Abstract
Purpose
Oxygen tension during the in vitro maturation (IVM) of oocytes is important for oocyte developmental competence. A conflict exists in the literature as to whether low oxygen during IVM is detrimental or beneficial to the oocyte. Many research and clinical labs use higher than physiological oxygen tension perhaps believing that low-oxygen tension is detrimental to oocyte development. Other studies show that glucose is important if low-oxygen tension is used during maturation. In this study, we look at the link between low oxygen and glucose availability during IVM to resolve misconceptions around low-oxygen tension during IVM.
Methods
Bovine cumulus oocyte complexes (COCs) were matured at 20% vs 7% oxygen in media containing differing glucose concentrations or varying availability. Cleavage and blastocyst rates were recorded. RT-PCR determined expression levels of metabolic, oxygen, and stress-responsive genes following IVM.
Results
Embryo development in 7% oxygen groups with 2.3mM glucose/low glucose availability was lower than 20% oxygen groups. Under 7% oxygen with 5.6mM glucose or higher glucose availability, rates were restored to those seen in 20% oxygen. Expressions of BNIP3, ENO1, GAPDH, and SLC2A1, were upregulated in 7% oxygen/low glucose, compared to 20% oxygen groups. BNIP3 expression was higher in 7% oxygen group with low glucose availability compared to the 20% groups.
Conclusion
Oocyte developmental competence is negatively impacted following IVM in low oxygen when glucose availability is limited. Glucose concentration and physical culture conditions need to be considered when comparing the effects of different oxygen concentrations during IVM.
Keywords: Oocyte, Cumulus oocyte complex, In vitro maturation, Bovine, Oxygen, Glucose, BNIP3, ENO1, GAPDH, SLC2A1
Introduction
In vitro maturation (IVM) of oocytes is used in human and livestock-assisted reproductive technology (ART). Reproductive pathologies that may benefit from IVM include polycystic ovary syndrome (PCOS) [1, 2], ovarian hyper-responsiveness [2], high antral follicle counts [3], and for fertility preservation in cases such as childhood cancer [4]. Normo-ovulatory women may choose IVM to avoid ovarian hyperstimulation, and large financial costs, while producing less adverse side effects than in standard in vitro fertilization (IVF) practices [5]. IVM is a necessary and valuable procedure in livestock industries for rapid genetic improvement of high value breeding stock [6]. IVM involves the collection of immature oocytes in the form of cumulus oocyte complexes (COCs) from ovaries, which are then matured, fertilised, and cultured in vitro, after which viable embryos can be either cryopreserved or transferred into a recipient. Despite decades of research into factors influencing oocyte developmental competence, IVM in both the clinical and livestock setting has been poorly translated into practice. Both reduced embryo production per collection [7, 8] and, more significantly, poorer pregnancy rates [9] compared to conventional approaches are generally observed, along with suspected aberrations in embryonic, fetal and post-partum growth [10, 11], and these have all constrained the adoption of IVM.
The oxygen concentration in the reproductive tract in many mammalian species is between 1.5 and 8.7% [12, 13], with other studies finding dissolved levels in follicular fluid to be between 1 and 5% [14, 15]. The oxygen concentration typically used during standard IVM in clinical and research laboratories and within the livestock industries is 20–21% (generally atmospheric levels, unless at altitude). Higher than physiological oxygen levels generates an increased risk of reactive oxygen species (ROS) formation [16–21], which may result in an imbalance in the ratio of pro-oxidants to anti-oxidants, leading to cell damage [22]. Varying the oxygen concentration can also influence many metabolic and apoptotic pathways, and deviation from physiological levels may result in aberrant long-term development [23]. There is also a risk that these unphysiological oxygen levels will confound the research surrounding oocyte maturation and development, giving an inaccurate picture of how these processes occur naturally.
Likewise, glucose levels that are too high or too low can have negative consequences during IVM and culture, affecting oocytes and embryos alike [24]. The glucose concentration in human follicular fluid is around 3.3 mM [25] and in cattle between 1.4 and 5 mM [26] yet most media utilised for human or cattle oocyte IVM contain 5.6 mM glucose (traditionally based on either TCM199, or MEM with Earle’s salts), while Waymouth medium contains 23 mM glucose. Metabolism of the COC changes under low oxygen, with glycolytic metabolism being the preferred pathway [27]. The oocyte is dependent on oxidative phosphorylation and has little capacity for glycolysis due to having low phosphofructokinase activity [28–32]. Under low oxygen levels, the cumulus cells increase conversion of glucose to pyruvate and L-lactate, which is then transported into the oocyte. Pyruvate is the key substrate for the tricarboxylic acid (TCA) cycle and oxidative phosphorylation for energy production (ATP) within the oocyte [28–33]. The high glycolytic activity in cumulus cells also produces ATP via lactate production. In this way, it has been proposed that cumulus cells “spare” the available oxygen for oxidative phosphorylation within the oocyte [34, 35]. Glucose is important for nuclear maturation [36–39] and cytoplasmic maturation [39] as its metabolism via the pentose phosphate pathway (PPP) produces substrates for purine nucleotide synthesis [40] and NADPH which is involved with cytoplasmic integrity during pro-nuclear formation [41]. A study by Bermejo-Alvarez [42] showed that low oxygen levels during IVM alters gene expression in bovine cumulus cells and oocytes, in particular, metabolic genes involved with anaerobic glycolysis. Glucose supply is also important for the expansion of the cumulus matrix following an ‘ovulatory’ signal, be it either in vivo or in vitro, as it contributes to the synthesis of hyaluronic acid, which is synthesised from glucose via the hexosamine biosynthesis pathway [43, 44], and is important for the capacitation and acrosome reaction during fertilisation [45–47]. This reliance on glucose during oocyte maturation under low oxygen suggests that an adequate concentration needs to be available per COC, which would be influenced by both the glucose concentration within the medium, as well as the density of COCs.
Previous literature investigating the optimal oxygen concentration during IVM has produced conflicting results, and this is likely due to differing IVM culture conditions. Early studies of mouse and hamster oocyte IVM suggest that low oxygen levels are optimal for nuclear maturation [48, 49]. Blastocyst development rate tended to decrease as oxygen concentration increased from 2 to 20% during IVM of mouse oocytes in one study [50], while no effect on development due to varying oxygen levels was found in another study [51]. Both positive and negative effects of reduced oxygen have been reported during IVM of bovine oocytes. Hashimoto et al. [20] reported that meiotic maturation and ATP content of bovine oocytes were lower when matured in 5% oxygen using a defined embryo culture medium (SOFaa) containing 1.5 mM glucose. However, both increased significantly as glucose levels were increased to up to 20 mM (from 0 mM, 1.5 mM, 5.56 mM, 20 mM, and 40 mM), with ATP content of oocytes significantly higher at 20 mM glucose than at 1.5 mM. Intracellular H2O2 was lower in COCs matured under 5% oxygen, compared with 20% oxygen, in 20 mM glucose, and subsequent blastocyst development rate was higher following IVM at the lower oxygen concentration. However, detrimental effects of low oxygen levels during bovine IVM have been reported in other studies. Pinyopummintr and Bavister [52] investigated bovine oocyte maturation and fertilisation under 5%, 10%, and 20% oxygen levels, concluding that low levels are unfavourable for IVM/IVF compared to atmospheric levels. However, there was no segregation between the influence of oxygen concentration during maturation and fertilisation in this study, while others have reported detrimental effects of low oxygen levels during IVF due to a higher oxygen demand in the presence of a high sperm density [42]. De Castro e Paula and Hansen [53] also found that blastocyst rate of bovine oocytes matured in 21% oxygen was higher than in 5% oxygen in medium containing 5.6 mM glucose; however, COCs were cultured at a density of 5 μl of media per COC, which has been shown to negatively impact glucose consumption [43]. Watson et al. [54] also reported lower blastocyst rates from oocytes matured in lower oxygen (7%) compared to 20%; however, this study used mSOFaa media, which typically contains 1.5 mM glucose concentration, a level shown by Hashimoto [20] to be inadequate in meeting the metabolic demands of the COC under low oxygen levels. These studies indicate that glucose concentration, as well as glucose availability in the maturation medium, when comparing the effects of high or low oxygen conditions during IVM could have influenced these conflicting results—this needs to be considered when looking at the effects of a low oxygen environment on the success of IVM.
We hypothesise that the suitability of a low oxygen concentration during IVM is determined by glucose availability, which in turn is a determinant of oocyte developmental competence. In this study, we carried out a thorough investigation into the effects of high or low oxygen concentration in combination with high or low glucose availability during IVM of bovine COCs. We varied glucose availability by altering 1) glucose concentration, 2) volume of maturation media/COC, 3) number of COCs per drop and 4) the effect of combining parameters 1-3. By increasing glucose concentration or the volume of medium/COC, more glucose would be available per COC. We also investigated whether this could be achieved by increasing access to glucose by decreasing the number of COCs per drop (i.e. having fewer COCs adjacent to one-another). Finally, we hypothesise that glucose availability is not as developmentally important when IVM is carried out in a high oxygen environment. To determine the potential mechanisms underlying the impact on oocyte developmental competence, we measured the expression of metabolic, oxygen responsive, and stress responsive genes in the COC following maturation. These genes were chosen to highlight the relationship between the effects of glucose and oxygen on COC metabolism.
Materials and methods
Unless otherwise stated, all chemicals and reagents were purchased from Sigma Aldrich (St Louis, MO, USA).
All media used were supplied as serum-free media (ART Labs Solutions, Adelaide, Australia), containing 0.05 g/L gentamycin and 4 mg/ml BSA (AlbumiNZ™ Low Fatty Acid BSA from MP Biomedicals), and comprised of: VitroWash: VitroMat; VitroFert (manufacturer’s recommended density = 10 μl/COC); VitroCleave (4 μl/embryo); and VitroBlast (4 μl/embryo). All media dishes were overlayed with paraffin oil (Merck Millipore, Darmstadt, Germany). Each replicate was performed with IVM occurring on separate days of ovary collection.
VitroFert was further supplemented with 12.5 μM penicillamine, 25 μM hypotaurine, 1.25 μM epinephrine, and 10 IU/ml heparin (DBL heparin sodium injection, Hospira Australia Pty Ltd, Melbourne, VIC, Supplier: Pacific Vet).
Collection and maturation of cumulus oocyte complexes
Bovine ovaries were obtained from a local abattoir. Ovaries were transported to the laboratory in a vacuum-sealed flask with physiological saline at 33 °C within 2–4 h of slaughter. COCs were aspirated from 3 to 8 mm antral follicles, using an 18 gauge needle. The undiluted follicular fluid aspirant was transferred into 14-ml conical tubes and held at approximately 38 °C for up to 1 h while aspiration occurred. The follicular fluid aspirant was transferred by glass pipette into a 100-mm diameter petri dish, and COCs were isolated using a dissecting microscope and immediately transferred into VitroWash medium. 40 ±5 COCs were selected for IVM for each treatment group on the basis of having a homogeneous cytoplasm and at least three layers of non-atretic and tightly packed cumulus cells. COCs were then randomly allocated to treatment groups for IVM. IVM conditions varied (oxygen concentration, glucose concentration, volume of media per COC, number of COCs per culture drop) as described below (Experiments 1–4). All IVM was performed in modifications of VitroMat media varying in glucose concentrations and supplemented with 0.1 IU/ml rhFSH (recombinant human Follicle Stimulating Hormone; Puregon®, Organon).
IVM experimental designs
For an overview of the IVM conditions, please refer to Table 1.
Table 1.
Experimental design. Bold entries indicate parameters altered within an experiment (n = 5 independent replicates per experiment, with 40±5 COCs per treatment per replicate)
| Experiment | Treatment group | Oxygen concentration (%) | Glucose (mM) | Volume of drop (ul) | Number of COCs per drop | Volume of available medium |
|---|---|---|---|---|---|---|
| 1 | 1 | 20 | 2.3 | 400 | 40 | 10 μl/COC |
| 2 | 20 | 5.6 | 400 | 40 | 10 μl/COC | |
| 3 | 7 | 2.3 | 400 | 40 | 10 μl/COC | |
| 4 | 7 | 5.6 | 400 | 40 | 10 μl/COC | |
| 2 | 1 | 20 | 2.3 | 400 | 40 | 10 μl/COC |
| 2 | 20 | 2.3 | 800 | 40 | 20 μl/COC | |
| 3 | 7 | 2.3 | 400 | 40 | 10 μl/COC | |
| 4 | 7 | 2.3 | 800 | 40 | 20 μl/COC | |
| 3 | 1 | 20 | 2.3 | 400 | 40 | 10 μl/COC |
| 2 | 20 | 2.3 | 100 | 10 | 10 μl/COC | |
| 3 | 7 | 2.3 | 400 | 40 | 10 μl/COC | |
| 4 | 7 | 2.3 | 100 | 10 | 10 μl/COC | |
| 4 | 1 | 20 | 2.3 | 400 | 40 | 10 μl/COC |
| 2 | 20 | 5.6 | 200 | 10 | 20 μl/COC | |
| 3 | 7 | 2.3 | 400 | 40 | 10 μl/COC | |
| 4 | 7 | 5.6 | 200 | 10 | 20 μl/COC |
Experiment 1: Effect of Oxygen and Glucose concentrations during IVM
In this experiment, we determined whether glucose concentration under low oxygen during IVM affects oocyte developmental competence. Maturation was performed in media containing either 2.3 mM glucose or 5.6 mM glucose in 20% or 7% oxygen at 38.5 °C in 6% CO2 with nitrogen (N2) balance, for 24 h. Standard culture conditions of 40 ± 5 COCs in 400 μl media were allocated to each treatment group (10 μl media per COC) in 4 well culture dishes (NUNC, Thermo Fisher Scientific, Waltham, MA, USA). Ten COCs were collected from each experimental group at 21 h and snap frozen for gene expression analysis. The experiment was replicated 5 times.
Experiment 2: Effect of oxygen and medium volume during IVM
In this experiment, we determined whether glucose availability is affected by volume of maturation medium per COC and whether the same affects are found as in Experiment 1. Maturation was performed in medium containing 2.3 mM glucose in either 20% or 7% oxygen at 38.5 °C in 6% CO2 with N2 balance, for 24 h. COCs (40 ± 5/treatment group) were cultured in volumes of either 10 μl medium per COC (400 μl) or 20 μl medium per COC (800 μl) in 60 mm petri dishes. The experiment was replicated 5 times.
Experiment 3: Effect of oxygen and number of COCs per drop during IVM
Here we determined whether glucose availability is affected by the number COCs per drop of maturation medium and whether the same affects are found as that for Experiment 1. Maturation was performed in medium containing 2.3 mM glucose in either 20% or 7% oxygen at 38.5 °C in 6% CO2 with N2 balance, for 24 h, with either 40 ± 5 COCs per drop or 10 COC per drop, in 10 μl medium per COC (40 ± 5/treatment group), using 35 mm Falcon dishes. The experiment was replicated 5 times.
Experiment 4: Effects of oxygen and combined media parameters
In this experiment, we determined whether physical culture conditions in combination with higher glucose has an additive effect on oocyte developmental competence under low oxygen concentration. Maturation was performed under 20% or 7% oxygen in medium containing either 2.3 mM glucose or 5.6 mM glucose at 38.5 °C in 6% CO2 with N2 balance, for 24 h. The control group was cultured in 20% oxygen, with 2.3 mM glucose, 40 ± 5 COCs per drop, 10 μl medium/COC. Group 2 was cultured in 20% oxygen, with 5.6 mM glucose, and 20 μl medium/COC, 10 COCs/drop. Group 3 was cultured in 7% oxygen, with 2.3 mM glucose, 10 μl medium/COC, 40 ± 5 COCs/group. Group 4 was cultured in 7% oxygen with 2.3 mM glucose, 10 COC/drop, 20 μl medium/COC, while Group 5 was cultured in 7% oxygen, 5.6 mM glucose, 10 COC/drop, 20 μl medium/COC. The experiment was replicated 5 times (40 ± 5/treatment group. From 3 of the replicates, 10 COCs were collected from each experimental group at 21 h and snap frozen for gene expression analysis.
Evaluation of cumulus expansion
For Experiment 1 and 4, COC expansion was graded at 21 h after the start of IVM according to a subjective scale ranging from 0 to 4 and a cumulus expansion index (CEI) was calculated as described previously [55, 56].
In vitro fertilisation (IVF) and in vitro culture of embryos (IVC)
Following IVM, 10 COCs per treatment group (in experiments 1 and 4) were processed for RNA extraction and the remainder were fertilised and cultured in vitro. In vitro fertilization of all mature oocytes was performed using frozen sperm from a single bull of proven in vitro fertility. A motile sperm fraction was separated from a thawed semen sample (from 0.25 ml straws) using a discontinuous silica-solution gradient (BoviPure/BoviDilute, Nidacon, Gothenburg, Sweden) and centrifuged at room temperature for 20–25 min at 774g relative centrifugal force (RCF). The supernatant was removed, the pellet washed with 1 ml of VitroWash, and centrifuged for 5 min at 194 RCF. Wash medium was removed, and the motile sperm pellet was resuspended with 200 μl of VitroFert medium then diluted to a concentration of 1×106 spermatozoa/ml within the fertilisation drops (10 μl/COC) and cultured for 24 h at 38.5 °C in 20% oxygen, 6% CO2.
Presumptive zygotes were manually denuded of cumulus cells in VitroWash medium at approximately 23-24 h post-insemination, and were washed and distributed into 20 μl drops (5 COCs/drop) of equilibrated VitroCleave medium and cultured at 38.5 °C in 7% oxygen, 6% CO2 and N2 balance for 5 days (Day 1 to Day 5). On Day 5, embryos were transferred into 20 μl drops of VitroBlast with 5 embryos per drop and further cultured at 38.5°C in 7% oxygen, 6% CO2, N2 balance to Day 8. Embryos were graded by a blinded experienced embryologist at Day 8 according to the definitions presented in the Manual of the International Embryo Transfer Society [57].
Gene expression
Quantification of mRNA abundance was performed on targeted genes. Expression of metabolic genes; Enolase 1 (ENO1), Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), Glucose-6-phosphate dehydrogenase (G6PD), and Glucose transporter 1 (SLC2A1); a specific oxygen regulated gene: Carbonic Anhydrase 9 (CA9), and two stress related genes: B-cell lymphoma 2 Associated X (BAX), and BCL2 B-cell lymphoma 2 Interacting Protein 3 (BNIP3) was measured in COCs collected from Experiments 1 and 4.
For RNA extraction, ten COCs from each treatment group were removed at 21 h maturation for each replicate, snap frozen in liquid nitrogen (LN2), and stored at −80 °C for later RNA extraction. Extraction of RNA was performed using an RNeasy Micro Kit (Qiagen Pty Ltd, Chadstone Place, Chadstone, VIC), according to the manufacturer’s instructions. RNA was eluted into ~14 μl RNase free water by centrifugation at 8161 RCF. The concentration of extracted RNA was determined using a NanoDrop 2000 (Thermo Fisher Scientific).
Reverse transcription was carried out using Superscript III First-Strand Synthesis System (Invitrogen-Thermo Fisher Scientific) for RT-PCR and random hexamer as a primer according to the manufacturer’s instructions. Negative controls included a no template control (NTC) and no Reverse Transcriptase (-RT) which were prepared at each cDNA synthesis. Once prepared, cDNA samples were stored at −20°C.
Real-time PCR
Quantification of cDNA was achieved using real time PCR (qRT-PCR). A Quant Studio 12k Flex system PCR machine (ThermoFisher Scientific Australia) was used and reactions were run in duplicate. TaqMan assay primers and probes with a FAM™ dye label (Life Technologies Australia Pty Ltd) were used to quantify cDNA. The PCR master mix contained (1x) 5 μl TaqMan Gene Expression Master Mix, 0.5 μl of primer set, and 2.5 μl water. The thermal profile was: Stage 1: 50 °C for 2 min, Stage 2: 95 °C for 10 min (Hot start), Stage 3: 95 °C for 15 s, and 60 °C for 1 min (40 cycles). The 2^DDCT was used to quantify gene expression levels. Quantification was normalised to the reference gene β-actin and controls included exclusion of cDNA template and reverse transcription enzyme in complete reactions. A calibration sample of cDNA was run with every plate/gene as a sample reference. Primer assay ID for each gene primer sequence can be found in Table 2.
Table 2.
TaqMan gene expression assay ID for gene expression analysis
| Gene description | Origin | Taqman gene expression Assay ID |
|---|---|---|
| ENO1 | Bovine | Bt03230937_m1 |
| GAPDH | Bovine | Bt03210913_g1 |
| G6PD | Bovine | Bt03649181_m1 |
| SLC2A1 | Bovine | APAAD7P (custom) |
| BAX | Bovine | Bt01016551_g1 |
| BNIP3 | Bovine | Bt03236550_m1 |
| CA9 | Bovine | APNKU6K (custom) |
| ACTB | Bovine | Bt03279174_g1 |
Statistical analysis
Developmental data were analysed by two-way analysis of variance (ANOVA) with Tukey’s multiple comparisons, on the transformed data (Arcsine(SQRT)) in GraphPad Prism 7.0 software (GraphPad Software, San Diego, CA, USA). Cleavage rate (transformed data) from experiment 4 was not normally distributed, and thereafter was analysed using a non-parametric test (Kruskal Wallis). CEI for experiment 1 was analysed by a Two-Way ANOVA, while CEI data for experiment 4 was not normally distributed, and thereafter was analysed using a non-parametric test (Kruskal Wallis). Gene expression differences found after qRT-PCR from Experiment 1 were analysed on the 2^DDCT values using a two-way ANOVA with Tukey’s multiple comparisons, while expression levels from Experiment 4 were analysed using a one-way ANOVA for non-parametric data (Kruskal Wallis test on log10 transformed data), with Dunn’s multiple comparisons, as the data were not normally distributed. Significance was accepted at P < 0.05, with biological trends accepted at P < 0.1. All data, where appropriate, are presented as mean ± the standard error of the mean (SEM).
Results
Cumulus expansion
Experiment 1: Effect of oxygen and glucose concentrations during IVM
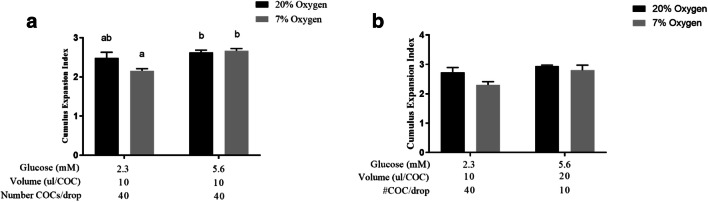
Cumulus expansion in Experiment 1 was reduced in COCs matured at 7% oxygen with 2.3 mM glucose compared with those matured in 5.6 mM glucose under 20% oxygen or 7% oxygen (Fig. 1a). Cumulus expansion in COCs matured in 7% oxygen, 2.3 mM glucose also tended to be lower than those matured in 20% oxygen, 2.3 mM glucose (0.1>P>0.05) (Fig. 1a).
Fig. 1.
Cumulus expansion of bovine COCs is negatively impacted when IVM occurs under low oxygen and low glucose levels. Average of cumulus expansion scores according to the Vanderhyden scoring system [56] following 21 h of IVM in two different oxygen and glucose concentrations (Experiment 1, 2×2 factorial design) (a), and with 4 varying treatment parameters (Experiment 4: oxygen; glucose concentration; volume of medium/COC; number of COCs/drop) (b). Data presented as mean ± SEM (n = 4 independent replicates with 40±5 COCs/treatment group/replicate). Means with different superscripts indicate significant differences between treatment groups (P < 0.05)
Experiment 4: Effect of oxygen and combined media parameters
Cumulus expansion did not differ between groups in Experiment 4 (Fig. 1b).
Embryo development
Experiment 1: Effect of oxygen and glucose concentrations during IVM
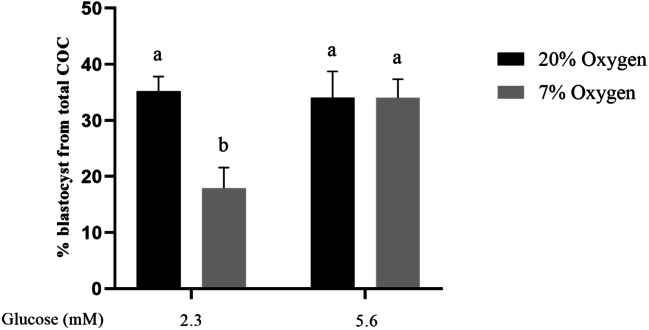
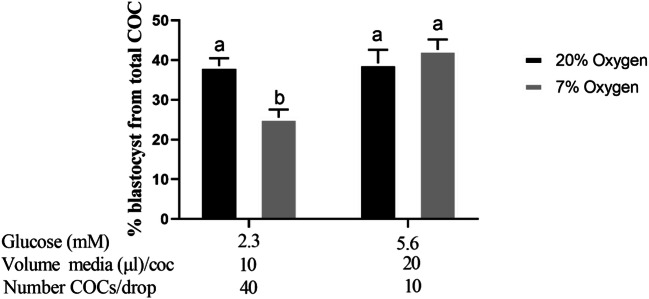
Cleavage rates did not differ between groups. Specifically, mean cleavage rates across replicates were 81% (control), 78% (20% oxygen/5.6mM glucose), 68% (7% oxygen/2.3mM glucose, and 83% (7% oxygen/5.6mM glucose). Blastocyst development rates, expressed as the percentage of total oocytes, were reduced following COC maturation in 7% oxygen with low glucose (2.3 mM), compared with 20% oxygen at either glucose level), and the group matured at 7% oxygen with 5.6 mM glucose (P < 0.05; Fig. 2). COCs matured in 7% oxygen with 5.6 mM glucose had comparable blastocyst rates to both 20% oxygen groups (Fig. 2). Hatching blastocyst rates from total oocytes were not different between groups; however, the 7% oxygen/2.3mM glucose group tended lower than the control (0.1>P>0.05).
Fig. 2.
During IVM, low oxygen in combination with low glucose significantly reduces subsequent development to blastocyst, which is rescued by increasing glucose concentration. Blastocyst rate (% from starting number of COCs) of embryos developed from COCs matured in two different oxygen concentrations, and two different glucose concentrations (Experiment 1, 2×2 factorial design). Data presented as mean ± SEM of (n = 5 independent replicates, 40±5 COCs/treatment group/replicate). Means with different superscripts indicate significant differences between treatment groups (P < 0.05)
Experiment 2: Effects of oxygen and media volume during IVM
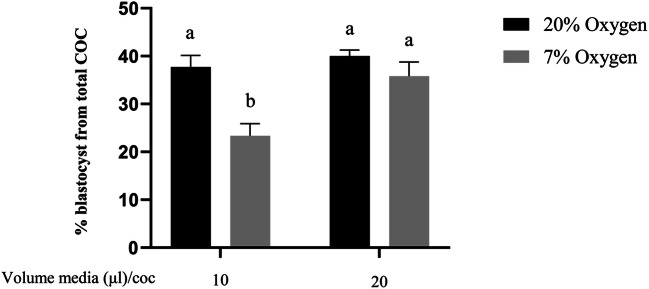
All COCs were matured in 2.3 mM glucose in this experiment. Cleavage rate did not differ between groups. Mean cleavage rates across reps were 80% (control), 79% (20% oxygen/20ul/COC), 65% (7% oxygen/10ul/COC, and 78% (7% oxygen/20ul/COC). Blastocyst development rates (% from total oocytes) were lower following IVM in 7% oxygen with 10 μl media per COC, compared to all other groups (P < 0.05; Fig. 3). Blastocyst development rates for COCs matured in 7% oxygen with 20 μl medium/COC were comparable to both groups matured at 20% oxygen. Hatching blastocyst rates from total oocytes were not different between groups.
Fig. 3.
During IVM, low oxygen with low glucose and a lower volume of medium per COC, significantly reduces oocyte developmental competence, which is rescued by increasing volume of medium available per COC. Blastocyst rate (% from starting number of COCs) of bovine embryos developed from oocytes matured in two different oxygen concentrations, and two different volumes of media per COC (Experiment 2, 2×2 factorial design). Data presented as mean ± SEM (n = 5 independent replicates with 40±5 COCs/treatment group/replicate). Means with different superscripts indicate significant differences between treatment groups (P < 0.05)
Experiment 3: Effect of oxygen and number of COCs per drop during IVM
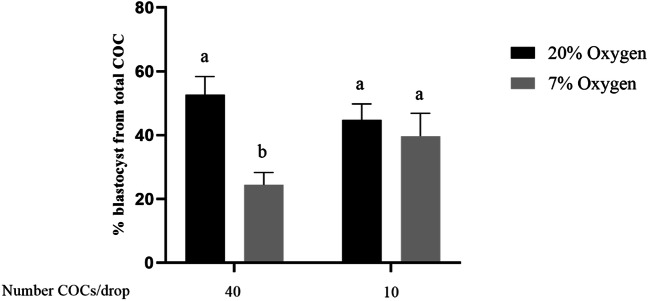
All COCs were matured in 2.3 mM glucose for this experiment. Cleavage rate did not differ between groups Mean cleavage rates across reps were 89% (control), 87% (20% oxygen/10COC/drop), 76% (7% oxygen/40COC/drop), and 78% (7% oxygen/10COC/drop). Blastocyst development rates (% from starting number of COCs) of COCs matured at 7% oxygen with 40 COCs per drop were lower than both 20% oxygen groups and the 7% oxygen groups with 10 COCs/drop (P < 0.05; Fig. 4). Blastocyst development rate from COCs matured at 7% oxygen with 10 COCs per drop was comparable to groups matured under 20% oxygen with either 40 or 10 COCs per drop. Hatching blastocyst rates from total oocytes were not different between groups; however, the 7% oxygen/40COC/drop group tended lower than the control (0.1>P>0.05).
Fig. 4.
During IVM, a high density of COCs in the presence of low oxygen and low glucose significantly reduces subsequent development to blastocyst. Blastocyst rate ((% from starting number of COCs) of bovine embryos developed from oocytes matured in two different oxygen concentrations, and two different numbers of COCs per IVM drop (2×2 factorial design). Mean ± SEM of % blastocyst rate was from five replicates 40 COCs/treatment group/replicate. #COC/drop is representative of 40 COCs for the 40 COC per drop group
Experiment 4: Effect of oxygen and combined media parameters
Blastocyst rate of COCs matured in 7% oxygen, 2.3 mM glucose, 40 COCs/drop, and 10 μl media/COC was lower than for COCs matured in all 20% oxygen groups (P < 0.05, Fig. 5). Blastocyst rate for COCs matured in 7% oxygen was restored, and not significantly different from the 20% oxygen groups, when IVM occured in 5.6 mM glucose, 10 COCs/drop and 20 μl media/COC (Fig. 5). Hatching blastocyst rates from total oocytes were not different between groups.
Fig. 5.
Development to the blastocyst stage is reduced when developed from COCs matured under low oxygen with low glucose, a low volume of medium per COC and a high density of COCs per drop. Blastocyst rate (% from starting number of COCs) of embryos developed from COCs matured in two different oxygen concentrations, with 3 differing treatment parameters (Experiment 4: glucose concentration; volume of medium/COC; number of COCs/drop). Data presented as mean ± SEM of % (n = 5 independent replicates with 40±5 COCs/treatment group/replicate). Superscripts indicate significant differences between treatment groups (P < 0.05)
Gene expression
Experiment 1: Effect of oxygen and glucose concentrations during IVM
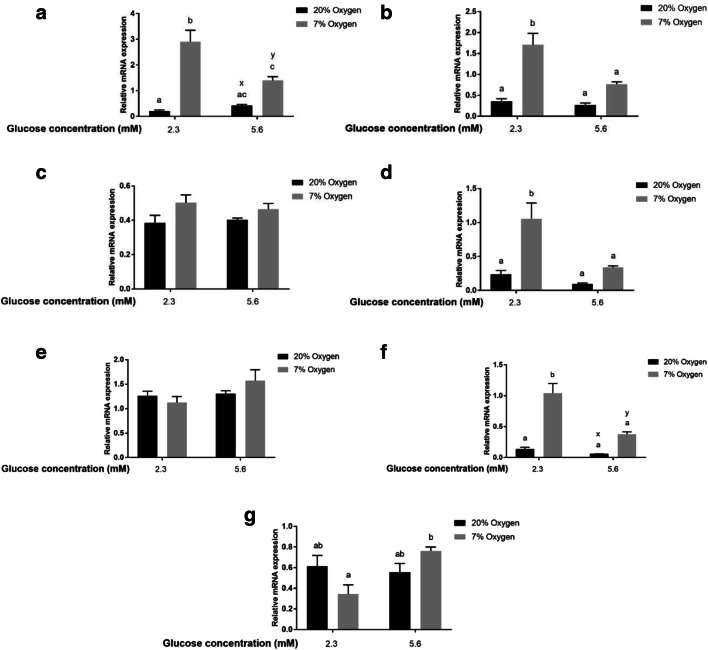
Metabolic genes: Abundance of mRNA for ENO1 (Fig. 6a), GAPDH (Fig. 6b), and SLC2A1 (Fig. 6d) was upregulated in COCs matured in 7% oxygen and low glucose compared to all other groups (P < 0.05), while no difference was found in the expression of G6PD (Fig. 6c). Apoptosis regulation genes: Abundance of mRNA for BNIP3 (Fig. 6f), was higher (P < 0.05) in COCs matured in 7% oxygen and 2.3 mM glucose compared to all other groups. Abundance of BNIP3 mRNA tended to be higher in COCs matured in 7% oxygen, 5.6 mM glucose than in those matured in 20% oxygen group with 5.6 mM glucose. BAX mRNA expression was not affected by oxygen or glucose concentration during IVM (Fig. 6e). Hypoxia/oxygen regulated genes: CA9 mRNA was lower (P< 0.05) in COCs matured in 7% oxygen and low glucose compared to those matured in 7% oxygen with higher glucose levels, but did not differ from COCs matured in 20% oxygen (Fig. 6g).
Fig. 6.
In vitro maturation of COCs in low glucose and low oxygen significantly upregulates expression of genes involved in anaerobic metabolism and apoptosis. Relative mRNA abundance of 7 genes related to metabolism (SLC2A1, GAPDH, ENO1 and G6PD), apoptosis genes (BAX and BNIP3), and a hypoxia response gene (CA9) in COCs matured for 21 hours in two different oxygen concentrations (20% or 7%), and two different glucose concentrations (2.3 mM or 5.6 mM) (Experiment 1, 2×2 factorial design). All COCs were matured in 10 μl media / COC and ~40 COCs per drop (between 35 and 42 COCs). Data presented as mean ± SEM (n = 4 independent replicates with 10 COC from at each replicate/treatment group utilised for analysis of gene expression). Means with different superscripts ab indicate significant differences between treatment groups (P < 0.05), while xy indicate a trend between treatment groups (0.1>P>0.05)
Experiment 4: Effect of oxygen and combined media parameters
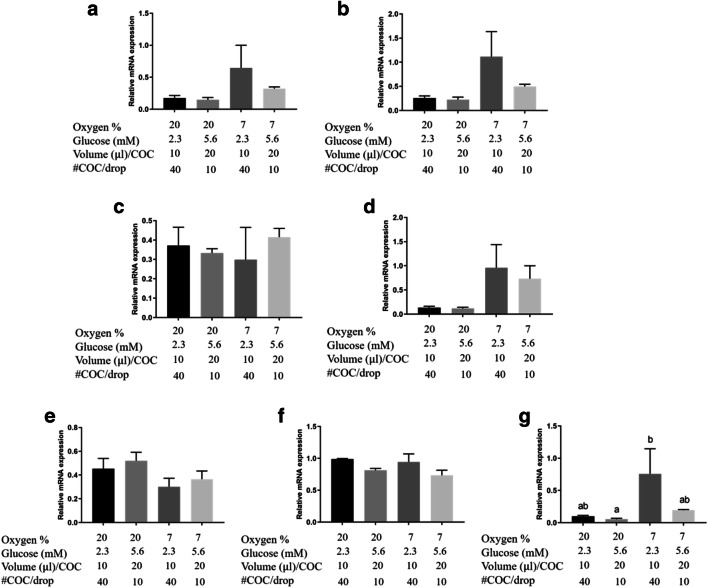
Metabolic genes: Expression of all metabolic genes (Fig. 7a, b, c, d) showed no differences across treatment groups. Apoptosis regulation genes: BNIP3 gene expression (Fig. 7g) was increased (P < 0.05) in COCs matured in 7% oxygen with low glucose availability (2.3 mM glucose; 10 μl/COC; 40 COCs /drop) compared to those matured at 20% oxygen in high glucose (5.6 mM with 20 μl/COC and 10 COC/drop). BNIP3 expression tended to be higher in COCs matured at 7% oxygen, 2.3 mM glucose with 20 μl/COC and 10 COC/drop compared to 20% oxygen, 5.6 mM glucose with 20 μl/COC and 10 COC/drop (Fig. 7g; 0.1>P>0.05). There was no difference in BAX gene expression between groups (Fig. 7f). Hypoxia/ oxygen regulated genes: CA9 gene expression was not different between groups (Fig. 7e).
Fig. 7.
Maturation of COCs in low oxygen, low glucose concentration, and reduced access to glucose significantly upregulates BNIP3 expression. Relative mRNA abundance of 7 genes related with metabolism (SLC2A1, GAPDH, ENO1, and G6PD), a hypoxia response gene (CA9), and apoptosis genes (BAX and BNIP3) in COCs matured for 21 h in two different oxygen concentrations (20% or 7%), with 3 differing treatment parameters (glucose; volume/COC; # of COCs/drop – Experiment 4). Data presented as mean ± SEM (n = 4 independent replicates with 10 COCs from each replicate/treatment group utilised for analysis of gene expression). Means with different superscripts indicate significant differences between treatment groups (P < 0.05)
Discussion
In this study, COCs matured in 7% oxygen with 2.3 mM (low) glucose, 10 μl media per COC, or 40 COCs per drop, had lower blastocyst rates than all other groups in all four experiments. The culture conditions within that group are likely to decrease the availability of glucose throughout IVM. Increasing the concentration of glucose in the IVM media to 5.6 mM, providing a higher volume ratio of culture media/COC (10 μl/COC vs 20 μl/COC), or reducing numbers of COCs in the media drop (10 vs 40/drop) restored blastocyst rates to levels observed in the 20% oxygen groups. Interestingly, altering the physical culture conditions alone, without increasing glucose, gave a bigger improvement in blastocyst development than when these parameters were adopted with the higher glucose concentration as well (Fig. 5). This illustrates the importance of physical culture conditions in a low-oxygen IVM setting, and may mean that glucose concentration plus the physical culture conditions may have an additive effect on how much glucose is accessible to the COC. Hatching rates only tended to be lower than control for the 7% oxygen groups with low glucose availability in experiment 1 and experiment 3, which is consistent with the findings for blastocyst rate in these experiments, with no difference found in hatching rates across groups for experiment 2 and 4. We found no evidence of improved blastocyst rate when COC maturation included a 7% oxygen environment compared to maturation at 20% oxygen. Hashimoto et al. [20] found similarly that developmental competence was related to both oxygen and glucose levels; however, a benefit of 5% oxygen above that of 20% oxygen was observed only in media containing 20 mM glucose. Whether a difference in developmental competence would be observed if a higher glucose level was used in our study during IVM at 7% oxygen requires further investigation.
The key observation made here is that glucose availability is crucial when bovine COCs are matured under low (7%) oxygen. We have previously published that a low oxygen environment activates the hypoxia-inducible factor 1 (HIF1) pathway within mouse cumulus cells during maturation under 5% and 2% oxygen [58], and HIF1 has been found to increase the expression of glycolytic genes and glucose transporters [59–61]. As cumulus cells have a high capacity for anaerobic metabolism, while the oocyte has a low capacity, activation of anaerobic glycolysis by low oxygen levels leads to greater glucose demand by cumulus cells [38, 62, 63], which will then produce pyruvate and l-lactate for use as TCA cycle substrates by the oocyte. Cumulus expansion is also a glucose-dependent event, requiring up-regulation of the cumulus cell hexosamine biosynthesis pathway (HBP) in response to the ovulatory stimulus (or via EGF/FSH stimulation during IVM) [43], and is an essential event for the gaining of oocyte competence [64–66]. The results for cumulus expansion in experiment 1 reveal that 7% oxygen levels with 2.3 mM glucose during IVM is likely resulting in the diversion of glucose away from cumulus expansion, with the CEI being significantly lower compared to the 5.6 mM glucose groups in (Fig. 1a). We speculate that the limited available glucose is being diverted away from the HBP to the glycolytic pathway in order to produce energy substrates for the oocyte, and that this is an important contributor to the resultant lack of developmental competence. It is likely also that there is inadequate glucose for metabolism of glucose by the cumulus cells through the pentose phosphate pathway (PPP), which is important in the regulation of meiotic progression via production of substrates for purine nucleotide synthesis [67]. Further to this, NADPH is also produced by the PPP, and is important in cytoplasmic maturation and pro-nuclear formation [67]. In regards to cumulus expansion in this study, we observed a 22% reduction in cumulus expansion in the 7% oxygen/2.3mM glucose treatment compared to 20% oxygen/5.6mM glucose treatment in Experiment 4 (Fig. 1b). This is similar to the 18% reduction in the comparable treatments in Experiment 1 (Fig. 1a) but failed to reach statistical significance in Experiment 4, likely due to higher variation. Increased variation between replicates in Experiment 4 may be due to a difference in the source of cattle ovaries or seasonal variation in oocyte quality observed in Australia.
Previous research has shown that pyruvate is the preferred energy substrate during IVM in atmospheric oxygen [20, 29] for aerobic metabolism of the COC. As the IVM medium used in this study contains 0.4 mM pyruvate, we suggest that depletion of available glucose causes dysregulation of a yet to be identified mechanism. One possibility is the recent hypothesis that lactate, in the form of lactide, has multiple beneficial functions [68], such as maintaining a high cytosolic NADH level, aiding in the redox state of the COC [69]. When glucose levels are adequate, cumulus cells produce significant amounts of lactic acid from their high glycolytic activity. This is also thought to partially annul the requirement for high oxygen consumption by cumulus cells, enabling most available oxygen to reach the oxidative phosphorylation-dependent oocyte [34].
No differences in developmental rates were observed across all experiments between groups matured in 20% oxygen with differing glucose conditions. This shows that under higher oxygen (20%) during bovine IVM, glucose availability is less critical to the developmental competence of COCs.
In Experiment 1, expression of the metabolic genes SLC2A1, GAPDH, and ENO1 was significantly upregulated (Fig. 6a, b, d) following maturation in 7% oxygen with 2.3 mM glucose, compared to all other groups. These genes are HIF responsive and may be upregulated in the cumulus cells via this mechanism, as shown in other studies [63]. This increased expression of glucose transporter and metabolic genes also suggests that both glucose uptake and glycolysis are increased in COCs when oxygen is low. However, expression of SLC2A1 and GAPDH was not upregulated in COCs matured in 7% oxygen, 5.6 mM glucose, indicating that low glucose availability may influence the upregulation of these genes in COCs matured in 7% oxygen. Expression of ENO1 was higher however in the 7% oxygen 5.6 mM glucose group compared to the control (20% oxygen, 2.3 mM glucose), which may indicate a HIF response for that gene. CA9 is thought to be a sensitive sensor of HIF1 activity; however, gene expression in the 7% oxygen groups was not different to the 20% oxygen group. It may be that carbonic anhydrase is acting more so in its role as a metabolic regulator of pH, and may not be as oxygen responsive within cumulus cells. Furthermore, expression of this gene was significantly higher in COCs matured in 7% oxygen, 5.6 mM glucose, compared to 7% oxygen, 2.3 mM glucose. Biggers et al [29] showed that pyruvate is the main substrate used by mouse oocytes matured under atmospheric oxygen, and Hashimoto et al [20] showed that glucose is the preferred substrate under 5% oxygen in bovine COCs.
BNIP3 is a member of the BCL2 family of proteins, and has dual roles, one being mitochondrial autophagy, which is essential for maintaining the quality of mitochondria [70, 71], and the other being a proapoptotic role. Both of these functions can be independently regulated [71]. In this study, BNIP3 was significantly upregulated in the 7% oxygen groups with 2.3mM glucose, suggesting that it is a possible pathway of apoptosis when both oxygen and glucose are low. However, further research is required to confirm this. As mitochondrial autophagy is regulated by nutrient availability, and stress responses [71], it is possible that the protein was upregulated for its role in mitochondrial autophagy, and this would need to be confirmed by investigating mitochondrial function and quality. Research findings indicate that hypoxia is a major trigger of increased BNIP3 expression, having two HIF-1α binding sites [72], suggesting that the low oxygen levels may have triggered upregulation of BNIP3; however, as with CA9, the expression of this gene was not upregulated in the 7% oxygen group compared to the control group when the glucose concentration was at 5.6 mM. The lack of upregulation of the oxygen sensitive genes examined in this study under 7% oxygen when glucose levels were at the higher concentration of 5.6 mM, suggests that perhaps this level of oxygen saturation during bovine IVM is not hypoxic, and that the upregulation of these genes observed when glucose was at the low level of 2.3 mM was due to a response to a challenged metabolic state. Other studies assessing the effects of low oxygen during IVM have typically looked at 5% oxygen levels. Our study looked at 7% oxygen in bovine IVM as we have routinely followed this protocol in bovine IVM based on a previous paper within our research group [73]. Although HIF1 supports anaerobic glycolysis and represses oxidative phosphorylation, hypoxia itself may not play a major role as HIF1 can be activated under normoxic conditions by phosphoinositide-3-kinase (PI3K) [74]. A recent study [75] found that inhibiting HIF1α leads to altered gene expression within cumulus cells, affecting genes involved in regulating cumulus cell function, and oocyte maturation in bovine COCs matured under 20% O2.
Gene expression results for experiment 4 also show a significant difference in the expression of BNIP3, in which the expression was upregulated in the 7% oxygen group with 2.3 mM glucose, 10 μl media/COC, and 40 COCs/drop compared to the 20% oxygen group with 5.6 mM glucose. Similar trends were observed with many of the other genes, with expression of the metabolic genes tending to be higher in the 7% oxygen, 2.3 mM glucose group for SLC2A1, ENO1 and GAPDH, however, expression was highly variable across replicates in these experiments, and no significant differences were found.
Conclusion
In conclusion, this study demonstrates that low oxygen during bovine IVM is not detrimental to oocyte developmental competence if adequate access to glucose is provided. Future studies attempting to compare high and low oxygen levels in IVM need to consider the culture conditions utilised, especially glucose concentration and availability, as determined by physical culture conditions. Based on the collective findings described herein, we recommend that when bovine IVM occurs under low oxygen (7%) that glucose be at 5.6mM (Experiment 1, Fig. 2). Alternatively, if using a lower glucose concentration (2.3 mM), then COCs be cultured in 20ul of media/COC (Experiment 2, Fig. 3) or at a density of 10 COCs/drop if maturation occurs in 10ul/COC (Experiment 3, Fig. 4). When culturing at 7% oxygen, higher glucose concentrations may increase developmental rates beyond that shown in this study. Further study of the mechanism behind the effects of low glucose availability under low oxygen on BNIP3 and other hypoxic-induced gene expression pathways is warranted.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Child TJ, Abdul-Jalil AK, Gulekli B, Tan SL. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil Steril. 2001;76(5):936–942. doi: 10.1016/s0015-0282(01)02853-9. [DOI] [PubMed] [Google Scholar]
- 2.Kumar P, Sait SF, Sharma A, Kumar M. Ovarian hyperstimulation syndrome. J Hum Reprod Sci. 2011;4(2):70–75. doi: 10.4103/0974-1208.86080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vuong LN, Ho VNA, Ho TM, Dang VQ, Phung TH, Giang NH, Le AH, Pham TD, Wang R, Norman RJ, Smitz J, Gilchrist RB, Mol BW. Effectiveness and safety of in vitro maturation of oocytes versus in vitro fertilisation in women with high antral follicle count: study protocol for a randomised controlled trial. BMJ Open. 2018;8(12):e023413-e023413. [DOI] [PMC free article] [PubMed]
- 4.Maman E, Meirow D, Brengauz M, Raanani H, Dor J, Hourvitz A. Luteal phase oocyte retrieval and in vitro maturation is an optional procedure for urgent fertility preservation. Fertil Steril. 2011;95(1):64–67. doi: 10.1016/j.fertnstert.2010.06.064. [DOI] [PubMed] [Google Scholar]
- 5.Ellenbogen A, Shavit T, Shalom-Paz E. IVM results are comparable and may have advantages over standard IVF. Facts, views & vision in ObGyn. 2014;6(2):77–80. [PMC free article] [PubMed] [Google Scholar]
- 6.Wu B, Zan L, Quan F, Wang H. A Novel Discipline in Embryology — Animal Embryo Breeding, in New Discoveries in Embryology. In: Wu B, editor. IntechOpen: Open Access; 2015.
- 7.Lonergan P, Fair T. Maturtion of oocytes in vitro. Anim Rev Annu Biosci. 2016;4(1):255–268. doi: 10.1146/annurev-animal-022114-110822. [DOI] [PubMed] [Google Scholar]
- 8.Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol Reprod Dev. 61(2):234–48. 10.1002/mrd.1153. [DOI] [PubMed]
- 9.Ealy A, Wooldridge LK, SR MC. Post-transfer consequences of in vitro-produced embryos in cattle. Anim Sci J, 2019. 97(6):2568. 10.1093/jas/skz116. [DOI] [PMC free article] [PubMed]
- 10.Lonergan P, Rizos D, Gutierrez-Adan A, Fair T, Boland MP. Oocyte and Embryo Quality: Effect of Origin, Culture Conditions and Gene Expression Patterns. Reprod Domest Anim. 2003;38(4):259–267. doi: 10.1046/j.1439-0531.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 11.Niemann H, Wrenzycki C. Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: Implications for subsequent development. Theriogenology. 2000;53(1):21–34. doi: 10.1016/s0093-691x(99)00237-x. [DOI] [PubMed] [Google Scholar]
- 12.Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. Reproduction (Cambridge, England) 1993;99(2):673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- 13.Mastroianni L, Jones R. Oxygen tension within the rabbit fallopian tube. Reproduction (Cambridge, England) 1965;9(1):99–102. doi: 10.1530/jrf.0.0090099. [DOI] [PubMed] [Google Scholar]
- 14.Van Blerkom J. Epigenetic influences on oocyte developmental competence: perifollicular vascularity and intrafollicular oxygen. J Assist Reprod Genet. 1998;15(5):226–234. doi: 10.1023/A:1022523906655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huey S, Abuhamad A, Barroso G, Kolm P, Mayer J, Oehninger S. Perifollicular blood flow doppler indices, but not follicular pO2, pCO2, or pH, predict oocyte developmental competence in in vitro fertilization. Fertil Steril. 1999;72(4):707–712. doi: 10.1016/s0015-0282(99)00327-1. [DOI] [PubMed] [Google Scholar]
- 16.Tatemoto H, Muto N, Sunagawa I, Shinjo A, Nakata T. Protection of porcine oocytes against cell damage caused by oxidative stress during in vitro maturation: role of superoxide dismutase activity in porcine follicular fluid. Biol Reprod. 2004;71(4):1150–1157. doi: 10.1095/biolreprod.104.029264. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79(4):829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal A, Prabakaran S, Allamaneni SSSR. Relationship between oxidative stress, varicocele and infertility: a meta-analysis. Reprod BioMed Online. 2006;12(5):630–633. doi: 10.1016/s1472-6483(10)61190-x. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol. 2006;18(3):325–332. doi: 10.1097/01.gco.0000193003.58158.4e. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto S, Minami N, Takakura R, Yamada M, Imai H, Kashima N. Low oxygen tension during in vitro maturation is beneficial for supporting the subsequent development of bovine cumulus–oocyte complexes. Mol Reprod Dev. 2000;57(4):353–360. doi: 10.1002/1098-2795(200012)57:4<353::AID-MRD7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Johnson MH, Nasresfahani MH. Radical solutions and cultural problems: Could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? BioEssays. 1994;16(1):31–38. doi: 10.1002/bies.950160105. [DOI] [PubMed] [Google Scholar]
- 22.Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol Lett. 2004;7(5):363–368. [Google Scholar]
- 23.Rinaudo PF, et al. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril. 2006;86(4):1265.e1–1265.e36. doi: 10.1016/j.fertnstert.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Sutton-McDowall M, Gilchrist R, Thompson J. Glucosamine supplementation during in vitro maturation leads to perturbed developmental capacity of bovine cumulus oocyte complexes. Reprod Fertil Dev. 2005;17(2):300. [Google Scholar]
- 25.Leese HJ, Lenton EA. Glucose and lactate in human follicular fluid: concentrations and interrelationships. Hum Reprod (Oxford) 1990;5(8):915–919. doi: 10.1093/oxfordjournals.humrep.a137219. [DOI] [PubMed] [Google Scholar]
- 26.Johnson AE, Lane M, Gardiner DK, Diekman MA, Krisher RL. Changes in follicular fluid environment between 5mm and 10mm follicles. Annual Conference of the Society for the Study of Reproduction, 2001.
- 27.Hashimoto S. Application of In Vitro Maturation to Assisted Reproductive Technology. J Reprod Dev. 2009;55(1):1–10. doi: 10.1262/jrd.20127. [DOI] [PubMed] [Google Scholar]
- 28.Cetica P, Pintos L, Dalvit G, Beconi M. Activity of key enzymes involved in glucose and triglyceride catabolism during bovine oocyte maturation in vitro. Reproduction (Cambridge, England) 2002;124(5):675–681. [PubMed] [Google Scholar]
- 29.Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oocyte and zygote. PNAS. 1967;58(2):560–567. doi: 10.1073/pnas.58.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rieger D, Loskutoff NM. Changes in the metabolism of glucose, pyruvate, glutamine and glycine during maturation of cattle oocytes in vitro. J Reprod Fertil. 1994;100(1):257–262. doi: 10.1530/jrf.0.1000257. [DOI] [PubMed] [Google Scholar]
- 31.Saito T, Hiroi M, Kato T. Development of glucose utilization studied in single oocytes and preimplantation embryos from mice. Biol Reprod. 1994;50(2):266–270. doi: 10.1095/biolreprod50.2.266. [DOI] [PubMed] [Google Scholar]
- 32.Dan-Goor M, Sasson S, Davarashvili A, Almagor M. Expression of glucose transporter and glucose uptake in human oocytes and preimplantation embryos. Hum Reprod (Oxford) 1997;12(11):2508–2510. doi: 10.1093/humrep/12.11.2508. [DOI] [PubMed] [Google Scholar]
- 33.Khurana NK. and. Niemann H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology. 2000;54(5):741–756. doi: 10.1016/S0093-691X(00)00387-3. [DOI] [PubMed] [Google Scholar]
- 34.Clark AR, Stokes YM, Lane M, Thompson JG. Mathematical modelling of oxygen concentration in bovine and murine cumulus–oocyte complexes. Reproduction (Cambridge, England) 2006;131(6):999–1006. doi: 10.1530/rep.1.00974. [DOI] [PubMed] [Google Scholar]
- 35.Thompson JG, Brown HM, Kind KL, Russell DL. The Ovarian Antral Follicle: Living on the Edge of Hypoxia or Not? Biol Reprod. 2015;92(6):153-153. [DOI] [PubMed]
- 36.Downs SM, Utecht AM. Metabolism of radiolabeled glucose by mouse oocytes and oocyte-cumulus cell complexes. Biol Reprod. 1999;60(6):1446–1452. doi: 10.1095/biolreprod60.6.1446. [DOI] [PubMed] [Google Scholar]
- 37.Spindler RE, Pukazhenthi BS, Wildt DE. Oocyte metabolism predicts the development of cat embryos to blastocyst in vitro. Mol Reprod Dev. 2000;56(2):163–171. doi: 10.1002/(SICI)1098-2795(200006)56:2<163::AID-MRD7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 38.Sutton-McDowall ML, Gilchrist RB, Thompson JG. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction (Cambridge, England) 2010;139(4):685–695. doi: 10.1530/REP-09-0345. [DOI] [PubMed] [Google Scholar]
- 39.Xie H-L, Wang YB, Jiao GZ, Kong DL, Li Q, Li H, Zheng LL, Tan JH. Effects of glucose metabolism during in vitro maturation on cytoplasmic maturation of mouse oocytes. Sci Rep. 2016;6(1):20764-20764. [DOI] [PMC free article] [PubMed]
- 40.Downs SM, Hudson ED. Energy substrates and the completion of spontaneous meiotic maturation. Zygote (Cambridge) 2000;8(4):339–351. doi: 10.1017/s0967199400001131. [DOI] [PubMed] [Google Scholar]
- 41.Urner F, Sakkas D. Influence of glucose on protein tyrosine phosphorylation in mouse spermatozoa. Hum Reprod (Oxford). 1999;14(Suppl_3):13-13.
- 42.Bermejo-Álvarez P, Lonergan P, Rizos D, Guttierez-Adan A. Low oxygen tension during IVM improves bovine oocyte competence and enhances anaerobic glycolysis. Reprod BioMed Online. 2009;20(3):341–349. doi: 10.1016/j.rbmo.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Sutton-McDowell ML, Gilchrist RB, Thompson JG. Cumulus expansion and glucose utilisation by bovine cumulus–oocyte complexes during in vitro maturation: the influence of glucosamine and follicle-stimulating hormone. Reproduction. 2004;128(3):313–319. doi: 10.1530/rep.1.00225. [DOI] [PubMed] [Google Scholar]
- 44.Dunning KR, Watson LN, Sharkey DJ, Brown HM, Norman RJ, Thompson JG, Robker RL, Russell DL. Molecular filtration properties of the mouse expanded cumulus matrix: controlled supply of metabolites and extracellular signals to cumulus cells and the oocyte. Biol Reprod. 2012;87(4):89-89. [DOI] [PubMed]
- 45.Tesarík J. Comparison of acrosome reaction-inducing activities of human cumulus oophorus, follicular fluid and ionophore A23187 in human sperm populations of proven fertilizing ability in vitro. J Reprod Fertil. 1985;74(2):383–388. doi: 10.1530/jrf.0.0740383. [DOI] [PubMed] [Google Scholar]
- 46.Hong S-J, Chiu PC-N, Lee K-F, Tse JY-M, Ho P-C, Yueng WS-B. Cumulus cells and their extracellular matrix affect the quality of the spermatozoa penetrating the cumulus mass. Fertil Steril. 2009;92(3):971–978. doi: 10.1016/j.fertnstert.2008.07.1760. [DOI] [PubMed] [Google Scholar]
- 47.Carrell DT, Middleton RG, Peterson CM, Jones KP, Urry RL. Role of the cumulus in the selection of morphologically normal sperm and induction of the acrosome reaction during human in vitro fertilization. Arch Androl. 1993;31(2):133–137. doi: 10.3109/01485019308988391. [DOI] [PubMed] [Google Scholar]
- 48.Haidri AA, Miller IM, Gwatkin RB. Culture of mouse oocytes in vitro, using a system without oil or protein. J Reprod Fertil. 1971;26(3):409–411. doi: 10.1530/jrf.0.0260409. [DOI] [PubMed] [Google Scholar]
- 49.Gwatkin RB, Haidri AA. Oxygen requirements for the maturation of hamster oocytes. J Reprod Fertil. 1974;37(1):127–129. doi: 10.1530/jrf.0.0370127. [DOI] [PubMed] [Google Scholar]
- 50.Banwell KM, Lane M, Russell DL, Kind KL, Thompson JG. Oxygen concentration during mouse oocyte in vitro maturation affects embryo and fetal development. Hum Reprod (Oxford) 2007;22(10):2768–2775. doi: 10.1093/humrep/dem203. [DOI] [PubMed] [Google Scholar]
- 51.Preis KA, Seidel GE, Gardner DK. Reduced oxygen concentration improves the developmental competence of mouse oocytes following in vitro maturation. Mol Reprod Dev. 2007;74(7):893–903. doi: 10.1002/mrd.20655. [DOI] [PubMed] [Google Scholar]
- 52.Pinyopummintr T, Bavister BD. Optimum gas atmosphere for in vitro maturation and in vitro fertilization of bovine oocytes. Theriogenology. 1995;44(4):471–477. doi: 10.1016/0093-691x(95)00219-x. [DOI] [PubMed] [Google Scholar]
- 53.de Castro e Paula LA, Hansen PJ. Interactions between oxygen tension and glucose concentration that modulate actions of heat shock on bovine oocytes during in vitro maturation. Theriogenology. 2007;68(5):763–770. doi: 10.1016/j.theriogenology.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Watson AJ, de Sousa P, Caveney A, Barcroft LC, Natale D, Urquhart J, Westhusin ME. Impact of bovine oocyte maturation media on oocyte transcript levels, blastocyst development, cell number, and apoptosis. Biol Reprod. 2000;62(2):355–364. doi: 10.1095/biolreprod62.2.355. [DOI] [PubMed] [Google Scholar]
- 55.Fagbohun CF, Down SM. Maturation of the mouse oocyte-cumulus cell complex: stimulation by lectins. Biol Reprod. 1990;42(3):413–423. doi: 10.1095/biolreprod42.3.413. [DOI] [PubMed] [Google Scholar]
- 56.Vanderhyden BC, Caron PJ, Buccione R, Eppig JJ. Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev Biol. 1990;140(2):307–317. doi: 10.1016/0012-1606(90)90081-s. [DOI] [PubMed] [Google Scholar]
- 57.Society, I.E.T.S, Manual of the International Embryo Transfer Society: A procedural guide and general information for the use of embryo transfer technology, emphasizing sanitary precautions. 1998, The Society, 1998.
- 58.Kind KL, Tam KKY, Banwell KM, Gauld AD, Russell DL, Macpherson AM, Brown HM, Frank LA, Peet DJ, Thompson JG. Oxygen-regulated gene expression in murine cumulus cells. Reprod Fertil Dev. 2015;27(2):407–418. doi: 10.1071/RD13249. [DOI] [PubMed] [Google Scholar]
- 59.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell (Cambridge) 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bashan N, Burdett E, Hundal HS, Klip A. Regulation of glucose transport and GLUT1 glucose transporter expression by O2 in muscle cells in culture. Am J Phys Cell Phys. 1992;262(3):682–690. doi: 10.1152/ajpcell.1992.262.3.C682. [DOI] [PubMed] [Google Scholar]
- 61.Ebert BL, Firth JD, Ratcliffe PJ. Hypoxia and Mitochondrial Inhibitors Regulate Expression of Glucose Transporter-1 via Distinct Cis-acting Sequences. J Biol Chem. 1995;270(49):29083–29089. doi: 10.1074/jbc.270.49.29083. [DOI] [PubMed] [Google Scholar]
- 62.Baddela VS, Sharma A, Michaelis M, Vanselow J. HIF1 driven transcriptional activity regulates steroidogenesis and proliferation of bovine granulosa cells. Sci Rep. 2020; 10(1):3906-3906. [DOI] [PMC free article] [PubMed]
- 63.Kind KL, Banwell KM, Gebhardt KM, Macpherson A, Gauld A, Russell DL, Thompson JG. Microarray analysis of mRNA from cumulus cells following in vivo or in vitro maturation of mouse cumulus-oocyte complexes. Reprod Fertil Dev. 2013;25(2):426–438. doi: 10.1071/RD11305. [DOI] [PubMed] [Google Scholar]
- 64.Thompson J, Lane M, Gilchrist R. Metabolism of the bovine cumulus-oocyte complex and influence on subsequent developmental competence. Society of Reproduction and Fertility supplement. 2007;64:179–190. doi: 10.5661/rdr-vi-179. [DOI] [PubMed] [Google Scholar]
- 65.Chen L, Russell PT, Larsen WJ. Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol Reprod Dev. 1993;34:87–93. doi: 10.1002/mrd.1080340114. [DOI] [PubMed] [Google Scholar]
- 66.Tirone E, Siracusa G, Hascall VC, Frajese G, Sulustri A. Oocytes preserve the ability of mouse cumulus cells in culture to synthesize hyaluronic acid and dermatan sulfate. Dev Biol. 1993;160(2):405–412. doi: 10.1006/dbio.1993.1316. [DOI] [PubMed] [Google Scholar]
- 67.Downs SM, Humpherson PG, Leese HJ. Meiotic induction in cumulus cell-enclosed mouse oocytes: involvement of the pentose phosphate pathway. Biol Reprod. 1998;58(4):1084–1094. doi: 10.1095/biolreprod58.4.1084. [DOI] [PubMed] [Google Scholar]
- 68.Gardner DK. Lactate production by the mammalian blastocyst: Manipulating the microenvironment for uterine implantation and invasion? BioEssays. 2015;37(4):364–371. doi: 10.1002/bies.201400155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dumollard R, Ward Z, Carroll J, Duchen MR. Regulation of redox metabolism in the mouse oocyte and embryo. Development (Cambridge) 2006;134(3):455–465. doi: 10.1242/dev.02744. [DOI] [PubMed] [Google Scholar]
- 70.Redmann M, Darley-Usmar V, Zhang J. The role of autophagy, mitophagy and lysosomal functions in modulating bioenergetics and survival in the context of redox and proteotoxic damage: implications for neurodegenerative diseases. Aging Dis. 2016;7(2):150–162. doi: 10.14336/AD.2015.0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Redmann M, Dodson M, Boyer-Guittaut M, Darley-Usmar V, Zhang J. Mitophagy mechanisms and role in human diseases. Int J Biochem Cell Biol. 2014;53:127–133. doi: 10.1016/j.biocel.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quinsay MN, Lee Y, Rikka S, Sayen RM, Molkentin JD, Gottleib RA, Gustaffsson AB. Bnip3 mediates permeabilization of mitochondria and release of cytochrome c via a novel mechanism. J Mol Cell Cardiol. 2009;48(6):1146–1156. doi: 10.1016/j.yjmcc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thompson JGE, Simpson AC, Pugh PA, Donnelly PE, Tervit HR. Effect of oxygen concentration on in-vitro development of preimplantation sheep and cattle embryos. J Reprod Fertil. 1990;89(2):573–578. doi: 10.1530/jrf.0.0890573. [DOI] [PubMed] [Google Scholar]
- 74.Stiehl DP, Jelkmann W, Wenger RH, Hellwig-Burgel T. Normoxic induction of the hypoxia-inducible factor 1α by insulin and interleukin-1β involves the phosphatidylinositol 3-kinase pathway. FEBS Lett. 2002;512(1):157–162. doi: 10.1016/s0014-5793(02)02247-0. [DOI] [PubMed] [Google Scholar]
- 75.Turhan A, Pereira MT, Schuler G, Bleul U, Kowalewski MP. Hypoxia-inducible factor (HIF1alpha) inhibition modulates cumulus cell function and affects bovine oocyte maturation in vitro. Biol Reprod. 2020. [DOI] [PMC free article] [PubMed]