Abstract
Aging is associated with stiffening of the large elastic arteries and consequent increases in systolic blood pressure (SBP), which together increase cardiovascular disease risk; however, the upstream mechanisms are incompletely understood. Using complementary translational approaches in mice and humans, we investigated the role of the gut microbiome-derived metabolite trimethylamine N-oxide (TMAO) in age-related aortic stiffening and increased SBP. Aortic stiffness was measured using carotid-femoral (c-f) or aortic (a) pulse wave velocity (PWV) in humans and mice, respectively. Study 1: Plasma TMAO concentrations were elevated (p<0.001) in healthy middle-aged to older (6.3±5.8 μM) vs. young (1.8±1.4 μM) humans and positively related to c-f PWV (r2=0.15, p<0.0001) and SBP (r2=0.09, p<0.001), independent of traditional cardiovascular risk factors. Study 2: Dietary supplementation with TMAO increased aPWV in young mice and exacerbated the already elevated aPWV of old mice, accompanied by increases in SBP of ~10 mmHg in both groups. TMAO-supplemented vs. control-fed mice also had higher intrinsic mechanical stiffness of the aorta (stress-strain testing) associated with higher aortic abundance of advanced glycation end-products (AGEs), which form crosslinks between structural proteins to promote aortic stiffening. Study 3: Ex vivo incubation of aortic rings with TMAO increased intrinsic stiffness, which was attenuated by the AGEs crosslink breaker alagebrium and prevented by inhibition of superoxide signaling. TMAO induces aortic stiffening and increases SBP via formation of AGEs and superoxide-stimulated oxidative stress, which together increase intrinsic wall stiffness. Increases in circulating TMAO with aging represent a novel therapeutic target for reducing risk of aortic stiffening-related clinical disorders.
Keywords: Arterial stiffness, microbiota, pulse wave velocity, advanced glycation end-products, superoxide
1. INTRODUCTION
Advancing age is the primary risk factor for cardiovascular diseases (CVD), which is in large part due to age-related stiffening of the large elastic arteries (i.e., the aorta and carotid arteries)1,2. Indeed, aortic stiffening, measured in humans by carotid-femoral pulse wave velocity (c-f PWV), is an established, independent predictor of future CVD-related mortality3,4. Aortic stiffening also contributes to increases in systolic blood pressure (BP) with aging, which further increases risk of CVD5. Together, these age-related changes in vascular health not only considerably increase CVD risk, but are also key risk factors for other major clinical disorders such as stroke and chronic kidney disease, and are implicated in cognitive declines with aging and risk of dementia/Alzheimer’s disease6-8. Age-related aortic stiffening occurs primarily due to structural changes in the arteries, in particular, fragmentation and degradation of elastin fibers and increased deposition of collagen9. In addition, there is greater accumulation of advanced glycation end-products (AGEs) that form crosslinks between these structural proteins to further increase stiffness10. However, the upstream mechanisms that drive these processes are incompletely understood.
Recent findings from our laboratory indicate that the gut microbiome plays a critical role in mediating aortic stiffening and increased systolic BP with aging11, possibly through altered production of metabolites that can enter the circulation and thereby influence host physiology. One such metabolite is trimethylamine N-oxide (TMAO), which is produced via gut microbe-dependent conversion of ingested precursors (e.g., choline and L-carnitine) into trimethylamine (TMA) that is subsequently converted in the liver to TMAO12. We and others have previously reported that plasma concentrations of TMAO increase with aging in mice and humans11,13,14. TMAO has been causally linked to the development of CVD in mice and humans15,16 and plasma levels of TMAO are predictive of CVD risk in humans17,18. Moreover, we recently demonstrated that increased plasma levels of TMAO promote vascular endothelial dysfunction – a determinant of aortic stiffness – with aging in mice and humans13. Thus, it follows that TMAO may also contribute to aortic stiffening and subsequent increases in systolic BP with aging; however, this possibility has not been addressed.
Here, we investigated whether elevated plasma TMAO contributes to age-related aortic stiffening. First, we showed that higher plasma concentrations of TMAO are positively related to aortic stiffness and systolic BP in healthy young and middle-aged to older (MA/O) adult humans, independent of traditional CVD risk factors. To better isolate effects of TMAO, we next used a mouse model to show that dietary supplementation with TMAO induces ‘aging-like’ aortic stiffening and increases in BP in young mice and exacerbates these age-related changes in old mice. Finally, because aortic stiffening appears to be the primary event leading to increases in systolic BP with aging19,20, we then investigated the mechanisms mediating TMAO-induced increases in aortic stiffness. In aorta rings from young mice, we then demonstrated that both dietary supplementation and ex vivo incubation with TMAO increase intrinsic mechanical stiffness of the aorta via stimulating the formation of AGEs. Overall, we have identified a novel upstream mechanism of age-related aortic stiffening that could have implications for the prevention of CVD, chronic kidney disease and cognitive disorders of aging.
2. METHODS
The data that support the findings of this study are available from the correspond author upon reasonable request.
2.1. Human Subjects Experiments
The Institutional Review Board at the University of Colorado Boulder approved all procedures involving human subjects, which adhered to the ethical principles defined in the Declaration of Helsinki. Written informed consent was obtained from all subjects prior to participation. A total of 21 young (18-27 years) and 101 middle-aged to older (MA/O; 45-79 years) adults who were enrolled in previously conducted studies21-25 were included in this retrospective analysis. All study participants were non-smokers and free from overt clinical disease, including CVD, as determined by medical history, physical examination by a physician, and graded exercise testing with simultaneous ECG and BP monitoring. Premenopausal women were studied during menses or in the low hormone phase if taking hormonal contraceptives. Body mass index was calculated via anthropometric measures of body mass and height. Peak oxygen consumption (VO2max) was measured on a separate day during incremental treadmill testing (Balke protocol).
Prior to vascular testing, all subjects had abstained from food for 8 to 12 hours, alcohol, caffeine or vigorous exercise for >20 hours, and dietary supplements or over-the-counter medications for >48 hours. Triplicate measures of casual brachial artery BP were obtained in a seated position with the arm supported at heart level using a semiautomated device (Dinamap XL, Johnson & Johnson, New Brunswick, NJ). Aortic stiffness was assessed in a subset of subjects (14 young, 83 MA/O) by carotid-femoral pulse wave velocity (c-f PWV) per American Heart Association guidelines26 using applanation tonometry (NIHem, Cardiovascular Engineering Inc., Norwood, MA). Blood samples were obtained for analysis of blood lipid and lipoprotein concentrations and fasting blood glucose by Boulder Community Hospital Clinical Laboratory (Boulder, CO; a Clinical Laboratory Improvement Amendments [CLIA]-certified laboratory) and analysis of inflammatory markers C-reactive protein (CRP) and interleukin-6 (IL-6) by commercial ELISA by the CU Anschutz Medical Campus Clinical Translational Research Center Core Laboratory. A separate plasma sample was obtained and stored at −80°C for later analysis of TMAO, choline, betaine, and L-carnitine using a stable isotope dilation liquid chromatography-tandem mass spectrometry (LC-MS) method against internal standards, as previously described14,27,28. Coefficients of variation for all analytes and representative mass spectrometry tracings are provided in Supplemental Table S1 and Supplemental Figure S2. Investigators were blinded to subject age and health status for plasma analyses. Further details on all procedures are included in the Online Supplemental Methods.
2.2. Animal Experiments
All animal protocols were approved by the University of Colorado Boulder Institutional Animal Care and Use Committee and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Young male C57Bl/6N mice were obtained from Charles River (at 2 months of age), and old male C57Bl/6N mice were obtained from the National Institute on Aging colony maintained by Charles River (at 18-19 months of age). Male, but not female, C57Bl/6 mice are an established model of human vascular aging and develop age-related aortic stiffening comparably to their human counterparts29,30. All mice acclimated to our facility for a minimum of 4 weeks prior to any testing. Mice were single housed (to avoid effects of group housing on the gut microbiome as mice are coprophagic) in a conventional facility on a 12-hour light/dark cycle and given ad libitum access to rodent chow and drinking water. Further details on animal numbers, including power calculations and attrition, are provided in the Online Supplemental Methods.
2.3. TMAO Dietary Supplementation in Mice
For the intervention, all mice were fed a defined low-choline diet (0.07%; to control for TMAO precursors, but still sufficient to avoid choline deficiency) and randomly assigned to receive chow that was either not supplemented (Control) or supplemented with 0.12% TMAO (customized diets from Envigo, Madison, WI; TMAO, Sigma-Aldrich Corp., St. Louis, MO), as established previously12,31. Young mice (12 months at sacrifice; equivalent to ~35 human years) received the intervention for 6 months, whereas old mice (24 months at sacrifice; ~70 human years) received the intervention for 3 months due to a higher rate of mortality observed in old TMAO-supplemented vs. Control mice.
Aortic stiffness was assessed in vivo at baseline and following 3 and 6 months (young mice only) of TMAO dietary supplementation using aortic pulse wave velocity (aPWV), as previously described30,32. In vivo BP was measured at baseline and each month throughout the intervention on three consecutive days using a non-invasive tail-cuff method (CODA; Kent Scientific, Torrington, CT), as previously described30.
All mice were euthanized by exsanguination via cardiac puncture while anesthetized with inhaled isoflurane (>5%), immediately followed by removal of the carotid arteries and heart. Heparinized plasma was frozen and stored at −80°C until later analysis of TMAO, choline, betaine, L-carnitine, and γ-butyrobetaine by LC-MS, as described above. The thoracic aortas from young mice were excised, dissected free of surrounding tissue, and segmented and stored appropriately for later stress-strain testing to determine aortic intrinsic mechanical stiffness and elasticity33,34 and for assessment of abundance of structural proteins (type-1 collagen, elastin) and AGEs by Western immunoblotting and immunohistochemistry (IHC). Investigators were blinded to treatment group for data collection and plasma and biochemical analyses. Detailed descriptions of all procedures are provided in the Online Supplemental Methods.
2.4. TMAO Incubations in Mouse Aortic Rings
To determine if TMAO directly affects aortic stiffness, segments (~1 mm) of thoracic aorta were obtained as described above from a separate cohort (N=18) of young (3-5 months) male C57BL/6N mice obtained from Charles River. Aortic segments were incubated under standard conditions (37°C, 5% CO2, 20% O2, humidified) for 72 hours in culture medium alone (Vehicle; Dulbecco’s Modified Eagle’s Medium [DMEM] supplemented with 10% fetal calf serum and 1% Penicillin-Streptomycin) or with the addition of 30 μM TMAO (Sigma-Aldrich Corp.), the average concentration measured in plasma from the dietary supplementation intervention. Vehicle- and TMAO-treated segments from a subset of mice were also treated with the AGEs inhibitor alagebrium35-37 (N=5; 2 mM; AstaTech, Inc., Bristol, PA) or the superoxide dismutase mimetic 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPOL; N=5 mice; 100 μM; Sigma-Aldrich, Corp) to suppress reactive oxygen species (ROS) production. Following the incubation, stress-strain testing was performed to assess intrinsic mechanical stiffness. Lastly, to determine if TMAO directly increases AGEs abundance and whether this may occur via ROS production, segments of thoracic aorta (~4 mm) from 4 additional young mice were incubated for 72 hours in culture medium alone, with the addition of 30 μM TMAO, or with 30 μM TMAO + 100 μM TEMPOL, and AGEs abundance was determined via Western immunoblotting. Detailed descriptions of all procedures, antibodies, and kits used are provided in the Online Supplemental Methods.
2.5. Statistical Analyses
Detailed descriptions of all statistical analyses performed are provided in the Online Supplemental Methods. Data are presented as mean ± S.E.M. in text, figures, and tables, unless specified otherwise. Statistical significance was set to α=0.05. All statistical analyses were performed using Prism, version 8 (GraphPad Software, Inc., La Jolla, CA) or R, version 1.2.5033 (The R Foundation, Vienna, Austria).
3. RESULTS
3.1. Plasma TMAO concentrations are positively related to aortic stiffness and systolic BP in healthy young and middle-aged to older (MA/O) adults
Casual (resting) BP and fasted plasma concentrations of TMAO and related metabolites were measured in healthy young (age 18-27; N=21) and middle-aged to older (MA/O; age 45-79; N=101) adults. Aortic stiffness was assessed in a subset of these subjects (young: N=14; MA/O: N=83) by the gold standard measure, carotid-femoral pulse wave velocity (c-f PWV). A histogram of the age distribution is provided in Supplemental Figure S3; age was normally distributed among MA/O adults (Shapiro-Wilk test: p=0.40). Subject characteristics, circulating inflammatory markers, and prescription medications are provided in Supplemental Table S2 and are also published elsewhere13. Compared to young adults, MA/O subjects had lower cardiorespiratory fitness (VO2max), higher serum total and LDL cholesterol, higher fasted blood glucose, and lower estimated glomerular filtration rate (eGFR) (all p≤0.0005), whereas body mass index was comparable between the groups (p=0.22). MA/O adults tended to have higher circulating concentrations of inflammatory markers C-reactive protein (CRP; p=0.13) and interleukin-6 (IL-6; p=0.07), but these did not reach statistical significance. Within each age group, there were no differences in any characteristics between the whole cohort and the subset of subjects with c-f PWV measurements. The types and number of subjects taking each type of medication were typical for healthy young and MA/O adults (subjects abstained from all medications the morning of testing).
Circulating (plasma) concentrations of TMAO were higher in MA/O compared to young adults (Figure 1A; p=0.0007), as we have previously reported13. Plasma concentrations of the TMAO precursor choline were also higher in MA/O vs. young adults (p<0.0001), but there were no age group differences in concentrations of other TMAO precursors betaine or L-carnitine (both p≥0.21) (Supplemental Table S2). Circulating TMAO (log-transformed to account for skewness) was inversely associated with eGFR (r2=0.07, p=0.003), a marker of kidney function, and positively associated with serum concentrations of the inflammatory marker CRP (r2=0.06, p=0.008). However, these relations were no longer significant when adjusted for age (eGFR: partial r2 of TMAO = 0.006, p=0.43; CRP: TMAO partial r2=0.02, p=0.12). There was no relation between TMAO and IL-6 (r2=0.03, p=0.08; partial r2 of TMAO when adjusted for age = 0.002, p=0.70).
Figure 1. Higher plasma concentrations of trimethylamine N-oxide (TMAO) with aging are related to increased arterial stiffness and systolic blood pressure (BP).
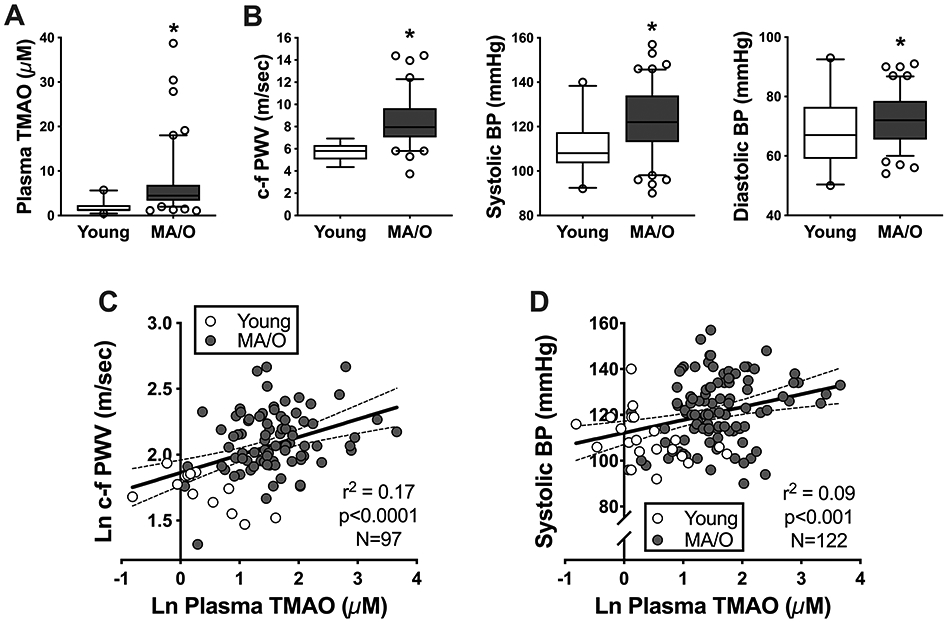
A) Plasma TMAO and B) arterial stiffness (assessed as carotid-femoral pulse wave velocity [c-f PWV]) and casual (resting) systolic and diastolic BP in young (N=14-21) and middle-aged to older (MA/O; N=83-101) adults. Data are mean ± S.E.M. *p<0.05 vs. young adults. C-D) Higher plasma concentrations of TMAO are positively related to c-f PWV (C) and casual SBP (D) in unadjusted linear regression models, with 95% confidence intervals (dashed lines).
MA/O adults had higher c-f PWV (p<0.0001), systolic BP (p=0.0004), and diastolic BP (p=0.007) compared to young adults (Figure 1B), typical of vascular aging. Pulse pressure was also higher in MA/O (50 ± 11 mmHg) vs. young (44 ± 4 mmHg, p=0.01) adults.
We performed multiple linear regression analyses to determine relations between circulating concentrations of TMAO and c-f PWV, systolic BP and diastolic BP. Full regression results are provided in Supplemental Table S3. For all analyses, both plasma TMAO and c-f PWV were log-transformed to account for skewness, whereas non-transformed values were used for systolic and diastolic BP as both were normally distributed (Shapiro-Wilk test for non-transformed data, TMAO: p<0.0001; c-f PWV: p=0.003; systolic BP: p=0.25; diastolic BP: p=0.21).
In unadjusted linear regression models, plasma TMAO was positively related to c-f PWV (r2=0.17, p<0.0001; Figure 1C) and systolic BP (r2=0.09, p=0.0008; Figure 1D), but was not related to diastolic BP (r2=0.02, p=0.11). Relations between TMAO and c-f PWV remained significant when analyses were conducted separately for each age group (young: r2=0.44, p=0.01: MA/O: r2=0.07, p=0.02). TMAO was positively related to systolic BP within MA/O adults only (r2=0.06, p=0.02), but not within young adults only r2=0.16, p=0.07).
When linear regression models were adjusted for traditional CVD risk factors (sex, body mass index, cardiorespiratory fitness, total and LDL cholesterol, and glucose), the independent effects of TMAO on c-f PWV (TMAO partial r2=0.09, p=0.004) and systolic BP (TMAO partial r2=0.05, p=0.02) remained significant, indicating that TMAO is associated with aortic stiffness and systolic BP independent of CVD risk factors. However, when linear regression models were further adjusted for age (in addition to traditional CVD risk factors), there were no longer significant independent effects of TMAO (for both c-f PWV and systolic BP, TMAO partial r2<0.01, p≥0.46), suggesting that the effect of TMAO on aortic stiffness and systolic BP in humans is concomitant with that of aging. We additionally ran statistical models for c-f PWV with mean arterial pressure (MAP) included as a covariate, as MAP represents the distending pressure that can augment c-f PWV. The effect of TMAO on c-f PWV remained significant when MAP was included as the only covariate (TMAO partial r2=0.15, p=0.0001) or along with CVD risk factors (TMAO partial r2=0.07, p=0.01), and disappeared, as before, when age was added to CVD risk factors and MAP (TMAO partial r2=0.004, p=0.57). Lastly, as a small number of MA/O subjects (14 out of 101) were taking anti-hypertensive medications, we also ran models with the number of anti-hypertensive medications included as a factor, but there was no significant effect of medication use on the relation between TMAO and systolic BP (p=0.08) nor c-f PWV (p=0.20). As such, these subjects were kept in the analyses.
Overall, these findings suggest that increases in plasma concentrations of TMAO with aging are associated with aortic stiffening and increases in systolic BP in adults free of clinical disease, independent of traditional CVD risk factors.
3.2. Chronic dietary supplementation with TMAO induces aortic stiffening and increases BP in mice
As it is difficult to isolate effects of TMAO in free-living humans, we next investigated whether dietary supplementation of TMAO induces and/or exacerbates age-related increases in aortic stiffness and systolic BP in male C57BL/6N mice. Male, but not female, mice of this strain demonstrate aortic stiffening with aging comparably to humans30,32. Young adult (12 months at sacrifice; equivalent to ~35 human years) and old (24 months at sacrifice; ~70 human years) mice were fed a defined-choline diet (0.07%; to control for TMAO precursors) that was either not supplemented (Control) or supplemented with 0.12% TMAO for either 6 months (young mice) or 3 months (old mice; shortened due to higher rate of mortality in TMAO-supplemented mice). Plasma TMAO concentrations did not differ between young and old Control mice (p>0.99), presumably due to the low-choline diet, but dietary supplementation of TMAO increased plasma TMAO concentrations vs. controls in both young adult (p=0.002) and old (p<0.0001) mice (Figure 2A). Plasma concentrations of TMAO-related metabolites and mouse characteristics are presented in Supplemental Table S4. Old mice had higher plasma concentrations of choline than young mice, consistent with our findings in humans and presumably due to differences in clearance of choline via the kidneys with aging; however, TMAO supplementation had no further effect on choline concentrations in either age group (both p>0.99), and there were no group differences in plasma levels of any other precursors of TMAO (all p>0.77). Old mice had lower body mass, liver mass, and visceral fat mass than young mice (all p<0.02), but there were no differences between Control and TMAO-supplemented mice within either age group. There were no differences in food intake or mass of other key organs (e.g., kidneys, heart) across age or treatment groups (all p≥0.44).
Figure 2. Chronic trimethylamine N-oxide (TMAO) supplementation increases plasma TMAO concentrations, aortic stiffness, and systolic blood pressure (BP) in young and old mice.
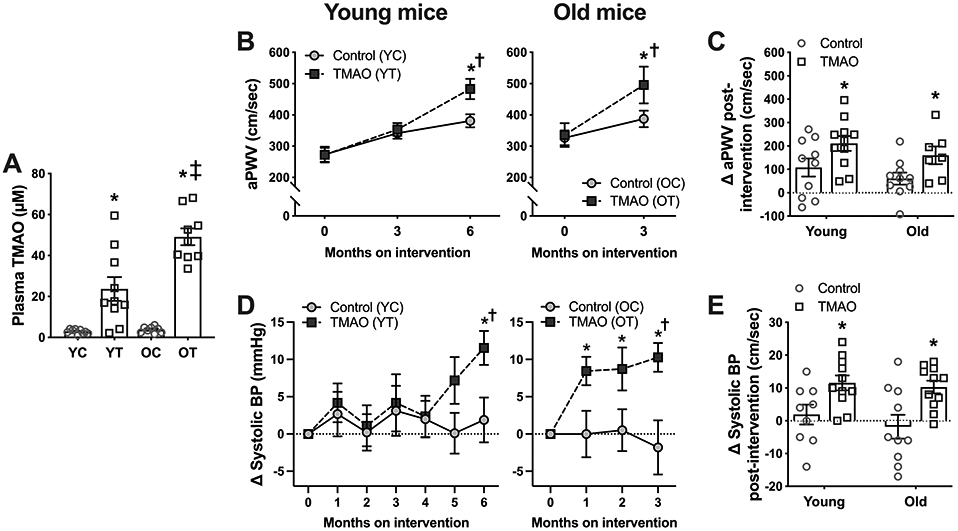
In young adult (6-12 months of age) and old (21-24 months) mice supplemented without (Control; YC, OC) or with 0.12% TMAO (YT, OT) for 3-6 months: A) Plasma concentrations of TMAO; B-C) in vivo aortic pulse wave velocity (aPWV); and D-E) in vivo tail cuff systolic BP. All data are mean ± S.E.M. N=7-11/group. *p<0.05 vs. Control, within time point/age group. †p<0.05 vs. baseline (0 months on intervention), within group. ‡p<0.05 OT vs. YC.
Aortic stiffness was assessed in vivo and non-invasively using the gold standard measure, aortic pulse wave velocity (aPWV), at baseline and following 3 (young and old mice) and 6 months (young mice only) of the intervention. Results are summarized in Figure 2B-C. In young adult mice, aPWV tended to increase across the 6-month intervention in Control mice (baseline vs. 6 mo: p=0.06). TMAO supplementation had no effect on aPWV after 3 months of the intervention (p=0.96 vs. Control) but induced a clear increase in aPWV by 6 months (p=0.0007, baseline vs. 6 months within TMAO group; p=0.04, Control vs. TMAO at 6 months), such that the increase in aPWV observed across the 6-month intervention was ~70% greater in TMAO-supplemented mice vs. Controls. At baseline, old mice had higher aPWV than young mice (330 ± 20 cm/sec vs. 273 ± 16 cm/sec, p=0.03). TMAO supplementation also exacerbated aortic stiffening in old mice (p=0.0005 vs. baseline; p=0.08 vs. Control at 3 months), but within half the time as was observed in young mice.
BP was measured in vivo and non-invasively by the tail cuff method on three consecutive days once per month throughout the interventions. Results are summarized in Figure 2D-E (systolic BP) and Supplemental Figure S4 (diastolic BP). Because of slight differences in tail cuff-assessed BP at baseline between the young (95 ± 2 / 67 ± 1 mmHg) and old (89 ± 2 / 62 ± 2 mmHg; both p=0.02) mice, all BP data are presented as a change from baseline within individual mice. There were no changes in either systolic or diastolic BP across the interventions in Control mice in both age groups. In young adult mice, both systolic and diastolic BP remained stable and comparable to Control mice through 4 months on the intervention but increased thereafter such that systolic and diastolic BP were, on average, 10 and 9 mmHg higher, respectively, in TMAO-supplemented vs. Control mice at 6 months (both p≤0.02 vs. baseline within TMAO mice and vs. Control at 6 months). In old mice, TMAO-induced increases in both systolic and diastolic BP were evident by 1 month into the intervention (both p=0.04 vs. baseline).
Overall, these data indicate that, although young mice had some initial resistance, dietary TMAO supplementation increases aortic stiffening and BP in both young adult and old mice. The temporal pattern of changes in aortic stiffness and BP, particularly in young mice followed over 6 months, suggest that these events may be linked as observed previously19,20.
3.3. Mechanisms of TMAO-induced aortic stiffening
We observed that dietary supplementation with TMAO induced aortic stiffening and increased BP in both young adult and old mice. Because aorta stiffening can precede and therefore contribute to increases in BP19,20, we next focused on discerning potential mechanisms of TMAO-induced aortic stiffening. To separate effects of TMAO from effects of aging, we performed mechanistic assessments in aortas from young adult mice that were either supplemented with TMAO in chow or not supplemented (Control).
3.3.1. Intrinsic properties.
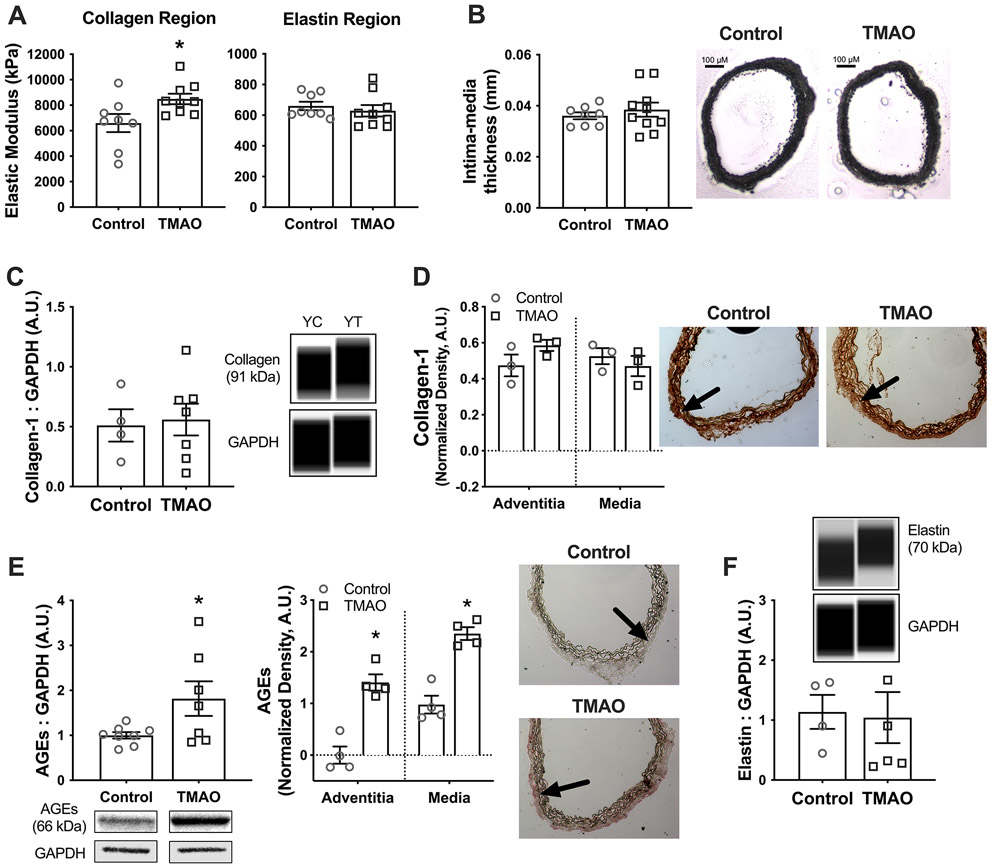
We measured aortic intrinsic mechanical stiffness and elasticity by conducting stress-strain testing in segments of thoracic aorta (see Supplemental Figure S1 for a representative tracing). The elastic modulus (EM) of the high-force region of the stress-strain curve, which is primarily dependent on collagen, was higher in young mice supplemented with TMAO (p=0.03 vs. Control; Figure 3A left), indicating greater intrinsic stiffness. However, there were no effects of TMAO supplementation on the EM of the elastin-dominant region of the stress-strain curve (low-force region where curvature is essentially zero) (p=0.87; Figure 3A right), a measure of elasticity. These data indicate that the TMAO-induced stiffening that we observed in vivo was due, at least in part, to increased intrinsic mechanical stiffness of the aorta but not accompanied by any changes in elasticity. This increase in stiffness was observed without any effects of TMAO supplementation on aortic intima-media thickness (p=0.77; Figure 3B) or diameter (YC: 0.66 ± 0.02 vs. YT: 0.65 ± 0.02 mm; p=0.79).
Figure 3. Chronic trimethylamine N-oxide (TMAO) supplementation increases aortic intrinsic mechanical stiffness and abundance of advanced glycation end-products.
In young adult mice (12 months at sacrifice) supplemented without (Control/YC) or with 0.12% TMAO (YT) for 6 months: A) Elastic modulus of the high-force collagen-dominant region (left) and the low-force elastin-dominant region (right) of the stress-strain curve, conducted in 1-2mm segments of thoracic aorta. B) Intima-media thickness measured in 7 μM segments of thoracic aorta, with representative images shown to the right. C-D) Protein abundance of mature type-1 collagen assessed by Western immunoblotting in aortic lysates, with representative Western blot images generated from WES electropherograms shown to the right (C), and assessed across vascular layers by immunohistochemistry in 7μm aorta sections, with representative images shown to the right (D). Arrows denote the medial-adventitial border. E) Protein abundance of advanced glycation end-products (AGEs) assessed by Western immunoblotting in aorta lysates (left) and across vascular layers by immunohistochemistry in 7 μM aorta sections (right), with representative blots/images below/to the right of the graphs. F) Protein abundance of elastin assessed by Western immunoblotting in aorta lysates, with representative Western blot images generated from WES electropherograms. All data are mean ± S.E.M. N=8-10/group for stress-strain testing. N=4-7/group for Western blotting and immunohistochemistry. *p<0.05 vs. Control. ‡p<0.10 vs. Control. AU, arbitrary units.
3.3.2. Abundance of structural proteins and AGEs.
Next, we measured aortic abundance of major arterial structural proteins and AGEs using both Western immunoblotting and IHC, which allows for discernment of protein abundance across vascular layers. Interestingly, we observed no effect of TMAO on whole aorta protein abundance of type-1 collagen (the major arterial isoform; p=0.82; Figure 3C), although we did observe a trend towards slightly higher type-1 collagen in the adventitia using IHC (p=0.09; Figure 3D). Abundance of AGEs, which increase aortic stiffness by forming crosslinks in structural proteins, was higher in aortas from TMAO-supplemented mice in the whole aorta (Western blot; p=0.04), and in both the adventitia and medial layers (IHC; both p<0.001) (Figure 3E). Together, these data suggest that TMAO supplementation increased intrinsic stiffness in part by increasing AGEs crosslinking of collagen fibers. Lastly, consistent with the lack of effect of TMAO supplementation on the EM of the elastin-dominant region of the stress-strain curve, there was no difference in whole aorta elastin abundance between Control and TMAO-supplemented mice (p=0.87; Figure 3F).
3.4. Incubation of aortic rings with TMAO directly induces aortic stiffening via AGEs crosslinking and oxidative stress
To determine whether TMAO induces aortic stiffening via direct effects on the arteries, we cultured matched aortic rings from young mice (aged 3-5 months) with 30 μM TMAO (average concentration measured in plasma from the dietary intervention) or with culture media only (vehicle condition), and then performed stress-strain testing to assess intrinsic mechanical stiffness. TMAO increased the EM of the collagen-dominant region of the stress-strain curve (p=0.04; Figure 4A), indicating greater intrinsic stiffness, by a similar magnitude as observed with dietary TMAO supplementation.
Figure 4. Acute ex vivo incubation with TMAO increases intrinsic mechanical stiffness via crosslinking of advanced glycation end-products (AGEs) and reactive oxygen species.
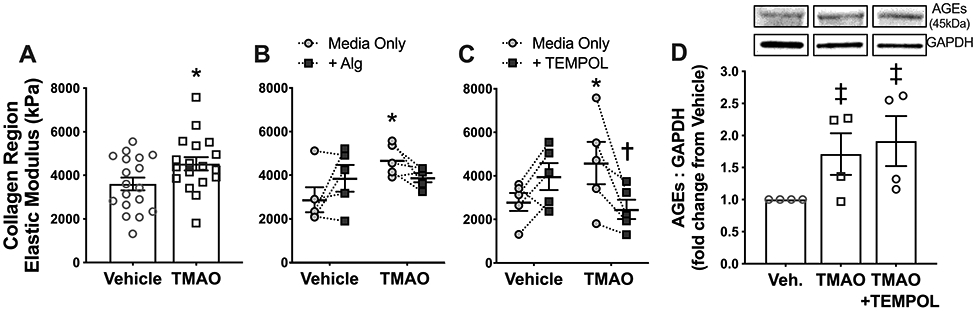
A-C) Elastic modulus of the high-force collagen-dominant region of the stress-strain curve (i.e., intrinsic mechanical stiffness) in multiple segments of thoracic aorta from the same young mice incubated for 72 h in control media (Vehicle) or media supplemented with 30 μM TMAO (A; N=18), with or without the addition of either the AGEs crosslink inhibitor alagebrium (B; N=5) or the superoxide dismutase mimetic TEMPOL (C; N=5). D) Abundance of AGEs in aorta rings from 4 additional young mice following 72 h incubation in control media (Vehicle) or media supplemented with 30 μM TMAO ± the superoxide dismutase mimetic TEMPOL. Data are mean ± S.E.M. *p≤0.05 vs. Vehicle (media only). †p<0.05 vs. TMAO (media only).
TMAO supplementation induced higher AGEs abundance, which increase aortic stiffness by crosslinking arterial structural proteins10. We have also shown that both dietary supplementation and ex vivo incubation with TMAO promote vascular oxidative stress13, which has been linked to AGEs in the context of aging38 and shown to promote stiffening independent of AGEs through various other pathways39,40. Thus, we tested whether TMAO increases aortic intrinsic mechanical stiffness via AGEs crosslinking and/or ROS signaling.
Matched aortic rings from a subset of the young mice were concomitantly incubated with TMAO or vehicle ± alagebrium, which specifically lowers or prevents AGES formation and therefore crosslinking of structural proteins caused by AGEs35,36. TMAO alone increased EM of the collagen-dominant region of the stress-strain curve, but the addition of alagebrium abolished this effect of TMAO (vehicle+Alg vs. TMAO+Alg: p>0.99) (Figure 4B).
Next, to determine whether TMAO-induced stiffening was mediated by greater ROS production, matched aortic rings from another subset of the young mice were concomitantly incubated with TMAO or vehicle ± the superoxide dismutase mimetic TEMPOL. Again, TMAO alone increased the collagen-dominant region EM (vehicle vs. TMAO in the absence of TEMPOL [media only]: p=0.05), and the addition of TEMPOL fully prevented this effect (TMAO vs. TMAO+TEMPOL: p=0.04; Figure 4C).
Lastly, to assess potential interactions between TMAO-induced AGEs accumulation and ROS production, we assessed AGEs abundance in aortic rings following incubation with TMAO in the absence or presence of TEMPOL. Abundance of AGEs was ~2-fold higher after incubation with TMAO vs. vehicle, but TEMPOL had no effect on TMAO-induced AGEs formation (Figure 4D). These data suggest that TMAO-induced ROS production occurs either downstream or independently of AGEs accumulation. These results also establish that the effects of TEMPOL on TMAO-induced intrinsic stiffness were unrelated to non-specific effects of the drug on AGEs.
4. DISCUSSION
Aortic stiffening is a key antecedent to the development of clinical CVD, including hypertension and atherosclerosis, and other age-related diseases, yet the upstream mechanisms driving this process are incompletely understood. In the present study, we show across multiple translational models that higher circulating concentrations of the gut microbiome-derived metabolite TMAO with aging are linked to aortic stiffening and increases in systolic BP. Specifically, our major findings are that: a) increases in plasma concentrations of TMAO with age are positively associated with aortic stiffness and systolic BP in healthy adult humans; b) dietary supplementation of TMAO in mice induces (in young adult mice) or exacerbates (in old mice) aortic stiffening and increases in BP; and c) both dietary supplementation with TMAO and incubation of aortic rings with TMAO increases the intrinsic stiffness of the aorta; and d) accumulation of AGEs and superoxide-stimulated oxidative stress contribute to TMAO-induced increases in intrinsic wall stiffness. Overall, these findings provide strong translational evidence that TMAO is an important upstream modulator of aortic stiffening with aging and offer novel insight into how TMAO may increase risk of CVD.
We have previously shown that, compared with young adult controls, plasma concentrations of TMAO are elevated in older humans13 and mice11 despite no differences in dietary intake of precursors of TMAO, including L-carnitine and choline, across age groups. Based on our data, age-related increases in circulating TMAO appear to be due to greater abundance of TMA-producing gut microbiota and/or the liver enzyme FMO3 that converts TMA to TMAO11. Expanding evidence implicates TMAO in the development of CVD18,31, particularly atherosclerosis12,15,16; however, to our knowledge, this is the first study to establish a link between TMAO and the development of arterial stiffness. As aortic stiffening and augmentation of systolic BP are key precipitating events in the development of clinical CVD1,41, it is possible that TMAO-related aortic stiffening is a primary contributor to later development of CVD.
In humans, TMAO predicted PWV and systolic BP independently of traditional CVD risk factors (e.g., cholesterol and blood glucose), but the relations between circulating TMAO and both aortic stiffness and systolic BP were closely linked to age. As such, we used an established mouse model of human vascular aging to isolate an independent role of TMAO in mediating aortic stiffening and increased BP. Young adult mice were initially resistant to TMAO supplementation, consistent with previous reports that young mice are inherently less responsive to stressors than older mice and humans42,43, but ultimately, both young adult and older mice exhibited aortic stiffening and increases in BP in response to directly supplementing TMAO in chow. Our combined data in humans and mice suggest a pathophysiological sequence of events whereby aging leads to increases in circulating TMAO, which then promote aortic stiffening and increases in systolic BP. By this notion, it is possible that intervening in mid-life (i.e., before the onset of aortic stiffening) to prevent increases in circulating TMAO with aging could also prevent, or at least attenuate, age-related aortic stiffening and increased systolic BP.
Mechanistically, we observed that dietary supplementation with TMAO induced greater intrinsic mechanical stiffness of the aorta that was accompanied by increased abundance of AGEs in the absence of changes in the major structural proteins collagen and elastin. Abundance of AGEs was also increased following incubation of aortic rings with TMAO. Consistent with our results, Tahara et al.44 found an independent relation between circulating levels of TMAO and the ratio of AGEs to soluble receptor for AGEs in healthy middle-aged and older humans. These authors attributed their findings to foods that promote TMAO (e.g., red meat) also having greater AGEs content. However, our data in mice indicate a direct effect of TMAO in promoting AGEs accumulation. To then establish a role of TMAO-induced AGEs accumulation in aortic stiffening, we co-incubated aortic rings with TMAO and the AGEs inhibitor alagebrium and found that the TMAO-induced increase in intrinsic stiffness was reduced by >50%, thus confirming an important role of AGEs-associated crosslinking of structural proteins in mediating TMAO-induced aortic wall stiffness.
In the present study, we also found that suppression of ROS production with TEMPOL prevented increases in intrinsic wall stiffness in response to TMAO. Greater ROS production can modulate arterial structure through various pathways40. However, in our TMAO dietary supplementation study, we found no evidence that TMAO increased aortic stiffness by increasing collagen deposition, degrading elastin or increasing intima-media wall thickness. A possible mechanism by which greater ROS production with TMAO may have increased stiffening is via upregulation of transglutaminase 2, which causes non-AGEs-dependent crosslinking of structural proteins45. Transglutaminase 2 activity is inhibited by the vasoprotective molecule nitric oxide46, and we have previously shown that exposure to TMAO reduces vascular nitric oxide bioavailability by stimulating ROS production13. Furthermore, genetic knockdown47 or pharmacological inhibition48 of transglutaminase 2 lowers BP and improves vascular endothelium-dependent dilation in old rodents, and, although the evidence is mixed47, may attenuate age-related increases in aortic stiffness49. Thus, although we were unable to assess transglutaminase activity due to limited availability of aortic lysate, this remains a potential mechanism of interest moving forward.
Lastly, TEMPOL had no effect on TMAO-induced increases in AGEs abundance, suggesting independent effects of ROS (superoxide) and AGEs in promoting aortic stiffening in response to TMAO. Alternatively, it is possible that TMAO evokes an accumulation of AGEs, which, in turn, stimulate ROS production. This latter possibility seems unlikely in our ex vivo model because AGEs appear to increase vascular oxidative stress via activation of surface receptors on endothelial and vascular smooth muscle cells50, and it is unclear if AGEs crosslinking structural proteins in the extracellular matrix of the aorta could elicit such effects. However, in vivo, TMAO may also increase free AGEs44 (e.g., circulating and/or interstitial), which could increase aortic stiffness via oxidative stress-related pathways.
4.1. Experimental Considerations and Limitations
The plasma concentrations of TMAO achieved following dietary supplementation in mice (~30 μM in young adult mice) were higher than the average plasma concentrations in MA/O humans. However, we observed a wide range of TMAO concentrations in humans (from ~1 to 45 μM). Therefore, the plasma TMAO concentrations achieved in mice were still within a physiological range for healthy aging.
We chose to use 30 μM TMAO in aortic ring experiments to match the average plasma TMAO concentrations observed in young adult mice following 6 months of dietary supplementation with TMAO. Although incubations were only 72 hours, we observed a similar magnitude increase in intrinsic mechanical stiffness (~30%) as following dietary supplementation, and our laboratory has previously shown that this duration of incubation is sufficient to alter stiffness in response to diverse stimuli30,51. It is possible that aortic stiffness can be altered more quickly ex vivo due to the absence of counter-regulatory functional (non-structural) factors that can influence PWV in vivo, including blood pressure, vascular tone, and/or sympathetic nervous system activity. Such influences may initially compensate for TMAO-induced changes in the extracellular matrix, thus delaying detectable differences in aortic PWV. Furthermore, it is possible the mechanisms of aortic stiffening may be slightly different between the chronic in vivo and short-term ex vivo conditions. For example, crosslinking of structural proteins by AGEs (i.e., a typically longer-term process) may have contributed to stiffening to a greater extent with dietary supplementation vs. ex vivo incubation, and interactions between AGEs and ROS production may have been somewhat different across models. Regardless, we show a clear role of TMAO-induced AGEs crosslinking in mediating aortic stiffening across our reverse translational experiments in mice.
The young adult control mice in our TMAO supplementation study exhibited some increases in aortic PWV over the 6-month intervention and had higher intrinsic mechanical stiffness than we have previously reported in young mice11,32. The long duration of the intervention, the defined (low) choline diet, and/or weight gain over the intervention all may have contributed to this increase in PWV. However, as all of these factors were matched across control and TMAO-treated mice, our study design still should isolate the effects of TMAO on aortic stiffening and the underlying mechanisms.
Only male mice were used in this study, as female mice do not exhibit increases in aortic stiffness until much later in life than male mice52 (and unpublished findings from our laboratory), likely due to differing sex hormone patterns across the lifespan. As such, female mice are not an appropriate model of vascular aging in women. We observed no sex differences in plasma TMAO concentrations in our human subjects and, thus, we believe our findings here in male mice are likely relevant to both men and women. That said, the absence of a biologically relevant mouse model for estrogen-deficient postmenopausal women is a limitation of our study.
Lastly, previous findings support the possibility that age-related aortic stiffening may precede increases in systolic BP19,20. In the present study, we observed largely parallel time courses for the respective increases in aPWV and systolic BP with dietary TMAO supplementation in mice, suggesting that those responses are temporally associated. However, neither these longitudinal observations in mice nor our cross-sectional comparisons in humans allow us to infer cause and effect. In all likelihood, the nature of the casual link between these events is bidirectional.
4.2. Conclusion
In the present study, we demonstrate a consistent and independent association between elevated circulating concentrations of TMAO and both aortic stiffening and increases in systolic BP with aging across both mice and humans. To our knowledge, this is the first study linking TMAO to arterial stiffening, as well as to BP in the context of aging. We also identify TMAO-induced accumulation of AGEs and consequent crosslinking of arterial structural proteins, as well as superoxide-related oxidative stress, as key mechanisms by which TMAO increases aortic stiffness.
5. PERSPECTIVES
Taken together, our results indicate that gut-targeted interventions to prevent the over-production of TMAO with aging may attenuate the development of aortic stiffening and increases in systolic BP with advancing age. Because aortic stiffness and above-normal systolic BP with aging are major risk factors for CVD and other age-related disorders, including cognitive dysfunction, Alzheimer’s disease, and chronic kidney disease, interventions aimed at reducing or preventing increases in circulating TMAO with aging may have the potential to reduce risk of CVD and other chronic conditions, thereby increasing human healthspan.
Certain dietary strategies may hold promise for reducing production of TMA by the gut microbiota, thus potentially minimizing increases in circulating TMAO concentrations with advancing age. For example, short-term feeding studies have shown that diets high in soluble fiber decrease plasma TMAO53, as does supplementation with specific probiotics54,55. Although the evidence is mixed on whether red meat and high saturated fat consumption increases TMAO levels56-58, humans adhering to a vegetarian/vegan diet have lower chronic urine concentrations of TMAO59 and exhibit a lesser rise in plasma TMAO following an oral carnitine challenge relative to omnivorous counterparts60. Therefore, such dietary-based strategies may help to suppress TMAO production in MA/O adults and thereby mitigate aortic stiffening and increases in systolic BP.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
We demonstrate a consistent association between higher circulating levels of the gut microbiome-derived metabolite TMAO in age-related arterial stiffening and augmentation of systolic BP in both mice and healthy humans.
We also identify accumulation of AGEs and consequent crosslinking of arterial structural proteins, as well as superoxide-related oxidative stress, as novel mechanisms mediating TMAO-induced arterial stiffening.
What is relevant?
Age-related arterial stiffening and augmentation of systolic BP are major antecedents to the development of CVD and other disorders of aging.
Emerging evidence suggests that age-related changes in the gut microbiome can influence the cardiovascular system.
This study identifies a novel upstream mechanism – higher circulating levels of TMAO – that contributes to age-related arterial stiffening and augmentation of systolic BP that could be targeted to improve vascular health with aging.
Summary
Using multiple complementary in vitro, ex vivo and in vivo translational models, we show that higher circulating levels of TMAO promote age-related arterial stiffening and increases in systolic BP by promoting accumulation of AGEs and excessive superoxide-related oxidative stress.
ACKNOWLEDGEMENTS
The authors would like to sincerely thank Jill Miyamoto-Ditmon, Danijel Djukovic, Zachary Condon, Zachary Cook, Christopher J Angiletta and Laura E Griffin for assistance with data collection and analysis.
SOURCES OF FUNDING
This work was supported by the National Institutes of Health [R01 HL143887 to D.R.S., F32 HL140875 to V.E.B., R21 AG058931 [K.P.D.], and Colorado CTSA UL1 TR002535]; and the Hatch Program of the National Institute of Food and Agriculture, U.S. Department of Agriculture to A.P.N.
Footnotes
CONFLICTS OF INTEREST / DISCLOSURES
None.
DATA AVAILABILITY
All data underlying this article will be shared upon reasonable request to the corresponding author.
REFERENCES
- 1.Palombo C, Kozàkovà M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol. 2016;77:1–7. [DOI] [PubMed] [Google Scholar]
- 2.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. [DOI] [PubMed] [Google Scholar]
- 3.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. [DOI] [PubMed] [Google Scholar]
- 4.Shokawa T, Imazu M, Yamamoto H, Toyofuku M, Tasaki N, Okimoto T, Yamane K, Kohno N. Pulse wave velocity predicts cardiovascular mortality: findings from the Hawaii-Los Angeles-Hiroshima study. Circ J. 2005;69:259–264. [DOI] [PubMed] [Google Scholar]
- 5.Franklin SS. Ageing and hypertension: the assessment of blood pressure indices in predicting coronary heart disease. J Hypertens Suppl. 1999;17:S29–36. [PubMed] [Google Scholar]
- 6.Nagai M, Hoshide S, Kario K. Hypertension and Dementia. Am J Hypertens. 2010;23:116–124. [DOI] [PubMed] [Google Scholar]
- 7.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse Pressure and Pulse Wave Velocity Are Related to Cognitive Decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. [DOI] [PubMed] [Google Scholar]
- 8.Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S, Mitchell GF. Aortic Stiffness and the Risk of Incident Mild Cognitive Impairment and Dementia. Stroke. 2016;47:2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. [DOI] [PubMed] [Google Scholar]
- 10.Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation - a mini-review. Gerontology. 2012;58:227–237. [DOI] [PubMed] [Google Scholar]
- 11.Brunt VE, Gioscia-Ryan RA, Richey JJ, Zigler MC, Cuevas LM, González A, Vázquez-Baeza Y, Battson ML, Smithson AT, Gilley AD, Ackermann G, Neilson AP, Weir T, Davy KP, Knight R, Seals DR. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J Physiol. 2019;597:2361–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, Wu Y, Schauer P, Smith JD, Allayee H, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunt VE, Gioscia-Ryan RA, Casso AG, VanDongen NS, Ziemba BP, Sapinsley ZJ, Richey JJ, Zigler MC, Neilson AP, Davy KP, Seals DR. Trimethylamine-N-Oxide Promotes Age-Related Vascular Oxidative Stress and Endothelial Dysfunction in Mice and Healthy Humans. Hypertension. 2020;76:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Levison BS, Hazen JE, Donahue L, Li X-M, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WHW, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015;163:1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang WHW, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisbrod RM, Shiang T, Sayah Al L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62:1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu S, Jin C, Li S, Zheng X, Zhang X, Cui L, Gao X. Aging, Arterial Stiffness, and Blood Pressure Association in Chinese Adults. Hypertension. 2019;73:893–899. [DOI] [PubMed] [Google Scholar]
- 21.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos-Parker JR, Strahler TR, Bassett CJ, Bispham NZ, Chonchol MB, Seals DR. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY). 2017;9:187–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, Seals DR. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension. 2018;71:1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeVan AE, Johnson LC, Brooks FA, Evans TD, Justice JN, Cruickshank-Quinn C, Reisdorph N, Bryan NS, McQueen MB, Santos-Parker JR, Chonchol MB, Bassett CJ, Sindler AL, Giordano T, Seals DR. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. J Appl Physiol. 2016;120:416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun. 2018;9:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T, American Heart Association Council on Hypertension. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boutagy NE, Neilson AP, Osterberg KL, Smithson AT, Englund TR, Davy BM, Hulver MW, Davy KP. Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity. 2015;23:2357–2363. [DOI] [PubMed] [Google Scholar]
- 28.Boutagy NE, Neilson AP, Osterberg KL, Smithson AT, Englund TR, Davy BM, Hulver MW, Davy KP. Short-term high-fat diet increases postprandial trimethylamine-N-oxide in humans. Nutr Res. 2015;35:858–864. [DOI] [PubMed] [Google Scholar]
- 29.Gioscia-Ryan RA, Clayton ZS, Fleenor BS, Eng JS, Johnson LC, Rossman MJ, Zigler MC, Evans TD, Seals DR. Late-life voluntary wheel running reverses age-related aortic stiffness in mice: a translational model for studying mechanisms of exercise-mediated arterial de-stiffening. Geroscience. 2020;63:636–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB, Seals DR. Sodium nitrite de-stiffening of large elastic arteries with aging: role of normalization of advanced glycation end-products. Exp Gerontol. 2012;47:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol [Internet]. 2018;124:1194–1202. Available from: 10.1152/japplphysiol.00670.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lammers SR, Kao PH, Qi HJ, Hunter K, Lanning C, Albietz J, Hofmeister S, Mecham R, Stenmark KR, Shandas R. Changes in the structure-function relationship of elastin and its impact on the proximal pulmonary arterial mechanics of hypertensive calves. Am J Physiol Heart Circ Physiol. 2008;295:H1451–H1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sokolis DP, Boudoulas H, Karayannacos PE. Assessment of the aortic stress-strain relation in uniaxial tension. J Biomech. 2002;35:1213–1223. [DOI] [PubMed] [Google Scholar]
- 35.Vasan S, Zhang X, Kapurniotu A, Bernhagen J, Teichberg S, Basgen J, Wagle D, Shih D, Terlecky I, Bucala R, Cerami A, Egan J, Ulrich P. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature. 1996;382:275–278. [DOI] [PubMed] [Google Scholar]
- 36.Chen NX, Srinivasan S, O'Neill K, Nickolas TL, Wallace JM, Allen MR, Metzger CE, Creecy A, Avin KG, Moe SM. Effect of Advanced Glycation End-Products (AGE) Lowering Drug ALT-711 on Biochemical, Vascular, and Bone Parameters in a Rat Model of CKD-MBD. J Bone Miner Res. 2020;35:608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toprak C, Sirmagul B, Yigitaslan S. Functional Effects of Alagebrium (ALT-711)–Isolated Rat Carotid Artery. Eurasian J Med. 2017;49:188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moldogazieva NT, Mokhosoev IM, Mel'nikova TI, Porozov YB, Terentiev AA. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid Med Cell Longev. 2019;2019:3085756–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyle AN, Raaz U. Killing Me Unsoftly: Causes and Mechanisms of Arterial Stiffness. Arterioscler Thromb Vasc Biol. 2017;37:e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lacolley P, Regnault V, Laurent S. Mechanisms of Arterial Stiffening: From Mechanotransduction to Epigenetics. Arterioscler Thromb Vasc Biol. 2020;40:1055–1062. [DOI] [PubMed] [Google Scholar]
- 41.Chirinos JA, Segers P, Hughes T, Townsend R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74:1237–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korou L-MA, Doulamis IP, Tzanetakou IP, Mikhailidis DP, Perrea DN. The effect of biological age on the metabolic responsiveness of mice fed a high-fat diet. Lab Anim. 2013;47:241–244. [DOI] [PubMed] [Google Scholar]
- 43.Lai M, Chandrasekera PC, Barnard ND. You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutr Diabetes. 2014;4:e135–e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tahara A, Tahara N, Yamagishi S-I, Honda A, Igata S, Nitta Y, Bekki M, Nakamura T, Sugiyama Y, Sun J, Takeuchi M, Shimizu M, Yamazaki H, Fukami K, Fukumoto Y. Ratio of serum levels of AGEs to soluble RAGE is correlated with trimethylamine-N-oxide in non-diabetic subjects. Int J Food Sci Nutr. 2017;68:1013–1020. [DOI] [PubMed] [Google Scholar]
- 45.Steppan J, Bergman Y, Viegas K, Armstrong D, Tan S, Wang H, Melucci S, Hori D, Park SY, Barreto SF, Isak A, Jandu S, Flavahan N, Butlin M, An SS, Avolio A, Berkowitz DE, Halushka MK, Santhanam L. Tissue Transglutaminase Modulates Vascular Stiffness and Function Through Crosslinking-Dependent and Crosslinking-Independent Functions. J Am Heart Assoc. 2017;6:L207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung SM, Jandu S, Steppan J, Belkin A, An SS, Pak A, Choi EY, Nyhan D, Butlin M, Viegas K, Avolio A, Berkowitz DE, Santhanam L. Increased tissue transglutaminase activity contributes to central vascular stiffness in eNOS knockout mice. Am J Physiol Heart Circ Physiol. 2013;305:H803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong DMF, Sikka G, Armstrong ADC, Saad KR, de Freitas WR, Berkowitz DE, Fagundes DJ, Santhanam L, Taha MO. Knockdown of transglutaminase-2 prevents early age-induced vascular changes in mice1. Acta Cir Bras. 2018;33:991–999. [DOI] [PubMed] [Google Scholar]
- 48.Pinilla E, Comerma-Steffensen S, Prat-Duran J, Rivera L, Matchkov VV, Buus NH, Simonsen U. Transglutaminase 2 Inhibitor LDN 27219 Age-Dependently Lowers Blood Pressure and Improves Endothelium-Dependent Vasodilation in Resistance Arteries. Hypertension. 2021;77:216–227. [DOI] [PubMed] [Google Scholar]
- 49.Santhanam L, Tuday EC, Webb AK, Dowzicky P, Kim JH, Oh YJ, Sikka G, Kuo M, Halushka MK, Macgregor AM, Dunn J, Gutbrod S, Yin D, Shoukas A, Nyhan D, Flavahan NA, Belkin AM, Berkowitz DE. Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ Res. 2010;107:117–125. [DOI] [PubMed] [Google Scholar]
- 50.Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD, Seals DR. Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell. 2014;13:576–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DuPont JJ, Kim SK, Kenney RM, Jaffe IZ. Sex differences in the time course and mechanisms of vascular and cardiac aging in mice: role of the smooth muscle cell mineralocorticoid receptor. Am J Physiol Heart Circ Physiol. 2021;320:H169–H180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Q, Wu T, Liu R, Zhang M, Wang R. Soluble Dietary Fiber Reduces Trimethylamine Metabolism via Gut Microbiota and Co-Regulates Host AMPK Pathways. Mol Nutr Food Res. 2017;61:1700473. [DOI] [PubMed] [Google Scholar]
- 54.Qiu L, Tao X, Xiong H, Yu J, Wei H. Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct. 2018;9:4299–4309. [DOI] [PubMed] [Google Scholar]
- 55.Qiu L, Yang D, Tao X, Yu J, Xiong H, Wei H. Enterobacter aerogenes ZDY01 Attenuates Choline-Induced Trimethylamine N-Oxide Levels by Remodeling Gut Microbiota in Mice. J Microbiol Biotechnol. 2017;27:1491–1499. [DOI] [PubMed] [Google Scholar]
- 56.Hamaya R, Ivey KL, Lee DH, Wang M, Li J, Franke A, Sun Q, Rimm EB. Association of diet with circulating trimethylamine-N-oxide concentration. American Journal of Clinical Nutrition. 2020;112:1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji Eun Park MM, Michael Miller MD, Jeffrey Rhyne BA, Zeneng Wang P, Stanley L Hazen MP. Differential Effect of Short-Term Popular Diets on TMAO and Other Cardio-metabolic Risk Markers. Nutrition, Metabolism and Cardiovascular Diseases. 2019;:1–23. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, Krauss RM, Hazen SL. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, Turroni S, Cocolin L, Brigidi P, Neviani E, Gobbetti M, O'Toole PW, Ercolini D. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. [DOI] [PubMed] [Google Scholar]
- 60.Wu W-K, Chen C-C, Liu P-Y, Panyod S, Liao B-Y, Chen P-C, Kao H-L, Kuo H-C, Kuo C-H, Chiu THT, Chen R-A, Chuang H-L, Huang Y-T, Zou H-B, Hsu C-C, Chang T-Y, Lin C-L, Ho C-T, Yu H-T, Sheen L-Y, Wu M-S. Identification of TMAO-producer phenotype and host-diet-gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut. 2019;68:1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data underlying this article will be shared upon reasonable request to the corresponding author.