Abstract
Purpose
To report the first successful application of in vitro maturation (IVM) of oocytes resulting in live births in two anovulatory women who had suffered oophorectomy following ovarian torsion after stimulation with gonadotropins.
Methods
Data abstraction was performed from medical records of two subfertile women with excessive functional ovarian reserve. Both women had previously received gonadotropins for ovulation induction or ovarian stimulation, resulting in ovarian torsion. They were offered IVM of oocytes retrieved from antral follicles after mild ovarian stimulation, insemination of mature oocytes using ICSI, and embryo transfer. Outcome measures were the incidence of complications and live birth after fertility treatment.
Results
Transvaginal retrieval of cumulus-oocyte complexes from a unique ovary was conducted. One patient had a singleton live birth after vitrified-warmed embryo transfer in the second IVM cycle. The other patient had a singleton live birth after transfer of a fresh blastocyst in her first IVM cycle.
Conclusions
Although approaches have been developed to prevent ovarian hyperstimulation syndrome (OHSS) and to increase the safety profile of fertility treatment in predicted high responders, women with an excessive functional ovarian reserve may have a non-negligible risk of ovarian torsion. For these patients, IVM should be considered as a safer alternative approach.
Keywords: IVF, IVM, Ovarian stimulation, Ovarian torsion, High responder
Introduction
Ovarian stimulation (OS) with gonadotropins is the cornerstone of assisted reproductive technologies. Whilst women who have an elevated functional ovarian reserve are at increased risk of ovarian hyperstimulation syndrome (OHSS) after OS, the emergence of OS protocols using GnRH agonists triggering final oocyte maturation and elective embryo vitrification has changed the landscape of modern reproductive medicine. Indeed, avoidance of severe OHSS is now a reality in high responders, and “freeze-only” strategies after GnRH agonist triggering can combine avoidance of severe OHSS with optimal cumulative live birth rates [1, 2]. Although OS in high responders generally results in increased cumulative live birth rates (CLBRs) compared to normal responders [3], ovarian enlargement in a proportion of these women may result in significant abdominal discomfort, or can even lead to ovarian torsion [4]. Ovarian stimulation with gonadotropins is also commonly used for ovulation induction in women with WHO I and II anovulation, including PCOS.
We here present two anovulatory patients with elevated functional ovarian reserve who had previously suffered ovarian torsion after OS with gonadotropins. Despite the general recommendation of conservative surgical management of ovarian torsion, both patients had undergone unilateral oophorectomy. After self-referral to our centre, they were offered in vitro maturation (IVM) of oocytes as a mild-approach alternative, which resulted in a live birth in both patients.
Methods
Data were obtained from chart review and reported without any patient identifiers. Patients signed informed consent regarding publishing their data. Publication of this case series was approved by the local ethical committee (no. B1432020000125).
Results
Patient 1
Patient 1 self-referred to our clinic with a history of primary subfertility for 2 years. She had experienced secondary amenorrhea after discontinuing the oral contraceptive pill. Before attending our clinic, she had previously undergone four cycles of ovulation induction prescribed by her gynaecologist. Because of resistance to clomiphene citrate, the patient had received HP-hMG (Menopur®; Ferring Pharmaceuticals A/S, Copenhagen, Denmark) at a daily dose of 75 IU, resulting in the recruitment of a single dominant follicle. Ovulation had been triggered using 5000 IU hCG (Pregnyl®, Organon, MSD, Haarlem, The Netherlands). After the third round of ovulation induction, the patient had presented at the emergency department with increasingly severe and persistent lower abdominal pain and nausea, 2 days after hCG administration. An explorative laparoscopy had been performed, which revealed two enlarged and rotated ovaries with a diameter of 12 cm and 10.5 cm. Because the right ovary and fallopian tube had shown persistent dark discolouration and complete absence of blood flow in the ovarian vessels after derotation, the gynaecologist had decided to perform a unilateral adnexectomy. The contralateral ovary had been derotated and recovered quickly. The histology report confirmed the diagnosis of necrosis of the right ovary. After self-referral to our fertility clinic, hormonal analysis was performed which was compatible with functional hypothalamic amenorrhea (WHO I anovulation, Table 1). Transvaginal ultrasound showed an antral follicle count (AFC) of 60 in the unique left ovary, a thin endometrium, and no ovarian cysts (Fig. 1). The BMI was 16.5 kg/m2 and sperm analysis in the partner was normal.
Table 1.
Baseline patient characteristics and IVM cycle outcome
| Patient 1 | Patient 2 | ||
|---|---|---|---|
| Baseline patient characteristics* | |||
| Age (years) | 25 | 30 | |
| BMI (kg/m2) | 16.5 | 19.4 | |
| AMH (ng/mL) | 24.5 | 12.3 | |
| AFC (N) | 60 | 30 | |
| FSH (IU/L) | <0.1 | 7.5 | |
| LH (IU/L) | <0.1 | 8.8 | |
| Progesterone (nmol/L) | 1.34 | 0.64 | |
| E2 (ng/L) | 32.0 | 28.0 | |
| IVM cycle outcome | Cycle 1 | Cycle 2 | |
| COC retrieved (N) | 70 | 77 | 30 |
| MII oocytes (N) | 35 | 37 | 25 |
| 2PN oocytes (N) | 25 | 22 | 21 |
| Cleavage-stage embryos (N) | 17 | 7 | 20 |
| Cryopreserved embryos (N/stage) | 0 | 7 (d3) | 7 (d5) |
| Fresh ET (N/stage) | 1/BL4BB (d5) | 0 | 1/BL4AA (d5)*** |
| eFET (N/stage)** | N/A | 4 | N/A |
| eFET no. 1 | 1/8c gr2 (d4) | ||
| eFET no. 2 | 1/C2 gr1 (d4) | ||
| eFET no. 3 | 1/5c gr3 (d4) | ||
| eFET no. 4 | 2/BL1 gr2, C2 gr2 (d4)*** | ||
| Live birth | 0 | 1 | 1 |
COC cumulus-oocyte complex, MII metaphase II, 2PN two pronuclei, ET embryo transfer, SET single embryo transfer, DET double embryo transfer, Y yes, N no
*Patient characteristics at intake (after unilateral oophorectomy)
**Cleavage-stage embryos were vitrified on day 3; embryo transfer was performed 1 day after embryo warming
***Resulting in live birth
Fig. 1.
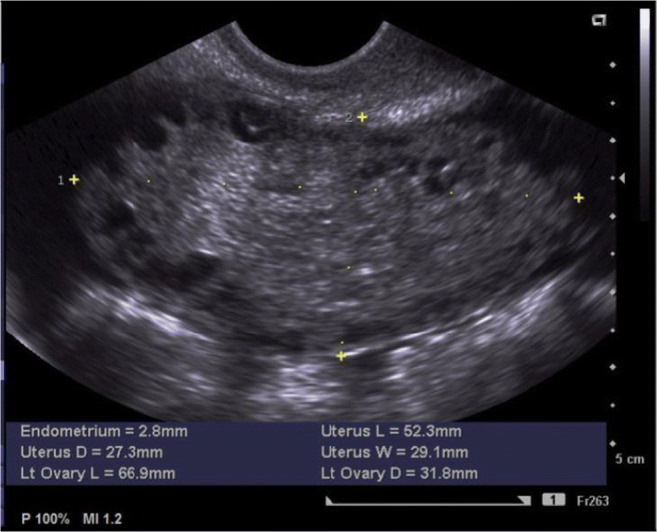
Baseline transvaginal ultrasound scan in patient 1 showing an AFC of 60 in the unique left ovary
Because of the history of ovarian torsion after OS with gonadotropins and hCG triggering, the patient declined further attempts of OS. In view of this, in vitro maturation (IVM) of oocytes was proposed, as previously described [5]. Briefly, ovarian stimulation involved administration of 225 IU HP-hMG (Menopur®) for four consecutive days. Transvaginal oocyte retrieval was performed 42 h after the last injection of HP-hMG. No hCG trigger was administered. Transvaginal ultrasound-guided oocyte retrieval was performed under general anaesthetic using a 17-gauge single-lumen needle on day 6 (K-OPS-1230-VUB; Cook Medical) at an aspiration pressure of −70 mmHg. No follicle flushing was performed. Follicular aspirates were collected in human tubal fluid (HTF) (IVF Basics® HTF HEPES, Gynotec B.V. Malden, The Netherlands) supplemented with heparin (5000 IU/mL, Heparin Leo, Leo Pharma, Belgium; final heparin concentration 20 IU/mL) and filtered through a cell strainer (Falcon®, 70-μm mesh size, BD Biosciences, CA, USA). In total, 70 cumulus-oocyte complexes (COCs) were harvested. After collection, COCs were washed and transferred to four-well dishes (Nunc, Thermo Fisher Scientific, MA, USA) containing IVM medium (IVM System, Medicult, Origio) supplemented with 75 mIU/mL HP-hMG (Menopur®), 100 mIU/mL hCG (Pregnyl®), and 10mg/mL human serum albumin (Vitrolife, Göteborg, Sweden), followed by 32 h of group culture of 10 COCs per well in 500 μL of IVM medium with an oil overlay (Ovoil, Vitrolife) at 37 °C under 6% CO2 and 20% O2. In total, 35 oocytes reached MII stage after IVM. Matured oocytes were inseminated using ICSI with partner sperm, and 25 oocytes fertilized normally. Embryos were cultured in individual 25-μL droplets of sequential media (Quinn’s Advantage™ Fertilisation, Fert™, Cleav™, Blast™ medium, Origio) and in G-TL™ monophasic culture medium (Vitrolife, Göteborg, Sweden) in the second cycle. Seventeen cleavage-stage embryos were observed on day 3 after ICSI, and embryo culture was continued to day 5.
Luteal-phase support for an IVM cycle with fresh embryo transfer consisted of transdermal estradiol (E2) gel (Oestrogel®; Besins Healthcare, Paris, France) at a dose of 2 mg, three times daily, which was started on the day before oocyte retrieval, and 600 mg daily of vaginal micronized progesterone (Utrogestan®, Besins Healthcare, Paris, France), starting on the evening of the day of the ICSI procedure. One blastocyst of good quality (BL4BB, as graded according to the Gardner and Schoolcraft scoring system [6]) was transferred freshly. No pregnancy ensued. Unfortunately, all other blastocysts were of insufficient developmental quality to be vitrified as surplus embryos.
A second IVM cycle was performed in this patient using the same protocol with 4 days of HP-hMG stimulation. Oocyte retrieval yielded 77 COCs; 37 oocytes reached MII stage after IVM, of which 22 were fertilized normally after ICSI. Seven embryos of good morphological quality were vitrified electively on day 3 after ICSI. In view of the poor embryo development beyond the cleavage stage in the previous IVM cycle, the embryos were not cultured to day 5. The patient went on to have HRT cycles for frozen embryo transfer (FET) when basal hormone levels were reached after the IVM cycle. Briefly, the endometrium was primed with transdermal Oestrogel® (two units administered three times a day). When an endometrial thickness of more than 6 mm was reached, luteal support was started using intravaginal micronized progesterone tablets (P, 200 mg three times a day; Utrogestan®, Besins Healthcare), and the embryo transfer was scheduled 5 days later. The transfer of day 3 vitrified embryos was performed 1 day after embryo warming. Administration of oestrogens and P was continued until a pregnancy test was performed and was continued until 7 weeks of gestation if the pregnancy test was positive, after which the dose was gradually reduced and discontinued 1 week later. Because no pregnancy was achieved after three consecutive HRT cycles with single vitrified-warmed embryo transfer, a diagnostic hysteroscopy with endometrium biopsy was performed which showed normal histology and no signs of endometritis. Two embryos that had been vitrified on day 3 were transferred 1 day after warming in a further HRT cycle, which resulted in a pregnancy leading to a healthy singleton live birth at term.
Patient 2
A 30-year-old woman self-referred to our clinic with primary subfertility for 3 years based on PCOS-related anovulation. Previous first-line fertility treatment with her gynaecologist had involved five cycles of ovulation induction with clomiphene citrate and intrauterine insemination (IUI), which had not resulted in pregnancy. She had gone on to have conventional ovarian stimulation for IVF using a GnRH antagonist protocol. Because of an increased risk of OHSS, she had been prescribed 0.2 mg of GnRH agonist triptorelin (Decapeptyl, Ipsen®, Merelbeke, Belgium) for final oocyte maturation. Fifteen cumulus-oocyte complexes had been retrieved and one good-quality blastocyst had been vitrified electively. After oocyte retrieval, the patient had presented severe pelvic pain whilst in the recovery room, not responding to standard analgesia. Upon laparoscopic exploration, gross enlargement of both ovaries had been observed and the right ovary had shown livid discolouration. The torsed right ovary had been derotated laparoscopically. However, because of signs of septicaemia on the next day, the patient had been operated again by laparoscopy 48 h later and had undergone unilateral right adnexectomy because of gangrenous changes of the ovary. After self-referral to our clinic, patient 2 was diagnosed with PCOS phenotype D, based on the extended Rotterdam criteria. Her basal hormonal profile is presented in Table 1.
Because of the history of ovarian torsion after OS using a GnRH antagonist protocol with GnRH agonist trigger, the patient declined further attempts of OS. In view of this, in vitro maturation (IVM) of oocytes was proposed, as described above. A short course of gonadotropins consisting of 225 IU HP-hMG (Menopur®) daily for three consecutive days was administered in patient 2. Transvaginal oocyte retrieval resulted in 30 COCs; 25 oocytes reached MII stage after IVM. All metaphase II oocytes were inseminated with ICSI, and 21 were fertilised normally. Embryos were cultured in individual 25-μL droplets of sequential media (Quinn’s Advantage™ Fertilisation, Fert™, Cleav™, Blast™ medium, Origio). On day 5 after ICSI, seven blastocysts of good or top quality were vitrified electively. Endometrium preparation for the fresh embryo transfer consisted of administration of Oestrogel® at a dose of 2 mg, three times daily and started on the day before oocyte retrieval, and 200 mg three times daily of intravaginal micronized progesterone (Utrogestan®) starting on the first day after oocyte retrieval. One top-quality blastocyst (BL4AA, as graded according to the Gardner and Schoolcraft scoring system [6]) was transferred freshly, which resulted in a healthy singleton live birth at term.
Discussion
We here illustrate the safe and successful use of IVM in patients at the severest side of the spectrum of elevated functional ovarian reserve. Not only do these patients have an increased risk of OHSS, the most common adverse event related to ovarian stimulation, but we would like to raise awareness of other potentially severe complications of fertility treatment in predicted high responders. With this report, we would like to warn against a reduced level of vigilance when treating these patients with gonadotropins in the current era, now that the incidence of severe OHSS, the worst enemy of reproductive medicine professionals, has reached an all-time low. Ovarian torsion may even occur after ovulation induction using gonadotropins in high responders, as illustrated by the first case in this report, and the combination of a GnRH agonist trigger with a freeze-only strategy prevented severe OHSS in the second case, but could not prevent ovarian torsion.
Adnexal torsion in the setting of fertility treatment has an incidence ranging from 0.08 to 0.2% and can lead to the loss of an ovary [7]. Its prevalence is probably underestimated, in view of typical under-reporting of poor results. Ovarian stimulation is a known risk factor for ovarian torsion due to ovarian enlargement. There is ample literature to recommend a conservative surgical approach when ovarian torsion occurs. Indeed, although population studies have indicated that unilateral oophorectomy does not lead to premature menopause, such a procedure may result in reduced success rates after fertility treatment in an IVF population [8]. Derotation of the ovary with or without oophoropexy has been advocated several decades ago and is considered the treatment of choice. Even when complete ischaemia has developed, detorsion of the ovary will often be successful in re-establishing reperfusion and normal ovarian function [9–11]. However, untimely diagnosis may lead to significant delay of surgical intervention, compromising the viability of the ovary.
Patients with polycystic ovarian morphology (PCOM) and those with polycystic ovary syndrome (PCOS) are predicted high responders and are particularly at risk for OHSS after OS [12]. In spite of well-defined criteria for the diagnosis of PCOS and a revised threshold for PCOM of ≥20 antral follicles per ovary [13], PCOS is a heterogeneous condition and there is no single best approach that will fit all patients with PCOS. Women with PCOS who undergo OS will exhibit a continuum of ovarian response intensity, depending on intrinsic ovarian parameters, including antral follicle count (AFC) and AMH serum levels, and patient characteristics such as BMI. Although pre-stimulation AFC and AMH has been found to be reliable predictors for high ovarian response, their utility to predict OHSS is limited [14] and there are no available literature data with regard to the prediction of the extent of ovarian enlargement and the risk of ovarian torsion in high responders. As far as the two patients described here are concerned, baseline AMH levels had not been analysed before the initial fertility treatment. However, because serum AMH levels were strongly elevated (24.5 ng/mL in patient 1; 12.3 ng/mL in patient 2) after oophorectomy, when the patients presented at our clinic, it is likely that these levels must have been even more elevated initially. Nevertheless, although a vast amount of literature has been produced with regard to ovarian response prediction, there is a lack of knowledge regarding the correlation between ovarian parameters, such as functional ovarian reserve and ovarian volume, and the risk of ovarian torsion. In a subset of high responders, OS may result in ovarian torsion even after moderate stimulation doses, such as in the setting of ovulation induction, as observed in patient 1 in this report.
We here illustrate the concept that for patients at the more severe side of the spectrum of functional ovarian reserve, IVM may be a safer alternative approach. Not only do these patients have an increased risk of side effects and adverse events related to ovarian stimulation, they also require intensified monitoring of ovarian stimulation, which may contribute to an increased level of stress during IVF treatment; on its turn, stress may lead to treatment termination before a successful pregnancy is achieved. In view of this and in spite of the existence of OHSS-free controlled ovarian stimulation protocols, a subset of high responders may be keen to embrace IVM as an alternative, lower-burden ART. To which extent these patients would accept a lower chance of pregnancy after IVM compared to standard IVF is currently unknown, although a recent survey among women with increase of OHSS has shown that about half of the patients are willing to accept a lower chance of pregnancy for a reduction of the OHSS rate [15].
Compared to conventional ovarian stimulation (OS) protocols, where oocytes are retrieved from large pre-ovulatory follicles, IVM involves the aspiration of cumulus-oocyte complexes (COCs) from antral follicles [16]. Hence, a shorter course of gonadotropins is administered, although the role of exogenous FSH has been controversial [17, 18] and FSH administration before oocyte retrieval has been omitted completely in some IVM clinics [19]. Nevertheless, even if gonadotropins are administered in an IVM cycle, very little monitoring is required. The cornerstone of an efficient IVM program is proper patient selection; women with elevated functional ovarian reserve parameters will yield sufficiently high numbers of immature oocytes to make up for the inherently lower efficiency of IVM compared to standard IVF [20–22]. Nevertheless, a specific AMH cut-off at which level the efficiency of IVM may approach or perhaps surpass that of OS followed by IVF/ICSI has not been established. Although IVM has initially been advocated as a method to eliminate OHSS, the development of OS strategies to dramatically reduce the risk of OHSS has mitigated the need for IVM as a strategy to avoid OHSS. Nevertheless, recent improvements to the IVM culture system [23] which may enhance the developmental potential of IVM embryos have refuelled the interest in IVM as a more patient-friendly approach in high responders. Although in centres with sufficient expertise cumulative live birth rates per started IVM cycle in women with PCOS reach ≈40% [24–26], embryo yield and success rates are still lower as compared to standard OS protocols. More specifically, IVM results in a relatively lower rate of embryos progressing to the blastocyst stage, but IVM embryos that do reach the blastocyst stage appear to have similar implantation potential as compared to blastocysts after OS [27]. Although the role of IVM in ART practice continues to be questioned in the modern era of agonist triggering and freeze-all strategies, we here illustrate the potential of IVM in selected patients who may have an increased risk of potentially severe complications when they undergo conventional ART. Nevertheless, future studies are required to compare the safety of IVM and conventional IVF/ICSI in a large cohort of predicted high responders.
Surprisingly, one case of ovarian torsion following IVM in a patient with PCOS has been reported [28]. However, in contrast with the patients presented here, the patient described by Giulini et al. had received a bolus of hCG before oocyte retrieval, which could have contributed to the development of this complication. In our opinion, and according to the recommendation by the International PCOS Network, IVM refers to the in vitro maturation of immature cumulus-oocyte complexes collected from antral follicles without the use of a hCG trigger [16].
Conclusion
Even though ovarian torsion is an infrequent complication of fertility treatment, with this report we would like to raise awareness of the risk of ovarian torsion after reproductive treatment in a subset of high responders to ovarian stimulation. We advocate that IVM should be considered as a safe and promising alternative ART in patients with strongly elevated markers of a high functional ovarian reserve, who are prone to develop adverse effects of gonadotropin stimulation. Moreover, ovarian stimulation in women with very high levels of AMH may also be cumbersome because of the non-linear dose-response curve. Further research should result in the identification of a specific category of high responders who have an elevated risk of ovarian torsion. As ovarian enlargement is more pronounced in women with high antral follicle counts, research efforts could be directed towards the establishment of a cut-off for AFC and AMH above which conventional ovarian stimulation may be too hazardous. In this category of patients, IVM may be considered as a safer option.
Author contribution
D.V. and M.D.V. drafted the manuscript. All the other authors critically reviewed the manuscript and approved the final version.
Funding
Dóra Vesztergom received an Erasmus+ grant as a contribution to her travel and subsistence costs (grant number: 19/1/KA103/060095/SMP /STT).
Data availability
All data were obtained from chart review and are available from the corresponding author (M.D.V.) on request.
Code availability
Not applicable.
Declarations
Ethics approval
Publication of this case series was approved by the local ethical committee (no. B1432020000125).
Consent to participate
Patients signed informed consent regarding publishing their data.
Consent for publication
Patients signed informed consent regarding publishing their data.
Conflict of interest
D.V., I.S., L.M., and C.B. declare no competing interests. M.D.V. has received speaker honoraria from MSD, Ferring and Gedeon Richter, and a grant from MSD, outside the submitted work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bodri D, Guillén JJ, Trullenque M, Schwenn K, Esteve C, Coll O. Early ovarian hyperstimulation syndrome is completely prevented by gonadotropin releasing-hormone agonist triggering in high-risk oocyte donor cycles: a prospective, luteal-phase follow-up study. Fertil Steril. 2010;93:2418–2420. doi: 10.1016/j.fertnstert.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Q, Chen Q, Wang L, Lu X, Lyu Q, Wang Y, Kuang Y. Live birth rates in the first complete IVF cycle among 20 687 women using a freeze-all strategy. Hum Reprod. 2018;33:924–929. doi: 10.1093/humrep/dey044. [DOI] [PubMed] [Google Scholar]
- 3.Drakopoulos P, Blockeel C, Stoop D, Camus M, De Vos M, Tournaye H, et al. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod. 2016;31:370–376. doi: 10.1093/humrep/dev316. [DOI] [PubMed] [Google Scholar]
- 4.Gorkemli H, Camus M, Clasen K. Adnexal torsion after gonadotrophin ovulation induction for IVF or ICSI and its conservative treatment. Arch Gynecol Obstet. 2002;267:4–6. doi: 10.1007/s00404-001-0251-x. [DOI] [PubMed] [Google Scholar]
- 5.Mostinckx L, Segers I, Belva F, Buyl R, Santos-Ribeiro S, Blockeel C, et al. Obstetric and neonatal outcome of ART in patients with polycystic ovary syndrome: IVM of oocytes versus controlled ovarian stimulation. Hum Reprod. 2019;34:1595–1607. doi: 10.1093/humrep/dez086. [DOI] [PubMed] [Google Scholar]
- 6.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–311. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Santos-Ribeiro S, Mackens S, Racca A, Blockeel C. Towards complication-free assisted reproduction technology. Best Pract Res Clin Endocrinol Metab. 2019;33:9–19. doi: 10.1016/j.beem.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Lind T, Holte J, Olofsson JI, Hadziosmanovic N, Gudmundsson J, Nedstrand E, et al. Reduced live-birth rates after IVF/ICSI in women with previous unilateral oophorectomy: results of a multicenter cohort study. Hum Reprod. 2018;33:238–247. doi: 10.1093/humrep/dex358. [DOI] [PubMed] [Google Scholar]
- 9.Mashiach S, Bider D, Moran O, Goldenberg M, Ben-Rafael Z. Adnexal torsion of hyperstimulated ovaries in pregnancies after gonadotropin therapy. Fertil Steril. 1990;53:76–80. doi: 10.1016/S0015-0282(16)53219-1. [DOI] [PubMed] [Google Scholar]
- 10.Oelsner G, Cohen SB, Soriano D, Admon D, Mashiach S, Carp H. Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum Reprod. 2003;18:2599–2602. doi: 10.1093/humrep/deg498. [DOI] [PubMed] [Google Scholar]
- 11.Weitzman VN, DiLuigi AJ, Maier DB, Nulsen JC. Prevention of recurrent adnexal torsion. Fertil Steril. 2008;90:2018–2020. doi: 10.1016/j.fertnstert.2008.02.144. [DOI] [PubMed] [Google Scholar]
- 12.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ, International PCOS Network Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364–379. doi: 10.1016/j.fertnstert.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nastri CO, Teixeira DM, Moroni RM, Leitão VM, Martins WP. Ovarian hyperstimulation syndrome: pathophysiology, staging, prediction and prevention. Ultrasound Obstet Gynecol. 2015;45:377–393. doi: 10.1002/uog.14684. [DOI] [PubMed] [Google Scholar]
- 15.Braam SC, de Bruin JP, Mol BWJ, van Wely M. The perspective of women with an increased risk of OHSS regarding the safety and burden of IVF: a discrete choice experiment. Hum Reprod Open. 2020;hoz034. 10.1093/hropen/hoz034. [DOI] [PMC free article] [PubMed]
- 16.De Vos M, Smitz J, Thompson JG, Gilchrist RB. The definition of IVM is clear-variations need defining. Hum Reprod. 2016;31:2411–2415. doi: 10.1093/humrep/dew208. [DOI] [PubMed] [Google Scholar]
- 17.Trounson A, Wood C, Kausche A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil Steril. 1994;62:353–362. doi: 10.1016/S0015-0282(16)56891-5. [DOI] [PubMed] [Google Scholar]
- 18.Fadini R, Dal Canto MB, Mignini Renzini M, Brambillasca F, Comi R, Fumagalli D, et al. Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: a prospective randomized study. Reprod BioMed Online. 2009;19:343–351. doi: 10.1016/S1472-6483(10)60168-X. [DOI] [PubMed] [Google Scholar]
- 19.Zheng X, Guo W, Zeng L, Zheng D, Yang S, Wang L, et al. Live birth after in vitro maturation versus standard in vitro fertilisation for women with polycystic ovary syndrome: protocol for a non-inferiority randomised clinical trial. BMJ Open. 2020;10:e035334. doi: 10.1136/bmjopen-2019-035334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fadini R, Comi R, Mignini Renzini M, Coticchio G, Crippa M, De Ponti E, et al. Anti-Mullerian hormone as a predictive marker for the selection of women for oocyte in vitro maturation treatment. J Assist Reprod Genet. 2011;28:501–508. doi: 10.1007/s10815-011-9589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzman L, Adriaenssens T, Ortega-Hrepich C, Albuz FK, Mateizel I, Devroey P, et al. Human antral follicles <6 mm: a comparison between in vivo maturation and in vitro maturation in non-hCG primed cycles using cumulus cell gene expression. Mol Hum Reprod. 2013;19:7–16. doi: 10.1093/molehr/gas038. [DOI] [PubMed] [Google Scholar]
- 22.Siristatidis C, Sergentanis TN, Vogiatzi P, Kanavidis P, Chrelias C, Papantoniou N, et al. In vitro maturation in women with vs. without polycystic ovarian syndrome: a systematic review and meta-analysis. PLoS One. 2015;10:e0134696. doi: 10.1371/journal.pone.0134696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sánchez F, Le AH, Ho VNA, Romero S, Van Ranst H, De Vos M, et al. Biphasic in vitro maturation (CAPA-IVM) specifically improves the developmental capacity of oocytes from small antral follicles. J Assist Reprod Genet. 2019;62:353–310. doi: 10.1007/s10815-019-01551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackens S, Pareyn S, Drakopoulos P, Deckers T, Mostinckx L, Blockeel C, et al. Outcome of in vitro oocyte maturation (IVM) in patients with PCOS: does phenotype have an impact? Hum Reprod. 2020;35:2272–2279. doi: 10.1093/humrep/deaa190. [DOI] [PubMed] [Google Scholar]
- 25.Vuong LN, Ho VNA, Ho TM, Dang VQ, Phung TH, Giang NH, et al. In-vitro maturation of oocytes versus conventional IVF in women with infertility and a high antral follicle count: a randomized non-inferiority controlled trial. Hum Reprod. 2020;35:2537–2547. doi: 10.1093/humrep/deaa240. [DOI] [PubMed] [Google Scholar]
- 26.Ho VNA, Braam SC, Pham TD, Mol BW, Vuong LN. The effectiveness and safety of in vitro maturation of oocytes versus in vitro fertilization in women with a high antral follicle count. Hum Reprod. 2019;34:1055–1064. doi: 10.1093/humrep/dez060. [DOI] [PubMed] [Google Scholar]
- 27.Walls ML, Ryan JP, Keelan JA, Hart R. In vitro maturation is associated with increased early embryo arrest without impairing morphokinetic development of useable embryos progressing to blastocysts. Hum Reprod. 2015;30:1842–1849. doi: 10.1093/humrep/dev125. [DOI] [PubMed] [Google Scholar]
- 28.Giulini S, Dante G, Xella S, La Marca A, Marsella T, Volpe A. Adnexal torsion during pregnancy after oocyte in vitro maturation and intracytoplasmic sperm injection cycle. Case Rep Med. 2010;2010:141875. 10.1155/2010/141875. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data were obtained from chart review and are available from the corresponding author (M.D.V.) on request.
Not applicable.


