon et al. 2018a) and share overlapping discriminative stimulus effects (Gatch et al. 2017; Gatch and Forster 2020). The non-pyrrolidine-containing synthetic cathinone methylone also produced MDPV-appropriate responding but produced a maximal effect slightly less than that of the pyrrolidine-containing cathinones or cocaine. Although these modest differences could be due to differences in their mechanism of action (i.e., inhibitor vs. substrate), or selectivity for dopamine relative to serotonin systems, it is also possible that the onset of rate decreasing effects by methylone interfered with our ability to observe similarly high levels of MDPV-appropriate responding. The latter is supported by the findings that, like MDPV, methylone has been shown to substitute for both methamphetamine and cocaine in drug discrimination assays (Gatch et al. 2013).
Interestingly, nicotine produced ≤20% MDPV-appropriate responding in the majority of subjects which is inconsistent with what has been observed in cocaine- (Desai et al. 1999, 2003) and methamphetamine-trained (Desai and Bergman 2010) rats. The underlying mechanism mediating the ability of nicotine to produce cocaine-appropriate responding is thought to be primarily dopaminergic in nature (Desai et al. 2003), so it is unclear why nicotine failed to increase MDPV-appropriate responding given its predominate actions on dopamine systems.
Although our laboratory has demonstrated that caffeine can enhance the discriminative stimulus properties of MDPV in rats trained to discriminate cocaine from saline (Collins et al. 2016), this is the first study to demonstrate that caffeine produces high levels of MDPV-appropriate responding in rats trained to discriminate MDPV from saline. This likely results from the ability of adenosine receptor antagonists, such as caffeine, to stimulate and/or enhance the activity of dopamine systems (Mumford and Holtzman 1991; Garrett and Holtzman 1994, 1996; Ferré 1997; Powell et al. 1999; Solinas et al. 2002).
The current studies utilized dopamine and noradrenergic receptor agonists to determine the mediators of the discriminative stimulus effects of MDPV. The dopamine D2-receptor-preferring agonist quinpirole produced high to moderate levels of MDPV-appropriate responding in the majority of animals whereas the dopamine D1-receptor-preferring agonist, SKF 82958, and the relatively non-selective dopamine receptor agonist, apomorphine, produced little to no responding on the MDPV-appropriate lever in the majority of rats suggesting that dopamine D2-like receptors primarily mediate the discriminative stimulus effects of MDPV. These data contrast (in part) previous studies demonstrating that the discriminative stimulus effects of MDPV can be blunted through dopamine D1 and D2 receptor antagonism, suggesting that both families of dopamine receptors are likely involved. This notion is also supported in rats trained to discriminate methamphetamine from saline, in which both D1 and D2 receptor agonists produce high levels of methamphetamine-appropriate responding (Munzar and Goldberg 2000). These discrepancies may be due, in part, to the use of dopaminergic agonists and antagonists to address the question. For instance, several conditions influence the discriminative stimulus effects of dopamine receptor agonists including feeding condition (Baladi and France 2010; Collins et al. 2014), schedule of reinforcement employed (Baladi et al. 2014), and/or the prominent rate decreasing effects of direct dopamine receptor agonists. Future studies could benefit by evaluating dopaminergic agonist substitution for MDPV under a stimulus shock termination schedule thereby circumventing the rate-decreasing effects.
To characterize the role of noradrenergic signaling in the discriminative stimulus effects of MDPV, direct α−1 (phenylephrine) and α−2 (clonidine), as well as indirect (desipramine) adrenoreceptor agonists were evaluated and demonstrated to be largely ineffective at producing MDPV-appropriate responding. Although desipramine produced 100% MDPV-appropriate responding in one subject, all other adrenergic drugs failed to produce MDPV-like discriminative stimulus effects. These data support previous findings demonstrating that desipramine does not produce MDPV-appropriate responding (Risca and Baker 2019). This is the first study to demonstrate that α-noradrenergic receptor agonists do not produce drug-lever responding in rats trained to discriminate MDPV from saline, consistent with what has been observed in rats trained to discriminate methamphetamine from saline (Munzar and Goldberg 1999). Although β-adrenergic receptor agonists were not evaluated in this study, one could surmise that agonism of these receptors would also be ineffective in producing MDPV-appropriate responding since it has been shown that antagonism of these receptors enhances the discriminative stimulus properties of cocaine (Kleven and Koek 1997). Interestingly, clonidine and desipramine have both been demonstrated to elicit responding on a cocaine-appropriate lever in rats trained to discriminate cocaine from saline (Wood et al. 1985; Quinton et al. 2006). It might be that the discriminative stimulus effects a lower training dose of MDPV might be conducive to noradrenergic agonists producing responding on the MDPV-appropriate lever, so the role of adrenergic signaling in the discriminative stimulus effects of MDPV cannot be entirely ruled out.
Due to the selectivity of MDPV to inhibit uptake at DAT relative to NET is only ~5-fold, it is difficult to parse out the relative contributions of dopamine and norepinephrine to the MDPV-like discriminative stimulus effects. We utilized a structure-activity approach through use of multiple synthetic cathinones whose slight structural differences convey differences in potency to inhibit uptake at DAT, NET, and SERT. For instance, pyrrolidine-containing cathinones containing a methylenedioxy moiety (e.g., MDPV versus α-PVP) or a longer α-alkyl side chain (e.g., MDPV versus MDPBP) have been demonstrated to exhibit increased potency to inhibit uptake at DAT. We observed a significant positive correlation between the potency of each pyrrolidine-containing cathinone and cocaine to produce MDPV-appropriate responding and potency to inhibit uptake at DAT (but not NET or SERT). While these correlations should be interpreted with caution given the relatively few data points, these data are consistent with previously published data from our laboratory, suggesting that both the reinforcing potency and potency to produce MDPV-like discriminative stimulus effects of these drugs are both positively correlated with their potency to inhibit uptake at DAT. Although the reinforcing effectiveness of these compounds was found to be positively correlated with selectivity to inhibit uptake at DAT relative to SERT, the relative selectivity of the cathinones for DAT over SERT (or NET) does not appear to be important to the MDPV-like discriminative stimulus effects of these compounds. This is in line with previous work that examined the discriminative stimulus effects of seven cathinones with various selectivities for DAT over SERT in rats trained to discriminate either methamphetamine or MDMA from saline and found that relative selectivity to inhibit DAT relative to SERT was not sufficient to predict the relative potency to substitute for methamphetamine compared to MDMA (Gatch et al. 2020). Taken together, these findings suggest that while serotonergic systems modulate the reinforcing effectiveness of monoamine inhibitors, a similar interaction between dopamine and serotonin signaling does not appear to exist with regard to the discriminative stimulus effects of MDPV.
In summary, the present data suggest that potency of the discriminative stimulus effects of stimulants are related to their potency to inhibit uptake at DAT rather than at NET or SERT. Furthermore, quinpirole producing MDPV-appropriate responding in most rats suggest a role for dopamine D2/D3 receptors in mediating the discriminative stimulus effects of MDPV. Finally, these data indicate that noradrenergic activity may not mediate the discriminative stimulus effects of MDPV. These findings are consistent with similar studies comparing the relative contributions of monoaminergic systems to the reinforcing effects of MDPV and related synthetic cathinones. Taken together, these results suggest that the reinforcing and discriminative stimulus effects of synthetic cathinones which impact their abuse liability are primarily mediated by their actions at DAT rather than NET or SERT.
Acknowledgements
The authors would like to thank Karen Jimenez and Melson Mesmin for their technical assistance in the completion of these studies. This research was supported by National Institutes of Health and National Institute on Drug Abuse (R01DA039146 [GTC] and R36DA050955 [MRD]), the jointly-sponsored National Institutes of Health Predoctoral Training Program in the Neurosciences (T32NS082145 [MRD]), and the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism (KCR).
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
References
- Baladi MG, France CP (2010) Eating high-fat chow increases the sensitivity of rats to quinpirole-induced discriminative stimulus effects and yawning. Behav Pharmacol 21:615–620. 10.1097/FBP.0b013e32833e7e5a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP (2014) Feeding condition and the relative contribution of different dopamine receptor subtypes to the discriminative stimulus effects of cocaine in rats. Psychopharmacology (Berl) 231:581–591. 10.1007/s00213-013-3271-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Partilla JS, et al. (2012) The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37:1192–1203. 10.1038/npp.2011.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, et al. (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacology 38:552–562. 10.1038/npp.2012.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquist MD, Baker LE (2017) Characterization of the discriminative stimulus effects of 3,4-methylenedioxypyrovalerone in male Sprague-Dawley rats. Behav Pharmacol 28:394–400. 10.1097/FBP.0000000000000310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquist MD, Thompson NA, Baker LE (2017) Evaluation of training dose in male Sprague-Dawley rats trained to discriminate 4-methylmethcathinone. Psychopharmacology (Berl) 234:3271–3278. 10.1007/s00213-017-4716-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Abbott M, Galindo K, et al. (2016) Discriminative Stimulus Effects of Binary Drug Mixtures: Studies with Cocaine, MDPV, and Caffeine. J Pharmacol Exp Ther 359:1–10. 10.1124/jpet.116.234252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Jackson JA, Koek W, France CP (2014) Effects of dopamine D(2)-like receptor agonists in mice trained to discriminate cocaine from saline: influence of feeding condition. Eur J Pharmacol 729:123–131. 10.1016/j.ejphar.2014.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Callahan PM (1991) Monoamine reuptake inhibitors enhance the discriminative state induced by cocaine in the rat. Psychopharmacology (Berl) 104:177–180. 10.1007/BF02244175 [DOI] [PubMed] [Google Scholar]
- DeLarge AF, Erwin LL, Winsauer PJ (2017) Atypical binding at dopamine and serotonin transporters contribute to the discriminative stimulus effects of mephedrone. Neuropharmacology 119:62–75. 10.1016/j.neuropharm.2017.04.006 [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P (1999) Asymmetric generalization between the discriminative stimulus effects of nicotine and cocaine. Behav Pharmacol 10:647–656. 10.1097/00008877-199911000-00011 [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P (2003) Dopaminergic and cholinergic involvement in the discriminative stimulus effects of nicotine and cocaine in rats. Psychopharmacology (Berl) 167:335–343. 10.1007/s00213-003-1426-x [DOI] [PubMed] [Google Scholar]
- Desai RI, Bergman J (2010) Drug discrimination in methamphetamine-trained rats: effects of cholinergic nicotinic compounds. J Pharmacol Exp Ther 335:807–816. 10.1124/jpet.110.173773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration, Department of Justice (2011) Schedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed Regist 76:65371–65375 [PubMed] [Google Scholar]
- Drug Enforcement Administration, Department of Justice (2014) Schedules of controlled substances: temporary placement of 10 synthetic cathinones into Schedule I. Final order. Fed Regist 79:12938–12943 [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, et al. (2013) Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol 85:1803–1815. 10.1016/j.bcp.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Reed JF, et al. (2017) Structure-Activity Relationships of Substituted Cathinones, with Transporter Binding, Uptake, and Release. J Pharmacol Exp Ther 360:33–47. 10.1124/jpet.116.236349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC (2013) In vivo effects of abused “bath salt” constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology 38:563–573. 10.1038/npp.2012.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S (1997) Adenosine-dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology (Berl) 133:107–120. 10.1007/s002130050380 [DOI] [PubMed] [Google Scholar]
- Gannon BM, Baumann MH, Walther D, et al. (2018a) The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology 43:2399–2407. 10.1038/s41386-018-0209-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Mesmin MP, et al. (2018b) Relative reinforcing effects of second-generation synthetic cathinones: Acquisition of self-administration and fixed ratio dose-response curves in rats. Neuropharmacology 134:28–35. 10.1016/j.neuropharm.2017.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, et al. (2016) Stereoselective Effects of Abused “Bath Salt” Constituent 3,4-Methylenedioxypyrovalerone in Mice: Drug Discrimination, Locomotor Activity, and Thermoregulation. J Pharmacol Exp Ther 356:615–623. 10.1124/jpet.115.229500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett BE, Holtzman SG (1994) D1 and D2 dopamine receptor antagonists block caffeine-induced stimulation of locomotor activity in rats. Pharmacol Biochem Behav 47:89–94. 10.1016/0091-3057(94)90115-5 [DOI] [PubMed] [Google Scholar]
- Garrett BE, Holtzman SG (1996) Comparison of the effects of prototypical behavioral stimulants on locomotor activity and rotational behavior in rats. Pharmacol Biochem Behav 54:469–477. 10.1016/0091-3057(95)02209-0 [DOI] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ (2015a) Comparative Behavioral Pharmacology of Three Pyrrolidine-Containing Synthetic Cathinone Derivatives. J Pharmacol Exp Ther 354:103–110. 10.1124/jpet.115.223586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ (2017) Locomotor activity and discriminative stimulus effects of a novel series of synthetic cathinone analogs in mice and rats. Psychopharmacology (Berl) 234:1237–1245. 10.1007/s00213-017-4562-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ (2019) Locomotor activity and discriminative stimulus effects of five novel synthetic cathinone analogs in mice and rats. Drug Alcohol Depend 199:50–58. 10.1016/j.drugalcdep.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ (2020) Methylenedioxymethamphetamine-like discriminative stimulus effects of seven cathinones in rats. Behav Pharmacol 31:378–384. 10.1097/FBP.0000000000000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ (2020) Methylenedioxymethamphetamine-like discriminative stimulus effects of pyrrolidinyl cathinones in rats. J Psychopharmacol (Oxford) 34:778–785. 10.1177/0269881120914213 [DOI] [PubMed] [Google Scholar]
- Gatch MB, Rutledge MA, Forster MJ (2015b) Discriminative and locomotor effects of five synthetic cathinones in rats and mice. Psychopharmacology (Berl) 232:1197–1205. 10.1007/s00213-014-3755-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ (2013) Locomotor stimulant and discriminative stimulus effects of “bath salt” cathinones. Behav Pharmacol 24:437–447. 10.1097/FBP.0b013e328364166d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EL, Baker LE (2016) Differential effects of 3,4-methylenedioxypyrovalerone (MDPV) and 4-methylmethcathinone (mephedrone) in rats trained to discriminate MDMA or a d-amphetamine + MDMA mixture. Psychopharmacology (Berl) 233:673–680. 10.1007/s00213-015-4142-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EL, Burroughs RL, Baker LE (2017) Effects of D1 and D2 receptor antagonists on the discriminative stimulus effects of methylendioxypyrovalerone and mephedrone in male Sprague-Dawley rats trained to discriminate D-amphetamine. Behav Pharmacol 28:586–589. 10.1097/FBP.0000000000000328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS, Johnson MW (2014) Investigation of “bath salts” use patterns within an online sample of users in the United States. J Psychoactive Drugs 46:369–378. 10.1080/02791072.2014.962717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Koek W (1997) Discriminative stimulus properties of cocaine: enhancement by beta-adrenergic receptor antagonists. Psychopharmacology (Berl) 131:307–312. 10.1007/s002130050297 [DOI] [PubMed] [Google Scholar]
- Kyle PB, Iverson RB, Gajagowni RG, Spencer L (2011) Illicit bath salts: not for bathing. J Miss State Med Assoc 52:375–377 [PubMed] [Google Scholar]
- Mumford GK, Holtzman SG (1991) Qualitative differences in the discriminative stimulus effects of low and high doses of caffeine in the rat. J Pharmacol Exp Ther 258:857–865 [PubMed] [Google Scholar]
- Munzar P, Nosál R, Goldberg SR (1998) Potentiation of the discriminative-stimulus effects of methamphetamine by the histamine H3 receptor antagonist thioperamide in rats. Eur J Pharmacol 363:93–101. 10.1016/s0014-2999(98)00789-4 [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg SR (1999) Noradrenergic modulation of the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology (Berl) 143:293–301. 10.1007/s002130050950 [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg SR (2000) Dopaminergic involvement in the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology 148:209–216. 10.1007/s002130050044 [DOI] [PubMed] [Google Scholar]
- Murray BL, Murphy CM, Beuhler MC (2012) Death following recreational use of designer drug “bath salts” containing 3,4-Methylenedioxypyrovalerone (MDPV). J Med Toxicol 8:69–75. 10.1007/s13181-011-0196-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for the Care and Use of Laboratory Animals, 8th edn. National Academies Press (US), Washington (DC) [Google Scholar]
- Powell KR, Koppelman LF, Holtzman SG (1999) Differential involvement of dopamine in mediating the discriminative stimulus effects of low and high doses of caffeine in rats. Behav Pharmacol 10:707–716. 10.1097/00008877-199912000-00001 [DOI] [PubMed] [Google Scholar]
- Quinton MS, Gerak LR, Moerschbaecher JM, Winsauer PJ (2006) Effects of pregnanolone in rats discriminating cocaine. Pharmacol Biochem Behav 85:385–392. 10.1016/j.pbb.2006.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risca HI, Baker LE (2019) Contribution of monoaminergic mechanisms to the discriminative stimulus effects of 3,4-methylenedioxypyrovalerone (MDPV) in Sprague-Dawley rats. Psychopharmacology (Berl) 236:963–971. 10.1007/s00213-018-5145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter MD (1997) Discriminative characteristics of high and low cocaine administration: effect of other psychostimulants. Pharmacol Biochem Behav 56:457–463. 10.1016/s0091-3057(96)00301-2 [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, et al. (2013) Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168:458–470. 10.1111/j.1476-5381.2012.02145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Hoener MC, Liechti ME (2014) Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology 79:152–160. 10.1016/j.neuropharm.2013.11.008 [DOI] [PubMed] [Google Scholar]
- Solinas M, Ferré S, You Z-B, et al. (2002) Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci 22:6321–6324. https://doi.org/20026640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD (1995) Noradrenergic involvement in the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 275:53–62 [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J (2011) Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila) 49:499–505. 10.3109/15563650.2011.590812 [DOI] [PubMed] [Google Scholar]
- Wood DM, Lal H, Yaden S, Emmett-Oglesby MW (1985) One-way generalization of clonidine to the discriminative stimulus produced by cocaine. Pharmacol Biochem Behav 23:529–533. 10.1016/0091-3057(85)90414-9 [DOI] [PubMed] [Google Scholar]


