Abstract
Aim:
Mitophagy is the regulated process that targets damaged or dysfunctional mitochondria for lysosomal-mediated removal. This process is an essential element of mitochondrial quality control, and dysregulation of mitophagy may contribute to a host of diseases, most notably neurodegenerative conditions such as Parkinson’s disease. Mitochondria targeted for mitophagic destruction are molecularly marked by the ubiquitination of several outer mitochondrial membrane (OMM) proteins. This ubiquitination is positively regulated, in part, by the mitochondrial-targeted kinase PINK1 and the E3 ubiquitin ligase Parkin. In contrast, the reverse phenomenon, deubiquitination, removes ubiquitin from Parkin substrates embedded in the OMM proteins, antagonizing mitophagy. Recent evidence suggests that the mitochondrial deubiquitinase USP30 negatively regulates Parkin mediated mitophagy, providing opportunities to identify USP30 inhibitors and test for their effects in augmenting mitophagy. Here we will characterize a USP30 inhibitor and demonstrate how the pharmacological inhibition of USP30 can augment stress-induced mitophagic flux.
Methods:
We have conducted mitophagy and mitochondrial analyses in cultured cells. We have determined the plasma pharmacokinetics of the USP30 inhibitor in mice and conducted analyses using the mt-Keima mice to measure in vivo mitophagy directly.
Results:
The compound has minimal mitochondrial toxicity in cultured cells and is tolerated well in mice. Interestingly, we demonstrated tissue-specific induction of mitophagy following USP30 pharmacological inhibition. In particular, pharmacological inhibition of USP30 induces a significant increase in cardiac mitophagy without detriment to cardiac function.
Conclusion:
Our data support the evidence that USP30 inhibition may serve as a specific strategy to selectively increase mitophagic flux, allowing for the development of novel therapeutic approaches.
Keywords: Mitophagy, USP30, mitochondrial deubiquitination, PINK1, Parkin, mt-Keima
Introduction
Mitophagy is emerging as a critical regulator of mitochondrial homeostasis at both the cellular and organismal level1–3. Mitophagy declines with age and, dysregulation of mitophagy is associated with a wide range of age-related human diseases, most notably neurodegenerative conditions such as Parkinson’s disease (PD)3–7. Our understanding of the mechanisms that govern mitophagy and regulate the removal of mitochondria upon mitochondrial damage has advanced vastly6,8. Evidence suggests that mitophagy requires the post-translational tagging of multiple outer mitochondrial membrane proteins (OMM) with ubiquitin to signal and recruit the autophagosomal machinery9–11. The E3-ubiquitin ligase, Parkin, has been implicated as a critical enzyme that catalyzes the ubiquitination of a wide range of mitochondrial proteins12. Parkin’s recruitment to the mitochondria involves the PTEN-induced putative kinase 1 (PINK1), a mitochondrial-targeted kinase whose stability is regulated, at least in part, by mitochondrial membrane potential, suggesting that PINK1 and Parkin function in the same biochemical pathway 13–15. Loss-of-function mutations in PINK1 or Parkin also have been identified as a cause for familial, early-onset Parkinson disease, strengthening the premise that these two proteins are critical for optimal mitochondrial quality control16,17. The use of innovative mouse models and patient-derived induced pluripotent stem cells (iPSCs) has dramatically enhanced our ability to further explore the clinical relevance of mitophagy and create therapies where mitophagy modulation may prove beneficial2,18–20.
Much remains to be understood regarding the additional inputs into the PINK1-Parkin’s regulatory hub, catalyzing the forward reaction to ubiquitinate a range of OMM proteins and controlling mitophagy. As yet, relatively little is known regarding the reverse phenomenon’s role, i.e., deubiquitination, during mitophagy activation, and how these effectors contribute to mitophagy regulation21–23. Deubiquitination is thought to be carried out enzymatically by a large family of proteins termed deubiquitinating enzymes or DUBs. Mitochondria have at least three DUBs, including the Ub-specific protease 8 (USP8), USP15, and USP30, which together appear to antagonize Parkin’s ability to regulate mitophagic flux19,22,24–26. Of these, perhaps the most convincing physiological evidence linking DUBs to mitophagy has come from the study of USP3021. USP30 is a deubiquitylase constitutively associated with the OMM21,22. Recent advances have demonstrated that USP30 can counteract Parkin-dependent mitophagy by deubiquitylating OMM proteins, including TOMM2021,22. In addition, genetic manipulations of USP30 have begun to elucidate how this enzyme contributes to the process of mitophagy21,22. In cultured cells, including neurons, USP30 overexpression inhibits mitophagy, and this effect is not seen when a catalytically inactive mutant of USP30 is employed21. Furthermore, the knockdown of USP30 enhances mitophagy in cultured cells21. Interestingly, knockdown of USP30 can rescue the defect in mitophagy seen in Parkin or PINK1-deficient flies21. These, and subsequent observations22,27, have suggested that inhibiting USP30 might provide a specific strategy to increase mitophagic flux selectively. Identifying specific small-molecule inhibitors of USP30 will offer valuable opportunities to dissect the role of mitophagy pharmacologically in health and disease28. Recent efforts have generated a few USP30 inhibitors, exemplified by some N-cyano pyrrolidines and a racemic phenylalanine derivative29,30, to lower the threshold for mitophagy induction and stimulate stress induced mitophagy. However, much of the current data rely on cells engineered to overexpress Parkin. Much less understood are the inhibitors of USP30 and whether inhibiting USP30 will activate mitophagy in vivo. Assessing the role of USP30 inhibition in mice may further enable the clinical development of USP30 inhibitors in a wide range of age-related human diseases.
Result
ST-539 inhibits USP30 and promotes mitophagy
The enzymatic activity of Parkin is to function as an E3 ubiquitin ligase12,31. Evidence suggests that Parkin can ubiquitinate a wide range of OMM proteins, including the translocase of outer membrane 20 (TOM20)19,21,31. To investigate TOM20 ubiquitination during mitophagy, we treated HeLa cells stably expressing YFP-Parkin (Hela-Parkin) for up to 18 h with both the mitochondrial complex III inhibitor antimycin A and the ATP synthase inhibitor oligomycin (antimycin A/oligomycin/ [A/O]; Figure 1a). Consistent with previous observations, we confirmed that in HeLa cells engineered to express Parkin, the addition of a cocktail of mitochondrial inhibitors triggered ubiquitination of TOM20, and the subsequent fall in TOM20 protein levels19,21,29 (Figure 1a). A/O treatment also results in a decline in the abundance of the OMM protein TOM40 and the inner mitochondrial protein TIM23 (Figure 1a). Previous results from cell culture experiments and flies suggest that USP30 can deubiquitylate OMM proteins and antagonize Parkin’s activity21. In this context, increased expression of USP30 blocked the accumulation of ubiquitinated TOM20 and prevented the destruction of this outer mitochondrial protein, as well as TOM40 and TIM23, after A/O treatment (Figure 1a). We then employed this cellular assay to monitor TOM20 turnover, and analyzed compounds known to be associated with the ubiquitination process for inhibitors of USP30 (data not shown). From our screen, the most potent mitophagy inducer was ST-539, a racemic phenylalanine derivative (Figure 1b). Previous studies report that this compound and similar structures selectively inhibit USP30 enzyme function in vitro30. We defined the effects of this chemical using biochemical assays. With ST-539 at 10 μg/ml, we found A/O treatment caused a ubiquitinated TOM20 and a loss of TOM20 protein level by ~90% (Figure 1c), confirming inhibited degradation of TOM20 in the presence of USP30. A close correlation exists between the TOM20 ubiquitination and the ST-539 dosage (Figure 1c). Initially to assess mitophagy in cells, we monitored the degradation of the inner membrane protein, the translocase of the inner membrane 23 (TIM23), by immunoblotting (Supplemental Figure 1a). Following 18h-A/O treatment, immunoblotting showed the protein levels of TIM23 and TOM40 decreased significantly in Hela-Parkin cells (Supplemental Figure 1a and 1b). USP30’s overexpression prevented a reduction in TIM23 and TOM40 levels. Moreover, ST-539 restored TIM23 and TOM40 degradation indicating ST-539 could promote mitophagy via USP30 inhibition (Supplemental Figure 1a and 1b).
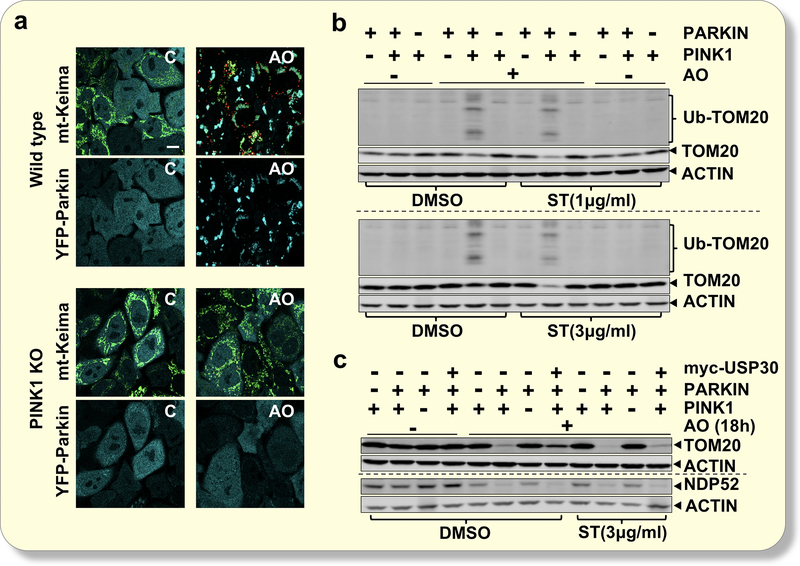
Figure 1. A USP30 inhibitor, St-539, promotes mitophagy.
Analysis of TOM20 ubiquitination, as well as levels of TOM20, TIM23, and TOM40 in the presence or absence of A/O treatment in Hela cells expressing Parkin or Parkin/Myc-tagged USP30. Shown is one representative western blot from three independent experiments, all providing similar results. (b) Chemical structure of ST-539. (c) Representative immunoblotting for TOM20 TOM40 and TIM23 in Hela cells expressing Parkin or Parkin/Myc-tagged USP30. Prior to A/O treatment, cultures were treated with ST-539 at indicated concentrations. (d) Representative FACS analysis of AO-induced mitophagy using mt-Keima fluorescence. Cells were treated with A/O for 2h and analyzed by FACS for lysosomal positive mt-Keima (pH4). Prior to A/O treatment, cultures were treated with DMSO or ST-539(3μg/ml).
We employed a sensitive fluorescence-activated cell sorting (FACS)-based method for detecting mitophagy using the pH-dependent fluorescent protein Keima31,32. Keima is a pH-sensitive, dual-excitation ratiometric fluorescent protein that can be targeted to the mitochondrial matrix utilizing the mitochondria-targeting sequence from COX VIII, allowing for the detection of mitophagy (mt-Keima)32. When mt-Keima is present in the physiological pH of the mitochondria (pH 8.0), the shorter-wavelength excitation predominates; mt-Keima undergoes a shift to longer-wavelength excitation in acidic conditions, such as in autolysosomes (pH 4.5)32. We exploited the mt-Keima probe and measured mitophagy in HeLa-Parkin cells and HeLa-Parkin cells that express USP30. Only ~1% of HeLa-Parkin cells display mitophagy under basal conditions. Consistent with previous observations, we confirmed that A/O induced a rapid and marked increase in overall red mt-Keima fluorescence, consistent with increased mitophagy (Figure 1d). HeLa-Parkin cells expressing USP30 showed minimal increase in mitophagy after A/O treatment. Interestingly, ST-539 treatment restored A/O induced mitophagy in HeLa-Parkin cells expressing USP30 (Figure 1d).
PINK1 and Parkin are required for ST-539 induced ubiquitination
Genetic suppression of USP30 in Parkin-overexpressing cells promotes the clearance of mitochondria in response to mitochondrial depolarizing agents19,21,29. Recent evidence suggests endogenous Parkin expression is sufficient to initiate depolarization-induced mitophagy19. To investigate if the PINK1/Parkin pathway is required for ST-539 induced mitophagy, we analyzed TOM20 ubiquitination in wild-type (WT) HeLa cells, which express little to no endogenous Parkin33, as well as in WT and PINK1 knockout (KO) HeLa cells stably expressing YFP-tagged Parkin31. In PINK1 KO cells, A/O induced Parkin mitochondrial translocation and mitophagy flux was inhibited (Figure 2a and Supplemental Figure 2). Moreover, the loss of PINK1 was sufficient to prevent A/O induced TOM20 ubiquitination and mitophagy (Figure 2b). Under the baseline, all three cell lines exhibited a similar degree of TOM20 ubiquitination and protein level (Figure 2b). However, A/O induced TOM20 ubiquitination and a decline in the TOM20 level only in cells expressing both ectopic Parkin and endogenous PINK1 (Figure 2b), which was further potentiated by the addition of ST-539 (Figure 2b). Long term treatment (18 h) of ST-539 did not promote TOM20 ubiquitination independent of the functional PINK1/Parkin pathway (Figure 2c). A more significant reduction in NDP52, a mitophagy receptor that signals autophagosome assembly proximal to the individual damaged mitochondria31, and mitochondrial protein TOM20 levels occurred in the presence of ST-539 in Parkin positive cells relative to the vehicle treated cells upon A/O, but not in WT Hela or PINK1 KO cells (Figure 2c). These results demonstrate that following A/O, although ST-539 promoted ubiquitination and mitophagy occurs, PINK1/Parkin activity is necessary for ST-539’s activity.
Figure 2. Inhibition of USP30 by ST-539 requires PINK1 and Parkin.
(a) Representative confocal images of wild type (WT) and PINK1 KO cells expressing YFP-Parkin and mt-Keima following 2h DMSO (C) or A/O treatment. The mt-Keima emission signal obtained after excitation with the 458-nm laser is shown in green, and that obtained after excitation with the 561-nm laser is shown in red. After treatment with A/O, recruitment of Parkin to the mitochondria (shown in cyan) and an increase in mitophagy (shown in red) can be observed in WT HeLa cells but not in PINK1 KO cells. Scale bar: 20 μm. (b) Analysis of TOM20 ubiquitination in Hela cells, Hela cells expressing YFP-Parkin and PINK1 KO Hela cells expressing YFP-Parkin. Prior to A/O treatment, cultures were treated with ST-539 at 1 or 3μg/ml. (c) Representative immunoblotting for TOM20 and NDP52 in Hela cells, Hela cells expressing YFP-Parkin and PINK1 KO Hela cells expressing YFP-Parkin or Parkin/Myc-tagged USP30 following A/O treatment for 18h. Prior to A/O treatment, cultures were treated with 3μg/ml ST-539.
Effects of USP30 inhibition on mitochondrial function
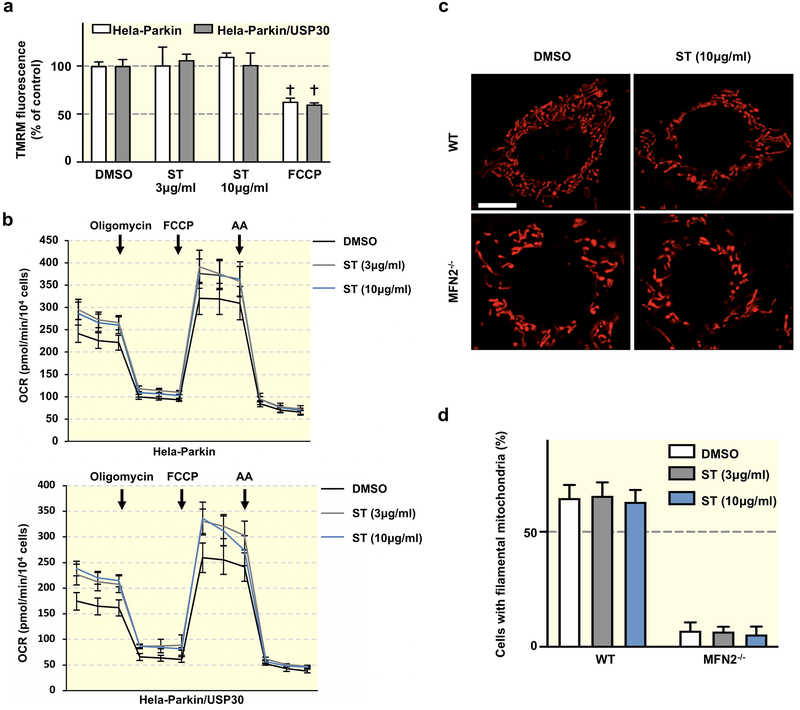
We next investigated if the pharmacological inhibition of USP30 by ST-539 could influence mitochondrial functions. We initially analyzed mitochondrial function using Tetramethylrhodamine (TMRM), a dye requiring mitochondrial membrane potential to accumulate within the mitochondria34. ST-539 treatment with concentrations of 3 to 10 μg/ml did not block TMRM staining, which is in contrast to the significant loss of staining when using Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) treatment (Figure 3a). The result indicates that ST-539 may not disrupt mitochondrial membrane potential. Next, we measured oxygen consumption using the Seahorse assay following 24 h of ST-539 treatment. Interestingly, we detected a slight increase in basal and maximal respiration in Hela-Parkin and Hela-Parkin cells expressing USP30 following ST-539 treatment at high dose (Figure 3b). Cell proliferation was maintained after ST-539 treatment for 24 hours (data not shown). Previous studies revealed that USP30 participates in maintaining mitochondrial morphology35,36. To investigate ST-539’s role in potential regulation of the mitochondrial dynamic processes, we treated Mfn2-knockout (Mfn2−/−) MEF cells containing a fragmented mitochondrial network characterized by short, rod-shaped mitochondria37. However, the addition of 3 or 10 μg /ml ST-539 did not induce the elongation of mitochondria significantly (Figure 3c). Similarly, ST-539 failed to change the mitochondrial morphology in the wild type MEFs (Figure 3c). Together the data imply that the pharmacological inhibition of USP30 by ST-539 marginally alters mitochondrial function, regardless of USP30 expression.
Figure 3. ST-539 has minimal effect on mitochondrial function in cells.
(a) The bar graph shows quantification of TMRM signal in Hela-Parkin or Hela-Parkin/USP30 cells in the presence or absence of ST-539 (ST, 3 or 10μg/ml) or FCCP (10μM). The intensity of TMRM reflects the level of mitochondrial membrane potential (ΔΨm). Values are normalized to the vehicle (DMSO) treated samples. Data represent mean ± SD of three independent experiments. (b) Hela-Parkin (upper) or Hela-Parkin/USP30 (lower) cells were analyzed using a Seahorse XF96 analyzer. Slightly increased basal respiration and spare respiratory capacity were observed in both cells after ST-539 treatment. A total of three different pair of MEFS were analyzed, all giving similar results. (c) Representative confocal images of WT and Mfn2−/− MEF cells untreated or treated with 10μg/ml ST-539 for 24 h. Scale bar, 20 μm. Cells were stained with Mito Tracker Red ™ for visualization of mitochondria. (d) Quantification of the indicated cells with connected and tubular mitochondria before and after 3 or 10μg/ml ST-539 treatment for 24 h. Data represent mean ± SD of three independent experiments, each with > 100 cells counted per condition.
ST-539 induced tissue specific mitophagy in vivo.
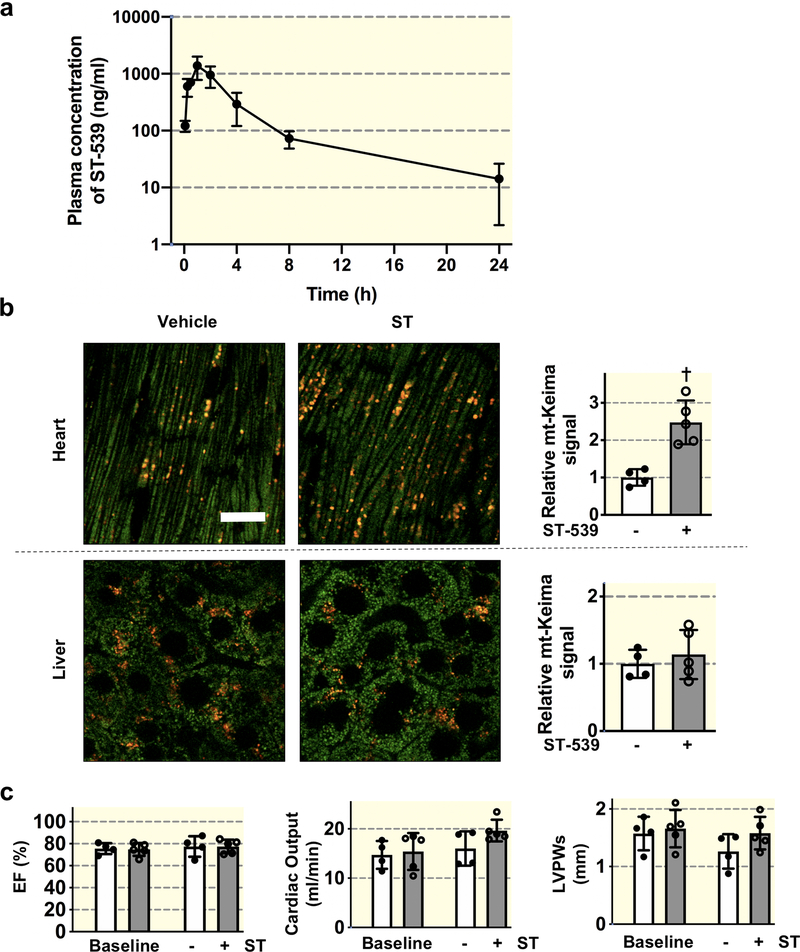
The correlation between USP30 and early-onset Parkinson’s disease (PD) associated PINK1 and Parkin pathway creates increased attention to this enzyme21. A set of USP30 inhibitors have been identified with specific USP30 binding activity in vitro29,30. Nonetheless, it remains unclear whether USP30 inhibition could regulate mitophagy in vivo. We initially attempted to define the plasma pharmacokinetic properties of ST-539 in mice. We administered the dose of 25 mg/kg ST-539 intraperitoneally (i.p). Figure 4a illustrates the post-administration plasma concentration-time curves. The maximum plasma concentration (Cmax) following the i.p. administration of ST-539 in mice was about 1.4μg/ml. The half-life (T1/2) of ST-539 is about 3.85 hr., with a time to reach peak plasma concentration (Tmax) value of 1.33 hr. (Table 1). We calculated the area under the plasma drug concentration-time curve (AUC), to reflect the extent of drug exposure, and obtained a value of ~ 4.2μg•h/ml (Table 1). These findings suggest that administrating daily i.p. doses of 25mg/kg could be used to test whether of ST-539 could activate mitophagy in vivo.
Figure 4. USP30 inhibition promotes cardiac mitophagy.
(a) Plasma ST-539 concentrations as a function of time following 25mg/kg i.p. injection into adult male mice. Concentration determined by LC-MS/MS. n=3 males/time point. Data presented as mean ± s.d. (b) Assessment of mitophagy using mt-Keima mice. Left, representative confocal images of heart and liver tissue from vehicle or ST-539 (25mg/kg/day for 5 days) treated mt-Keima mice; right, quantification of cardiac and hepatic mitophagy. The emission signal obtained after excitation with the 458-nm laser is shown in green, and that obtained after excitation with the 561-nm laser is shown in red. Individual mouse data points are shown. Values are normalized to control levels of mitophagy (n=4 for control and n=5 for ST-539). Scale bars, 20 μm. † p < 0.01. (c) Echocardiographic quantification showing similar percentage ejection fraction (EF) cardiac output and left ventricular posterior wall thickness at end-systole (LVPWs) for vehicle or ST-539 treated mt-Keima mice (n=4 for control and n=5 for ST-539). Data are mean ± s.d. Individual mouse data points are shown.
Table 1:
Pharmacokinetic parameters of the plasma ST-539 concentration-time curve.
| PK Parameters | Estimate |
|---|---|
| Cmax (ng/mL) | 1427 |
| Tmax (h) | 1.33 |
| T1/2 (h) | 3.85 |
| Tlast (h) | 24.0 |
| AUC0-last (ng·h/mL) | 4162 |
| AUC0-inf (ng·h/mL) | 4240 |
| MRT0-last (h) | 3.86 |
| MRT0-inf (h) | 4.27 |
Cmax: maximum plasma concentration; Tmax: time to reach Cmax; T1/2: half-life; AUClast: area under the curve from t=0 to the time of the last quantifiable concentration; AUCinf: AUC from t=0 to infinity; MRTinf: mean residence time.
We sought to provide the first direct measurements of mitophagy following ST-539 treatment, employing the pH-sensitive fluorescent protein mt-Keima32, which can provide rapid and faithful determination of mitophagy in a mouse model2,38. The mt-Keima mice received daily injections of ST-539 or a vehicle for five days. We visualized the basal mitophagic levels via tissues obtained from mt-Keima mice, which presented as red and green punctate within the cardiac and hepatic sections (Figure 4b). Treating mt-Keima mice with ST-539 resulted in a marked induction of the red signal in the heart tissue (Figure 4b), suggesting an augmented cardiac mitophagy. Consistent with these observations, the hearts following ST-539 treatment demonstrated an increase in LC3-II/LC3-I ratio compared to control hearts (Supplemental Figure 3). To verify that the mitophagy activation was not associated with cardiac pathology, we performed an echocardiographic analysis in the treated mice to monitor cardiac morphology and function following ST-539 treatment. We observed minimal differences in left ventricle systolic function represented by the ejection fraction (EF) (Figure 4c). Moreover, ST-539 treatment does not appear to impact cardiac output or left ventricular posterior wall thickness (LVPWs) (Figure 4c). The results suggest that pharmacologically manipulating USP30 activity can stimulate mitophagy in the heart independent of cardiac pathological responses. However, mitophagic flux in metabolically different organs such as the liver (Figure 4b and Supplemental Figure 3) or the hippocampus of the brain (Data not shown) remains unchanged following the ST-539 administration. As such, the current dosage of ST-539 appears to be safe in mice, and ST-539 represents a potential USP30 inhibitor to regulate in vivo mitophagy in a tissue specific manner. This may reflect variation in USP30 expression levels among tissues39,40, although we cannot exclude the possibility that the lack of mitophagy activation in the brain tissue is due to insufficient exposure to ST-539.
Overall, our results demonstrate the pharmacological inhibition of USP30 by ST-539 modulates PINK1/Parkin dependent mitophagy, and efficiently induces cardiac mitophagy. Further investigation of ST-539, to determine its detailed Pharmacokinetic-Pharmacodynamic (PKPD) hopefully leads to potential therapies for multiple diseases.
Discussion
Maintaining mitochondrial quality through mitophagy to selectively eliminate dysfunctional mitochondria could be particularly crucial under disease conditions41–43. Multiple pathophysiological processes have been implicated in the regulation of mitophagy, and the mitophagic removal may require exquisite regulatory controls6,44. For instance, if the removal is too exuberant, the energetically demanding tissues such as the heart and brain, may become maladapted to its environment. The complexity of mitophagy regulation has impeded identification of a rate-limiting therapeutic target. The benign effects of USP30 inhibition may afford a means to enhance suitable mitophagy, making it a promising target candidate. Our study, both in vitro and in a mouse model, reports that ST-539 inhibits USP30, effectively activating mitophagy in cells and in the heart. Future research will explore the use of this compound and its derivatives in disease models, such as myocardial infarction and age-related heart failure, both of which implicate mitophagy dysregulation45–47.
It is well established that the USP30 dependent suppression of mitophagy may rely on over-expressed Parkin together with an overt depolarization21,22,27. Nevertheless, deletion of USP30 from cells with endogenous Parkin only results in a modest effect on mitophagic flux, and Parkin can rapidly overcome USP30’s activity to allow maximal activation of the Parkin dependent mitophagy19. Moreover, the ubiquitylation of the majority of Parkin targets is unaffected upon USP30 deletion19. Further studies are required to understand the role of USP30 in buffering Parkin activation and mitophagy regulation. Interestingly, recent studies have provided a comprehensive analysis of the impact of USP30 on mitochondrial ubiquitylation dynamics, establishing a model proposing that USP30 loss or inhibition may boost mitophagy by lowering the threshold of mitochondrial damage19,29,48.
The inability of ST-539 to induce hepatic mitophagy is somewhat unexpected. We hypothesize that this reflects the differences of basal mitophagy threshold in hepatocytes compared to cardiomyocytes following USP30 inhibition. This result is consistent with previous work comparing the basal levels of mitophagy in various mouse tissues2,20, where we observed tissue-specific differences in mitophagy, with relatively high mitophagic rates seen in organs such as the heart2. Our cellular studies reveal a minimal change in mitophagy following ST-539 treatment in the absence of mitochondrial stress. As hepatic mitophagy is sensitive to environmental and genetic perturbations2, it is possible that ST-539 may stimulate mitophagy in the liver when animals are exposed to specific stressors. Moreover, the liver and the brain exhibit a higher level of USP30 expression than cardiac muscle39,40. An increased dose and/or the duration of ST-539 administration may be required to effectively induce mitophagy in the liver or brain. Of note, we have detected ST-539 in modest concentrations from the mouse brain homogenate, suggesting a potential brain distribution of ST-539 (Table 2). Though untested, mitophagy induction following USP30 inhibition may differ substantially among tissues and vary widely between cell types in the same tissue.
Table 2:
Tissue concentrations of ST-539 24 Hours Post-dose in Male C57BL/6 Mice (ng/g).
| Tissue | Mean |
|---|---|
| Brain | 17.4 |
| Heart | 37.0 |
Based on our studies, ST539 emerges as a promising compound for the in vivo evaluation of USP30 inhibition. However, it is not clear what dose or duration of ST539 is required to see the beneficial effects of mitophagy induction in diseased models. Therefore, altering the dose and the duration of ST-539 administration could modulate the tissue-specific effects of USP30 inhibition. A successful result would prompt additional tests of the compound’s specificity and any off-target effects in vivo. Additionally, the PK/PD and toxicity profiles of ST-539 should allow further preclinical assessment of USP30 inhibition as a therapeutic strategy for a wide variety of diseases. Encouragingly, the crystal structure of human USP30 bound to monoubiquitin and Lys6-linked di-ubiquitin was reported recently 28, providing insights into the activity and regulation of USP30 to facilitate drug design against this enzyme.
Materials and methods
Cell culture and reagents
HeLa cells and mouse embryonic fibroblasts (MEFs) were grown in Dulbecco’s minimum essential medium (DMEM) with 10% fetal bovine serum (FBS) supplemented with penicillin-streptomycin. HeLa Parkin, PINK1 KO cells, WT and MFN2 KO cells were previously described31,37,49. Cells stably expressing USP30 were generated using lentiviral vectors of pLVX-Puro-Myc-USP30. The USP30 plasmid50 was obtained from Addgene. The plasmid was transferred into a lentiviral vector and viral particles were prepared by transiently transfecting HEK293T using standard methods. For the drug treatment experiments, cells were incubated in medium containing a mixture of 5 μM oligomycin and 5 μM antimycin A (A/O; see figures for treatment times). In 18 h treatment, A/O were used in combination with 10 μM quinolyl-valyl-O-methylaspartyl-[−2,6-difluorophenoxy]-methyl ketone (QVD), a broad-spectrum caspase inhibitor. ST51000539 (ST-539) was purchased from TimTec, Inc. Other chemicals were from Sigma-Aldrich (St. Louis, MO, USA). For FACS analysis, cells stably expressing mt-Keima were incubated in medium containing a mixture of 1 μM oligomycin and 1 μM antimycin A for 1.5 h. Prior to A/O treatment, cultures were treated with DMSO or ST-539 at 3μg/ml. Cells were trypsinized, washed once with PBS buffer and then resuspended into 500 μl of PBS prior to analysis using a BD Fortessa flow cytometer as previously described2.
Western blotting
Cells were lysed in RIPA buffer (50 mM Tris-HCl, at pH 8.0; 150 mM NaCl; 1% (vol/vol) Nonidet P-40; 0.5% sodium deoxycholate, 0.1% SDS and protease inhibitor cocktail (Roche)) on ice. Primary antibodies were used at the following concentrations: USP30 (Santa Cruz, sc-515235, 1:200); TOM20 (Cell Signaling Technology, 42406S, 1:1000); TOM40 (Proteintech, 18409–1-AP, 1:1000); NDP52 (Cell Signaling Technology, 60732S, 1:1000); TIM23 (Proteintech, 11123–1-AP, 1:500); LC3A/B (Cell Signaling Technology, 4108S, 1:1000); GAPDH (Cell Signaling Technology, 5175S, 1:1000); PINK1 (Cell Signaling Technology, 6946S, 1:1000); ACTIN (Cell Signaling Technology, 3700S, 1:1000). The membranes were incubated with anti-rabbit (LI-COR, 926–32211, 1:15000) or anti-mouse (LI-COR, 926–68072, 1:15000) IgG secondary antibodies for 1 h at room temperature. Images were captured using the Odyssey system (LI-Cor). One representative blot is shown of three independent experiments.
Seahorse assay
Measurement of intact cellular respiration was performed using the Seahorse XFe96 Analyzer as previously described2. In brief, 2 × 104 cells per well were seeded in the Seahorse XF Cell Culture Microplate (Agilent) using high-glucose DMEM at 37°C, with a humidified atmosphere of 5% CO2, overnight. And a sensor cartridge was hydrated in Seahorse XF Calibrant in a non-CO2 incubator overnight at 37 °C. The high-glucose DMEM in Cell Culture Microplate was changed to warmed assay medium and placed into a 37 °C non-CO2 incubator for 45 minutes prior to the assay. Oligomycin (1.5μM/well), FCCP (1μM/well) and Antimycin A (0.5μM/well) were supplemented at 18 minutes, 36 minutes and 54 minutes, respectively. The results were analyzed using the Seahorse Wave software.
Measurement of ΔΨm
ΔΨm was measured using a fluorescence microplate reader (BioTek Instruments) in cells preincubated with Tetramethylrhodamine methyl ester (TMRM, Thermo Scientific) following manufacturer’s instructions. Cells were washed in PBS before resuspension in Hank’s balanced salt solution (HBSS; 156 mM NaCl, 3 mM KCl, 2 mM MgSO4, 1.25 mM KH2PO4, 2 mM CaCl2, 10 mM glucose and 10 mM HEPES; pH adjusted to 7.35 with NaOH) (1.0×106 cells/ml) containing 50 nM TMRM. For each individual experiment, average TMRM fluorescence was normalized to control cells.
Confocal Microscopy
Fluorescent samples were examined with a Zeiss LSM 780 confocal microscope (Carl Zeiss MicroImaging). As previously described2, fluorescence of mt-Keima was imaged in two channels via two sequential excitations (458 nm, green; 561 nm, red) and using a 570- to 695-nm emission range. Confocal experiments for parkin translocation were performed using a HeLa cell line stably expressing YFP-Parkin plated on 35mm coverglass #1.5 chamber dishes (MatTek). These cells were treated for 2 h with DMSO as vehicle control or with A/O. YFP was imaged with a 514-nm excitation and 520- to 570-emission filters. Representative confocal images were processed using Imaris software by contrast linear stretch only. Calculation of mitophagy based on mt-Keima signal was performed using Zeiss ZEN software as previously described2. The average of four images from each tissue sample was taken, and the values were normalized to the average value seen in the controls, assigned the value of one.
Animals
We have previously described the mt-Keima mouse2. ST-539 was dissolved into 1% DMSO in Sesame oil and injected intraperitoneally (i.p.) at doses of 25 mg/kg/day with five total doses. Both male and female mice were used in this study. The control group received vehicle only. Echocardiographic measurements were taken using a Vevo3100 Visual Sonics (Visual Sonics) system. The mice were lightly anesthetized with isoflurane and the ejection fraction, fractional shortening, and ventricular chamber dimensions were determined using 2-D-guided M mode images. Ejection fraction, fractional shortening, ventricular chamber dimensions, and left ventricular mass were calculated automatically using the VevoLAB program. All experiments involving animals were approved by the Institutional Animal Care and Use Committee at The Ohio State University.
Pharmacokinetics
ST-539 was dissolved into 1% DMSO in Sesame oil and injected intraperitoneally (i.p.) at doses of 25 mg/kg. Serial blood samples were collected at 0.083, 0.25, 0.5, 1, 2, 4 and 8 hours post dosing. Mice were euthanized at 24 hours post dosing. A terminal blood sample was collected by cardiac puncture followed by harvesting the brain and heart. Transcardial perfusion was performed prior to brain collection. The blood samples were placed in microtubes pretreated with K2EDTA as an anticoagulant and kept on ice until centrifugation. The tissue samples were rinsed using cold distilled water to remove blood, blotted dry, weighed and stored on dry ice until LC/MS/MS analysis. Plasma was transferred into polypropylene tubes or 96-well plates, quick frozen on dry ice, and stored at − 70 ± 10°C until LC/MS/MS analysis.
Statistics
Statistical analysis was performed with an unpaired 2-tailed t test. P values <0.05 were considered significant. Results are presented as the mean ± SD.
Supplementary Material
Supplemental Figure 2
Representative immunoblotting for expression of PINK1 in Hela WT cells or PINK1 KO cells expressing Parkin. ACTIN is shown as a loading control.
Supplemental Figure 1
Representative immunoblotting for (a) TOM20 and TIM23 or (b) TOM40 and LC3 in Hela cells expressing Parkin or Parkin/Myc-tagged USP30 following A/O treatment for 18h. Prior to A/O treatment, cultures were treated with ST-539 at indicated concentrations.
Supplemental Figure 3
Representative immunoblotting for LC3 I/II of (a) heart and (b) liver tissue from vehicle or ST-539 (25mg/kg/day for 5 days) treated mice. ST-539 treated hearts exhibited increased levels of LC3-II compared to vehicle treated hearts, consistent with augmented cardiac mitophagy following ST-539 treatment. Data are mean ± s.d. *p < 0.05.
Acknowledgements
We thank Dr. Toren Finkel for kindly providing plasmids. We thank Dr. Richard Youle and Dr. David Chan for generously providing us with the Hela Parkin cell lines, PINK1 KO cells and MFN2 MEF cells.
Funding
This work was supported by grant from the National Institutes for Health to N.S. (K22-HL135051).
Abbreviations:
- OMM
Outer mitochondrial membrane
- PINK1
PTEN-induced putative kinase 1
- USP
Ub-specific protease
- DUBs
deubiquitinating enzymes
- TOM20
Translocase of outer membrane 20
- A/O
Antimycin A/Oligomycin
- WT
Wild-type
- KO
Knockout
- TMRM
Tetramethylrhodamine
- FCCP
Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References:
- 1.McWilliams TG, Prescott AR, Montava-Garriga L, et al. Basal Mitophagy Occurs Independently of PINK1 in Mouse Tissues of High Metabolic Demand. Cell metabolism. 2018;27(2):439–449 e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun N, Yun J, Liu J, et al. Measuring In Vivo Mitophagy. Molecular cell. 2015;60(4):685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nature reviews Molecular cell biology. 2011;12(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Ham A, Ma TC, et al. Mitochondrial dysfunction and mitophagy defect triggered by heterozygous GBA mutations. Autophagy. 2019;15(1):113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun N, Youle RJ, Finkel T. The Mitochondrial Basis of Aging. Mol Cell. 2016;61(5):654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickles S, Vigie P, Youle RJ. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Current biology : CB. 2018;28(4):R170–R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fivenson EM, Lautrup S, Sun N, et al. Mitophagy in neurodegeneration and aging. Neurochem Int. 2017;109:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine B, Kroemer G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell. 2019;176(1–2):11–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McWilliams TG, Muqit MM. PINK1 and Parkin: emerging themes in mitochondrial homeostasis. Curr Opin Cell Biol. 2017;45:83–91. [DOI] [PubMed] [Google Scholar]
- 10.Ordureau A, Paulo JA, Zhang W, et al. Dynamics of PARKIN-Dependent Mitochondrial Ubiquitylation in Induced Neurons and Model Systems Revealed by Digital Snapshot Proteomics. Mol Cell. 2018;70(2):211–227 e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasson SA, Kane LA, Yamano K, et al. High-content genome-wide RNAi screens identify regulators of parkin upstream of mitophagy. Nature. 2013;504(7479):291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarraf SA, Raman M, Guarani-Pereira V, et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496(7445):372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin-induced mitophagy in the pathogenesis of Parkinson disease. Autophagy. 2009;5(5):706–708. [DOI] [PubMed] [Google Scholar]
- 14.Narendra DP, Jin SM, Tanaka A, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS biology. 2010;8(1):e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark IE, Dodson MW, Jiang C, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441(7097):1162–1166. [DOI] [PubMed] [Google Scholar]
- 16.Narendra D, Walker JE, Youle R. Mitochondrial quality control mediated by PINK1 and Parkin: links to parkinsonism. Cold Spring Harb Perspect Biol. 2012;4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan PY, Yue Z. Genetic causes of Parkinson’s disease and their links to autophagy regulation. Parkinsonism Relat Disord. 2014;20 Suppl 1:S154–157. [DOI] [PubMed] [Google Scholar]
- 18.Fang EF, Hou Y, Palikaras K, et al. Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci. 2019;22(3):401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ordureau A, Paulo JA, Zhang J, et al. Global Landscape and Dynamics of Parkin and USP30-Dependent Ubiquitylomes in iNeurons during Mitophagic Signaling. Molecular cell. 2020;77(5):1124–1142 e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McWilliams TG, Prescott AR, Allen GF, et al. mito-QC illuminates mitophagy and mitochondrial architecture in vivo. J Cell Biol. 2016;214(3):333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bingol B, Tea JS, Phu L, et al. The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature. 2014;510(7505):370–375. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham CN, Baughman JM, Phu L, et al. USP30 and parkin homeostatically regulate atypical ubiquitin chains on mitochondria. Nature cell biology. 2015;17(2):160–169. [DOI] [PubMed] [Google Scholar]
- 23.Bingol B, Sheng M. Mechanisms of mitophagy: PINK1, Parkin, USP30 and beyond. Free Radic Biol Med. 2016;100:210–222. [DOI] [PubMed] [Google Scholar]
- 24.Torre S, Polyak MJ, Langlais D, et al. USP15 regulates type I interferon response and is required for pathogenesis of neuroinflammation. Nat Immunol. 2017;18(1):54–63. [DOI] [PubMed] [Google Scholar]
- 25.Durcan TM, Fon EA. The three ‘P’s of mitophagy: PARKIN, PINK1, and post-translational modifications. Genes Dev. 2015;29(10):989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durcan TM, Tang MY, Perusse JR, et al. USP8 regulates mitophagy by removing K6-linked ubiquitin conjugates from parkin. EMBO J. 2014;33(21):2473–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang JR, Martinez A, Lane JD, Mayor U, Clague MJ, Urbé S. USP30 deubiquitylates mitochondrial Parkin substrates and restricts apoptotic cell death. EMBO reports. 2015;16(5):618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gersch M, Gladkova C, Schubert AF, Michel MA, Maslen S, Komander D. Mechanism and regulation of the Lys6-selective deubiquitinase USP30. Nat Struct Mol Biol. 2017;24(11):920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rusilowicz-Jones EV, Jardine J, Kallinos A, et al. USP30 sets a trigger threshold for PINK1-PARKIN amplification of mitochondrial ubiquitylation. Life science alliance. 2020;3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kluge AF, Lagu BR, Maiti P, et al. Novel highly selective inhibitors of ubiquitin specific protease 30 (USP30) accelerate mitophagy. Bioorganic & medicinal chemistry letters. 2018;28(15):2655–2659. [DOI] [PubMed] [Google Scholar]
- 31.Lazarou M, Sliter DA, Kane LA, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524(7565):309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katayama H, Kogure T, Mizushima N, Yoshimori T, Miyawaki A. A sensitive and quantitative technique for detecting autophagic events based on lysosomal delivery. Chem Biol. 2011;18(8):1042–1052. [DOI] [PubMed] [Google Scholar]
- 33.Denison SR, Wang F, Becker NA, et al. Alterations in the common fragile site gene Parkin in ovarian and other cancers. Oncogene. 2003;22(51):8370–8378. [DOI] [PubMed] [Google Scholar]
- 34.Poot M, Zhang YZ, Kramer JA, et al. Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. J Histochem Cytochem. 1996;44(12):1363–1372. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura N, Hirose S. Regulation of mitochondrial morphology by USP30, a deubiquitinating enzyme present in the mitochondrial outer membrane. Mol Biol Cell. 2008;19(5):1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yue W, Chen Z, Liu H, et al. A small natural molecule promotes mitochondrial fusion through inhibition of the deubiquitinase USP30. Cell Res. 2014;24(4):482–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160(2):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun N, Malide D, Liu J, Rovira II, Combs CA, Finkel T. A fluorescence-based imaging method to measure in vitro and in vivo mitophagy using mt-Keima. Nat Protoc. 2017;12(8):1576–1587. [DOI] [PubMed] [Google Scholar]
- 39.Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science (New York, NY). 2015;347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- 40.Fagerberg L, Hallström BM, Oksvold P, et al. Analysis of the Human Tissue-specific Expression by Genome-wide Integration of Transcriptomics and Antibody-based Proteomics. Molecular & cellular proteomics : MCP. 2014;13(2):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang R, Krigman J, Luo H, Ozgen S, Yang M, Sun N. Mitophagy in cardiovascular homeostasis. Mech Ageing Dev. 2020;188:111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Killackey SA, Philpott DJ, Girardin SE. Mitophagy pathways in health and disease. J Cell Biol. 2020;219(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sliter DA, Martinez J, Hao L, et al. Parkin and PINK1 mitigate STING-induced inflammation. Nature. 2018;561(7722):258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nature cell biology. 2018;20(9):1013–1022. [DOI] [PubMed] [Google Scholar]
- 45.Bravo-San Pedro JM, Kroemer G, Galluzzi L. Autophagy and Mitophagy in Cardiovascular Disease. Circ Res. 2017;120(11):1812–1824. [DOI] [PubMed] [Google Scholar]
- 46.Kubli DA, Zhang X, Lee Y, et al. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. The Journal of biological chemistry. 2013;288(2):915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eisenberg T, Abdellatif M, Schroeder S, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22(12):1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phu L, Rose CM, Tea JS, et al. Dynamic Regulation of Mitochondrial Import by the Ubiquitin System. Mol Cell. 2020;77(5):1107–1123 e1110. [DOI] [PubMed] [Google Scholar]
- 49.Nezich CL, Wang C, Fogel AI, Youle RJ. MiT/TFE transcription factors are activated during mitophagy downstream of Parkin and Atg5. J Cell Biol. 2015;210(3):435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138(2):389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 2
Representative immunoblotting for expression of PINK1 in Hela WT cells or PINK1 KO cells expressing Parkin. ACTIN is shown as a loading control.
Supplemental Figure 1
Representative immunoblotting for (a) TOM20 and TIM23 or (b) TOM40 and LC3 in Hela cells expressing Parkin or Parkin/Myc-tagged USP30 following A/O treatment for 18h. Prior to A/O treatment, cultures were treated with ST-539 at indicated concentrations.
Supplemental Figure 3
Representative immunoblotting for LC3 I/II of (a) heart and (b) liver tissue from vehicle or ST-539 (25mg/kg/day for 5 days) treated mice. ST-539 treated hearts exhibited increased levels of LC3-II compared to vehicle treated hearts, consistent with augmented cardiac mitophagy following ST-539 treatment. Data are mean ± s.d. *p < 0.05.