Abstract
Gene therapy encompasses the transfer of exogenous genetic materials into the patient’s target cells to treat or prevent diseases. Nevertheless, the transfer of genetic material into desired cells is challenging and often requires specialized tools or delivery systems. For the past 40 years, scientists are mainly pursuing various viruses as gene delivery vectors, and the overall progress has been slow and far from the expectation. As an alternative, nonviral vectors have gained substantial attention due to their several advantages, including superior safety profile, enhanced payload capacity, and stealth abilities. Since nonviral vectors encounter multiple extra- and intra-cellular barriers limiting the transfer of genetic payload into the target cell nucleus, we have discussed these barriers in detail in this review. A direct approach, utilizing physical methods like electroporation, sonoporation, gene gun, eliminate the requirement for a specific carrier for gene delivery. In contrast, chemical methods of gene transfer exploit natural or synthetic compounds as carriers to increase cellular targeting and gene therapy effectiveness. We have also emphasized the recent advancements toward enhancing the current nonviral approaches. Therefore, in this review, we have focused on discussing the current evolving nonviral gene delivery systems and their future perspectives.
Keywords: Gene delivery, nonviral vector, cationic lipid
1. Introduction
Gene therapy is an emerging therapeutic strategy for the curative treatment of genetic disorders and numerous other diseases. The basic concept of gene therapy involves delivering a functioning copy of a gene into specific host cells to compensate for the missing or mutated endogenous counterpart or produce a beneficial protein [1]. In addition, the sequence-specific inhibition of a disease-causing gene with siRNA or antisense DNA has also been considered a type of gene therapy. Transgenes as a therapeutic modality facilitate in situ expression of bioactive molecules at the target site in their native form [2] and offer better flexibility in their applications than the delivery of exogenous proteins or bioactive molecules. Furthermore, the sustained transgene expression ensures synchronization between the kinetics of signaling receptor expression and the availability of bioactive molecules [3].
Initially, gene therapy focused mainly on life-threatening monogenic orphan diseases that are hard to treat with conventional therapies such as adenosine deaminase-severe combined immunodeficiency, cystic fibrosis, and hemophilia [4–7]. However, the success of the Human Genome Project provided a better understanding of the role of genetics in various diseases and enabled the scope of gene-based medicine [8]. As a result, gene therapy is now evolving as a potential therapeutic modality against a large spectrum of diseases, including different types of cancers [9–12], cardiovascular diseases [13, 14], neurodegenerative or metabolic disorders such as Parkinson’s [15], Alzheimer’s [16, 17], Huntington’s disease [18], and diabetes [19]. Further, gene therapy could prevent infectious diseases through genetic immunization [20, 21].
Cancer remains the most popular for gene-based therapeutics out of various disorders as its development is associated with various mutations and genetic disorders. Gene therapy for cancer has explored various strategies, including immunostimulation against cancer, oncogene suppression, activation of suicidal genes, mutation correction, tumor suppressor gene activation, and downregulation of genes for angiogenesis. More information can be found in recent comprehensive reviews on nonviral gene therapy for cancer [22]. By February 2021, a total of 3180 gene therapy clinical trials had been approved worldwide; the majority (67.4%) accounts for cancers [23].
Over the last few decades, gene therapy has been extensively studied to prevent and treat a wide range of diseases. Based on the delivery techniques, gene therapy can be classified as in vivo gene therapy and ex vivo gene therapy. With the in vivo gene therapy, the targeted cells remain in the patient’s body, and genetic material is administered directly to the patient using various physical and chemical approaches. Examples of in vivo gene therapy include treatment of ADA-SCID or Leber congenital amaurosis. However, in ex vivo gene therapy, the targeted cells are first collected from the patient. Then, genetic material is administered to the cells in vitro before being readministered to the patient’s body—for instance, highly personalized cancer immunotherapy using CAR-T cells.
The clinical outcomes of gene therapies are often limited due to several technical barriers associated with gene delivery. The most critical challenge for successful in vivo gene therapy is transferring nucleic acid therapeutics into target tissue efficiently. Systemic administration of unprotected genes is impractical because of their vulnerability towards nuclease degradation [24]. While for both in vivo and ex vivo gene transfer, the genetic materials have to be delivered into the appropriate cellular machinery. The high negative charge density, large size, and strong hydrophilicity of these macromolecules make them difficult for intracellular delivery [25]. Even after endocytosis, these macromolecules are transported to lysosomes, where different hydrolytic enzymes degrade them. These factors together diminish the activity of gene-based therapeutics and requiring large quantities to be effective. Therefore, the development of safe and efficient gene delivery systems is the cornerstone of any successful gene therapy.
2. Barriers to nonviral gene delivery
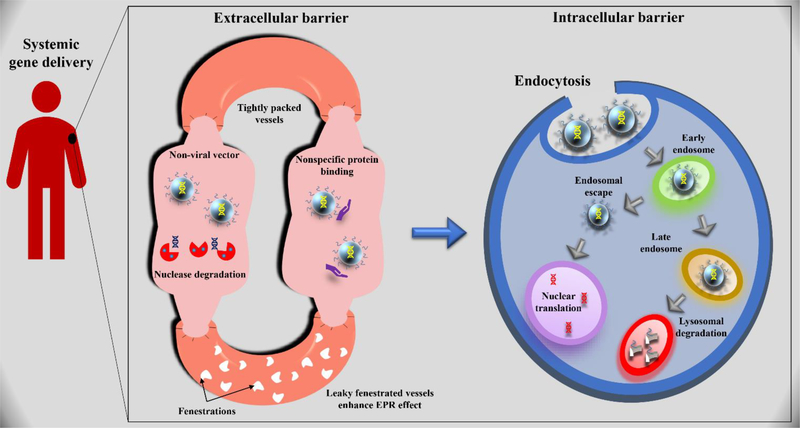
The success of gene therapy is largely dependent on the efficient delivery of the genetic cargo to the body’s target cell population in a safe manner. However, nonviral vectors encounter multiple extra- and intra-cellular barriers limiting genetic payload transfer into the target cells (Figure 1).
Figure 1.
Extra- and intracellular barriers for nonviral gene transfer of DNA-based therapeutics.
2.1. Extracellular barrier
Interaction of nonviral vectors with the extracellular environment is unavoidable irrespective of the administration route. Several factors in the extracellular environment are responsible for extensive clearance and degradation of the delivery system before reaching its target area. These factors include transportation across blood vessels, stability in biological fluids, and binding to the target ligand of interest. Moreover, blood cells and proteins also pose a significant challenge in efficiently delivering a therapeutic gene to its target site.
2.1.1. Stability in extracellular compartments
The extracellular stability implies both the chemical stability of nucleic acid and the physical stability of the delivery systems. The endo- and exonucleases in physiological fluids and extracellular space can degrade unprotected DNA within ten minutes following systemic administration [26]. This degradation can minimize by formulating genetic materials in a suitable delivery system, thereby protecting them from the extracellular enzymatic environment.
Colloidal instability of DNA complexes in an extracellular environment is a critical concern for carrier-mediated nonviral gene delivery. The increased salt concentration diminishes the electrostatics interactions between DNA and polycations and shields the interparticle electrostatic repulsive forces, leading to precipitation of gene carriers in physiological fluids [27]. Moreover, positively charged DNA-polycation complexes exhibit instability in the extracellular milieu due to their interactions with negatively charged blood components. These precipitates are promptly cleared from circulation by the mononuclear phagocytic system, preventing localization at target sites [28]. Several approaches have been adopted to improve the colloidal stability of nonviral gene delivery systems. PEGylation is one of the most effective strategies for improving colloidal stability and minimizing aggregation of nanoparticle-based delivery systems [29, 30]. In addition, the incorporation of neutral helper lipids such as 1,2-dioleoylphosphatidylethanpolymerolamine (DOPE) and cholesterol have been shown to enhance the colloidal stability of cationic lipid/DNA complexes [31, 32]. Alternatively, hydrophilic neutral polymers such as polyvinyl alcohol or polyvinylpyrrolidone can be used to form reversible neutral or anionic polyplexes and inhibits its degradation by nucleases [33, 34].
2.1.2. Extravasation
Extravasation of DNA complexes through capillary walls into the target tissues following systemic administration is another barrier to efficient gene delivery. The extravasation of DNA complexes depends on the physicochemical properties of the complexes like size, shape, and permeability through the vascular layers they encounter and the biological parameters such as regional variations in the target tissue’s capillary structure and disease state. For example, contrary to most tissues, the liver and spleen capillaries have fenestrated and discontinuous endothelium with a pore size of up to 150 nm, thus favoring leakage of DNA complexes in these organs [35]. However, direct injection of DNA complexes into the target tissue has also been tried to overcome the extravasation barrier [36].
2.1.3. Cellular association and internalization
Once the delivery system reaches the target cells, the next significant challenge is crossing the cell membrane. Nevertheless, the high negative charges of naked DNA prevent its association with the anionic cell membranes. Thus, the use of polycations could significantly neutralize the negative charges on DNA and enhance its association to the cell membrane. The association of cationic delivery systems is thought to be mediated by its interaction with the membrane-bound heparin sulfate proteoglycans (HSPGs), promoting endocytosis of the DNA complexes. Additionally, the conjugation of cell-specific ligands to the delivery systems has been shown to enhance the cell surface binding of DNA in vitro [37, 38] and in vivo [39–41]. Adsorptive endocytosis [42], receptor mediated-endocytosis [43], macropinocytosis [25, 44], and phagocytosis [45] are the major pathways responsible for cellular uptake of nonviral gene delivery systems. However, physical gene delivery techniques can also promote intracellular delivery of genetic materials.
2.2. Intracellular barrier
Intracellular barriers, notably the physical barrier like membranes, help compartmentalize the biological functions of a cell. However, these barriers pose a set of new challenges for efficient gene delivery. Typically, cells can internalize gene delivery vehicles efficiently in vitro (> 95%); however, the conversion rate of these genes into proteins is abysmal (<50%). Furthermore, the endocytosis pathway, the major pathway for the internalization of nonviral vectors, leads to vesicles with acidic pH and degradation enzymes. Therefore, efficient gene delivery heavily relies on the escape of gene delivery vector from endosomes into the cytoplasm, crossing the nuclear membrane and release the desired gene for its transfection.
2.2.1. Endosomal escape
Following endocytosis, the intracellular vesicles carrying the vector-DNA complexes are fused with the cytosolic organelles to form early endosomes. The failure of vector-DNA complexes to escape from the endocytic pathway presumably leads to their trafficking via late endosomes to lysosomes. The harsh lysosomal environment characterized by low pH (approximately 4.5) and the presence of various hydrolytic enzymes such as nucleases, proteinases, and lipases can rapidly degrade the complexes and their attached cargos [24]. Therefore, effective endosomal escape of the DNA into the cytoplasm is necessary for efficient gene delivery and subsequent gene expression.
Lipoplexes (cationic lipid/DNA complexes) can escape from endosomes by fusing the cationic lipid membrane with the endosomal membranes, ultimately disrupting the endosomal membrane and facilitating DNA release into the cytoplasm [46]. Moreover, incorporating dioleoylphosphatidylethanolamine (DOPE) and cholesterol have demonstrated enhanced endosomal escape because of their transformation from bilayer to non-bilayer inverted hexagonal-II structures in the acidic pH range of 5–6 [47]. However, it is worth mentioning that the membrane disruption resulting from lipid mixing amongst anionic membranes and cationic lipids occurs presumably at the endosomes rather than at the cell surface [27]. Unlike cationic lipids, the exact mechanisms involved in the endosomal escape of cationic polymer/DNA complexes are less clear, and two possible mechanisms have been proposed. One hypothesis suggests cationic polymer-mediated physical disruption of endosomes [48]. This mechanism is generally proposed for poly-L-lysine (PLL), polyamidoamine (PAMAM) dendrimers, polyornithine. Alternatively, cationic polymers with ionizable amines such as polyethyleneimine (PEI), PAMAM, and PLL are thought to release their contents via the proton sponge effect [49]. Although widely accepted, numerous reports have questioned the proton sponge theory primarily due to insufficient data in favor of polyplex-mediated endosomal escape through pH buffering [50, 51]. Alternatively, endosomal escape is proposed to rely on a time-dependent proton-mediated membrane perturbation due to strong binding of the inner endosomal membrane and the polyplexes.
Many other strategies have been used to enhance the endosomal escape of DNA. For example, pretreatment of cells with lysosomotropic agents (chloroquine and ammonium chloride) enhances endosomal escape through buffering endosome/lysosome pH [46, 52]. Similarly, high sucrose concentrations, glycerol, or polyvinylpyrrolidone have exhibited enhanced transfection, apparently by osmotic rupture of vesicles [53, 54]. However, these strategies are impractical for in vivo gene delivery applications and are limited to in vitro experiments.
2.2.2. Cytoplasmic trafficking
Upon successful release into the cytoplasm, nucleic acids must be transported to their target intracellular compartment to exert desired biological response. Thus, the final target of antisense oligonucleotides, mRNA, siRNA, and miRNA/miRNA mimics is the cytoplasm, whereas plasmid DNA must be traversed the cytoplasm to access the transcriptional machinery in the nucleus. However, it has been demonstrated that the diffusion of DNA in the cytoplasm is size-dependent, and DNAs of 3000 base pairs or larger are virtually immobile [55]. Additionally, the naked DNA is rapidly degraded by the cytosolic nucleases with an apparent half-life of ~1–1.5 h [56]. This degradation presents a significant challenge for transferring free DNA and lipoplexes, which are thought to be detached before entering the nucleus.
Several studies demonstrated that DNA utilizes dynein motor proteins and microtubule networks for translocation through the cytoplasm to the nucleus [57, 58]. It has also been reported that transcription factors are the crucial components of microtubules-mediated DNA migration. Nevertheless, the inclusion of specific transcription factor binding sites within plasmids, such as cyclic AMP response-element binding protein (CREB) or modulating microtubule acetylation through histone deacetylase 6 (HDAC6) inhibition, enhances the rate of DNA migration [57]. Similarly, positively charged polyplexes could migrate along microtubules via nonspecific interaction with anionic microtubules or motor proteins. However, both free DNA and polyplexes could be redistributed throughout the cell during mitosis, and some might accumulate inside the nucleus.
2.2.3. Nuclear localization
The nuclear entry of exogenous DNA is one of the significant limiting steps in nonviral gene transfer. It has been demonstrated that direct microinjection of the thymidine kinase (TK) gene inside TK-deficient cell nuclei leads to kinase expression in 50–100% of the cells [59]. In contrast, no thymidine kinase activity was detected in cells that received a cytosolic injection of the same DNA. These results suggest that cytoplasmic elements sequester most of the DNA that enters the cytoplasm, and only a small fraction successfully enters the nucleus. Therefore, studies designed to understand DNA nuclear translocation mechanism to enhance this process have been vital to improving nonviral gene delivery efficiency.
Nuclear transport of DNA occurs via three possible pathways: i) nuclear membrane disruption during mitosis, ii) entry via nuclear pores and iii) kariophilic proteins mediated nuclear entry [57]. In actively dividing cells, the nuclear membrane disintegrates during mitosis, thus allowing DNA passage into the nucleus [57]. However, in non-dividing cells, the nuclear transport of DNA occurs primarily via nuclear pore complex (NPC), which allows the free passage of molecules less than 9 nm in diameter (i.e., nucleic acids of ~300 bp or proteins <60 kDa), but limits larger macromolecules [60]. Nuclear transport is energy-dependent for larger macromolecules mediated through nuclear localization sequence (NLS) and their nuclear receptors [57]. Therefore, adding a karyophilic protein binding site to the DNA sequence can greatly enhance DNA delivery into the nucleus. For instance, the addition of SV40 enhancers to plasmids can help the localization of plasmids into the nucleus of quiescent cells within a few hours [61]. Several other approaches have been adopted to enhance nuclear localization of the DNA, including covalent modification of DNA for binding of NLS peptide, conjugation of NLS peptide to nonviral vectors, nuclear proteins, and small molecules ligands [57].
2.2.4. Vector unpacking
The release of genetic materials from its delivery vector is considered to be crucial for subsequent gene expression. However, the effect of vector unpacking on the degree of gene expression remains unclear. In the case of cationic lipid/DNA complexes, the DNA release is facilitated by the fusion of positively charged lipid with the endosomal membrane [62]. On the other hand, polyplexes are known to internalize in an intact form inside the nucleus, where dissociation of the polyplexes occurs. It has also been reported that the slow release of DNA from its carrier resulted in diminished gene expression. Overall, lipoplexes are considered more efficient in transfection than their polymeric counterparts due to their better membrane interaction ability and superior biocompatibility [63].
Several additional strategies have been attempted to improve vector stability in the extracellular environment while ensuring the intracellular release of genetic cargos. For example, disulfide linkages are often incorporated into the polymer chain to form highly cross-linked polyplexes and prevent dissociation in the extracellular milieu [64–68]. However, in intracellular reductive environments where the glutathione concentration is ~50−1000 fold greater than in the extracellular environment, these polyplexes dissociate to facilitate genetic cargo release. Researchers have shown more than 10-fold enhancement in transfection efficiency utilizing this technique in vitro [69]. The use of fusogenic cationic lipid is another strategy to promote cytosolic vector unpacking [70]. This fusogenic lipidic polyplex vector fuses with the cell membranes and emits the polyplex into the cytoplasm. Subsequently, the cationic polymer undergoes oxidation by the intracellular reactive oxygen species (ROS) to become negatively charged, leading to the efficient release of the DNA. Similarly, the cationic polymer that can degrade into neutral thioether fragments in response to intracellular ROS has also been investigated for efficient DNA release [71].
3. Gene delivery systems
Numerous carriers and delivery techniques have been utilized in clinical trials that are broadly classified as viral and nonviral vectors. Viruses are highly efficient in carrying their genome to host cells and exploiting cellular machinery to initiate their genome expression. Several viruses have been transformed into gene-delivery carriers by replacing all or a part of the viral coding regions with a therapeutic gene. Nevertheless, viral vectors remain the most prevalent gene carriers, having been used in more than two-thirds of the clinical trials carried out to date [23]. The most commonly used viral vectors in clinical trials include adenovirus, retrovirus, adeno-associated virus, lentivirus, herpes simplex virus, and poxvirus. Each of these viral vectors exhibits specific features with unique advantages for clinical gene transfer but also accompanied by several intrinsic drawbacks, including immunogenicity, carcinogenesis, severe inflammatory responses, low target specificity, and limited DNA packaging capacity [72, 73]. Detailed information on recombinant viral-based gene delivery vectors has been explicitly discussed in several excellent reviews [74–76].
Nonviral vectors are capable of addressing many of these concerns, especially regarding biosafety. They are less toxic and far less immunogenic than their viral counterpart. Other potential benefits of nonviral vectors include the ability to delivering a larger genetic payload, ease of large-scale production, and the possibility of repetitive administrations. Therefore, numerous nonviral strategies, including various chemical carriers or physical techniques, have been explored for nucleic acid transport [77–81]. Nevertheless, the clinical applications of these systems are limited owing to their poor transduction efficiency compared to viral vectors. In addition, nonviral vectors are unable to overcome multiple extra- and intra-cellular barriers encountered prior to transferring their genetic payloads into their site of action.
3.1. Nonviral delivery systems
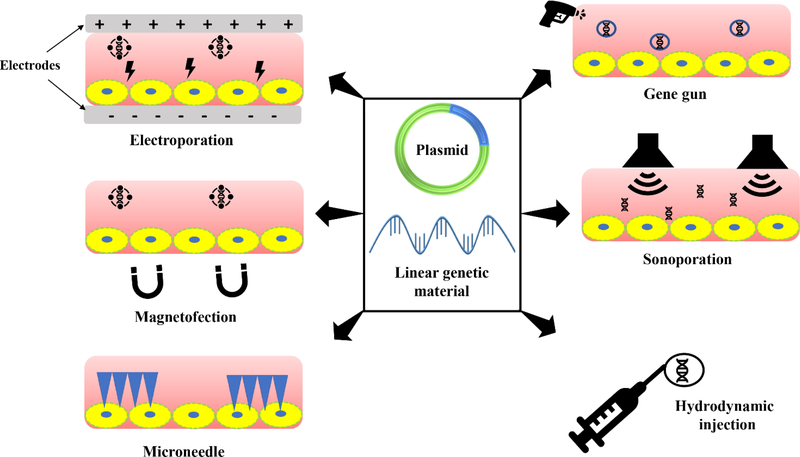
Nonviral gene delivery approaches have drawn increasing interest due to their excellent biosafety profile. Advances in transfection efficiency, specificity, and safety led to an increased number of nonviral vectors in gene therapy clinical trials [82]. Nonviral vectors can be broadly classified into physical methods and chemical carriers. Physical methods are used to remove the requirement for a specific gene carrier to deliver exogenous DNA into target cells. These approaches utilize physical force to make transient pores on cell membranes, allowing DNA entry into cells via diffusion. Figure 2 shows the pictorial representation of different physical transfection methods. Physical methods provide several benefits over other methods including, simplicity, safety, and, importantly, their potential to control process parameters toward specific therapeutic needs. However, the gene transfection efficiency of these techniques is low as compared to viral vectors. Furthermore, gene delivery to internal organs is challenging since it requires surgical procedures to access target tissue.
Figure 2.
Pictorial representation of various physical methods of gene transfer.
Chemical methods use natural or synthetic compounds as carriers to deliver exogenous genetic materials into cells. The key benefits of chemical methods include simplicity, low cytotoxicity, and ease of large-scale production. Numerous chemical compounds have been developed since DEAE-dextran was first used as a nonviral vector in 1969 [83]. Table 1. summarizes the most utilized nonviral vectors.
Table 1.
Nonviral gene delivery systems.
| Category | Gene delivery systems |
|---|---|
| Physical methods | 1. Electroporation 2. Gene gun 3. Hydrodynamic injection 4. Sonoporation 5. Microneedle 6. Magnetofection |
| Chemical methods | 1. Cationic lipids: 1,2-dioleoyl-3-trimethylammonium propane (DOTAP); 1,2-di-O-octadecenyl-3-trimethylammonium propane (DOTMA); 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE); Dimethyldidodecylammonium bromide (DDAB) 2. Cationic polymers: PEI, chitosan, PLL 3. Dendrimers: PAMAM, polypropylenimine (PPI), PLL, triazine based dendrimers 4. Polypeptide-based vectors: trans-activating transcriptional activators (TAT) peptide, MPG, model amphipathic peptide (MAP) 5. Inorganic, polymeric, and lipid nanoparticles: Carbon nanotubes, quantum dots, silica nanoparticles, gold nanoparticles, poly (d,l-lactide-co-glycolide) (PLGA) nanoparticles, solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs) 6. Gemini surfactant |
The following section describes an overview of the prevailing nonviral gene delivery systems with their specific benefits and limitations.
3.1.1. Physical methods
3.1.1.1. Electroporation
Electroporation was initially developed as a laboratory technique for introducing DNA to bacteria and mammalian cells in culture. Electroporation uses a high-voltage pulse of electricity to create transient and reversible openings in the cell membranes. This temporary permeabilized state facilitates the delivery of normally impermeable macromolecules such as DNA into the cytoplasm. Once the electrical field is withdrawn, the pores destabilize and anneal over time (minute scale), allowing the membrane to return to its normal permeability state. Neumann and colleagues demonstrated for the first time that electroporation could be utilized to transfect mammalian cells in culture [84]. In the last four decades, tremendous advances have been achieved in the field, particularly in electrode development, allowed this technique to be appropriate for in vivo applications [85].
Electroporation has been proven safe and highly efficient for in vivo gene transfer among the different nonviral approaches. For in vivo purposes, DNA is usually injected locally, and then electric pulses applied surrounding the injection site. Several factors must be considered when selecting in vivo electroporation parameters, including electrode design, tissue type, size of the delivered DNA, and the pulse protocol used [86, 87]. The pulse parameters such as pulse type, length, magnitude, number, and frequency can be fine-tuned to control transgene expression levels and duration. Furthermore, proper optimization of the electroporation parameters leads to efficient gene transfection comparable to viral vectors [88]. However, no universal protocol results in the highest efficacy in every condition; tailoring is necessary for each new setting.
Although electroporation has been used in numerous in vitro gene transfer studies, it has several drawbacks regarding its potential for clinical gene therapy. First, electroporation is effective only within ~1 cm range between the electrodes, making it unlikely to transfect cells in a larger area of tissues. Second, electroporation of internal organs is a challenging task; surgical interventions are necessary for the implantation and removal of electrodes. Additionally, high voltage electric pulses usage result in the degradation of genomic DNA and permanent tissue damage [89]. However, some of these problems can be overcome by developing suitable electrodes, proper spatial arrangement, and selecting optimal pulse parameters such as applied voltage, pulse type, pulse length, and pulse numbers. Electroporation has recently been successfully used in numerous DNA vaccine and gene therapy applications.
3.1.1.2. Gene gun
Biolistic particle delivery system or gene gun was initially developed for genetic transformation of plant cells [90] and later successfully employed for gene delivery in mammalian cells in vitro and in vivo [91, 92]. In this method, target cells are bombarded with high-speed DNA-coated heavy metal microparticles accelerated by a compressed inert gas such as helium. Generally, tungsten, gold, or silver microparticles in the range of 0.5 to 1 μm diameter are employed as the gene carrier [93, 94]. Particle size and density of the gene carrier, bombardment force, particles to DNA ratio, dosing frequency, and gene gun instrumentation strongly influence the penetration capacity, degree of tissue damage, and transfection efficiency of a biolistic procedure. Thus, optimizing these parameters is essential to deliver genes while minimizing cell/tissue damage efficiently. Further, the types of cells or tissues to be transfected can significantly influence these parameters.
The biolistic gene delivery is beneficial for being simple, fast, reliable, and highly efficient. It enables direct delivery of the transgene in target tissues without requiring any toxic chemical adjuvants as gene carriers, and the gene transfer is independent of DNA size, cell types, and cell surface receptors. Further, the gene is forced to enter the cytosol and nucleus of the target cells and evade the enzymes present in endolysosomal vesicles, substantially reducing the doses of transgenes required. Additionally, gene gun permits multiple gene delivery simultaneously to the same tissues, enabling investigation of interaction among various gene products. Finally, since gene guns can produce an adequate immune response with a minuscule amount of DNA, it is a promising genetic immunization technique.
Gene gun is frequently used for intramuscular, intradermal, and intratumoral DNA vaccination in numerous animal models and human clinical trials. Besides DNA vaccination, biolistic gene delivery has been modestly used in other gene therapy protocols where a small amount of therapeutic protein is sufficient to produce the desired response. However, the applications of gene guns are mainly limited to superficial tissues such as skin and muscles. This is because a gene gun can transport DNA to a shallow depth of 100–500μm, and it would not be easy to transfect entire organs using this technique. Furthermore, though the use of higher gas pressure delivers genes into deeper areas, it also results in cell damages at the surface. Hence, there is always a trade-off between penetration depth and cell/tissue damage. The other disadvantages are short-term gene expression, high input cost, and lack of cellular target (i.e., cytoplasm or nucleus).
3.1.1.3. Hydrodynamic injection
Hydrodynamic gene delivery utilizes the rapid injection of high-volume DNA solution (8–12% of body weight), which leads to momentary cardiac congestion and increases hydrodynamic pressure in the inferior vena cava. Consequently, DNA solution flows back to the kidney and liver through the renal and hepatic veins, respectively [95]. This raised pressure expands the liver’s fenestrae and creates pores on the hepatocyte membrane, facilitating intracellular delivery of DNA molecules. The hepatocytes reseal with time (< 2 minutes), trapping the DNA molecules inside [95, 96]. Notably, the effect of hydrodynamic pressure on the liver is reversible and short-lived and disrupted sinusoids recover their original structure and function between 24 – 36 h of injection [97]. The effectiveness of hydrodynamic gene delivery depends on several parameters such as capillary structure (fenestrated vs. continuous), the architecture of cells adjacent to the capillary, and the applied hydrodynamic force [98, 99].
The hydrodynamic procedure is considered the most efficient nonviral technique for gene delivery into rodents because of its high efficiency, simplicity, safety, and reproducibility. This procedure has been widely used for improving gene delivery to the liver [100], kidney [101], lungs [102], skeletal muscle [103], heart [104], and pancreas [105]. While all of these organs display considerable transgene expression, but especially the liver shows the highest level of transgene expression [106, 107]. The higher gene expression in the liver following hydrodynamic gene delivery via the tail vein could be attributed to several unique features of the liver, including big size, presence of fenestrated sinusoids, lack of basement membrane, proximity to inferior vena cava, low blood pressure, and high gene expression capacity of hepatocytes [108].
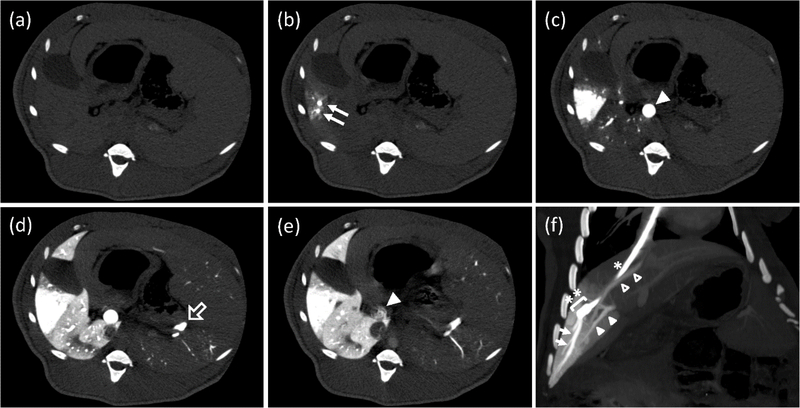
Though the hydrodynamic method is an effective gene transfer technique, its clinical application is mainly restricted due to high volume injection. Nevertheless, various modifications have been made to basic hydrodynamic techniques to meet clinical and experimental needs. For instance, the image-guided catheterization technique has been used for hydrodynamic gene transfer into the swine liver (Figure 3) [109]. These results suggest that hydrodynamic gene transfer is feasible for large animals, including humans with minimal tissue damage. However, the transgene expression level was not controllable even though the hydrodynamic procedure was performed by the same surgeon using the same procedure [110]. This is primarily due to the complication associated with placing a catheter at the same site precisely, resulting in variations in hydrodynamic pressure and consequent alterations in gene transfer efficiency. A computer-assisted automated injection device has been developed to overcome this issue [111]. This device uses real-time intravascular pressure as a controller for automatic adjustments of injection parameters. These results suggest that hydrodynamic-based gene delivery is feasible for human uses, especially after developing computer-controlled injection devices combined with image-guided catheterization.
Figure 3.
Computed tomography (CT) study during a hydrodynamic injection from a catheter, which was placed at a branch of the hepatic vein. (a–e) CT images at the same axial level were repeatedly obtained with a 0.5 s interval before, during, and after a regional hydrodynamic injection of contrast medium at a constant speed of 20 mL/s in 7.5 s. Each image was taken before the injection, (a); 1 s, (b); 2.5 s, (c); 7.5 s, (d); and 12 s, (e); after the injection. Closed arrows: hepatic vein (HV) branches in the target area; closed arrowhead: main trunk of the portal vein (PV); open arrow: splenic vein. (f) An oblique multi-planar reconstruction image immediately after the end of a hydrodynamic injection before removing a balloon occlusion. The coronal image was reconstituted with a 17.7-mm thickness. A catheter (*) with a balloon (**) was placed in the branch of hepatic vein via the right jugular vein. Arrows: periphery of the target HV; closed arrowheads: portal vein of the target area; open arrowheads: another hepatic vein beginning from the inside of the target area. (Reprinted from T. Yokoo, T. Kanefuji, T. Suda, K. Kamimura, D. Liu, S. Terai, Site-Specific Impact of a Regional Hydrodynamic Injection: Computed Tomography Study during Hydrodynamic Injection Targeting the Swine Liver, Pharmaceutics 7(3) (2015) 334–343 under the terms of the Creative Commons CC BY license).
3.1.1.4. Sonoporation
Sonoporation uses ultrasound waves for intracellular transport of macromolecules such as proteins and genes [112]. Although the exact sonoporation mechanism is not entirely identified, it is believed that ultrasound induces hyperthermia and microbubble cavitation, resulting in temporary openings on the membrane of the target cells [112, 113]. The biophysical and biological mechanisms contributing to enhanced cell permeabilization depend primarily on the variety of ultrasound settings (e.g., high-intensity ultrasound causes inertial cavitation, whereas low-intensity ultrasound leads to stable cavitation) [114]. However, most gene transfer studies use 1–3 MHz ultrasound with an intensity of 0.5–2.5 W/cm2) effectively delivering macromolecules in vitro and in vivo [115, 116].
Though less effective than hydrodynamic injection and electroporation, sonoporation has received increasing attention due to its noninvasiveness, safety, simplicity, and flexibility compared to other gene transfer methods [117]. Further, the efficacy of sonoporation can improve significantly in combination with microbubbles or echo-contrast agents. Microbubbles act as cavitation nuclei and enhance membrane permeability by minimizing the cavitation threshold of ultrasound [118]. One of the most frequently employed contrast agents is Optison, which consists of albumin-coated octafluoropropane gas-filled microspheres. The overall efficacy of sonoporation depends on several factors, including ultrasonic parameters (e.g., pulse length, frequency, repetition rate, duty cycle, and acoustic pressure), microbubble parameters (e.g., size, surface rigidity, gas species, and shell material), DNA concentration, cells, and tissue types, and even the ambient temperature [119]. For example, it has been shown in comparison to neutral microbubbles, pDNA bound cationic microbubbles are a more effective gene carrier [120]. Therefore, these parameters need to be carefully optimized depending on the target tissues, models, and therapeutic needs. Sonoporation technique has been used for gene delivery to different tissues and organs such as muscles [121], brain [122], liver [123, 124], heart [125], kidney [126], lungs [127], and solid tumors [128, 129].
3.1.1.5. Microneedle
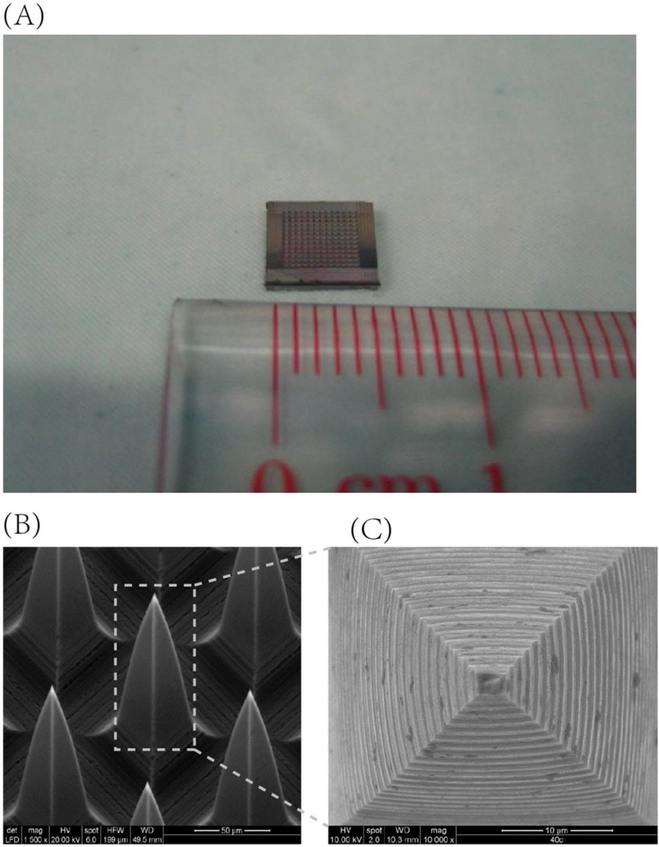
Microneedle-mediated delivery is an innovative strategy to deliver DNA therapeutics across the skin in a minimally invasive and patient-friendly manner. The microneedle-based delivery system consists of solid or hollow micro-projections typically ranging from 25–2000 μm in length, which can be fabricated into varying shapes, heights, and densities using diverse materials such as steel, ceramics, silicon, glass, polymers, amongst others (Figure 4) [130–132]. These projections penetrate the stratum corneum, a significant physical barrier for delivery through the skin, to form transient pores that enable the delivery of target DNA intra- or trans-dermally, a region rich in antigen-presenting cells such as Langerhans cells, dendritic cells, and macrophages) [133, 134]. The primary application of microneedles mediated delivery of nucleic acids is reported to treat genetic skin conditions (e.g., alopecia, allergy, psoriasis, hyperpigmentation), cancers, wounds, hyper-proliferative diseases, and vaccination [131, 135]. Mikszta et al. [136] were the first to report in vivo delivery of plasmid DNA encoding hepatitis B surface antigen using microenhancer arrays (MEAs) consisting of silicon projections. MEA-based topical immunization using naked DNA resulted in stronger and less variable immune responses and lowered number of immunizations required for full seroconversion. In 2016, Deng et al. [137] demonstrated non-invasive and efficient delivery of siRNA (GAPDH gene) in mouse skin through microneedle arrays reducing GAPDH expression up to 66% in the skin without accumulation in major organs.
Figure 4.
Microneedle array patch. (A) The size of the microneedle array patch; (B) Configuration of the microneedle array patch and (C) Structure of a single microneedle model. (Reprint from Y. Deng, J. Chen, Y. Zhao, X. Yan, L. Zhang, K. Choy, J. Hu, H.J. Sant, B.K. Gale, T. Tang, Transdermal Delivery of siRNA through Microneedle Array, Scientific Reports 6 (2016) 21422, under the terms of the Creative Commons CC BY license).
Combining a microneedle-based delivery system with other technologies such as electroporation and gene gun has also been investigated to increase gene expression efficiency. Pioneer studies utilizing solid microneedle array in conjugation with electrical pulses to aid gene delivery were conducted by Hooper et al. [138]. Plasmid DNA for live vaccinia virus was dried on the microneedles’ tips (≤1mm long) and delivered in adult BALB/c mice using an Easy Vax™ DNA vaccine delivery system. Vaccinated mice demonstrated higher neutralizing antibody titers and protection against a lethal dose of vaccinia virus. Subsequently, researchers demonstrated highly efficient siRNA delivery into tumor xenograft and plasmid DNA delivery in healthy muscle tissue of C57BL/6 mice using microneedle array technique in conjunction with low-voltage electroporation [139]. Moreover, a broad range of clinical trials has outlined safe and efficient use of microneedles for the delivery of biologics, which supports the potential to produce microneedle arrays on a large scale for clinically acceptable nucleic acid delivery [140]. However, limitations such as inability to incorporate high loading dose, difficulty in accurately estimating the administered dose, and significant loss of DNA cargo through residual formulation remaining on microneedle surface, and incomplete migration of nucleic acid in contact with the skin are significant issues that need to be resolved before clinical application of such delivery system [133].
3.1.1.6. Magnetofection
Magnetofection is an approach to transfect cells or tissues which is based on magnetic nanoparticles. Magnetic nanoparticles complexed with the therapeutic gene are directed to targeted cells under the influence of an external magnetic field [141]. This technique has already proven as an efficient tool for in vitro and in vivo gene delivery with high transfection efficiency [142, 143]. The high transfection efficiency of this technique is primarily due to the presence of an electric field gradient generated by placing electromagnets below the cell culture device, which results in increasing penetration of the magnetic nanocomplexes into the cells. Efficient in vivo transfection efficiency can be achieved upon intravenous administration of these magnetic nanoparticles. Under the influence of high gradient electromagnetic fields, the nanoparticles are held at a target site to release therapeutic genes via enzymatic cleavage, electrostatic interactions, or matrix degradation. Therefore, the underlying principle of magnetofection is similar to that for drug-targeted magnetic nanoparticles.
Magnetofection majorly employs the use of iron oxide particles. These iron oxide particles are usually dispersed in a polymer matrix like dextran or encapsulated in a metallic or polymeric shell [144]. Commonly, supramagnetic iron oxide particles are used due to their strong movement along the external field gradient. Polyethyleneimine is majorly used to form a shell around iron oxide particles as it can easily form an electrostatic complex with the therapeutic gene. One of the major advantages of superparamagnetic particles is that even in the absence of an external magnetic field, these nanoparticles have low chances of aggregating due to magnetic dipole interactions. Magnetofection is capable of transfecting primary cells, which is challenging to transfect with other methods [145, 146]. This technique has been investigated to treat breast cancer and other solid tumors [147, 148]. It has also been used in intradermal gene therapy for ischemic skin flaps in rats [149]. Even though magnetofection has shown promising results in various disorders, several challenges remain to overcome: low in vivo transfection efficiency, accumulation of iron oxide on multiple dosing, and rapid clearance of magnetic particles from systemic circulation upon intravenous administration. Also, particles with a size less than 50 nm or larger than 5 μm cannot be used for this technique [150]. Furthermore, the presence of blood flow in the human aorta and the reduction in magnetic flux density and gradient as distance increases from magnetic poles limit the transfection efficiency of the therapeutic gene [150].
3.1.2. Chemical methods
Chemical gene transfer methods use cationic lipids, cationic polymers, or polypeptides, condense DNA into nanosized complexes and provide adequate protection against nucleases. It also aids in the cellular uptake of DNA and its migration towards the nucleus. Here we briefly discuss various chemical-based gene delivery methods.
3.1.2.1. Cationic lipids
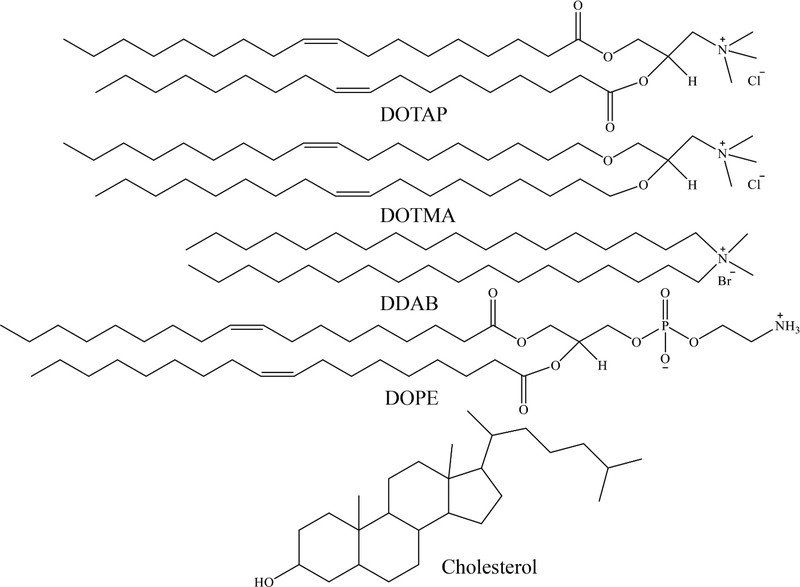
Cationic lipids represent the most frequently used alternative to viral-based gene delivery vectors. To date, numerous cationic lipids with diverse chemical structures have been investigated for gene transfer. Chemical structures of a few commonly used cationic lipids are shown in (Figure 5). However, cationic lipids used for gene delivery purposes consist of three essential components: polar headgroup, linker, and a hydrophobic tail. The cationic headgroup binds to negatively charged nucleic acids via electrostatic interaction. Although primary, secondary, tertiary, or quaternary amines are the most prevalent cationic headgroups, guanidine, imidazole, pyridinium, and phosphorus groups have also been studied. The hydrophobic tail is typically composed of aliphatic chains (saturated or unsaturated), cholesterol, or other steroid rings. The linkers between the hydrophobic domain and polar head group are usually amino, carbamate, ether, or ester bonds and often influence the stability, transfection efficiency, and biocompatibility of the lipid.
Figure 5.
Structures of frequently used lipids for gene transfer. DOTAP: 1,2-dioleoyl-3-trimethylammonium propane (chloride salt); DOTMA: 1,2-di-O-octadecenyl-3-trimethylammonium propane (chloride salt); DDAB: dimethyldidodecylammonium (bromide salt); DOPE: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine.
The charges on cationic lipids allow their complexation with the anionic nucleic acid to form lipid/DNA complexes or lipoplexes. The transfection efficiency of lipoplexes depends on several parameters, including the lipids’ structure (e.g., the number of cationic charge molecules, anchor types, or overall geometric shape), natures of co-lipids used, and lipid/DNA charge ratio [151, 152]. The cationic lipids also provide efficient protection of genetic cargos from nuclease degradation and enabling the endosomal escape of the contents [152, 153]. Moreover, the positive charges on the lipoplexes facilitate their interaction with proteoglycans of cell membranes, promoting adsorption mediated cellular uptake of the lipoplexes. Overall, cationic lipids have emerged as attractive gene carriers since they can easily synthesize, formulate, and have a relatively simple transfection procedure. Nevertheless, cationic lipids can be easily modulated to impart cell specificity and environment-specific DNA release [154, 155]. Although cationic lipids are the most efficient nonviral gene carriers, their transfection efficiency needs to be further improved to become clinically relevant.
3.1.2.2. Cationic polymers
The ability of cationic polymers to form polyelectrolyte complexes with DNA has enabled their application as gene carriers. These polymeric/DNA complexes (polyplexes) enhance the complexed gene’s hydrodynamic characteristics and offer protection against nucleases. Since these cationic polymers do not comprise any hydrophobic moieties, they are generally water-soluble [156]. These polymers can condense DNA more efficiently into smaller complexes than their lipid counterparts [157]. This characteristic is crucial for gene transfer, as smaller-sized complexes are favored for cellular internalization and subsequent gene expression. PEI, PLL, and chitosan are some of the most commonly used cationic polymers for gene therapy.
PEI has been widely investigated for in vitro and in vivo gene transfer and is considered the gold standard for nonviral gene carriers. Commercially, PEI is available as both linear or branched structures with different molecular weights. PEI comprises a very high density of amine groups (primary, secondary, or tertiary). The majority (~80%) of these amine groups remain uncharged at physiological pH and provide substantial buffering capacity over an extended pH range. It enables polyplexes to escape from endosomes efficiently, leading to improved gene expression [49]. Additionally, the higher charge density of PEI than PLL and other cationic polymers confer it with better complex formation, DNA protection, and transfection efficiency under the physiological environment. However, this high charge density is also responsible for its high cytotoxicity. Numerous parameters such as the degree of branching and molecular weight of PEI, the PEI/DNA weight ratio, and polyplex size dictates the cytotoxicity and gene expression efficiency of PEI-based formulation [158]. The non-biodegradable nature and high toxicity of this polymer is the major obstacle for its clinical applications. Various approaches such as the use of amino acid-conjugated low molecular weight PEI [159], linking targeting ligands to PEI [160–162], steric stabilization of PEI with inert polymers such as dextran [163], polyethylene glycol (PEG) [164, 165], pluronic triblock polymers [166], or PLL [167] have been utilized to improve transfection efficiency while reducing its cytotoxicity. Similarly, conjugation of lithocholic acid with PEI demonstrated improved gene transfection efficacy and safety [168].
PLL, a polypeptide of L-lysine, is another cationic polymer commonly used for in vitro and in vivo gene delivery [169, 170]. It is biodegradable and produced from the polymerization of N-carboxyanhydride of lysine. However, the PLL-based polymers are less efficient than PEI, primarily due to their inability to facilitate the endolysosomal escape of the genetic cargo [171]. The use of high molecular weight PLL results in higher DNA condensation and subsequent gene transfection. However, it is also associated with higher cytotoxicity. Therefore, numerous strategies are employed to enhance transfection and reduce the toxicity of PLL, such as the incorporation of buffering moieties to the PLL backbone [172, 173], design of PEG conjugated PLL [174], and addition of targeting ligand [175]. In a recent study, agmatine-grafted bioreducible PLL polymer has been used for highly efficient gene delivery with low cytotoxicity [176]. SPION-loaded PLL/hyaluronic acid micelles have also been explored as a magnetic resonance imaging and gene delivery carrier for cancer theranostics [177].
Chitosan is a natural polysaccharide of D-glucosamine and β-(1–4)-linked N-acetyl-D-glucosamine residues [178]. It is synthesized from the deacetylation of chitin at alkaline pH. Chitin is obtained from the exoskeleton of crustaceans (e.g., shrimp, crab, and lobster) and the cell wall of fungi [179–181]. The relative percentage of D-glucosamine units in a chitosan molecule is denoted as the degree of deacetylation (DDA). In general, the DDA of chitosan varies between 60–100%. The pKa of primary amino groups of chitosan is ~6.5, enabling their protonation at pH ≤ 6.5. Chitosan being positively charged can form polyelectrolyte complexes with genetic materials and imparts improved stability by protecting them from enzymatic degradation [182, 183]. Several factors, such as molecular weight and DDA of chitosan, pH of the transfection medium, and N/P ratio (chitosan amines: nucleic acid phosphates), majorly influence the stability and transfection efficiency of these complexes.
Over the last few years, chitosan and its derivatives have been used widely to transfer genetic materials due to their excellent safety profile and biodegradability. However, the gene delivery potential of unmodified chitosan is inadequate and clinically irrelevant. Thus, structural amendments of chitosan with various chemical moieties, including lipids [184], fatty acids [185], and cell-penetrating peptides [186], have been tried to enhance its transfection ability. Furthermore, chitosan-based polymers have also functionalized with numerous ligands to impart target specificity [39, 183].
3.1.2.3. Dendrimer-based vectors
Dendrimers are a class of nanoscale, radially symmetric three-dimensional macromolecules with a well-defined, homogenous, tree-like structure consisting of a central core, inner branches, and functional surface groups [187, 188]. PAMAM, polypropylenimine (PPI), PLL, triazine, phosphorus, carbosilane, and viologen dendrimers have been explored as gene carriers [189]. PAMAM and PPI are the most frequently used cationic dendrimers owing to their high gene transfection efficiency and low cytotoxicity [190]. The presence of free amine groups on cationic dendrimers’ surface allows them to efficiently condense nucleic acids, protect them from enzymatic degradation, and increase their internalization via adsorption mediated endocytosis [189]. Numerous tertiary amine groups in the core of these dendrimers confer strong pH buffering capacity (pKa ~6.0), facilitating the endosomal escape of DNA-dendrimer polyplexes through the proton-sponge mechanism [191]. Different alterations to PAMAM dendrimer structures have been investigated to reduce cytotoxicity, optimize complex formation with nucleic acids and endosomal release, and promote cell binding and targeting [192, 193]. For instance, histidine and arginine were conjugated to PAMAM generation 2 dendrimer to enhance its gene delivery efficiency while reducing cytotoxicity [194]. PPI-based dendrimers consist of basic primary amine groups on their surface, which form polyplexes with DNA, and comparatively acidic core with tertiary amine groups available to act as a proton sponge [195]. Like PAMAM dendrimers, various surface functionalities have been investigated to overcome limitations by improving cytocompatibility, cellular transfection, and cell-specific targeting of PPI dendrimers [196–198].
3.1.2.4. Polypeptide-based vectors
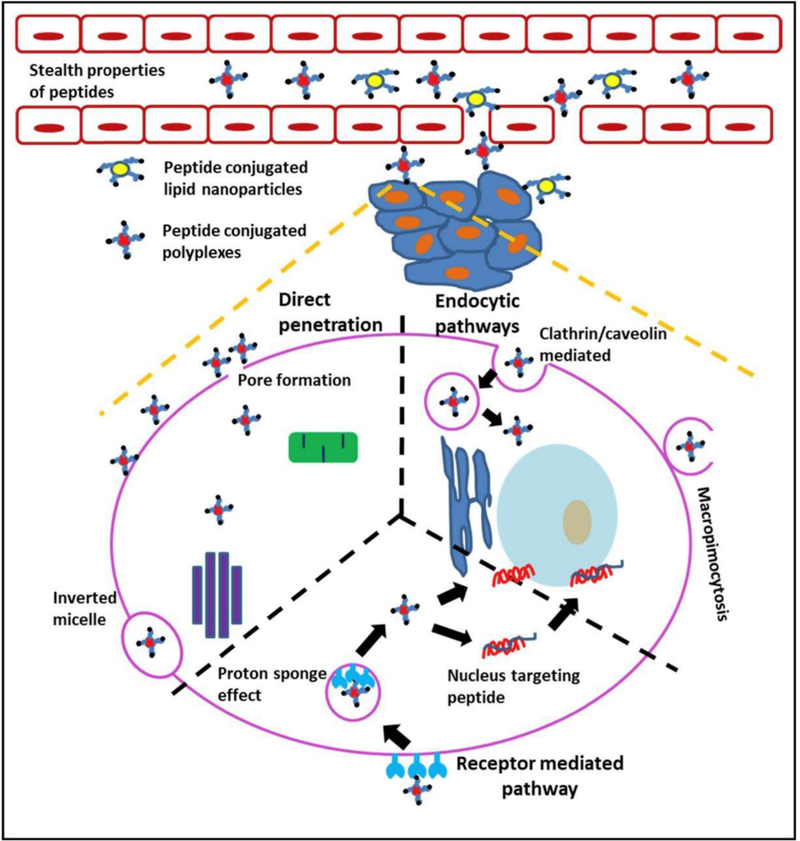
Peptide-nucleic acid conjugates have been shown to deliver DNA and siRNA with high transfection efficacy alongside cellular targeting [199]. Most peptide-based vectors contain short basic amino acid sequences termed as protein transduction domains or cell-penetrating peptides, which are usually lysine-rich such as model amphipathic peptide (MAP), MPG, transportan peptide, or arginine-rich such as Antennapedia homeodomain peptide, trans-activating transcriptional activators (TAT) peptide [200]. These peptides can either be directly covalently conjugated to oligonucleotide sequences [199, 201] or attached to nanocarriers, forming electrostatic polyplexes with nucleic acid [202]. Various researchers have investigated the mechanisms of cellular uptake for these polypeptide-DNA complexes. Endocytosis by caveolae-dependent for TAT-DNA conjugates, micropinocytosis for transportan-DNA complexes, direct insertion into cellular transmembrane by MPG-like peptides, and energy-dependent pathway for Antp-DNA complexes have been reported (Figure 6) [190].
Figure 6.
Schematic representation of pathways for peptide-mediated penetration of biological barriers to address key issues in non-viral gene carriers. Peptides can provide stealth properties to prevent opsonization while in circulation. Furthermore, peptides can facilitate the penetration of non-viral carriers across various cellular and sub-cellular barriers. Peptide-mediated transport mechanisms across the plasma membrane include uptake through endocytosis or macropinocytosis (right); active uptake through receptor-mediated endocytosis (bottom); and pore formation (left). Some peptides can mediate transcytosis through sequential endocytic uptake, subcellular transport, and exocytosis. Within cells, peptides can facilitate endosomal escape or nuclear targeting, leading to increased RNA delivery or gene transfection. (Reprint from R.K. Thapa, M.O. Sullivan, Gene delivery by peptide-assisted transport, Current Opinion in Biomedical Engineering 7 (2018) 71–82 under the terms of the Creative Commons CC BY license).
Furthermore, amino acid alterations, mutations, and conjugation to other peptides like RGD or mu (μ) have been investigated to achieve improved cellular uptake and gene transfection efficiency of such peptide-based vectors [203–205]. Although peptide-based vectors allow high cell specificity and uptake, the primary factor limiting gene transfection is inefficient endosomal release. Conjugation to basic amino acid residues like cysteine or histidine or cationic polymers like lipofectamine and PEI have been reported to increase gene transfection efficacy using such vectors [203, 204, 206].
3.1.2.5. Inorganic, polymeric, and lipid nanoparticles
Remarkable progress in nanotechnology has made nanoparticle-mediated gene delivery an attractive strategy for the treatment of genetic disorders. The incorporation of nucleic acids (plasmids DNA, mRNA, siRNA) into functionalized nanoparticles has been continuously investigated to deliver genes selectively to tissues and cells [207]. Nanoparticles can be easily tuned to obtain desirable properties, including biodegradability, biocompatibility, non-immunogenicity, high payload carrying capacity alongside suitable cellular internalization and transfection. Various types of nanoparticles have been evaluated as vectors for gene delivery, such as inorganic nanoparticles like carbon nanotubes [208], quantum dots [209], magnetic nanoparticles [210], gold nanoparticles [211], and silica nanoparticles [212], polymer-based nanoparticles [213, 214], and lipid-based nanoparticles [215].
Quantum dots can be covalently conjugated to plasmid DNA for gene delivery [216]. They provide the simultaneous benefit of labeling and tracking nanoparticles in vivo attributed to their function as an efficient fluorescent probe [216]. However, chemical modifications to DNA can negatively affect transfection efficiency. Carbon nanotubes are cylindrical graphene structures with unique physicochemical properties. They have been investigated for gene therapy due to their small size and chemical inertness. However, covalent or non-covalent surface functionalization is necessary to make them suitable for biological application [207]. Magnetic nanoparticles prepared from iron oxides can be used for magnetic resonance imaging-assisted tracking [207]. They can also be coated with natural (e.g., proteins, carbohydrates) or synthetic polymers (e.g., PEI) for enhancing DNA complexation and cellular uptake [217]. Gold and silica-based nanoparticles are attractive owing to their robust stability, inertness, and low cellular toxicity. These nanoparticles are often modified with cationic functional groups or polymers to modulate their function and effectiveness [212, 218].
Lipid-based nanoparticles such as SLNs and NLCs are an extensively used category of nonviral gene delivery strategies. NLCs differ from SLNs in that the composition of NLCs is a mixture of solid and liquid lipids [219]. They are well recognized for their ability to be easily processed and stored in a stable form, protect the genetic material from enzymatic degradation, and be functionalized to improve cell-specific targeting, internalization, and gene transfection [220, 221]. SLN and liposomes prepared using the same cationic lipids showed equipotent transfection efficiencies [222]. Asasutjarit et al. reported that cationic SLN formed electrostatic complexes with plasmid DNA and demonstrated higher transfection efficiency than naked DNA, although significantly lower than commercially available transfection reagent Fugene [223]. Gene delivery potential of lipid-based nanoparticles can further be improved by functionalization with targeting ligands and pH-sensitive lipids to enhance cellular internalization and endosomal escape, respectively [220, 224].
Polymer-based nanoparticles prepared from natural or synthetic polymers have been widely investigated for gene delivery due to the versatility of physicochemical properties through variation in structure, molecular weight, nature of the monomers, and complete entrapment of genetic material, unlike lipoplexes and polyplexes [190, 207, 225]. Natural polymers, including cyclodextrins and lignin, have been investigated for pDNA and siRNA delivery, with and without modifications by different groups [226–228]. Chen et al. demonstrated amine-functionalized cationic polylactides to possess superior transfection ability compared to FuGENE 6 [229]. Similar to lipid-based nanoparticles, they offer the advantages of surface functionalization, efficient internalization, endosomal escape, and low cell toxicity and immunogenicity [230]. Additionally, polymeric nanoparticles have been shown to possess excellent stability under physiological conditions making them an attractive candidate for oral gene delivery.
3.1.2.6. Gemini Surfactants
Gemini surfactants are a relatively new class of amphiphiles with a general structure of two surfactant monomers connected by a rigid spacer group [231]. Typically, they consist of two hydrophilic cationic head groups, two hydrophobic tails, and a linker between head groups. This distinct structure enables gemini surfactants to bind and condense nucleic acid and subsequently facilitate their cellular uptake [232, 233]. In a study, Cardoso et al. [234] demonstrated successful mitochondrial delivery of plasmid DNA in HeLa cells using conventional bis-quaternary gemini surfactant 14–2–14 (A) and serine-derived bis-quaternary gemini surfactants (nSer) 2N5 (n = 14 and 16) in combination with the helper lipids DOPE and cholesterol. Furthermore, the transfection efficiency and toxicity of gemini surfactants can be significantly modulated by varying the head group, spacer, and length of the hydrophobic tail. For instance, the use of bis-transfection efficiency of gemini surfactants [235, 236]. Also, unsaturated alkyl tails increase the transfection efficiency of the pyridinium-based gemini surfactant [235]. At the same time, amino acid moieties have been attached to the spacer region to improve biocompatibility and transfection efficiencies [237]. A recent review by Damen et al. [238] details the physicochemical and transfection properties of gemini surfactants.
Table 2 represents a brief list of chemical vectors used in gene delivery.
Table 2.
Chemical vectors used for nonviral gene delivery
| Delivery system | Nucleic acid | Cells/Model | Effects | Reference |
|---|---|---|---|---|
| Cationic lipid-based | ||||
| DOTMA-based ether and DOTAP-based ester trichain | gWIZ-luciferase plasmid | B104 cells | • The third hydrophobic chain of at least 12 carbon had a substantial influence on lipid packing in the presence of DNA • Transfection efficacies of trichain lipids were found to be higher than dichain analogs |
[239] |
| Cationic liposomes composed of DOTAP, DOPE and Cholesterol (3:4:3 molar ratio) | CYP1A1 siRNA | In vitro: A549 cells In vivo: A549 tumor-bearing BALB/c nude mice |
• Downregulation of CYP1A1 mRNA, protein, and enzymatic activity • Reduced in vivo tumor growth upon silencing of CYP1A1 gene |
[240] |
| PEGylated cationic liposomes (PCL) composed of DOPE: POPC: Cholesterol: DC-6-14 (3:2:3:2 molar ratio) | pEGFP-N1 or siGFP | Male BALB/c mice (healthy mice), male BALB/c nude mice (T cell-deficient mice), and female MRL/lpr mice (SLE-prone mice) | • The intravenous injection of PCL-associated nucleic acids (pDNA or siRNA) induced anti-nucleic acids (IgM) in healthy mice but did not cause SLE and did not trigger lupus nephritis • PCL-associated nucleic acids might exacerbate SLE symptoms in SLE-prone mice with pre-existing anti-nuclear antibodies |
[241] |
| Cationic polymer-based | ||||
| Poly(3-hydroxybutyrate) (PHB)-co-PEI nanoparticles | miR-128-encoding plasmid | U87 cells | • Adequate protection of plasmid DNA against serum nucleases • Efficiently transfected U87 cells and initiated apoptosis |
[242] |
| Low molecular weight PEI conjugated guar gum (GG-g-LPEI) | EGFP-N1 plasmid DNA | MDA-MB-231 and HeLa cells | • The GG-g-LPEI polymer displayed excellent biocompatibility and blood compatibility • The GG-g-LPEI polymer demonstrated high in vitro transfection efficiency in MDA-MB-231 cells |
[243] |
| PLL-g-PEG polymer | HIF-1α plasmid | Healthy and diabetic rats | • PLL-g-PEG polymer effectively condensed plasmid DNA • PLL-g-PEG mediated HIF-1α delivery led to differential gene expression in vivo and initiated angiogenesis in cutaneous wounds |
[170] |
| Methylated 4-N,N dimethyl aminobenzyl N,O carboxymethyl chitosan (MABCC), thiolated trimethyl chitosan (TMC-Cys), and thiolated trimethyl aminobenzyl chitosan (MABC-Cys) | EGFP-N1 plasmid | HEK-293T, SKOV-3, and MCF-7 cells | • All three polymers effectively condensed plasmid DNA at N/P ≥2, and polyplexes had negligible cytotoxicity • MABC-Cys exhibited the maximum gene expression in HEK-293T cells, while TMC-Cys was most effective in SKOV-3 and MCF-7 cells |
[244] |
| Linoleic acid and penetratin dual-functionalized chitosan (CS-Lin-Pen) | gWiz-βGal and gWiz-GFP plasmids | HEK293, CHO, and HeLa cells | • Enhanced DNA protection, cell uptake, and endosomal escape • Improved gene transfection |
[245] |
| Folate-conjugated amphiphilic cationic methoxy- poly(ethylene glycol)- b- poly(ε-caprolactone)- b- poly(ethylene imine) copolymer (MPEG–PCL–PEI-FA) | pLuc and Nur77 gene | HEK293 and HeLa/Bcl-2 cells | • MPEG-PCL-PEI-FA copolymer showed lower cytotoxicity and better gene transfection efficiency than PEI • Exhibited effective growth inhibition of therapeutic resistant HeLa/Bcl-2 cancer cells |
[246] |
| Thiolated trimethylated chitosan/pDNA/folate conjugated cis-aconitic amide-PEI ternary complex (FA-PEI-AcO/TMC-SS/pDNA) | pEGFP | HeLa cells | • TMC-SS acquired good pDNA condensation and redox-responsive pDNA release. • FA-PEI-AcO/TMC-SS/pDNA demonstrated lowered cytotoxicity and enhanced gene transfection than TMC-SS/pDNA polyplexes in folate receptor positive tumor cells |
[247] |
| TAT-functionalized PEI-grafting rice bran polysaccharides (PRBP-TAT) | EGFP plasmid | HEK293T, BRL, and HepG2 cells | • Effective DNA condensation and protection at low N/P ratio (i.e., 1–3.5) • PRBP-TAT showed lower cytotoxicity and higher gene transfection efficiency than PEI |
[80] |
| Hydroxyl-rich poly (glycidyl methacrylate) (PGMA)-based cationic glycopolymers | EGFR-siRNA | HeLa cells | • The optimized formulation showed ∼52% knockdown efficiency • The polymer was found to be cytocompatible |
[248] |
| Dendrimer-based | ||||
| G5 PAMAM dendrimers, and L-PEI | GFP plasmid | HEK 293A cells | • pDNA loaded in cells using PAMAM dendrimer and endosomal release was mediated by L-PEI • The addition of L-PEI led to a 10-fold higher gene expression |
[249] |
| Coumarin-anchored PAMAM dendrimers | TRAIL plasmid | MDA-MB-231 and HEK293 cells | • Light-responsive drug release behavior and light-enhanced anticancer activity • TRAIL plasmid and 5-Fu combination therapy resulted in improved anticancer activity |
[250] |
| Polypeptide-based | ||||
| Tat–RGD peptide | Fibroblasts (FB) and smooth muscle cells (SMC) derived from pulmonary arteries and A549 cells | • The improvement of DNA internalization by Tat–RGD polymer was primarily mediated by caveolae-dependent endocytosis • Efficient gene delivery to human pulmonary cells |
[204] | |
| Inorganic nanoparticles | ||||
| Carbon quantum dots (CQDs) | TGFβ1 plasmid | 3T6 cells | • CQDs played dual roles as gene vectors and bioimaging probes at the same time. • CQDs displayed strong DNA condensing capacity, good biocompatibility, and high transfection efficiency |
[251] |
| Gold nanoparticles | pCMVp53 plasmid | In vitro: SKOV-3 cells In vivo: SKOV-3 tumor xenograft mouse model |
• The nanoparticle/pDNA complex was serum-stable and protected complexed pDNA from DNase-I digestion. • The nanoparticle/pDNA complex demonstrated considerable tumor targeting and tumor regression |
[252] |
| Zwitterion-functionalized dendrimer-entrapped gold nanoparticles | pGFP and pLuc | HeLa cells | • Compaction of pDNA to form polyplexes • Effectively transfected cancer cells at an N/P ratio of 4 |
[253] |
| Lipid nanoparticles | ||||
| iRGD functionalized solid lipid nanoparticle | siPDL1 and siEGFR | In vitro: U87 and GL261 cells In vivo: orthotopic glioblastoma (GL261) xenograft mouse model |
• Nanoparticles provided efficient protection of siRNAs against the harsh biological environment • Significant tumor inhibition led to prolonged mouse survival |
[254] |
DOTMA: 1,2-di-O-octadecenyl-3-trimethylammonium propane; DOTAP: 1,2-dioleoyl-3-trimethylammonium propane; DOPE: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; POPC: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; DC-6-14: O,O′-ditetradecanoyl-N-(alpha-trimethylammonio acetyl) diethanolamine chloride; SLE; systemic lupus erythematosus.
4. Nonviral vectors used in clinical trials
Numerous nonviral gene delivery approaches have been explored to enhance the transfer of nucleic acids to the target site for achieving clinically relevant protein expressions. Polymeric and lipid nanoparticles remain popular as nonviral vectors and have made substantial advancements for delivering various types of genetic materials to treat a diverse range of disorders [255]. This fact can be validated by the sheer number of these vectors under clinical trials (Tables 3 and 4). Moreover, due to the recent COVID-19 pandemic, these advancements have been accelerated tremendously, resulting in the approval of nonviral gene therapies against severe acute respiratory syndrome coronavirus-2 [256]. It resulted in the emergency approval of the first mRNA-based vaccines developed by BioNtech/Pfizer and Moderna therapeutics. The vaccine developed by Moderna Therapeutics (mRNA- 1273) is a lipid nanoparticle-based formulation consisting of one proprietary (SM-102) and three commercially available (DSPC, cholesterol, and PEG2000- DMG) lipids [257]. On the other hand, BNT162b2 vaccine developed by BioNtech/Pfizer used 4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis (2-hexyldecanoate), 2 [(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, DSPC, and cholesterol [258]. These vaccines claimed efficacy over 94% due to their robust cellular response and higher antibody titers [256]. Moreover, these nonviral vectors are versatile and scalable and can be adapted rapidly for mutations or future epidemics. Besides, mRNA delivery is considered safer than their viral counterparts due to their inability to integrate into the host genome and be non-infectious. However, long-term storage of these formulations requires subzero temperatures leading to logistic barriers for their administration and distribution worldwide. Nevertheless, these therapeutics’ development represents a huge milestone for biotechnology and nanomedicine by overcoming the translation challenges.
Table 3.
Current status of nonviral vector-based DNA therapies in clinical trials.
| Delivery system | Gene (DNA) | Delivery route | Sponsor | Indications | Development phase | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|
| Cationic liposome (DMRIE/DOPE) | Cystic fibrosis gene (pGT-1) | Nasal instillation | University of Alabama at Birmingham | Cystic fibrosis | I | NCT00004471 |
| Cationic liposome (DMRIE/DOPE) | Cystic fibrosis transmembrane conductance regulator (CFTR) gene | Intranasal | University of Alabama at Birmingham | Cystic fibrosis | I | NCT00004806 |
| DC-cholesterol liposome | EGFR antisense DNA | Intratumoral | University of Pittsburgh | Head and neck cancer | I | NCT00009841 |
| DOTAP:Cholesterol | FUS 1 | Intravenous | M.D. Anderson Cancer Center | Non-Small-Cell Lung Cancer | I | NCT00059605 |
| GL67A (a mixture of GL67–DOPE–DMPE-PEG5000) | CFTR gene | Nebulization | Imperial College London | Cystic fibrosis | II | NCT01621867 |
| DOTAP: DOPE cationic liposome | Normal human wild type (wt) p53 DNA | Intravenous | SynerGene Therapeutics, Inc. | Solid tumors | I | NCT00470613 |
| DOTAP: DOPE cationic liposome | Normal human wild type p53 DNA | Intravenous | SynerGene Therapeutics, Inc. | Metastatic pancreatic cancer | II | NCT02340117 |
| DOTAP: DOPE cationic liposome | Normal human wild type (wt) p53 DNA | Intravenous | SynerGene Therapeutics, Inc. | Refractory or recurrent solid tumors | I | NCT02354547 |
| PEI | DTA-H19 | Intravesical | Hebrew University of Jerusalem | Bladder neoplasms | I/II | NCT00393809 |
| PEI | DTA-H19 | Intravesical | BioCancell Ltd. | Superficial bladder cancer | II | NCT00595088 |
| PEI | BC-819 BCG vaccine |
Intravesical | BioCancell Ltd. | Transitional cell carcinoma of bladder | I | NCT01878188 |
| PEI | HIV DNA Vaccine | Intradermal | Swedish Institute for Infectious Disease Control | HIV-1 | I | NCT01140139 |
| PEG-PEI-Cholesterol | EGEN-001-301 | Intraperitoneal | EGEN, Inc. | Colorectal cancer | I/II | NCT01300858 |
| PEG-PEI-Cholesterol | EGEN-001 (IL-12 DNA Plasmid Vector GEN-1) |
Intraperitoneal | Gynecologic Oncology Group | Fallopian tube, primary peritoneal, and recurrent ovarian carcinoma | II | NCT01118052 |
| PEG-PEI-Cholesterol | EGEN-001 (IL-12 DNA Plasmid Vector GEN-1) |
Intraperitoneal | Gynecologic Oncology Group | Ovarian, recurrent ovarian, undifferentiated ovarian, recurrent fallopian tube, and recurrent primary peritoneal cancer | I | NCT01489371 |
| PEG-PEI-Cholesterol | GEN-1 (IL-12 Plasmid) | Intraperitoneal | Celsion | Epithelial ovarian, fallopian tube, and primary peritoneal cancer | I | NCT02480374 |
| Poloxamer CRL1005/benzalkonium chloride | ASP0113 | Intramuscular | Astellas Pharma Global Development, Inc. | Cytomegalovirus (CMV) therapeutic vaccine | III | NCT01877655 |
| Poloxamer CRL1005/benzalkonium chloride | VCL-CB01 | Intramuscular | Astellas Pharma Inc. | CMV therapeutic vaccine | II | NCT00285259 |
Table 4.
Current status of nonviral vector-based RNA therapies in clinical trials.
| Delivery system | Intervention | RNA | Target gene | Delivery route | Sponsor | Indications | Development phase | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|---|---|
| Naked siRNA | ALN-RSV01 | siRNA | Nucleocapsid gene of RSV | Nebulization | Alnylam Pharmaceuticals | RSV infections | II | NCT00658086 |
| Lipid nanoparticles | TKM-080301 | siRNA | Polo-like kinase 1 (PLK1) | Hepatic intra-arterial | National Cancer Institute | Primary or secondary liver cancer | I | NCT01437007 |
| Lipid nanoparticles | TKM-080301 | siRNA | PLK1 | Intravenous | Arbutus Biopharma Corporation | Neuroendocrine tumors, adrenocortical carcinoma | I/II | NCT01262235 |
| Lipid nanoparticles | TKM-080301 | siRNA | PLK1 | Intravenous | Arbutus Biopharma Corporation | Advanced hepatocellular carcinoma | I/II | NCT02191878 |
| DOPC liposomes | siRNA-EphA2-DOPC | siRNA-EphA2 | Ephrin type-A receptor 2 (EphA2) | Intravenous | M.D. Anderson Cancer Center | Advanced Cancers | I | NCT01591356 |
| Lipid-based formulation (Cationic lipid AtuFECT, fusogenic lipid, PEG-LIPID) | Atu027 + gemcitabine | siRNA (Atu027) | Protein kinase N3 (PKN3) | Intravenous | Silence Therapeutics GmbH | Advanced or metastatic pancreatic cancer | I/II | NCT01808638 |
| PEI | RGT100-PEI | RGT100 | RIG-I | Intratumorally/intralesionally | Rigontec GMBH | Advanced Solid Tumors | I/II | NCT03065023 |
| Vitamin A-coupled lipid nanoparticle | ND-L02-s0201 | siRNA | Serpin peptidase inhibitor clade H member 1 (SERPINH1) (Hsp47) | Intravenous | Bristol-Myers Squibb | Moderate to extensive hepatic fibrosis | I | NCT02227459 |
| EnCore lipid nanoparticles | DCR-MYC | siRNA | MYC | Intravenous | Dicerna Pharmaceuticals, Inc. | Solid tumors, multiple myeloma, or Non-Hodgkins lymphoma | I | NCT02110563 |
| EnCore lipid nanoparticles | DCR-MYC | siRNA | MYC | Intravenous | Dicerna Pharmaceuticals, Inc. | Hepatocellular carcinoma | I/II | NCT02314052 |
| PLGA matrix | siG12D LODER | siRNA | G12D-mutated KRAS | Intratumoral implant | Silenseed Ltd | Unresectable locally advanced pancreatic cancer | I | NCT01188785 |
| PLGA matrix | siG12D-LODER | siRNA | G12D-mutated KRAS | Intratumoral implant | Silenseed Ltd | Unresectable locally advanced pancreatic cancer | II | NCT01676259 |
5. Conclusion and future perspective
Genes are the blueprint for proteins that serve as building blocks for tissue and crucial regulators of biochemical reactions inside all living cells. Thus, genetic defects can often disrupt the expression of essential proteins resulting in various diseases. While conventional therapies can only alleviate disease symptoms, gene therapy has the potential to eradicate the underlying cause by repairing or replacing the genetic code of the patients. However, the transfer of genetic materials to the target cells is challenging and often required specialized delivery systems or techniques. The current gene delivery methods are broadly classified as viral and nonviral systems. The viral gene carriers include retrovirus, lentivirus, adenovirus, adeno-associated virus, poxvirus, and herpes simplex virus. The nonviral gene transfer can be achieved through various physical methods (e.g., gene gun, electroporation, sonoporation, hydrodynamic injection, microneedles, and magnetofection) or chemical methods (e.g., cationic lipids, cationic polymers, dendrimers, polypeptides, and nanoparticles of various compositions). Recently, nonviral gene delivery systems have been increasingly utilized in gene delivery as an alternative to viral vectors due to their superior safety profile and ease of development. Although nonviral vectors’ transfection ability has improved significantly over the years, they still lack a lot compared to their highly efficient viral counterparts. Currently, viral vectors are prevailing in clinical trials, and they consist of more than two-thirds of the total gene therapy protocols. However, there has been a gradual reduction in these gaps with continuous improvement in nonviral approaches due to the better understanding of their limitations in vivo.
There are multiple ways to improve the transfection potential of nonviral gene delivery systems ranging from exploring the topology of genetic material being transferred to changing the composition of the delivery system. The topology of the genetic material (e.g., linear, open circular, and supercoiled) plays a significant role in gene expression. For example, it has been shown that the supercoiled and circular form of the DNA induced superior transgene expression compared to their linear counterpart [259, 260]. However, the exact mechanism by which the DNA topology affects overall gene expression is poorly understood. Nonetheless, supercoiled DNA is least susceptible to intracellular degradation [260], and the smaller size of supercoiled DNA is believed to be responsible for their improved intracellular mobility to reach the nucleus [260–262]. Additionally, promoters used in the plasmid design should be chosen carefully according to the cell line being targeted. For instance, human cytomegalovirus (CMV) demonstrates poor transgene expression in stem cells but showed high transfection efficiency in various immortalized cell lines [263, 264].
The design of safe and efficient delivery systems is crucial for the desired level of gene expression. Over the decades, tremendous efforts have been made to design and engineering polymers and lipids to overcome major barriers to gene therapy. However, few features of these moieties remain constant for achieving high transfection efficiencies, such as high cationic charge, good buffering capacity, and low cytotoxicity. On the other hand, there has not been a consensus on the influence of the molecular weight on transfection efficiency since various researchers have demonstrated conflicting results [265, 266]. Additionally, the N/P ratio, composition of the complexation buffer, and incubation period should be evaluated thoroughly to achieve the best transfection. Recently, lipid and polymer mixtures have also been used to achieve superior transfection efficacy [63, 267].
Highlights:
Gene therapy provides curative treatment of genetic disorders and many other diseases
Nonviral gene delivery vectors can overcome the safety concerns of viral vectors
Lipid and polymer-based carriers have been extensively used for targeted gene therapy
Development of nonviral COVID-19 mRNA vaccines have augmented nonviral gene delivery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Culver KW, Gene Therapy. A Primer For Physicians, 2nd Edition ed., Mary Ann Liebert, Larchmont, NY, 1996. [Google Scholar]
- [2].Winn SR, Hu Y, Sfeir C, Hollinger JO, Gene therapy approaches for modulating bone regeneration, Advanced Drug Delivery Reviews 42(1) (2000) 121–138. [DOI] [PubMed] [Google Scholar]
- [3].Howell TH, Fiorellini J, Jones A, Alder M, Nummikoski P, Lazaro M, Lilly L, Cochran D, A feasibility study evaluating rhBMP-2/absorbable collagen sponge device for local alveolar ridge preservation or augmentation, Int J Periodontics Restorative Dent 17(2) (1997) 124–139. [PubMed] [Google Scholar]
- [4].Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, Shearer G, Chang L, Chiang Y, Tolstoshev P, Greenblatt JJ, Rosenberg SA, Klein H, Berger M, Mullen CA, Ramsey WJ, Muul L, Morgan RA, Anderson WF, T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years, Science (New York, N.Y.) 270(5235) (1995) 475–80. [DOI] [PubMed] [Google Scholar]
- [5].Silver JN, Elder M, Conlon T, Cruz P, Wright AJ, Srivastava A, Flotte TR, Recombinant adeno-associated virus-mediated gene transfer for the potential therapy of adenosine deaminase-deficient severe combined immune deficiency, Human gene therapy 22(8) (2011) 935–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sharma A, Easow Mathew M, Sriganesh V, Reiss UM, Gene therapy for haemophilia, The Cochrane database of systematic reviews 4(4) (2020) Cd010822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yan Z, McCray PB Jr., Engelhardt JF, Advances in gene therapy for cystic fibrosis lung disease, Human molecular genetics 28(R1) (2019) R88–r94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hood L, Rowen L, The Human Genome Project: big science transforms biology and medicine, Genome Med 5(9) (2013) 79–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Buscail L, Bournet B, Vernejoul F, Cambois G, Lulka H, Hanoun N, Dufresne M, Meulle A, Vignolle-Vidoni A, Ligat L, Saint-Laurent N, Pont F, Dejean S, Gayral M, Martins F, Torrisani J, Barbey O, Gross F, Guimbaud R, Otal P, Lopez F, Tiraby G, Cordelier P, First-in-man phase 1 clinical trial of gene therapy for advanced pancreatic cancer: safety, biodistribution, and preliminary clinical findings, Molecular therapy : the journal of the American Society of Gene Therapy 23(4) (2015) 779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liang Z, Du L, Zhang E, Zhao Y, Wang W, Ma P, Dai M, Zhao Q, Xu H, Zhang S, Zhen Y, Targeted-delivery of siRNA via a polypeptide-modified liposome for the treatment of gp96 over-expressed breast cancer, Materials Science and Engineering: C 121 (2021) 111847. [DOI] [PubMed] [Google Scholar]
- [11].Liu X, Chen X, Chua MX, Li Z, Loh XJ, Wu Y-L, Injectable Supramolecular Hydrogels as Delivery Agents of Bcl-2 Conversion Gene for the Effective Shrinkage of Therapeutic Resistance Tumors, 6(11) (2017) 1700159. [DOI] [PubMed] [Google Scholar]
- [12].Liu X, Li Z, Loh XJ, Chen K, Li Z, Wu YL, Targeted and Sustained Corelease of Chemotherapeutics and Gene by Injectable Supramolecular Hydrogel for Drug-Resistant Cancer Therapy, Macromolecular rapid communications 40(5) (2019) e1800117. [DOI] [PubMed] [Google Scholar]
- [13].Deng J, Guo M, Li G, Xiao J, Gene therapy for cardiovascular diseases in China: basic research, Gene Therapy 27(7) (2020) 360–369. [DOI] [PubMed] [Google Scholar]
- [14].Wu Q-Q, Xiao Y, Liu C, Duan M, Cai Z, Xie S, Yuan Y, Wu H, Deng W, Tang Q, The protective effect of high mobility group protein HMGA2 in pressure overload-induced cardiac remodeling, Journal of Molecular and Cellular Cardiology 128 (2019) 160–178. [DOI] [PubMed] [Google Scholar]
- [15].Chen Y, Shen J, Qi G, Zha Q, Zhang C, Yao W, Gao X, Chen S, Potential therapeutic role of fibroblast growth factor 21 in neurodegeneration: Evidence for ameliorating parkinsonism via silent information regulator 2 homolog 1 and implication for gene therapy, Neuropharmacology 181 (2020) 108335. [DOI] [PubMed] [Google Scholar]
- [16].Bakay RAE, Arvanitakis Z, Tuszynski M, Potkin S, Bartus R, Bennett D, Analyses of a Phase 1 Clinical Trial of Adeno- associated Virus-Nerve Growth Factor (CERE-110) Gene Therapy in Alzheimer’s Disease866, Neurosurgery 61(1) (2007) 216–216. [Google Scholar]
- [17].Arora S, Layek B, Singh J, Design and Validation of Liposomal ApoE2 Gene Delivery System to Evade Blood–Brain Barrier for Effective Treatment of Alzheimer’s Disease, Molecular Pharmaceutics 18(2) (2021) 714–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jeon J, Kim W, Jang J, Isacson O, Seo H, Gene therapy by proteasome activator, PA28γ, improves motor coordination and proteasome function in Huntington’s disease YAC128 mice, Neuroscience 324 (2016) 20–28. [DOI] [PubMed] [Google Scholar]
- [19].Erendor F, Eksi YE, Sahin EO, Balci MK, Griffith TS, Sanlioglu S, Lentivirus Mediated Pancreatic Beta-Cell-Specific Insulin Gene Therapy for STZ-Induced Diabetes, Molecular Therapy 29(1) (2021) 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ivacik D, Ely A, Ferry N, Arbuthnot P, Sustained inhibition of hepatitis B virus replication in vivo using RNAi-activating lentiviruses, Gene therapy 22(2) (2015) 163–71. [DOI] [PubMed] [Google Scholar]
- [21].Kumar R, Nyakundi R, Kariuki T, Ozwara H, Nyamongo O, Mlambo G, Ellefsen B, Hannaman D, Kumar N, Functional evaluation of malaria Pfs25 DNA vaccine by in vivo electroporation in Olive baboons, Vaccine 31(31) (2013) 3140–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhou Z, Liu X, Zhu D, Wang Y, Zhang Z, Zhou X, Qiu N, Chen X, Shen Y, Nonviral cancer gene therapy: Delivery cascade and vector nanoproperty integration, Advanced Drug Delivery Reviews 115 (2017) 115–154. [DOI] [PubMed] [Google Scholar]
- [23].Gene Therapy Clinical Trial Databases, The Journal of Gene Medicine, 2021. [Google Scholar]
- [24].Layek B, Singh J, 8 - Chitosan for DNA and gene therapy, in: Jennings JA, Bumgardner JD (Eds.), Chitosan Based Biomaterials Volume 2, Woodhead Publishing; 2017, pp. 209–244. [Google Scholar]
- [25].Layek B, Lipp L, Singh J, Cell Penetrating Peptide Conjugated Chitosan for Enhanced Delivery of Nucleic Acid, International Journal of Molecular Sciences 16(12) (2015) 26142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kawabata K, Takakura Y, Hashida M, The fate of plasmid DNA after intravenous injection in mice: involvement of scavenger receptors in its hepatic uptake, Pharmaceutical research 12(6) (1995) 825–30. [DOI] [PubMed] [Google Scholar]
- [27].Wiethoff CM, Middaugh CR, Barriers to nonviral gene delivery, Journal of pharmaceutical sciences 92(2) (2003) 203–17. [DOI] [PubMed] [Google Scholar]
- [28].Morille M, Passirani C, Vonarbourg A, Clavreul A, Benoit JP, Progress in developing cationic vectors for non-viral systemic gene therapy against cancer, Biomaterials 29(24–25) (2008) 3477–96. [DOI] [PubMed] [Google Scholar]
- [29].Layek B, Haldar MK, Sharma G, Lipp L, Mallik S, Singh J, Hexanoic acid and polyethylene glycol double grafted amphiphilic chitosan for enhanced gene delivery: influence of hydrophobic and hydrophilic substitution degree, Molecular pharmaceutics 11(3) (2014) 982–94. [DOI] [PubMed] [Google Scholar]
- [30].Suk JS, Xu Q, Kim N, Hanes J, Ensign LM, PEGylation as a strategy for improving nanoparticle-based drug and gene delivery, Adv Drug Deliv Rev 99(Pt A) (2016) 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chan C-L, Ewert KK, Majzoub RN, Hwu Y.-k., Liang KS, Leal C, Safinya CR, Optimizing Cationic and Neutral Lipids for Efficient Gene Delivery at High Serum Content, The journal of gene medicine 16(0) (2014) 84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zylberberg C, Gaskill K, Pasley S, Matosevic S, Engineering liposomal nanoparticles for targeted gene therapy, Gene Ther 24(8) (2017) 441–452. [DOI] [PubMed] [Google Scholar]
- [33].Del Prado A, Civantos A, Martínez-Campos E, Levkin PA, Reinecke H, Gallardo A, Elvira C, Efficient and Low Cytotoxicity Gene Carriers Based on Amine-Functionalized Polyvinylpyrrolidone, Polymers 12(11) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nguyen J, Reul R, Roesler S, Dayyoub E, Schmehl T, Gessler T, Seeger W, Kissel TH, Amine-modified poly(vinyl alcohol)s as non-viral vectors for siRNA delivery: effects of the degree of amine substitution on physicochemical properties and knockdown efficiency, Pharmaceutical research 27(12) (2010) 2670–82. [DOI] [PubMed] [Google Scholar]
- [35].Takakura Y, Mahato RI, Hashida M, Extravasation of macromolecules, Adv Drug Deliv Rev 34(1) (1998) 93–108. [DOI] [PubMed] [Google Scholar]
- [36].Sendra L, Herrero MJ, Aliño SF, Translational Advances of Hydrofection by Hydrodynamic Injection, 9(3) (2018) 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cao M, Gao Y, Qiu N, Shen Y, Shen P, Folic acid directly modified low molecular weight of polyethyleneimine for targeted pDNA delivery, Journal of Drug Delivery Science and Technology 56 (2020) 101522. [Google Scholar]
- [38].Lin WJ, Lee W-C, Shieh M-J, Hyaluronic acid conjugated micelles possessing CD44 targeting potential for gene delivery, Carbohydrate Polymers 155 (2017) 101–108. [DOI] [PubMed] [Google Scholar]
- [39].Layek B, Lipp L, Singh J, APC targeted micelle for enhanced intradermal delivery of hepatitis B DNA vaccine, J Control Release 207 (2015) 143–53. [DOI] [PubMed] [Google Scholar]
- [40].Yi A, Sim D, Lee YJ, Sarangthem V, Park RW, Development of elastin-like polypeptide for targeted specific gene delivery in vivo, Journal of nanobiotechnology 18(1) (2020) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Perrone F, Craparo EF, Cemazar M, Kamensek U, Drago SE, Dapas B, Scaggiante B, Zanconati F, Bonazza D, Grassi M, Truong N, Pozzato G, Farra R, Cavallaro G, Grassi G, Targeted delivery of siRNAs against hepatocellular carcinoma-related genes by a galactosylated polyaspartamide copolymer, Journal of Controlled Release (2020). [DOI] [PubMed] [Google Scholar]
- [42].Wang Y, Xu Z, Zhang R, Li W, Yang L, Hu Q, A facile approach to construct hyaluronic acid shielding polyplexes with improved stability and reduced cytotoxicity, Colloids Surf B Biointerfaces 84(1) (2011) 259–66. [DOI] [PubMed] [Google Scholar]
- [43].Singh B, Maharjan S, Kim YK, Jiang T, Islam MA, Kang SK, Cho MH, Choi YJ, Cho CS, Targeted gene delivery via N-acetylglucosamine receptor mediated endocytosis, J Nanosci Nanotechnol 14(11) (2014) 8356–64. [DOI] [PubMed] [Google Scholar]
- [44].Layek B, Singh J, Caproic acid grafted chitosan cationic nanocomplexes for enhanced gene delivery: effect of degree of substitution, International journal of pharmaceutics 447(1–2) (2013) 182–91. [DOI] [PubMed] [Google Scholar]
- [45].El-Sayed A, Harashima H, Endocytosis of gene delivery vectors: from clathrin-dependent to lipid raft-mediated endocytosis, Molecular therapy : the journal of the American Society of Gene Therapy 21(6) (2013) 1118–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Brock DJ, Kondow-McConaghy HM, Hager EC, Pellois J-P, Endosomal Escape and Cytosolic Penetration of Macromolecules Mediated by Synthetic Delivery Agents, Bioconjug Chem 30(2) (2019) 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kamiya H, Tsuchiya H, Yamazaki J, Harashima H, Intracellular trafficking and transgene expression of viral and non-viral gene vectors, Advanced Drug Delivery Reviews 52(3) (2001) 153–164. [DOI] [PubMed] [Google Scholar]
- [48].Pei D, Buyanova M, Overcoming Endosomal Entrapment in Drug Delivery, Bioconjug Chem 30(2) (2019) 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Behr JP, The proton sponge: A trick to enter cells the viruses did not exploit, Chimia 51(1–2) (1997) 34–36. [Google Scholar]
- [50].Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL, The possible “proton sponge “ effect of polyethylenimine (PEI) does not include change in lysosomal pH, Molecular therapy : the journal of the American Society of Gene Therapy 21(1) (2013) 149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Funhoff AM, van Nostrum CF, Koning GA, Schuurmans-Nieuwenbroek NM, Crommelin DJ, Hennink WE, Endosomal escape of polymeric gene delivery complexes is not always enhanced by polymers buffering at low pH, Biomacromolecules 5(1) (2004) 32–9. [DOI] [PubMed] [Google Scholar]
- [52].Varkouhi AK, Scholte M, Storm G, Haisma HJ, Endosomal escape pathways for delivery of biologicals, J Control Release 151(3) (2011) 220–8. [DOI] [PubMed] [Google Scholar]
- [53].Ciftci K, Levy RJ, Enhanced plasmid DNA transfection with lysosomotropic agents in cultured fibroblasts, Int J Pharm 218(1–2) (2001) 81–92. [DOI] [PubMed] [Google Scholar]
- [54].Zauner W, Kichler A, Schmidt W, Sinski A, Wagner E, Glycerol enhancement of ligand-polylysine/DNA transfection, Biotechniques 20(5) (1996) 905–13. [DOI] [PubMed] [Google Scholar]
- [55].Lukacs GL, Haggie P, Seksek O, Lechardeur D, Freedman N, Verkman AS, Size-dependent DNA mobility in cytoplasm and nucleus, J Biol Chem 275(3) (2000) 1625–9. [DOI] [PubMed] [Google Scholar]
- [56].Lechardeur D, Sohn KJ, Haardt M, Joshi PB, Monck M, Graham RW, Beatty B, Squire J, O’Brodovich H, Lukacs GL, Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer, Gene Ther 6(4) (1999) 482–97. [DOI] [PubMed] [Google Scholar]
- [57].Bai H, Lester GMS, Petishnok LC, Dean DA, Cytoplasmic transport and nuclear import of plasmid DNA, Biosci Rep 37(6) (2017) BSR20160616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Vaughan EE, Dean DA, Intracellular trafficking of plasmids during transfection is mediated by microtubules, Molecular therapy : the journal of the American Society of Gene Therapy 13(2) (2006) 422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Capecchi MR, High efficiency transformation by direct microinjection of DNA into cultured mammalian cells, Cell 22(2, Part 2) (1980) 479–488. [DOI] [PubMed] [Google Scholar]
- [60].Bastos R, Pante N, Burke B, Nuclear pore complex proteins, International review of cytology 162B (1995) 257–302. [DOI] [PubMed] [Google Scholar]
- [61].Dean DA, Import of Plasmid DNA into the Nucleus Is Sequence Specific, Experimental Cell Research 230(2) (1997) 293–302. [DOI] [PubMed] [Google Scholar]
- [62].Zuhorn IS, Bakowsky U, Polushkin E, Visser WH, Stuart MC, Engberts JB, Hoekstra D, Nonbilayer phase of lipoplex-membrane mixture determines endosomal escape of genetic cargo and transfection efficiency, Molecular therapy : the journal of the American Society of Gene Therapy 11(5) (2005) 801–10. [DOI] [PubMed] [Google Scholar]
- [63].Gigante A, Li M, Junghänel S, Hirschhäuser C, Knauer S, Schmuck C, Non-viral transfection vectors: are hybrid materials the way forward?, MedChemComm 10(10) (2019) 1692–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Miyata K, Kakizawa Y, Nishiyama N, Harada A, Yamasaki Y, Koyama H, Kataoka K, Block Catiomer Polyplexes with Regulated Densities of Charge and Disulfide Cross-Linking Directed To Enhance Gene Expression, Journal of the American Chemical Society 126(8) (2004) 2355–2361. [DOI] [PubMed] [Google Scholar]
- [65].Wan L, Manickam DS, Oupický D, Mao G, DNA Release Dynamics from Reducible Polyplexes by Atomic Force Microscopy, Langmuir 24(21) (2008) 12474–12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Choi S, Lee K-D, Enhanced gene delivery using disulfide-crosslinked low molecular weight polyethylenimine with listeriolysin o-polyethylenimine disulfide conjugate, Journal of Controlled Release 131(1) (2008) 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Takae S, Miyata K, Oba M, Ishii T, Nishiyama N, Itaka K, Yamasaki Y, Koyama H, Kataoka K, PEG-Detachable Polyplex Micelles Based on Disulfide-Linked Block Catiomers as Bioresponsive Nonviral Gene Vectors, Journal of the American Chemical Society 130(18) (2008) 6001–6009. [DOI] [PubMed] [Google Scholar]
- [68].Zhang H, Vinogradov SV, Short biodegradable polyamines for gene delivery and transfection of brain capillary endothelial cells, J Control Release 143(3) (2010) 359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sun W, Davis PB, Reducible DNA nanoparticles enhance in vitro gene transfer via an extracellular mechanism, J Control Release 146(1) (2010) 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Liu X, Xiang J, Zhu D, Jiang L, Zhou Z, Tang J, Liu X, Huang Y, Shen Y, Fusogenic Reactive Oxygen Species Triggered Charge-Reversal Vector for Effective Gene Delivery, 28(9) (2016) 1743–1752. [DOI] [PubMed] [Google Scholar]
- [71].Zhu D, Yan H, Liu X, Xiang J, Zhou Z, Tang J, Liu X, Shen Y, Intracellularly Disintegratable Polysulfoniums for Efficient Gene Delivery, 27(16) (2017) 1606826. [Google Scholar]
- [72].Thomas CE, Ehrhardt A, Kay MA, Progress and problems with the use of viral vectors for gene therapy, Nat Rev Genet 4(5) (2003) 346–58. [DOI] [PubMed] [Google Scholar]
- [73].Shirley JL, de Jong YP, Terhorst C, Herzog RW, Immune Responses to Viral Gene Therapy Vectors, Molecular therapy : the journal of the American Society of Gene Therapy 28(3) (2020) 709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kotterman MA, Chalberg TW, Schaffer DV, Viral Vectors for Gene Therapy: Translational and Clinical Outlook, Annu Rev Biomed Eng 17 (2015) 63–89. [DOI] [PubMed] [Google Scholar]
- [75].Yi Y, Jong Noh M, Hee Lee K, Current Advances in Retroviral Gene Therapy, Current Gene Therapy 11(3) (2011) 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Naso MF, Tomkowicz B, Perry WL, Strohl WR, Adeno-Associated Virus (AAV) as a Vector for Gene Therapy, Biodrugs 31(4) (2017) 317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ke L, Cai P, Wu Y-L, Chen X, Polymeric Nonviral Gene Delivery Systems for Cancer Immunotherapy, 3(6) (2020) 1900213. [Google Scholar]
- [78].Xu C, Wu Y-L, Li Z, Loh XJ, Cyclodextrin-based sustained gene release systems: a supramolecular solution towards clinical applications, Materials Chemistry Frontiers 3(2) (2019) 181–192. [Google Scholar]
- [79].Jinturkar KA, Rathi MN, Misra A, 3 - Gene Delivery Using Physical Methods, in: Misra A (Ed.), Challenges in Delivery of Therapeutic Genomics and Proteomics, Elsevier, London, 2011, pp. 83–126. [Google Scholar]
- [80].Liu L, Yan Y, Ni D, Wu S, Chen Y, Chen X, Xiong X, Liu G, TAT-functionalized PEI-grafting rice bran polysaccharides for safe and efficient gene delivery, International Journal of Biological Macromolecules 146 (2020) 1076–1086. [DOI] [PubMed] [Google Scholar]
- [81].Jaleel JA, Ashraf SM, Rathinasamy K, Pramod K, Carbon dot festooned and surface passivated graphene-reinforced chitosan construct for tumor-targeted delivery of TNF-α gene, International Journal of Biological Macromolecules 127 (2019) 628–636. [DOI] [PubMed] [Google Scholar]
- [82].Mohammadinejad R, Dehshahri A, Sagar Madamsetty V, Zahmatkeshan M, Tavakol S, Makvandi P, Khorsandi D, Pardakhty A, Ashrafizadeh M, Ghasemipour Afshar E, Zarrabi A, In vivo gene delivery mediated by non-viral vectors for cancer therapy, Journal of Controlled Release 325 (2020) 249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rigby PG, Prolongation of Survival of Tumour-bearing Animals by Transfer of “Immune” RNA with DEAE Dextran, Nature 221 (1969) 968. [DOI] [PubMed] [Google Scholar]
- [84].Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH, Gene transfer into mouse lyoma cells by electroporation in high electric fields, EMBO J 1(7) (1982) 841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Shi J, Ma Y, Zhu J, Chen Y, Sun Y, Yao Y, Yang Z, Xie J, A Review on Electroporation-Based Intracellular Delivery, 23(11) (2018) 3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Medi BM, Layek B, Singh J, Electroporation for Dermal and Transdermal Drug Delivery, in: Dragicevic N, Maibach HI (Eds.), Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement, Springer Berlin Heidelberg, Berlin, Heidelberg, 2017, pp. 105–122. [Google Scholar]
- [87].Kim HB, Lee S, Shen Y, Ryu P-D, Lee Y, Chung JH, Sung CK, Baik KY, Physicochemical factors that affect electroporation of lung cancer and normal cell lines, Biochemical and Biophysical Research Communications 517(4) (2019) 703–708. [DOI] [PubMed] [Google Scholar]
- [88].Andre F, Mir LM, DNA electrotransfer: its principles and an updated review of its therapeutic applications, Gene Ther 11 Suppl 1 (2004) S33–42. [DOI] [PubMed] [Google Scholar]
- [89].Young JL, Dean DA, Chapter Three - Electroporation-Mediated Gene Delivery, in: Huang L, Liu D, Wagner E (Eds.), Advances in Genetics, Academic Press; 2015, pp. 49–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Klein TM, Wolf ED, Wu R, Sanford JC, High-velocity microprojectiles for delivering nucleic acids into living cells, Nature 327 (1987) 70. [PubMed] [Google Scholar]
- [91].Tsai S-W, Tung Y-T, Chen H-L, Yang S-H, Liu C-Y, Lu M, Pai H-J, Lin C-C, Chen C-M, Myostatin propeptide gene delivery by gene gun ameliorates muscle atrophy in a rat model of botulinum toxin-induced nerve denervation, Life Sciences 146 (2016) 15–23. [DOI] [PubMed] [Google Scholar]
- [92].Peking P, Koller U, Hainzl S, Kitzmueller S, Kocher T, Mayr E, Nyström A, Lener T, Reichelt J, Bauer JW, Murauer EM, A Gene Gun-mediated Nonviral RNA trans-splicing Strategy for Col7a1 Repair, Molecular Therapy - Nucleic Acids 5 (2016) e287. [DOI] [PubMed] [Google Scholar]
- [93].Mhashilkar A, Chada S, Roth JA, Ramesh R, Gene therapy. Therapeutic approaches and implications, Biotechnol Adv 19(4) (2001) 279–97. [DOI] [PubMed] [Google Scholar]
- [94].Miyazaki M, Obata Y, Abe K, Furusu A, Koji T, Tabata Y, Kohno S, Gene transfer using nonviral delivery systems, Perit Dial Int 26(6) (2006) 633–40. [PubMed] [Google Scholar]
- [95].Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ, Dean DA, Liu D, Hydroporation as the mechanism of hydrodynamic delivery, Gene Ther 11(8) (2004) 675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kobayashi N, Nishikawa M, Hirata K, Takakura Y, Hydrodynamics-based procedure involves transient hyperpermeability in the hepatic cellular membrane: implication of a nonspecific process in efficient intracellular gene delivery, J Gene Med 6(5) (2004) 584–92. [DOI] [PubMed] [Google Scholar]
- [97].Suda T, Gao X, Stolz DB, Liu D, Structural impact of hydrodynamic injection on mouse liver, Gene Ther 14(2) (2007) 129–37. [DOI] [PubMed] [Google Scholar]
- [98].Huang M, Sun R, Huang Q, Tian Z, Technical Improvement and Application of Hydrodynamic Gene Delivery in Study of Liver Diseases, 8(591) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Suda T, Liu D, Chapter Four - Hydrodynamic Delivery, in: Huang L, Liu D, Wagner E (Eds.), Advances in Genetics, Academic Press; 2015, pp. 89–111. [DOI] [PubMed] [Google Scholar]
- [100].Kumbhari V, Li L, Piontek K, Ishida M, Fu R, Khalil B, Garrett CM, Liapi E, Kalloo AN, Selaru FM , Successful liver-directed gene delivery by ERCP-guided hydrodynamic injection (with videos), Gastrointestinal Endoscopy 88(4) (2018) 755–763.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Woodard LE, Cheng J, Welch RC, Williams FM, Luo W, Gewin LS, Wilson MH, Kidney-specific transposon-mediated gene transfer in vivo, Scientific Reports 7 (2017) 44904–44904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Pan X, Xu K, Li Y, Wang X, Peng X, Li M, Li Y, Interleukin-35 expression protects against cigarette smoke-induced lung inflammation in mice, Biomedicine & Pharmacotherapy 110 (2019) 727–732. [DOI] [PubMed] [Google Scholar]
- [103].Yasuzaki Y, Yamada Y, Kanefuji T, Harashima H, Localization of exogenous DNA to mitochondria in skeletal muscle following hydrodynamic limb vein injection, Journal of Controlled Release 172(3) (2013) 805–811. [DOI] [PubMed] [Google Scholar]
- [104].Abe S, Hanawa H, Hayashi M, Yoshida T, Komura S, Watanabe R, Lie H, Chang H, Kato K, Kodama M, Maruyama H, Nakazawa M, Miyazaki J, Aizawa Y, Prevention of Experimental Autoimmune Myocarditis by Hydrodynamics-Based Naked Plasmid DNA Encoding CTLA4-Ig Gene Delivery, Journal of Cardiac Failure 11(7) (2005) 557–564. [DOI] [PubMed] [Google Scholar]
- [105].Ogawa K, Kamimura K, Kobayashi Y, Abe H, Yokoo T, Sakai N, Nagoya T, Sakamaki A, Abe S, Hayashi K, Ikarashi S, Kohisa J, Tsuchida M, Aoyagi Y, Zhang G, Liu D, Terai S, Efficacy and Safety of Pancreas-Targeted Hydrodynamic Gene Delivery in Rats, Molecular Therapy - Nucleic Acids 9 (2017) 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Song YK, Liu F, Zhang G, Liu D, Hydrodynamics-based transfection: simple and efficient method for introducing and expressing transgenes in animals by intravenous injection of DNA, Methods Enzymol 346 (2002) 92–105. [DOI] [PubMed] [Google Scholar]
- [107].Zhang G, Budker V, Wolff JA, High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA, Human gene therapy 10(10) (1999) 1735–7. [DOI] [PubMed] [Google Scholar]
- [108].Kamimura K, Yokoo T, Abe H, Kobayashi Y, Ogawa K, Shinagawa Y, Inoue R, Terai S, Image-Guided Hydrodynamic Gene Delivery: Current Status and Future Directions, Pharmaceutics 7(3) (2015) 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Yokoo T, Kanefuji T, Suda T, Kamimura K, Liu D, Terai S, Site-Specific Impact of a Regional Hydrodynamic Injection: Computed Tomography Study during Hydrodynamic Injection Targeting the Swine Liver, Pharmaceutics 7(3) (2015) 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Yoshino H, Hashizume K, Kobayashi E, Naked plasmid DNA transfer to the porcine liver using rapid injection with large volume, Gene therapy 13(24) (2006) 1696–1702. [DOI] [PubMed] [Google Scholar]
- [111].Suda T, Suda K, Liu D, Computer-assisted Hydrodynamic Gene Delivery, Molecular Therapy 16(6) (2008) 1098–1104. [DOI] [PubMed] [Google Scholar]
- [112].Bouakaz A, Zeghimi A, Doinikov AA, Sonoporation: Concept and Mechanisms, in: Escoffre J-M, Bouakaz A (Eds.), Therapeutic Ultrasound, Springer International Publishing, Cham, 2016, pp. 175–189. [DOI] [PubMed] [Google Scholar]
- [113].Yang Y, Li Q, Guo X, Tu J, Zhang D, Mechanisms underlying sonoporation: Interaction between microbubbles and cells, Ultrasonics Sonochemistry 67 (2020) 105096. [DOI] [PubMed] [Google Scholar]
- [114].Lentacker I, De Cock I, Deckers R, De Smedt SC, Moonen CTW, Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms, Advanced Drug Delivery Reviews 72 (2014) 49–64. [DOI] [PubMed] [Google Scholar]
- [115].Kim HJ, Greenleaf JF, Kinnick RR, Bronk JT, Bolander ME, Ultrasound-mediated transfection of mammalian cells, Human gene therapy 7(11) (1996) 1339–46. [DOI] [PubMed] [Google Scholar]
- [116].Mitragotri S, Blankschtein D, Langer R, Transdermal drug delivery using low-frequency sonophoresis, Pharm Res 13(3) (1996) 411–20. [DOI] [PubMed] [Google Scholar]
- [117].Rychak JJ, Klibanov AL, Nucleic acid delivery with microbubbles and ultrasound, Advanced Drug Delivery Reviews 72 (2014) 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Endoh M, Koibuchi N, Sato M, Morishita R, Kanzaki T, Murata Y, Kaneda Y, Fetal gene transfer by intrauterine injection with microbubble-enhanced ultrasound, Molecular therapy : the journal of the American Society of Gene Therapy 5(5 Pt 1) (2002) 501–8. [DOI] [PubMed] [Google Scholar]
- [119].Alsaggar M, Liu D, Chapter One - Physical Methods for Gene Transfer, in: Huang L, Liu D, Wagner E (Eds.), Advances in Genetics, Academic Press; 2015, pp. 1–24. [DOI] [PubMed] [Google Scholar]
- [120].Wang DS, Panje C, Pysz MA, Paulmurugan R, Rosenberg J, Gambhir SS, Schneider M, Willmann JK , Cationic versus neutral microbubbles for ultrasound-mediated gene delivery in cancer, Radiology 264(3) (2012) 721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Li Y, Wang Y, Wang J, Chong KY, Xu J, Liu Z, Shan C, Expression of Neprilysin in Skeletal Muscle by Ultrasound-Mediated Gene Transfer (Sonoporation) Reduces Amyloid Burden for AD, Molecular Therapy - Methods & Clinical Development 17 (2020) 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Stavarache MA, Petersen N, Jurgens EM, Milstein ER, Rosenfeld ZB, Ballon DJ, Kaplitt MG, Safe and stable noninvasive focal gene delivery to the mammalian brain following focused ultrasound, J Neurosurg (2018) 1–10. [DOI] [PubMed] [Google Scholar]
- [123].Yang D, Gao YH, Tan KB, Zuo ZX, Yang WX, Hua X, Li PJ, Zhang Y, Wang G, Inhibition of hepatic fibrosis with artificial microRNA using ultrasound and cationic liposome-bearing microbubbles, Gene therapy 20 (2013) 1140. [DOI] [PubMed] [Google Scholar]
- [124].Rinaldi L, Folliero V, Palomba L, Zannella C, Isticato R, Di Francia R, Berretta M, de Sio I, Adinolfi LE, Morelli G, Lastoria S, Altucci L, Pedone C, Galdiero M, Franci G, Sonoporation by microbubbles as gene therapy approach against liver cancer, Oncotarget 9(63) (2018) 32182–32190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Fujii H, Li S-H, Wu J, Miyagi Y, Yau TM, Rakowski H, Egashira K, Guo J, Weisel RD, Li R-K, Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair, European Heart Journal 32(16) (2010) 2075–2084. [DOI] [PubMed] [Google Scholar]
- [126].Ishida R, Kami D, Kusaba T, Kirita Y, Kishida T, Mazda O, Adachi T, Gojo S, Kidney-specific Sonoporation-mediated Gene Transfer, Molecular therapy : the journal of the American Society of Gene Therapy 24(1) (2016) 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Xenariou S, Griesenbach U, Liang HD, Zhu J, Farley R, Somerton L, Singh C, Jeffery PK, Ferrari S, Scheule RK, Cheng SH, Geddes DM, Blomley M, Alton EWFW, Use of ultrasound to enhance nonviral lung gene transfer in vivo, Gene therapy 14 (2007) 768. [DOI] [PubMed] [Google Scholar]
- [128].Zhou Y, Ultrasound-Mediated Drug/Gene Delivery in Solid Tumor Treatment, Journal of Healthcare Engineering 4(2) (2013). [DOI] [PubMed] [Google Scholar]
- [129].Haag P, Frauscher F, Gradl J, Seitz A, Schäfer G, Lindner JR, Klibanov AL, Bartsch G, Klocker H, Eder IE, Microbubble-enhanced ultrasound to deliver an antisense oligodeoxynucleotide targeting the human androgen receptor into prostate tumours, The Journal of Steroid Biochemistry and Molecular Biology 102(1) (2006) 103–113. [DOI] [PubMed] [Google Scholar]
- [130].McCaffrey J, Donnelly RF, McCarthy HO, Microneedles: an innovative platform for gene delivery, Drug Delivery and Translational Research 5(4) (2015) 424–437. [DOI] [PubMed] [Google Scholar]
- [131].Chen W, Li H, Shi D, Liu Z, Yuan W, Microneedles As a Delivery System for Gene Therapy, Frontiers in Pharmacology 7(137) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Seok H.-y., Suh H, Baek S, Kim Y-C, Microneedle Applications for DNA Vaccine Delivery to the Skin, in: Rinaldi M, Fioretti D, Iurescia S (Eds.), DNA Vaccines: Methods and Protocols, Springer New York, New York, NY, 2014, pp. 141–158. [DOI] [PubMed] [Google Scholar]
- [133].Ita K, Transdermal delivery of vaccines - Recent progress and critical issues, Biomed Pharmacother 83 (2016) 1080–1088. [DOI] [PubMed] [Google Scholar]
- [134].Kim NW, Lee MS, Kim KR, Lee JE, Lee K, Park JS, Matsumoto Y, Jo D-G, Lee H, Lee DS, Jeong JH, Polyplex-releasing microneedles for enhanced cutaneous delivery of DNA vaccine, Journal of Controlled Release 179 (2014) 11–17. [DOI] [PubMed] [Google Scholar]
- [135].McLean WHI, Moore CBT, Keratin disorders: from gene to therapy, Human molecular genetics 20(R2) (2011) R189–R197. [DOI] [PubMed] [Google Scholar]
- [136].Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG, Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery, Nature Medicine 8 (2002) 415. [DOI] [PubMed] [Google Scholar]
- [137].Deng Y, Chen J, Zhao Y, Yan X, Zhang L, Choy K, Hu J, Sant HJ, Gale BK, Tang T, Transdermal Delivery of siRNA through Microneedle Array, Scientific Reports 6 (2016) 21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Hooper JW, Golden JW, Ferro AM, King AD, Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge, Vaccine 25(10) (2007) 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wei Z, Zheng S, Wang R, Bu X, Ma H, Wu Y, Zhu L, Hu Z, Liang Z, Li Z, A flexible microneedle array as low-voltage electroporation electrodes for in vivo DNA and siRNA delivery, Lab on a Chip 14(20) (2014) 4093–4102. [DOI] [PubMed] [Google Scholar]
- [140].Bhatnagar S, Dave K, Venuganti VVK, Microneedles in the clinic, Journal of Controlled Release 260 (2017) 164–182. [DOI] [PubMed] [Google Scholar]
- [141].Bi Q, Song X, Hu A, Luo T, Jin R, Ai H, Nie Y, Magnetofection: Magic magnetic nanoparticles for efficient gene delivery, Chinese Chemical Letters 31(12) (2020) 3041–3046. [Google Scholar]
- [142].Prijic S, Prosen L, Cemazar M, Scancar J, Romih R, Lavrencak J, Bregar VB, Coer A, Krzan M, Znidarsic A, Sersa G, Surface modified magnetic nanoparticles for immuno-gene therapy of murine mammary adenocarcinoma, Biomaterials 33(17) (2012) 4379–4391. [DOI] [PubMed] [Google Scholar]
- [143].Prijic S, Sersa G, Magnetic nanoparticles as targeted delivery systems in oncology, Radiology and oncology 45(1) (2011) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Neuberger T, Schöpf B, Hofmann H, Hofmann M, von Rechenberg B, Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system, Journal of Magnetism and Magnetic Materials 293(1) (2005) 483–496. [Google Scholar]
- [145].Dobson J, Gene therapy progress and prospects: magnetic nanoparticle-based gene delivery, Gene Therapy 13(4) (2006) 283–287. [DOI] [PubMed] [Google Scholar]
- [146].Jones CH, Chen CK, Ravikrishnan A, Rane S, Pfeifer BA, Overcoming nonviral gene delivery barriers: perspective and future, Mol Pharm 10(11) (2013) 4082–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Majee S, Avlani D, Roy Biswas G, Magnet-guided nanovectors as agents for magnetofection in therapeutic management of solid tumors, World Journal of Pharmaceutical Research 5 (2016) 469. [Google Scholar]
- [148].Zuvin M, Kuruoglu E, Kaya VO, Unal O, Kutlu O, Yagci Acar H, Gozuacik D, Koşar A, Magnetofection of Green Fluorescent Protein Encoding DNA-Bearing Polyethyleneimine-Coated Superparamagnetic Iron Oxide Nanoparticles to Human Breast Cancer Cells, ACS Omega 4(7) (2019) 12366–12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Holzbach T, Vlaskou D, Neshkova I, Konerding MA, Wörtler K, Mykhaylyk O, Gänsbacher B, Machens HG, Plank C, Giunta RE, Non-viral VEGF(165) gene therapy--magnetofection of acoustically active magnetic lipospheres (‘magnetobubbles’) increases tissue survival in an oversized skin flap model, Journal of cellular and molecular medicine 14(3) (2010) 587–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Misra A, Challenges in Delivery of Therapeutic Genomics and Proteomics, 2011. [Google Scholar]
- [151].Zhi D, Zhang S, Wang B, Zhao Y, Yang B, Yu S, Transfection Efficiency of Cationic Lipids with Different Hydrophobic Domains in Gene Delivery, Bioconjug Chem 21(4) (2010) 563–577. [DOI] [PubMed] [Google Scholar]
- [152].Ponti F, Campolungo M, Melchiori C, Bono N, Candiani G, Cationic lipids for gene delivery: many players, one goal, Chemistry and Physics of Lipids 235 (2021) 105032. [DOI] [PubMed] [Google Scholar]
- [153].Arora S, Sharma D, Singh J, GLUT-1: An Effective Target To Deliver Brain-Derived Neurotrophic Factor Gene Across the Blood Brain Barrier, ACS Chemical Neuroscience 11(11) (2020) 1620–1633. [DOI] [PubMed] [Google Scholar]
- [154].Sharma G, Modgil A, Layek B, Arora K, Sun C, Law B, Singh J, Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: Biodistribution and transfection, Journal of Controlled Release 167(1) (2013) 1–10. [DOI] [PubMed] [Google Scholar]
- [155].Kimura S, Khalil IA, Elewa YHA, Harashima H, Novel lipid combination for delivery of plasmid DNA to immune cells in the spleen, Journal of Controlled Release 330 (2021) 753–764. [DOI] [PubMed] [Google Scholar]
- [156].Elouahabi A, Ruysschaert J-M, Formation and Intracellular Trafficking of Lipoplexes and Polyplexes, Molecular Therapy 11(3) (2005) 336–347. [DOI] [PubMed] [Google Scholar]
- [157].Ruponen M, Yla-Herttuala S, Urtti A, Interactions of polymeric and liposomal gene delivery systems with extracellular glycosaminoglycans: physicochemical and transfection studies, Biochim Biophys Acta 1415(2) (1999) 331–41. [DOI] [PubMed] [Google Scholar]
- [158].Crespo-Barreda A, Encabo-Berzosa MM, González-Pastor R, Ortíz-Teba P, Iglesias M, Serrano JL, Martin-Duque P, Chapter 11 - Viral and Nonviral Vectors for In Vivo and Ex Vivo Gene Therapies, in: Laurence J (Ed.), Translating Regenerative Medicine to the Clinic, Academic Press, Boston, 2016, pp. 155–177. [Google Scholar]
- [159].Wu X-R, Zhang J, Zhang J-H, Xiao Y-P, He X, Liu Y-H, Yu X-Q, Amino Acid-Linked Low Molecular Weight Polyethylenimine for Improved Gene Delivery and Biocompatibility, Molecules (Basel, Switzerland) 25(4) (2020) 975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Gabrielson NP, Pack DW, Efficient polyethylenimine-mediated gene delivery proceeds via a caveolar pathway in HeLa cells, Journal of Controlled Release 136(1) (2009) 54–61. [DOI] [PubMed] [Google Scholar]
- [161].Tian H, Lin L, Chen J, Chen X, Park TG, Maruyama A, RGD targeting hyaluronic acid coating system for PEI-PBLG polycation gene carriers, Journal of Controlled Release 155(1) (2011) 47–53. [DOI] [PubMed] [Google Scholar]
- [162].Lee J, Oh J, Lee E.-s., Kim Y-P, Lee M, Conjugation of prostate cancer-specific aptamers to polyethylene glycol-grafted polyethylenimine for enhanced gene delivery to prostate cancer cells, Journal of Industrial and Engineering Chemistry 73 (2019) 182–191. [Google Scholar]
- [163].Jiang D, Salem AK, Optimized dextran-polyethylenimine conjugates are efficient non-viral vectors with reduced cytotoxicity when used in serum containing environments, International journal of pharmaceutics 427(1) (2012) 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Saqafi B, Rahbarizadeh F, Polyethyleneimine-polyethylene glycol copolymer targeted by anti-HER2 nanobody for specific delivery of transcriptionally targeted tBid containing construct, Artificial Cells, Nanomedicine, and Biotechnology 47(1) (2019) 501–511. [DOI] [PubMed] [Google Scholar]
- [165].Tang X, Lu P, Qiu M, Chen J, Ma L, Sun Y, Zheng F, Xu E, Sheng J, Su J, Screening PEGylated polyethylenimine derivatives for safe and efficient delivery of gene materials, RSC Advances 6(108) (2016) 106316–106326. [Google Scholar]
- [166].Wang M, Lu P, Wu B, Tucker JD, Cloer C, Lu Q, High efficiency and low toxicity of polyethyleneimine modified Pluronics (PEI–Pluronic) as gene delivery carriers in cell culture and dystrophic mdx mice, Journal of Materials Chemistry 22(13) (2012) 6038–6046. [Google Scholar]
- [167].Sheikh MA, Malik YS, Xing Z, Guo Z, Tian H, Zhu X, Chen X, Polylysine-modified polyethylenimine (PEI-PLL) mediated VEGF gene delivery protects dopaminergic neurons in cell culture and in rat models of Parkinson’s Disease (PD), Acta Biomaterialia 54 (2017) 58–68. [DOI] [PubMed] [Google Scholar]
- [168].Wang J, Meng F, Kim B-K, Ke X, Yeo Y, In-vitro and in-vivo difference in gene delivery by lithocholic acid-polyethyleneimine conjugate, Biomaterials 217 (2019) 119296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Mandal H, Katiyar SS, Swami R, Kushwah V, Katare PB, Kumar Meka A, Banerjee SK, Popat A, Jain S, ε-Poly-l-Lysine/plasmid DNA nanoplexes for efficient gene delivery in vivo, International Journal of Pharmaceutics 542(1) (2018) 142–152. [DOI] [PubMed] [Google Scholar]
- [170].Thiersch M, Rimann M, Panagiotopoulou V, Öztürk E, Biedermann T, Textor M, Lühmann TC, Hall H, The angiogenic response to PLL-g-PEG-mediated HIF-1α plasmid DNA delivery in healthy and diabetic rats, Biomaterials 34(16) (2013) 4173–4182. [DOI] [PubMed] [Google Scholar]
- [171].Akinc A, Langer R, Measuring the pH environment of DNA delivered using nonviral vectors: Implications for lysosomal trafficking, 78(5) (2002) 503–508. [DOI] [PubMed] [Google Scholar]
- [172].Hwang HS, Hu J, Na K, Bae YH, Role of polymeric endosomolytic agents in gene transfection: a comparative study of poly(L-lysine) grafted with monomeric L-histidine analogue and poly(L-histidine), Biomacromolecules 15(10) (2014) 3577–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Urello MA, Xiang L, Colombo R, Ma A, Joseph A, Boyd J, Peterson N, Gao C, Wu H, Christie RJ, Metabolite-Based Modification of Poly(l-lysine) for Improved Gene Delivery, Biomacromolecules 21(9) (2020) 3596–3607. [DOI] [PubMed] [Google Scholar]
- [174].Kim Y, Uthaman S, Nurunnabi M, Mallick S, Oh KS, Kang S-W, Cho S, Kang HC, Lee Y.-k., Huh KM, Synthesis and characterization of bioreducible cationic biarm polymer for efficient gene delivery, International Journal of Biological Macromolecules 110 (2018) 366–374. [DOI] [PubMed] [Google Scholar]
- [175].Hu B, Wang SS, Li J, Zeng XX, Huang QR, Assembly of bioactive peptide-chitosan nanocomplexes, J Phys Chem B 115(23) (2011) 7515–23. [DOI] [PubMed] [Google Scholar]
- [176].Zhao J, Ullah I, Gao B, Guo J, Ren X.-k., Xia S, Zhang W, Feng Y, Agmatine-grafted bioreducible poly(l-lysine) for gene delivery with low cytotoxicity and high efficiency, Journal of Materials Chemistry B 8(12) (2020) 2418–2430. [DOI] [PubMed] [Google Scholar]
- [177].Thomas RG, Muthiah M, Moon M, Park I-K, Jeong YY, SPION loaded poly(L-lysine)/hyaluronic acid micelles as MR contrast agent and gene delivery vehicle for cancer theranostics, Macromolecular Research 25(5) (2017) 446–451. [Google Scholar]
- [178].Layek B, Das S, Chapter 8 - Chitosan-based nanomaterials in drug delivery applications, in: Bera H, Hossain CM, Saha S (Eds.), Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications, Academic Press; 2021, pp. 185–219. [Google Scholar]
- [179].Layek B, Mandal S, Natural polysaccharides for controlled delivery of oral therapeutics: a recent update, Carbohydrate Polymers 230 (2020) 115617. [DOI] [PubMed] [Google Scholar]
- [180].Kumar D, Gihar S, Shrivash MK, Kumar P, Kundu PP, A review on the synthesis of graft copolymers of chitosan and their potential applications, International Journal of Biological Macromolecules 163 (2020) 2097–2112. [DOI] [PubMed] [Google Scholar]
- [181].Shanmuganathan R, Edison TNJI, LewisOscar F, Kumar P, Shanmugam S, Pugazhendhi A, Chitosan nanopolymers: An overview of drug delivery against cancer, International Journal of Biological Macromolecules 130 (2019) 727–736. [DOI] [PubMed] [Google Scholar]
- [182].Banerjee A, Sharma D, Trivedi R, Singh J, Treatment of insulin resistance in obesity-associated type 2 diabetes mellitus through adiponectin gene therapy, International journal of pharmaceutics 583 (2020) 119357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Sharma D, Arora S, Banerjee A, Singh J, Improved insulin sensitivity in obese-diabetic mice via chitosan Nanomicelles mediated silencing of pro-inflammatory Adipocytokines, Nanomedicine: Nanotechnology, Biology and Medicine 33 (2021) 102357. [DOI] [PubMed] [Google Scholar]
- [184].Baghdan E, Pinnapireddy SR, Strehlow B, Engelhardt KH, Schäfer J, Bakowsky U, Lipid coated chitosan-DNA nanoparticles for enhanced gene delivery, International journal of pharmaceutics 535(1–2) (2018) 473–479. [DOI] [PubMed] [Google Scholar]
- [185].Sharma D, Singh J, Synthesis and Characterization of Fatty Acid Grafted Chitosan Polymer and Their Nanomicelles for Nonviral Gene Delivery Applications, Bioconjug Chem 28(11) (2017) 2772–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Wu D, Zhang Y, Xu X, Guo T, Xie D, Zhu R, Chen S, Ramakrishna S, He L, RGD/TAT-functionalized chitosan-graft-PEI-PEG gene nanovector for sustained delivery of NT-3 for potential application in neural regeneration, Acta Biomaterialia 72 (2018) 266–277. [DOI] [PubMed] [Google Scholar]
- [187].Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, Hanifehpour Y, Nejati-Koshki K, Pashaei-Asl R, Dendrimers: synthesis, applications, and properties, Nanoscale research letters 9(1) (2014) 247–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Dias AP, da Silva Santos S, da Silva JV, Parise-Filho R, Igne Ferreira E, Seoud OE, Giarolla J, Dendrimers in the context of nanomedicine, International journal of pharmaceutics 573 (2020) 118814. [DOI] [PubMed] [Google Scholar]
- [189].Yang J, Zhang Q, Chang H, Cheng Y, Surface-Engineered Dendrimers in Gene Delivery, Chemical Reviews 115(11) (2015) 5274–5300. [DOI] [PubMed] [Google Scholar]
- [190].Mintzer MA, Simanek EE, Nonviral vectors for gene delivery, Chem Rev 109(2) (2009) 259–302. [DOI] [PubMed] [Google Scholar]
- [191].Wang X, Shao N, Zhang Q, Cheng Y, Mitochondrial targeting dendrimer allows efficient and safe gene delivery, Journal of Materials Chemistry B 2(17) (2014) 2546–2553. [DOI] [PubMed] [Google Scholar]
- [192].Li J, Liang H, Liu J, Wang Z, Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy, International journal of pharmaceutics 546(1) (2018) 215–225. [DOI] [PubMed] [Google Scholar]
- [193].Luong D, Kesharwani P, Deshmukh R, Mohd Amin MCI, Gupta U, Greish K, Iyer AK, PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery, Acta Biomaterialia 43 (2016) 14–29. [DOI] [PubMed] [Google Scholar]
- [194].Lee Y, Lee J, Kim M, Kim G, Choi JS, Lee M, Brain gene delivery using histidine and arginine-modified dendrimers for ischemic stroke therapy, Journal of Controlled Release 330 (2021) 907–919. [DOI] [PubMed] [Google Scholar]
- [195].Ariaee FM, Hashemi M, Farzad SA, Abnous K, Ramezani M, Alkyl cross-linked low molecular weight polypropyleneimine dendrimers as efficient gene delivery vectors, Iranian journal of basic medical sciences 19(10) (2016) 1096–1104. [PMC free article] [PubMed] [Google Scholar]
- [196].Malaekeh-Nikouei B, Rezaee M, Gholami L, Sanjar Mousavi N, Kazemi Oskuee R, Synthesis, characterization and evaluation of transfection efficiency of dexamethasone conjugated poly(propyleneimine) nanocarriers for gene delivery#(), Pharmaceutical biology 56(1) (2018) 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Laskar P, Somani S, Altwaijry N, Mullin M, Bowering D, Warzecha M, Keating P, Tate RJ, Leung HY, Dufès C, Redox-sensitive, cholesterol-bearing PEGylated poly(propylene imine)-based dendrimersomes for drug and gene delivery to cancer cells, Nanoscale 10(48) (2018) 22830–22847. [DOI] [PubMed] [Google Scholar]
- [198].Somani S, Blatchford DR, Millington O, Stevenson ML, Dufès C, Transferrin-bearing polypropylenimine dendrimer for targeted gene delivery to the brain, Journal of controlled release : official journal of the Controlled Release Society 188 (2014) 78–86. [DOI] [PubMed] [Google Scholar]
- [199].Chen J, Guan X, Hu Y, Tian H, Chen X, Peptide-Based and Polypeptide-Based Gene Delivery Systems, in: Cheng Y (Ed.), Polymeric Gene Delivery Systems, Springer International Publishing, Cham, 2018, pp. 85–112. [Google Scholar]
- [200].Gupta B, Levchenko TS, Torchilin VP, Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides, Advanced Drug Delivery Reviews 57(4) (2005) 637–651. [DOI] [PubMed] [Google Scholar]
- [201].Klein AF, Varela MA, Arandel L, Holland A, Naouar N, Arzumanov A, Seoane D, Revillod L, Bassez G, Ferry A, Jauvin D, Gourdon G, Puymirat J, Gait MJ, Furling D, Wood MJ, Peptide-conjugated oligonucleotides evoke long-lasting myotonic dystrophy correction in patient-derived cells and mice, The Journal of clinical investigation 129(11) (2019) 4739–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Thapa RK, Sullivan MO, Gene delivery by peptide-assisted transport, Current Opinion in Biomedical Engineering 7 (2018) 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Lo SL, Wang S, An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection, Biomaterials 29(15) (2008) 2408–2414. [DOI] [PubMed] [Google Scholar]
- [204].Renigunta A, Krasteva G, König P, Rose F, Klepetko W, Grimminger F, Seeger W, Hänze J, DNA Transfer into Human Lung Cells Is Improved with Tat−RGD Peptide by Caveoli-Mediated Endocytosis, Bioconjugate Chemistry 17(2) (2006) 327–334. [DOI] [PubMed] [Google Scholar]
- [205].Fu S, Xu X, Ma Y, Zhang S, Zhang S, RGD peptide-based non-viral gene delivery vectors targeting integrin αvβ3 for cancer therapy, Journal of Drug Targeting 27(1) (2019) 1–11. [DOI] [PubMed] [Google Scholar]
- [206].Kilk K, El-Andaloussi S, Järver P, Meikas A, Valkna A, Bartfai T, Kogerman P, Metsis M, Langel Ü, Evaluation of transportan 10 in PEI mediated plasmid delivery assay, Journal of Controlled Release 103(2) (2005) 511–523. [DOI] [PubMed] [Google Scholar]
- [207].Riley MK, Vermerris W, Recent Advances in Nanomaterials for Gene Delivery-A Review, Nanomaterials (Basel, Switzerland) 7(5) (2017) 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Zheng Y-Y, Qiu D-K, Guo Z-R, Gong Y-M, Wang G-X, Zhu B, Evaluation of SWCNTs-loaded DNA vaccine encoding predominant antigen epitope VP4–3 against type II GCRV, Aquaculture 534 (2021) 736197. [Google Scholar]
- [209].Uğurlu Ö, Barlas FB, Evran S, Timur S, The cell-penetrating YopM protein-functionalized quantum dot-plasmid DNA conjugate as a novel gene delivery vector, Plasmid 110 (2020) 102513. [DOI] [PubMed] [Google Scholar]
- [210].Cristofolini T, Dalmina M, Sierra JA, Silva AH, Pasa AA, Pittella F, Creczynski-Pasa TB, Multifunctional hybrid nanoparticles as magnetic delivery systems for siRNA targeting the HER2 gene in breast cancer cells, Materials Science and Engineering: C 109 (2020) 110555. [DOI] [PubMed] [Google Scholar]
- [211].Pędziwiatr-Werbicka E, Gorzkiewicz M, Michlewska S, Ionov M, Shcharbin D, Klajnert-Maculewicz B, Peña-González CE, Sánchez-Nieves J, Gómez R, de la Mata FJ, Bryszewska M, Evaluation of dendronized gold nanoparticles as siRNAs carriers into cancer cells, Journal of Molecular Liquids 324 (2021) 114726. [Google Scholar]
- [212].Zhou Y, Quan G, Wu Q, Zhang X, Niu B, Wu B, Huang Y, Pan X, Wu C, Mesoporous silica nanoparticles for drug and gene delivery, Acta Pharmaceutica Sinica B 8(2) (2018) 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Chen Q, Gao M, Li Z, Xiao Y, Bai X, Boakye-Yiadom KO, Xu X, Zhang X-Q, Biodegradable nanoparticles decorated with different carbohydrates for efficient macrophage-targeted gene therapy, Journal of Controlled Release 323 (2020) 179–190. [DOI] [PubMed] [Google Scholar]
- [214].Ramezani M, Ebrahimian M, Hashemi M, Current Strategies in the Modification of PLGA-based Gene Delivery System, Current medicinal chemistry 24(7) (2017) 728–739. [DOI] [PubMed] [Google Scholar]
- [215].Gokita K, Inoue J, Ishihara H, Kojima K, Inazawa J, Therapeutic Potential of LNP-Mediated Delivery of miR-634 for Cancer Therapy, Molecular Therapy - Nucleic Acids 19 (2020) 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Ghafary SM, Nikkhah M, Hatamie S, Hosseinkhani S, Simultaneous Gene Delivery and Tracking through Preparation of Photo-Luminescent Nanoparticles Based on Graphene Quantum Dots and Chimeric Peptides, Scientific Reports 7(1) (2017) 9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Majidi S, Zeinali Sehrig F, Samiei M, Milani M, Abbasi E, Dadashzadeh K, Akbarzadeh A, Magnetic nanoparticles: Applications in gene delivery and gene therapy, Artif Cells Nanomed Biotechnol 44(4) (2016) 1186–93. [DOI] [PubMed] [Google Scholar]
- [218].Tian H, Guo Z, Chen J, Lin L, Xia J, Dong X, Chen X, PEI Conjugated Gold Nanoparticles: Efficient Gene Carriers with Visible Fluorescence, Advanced Healthcare Materials 1(3) (2012) 337–341. [DOI] [PubMed] [Google Scholar]
- [219].Naseri N, Valizadeh H, Zakeri-Milani P, Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application, Advanced pharmaceutical bulletin 5(3) (2015) 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].del Pozo-Rodríguez A, Solinís MÁ, Rodríguez-Gascón A, Applications of lipid nanoparticles in gene therapy, European Journal of Pharmaceutics and Biopharmaceutics 109 (2016) 184–193. [DOI] [PubMed] [Google Scholar]
- [221].Battaglia L, Serpe L, Foglietta F, Muntoni E, Gallarate M, Del Pozo Rodriguez A, Solinis MA, Application of lipid nanoparticles to ocular drug delivery, Expert Opinion on Drug Delivery 13(12) (2016) 1743–1757. [DOI] [PubMed] [Google Scholar]
- [222].Tabatt K, Kneuer C, Sameti M, Olbrich C, Müller RH, Lehr C-M, Bakowsky U, Transfection with different colloidal systems: comparison of solid lipid nanoparticles and liposomes, Journal of Controlled Release 97(2) (2004) 321–332. [DOI] [PubMed] [Google Scholar]
- [223].Asasutjarit R, Lorenzen S-I, Sirivichayakul S, Ruxrungtham K, Ruktanonchai U, Ritthidej GC, Effect of Solid Lipid Nanoparticles Formulation Compositions on Their Size, Zeta Potential and Potential for In Vitro pHIS-HIV-Hugag Transfection, Pharmaceutical Research 24(6) (2007) 1098–1107. [DOI] [PubMed] [Google Scholar]
- [224].Zhao Y, Huang L, Chapter Two - Lipid Nanoparticles for Gene Delivery, in: Huang L, Liu D, Wagner E (Eds.), Advances in Genetics, Academic Press; 2014, pp. 13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Xiang Y, Oo NNL, Lee JP, Li Z, Loh XJ, Recent development of synthetic nonviral systems for sustained gene delivery, Drug Discovery Today 22(9) (2017) 1318–1335. [DOI] [PubMed] [Google Scholar]
- [226].Evans JC, Malhotra M, Guo J, O’Shea JP, Hanrahan K, O’Neill A, Landry WD, Griffin BT, Darcy R, Watson RW, O’Driscoll CM, Folate-targeted amphiphilic cyclodextrin.siRNA nanoparticles for prostate cancer therapy exhibit PSMA mediated uptake, therapeutic gene silencing in vitro and prolonged circulation in vivo, Nanomedicine: Nanotechnology, Biology and Medicine 12(8) (2016) 2341–2351. [DOI] [PubMed] [Google Scholar]
- [227].Ten E, Ling C, Wang Y, Srivastava A, Dempere LA, Vermerris W, Lignin Nanotubes As Vehicles for Gene Delivery into Human Cells, Biomacromolecules 15(1) (2014) 327–338. [DOI] [PubMed] [Google Scholar]
- [228].Schneider WDH, Dillon AJP, Camassola M, Lignin nanoparticles enter the scene: A promising versatile green tool for multiple applications, Biotechnology Advances 47 (2021) 107685. [DOI] [PubMed] [Google Scholar]
- [229].Chen C-K, Law W-C, Aalinkeel R, Nair B, Kopwitthaya A, Mahajan SD, Reynolds JL, Zou J, Schwartz SA, Prasad PN, Cheng C, Well-Defined Degradable Cationic Polylactide as Nanocarrier for the Delivery of siRNA to Silence Angiogenesis in Prostate Cancer, Advanced Healthcare Materials 1(6) (2012) 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Lin G, Zhang H, Huang L, Smart polymeric nanoparticles for cancer gene delivery, Mol Pharm 12(2) (2015) 314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Menger FM, Keiper JS, Gemini Surfactants, 39(11) (2000) 1906–1920. [DOI] [PubMed] [Google Scholar]
- [232].Bombelli C, Borocci S, Diociaiuti M, Galantini L, Luciani P, Mancini G, Sacco MG, Role of the Spacer of Cationic Gemini Amphiphiles in the Condensation of DNA, Langmuir 21(23) (2005) 10271–10274. [DOI] [PubMed] [Google Scholar]
- [233].Grueso E, Kuliszewska E, Roldan E, Perez-Tejeda P, Prado-Gotor R, Brecker L, DNA conformational changes induced by cationic gemini surfactants: the key to switching DNA compact structures into elongated forms, RSC Advances 5(37) (2015) 29433–29446. [Google Scholar]
- [234].Cardoso AM, Morais CM, Cruz AR, Cardoso AL, Silva SG, do Vale ML, Marques EF, Pedroso de Lima MC, Jurado AS, Gemini Surfactants Mediate Efficient Mitochondrial Gene Delivery and Expression, Molecular Pharmaceutics 12(3) (2015) 716–730. [DOI] [PubMed] [Google Scholar]
- [235].van der Woude I, Wagenaar A, Meekel AA, ter Beest MB, Ruiters MH, Engberts JB, Hoekstra D, Novel pyridinium surfactants for efficient, nontoxic in vitro gene delivery, Proceedings of the National Academy of Sciences of the United States of America 94(4) (1997) 1160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Pérez L, Pinazo A, Pons R, Infante M, Gemini surfactants from natural amino acids, Advances in colloid and interface science 205 (2014) 134–55. [DOI] [PubMed] [Google Scholar]
- [237].Singh J, Yang P, Michel D, Verrall RE, Foldvari M, Badea I, Amino acid-substituted gemini surfactant-based nanoparticles as safe and versatile gene delivery agents, Current drug delivery 8(3) (2011) 299–306. [DOI] [PubMed] [Google Scholar]
- [238].Damen M, Groenen AJJ, van Dongen SFM, Nolte RJM, Scholte BJ, Feiters MC, Transfection by cationic gemini lipids and surfactants, MedChemComm 9(9) (2018) 1404–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Kudsiova L, Mohammadi A, Mustapa MFM, Campbell F, Welser K, Vlaho D, Story H, Barlow DJ, Tabor AB, Hailes HC, Lawrence MJ, Trichain cationic lipids: the potential of their lipoplexes for gene delivery, Biomaterials Science 7(1) (2019) 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Zhang M, Wang Q, Wan K-W, Ahmed W, Phoenix DA, Zhang Z, Elrayess MA, Elhissi A, Sun X, Liposome mediated-CYP1A1 gene silencing nanomedicine prepared using lipid film-coated proliposomes as a potential treatment strategy of lung cancer, International journal of pharmaceutics 566 (2019) 185–193. [DOI] [PubMed] [Google Scholar]
- [241].Takata H, Shimizu T, Kawaguchi Y, Ueda H, Elsadek NE, Ando H, Ishima Y, Ishida T, Nucleic acids delivered by PEGylated cationic liposomes in systemic lupus erythematosus-prone mice: A possible exacerbation of lupus nephritis in the presence of pre-existing anti-nucleic acid antibodies, International Journal of Pharmaceutics 601 (2021) 120529. [DOI] [PubMed] [Google Scholar]
- [242].Memari E, Maghsoudi A, Yazdian F, Yousefi M, Mohammadi M, Synthesis of PHB-co-PEI nanoparticles as gene carriers for miR-128-encoding plasmid delivery to U87 glioblastoma cells, Colloids and Surfaces A: Physicochemical and Engineering Aspects 599 (2020) 124898. [Google Scholar]
- [243].Jana P, Ghosh S, Sarkar K, Low molecular weight polyethyleneimine conjugated guar gum for targeted gene delivery to triple negative breast cancer, International Journal of Biological Macromolecules 161 (2020) 1149–1160. [DOI] [PubMed] [Google Scholar]
- [244].Rahmani S, Hakimi S, Esmaeily A, Samadi FY, Mortazavian E, Nazari M, Mohammadi Z, Tehrani NR, Tehrani MR, Novel chitosan based nanoparticles as gene delivery systems to cancerous and noncancerous cells, International journal of pharmaceutics 560 (2019) 306–314. [DOI] [PubMed] [Google Scholar]
- [245].Layek B, Singh J, Cell Penetrating Peptide Conjugated Polymeric Micelles as a High Performance Versatile Nonviral Gene Carrier, Biomacromolecules 14(11) (2013) 4071–4081. [DOI] [PubMed] [Google Scholar]
- [246].Li Z, Liu X, Chen X, Chua MX, Wu Y-L, Targeted delivery of Bcl-2 conversion gene by MPEG-PCL-PEI-FA cationic copolymer to combat therapeutic resistant cancer, Materials Science and Engineering: C 76 (2017) 66–72. [DOI] [PubMed] [Google Scholar]
- [247].Liu Q, Jin Z, Huang W, Sheng Y, Wang Z, Guo S, Tailor-made ternary nanopolyplexes of thiolated trimethylated chitosan with pDNA and folate conjugated cis-aconitic amide-polyethylenimine for efficient gene delivery, International Journal of Biological Macromolecules 152 (2020) 948–956. [DOI] [PubMed] [Google Scholar]
- [248].Chen Y, Diaz-Dussan D, Peng Y-Y, Narain R, Hydroxyl-Rich PGMA-Based Cationic Glycopolymers for Intracellular siRNA Delivery: Biocompatibility and Effect of Sugar Decoration Degree, Biomacromolecules 20(5) (2019) 2068–2074. [DOI] [PubMed] [Google Scholar]
- [249].Vaidyanathan S, Chen J, Orr BG, Banaszak Holl MM, Cationic Polymer Intercalation into the Lipid Membrane Enables Intact Polyplex DNA Escape from Endosomes for Gene Delivery, Molecular Pharmaceutics 13(6) (2016) 1967–1978. [DOI] [PubMed] [Google Scholar]
- [250].Wang H, Miao W, Wang F, Cheng Y, A Self-Assembled Coumarin-Anchored Dendrimer for Efficient Gene Delivery and Light-Responsive Drug Delivery, Biomacromolecules 19(6) (2018) 2194–2201. [DOI] [PubMed] [Google Scholar]
- [251].Zhou J, Deng W, Wang Y, Cao X, Chen J, Wang Q, Xu W, Du P, Yu Q, Chen J, Spector M, Yu J, Xu X, Cationic carbon quantum dots derived from alginate for gene delivery: One-step synthesis and cellular uptake, Acta Biomaterialia 42 (2016) 209–219. [DOI] [PubMed] [Google Scholar]
- [252].Kotcherlakota R, Vydiam K, Jeyalakshmi Srinivasan D, Mukherjee S, Roy A, Kuncha M, Rao TN, Sistla R, Gopal V, Patra CR, Restoration of p53 Function in Ovarian Cancer Mediated by Gold Nanoparticle-Based EGFR Targeted Gene Delivery System, ACS biomaterials science & engineering 5(7) (2019) 3631–3644. [DOI] [PubMed] [Google Scholar]
- [253].Xiong Z, Alves CS, Wang J, Li A, Liu J, Shen M, Rodrigues J, Tomás H, Shi X, Zwitterion-functionalized dendrimer-entrapped gold nanoparticles for serum-enhanced gene delivery to inhibit cancer cell metastasis, Acta Biomaterialia 99 (2019) 320–329. [DOI] [PubMed] [Google Scholar]
- [254].Erel-Akbaba G, Carvalho LA, Tian T, Zinter M, Akbaba H, Obeid PJ, Chiocca EA, Weissleder R, Kantarci AG, Tannous BA, Radiation-Induced Targeted Nanoparticle-Based Gene Delivery for Brain Tumor Therapy, ACS Nano 13(4) (2019) 4028–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Wahane A, Waghmode A, Kapphahn A, Dhuri K, Gupta A, Bahal R, Role of Lipid-Based and Polymer-Based Non-Viral Vectors in Nucleic Acid Delivery for Next-Generation Gene Therapy, 25(12) (2020) 2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Nanomedicine and the COVID-19 vaccines, Nature Nanotechnology 15(12) (2020) 963–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Ho W, Gao M, Li F, Li Z, Zhang X-Q, Xu X, Next-Generation Vaccines: Nanoparticle-Mediated DNA and mRNA Delivery, n/a(n/a) 2001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Pfizer-BioNTech COVID-19 vaccine EUA fact sheet for recipients and caregivers, U.S. Food and Drug Administration, 2021.
- [259].Hsu CYM, Uludağ H, Effects of size and topology of DNA molecules on intracellular delivery with non-viral gene carriers, BMC Biotechnology 8(1) (2008) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Cherng JY, Schuurmans-Nieuwenbroek NM, Jiskoot W, Talsma H, Zuidam NJ, Hennink WE, Crommelin DJ, Effect of DNA topology on the transfection efficiency of poly((2-dimethylamino)ethyl methacrylate)-plasmid complexes, J Control Release 60(2–3) (1999) 343–53. [DOI] [PubMed] [Google Scholar]
- [261].Mairhofer J, Grabherr R, Rational vector design for efficient non-viral gene delivery: challenges facing the use of plasmid DNA, Molecular biotechnology 39(2) (2008) 97–104. [DOI] [PubMed] [Google Scholar]
- [262].Tudini E, Burke LJ, Whiley PJ, Sevcik J, Spurdle AB, Brown MA, Caution: Plasmid DNA topology affects luciferase assay reproducibility and outcomes, 67(3) (2019) 94–96. [DOI] [PubMed] [Google Scholar]
- [263].Raup A, Jérôme V, Freitag R, Synatschke CV, Müller AHE, Promoter, transgene, and cell line effects in the transfection of mammalian cells using PDMAEMA-based nano-stars, Biotechnology Reports 11 (2016) 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Chung S, Andersson T, Sonntag K-C, Björklund L, Isacson O, Kim K-S, Analysis of Different Promoter Systems for Efficient Transgene Expression in Mouse Embryonic Stem Cell Lines, 20(2) (2002) 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Malloggi C, Pezzoli D, Magagnin L, De Nardo L, Mantovani D, Tallarita E, Candiani G, Comparative evaluation and optimization of off-the-shelf cationic polymers for gene delivery purposes, Polymer Chemistry 6(35) (2015) 6325–6339. [Google Scholar]
- [266].Fischer D, Bieber T, Li Y, Elsässer H-P, Kissel T, A Novel Non-Viral Vector for DNA Delivery Based on Low Molecular Weight, Branched Polyethylenimine: Effect of Molecular Weight on Transfection Efficiency and Cytotoxicity, Pharmaceutical Research 16(8) (1999) 1273–1279. [DOI] [PubMed] [Google Scholar]
- [267].Siewert CD, Haas H, Cornet V, Nogueira SS, Nawroth T, Uebbing L, Ziller A, Al-Gousous J, Radulescu A, Schroer MA, Blanchet CE, Svergun DI, Radsak MP, Sahin U, Langguth P, Hybrid Biopolymer and Lipid Nanoparticles with Improved Transfection Efficacy for mRNA, 9(9) (2020) 2034. [DOI] [PMC free article] [PubMed] [Google Scholar]