Abstract
The success of transgenic mosquito vector control approaches relies on well-targeted gene expression, requiring the identification and characterization of a diverse set of mosquito promoters and transcriptional enhancers. However, few enhancers have been characterized in Anopheles gambiae to date. Here, we employ the SCRMshaw method we previously developed to predict enhancers in the A. gambiae genome, preferentially targeting vector-relevant tissues such as the salivary glands, midgut, and nervous system. We demonstrate a high overall success rate, with at least eight of eleven (73%) tested sequences validating as enhancers in an in vivo xenotransgenic assay. Four tested sequences drive expression in either the salivary gland or the midgut, making them directly useful for probing the biology of these infection-relevant tissues. The success of our study suggests that computational enhancer prediction should serve as an effective means for identifying A. gambiae enhancers with activity in tissues involved in malaria propagation and transmission.
Keywords: malaria, mosquito control, cross-species enhancer discovery, computational enhancer prediction, Drosophila melanogaster, regulatory genomics, cis-regulatory element, vector biology
Graphical Abstract
The success of transgenic mosquito vector control approaches relies on well-targeted gene expression, requiring the identification and characterization of transcriptional enhancers.
We employ a computational enhancer-discovery method to predict enhancers in the A. gambiae genome, targeting vector-relevant tissues such as the salivary glands, midgut, and nervous system.
We demonstrate a high overall success rate, with at least eight of eleven (73%) tested sequences validating as enhancers in an in vivo xenotransgenic assay.
Introduction
Mosquito-borne diseases present one of the world’s greatest challenges to global health. Malaria, despite recent declines, affects more than 225 million people and causes approximately 400,000 deaths each year (WHO, 2019). While efforts in vaccine development and insecticides continue to save millions of humans infected with malaria, vector control remains the most cost-effective method to protect populations from malaria epidemics (Bhatt et al., 2015). With the control of malaria threatened with widespread increases in insecticide resistance, research efforts to develop genetic strategies to control malaria transmission have taken on renewed importance (Ranson and Lissenden, 2016).
In sub-Saharan Africa, the malaria parasite, Plasmodium falciparum, is primarily transmitted by mosquitoes of the Anopheles gambiae species complex. The Anopheles host plays a vital role in the life cycle of the parasite, beginning with the ingestion of sexual-stage gametocytes during blood feeding from an infected host (Sinden, 1984). For successful completion of the life cycle in the mosquito, Plasmodium must cross the epithelial barriers of both the midgut and the salivary gland. Following ingestion, Plasmodium makes its way to the mosquito midgut where the reproductive forms fuse and form a zygote, which develops into an ookinete (Sinden, 1984, Touray, 1992). The ookinetes traverse the midgut epithelium and develop into oocysts that lodge between the epithelium and basal lamina (Sinden, 1984, Touray, 1992). After ~10 days, the oocysts start to rupture and release thousands of motile sporozoites that disperse throughout the mosquito (Sinden, 1984, Touray, 1992). A fraction of the released sporozoites invade the distal lateral and medial lobes of the salivary glands where they complete their time-dependent maturation and are transmitted to the next host via mosquito bite (Choumet, 2007).
Given the critical obligate role played by A. gambiae in both the life cycle of Plasmodium and the transmission of malaria from an infected to an uninfected host, disrupting the ability of the host to support the life cycle and transmission of the parasite creates attractive targets for malaria control. Moreira et al. (2000) generated transgenic Anopheles stephensi that, for the first time, were impaired in the transmission of the mouse malaria parasite, Plasmodium berghei, using an A. gambiae-derived blood-meal-inducible gut-specific promoter to induce the A. gambiae carboxypeptidase gene. CRISPR/Cas9 gene-editing technology was used by Dong et al. (2018) to create a knockout of FREP1, which plays an essential role duing the parasite’s midgut infection stage, resulting in malaria-resistant A. gambiae mosquitoes. Similar results have been achieved using a variety of other effector molecules (Caragata et al., 2020, Dong et al., 2011, Abraham et al., 2005, Isaacs et al., 2011). These studies constitute proof-of-principle that mosquitoes can be engineered to reduce malaria transmission via targeted transgene expression. Along the same lines, manipulating the immune system of A. gambiae has been proposed as a way to reduce malaria transmission (Caragata et al., 2020, Terenius et al., 2008).
Controlling the mosquito population is another potential route for breaking the malaria cycle. Improving the sterile insect technique (SIT), which is based on the sterilization of males before mass release and has been used to successfully suppress field populations of many insect species of agricultural importance (Carvalho et al., 2015, Dame et al., 2009, Dyck, 2005, Helinski et al., 2009, Lees et al., 2015), through genetically-engineered mosquitoes is one area showing promise. For example, a sex-specific dominant lethal gene can be introduced into a population, a method known as RIDL (release of insects with a dominant lethal). Trials of this approach to suppress Aedes aegypti populations have successfully been carried out in the field (Caragata et al., 2020, Carvalho et al., 2015, Garziera et al., 2017, Evans et al., 2019). Engineered gene drive systems have also been proposed as a method to prevent malaria by causing rapid spread of a deletrious gene to knock out mosqutio populations (Alphey et al., 2013, Champer et al., 2016, Sinkins and Gould, 2006). Windbichler et al. (2011) explored a sex-ratio distorting system based on homing exonucleases for A. gambiae to reduce or eliminate certain local populations, and CRISPR-based gene drives have been tested in the laboratory (Caragata et al., 2020, Gantz et al., 2015, Kyrou et al., 2018).
A common element in each of these approaches is the need for well-targeted gene expression, requiring the identification and characterization of a diverse set of mosquito promoters and enhancers (distal positive-acting gene regulatory elements). However, the importance of characterizing mosquito enhancers goes well beyond their utility for such biotechnology-based approaches. Identifying and studying mosquito enhancers acting at all stages of the life cycle can provide valuable insights into mosquito biology. For instance, polymorphisms in enhancers have been shown to affect mating success (Drapeau et al., 2006, Massey et al., 2019) and resistance to insecticides (Daborn et al., 2002). Enhancer studies are critical for improved understanding of embryonic and larval development, which in turn may spur new strategies and targets for mosquito biocontrol. With many Anopheles species now sequenced, enhancer identification will enable in-depth study of the roles of non-coding polymorphisms and of mechanisms driving evolution and speciation. Unfortunately, few A. gambiae enhancers have been characterized to date, with most targeted gene expression in this species depending on either using promoters of genes with desired expression, or creating enhancer-trap lines that target gene expression without concomitant identification of the relevant functional sequences (O’Brochta et al., 2012). While useful, such approaches leave unexplored the vast majority of the mosquito regulatory sequence landscape.
Comparative genomics has allowed for identification of transcription factor binding sites in mosquitoes (Sieglaff et al., 2009), but the sequence divergence between Drosophila and Anopheles prevents reliable enhancer discovery based on alignment (Kazemian et al., 2014), and binding-site data alone have limited effectiveness (Suryamohan and Halfon, 2015). Although the recent availability of 20+ related Anopheline genomes improves the ability to detect potential enhancer regions based on sequence conservation (e.g., using EvoPrinter (Brody et al., 2020)), such methods need to be targeted to a particular locus, and while conservation can suggest that a given sequence might be functionally important, it does not provide any indication as to what the tissue-specificity of a putative enhancer might be. Similarly, while genome-wide methods such as ATAC-seq or FAIRE-seq (Simon et al., 2012, Buenrostro et al., 2015) can identify potential enhancers, unless they are performed in a tissue-specific fashion, many candidate sequences need to be assessed to determine actual function.
We have previously shown that our computational SCRMshaw method, which uses training data from the fly, Drosophila melanogaster, works for enhancer discovery in diverged insects including mosquitoes (Kazemian et al., 2014, Suryamohan et al., 2016). SCRMshaw provides three key advantages: one, as a computational approach, is it rapid and low-cost; two, it provides genome-wide results; and three, because it uses tissue-specific training input, it predicts enhancers in a tissue-specific manner. Here, we use SCRMshaw to predict enhancers in the A. gambiae genome, with a particular focus on those predicted to regulate gene expression in the salivary gland and the midgut, two tissues important for successful Plasmodium transmission. We demonstrate a high overall success rate, with at least eight of eleven tested sequences validating as enhancers. Four tested sequences drive expression in either the salivary gland or the midgut, making them directly useful for probing the biology of these infection-relevant tissues.
Results and Discussion
In previous work we developed SCRMshaw (for “Supervised cis-Regulatory Module prediction”), an approach for accurate prediction of enhancers in D. melanogaster and other insects (Kantorovitz et al., 2009, Kazemian et al., 2014, Kazemian et al., 2011). Briefly, SCRMshaw uses a training set of known D. melanogaster enhancers that are defined by common functional characterization to build a statistical model that captures their short DNA subsequence (k-mer) count distributions. The trained model is used to score overlapping windows in the “target genome,” and the highest-scoring regions are predicted to be enhancers (Fig. 1). In Kazemian et al. (2014), we showed that the target genome does not need to be D. melanogaster but rather can be any holometabolous insect. In that study, two candidate Anopheles gambiae enhancers, both from an early embryo patterning gene training set, were chosen for testing and successfully validated, suggesting that SCRMshaw performs strongly for this species.
Figure 1.

Supervised motif-blind CRM discovery (SCRMshaw). (A) SCRMshaw uses a training set of known Drosophila melanogaster enhancers (“Training CRM set”) that are defined by common functional characterization, along with a background set of similarly-sized non-enhancer sequences (“BKG sequences”). (B) The short DNA subsequence (k-mer) count distributions of these sequences are then used to train a statistical model. The trained model (C) is used to score overlapping windows in the “target genome”—here, Anopheles gambiae—and the highest scoring regions are predicted to be enhancers (D, asterisks). Figure adapted from Suryamohan and Halfon (2015).
Here, we used SCRMshaw to perform a more extensive prediction of enhancers in Anopheles gambiae (see Methods). For this initial study we relied on established and already-proven training sets, which primarily target embryonic tissues (Kazemian et al., 2014), but with a focus on tissues more reflective of vector biology: gut, salivary gland, and nervous system, as well as imaginal discs, mesoderm, and ectoderm (Table 1). The top 500 ranked predictions from each training set were considered as potential true-positive predictions; from these, candidate sequences for in-vivo validation were chosen by prioritizing those whose nearest annotated A. gambiae gene had a known D. melanogaster ortholog with expression in tissues of interest (e.g., salivary gland or midgut; see Methods) (Table S1). We selected a total of eleven predicted A. gambiae enhancers and tested them for regulatory activity using reporter gene assays in transgenic D. melanogaster (Table 1).
Table 1.
Results of in vivo A. gambiae enhancer validations.
| Predicted Enhancer | Drosophila melanogaster ortholog(s)a | Tested Coordinatesb | Training set | Expected expressionc | Observed expression | Match gene?d | Match training?e |
|---|---|---|---|---|---|---|---|
| C01-800 | Inside CG7611 | 2L:25564979-25565526 | mapping2.salivary_gland | Expressed ubiquitously in the embryo, high levels of expression in the larval salivary glands and midgut | clypeolabrum (st. 14); anterior pharynx (st. 15–16); midgut (starting st. 14); subset cells in amnioserosa; weak hindgut | U | N |
| C02-355 | Inside of Atg5 | 3L:13487751-13488250 | mapping1.salivary_gland | Pupal salivary glands | Salivary gland (possibly due to vector-driven expression) | U | U |
| C02-783 | Downstream of β-Man | X:2179509-2180000 | mapping1.salivary_gland | Salivary gland and midgut | Fat body, gut, salivary gland, other | Y | U |
| C02-221 | Inside of Hnf4 | 2R:16555950-16556550 | mapping1.endoderm and mapping1.salivary_gland | Embryonic salivary gland, fat body, gut, oenocyte | Subset of cells in stomatogastric nervous system; lateral segmentally repeated cells (probable oenocyte precursors) | Y | Y |
| C02-333 | Exonic region of CG30460 | 3L:3138501-3139001 | mapping1.endoderm | Midgut and muscle | Proventriculus | Y | Y |
| C02-188 | Downstream of Ncc69 and upstream of CrzR | 2R:6200001-6200501 | mapping1.endoderm | Midgut, foregut, and late salivary gland | No expression | N | N |
| C01-333 | Upstream of Stacl | 2L:13256901-13257589 | mapping1.pns | Embryonic peripheral and central nervous systems | Peripheral nervous system (chordotonal organs) | Y | Y |
| C01-4868 | Inside of eyg | 3L:24132710-24134004 | mapping1.imaginal_disc also: mapping1.trachea, mapping2.neuronal | Embryonic nervous system expression | Central nervous system, brain, posterior, faint dorsal epidermis | Y | Y |
| C01-2915 | Upstream of CG6154 | 2R:24703930-24704594 | mapping1.ventral_ectoderm | Not well characterized | Tracheal system - different subset of cells then C01-4888 | U | N |
| C01-4888 | Inside of kn | 3L:24700700-24701525 | mapping1.somatic_muscle & mapping2.mesoderm also: neuroectoderm, mesectoderm | Imaginal tissue, sensory system, nervous system, digestive system | Tracheal system - different subset of cells then C01-2915 | N | N |
| C01-2230 | Inside of Desat1 | 2R:8869422-8870077 | mapping0.dv_neurogenicectoderm | Ubiquitous, strong expression in midgut | No expression | N | N |
Based on closest D. melanogaster gene; assignments as per FlyBase (Thurmond et al., 2019)
Anopheles gambiae PEST P4 coordinates
Based on FlyBase (Thurmond et al., 2019) gene report
Observed expression is broadly consistent with that of putative target gene. Y, yes; N, no; U, unable to determine (target gene expression is unknown or ubiquitous, or reporter gene expression is confounded with vector-only expression).
Observed expression is consistent with expectation based on training set. Y, yes; N, no; U, unable to determine
Six of the eleven candidate enhancer sequences were cloned into the pGreenRabbit GFP reporter vector; the other five were cloned into pGHEEP, a φC31- and piggyBac enabled transformation vector with a Gal4 reporter gene (see Methods). We developed pGHEEP to allow for ease of evaluation of enhancer activity in either transgenic D. melanogaster or A. gambiae, using the same reporter construct. However, we found that the pGHEEP vector, which uses promoter sequences subcloned from pRed-H-Stinger (Barolo et al., 2004), itself mediates late embryonic, larval, and pupal salivary gland reporter gene expression (Fig. 2A). This is consistent with previous reports that the Stinger series of P-element transformation vectors can drive reporter gene expression in the larval and pupal salivary gland (Zhu and Halfon, 2007), although the embryonic component of the expression was unexpected. This vector-dependent expression unfortunately prevented us from reliably scoring potential activity of the candidate enhancers in the salivary glands.
Figure 2.
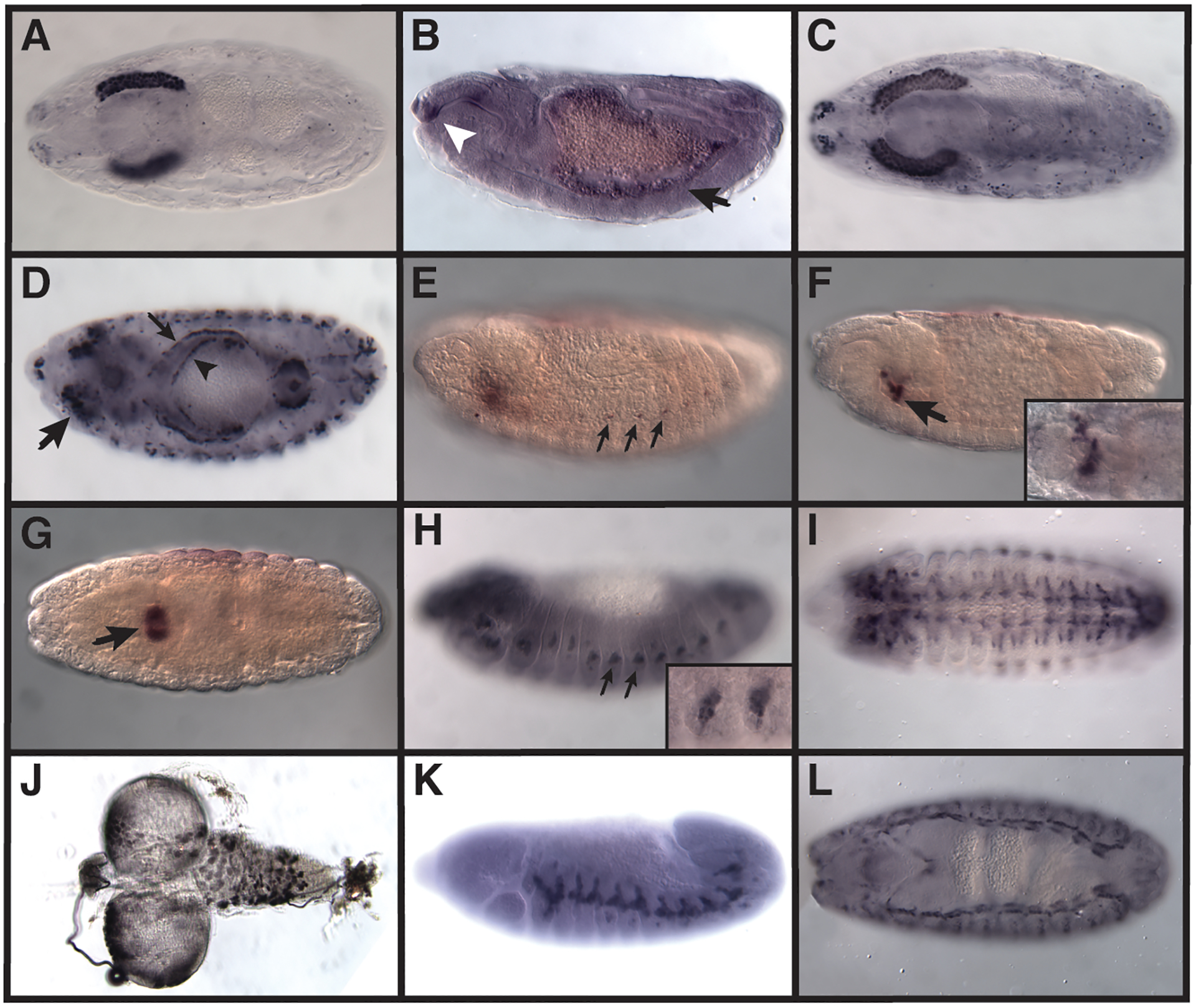
Predicted Anopheles gambiae enhancer sequences were used to drive reporter gene expression in transgenic Drosophila melanogaster. (A, C, D) expression was visualized by crossing each line with UAS-LacZ and staining with anti-β-galactosidase. (B, H-L) expression was visualized by staining with anti-GFP. (E-G) expression was visualized by in situ hybridization for detection of Gal4 transcripts. (A) The pGHEEP vector alone mediates late embryonic salivary gland reporter gene expression. (B) C01–800 drove expression in the clypeolabrum (arrowhead), with weaker expression in the midgut (arrow). (C) C02–355 drove expression in the embryonic salivary gland (consistent with the pGHEEP vector (compare with panel A)). (D) C02–783 drove expression in the embryonic salivary gland (thick arrow) (consistent with the empty pGHEEP vector), the fat body (thin arrow), and the midgut (arrowhead). (E, F) C02–221 drove expression in a small set of laterally-positioned cells in the abdominal segments (arrows) (E) and in a subset of cells in the embryonic stomatogastric nervous system (arrow and insert) (F). (G) C02–333 drove expression in the proventriculus (arrow). (H) C01–333 drove expression in ventral chordotonal organs (arrows and insert). (I, J) C01–4868 drove expression in both the embryonic (I) and larval (J) central nervous system. (K) C01–2915 drove expression in the tracheal system, as did C01–4888 (L). Embryos are pictured as dorsal views (A, C, G, L, D) or as sagittal views with dorsal to the top (B, E, F, H, K); panel I is a ventral view. All embryos are shown with anterior to the left.
Eight of the eleven (73%) candidate enhancer sequences (plus one which could not be evaluated due to possible vector-dependent expression) showed activity in the transgenic fly assay, consistent with our previously-reported enhancer-discovery rates (75%) (Kantorovitz et al., 2009, Kazemian et al., 2014, Kazemian et al., 2011). We assayed embryos at all stages; for putative enhancers cloned into pGHEEP we also assayed larvae and pupae.
We evaluated three candidate enhancers (C01–800, C02–355, and C02–783) from the salivary gland training sets (Table 1). In the embryo, C01–800 drove expression in the clypeolabrum, persisting into the anterior portion of the pharynx. Additional weak expression could be observed in the midgut and part of the hindgut, and in a small number of cells in the amnioserosa (Table 1, Fig. 2B and data not shown). No larval expression was observed. The enhancer lies within an intron of gene AGAP006042, a putative ortholog of the Drosophila gene CG7611. CG7611 is expressed ubiquitously in the embryo, but has high levels of expression in the larval salivary glands and midgut (Hammonds et al., 2013, Tomancak et al., 2002, Tomancak et al., 2007). Both C02–355 (Fig. 2C) and C02–783 (Fig. 2D) displayed strong expression in the embryonic salivary gland. C02–355 lies in an intron of AGAP01939, orthologous to Drosophila Atg5. Atg5 is not strongly expressed in embryos (Hammonds et al., 2013, Tomancak et al., 2002, Tomancak et al., 2007, Lee et al., 2003), but interestingly, the Atg5 intron has been shown to contain at least one enhancer targeting the nearby gene brk (Hong et al., 2008). A. gambiae maintains the syntenic relationship of these two genes, raising the possibility that similarly, the tested putative enhancer could target the brk ortholog; brk is expressed in the embryonic salivary glands. C02–783 was originally chosen due to its location roughly 1 kb downstream of AGAP000143, orthologous to β-Man. β-Man is expressed in both salivary gland and midgut (Hammonds et al., 2013, Tomancak et al., 2002, Tomancak et al., 2007). However, updated gene models (Vectorbase release 48 (Giraldo-Calderon et al., 2015, Sharakhova et al., 2007)) now suggest that the tested sequence lies within the 3’ UTR of the gene, rather than in the downstream intergenic region. C02–783 drives extensive expression in addition to that in the salivary glands, including in the fat body, gut, and additional segmentally-repeated cells (Table 1, Fig. 2D). Although we cannot be certain that the observed salivary gland expression for C02–355 and C02–783 results from the putative enhancer sequences rather than the plasmid vector, we note that regardless, we have created strong salivary-gland-targeted expression constructs that may prove to be useful reagents with respect to this malaria-relevant tissue.
Three putative enhancers (C02–221, C02–333, and C02–188) were evaluated from the endoderm training set (Table 1). C02–221 (which was also predicted by the salivary gland training set, with a lower score) maps to an intron of AGAP002155, orthologous to Drosophila Hnf4, and drove expression in a subset of cells in the embryonic stomatogastric nervous system as well as a small set of laterally-positioned cells in the abdominal segments (Table 1, Fig. 2E, F). The location of the latter suggests that they might be oenocyte precursors, a known site of Hnf4 expression, although the weak expression precluded a definitive identification (Hammonds et al., 2013, Tomancak et al., 2002, Tomancak et al., 2007). C02–333 drove expression in the proventriculus (Table 1, Fig. 2G). This sequence was originally selected as lying in an intergenic region between AGAP010424 and AGAP01425, but updated annotation places it overlapping an exon of AGAP028496. This gene is most closely related to Drosophila CG30460, which is expressed in both midgut and muscle (Hammonds et al., 2013, Tomancak et al., 2002, Tomancak et al., 2007). C02–188, which overlaps the promoter of AGAP001558, showed no activity (Table 1).
We evaluated one candidate (C01–333) from the peripheral nervous system (PNS) training set and found that, as predicted, it drove expression in a subset of the PNS (Table 1, Fig. 2H). C01–333 sits just upstream of AGAP029530, the expression of whose Drosophila ortholog, Stacl, is not well-characterized but does include lateral PNS expression (Hammonds et al., 2013, Tomancak et al., 2002, Tomancak et al., 2007). C01–4868 was predicted both from our imaginal disc and neuronal training sets, and drove expression in the embryonic and larval central nervous systems (Table 1, Fig. 2I, J). The sequence lies in an intron of AGAP011417, which is orthologous to Drosophila eyg, a gene with known embryonic nervous system expression (Hammonds et al., 2013, Tomancak et al., 2002, Tomancak et al., 2007).
Candidate sequences from additional training sets also proved to be bona fide enhancers, but regulated expression in patterns other than what was predicted from the initial training data. An enhancer (C01–2915) from the ventral ectoderm training set drove expression in the tracheal system (Table 1, Fig. 2K), as did one (C01–4888) from the somatic muscle/mesoderm and neuroectoderm training sets (Table 1, Fig. 2L). A candidate enhancer (C01–2230) from the neurogenic ectoderm training set showed no activity, suggesting a false-positive prediction result (Table 1).
Overall, 63% of the tested sequences (five of eight that could be evaluated) drove tissue-specific expression in a pattern that reasonably overlapped that of the Drosophila ortholog of their closest gene, and 44%−55% acted in tissues consistent with prediction. (Not all of the eleven lines could be evaluated for each criterion, either due to possible vector-dependent expression or because expression of orthologs is not known; the range for predicted expression matching represents the lower/upper bounds depending on whether observed expression is enhancer-driven or vector-driven.) Although these numbers are moderately weaker than what we have observed in previous applications of SCRMshaw (Kantorovitz et al., 2009, Kazemian et al., 2014, Kazemian et al., 2011), they are well above random expectation (Asma and Halfon, 2019). SCRMshaw performance is highly dependent on the training data used, and a relatively narrow selection of training sets were used here. Repeating the analysis with a refined set of training data may lead to a higher validation rate, as may combining SCRMshaw analysis with data from a chromatin-profiling method such as ATAC-seq or FAIRE-seq (Lai et al., 2018). The state of the A. gambiae genome annotation at the time the initial SCRMshaw analysis was performed may also have impacted performance; for instance, the intergenic sequence selected for C02–783 was subsequently reannotated as a 3’ untranslated region, and C01–333 was reannotated as overlapping a coding exon. Although it is not unheard of for enhancers to lie in such sequences—and even to regulate genes other than the ones in which they lie (Birnbaum et al., 2012)—SCRMshaw does not perform as well when coding sequences are included in the analysis (B. Yuen, H. Asma, and MSH, unpublished data), as coding sequence has distinct sequence properties. This highlights the importance of having an accurate and comprehensive genome annotation for enabling robust enhancer discovery. We note as well that A. gambiae genes are not always expressed in the same pattern as their Drosophila orthologs (although in most known cases expression is grossly, even if not exactly, similar). Finally, assignment of enhancer target genes here are based primarily on the identity of the closest flanking gene, a method that while simple to apply is known to be of questionable accuracy. Nevertheless, most described Anopheles enhancers have been tested using only a transgenic Drosophila model, as we have done here (e.g., Cande et al., 2009, Skavdis et al., 1996, Erives and Levine, 2004, Goltsev et al., 2007, Markstein et al., 2004, Kazemian et al., 2014, Ahanger et al., 2013, Simpson et al., 2006, Rebeiz et al., 2012), with studies using transgenic mosquitoes focused primarily on extended promoter sequences (e.g., Ye et al., 2020, Lynd and Lycett, 2012, Lombardo et al., 2005, Chen et al., 2007, Nolan et al., 2011, Dong et al., 2020, Volohonsky et al., 2015). In the rare cases where the same sequences have been tested in both fly and mosquito, expression has been essentially identical (e.g., Lombardo et al., 2005). Our own previous studies have demonstrated strong congruence in the expression patterns driven by putative enhancers tested xenotransgenically in Drosophila with expression driven by transgenes in the native species (Lai et al., 2018), or based on in situ hybridization to the target gene mRNA (Kazemian et al., 2014). Ultimately, it will still be necessary to assay our identified sequences directly in transgenic A. gambiae to verify their native tissue specificity.
At least four of the constructs we tested (36%) were capable of regulating gene expression in embryonic tissues for which the adult tissue is of potential interest for vector control, suggesting that over one-third of our predicted sequences may be relevant tissue-specific A. gambiae enhancers. Although we focused here mainly on embryonic tissues, we expect that, given our ability to target these tissue types, properly constructed training sets based on the related adult-specific enhancer activity should work equally well. Training sets for many adult tissues of interest, especially midgut and nervous system, should be available soon (H. Asma and MSH, unpublished data). Computational enhancer prediction, using carefully crafted training data based on well-characterized Drosophila regulatory sequences, should therefore serve as an effective means for identifying A. gambiae enhancers with activity in tissues involved in Plasmodium’s life cycle in the mosquito, or its subsequent transmission.
Experimental Procedures
Selection of candidate enhancers
SCRMshaw was run as previously described (Kazemian and Halfon, 2019) with the following parameters: --thitg 300 --imm --pac --hexmcd, and the A. gambiae PEST P4 genome assembly and P4.4 annotation version (downloaded from Vectorbase (Giraldo-Calderon et al., 2015)). Training data were drawn from (Kazemian et al., 2014), except that only the following sets were used: mapping2.mesoderm, mapping1.glia, mapping0.dv, mapping1.malpighian_tubules, mapping2.neuroectoderm, mapping1.mesectoderm, mapping1.salivary_gland, mapping1.imaginal_disc, mapping1.mesoderm, mapping1.larval_mesoderm, mapping2.salivary_gland, mapping1.pns, mapping1.visceral_mesoderm, mapping1.male_gonad, mapping0.ap, mapping1.female_gonad, mapping0.dv_earlymesoderm, mapping1.neuroectoderm, mapping1.ventral_ectoderm, mapping1.tracheal_system, mapping2.mesectoderm, mapping2.reproductive_system, mapping0.dv_neurogenicectoderm, mapping1.somatic_muscle, mapping2.tracheal_system. Training sets mapping1.salivary_gland and mapping1.endoderm were evaluated using the same parameters except with the P4.9 annotation version (downloaded from Vectorbase (Giraldo-Calderon et al., 2015)). After running SCRMshaw, the top 500 SCRMshaw results for each training set were merged to account for overlapping regions and/or duplicate predictions (Table S1). From these top SCRMshaw predictions, candidates for validation were selected based on high SCRMshaw scores, the identity of the nearest annotated gene(s), and the relevance of the training set to malaria vector biology (Table 1, S2). Expression profiles for the orthologous Drosophila genes were obtained from FlyBase and manually inspected for expression of interest (e.g., salivary gland and midgut).
Reporter constructs and transgenic Drosophila
Genomic sequences were amplified by PCR from A. gambiae genomic DNA, cloned into pJet1.2blunt (Fermentas), and confirmed by sequencing. Primer sequences are provided in Table S2. Six putative enhancer sequences were subcloned into plasmid pGreenRabbit (Table S2), a φC31-enabled Drosophila transformation vector containing enhanced green fluorescent protein (EGFP) under the control of a minimal hsp70 promoter (Housden, 2012). The remaining five putative enhancer sequences were subcloned into pGHEEP (Table S2), a derivative of the piggyBac-Gal4 construct described in Mysore et al. (2018) (provided by M. Duman-Scheel) modified through insertion of the upstream hsp70 minimal promoter from plasmid pRed-H-Stinger (Barolo et al., 2004). Transgenic flies were produced by Rainbow Transgenic Flies (Camarillo, CA) by injection into lines attP2 R8622 or 8622.
Immunohistochemistry and in situ hybridization
For all analyses, a minimum of ten embryos were analyzed in detail. Immunohistochemistry was performed using standard methods (Muller, 2008). Primary antibodies used were rabbit anti-GFP (1:500; Ab-cam, ab290) and mouse anti-β-galactosidase (1:500; Ab-cam). The ABC kit (Vector Laboratories) was used for immunohistochemical staining. Differential interference contrast (DIC) microscopy was performed using a Zeiss Axioskop 2 microscope and Openlab software (PerkinElmer) for image capture. In situ hybridization for detection of Gal4 transcripts was performed using standard methods (Tautz and Pfeifle, 1989).
Image credits
The following graphical elements were obtained from The Noun Project (thenounproject.com) under a Creative Commons CCBY license: fly, Georgiana Ionescu; mosquito, Cristiano Zoucas; analytics, Wilson Joseph.
Supplementary Material
Table S1. Predicted A. gambiae enhancers. The top 500 SCRMshaw predicted enhancers for A. gambiae.
Acknowledgements
We thank Molly Duman-Scheel for the gift of the piggyBac-Gal4 plasmid and for helpful comments on the manuscript, Hasiba Asma for assistance with bioinformatics analysis, Jack Leatherbarrow for assistance with cloning and Drosophila genetics, and Caila Wagar for assistance with Drosophila embryo collection. This work was supported by grant NIH R21 AI125918.
References
- ABRAHAM EG, DONNELLY-DOMAN M, FUJIOKA H, GHOSH A, MOREIRA L & JACOBS-LORENA M 2005. Driving midgut-specific expression and secretion of a foreign protein in transgenic mosquitoes with AgAper1 regulatory elements. Insect Mol Biol, 14, 271–9. [DOI] [PubMed] [Google Scholar]
- AHANGER SH, SRINIVASAN A, VASANTHI D, SHOUCHE YS & MISHRA RK 2013. Conserved boundary elements from the Hox complex of mosquito, Anopheles gambiae. Nucleic Acids Res, 41, 804–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALPHEY L, MCKEMEY A, NIMMO D, NEIRA OVIEDO M, LACROIX R, MATZEN K & BEECH C 2013. Genetic control of Aedes mosquitoes. Pathog Glob Health, 107, 170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASMA H & HALFON MS 2019. Computational enhancer prediction: evaluation and improvements. BMC Bioinformatics, 20, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAROLO S, CASTRO B & POSAKONY JW 2004. New Drosophila transgenic reporters: insulated P-element vectors expressing fast-maturing RFP. Biotechniques, 36, 436–40, 442. [DOI] [PubMed] [Google Scholar]
- BHATT S, WEISS DJ, CAMERON E, BISANZIO D, MAPPIN B, DALRYMPLE U, BATTLE KE, MOYES CL, HENRY A, ECKHOFF PA, WENGER EA, BRIET O, PENNY MA, SMITH TA, BENNETT A, YUKICH J, EISELE TP, GRIFFIN JT, FERGUS CA, LYNCH M, LINDGREN F, COHEN JM, MURRAY CLJ, SMITH DL, HAY SI, CIBULSKIS RE & GETHING PW 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature, 526, 207–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRNBAUM RY, CLOWNEY EJ, AGAMY O, KIM MJ, ZHAO JJ, YAMANAKA T, PAPPALARDO Z, CLARKE SL, WENGER AM, NGUYEN L, GURRIERI F, EVERMAN DB, SCHWARTZ CE, BIRK OS, BEJERANO G, LOMVARDAS S & AHITUV N 2012. Coding exons function as tissue-specific enhancers of nearby genes. Genome Research, 22, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODY T, YAVATKAR A, KUZIN A & ODENWALD WF 2020. Ultraconserved Non-coding DNA Within Diptera and Hymenoptera. G3-Genes Genomes Genetics, 10, 3015–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUENROSTRO JD, WU B, CHANG HY & GREENLEAF WJ 2015. ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr Protoc Mol Biol, 109, 21 29 1–21 29 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANDE J, GOLTSEV Y & LEVINE MS 2009. Conservation of enhancer location in divergent insects. Proc Natl Acad Sci U S A, 106, 14414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARAGATA EP, DONG S, DONG Y, SIMOES ML, TIKHE CV & DIMOPOULOS G 2020. Prospects and Pitfalls: Next-Generation Tools to Control Mosquito-Transmitted Disease. Annu Rev Microbiol, 74, 455–475. [DOI] [PubMed] [Google Scholar]
- CARVALHO DO, MCKEMEY AR, GARZIERA L, LACROIX R, DONNELLY CA, ALPHEY L, MALAVASI A & CAPURRO ML 2015. Suppression of a Field Population of Aedes aegypti in Brazil by Sustained Release of Transgenic Male Mosquitoes. PLoS Negl Trop Dis, 9, e0003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMPER J, BUCHMAN A & AKBARI OS 2016. Cheating evolution: engineering gene drives to manipulate the fate of wild populations. Nat Rev Genet, 17, 146–59. [DOI] [PubMed] [Google Scholar]
- CHEN XG, MARINOTTI O, WHITMAN L, JASINSKIENE N, JAMES AA & ROMANS P 2007. The Anopheles gambiae vitellogenin gene (VGT2) promoter directs persistent accumulation of a reporter gene product in transgenic Anopheles stephensi following multiple bloodmeals. Am J Trop Med Hyg, 76, 1118–24. [PubMed] [Google Scholar]
- CHOUMET V, CARRMI-LEROY A, LAURENT C, LENORMAND P, ROUSSELLE JC, NAMANE A, ROTH C, BREY PT 2007. The salivary glands and saliva of Anopheles gambiae asan essential step in the Plasmodium life cycle: A globalproteomic study. Proteomics, 7. [DOI] [PubMed] [Google Scholar]
- DABORN PJ, YEN JL, BOGWITZ MR, LE GOFF G, FEIL E, JEFFERS S, TIJET N, PERRY T, HECKEL D, BATTERHAM P, FEYEREISEN R, WILSON TG & FFRENCH-CONSTANT RH 2002. A single p450 allele associated with insecticide resistance in Drosophila. Science, 297, 2253–6. [DOI] [PubMed] [Google Scholar]
- DAME DA, CURTIS CF, BENEDICT MQ, ROBINSON AS & KNOLS BG 2009. Historical applications of induced sterilisation in field populations of mosquitoes. Malar J, 8 Suppl 2, S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONG Y, DAS S, CIRIMOTICH C, SOUZA-NETO JA, MCLEAN KJ & DIMOPOULOS G 2011. Engineered anopheles immunity to Plasmodium infection. PLoS Pathog, 7, e1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONG Y, SIMOES ML & DIMOPOULOS G 2020. Versatile transgenic multistage effector-gene combinations for Plasmodium falciparum suppression in Anopheles. Sci Adv, 6, eaay5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONG Y, SIMOES ML, MAROIS E & DIMOPOULOS G 2018. CRISPR/Cas9 -mediated gene knockout of Anopheles gambiae FREP1 suppresses malaria parasite infection. PLoS Pathog, 14, e1006898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAPEAU MD, CYRAN SA, VIERING MM, GEYER PK & LONG AD 2006. A cis-regulatory sequence within the yellow locus of Drosophila melanogaster required for normal male mating success. Genetics, 172, 1009–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYCK VA, HENDRICHS J & ROBINSON AS 2005. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, Springer; Netherlands. [Google Scholar]
- ERIVES A & LEVINE M 2004. Coordinate enhancers share common organizational features in the Drosophila genome. Proc Natl Acad Sci U S A, 101, 3851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS BR, KOTSAKIOZI P, COSTA-DA-SILVA AL, IOSHINO RS, GARZIERA L, PEDROSA MC, MALAVASI A, VIRGINIO JF, CAPURRO ML & POWELL JR 2019. Transgenic Aedes aegypti Mosquitoes Transfer Genes into a Natural Population. Scientific Reports, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANTZ VM, JASINSKIENE N, TATARENKOVA O, FAZEKAS A, MACIAS VM, BIER E & JAMES AA 2015. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci U S A, 112, E6736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARZIERA L, PEDROSA MC, DE SOUZA FA, GOMEZ M, MOREIRA MB, VIRGINIO JF, CAPURRO ML & CARVALHO DO 2017. Effect of interruption of over-flooding releases of transgenic mosquitoes over wild population of Aedes aegypti: two case studies in Brazil. Entomologia Experimentalis Et Applicata, 164, 327–339. [Google Scholar]
- GIRALDO-CALDERON GI, EMRICH SJ, MACCALLUM RM, MASLEN G, DIALYNAS E, TOPALIS P, HO N, GESING S, VECTORBASE C, MADEY G, COLLINS FH & LAWSON D 2015. VectorBase: an updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res, 43, D707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLTSEV Y, FUSE N, FRASCH M, ZINZEN RP, LANZARO G & LEVINE M 2007. Evolution of the dorsal-ventral patterning network in the mosquito, Anopheles gambiae. Development, 134, 2415–24. [DOI] [PubMed] [Google Scholar]
- HAMMONDS AS, BRISTOW CA, FISHER WW, WEISZMANN R, WU S, HARTENSTEIN V, KELLIS M, YU B, FRISE E & CELNIKER SE 2013. Spatial expression of transcription factors in Drosophila embryonic organ development. Genome Biol, 14, R140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELINSKI ME, PARKER AG & KNOLS BG 2009. Radiation biology of mosquitoes. Malar J, 8 Suppl 2, S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONG JW, HENDRIX DA & LEVINE MS 2008. Shadow enhancers as a source of evolutionary novelty. Science, 321, 1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUSDEN BE, MILLEN K, BRAY SJ 2012. Drosophila reporter vectors compatible with PhiC31 integrase transgenesis techniques and their use to generate new notch reporter fly lines. G3: Genes, Genomes, Genetics, 2, 79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAACS AT, LI F, JASINSKIENE N, CHEN X, NIRMALA X, MARINOTTI O, VINETZ JM & JAMES AA 2011. Engineered resistance to Plasmodium falciparum development in transgenic Anopheles stephensi. PLoS Pathog, 7, e1002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANTOROVITZ MR, KAZEMIAN M, KINSTON S, MIRANDA-SAAVEDRA D, ZHU Q, ROBINSON GE, GOTTGENS B, HALFON MS & SINHA S 2009. Motif-blind, genome-wide discovery of cis-regulatory modules in Drosophila and mouse. Dev Cell, 17, 568–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAZEMIAN M & HALFON MS 2019. CRM Discovery Beyond Model Insects. Methods Mol Biol, 1858, 117–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAZEMIAN M, SURYAMOHAN K, CHEN JY, ZHANG Y, SAMEE MA, HALFON MS & SINHA S 2014. Evidence for deep regulatory similarities in early developmental programs across highly diverged insects. Genome Biol Evol, 6, 2301–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAZEMIAN M, ZHU Q, HALFON MS & SINHA S 2011. Improved accuracy of supervised CRM discovery with interpolated Markov models and cross-species comparison. Nucleic Acids Res, 39, 9463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KYROU K, HAMMOND AM, GALIZI R, KRANJC N, BURT A, BEAGHTON AK, NOLAN T & CRISANTI A 2018. A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat Biotechnol, 36, 1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAI YT, DEEM KD, BORRAS-CASTELLS F, SAMBRANI N, RUDOLF H, SURYAMOHAN K, EL-SHERIF E, HALFON MS, MCKAY DJ & TOMOYASU Y 2018. Enhancer identification and activity evaluation in the red flour beetle, Tribolium castaneum. Development, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE CY, CLOUGH EA, YELLON P, TESLOVICH TM, STEPHAN DA & BAEHRECKE EH 2003. Genome-wide analyses of steroid- and radiation-triggered programmed cell death in Drosophila. Curr Biol, 13, 350–7. [DOI] [PubMed] [Google Scholar]
- LEES RS, GILLES JR, HENDRICHS J, VREYSEN MJ & BOURTZIS K 2015. Back to the future: the sterile insect technique against mosquito disease vectors. Curr Opin Insect Sci, 10, 156–162. [DOI] [PubMed] [Google Scholar]
- LOMBARDO F, NOLAN T, LYCETT G, LANFRANCOTTI A, STICH N, CATTERUCCIA F, LOUIS C, COLUZZI M & ARCA B 2005. An Anopheles gambiae salivary gland promoter analysis in Drosophila melanogaster and Anopheles stephensi. Insect Mol Biol, 14, 207–16. [DOI] [PubMed] [Google Scholar]
- LYND A & LYCETT GJ 2012. Development of the bi-partite Gal4-UAS system in the African malaria mosquito, Anopheles gambiae. PLoS One, 7, e31552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKSTEIN M, ZINZEN R, MARKSTEIN P, YEE KP, ERIVES A, STATHOPOULOS A & LEVINE M 2004. A regulatory code for neurogenic gene expression in the Drosophila embryo. Development, 131, 2387–94. [DOI] [PubMed] [Google Scholar]
- MASSEY JH, CHUNG D, SIWANOWICZ I, STERN DL & WITTKOPP PJ 2019. The yellow gene influences Drosophila male mating success through sex comb melanization. Elife, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOREIRA LA, EDWARDS MJ, ADHAMI F, JASINSKIENE N, JAMES AA & JACOBS-LORENA M 2000. Robust gut-specific gene expression in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A, 97, 10895–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER HA 2008. Immunolabeling of embryos. Methods Mol Biol, 420, 207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYSORE K, LI P & DUMAN-SCHEEL M 2018. Identification of Aedes aegypti cis-regulatory elements that promote gene expression in olfactory receptor neurons of distantly related dipteran insects. Parasites & Vectors, 11, 406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOLAN T, PETRIS E, MULLER HM, CRONIN A, CATTERUCCIA F & CRISANTI A 2011. Analysis of two novel midgut-specific promoters driving transgene expression in Anopheles stephensi mosquitoes. PLoS One, 6, e16471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’BROCHTA DA, PILITT KL, HARRELL RA 2ND, ALUVIHARE C & ALFORD RT 2012. Gal4-based enhancer-trapping in the malaria mosquito Anopheles stephensi. G3 (Bethesda), 2, 1305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANSON H & LISSENDEN N 2016. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends in Parasitology, 32, 187–196. [DOI] [PubMed] [Google Scholar]
- REBEIZ M, CASTRO B, LIU F, YUE F & POSAKONY JW 2012. Ancestral and conserved cis-regulatory architectures in developmental control genes. Dev Biol, 362, 282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARAKHOVA MV, HAMMOND MP, LOBO NF, KRZYWINSKI J, UNGER MF, HILLENMEYER ME, BRUGGNER RV, BIRNEY E & COLLINS FH 2007. Update of the Anopheles gambiae PEST genome assembly. Genome Biol, 8, R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGLAFF DH, DUNN WA, XIE XS, MEGY K, MARINOTTI O & JAMES AA 2009. Comparative genomics allows the discovery of cis-regulatory elements in mosquitoes. Proc Natl Acad Sci U S A, 106, 3053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON JM, GIRESI PG, DAVIS IJ & LIEB JD 2012. Using formaldehyde-assisted isolation of regulatory elements (FAIRE) to isolate active regulatory DNA. Nature Protocols, 7, 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMPSON P, LEWIS M & RICHARDSON J 2006. Conservation of upstream regulators of scute on the notum of cyclorraphous Diptera. Dev Genes Evol, 216, 363–71. [DOI] [PubMed] [Google Scholar]
- SINDEN RE 1984. The biology of Plasmodium in the mosquito. Experientia, 40, 1330–43. [DOI] [PubMed] [Google Scholar]
- SINKINS SP & GOULD F 2006. Gene drive systems for insect disease vectors. Nat Rev Genet, 7, 427–35. [DOI] [PubMed] [Google Scholar]
- SKAVDIS G, SIDEN-KIAMOS I, MULLER HM, CRISANTI A & LOUIS C 1996. Conserved function of anopheles gambiae midgut-specific promoters in the fruitfly. EMBO J, 15, 344–50. [PMC free article] [PubMed] [Google Scholar]
- SURYAMOHAN K & HALFON MS 2015. Identifying transcriptional cis-regulatory modules in animal genomes. Wiley Interdiscip Rev Dev Biol, 4, 59–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SURYAMOHAN K, HANSON C, ANDREWS E, SINHA S, SCHEEL MD & HALFON MS 2016. Redeployment of a conserved gene regulatory network during Aedes aegypti development. Dev Biol, 416, 402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUTZ D & PFEIFLE C 1989. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma, 98, 81–5. [DOI] [PubMed] [Google Scholar]
- TERENIUS O, MARINOTTI O, SIEGLAFF D & JAMES AA 2008. Molecular genetic manipulation of vector mosquitoes. Cell Host Microbe, 4, 417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THURMOND J, GOODMAN JL, STRELETS VB, ATTRILL H, GRAMATES LS, MARYGOLD SJ, MATTHEWS BB, MILLBURN G, ANTONAZZO G, TROVISCO V, KAUFMAN TC, CALVI BR & FLYBASE C 2019. FlyBase 2.0: the next generation. Nucleic Acids Res, 47, D759–D765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMANCAK P, BEATON A, WEISZMANN R, KWAN E, SHU S, LEWIS SE, RICHARDS S, ASHBURNER M, HARTENSTEIN V, CELNIKER SE & RUBIN GM 2002. Systematic determination of patterns of gene expression during Drosophila embryogenesis. Genome Biol, 3, RESEARCH0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMANCAK P, BERMAN BP, BEATON A, WEISZMANN R, KWAN E, HARTENSTEIN V, CELNIKER SE & RUBIN GM 2007. Global analysis of patterns of gene expression during Drosophila embryogenesis. Genome Biol, 8, R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOURAY MG, WARBURG A, LAUHINGHOUSE A, KRETTLI AU, MILLER LH 1992. Developmentally Regulated !nfectivity of Malaria Sporozoites for Mosquito Salivary Glands and the Vertebrate Host The Journal of Experimental Medicine, 175, 1607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLOHONSKY G, TERENZI O, SOICHOT J, NAUJOKS DA, NOLAN T, WINDBICHLER N, KAPPS D, SMIDLER AL, VITTU A, COSTA G, STEINERT S, LEVASHINA EA, BLANDIN SA & MAROIS E 2015. Tools for Anopheles gambiae Transgenesis. G3 (Bethesda), 5, 1151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO 2019. World malaria report: 2019. Geneva; WHO. [Google Scholar]
- WINDBICHLER N, MENICHELLI M, PAPATHANOS PA, THYME SB, LI H, ULGE UY, HOVDE BT, BAKER D, MONNAT RJ JR., BURT A & CRISANTI A 2011. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature, 473, 212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YE Z, LIU F, SUN H, BARKER M, PITTS RJ & ZWIEBEL LJ 2020. Heterogeneous expression of the ammonium transporter AgAmt in chemosensory appendages of the malaria vector, Anopheles gambiae. Insect Biochem Mol Biol, 120, 103360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHU Q & HALFON MS 2007. Vector-dependent gene expression driven by insulated P-element reporter vectors. Fly, 1, 55–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Predicted A. gambiae enhancers. The top 500 SCRMshaw predicted enhancers for A. gambiae.


