Abstract
Mesenchymal stem/stromal cells (MSCs) are the most utilized cell type for cellular therapy, partly due to their important proliferative potential and ability to differentiate into various cell types. MSCs produce large amounts of extracellular vesicles (EVs), which carry genetic and protein cargo to mediate MSC paracrine function. Recently, MSC-derived EVs have been successfully employed in several preclinical models of chronic kidney disease. However, uncertainty remains regarding EV fate, safety, and long-term effects, which might impose important limitations on their path to clinical translation. This review discusses the therapeutic application of MSC-derived EV therapy for renal disease, with particular emphasis on potential mechanisms of kidney repair and major translational barriers. Emerging evidence indicates that the cargo of MSC-derived EVs is capable of modulating several pathways responsible for renal injury, including inflammation, oxidative stress, apoptosis, fibrosis, and microvascular remodeling. EV-induced modulation of these pathways has been associated with important renoprotective effects in experimental studies. However, scarce clinical data are available, and several challenges need to be addressed as we move towards clinical translation, including standardization of methods for EV isolation and characterization, EV fate, duration of EV effects, and effects of cardiovascular risk factors. MSC-derived EVs have potential to preserve renal structure and function, but further experimental and clinical evidence is needed to confirm their protective effects in patients with chronic kidney disease.
Keywords: mesenchymal stem cells, extracellular vesicles, microvesicles, exosomes, kidney
Introduction
Chronic kidney disease (CKD) remains a growing health concern that leads to progressive and irreversible deterioration of renal function and end-stage renal disease (ESRD), requiring dialysis or kidney transplantation. The prevalence of hypertension is significantly higher in patients with CKD compared to the general population1, 2. Hypertension is one of the leading causes for CKD and they possess a mutually perpetuation potential. CKD affects almost 15% of the United States population3, but its prevalence is projected to increase to 17% by 20304, underscoring the need of novel treatment approaches to retard its progression of ESRD. Similarly, hypertension affects approximately one-third of the United States population and contributes to morbidity and mortality5. In this context, the advent of regenerative therapies that support repair, regeneration, and restoration of damaged tissues emerged as promising approaches to preserve the structure and function of the kidney.
Mesenchymal stem/stromal cells (MSCs) are the most utilized cell type for cellular therapy. These multipotent stem cells are found in multiple tissues, including bone marrow, umbilical cord, and adipose tissue, display broad pro-angiogenic and immunomodulatory properties, and can differentiate into several cell types. Minimal criteria to define human MSCs include plastic-adherence in standard culture conditions, expression of CD105, CD73 and CD90, lack expression of CD45, CD34, CD14 or CD11b, CD79alpha or CD19 and HLA-DR surface molecules, and transdifferentiation into osteoblasts, adipocytes, and chondroblasts in vitro6.
Currently, preclinical evidence suggests that MSCs can foster renal regeneration and repair in several forms of CKD7–9. Hundreds of clinical trials involving MSCs are listed in www.clinicaltrials.gov. Clinical trials in patients with hypertension and diabetic nephropathy, entities responsible for up to two-thirds of CKD cases, underscore the safety and efficacy of MSCs to ameliorate kidney injury and dysfunction10, 11, positioning MSCs as propitious candidates for cell-based therapy in patients with CKD.
It is now well-accepted that MSCs exert their paracrine activity partly by releasing extracellular vesicles (EVs), lipid bilayer-delimited particles. These include microvesicles (0.1–1μm diameter, shed by outward blebbing of the plasma membrane) and exosomes (30–100nm released by the fusion of multi-vesicular bodies with the plasma membrane) (Figure 1). EVs carry messages in the form of genes, proteins, and micro-RNAs (miRNAs) capable of modulating several pathways responsible for cellular injury in recipient cells, including inflammation, oxidative stress, apoptosis, angiogenesis, and fibrosis, all of which contribute to the development and progression of CKD12. Indeed, experimental studies in several models of CKD uncovered the feasibility and efficacy of MSC-derived EVs to preserve renal structure and function13, forging a paradigm shift towards cell-free therapeutic interventions for renal repair.
Figure 1. MSCs release extracellular vesicles.
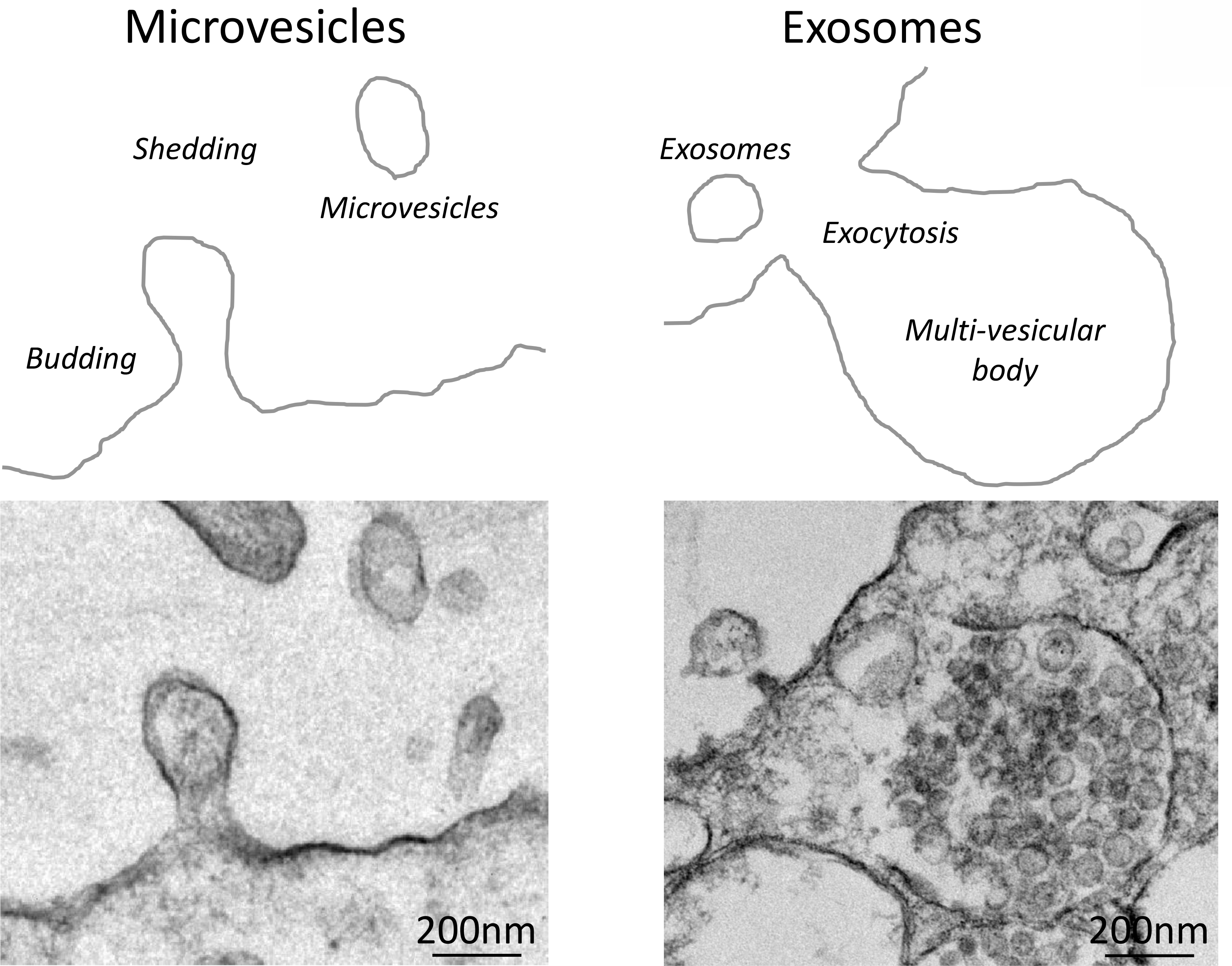
Top: Schematic of the mechanisms of formation of microvesicles (left) and exosomes (right). Bottom: Representative transmission electron microscopy images of MSCs releasing microvesicles (left) and exosomes (right).
To date, few published and ongoing clinical trials have tested the safety and efficacy of MSC-derived EVs. This includes studies using allogeneic cord tissue MSC-derived EVs on B-cell mass in type-1 diabetes (NCT02138331), macular degeneration (NCT03437759), CKD14, graft-versus-host disease15, as well as bone marrow MSC-derived EVs containing miR-124 for ischemic stroke (NCT03384433).
Although therapeutic MSC-derived EVs are of great potential interest, challenges regarding standardization of methods for EV isolation and characterization, EV fate, and duration of EV effects, among others, represent important hurdles that need to be overcome before widespread adoption of this therapy in the clinical arena. This review discusses the therapeutic application of MSC-derived EV therapy for renal disease, with particular emphasis on potential mechanisms of kidney repair and major translational barriers.
MSC-derived EVs in CKD models
In recent years, the renoprotective effects of MSC-derived EVs have been investigated in several in-vivo models of CKD, including diabetic and hypertensive nephropathy, ischemia-reperfusion injury (IRI), unilateral ureteral obstruction (UUO), environmental exposure to heavy metals, and subtotal nephrectomy (STN). For example, Grange and colleagues investigated the potential therapeutic effect of EVs, isolated from human bone marrow MSCs, on the progression and reversal of fibrosis in a murine model of diabetic nephropathy16. Twenty-eight days after the onset of streptozotocin-induced diabetes, EVs (1×10^10 EVs/injection) were administered intravenously weekly to mice for four consecutive weeks, resulting in a 0.25mg/dl improvement in plasma creatinine and >20% reductions in tubular injury and glomerular fibrosis. The anti-fibrotic effects of EVs were supported by their enrichment with miRNAs capable of targeting biological pathways involved in the development of fibrosis. Likewise, after establishment of streptozotocin-induced diabetes in rats, weekly intravenous injection of EVs (100μg in PBS) isolated from human urinary MSCs prevented kidney complications by inhibiting by 70% podocyte apoptosis and promoting vascular regeneration and cell survival (increase CD31+/Ki-67+ endothelial area)17. Analysis of the cargo of EVs revealed that they contained important pro-angiogenic and pro-survival factors, such as vascular endothelial growth factor (VEGF) and bone morphogenetic protein (BMP)-7, which might have partly accounted for their reno-protective effects. Similarly, renal subcapsular administration of bone-marrow MSC-derived EVs (5.3×10^7 in 200μl PBS) 28 days after the onset of diabetes decreased urinary albumin-creatinine ratio and exerted important anti-fibrotic (almost 80% reduction in glomerular score), anti-apoptotic, and anti-inflammatory effects in the kidneys of high-fat diet-induced type-2 diabetic mice 2 weeks later18, suggesting that MSC-derived EV therapy might be a promising tool to ameliorate the renal damage of diabetes.
The efficacy of MSC-derived EVs has been also tested in models of renovascular hypertension. In 2-kidneys, 1-clip (2K-1C) rats, two intravenous injections of EVs (100μg on 3rd and 5th weeks after clamping) harvested from adipose-tissue MSCs decreased systolic blood pressure by 40mmHg, proteinuria by 30mg/24h, and renal expression of pro-fibrotic factors, including transforming growth factor (TGF)-β and collagen-I, but increased expression of the anti-inflammatory cytokine interleukin (IL)-1019 6 weeks later. Our group has also shown in a swine model of endovascularly-induced renal artery stenosis (RAS) that a single intra-renal infusion of autologous MSC-derived EVs (10×10^7) preserved the structure and function of the post-ischemic kidney four weeks later20. Delivery of EVs decreased renal vein levels of several pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α and IL-6, but increased levels of IL-10 and the number of reparative M2 macrophages in the renal parenchyma. Importantly, the immunomodulatory effects of EVs were associated with increased post-stenotic kidney expression of pro-angiogenic factors, restoration of the intra-renal microcirculation, improvements in medullary oxygenation and fibrosis by 12%, and in turn in stenotic-kidney GFR (+28.4ml/min)21. However, their salutary effects were attenuated in animals treated with IL-10 knock-down EVs, implicating this anti-inflammatory cytokine in the protective effects of EVs. Although MSC-derived EVs were also enriched with anti-fibrotic factors22, modulation of renal inflammation seems to be upstream of many other benefits (e.g. reduction of fibrosis). Importantly, delivery of IL-10 enriched MSC-derived EVs might be safer and more effective than delivery of IL-10, which may induce untoward immunological changes23, 24.
MSC-derived EVs are also effective in ameliorating chronic kidney damage consequent to IRI. Intravenous injection of EVs (30μg) isolated from human bone-marrow MSCs in rats immediately after a 45 min IRI and unilateral nephrectomy decreased creatinine levels (−1.3mg/dl) and glomerular fibrosis (−12%) 6 months later25. Acute effects of EVs included inhibition of apoptosis and stimulation of tubular epithelial cell proliferation, which reduced acute kidney injury (AKI), and in turn protected these kidneys from later CKD. However, inactivation of their mRNA cargo abrogated these protective effects, implying that the efficacy of EVs relies in part on the transfer of genes to target damaged cells. In a similar model in rats, a single administration of MSC-derived EVs (100μg) immediately after IRI ameliorated by 2 days later renal injury (decreased >2 points in histopathological scoring) in both the acute and chronic stages by reducing macrophage accumulation and fibrosis (decreased fibrosis score by 44%) through suppression of the pro-inflammatory and pro-fibrotic protein fractalkine (CX3CL1)26.
Beneficial effects of MSC-derived EVs were also reported in experimental UUO models. Intravenous administration of EVs (100μg) obtained from MSC supernatants reduced tubular injury and fibrosis (−20%) and improved kidney function 14 days after UUO27. An important mechanism of action of EVs could have been the transfer miRNAs implicated in modulating of fibrosis and epithelial-to-mesenchymal transition (EMT). Congruently, a single intravenous delivery of MSC-derived EVs (2×10^7) in mice with UUO ameliorated peritubular capillary rarefaction via inhibition of EMT and protected against progression of renal damage by decreasing tubulointerstitial fibrosis (−70%) 7 days after UUO28. These observations highlight important anti-fibrotic renoprotective properties of EVs in experimental UUO, and may provide a gateway for new therapeutics in this condition.
More recently, renoprotective effects of MSC-derived EVs were studied in a model that mimics CKD secondary to long-term environmental exposure to heavy metals. Medakas fish were exposed to high cadmium for 4 days and 4×10^7 EVs isolated from human bone-marrow intravenously injected 1 day later29. EVs repaired the damage to apical and basolateral membranes and mitochondria of kidney proximal tubules, and improved renal function and survival by 20%. Additional studies are needed to test whether EV therapy might be useful to other forms of toxic-induced CKD. MSC-derived EVs have also shown promise in murine models of STN. In a mouse remnant kidney model, intravenous delivery of EVs (30μg) 2 days after STN ameliorated interstitial lymphocyte infiltration, prevented tubular atrophy (−1.46 points in histological score) and fibrosis, and decreased proteinuria and serum creatinine levels 7 days later30, underscoring the potential of EV therapy in preserving the kidney in these models of CKD.
However, despite robust preclinical data proclaiming that MSC-derived EVs promote tissue repair and preserve renal function, their safety and efficacy in patients with CKD remain to be determined. A pilot study in CKD patients suggested that administration of cell-free cord-blood MSC-derived EVs ameliorated inflammatory immune reaction and improved overall kidney function14. Forty patients with estimated-GFR (eGFR) between 15–60ml/min were randomized to receive either placebo or two doses of EVs. One year after EV administration, eGFR, serum creatinine, blood urea nitrogen levels, and urinary albumin/creatinine ratio improved, circulating levels of TNF-α decreased, but levels of IL-10 increased. Cell regeneration and differentiation markers improved 3-months after therapy and no significant adverse events occurred during EV infusion or throughout the follow-up period. Although these observations suggest that MSC-derived EV therapy might safely and effectively attenuate renal inflammation and dysfunction in CKD, additional long-term follow-up trials are needed to confirm these findings.
Are EVs superior to MSCs?
Based on the available experimental data, it is reasonable to ponder whether delivery of EVs would confer more efficient reno-protection compared to their parent MSCs. Evidently, EVs possess several advantages over MSCs31 (Table 1). Their phospholipid bilayer makes them more stable after freezing and thawing, protecting their genetic and protein cargo. EVs can be more easily prepared in a standard manner and stored for a long time compared to MSCs, allowing their use as “off-the-shelf” products. Furthermore, they can be modified to function as delivery carriers to target specific molecules, proteins, or signaling pathways in recipient cells.
Table 1.
Advantages and disadvantages of EVs over MSCs for CKD.
| Therapy | MSCs | EVs |
|---|---|---|
| In-vitro: | ||
| Half-life (following freezing and thawing) | + | ++ |
| Stability (in inflammatory microenvironment) | − | + |
| Utility (use as “off-the-shelf” products or delivery carriers) | +/− | + |
| In-vivo: | ||
| Tumorigenicity (ability to give rise to benign or malignant tumors) | +/− | − |
| Immunogenicity (ability to induce humoral or cell immune responses) | + | − |
| Efficacy (preservation of renal structure and function in CKD) | ++ | ++ |
| Efficiency (reaching damaged sites within the neprhon) | + | ++ |
| Targeting (ability to ensure renal homing) | + | + |
Cardiovascular risk factors can alter the transcriptome and proteome of swine adipose tissue-derived MSCs32–34, which might limit their reparative function and utility as an exogenous regenerative therapy. In contrast, EVs are more resistant to the deleterious effects of an inflammatory microenvironment35, increasing their half-life following injection (Table 1).
Despite their excellent safety profile, few reports of sarcomas36 and teratomas37 following exogenous MSC administration underscore their potential for transformation into tumors. Unlike MSCs, EVs are acellular and do not self-replicate, which lowers the potential for endogenous tumor-formation. Likewise, EVs have lower immunogenicity than their parent MSCs, which is partly due to their small size and lower expression of membrane histocompatibility molecules38.
In terms of in-vivo efficacy, these small particles easily circulate and may reach distant damaged sites of the nephron. Like MSCs, injected EVs are retained in damaged kidneys and can engraft into several cell types, including proximal and distal tubular cells, endothelial cells, and macrophages20, 39. Yet, while MSCs primarily exert their protective effects by paracrine mechanisms, like release of EVs, growth factors, and cytokines, they may also replace damaged cells, which might extend and prolong their in-vivo efficacy compared to EVs.
Unfortunately, few experimental studies have compared the efficacy of MSCs and EVs in kidney repair. In murine IRI and STN models, delivery of MSCs and EVs exhibited similar potential to preserve renal structure and function30, 40. However, EVs in mice with UUO conferred reno-protective effects exceeding those of their parent MSCs27. In acute IRI, the combination of MSCs and MSC-derived EVs was more effective to either one alone in reducing proteinuria and preserving kidney function, suggesting additive effects41. More recently, we found that intra-renal delivery of either adipose tissue-derived MSCs or their daughter EVs improved stenotic-kidney function and decreased injury in swine RAS, albeit by slightly different mechanisms42. MSCs were more effective in suppressing inflammatory cytokines and preserving the intra-renal microcirculation, whereas EVs bestowed better preservation of renal cellular integrity. Yet, both strategies recovered renal structure and function, supporting the notion that EVs recapitulate the salutary effects of MSCs.
Obviously, longer-term studies would be useful for comparing MSCs and EVs for CKD. There are currently robust experimental data and several ongoing or completed clinical trials testing the safety and efficacy of MSCs for renal disease, some in common forms of CKD like diabetic nephropathy and hypertension43. No doubt, additional experimental research is needed to determine whether EVs confer more efficient reno-protection than MSCs.
Specific renal injury pathways modulated by MSC-derived EVs
Accumulating experimental evidence suggests that MSC-derived EVs are capable of modulating several pathways responsible for renal injury in CKD, including inflammation, oxidative stress, apoptosis, microvascular damage, and fibrosis (Figure 2). Circulating pro-inflammatory cytokines and subsequent inflammatory cell infiltration favor production of reactive oxygen species (ROS) and microvascular damage, leading to tubular injury and fibrosis44. In patients with CKD, increased ROS production contributes to the acceleration of GFR decline and several complications, such as hypertension, atherosclerosis, and anemia45. Similarly, intra-renal microvascular rarefaction and dysfunction are major determinants of the progression of CKD46, and loss of renal tubular cells from apoptosis leads to expansion of the extracellular matrix and fibrosis, and in turn progression of CKD47. MSCs pass on important anti-inflammatory, anti-oxidant, anti-apoptotic, anti-fibrotic, and pro-angiogenic properties to their daughter EVs, rendering them an attractive tool to treat and improve outcomes in CKD12, 13.
Figure 2. MSC-derived EVs modulate renal injury pathways in recipient cells.
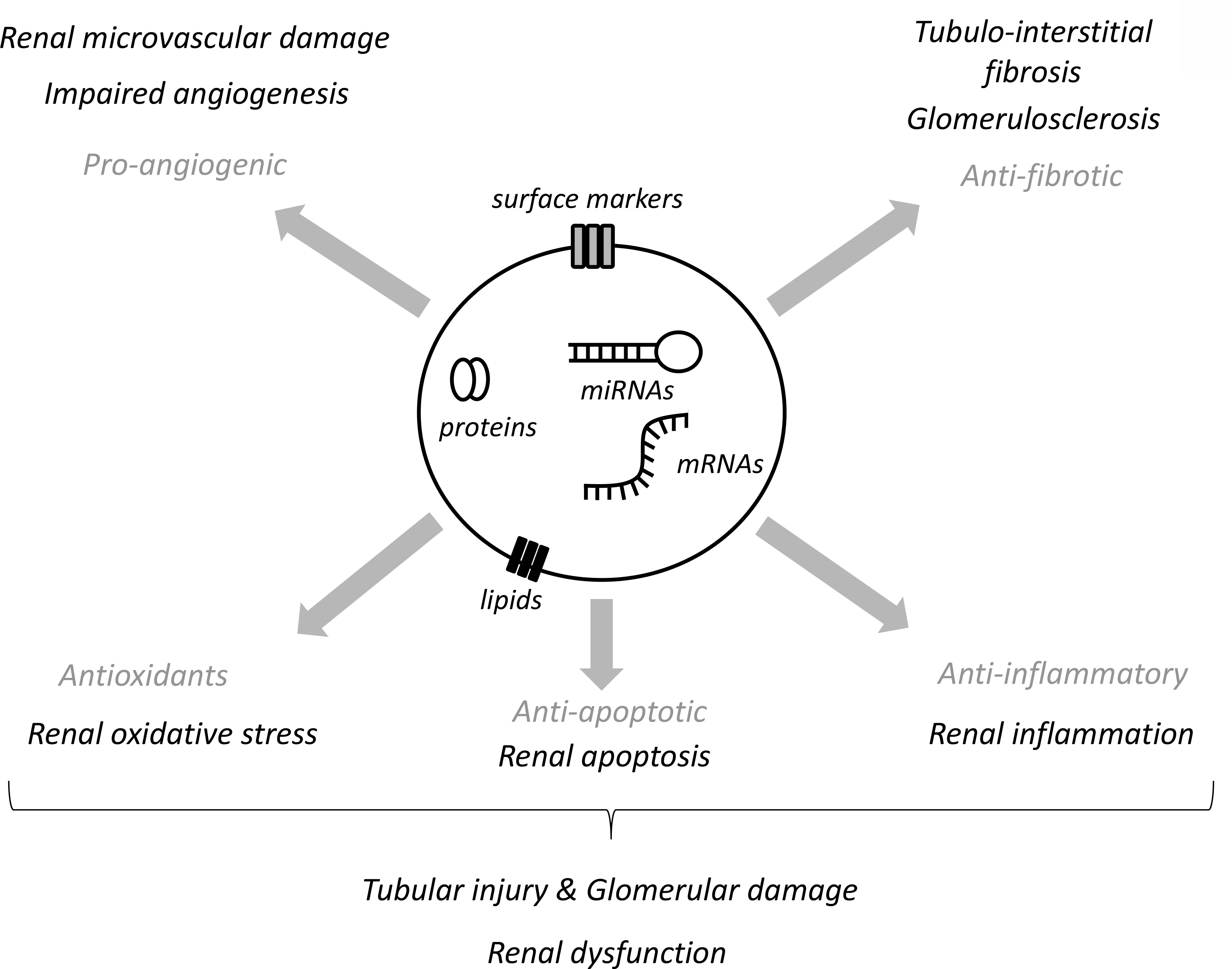
MSC-derived EVs contain mRNAs, proteins, and micro-RNAs (miRNAs) capable of targeting pathways responsible for renal injury, including the renal microvasculature damage and defective angiogenesis, tubulo-interstitial and glomerular fibrosis, as well as renal inflammation, apoptosis, and oxidative stress.
The primary mode by which EVs modulate renal injury pathways involves their renal engraftment and transfer of their cargo to injured renal cells. Studies described the biological signature of MSC-derived EVs and found that they are selectively packed with genes and proteins involved in immune regulation, extracellular matrix remodeling, apoptosis, angiogenesis, and redox cellular response12. For example, EVs derived from human umbilical cord MSCs are packed with the mitochondria-located antioxidant enzyme manganese superoxide dismutase (MnSOD)48, and their delivery decreases renal oxidative stress49. Similarly, EVs released from bone marrow-derived MSCs transfer anti-apoptotic mRNAs to recipient cells and exert a pro-survival benefit on renal cells in-vitro and in-vivo50. We have shown that EVs harvested from swine adipose tissue contain several genes and proteins involved in angiogenesis, including the proangiogenic genes hepatocyte growth-factor, as well as VEGF and von Willebrand factor proteins22, 51. The genetic and protein cargo of swine MSC-derived EVs included modulators of TNF-α and TGFβ signaling52, implying regulation of inflammatory response and extracellular matrix remodeling, as well as several anti-oxidants proteins, including MnSOD, catalase, and glutathione peroxidases. Importantly, delivery of these EVs into a stenotic swine kidney ameliorated inflammation and oxidative stress, and preserved its microcirculation and GFR20, 21, underscoring the therapeutic potential of MSC-derived EVs to modulate renal injury pathways and improve kidney function in experimental CKD.
In addition to genes and proteins, MSC-derived EVs possess an important cargo of miRNAs, small non-coding RNAs which control gene expression post-transcriptionally and modulate important pathways responsible for renal injury in CKD. Among them are miR-24, an important modulator of vascular inflammation53 implicated in EV-induced renal repair after ischemia54, and miR29, which suppresses TNF-α production55. Swine MSC-derived EVs are enriched with miR148a-3p, which targets genes that regulate apoptosis and angiogenesis51. EVs decrease apoptosis and restore expression of angiogenic factors in the stenotic kidney39, suggesting that their renoprotective effects might be attributed partly to their miRNA cargo.
Challenges
Experimental studies have greatly expanded our knowledge on the cargo, mechanisms of action, and reno-protective properties of MSC-derived EVs, yet clinical translation of this novel approach for patients with CKD remains associated with several challenges. In preclinical studies EVs were isolated by variable methods, including differential ultracentrifugation, filtration, and size exclusion chromatography among others56. Similarly, handling and storage of isolated EVs widely vary among studies. While each method has advantages and disadvantages for a given research hypothesis, the purity, concentration, morphology, size range, and even functional activity of EVs might differ with each different method or protocol for handling and storage57, 58. For example, preparations may contain different proportions of microvesicles and exosomes. Therefore, it is critical to define and standardize accurate, reliable, and easily implemented techniques for EV isolation before widespread use in CKD patients. The recent guidelines from International Society for Extracellular Vesicles59 are an important step in this direction.
Despite the growing number of experimental studies testing the efficacy of MSC-derived EVs in many forms of CKD, uncertainty remains regarding their fate. Unfortunately, their small size is not conducive to tracking injected EVs. In AKI and CKD models, systemically injected EVs primarily engrafted into damaged kidneys28, 60, with some retention in healthy kidneys26. EVs were taken up by several renal cells types, but tubular cells and peritubular capillaries exhibited higher retention rates compared to glomeruli28, 60. EVs also lodge preferentially in injured kidneys. Intra-renal administration in swine RAS was associated with higher EV retention in stenotic than contralateral kidneys four weeks after delivery, with significant uptake by tubular epithelial cells, peritubular capillary endothelial cells, and macrophages20, 39. Indeed, renovascular hypertensive kidneys release adhesion molecules and chemoattractants, like stromal-derived factor-1 and stem-cell factor, to promote migration and retention of progenitor cells61. Congruently, targeting murine MSCs to kidney injury molecule (KIM)-1 improves their therapeutic efficacy in murine chronic ischemic kidney injury62, suggesting organ-specific recruitment strategies (e.g., injury signals) to attract MSCs and their EVs to the site of injury. The mechanisms by which EVs reach renal tubules may involve endocytosis in glomerular endothelial cells63 or vascular extravasation via endothelial cell fenestrations or inter-cellular junctions, as we have shown in experimental RAS21. Nevertheless, studies are needed to clarify the mechanisms underlying renal cell uptake of EVs and establish the preferred route of delivery for clinical trials.
Additional research is also needed to establish the best timing window for EV administration. In rats, delivery of MSC-derived EVs prior to treatment with cisplatin prevents nephrotoxicity64, yet their administration in mice after cisplatin-induced nephrotoxicity also ameliorates tubular injury and improves kidney function50, suggesting that this intervention may both prevent and reverse established disease. EV efficacy also likely depends of the disease severity. Our previous studies in swine RAS suggest that MSC-derived EVs prevent rather than reverse further development of tubulo-interstitial fibrosis and glomerulosclerosis20. Clearly, more studies are needed to identify optimal timing for EV delivery.
Likewise, studies are needed to determine the duration, long-term effects, and optimal regimen of MSC-derived EVs. Most experimental studies using MSC-derived EVs were performed in AKI models followed ≤2 weeks after injection. However, in rats with IRI, MSC-derived EVs lowered the incidence of CKD 6 months after therapy25. Although these observations suggest that the beneficial effects might be sustained for at least few months, repeated doses might be necessary. In mice with AKI, multiple EVs injections decreased mortality and improved renal function compared to a single injection regimen50. Further studies should explore the duration of reno-protective effects of EVs and the efficacy of escalating doses to determine the adequate dose regimen for future clinical trials.
The source of MSCs may also impact the immunomodulatory or therapeutic effects of EVs. In diabetic mice, EVs derived from adipose tissue MSCs promote wound healing better than those derived from bone-marrow MSCs65, probably thanks to different protein and miRNA cargo. Furthermore, liver-derived human MSCs produce more pro-angiogenic, anti-inflammatory, and anti-apoptotic cytokines than bone marrow-derived MSCs66, but are comparable to adipose-tissue MSCs67, and their EVs accelerate hepatic regeneration68, underscoring the importance of the choice of parent MSC for EV-based therapy.
Studies are also needed to explore potential long-term detrimental effects of EVs. Immune response is not commonly observed following MSC or EV administration at standard doses. MSCs are hypoimmunogenic or ‘immune-privileged’, expressing low levels of MHC class-I and no MHC class-II or co-stimulatory molecules, enabling MSC transplantation across major histocompatibility barriers69. Indeed, cord-blood MSC-derived EVs ameliorate inflammatory immune reaction and improve kidney function in patients with CKD14. Furthermore, being acellular, EVs do not proliferate and are likely devoid of potential tumor growth, malformation, or microinfarctions attributed to MSCs36, 70.
Emerging experimental evidence suggests that comorbidities and cardiovascular risk factors can impair the in-vitro and in-vivo functionality of autologous MSC-derived EVs. EVs released from adipose tissue-derived stem cells of obese individuals have limited pro-angiogenic potential, partly attributed to their reduced content of VEGF and miR-12671. We have shown that diet-induced metabolic syndrome (MetS) in swine alters the packaging of genes, proteins, and miRNAs of MSC-derived EVs, which contained genes involved in modulation of inflammation, but lacked mRNAs related to TGF-β signaling72. Additionally, target genes of miRNAs enriched in swine MetS-EVs were implicated in the development of MetS and its complications73, whereas proteins were linked to pro-inflammatory pathways74. Notably, MetS-induced changes in MSC-derived EV cargo impaired their ability to support angiogenic function of endothelial cells in-vitro, and blunted their capacity to preserve the microvasculature in the post-stenotic kidney in-vivo52, 75, 76. Interestingly, MetS also induces release of smaller EVs packed with genes commonly associated with exosomes, whereas Lean EVs were larger (macrovesicles)34. Although the functions of these EV subsets might overlap, their biogenesis and molecular contents are different, warranting further studies to determine the impact of EV size distribution on their function. Overall, our observations may raise concerns about the use of autologous MSC-derived EVs in patients with cardiovascular risk factors, and need to be considered when designing clinical trials in CKD.
Summary and future perspectives: Are we there yet?
Hypertension and CKD are closely associated pathophysiologic states, and warrant novel treatment approaches to retard their progression. Experimental evidence demonstrates that MSC-derived EVs might confer protective effects in kidneys exposed to different forms of CKD, whereas clinical data supporting their safety and feasibility are clearly very limited, raising a concern about potential impediments for the translational process. First, we must ensure that important hurdles are overcome before moving towards designing clinical trials. This includes standardization of methods for EV isolation and characterization, which may increase reproducibility and facilitate interpretation of preclinical data. The International Society for Extracellular Vesicles proposed minimum criteria for characterization of EVs (e.g. nanocyte tracking analysis, western blotting, electron microscopy, and flow cytometry analysis), and suggested protocols and steps to document specific EV-associated functional activities59. At the same time, we need to elucidate the complex mechanisms of EV-induced reno-protection. Experimental evidence suggests that following systemic or intra-renal injection EVs are taken up by renal tubular cells and peritubular capillaries. In-vitro data support the transfer of protein, mRNA, and miRNA content into recipient cells, which contribute to modulate pathways implicated in renal damage in CKD. The ability to target EVs to the kidney could increase their engraftment and minimize off target effects77. Therefore, experimental data suggest that delivery of MSC-derived EVs might be an attractive therapy for individuals with stage 3–4 CKD to delay or decrease the need for renal replacement therapy. However, unmet needs remain in the field regarding EV fate, duration of their effects, and impact of cardiovascular risk factors. There is definitely a need for additional experimental studies to develop sound evidence-based guidelines for EV therapy. The future of MSC-derived EV therapy for renal repair is in its infancy, and will hopefully mature to establish this promising intervention in patients suffering from CKD.
Sources of Funding
This work was partly supported by the National Institutes of Health (DK106427, DK122137, DK122734, DK120292, AG062104, and DK102325).
Footnotes
Disclosures
Dr. Lerman is an advisor to AstraZeneca, Janssen Pharmaceuticals, and Butterfly Biosciences. The authors declared no conflicts of interest.
References
- 1.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI and Chronic Renal Insufficiency Cohort Study G. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 2009;4:1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egan BM, Zhao Y and Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–50. [DOI] [PubMed] [Google Scholar]
- 3.Ojo A. Addressing the global burden of chronic kidney disease through clinical and translational research. Trans Am Clin Climatol Assoc 2014;125:229–43; discussion 243–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Hoerger TJ, Simpson SA, Yarnoff BO, Pavkov ME, Rios Burrows N, Saydah SH, Williams DE and Zhuo X. The future burden of CKD in the United States: a simulation model for the CDC CKD Initiative. Am J Kidney Dis 2015;65:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park S, Gillespie C, Baumgardner J, Yang Q, Valderrama AL, Fang J, Loustalot F and Hong Y. Modeled state-level estimates of hypertension prevalence and undiagnosed hypertension among US adults during 2013–2015. J Clin Hypertens (Greenwich). 2018;20:1395–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D and Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. [DOI] [PubMed] [Google Scholar]
- 7.Eirin A and Lerman LO. Mesenchymal stem cell treatment for chronic renal failure. Stem Cell Res Ther 2014;5:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roushandeh AM, Bahadori M and Roudkenar MH. Mesenchymal Stem Cell-based Therapy as a New Horizon for Kidney Injuries. Arch Med Res 2017;48:133–146. [DOI] [PubMed] [Google Scholar]
- 9.Yun CW and Lee SH. Potential and Therapeutic Efficacy of Cell-based Therapy Using Mesenchymal Stem Cells for Acute/chronic Kidney Disease. Int J Mol Sci 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Packham DK, Fraser IR, Kerr PG and Segal KR. Allogeneic Mesenchymal Precursor Cells (MPC) in Diabetic Nephropathy: A Randomized, Placebo-controlled, Dose Escalation Study. EBioMedicine. 2016;12:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saad A, Dietz AB, Herrmann SMS, Hickson LJ, Glockner JF, McKusick MA, Misra S, Bjarnason H, Armstrong AS, Gastineau DA, Lerman LO and Textor SC. Autologous Mesenchymal Stem Cells Increase Cortical Perfusion in Renovascular Disease. J Am Soc Nephrol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nargesi AA, Lerman LO and Eirin A. Mesenchymal Stem Cell-derived Extracellular Vesicles for Renal Repair. Curr Gene Ther 2017;17:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aghajani Nargesi A, Lerman LO and Eirin A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther 2017;8:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, Temraz M, Saad AN, Essa W and Adel H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res 2016;20:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW and Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–3. [DOI] [PubMed] [Google Scholar]
- 16.Grange C, Tritta S, Tapparo M, Cedrino M, Tetta C, Camussi G and Brizzi MF. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci Rep 2019;9:4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang ZZ, Liu YM, Niu X, Yin JY, Hu B, Guo SC, Fan Y, Wang Y and Wang NS. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther 2016;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Konari N and Fujimiya M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep 2016;6:34842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishiy C, Ormanji MS, Maquigussa E, Ribeiro RS, da Silva Novaes A and Boim MA. Comparison of the Effects of Mesenchymal Stem Cells with Their Extracellular Vesicles on the Treatment of Kidney Damage Induced by Chronic Renal Artery Stenosis. Stem Cells Int. 2020;2020:8814574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A and Lerman LO. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int 2017;92:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eirin A, Zhu XY, Jonnada S, Lerman A, van Wijnen AJ and Lerman LO. Mesenchymal Stem Cell-Derived Extracellular Vesicles Improve the Renal Microvasculature in Metabolic Renovascular Disease in Swine. Cell Transplant 2018;27:1080–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eirin A, Zhu XY, Puranik AS, Woollard JR, Tang H, Dasari S, Lerman A, van Wijnen AJ and Lerman LO. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci Rep 2016;6:36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore KW, de Waal Malefyt R, Coffman RL and O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
- 24.Tilg H, Ulmer H, Kaser A and Weiss G. Role of IL-10 for induction of anemia during inflammation. J Immunol 2002;169:2204–9. [DOI] [PubMed] [Google Scholar]
- 25.Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C and Camussi G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant 2011;26:1474–83. [DOI] [PubMed] [Google Scholar]
- 26.Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G, Miao S, Liu G, Lu M and Zhu Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther 2014;5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He J, Wang Y, Lu X, Zhu B, Pei X, Wu J and Zhao W. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology (Carlton). 2015;20:591–600. [DOI] [PubMed] [Google Scholar]
- 28.Choi HY, Lee HG, Kim BS, Ahn SH, Jung A, Lee M, Lee JE, Kim HJ, Ha SK and Park HC. Mesenchymal stem cell-derived microparticles ameliorate peritubular capillary rarefaction via inhibition of endothelial-mesenchymal transition and decrease tubulointerstitial fibrosis in unilateral ureteral obstruction. Stem Cell Res Ther 2015;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsukura T, Inaba C, Weygant EA, Kitamura D, Janknecht R, Matsumoto H, Hyink DP, Kashiwada S and Obara T. Extracellular vesicles from human bone marrow mesenchymal stem cells repair organ damage caused by cadmium poisoning in a medaka model. Physiol Rep 2019;7:e14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He J, Wang Y, Sun S, Yu M, Wang C, Pei X, Zhu B, Wu J and Zhao W. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology (Carlton). 2012;17:493–500. [DOI] [PubMed] [Google Scholar]
- 31.Lai P, Weng J, Guo L, Chen X and Du X. Novel insights into MSC-EVs therapy for immune diseases. Biomark Res 2019;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pawar AS, Eirin A, Krier JD, Woollard JR, Zhu XY, Lerman A, van Wijnen AJ and Lerman LO. Alterations in genetic and protein content of swine adipose tissue-derived mesenchymal stem cells in the metabolic syndrome. Stem Cell Res 2019;37:101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawar AS, Eirin A, Tang H, Zhu XY, Lerman A and Lerman LO. Upregulated tumor necrosis factor-alpha transcriptome and proteome in adipose tissue-derived mesenchymal stem cells from pigs with metabolic syndrome. Cytokine. 2020;130:155080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conley SM, Shook JE, Zhu XY, Eirin A, Jordan KL, Woollard JR, Isik B, Hickson LJ, Puranik AS and Lerman LO. Metabolic Syndrome Induces Release of Smaller Extracellular Vesicles from Porcine Mesenchymal Stem Cells. Cell Transplant 2019;28:1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Wang S, Zhu R, Li H, Han Q and Zhao RC. Lung tumor exosomes induce a pro-inflammatory phenotype in mesenchymal stem cells via NFkappaB-TLR signaling pathway. J Hematol Oncol 2016;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai K, Oseth L, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE and Blazar BR. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–9. [DOI] [PubMed] [Google Scholar]
- 37.Thirabanjasak D, Tantiwongse K and Thorner PS. Angiomyeloproliferative lesions following autologous stem cell therapy. J Am Soc Nephrol 2010;21:1218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reis M, Ogonek J, Qesari M, Borges NM, Nicholson L, Preussner L, Dickinson AM, Wang XN, Weissinger EM and Richter A. Recent Developments in Cellular Immunotherapy for HSCT-Associated Complications. Front Immunol 2016;7:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eirin A, Zhu XY, Ebrahimi B, Krier JD, Riester SM, van Wijnen AJ, Lerman A and Lerman LO. Intrarenal Delivery of Mesenchymal Stem Cells and Endothelial Progenitor Cells Attenuates Hypertensive Cardiomyopathy in Experimental Renovascular Hypertension. Cell Transplant 2015;24:2041–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang R, Lin M, Li L, Li L, Qi G, Rong R, Xu M and Zhu T. [Bone marrow mesenchymal stem cell-derived exosome protects kidney against ischemia reperfusion injury in rats]. Zhonghua Yi Xue Za Zhi. 2014;94:3298–303. [PubMed] [Google Scholar]
- 41.Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, Yang CC, Sun CK, Kao GS, Chen SY, Chai HT, Chang CL, Chen CH and Lee MS. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol 2016;216:173–85. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y, Zhu X, Zhang L, Ferguson CM, Song T, Jiang K, Conley SM, Krier JD, Tang H, Saadiq I, Jordan KL, Lerman A and Lerman LO. Mesenchymal Stem/Stromal Cells and their Extracellular Vesicle Progeny Decrease Injury in Poststenotic Swine Kidney Through Different Mechanisms. Stem Cells Dev. 2020;29:1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hickson LJ, Eirin A and Lerman LO. Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int 2016;89:767–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mihai S, Codrici E, Popescu ID, Enciu AM, Albulescu L, Necula LG, Mambet C, Anton G and Tanase C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J Immunol Res 2018;2018:2180373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F and Bammens B. Oxidative stress in chronic kidney disease. Pediatr Nephrol 2019;34:975–991. [DOI] [PubMed] [Google Scholar]
- 46.Chade AR. Renal vascular structure and rarefaction. Compr Physiol 2013;3:817–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Y, Cui H, Xia Y and Gan H. RIPK3-Mediated Necroptosis and Apoptosis Contributes to Renal Tubular Cell Progressive Loss and Chronic Kidney Disease Progression in Rats. PLoS One. 2016;11:e0156729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao J, Zheng J, Cai J, Zeng K, Zhou C, Zhang J, Li S, Li H, Chen L, He L, Chen H, Fu H, Zhang Q, Chen G, Yang Y and Zhang Y. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J 2019;33:1695–1710. [DOI] [PubMed] [Google Scholar]
- 49.Zhang G, Zou X, Miao S, Chen J, Du T, Zhong L, Ju G, Liu G and Zhu Y. The anti-oxidative role of micro-vesicles derived from human Wharton-Jelly mesenchymal stromal cells through NOX2/gp91(phox) suppression in alleviating renal ischemia-reperfusion injury in rats. PLoS One. 2014;9:e92129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C and Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O’Brien D, van Wijnen AJ and Lerman LO. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song T, Eirin A, Zhu X, Zhao Y, Krier JD, Tang H, Jordan KL, Woollard JR, Taner T, Lerman A and Lerman LO. Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Regulatory T Cells to Ameliorate Chronic Kidney Injury. Hypertension. 2020;75:1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maegdefessel L, Spin JM, Raaz U, Eken SM, Toh R, Azuma J, Adam M, Nakagami F, Heymann HM, Chernogubova E, Jin H, Roy J, Hultgren R, Caidahl K, Schrepfer S, Hamsten A, Eriksson P, McConnell MV, Dalman RL and Tsao PS. miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun 2014;5:5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collino F, Bruno S, Incarnato D, Dettori D, Neri F, Provero P, Pomatto M, Oliviero S, Tetta C, Quesenberry PJ and Camussi G. AKI Recovery Induced by Mesenchymal Stromal Cell-Derived Extracellular Vesicles Carrying MicroRNAs. J Am Soc Nephrol 2015;26:2349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gebeshuber CA, Zatloukal K and Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep 2009;10:400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, Emanueli C, Gasecka A, Hendrix A, Hill AF, Lacroix R, Lee Y, van Leeuwen TG, Mackman N, Mager I, Nolan JP, van der Pol E, Pegtel DM, Sahoo S, Siljander PRM, Sturk G, de Wever O and Nieuwland R. Methodological Guidelines to Study Extracellular Vesicles. Circ Res 2017;120:1632–1648. [DOI] [PubMed] [Google Scholar]
- 57.Yuana Y, Boing AN, Grootemaat AE, van der Pol E, Hau CM, Cizmar P, Buhr E, Sturk A and Nieuwland R. Handling and storage of human body fluids for analysis of extracellular vesicles. Journal of Extracellular Vesicles. 2015;4:29260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eldh M, Lotvall J, Malmhall C and Ekstrom K. Importance of RNA isolation methods for analysis of exosomal RNA: evaluation of different methods. Mol Immunol 2012;50:278–86. [DOI] [PubMed] [Google Scholar]
- 59.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom K, El Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gamez-Valero A, Gardiner C, Gartner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Gorgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanovic MM, Kovacs AF, Kramer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lasser C, Laurent LC, Lavieu G, Lazaro-Ibanez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Line A, Linnemannstons K, Llorente A, Lombard CA, Lorenowicz MJ, Lorincz AM, Lotvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr., Meehan KL, Mertens I, Minciacchi VR, Moller A, Moller Jorgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Ostergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saa P, Sahoo S, Salas-Huenuleo E, Sanchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schoyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sodar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr., Veit TD, Vella LJ, Velot E, Verweij FJ, Vestad B, Vinas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yanez-Mo M, Yin H, Yuana Y, Zappulli V, Zarubova J, Zekas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D and Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi HY, Moon SJ, Ratliff BB, Ahn SH, Jung A, Lee M, Lee S, Lim BJ, Kim BS, Plotkin MD, Ha SK and Park HC. Microparticles from kidney-derived mesenchymal stem cells act as carriers of proangiogenic signals and contribute to recovery from acute kidney injury. PLoS One. 2014;9:e87853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eirin A, Gloviczki ML, Tang H, Gossl M, Jordan KL, Woollard JR, Lerman A, Grande JP, Textor SC and Lerman LO. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J 2013;34:540–548a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zou X, Jiang K, Puranik AS, Jordan KL, Tang H, Zhu X and Lerman LO. Targeting Murine Mesenchymal Stem Cells to Kidney Injury Molecule-1 Improves Their Therapeutic Efficacy in Chronic Ischemic Kidney Injury. Stem Cells Transl Med 2018;7:394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stahl AL, Arvidsson I, Johansson KE, Chromek M, Rebetz J, Loos S, Kristoffersson AC, Bekassy ZD, Morgelin M and Karpman D. A novel mechanism of bacterial toxin transfer within host blood cell-derived microvesicles. PLoS Pathog 2015;11:e1004619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang B, Jia H, Zhang B, Wang J, Ji C, Zhu X, Yan Y, Yin L, Yu J, Qian H and Xu W. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res Ther 2017;8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pomatto M, Gai C, Negro F, Cedrino M, Grange C, Ceccotti E, Togliatto G, Collino F, Tapparo M, Figliolini F, Lopatina T, Brizzi MF and Camussi G. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int J Mol Sci 2021;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Yu X, Chen E and Li L. Liver-derived human mesenchymal stem cells: a novel therapeutic source for liver diseases. Stem Cell Res Ther 2016;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yigitbilek F, Conley SM, Tang H, Saadiq IM, Jordan KL, Lerman LO and Taner T. Comparable in vitro Function of Human Liver-Derived and Adipose Tissue-Derived Mesenchymal Stromal Cells: Implications for Cell-Based Therapy. Front Cell Dev Biol 2021;9:641792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C and Camussi G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med 2010;14:1605–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E and Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 2003;31:890–6. [DOI] [PubMed] [Google Scholar]
- 70.Kunter U, Rong S, Boor P, Eitner F, Muller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, Milovanceva-Popovska M, Kerjaschki D and Floege J. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol 2007;18:1754–64. [DOI] [PubMed] [Google Scholar]
- 71.Togliatto G, Dentelli P, Gili M, Gallo S, Deregibus C, Biglieri E, Iavello A, Santini E, Rossi C, Solini A, Camussi G and Brizzi MF. Obesity reduces the pro-angiogenic potential of adipose tissue stem cell-derived extracellular vesicles (EVs) by impairing miR-126 content: impact on clinical applications. Int J Obes (Lond). 2016;40:102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meng Y, Eirin A, Zhu XY, O’Brien DR, Lerman A, van Wijnen AJ and Lerman LO. The metabolic syndrome modifies the mRNA expression profile of extracellular vesicles derived from porcine mesenchymal stem cells. Diabetol Metab Syndr. 2018;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meng Y, Eirin A, Zhu XY, Tang H, Chanana P, Lerman A, Van Wijnen AJ and Lerman LO. The metabolic syndrome alters the miRNA signature of porcine adipose tissue-derived mesenchymal stem cells. Cytometry A. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eirin A, Zhu XY, Woollard JR, Tang H, Dasari S, Lerman A and Lerman LO. Metabolic Syndrome Interferes with Packaging of Proteins within Porcine Mesenchymal Stem Cell-Derived Extracellular Vesicles. Stem Cells Transl Med 2019;8:430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eirin A, Ferguson CM, Zhu XY, Saadiq IM, Tang H, Lerman A and Lerman LO. Extracellular vesicles released by adipose tissue-derived mesenchymal stromal/stem cells from obese pigs fail to repair the injured kidney. Stem Cell Res. 2020;47:101877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farahani RA, Zhu XY, Tang H, Jordan KL, Lerman A, Lerman LO and Eirin A. Metabolic Syndrome Alters the Cargo of Mitochondria-Related microRNAs in Swine Mesenchymal Stem Cell-Derived Extracellular Vesicles, Impairing Their Capacity to Repair the Stenotic Kidney. Stem Cells Int. 2020;2020:8845635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen XJ, Jiang K, Ferguson CM, Tang H, Zhu X, Lerman A and Lerman LO. Augmented efficacy of exogenous extracellular vesicles targeted to injured kidneys. Signal Transduct Target Ther 2020;5:199. [DOI] [PMC free article] [PubMed] [Google Scholar]


