Abstract
Purpose
To investigate the developmental competence of ovarian tissue oocytes from patients with gynecological tumors using a biphasic in vitro maturation system with capacitation (CAPA-IVM) in comparison with standard IVM.
Methods
This sibling pilot study included 210 oocytes in 10 patients with gynecological malignancies. After ovariectomies, ovaries were cut into even halves and immature cumulus-oocyte complexes (COCs) were retrieved from the ovarian tissue. COCs were separately cultured in either a biphasic CAPA-IVM system for 53 h or in standard IVM for 48 h. After IVM, all COCs were denuded and mature oocytes were either vitrified (N=5) or used for ICSI (N=5). Embryos were cultured for 5–6 days and obtained blastocysts were vitrified.
Results
Use of the CAPA-IVM system led to a higher meiotic maturation rate in ovarian tissue oocytes (OTO) compared to standard IVM (56 vs 35%, p=0.0045) and had a tendency to result in lower degeneration after IVM. Only the CAPA-IVM method supported blastocyst formation.
Conclusions
The biphasic in vitro maturation system improved the competence of OTO in comparison to the standard IVM method. The study suggests that fertility preservation programs could become more efficient using IVM after capacitation culture.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02118-z.
Keywords: IVM, Fertility preservation, Gynecological cancer, CNP, Oocyte vitrification
Background
Ovarian tissue oocytes in vitro maturation (OTO IVM) is a promising method of fertility preservation for oncological patients. It allows immature oocyte retrieval without any ovarian stimulation, simultaneously with other surgical interventions, and can be combined with ovarian tissue cryopreservation. Most importantly, this method is safe for patients with ovarian malignancies [1]. This group of patients in the course of oncological treatment has to go through radical ovariectomies and irrevocably lose fertility. As part of the cryopreservation of ovarian cortical tissue, immature oocytes can be isolated from small (0.5–6 mm in diameter) and mid-sized follicles (7–11 mm in diameter) from the ovaries after their removal. These have a potential for in vitro maturation. Few live births have already been reported using this technology [2–5]. However, the maturation rate of ovarian tissue oocytes remains low [6] and usually does not surpass 40% [2, 5, 7, 8]. Taking into consideration the fact that this fertility preservation method is time-consuming [9] and that no culture system is currently available for 0.5–6 mm follicles, OTO IVM is not yet part of routine clinical practice.
Our hypothesis is that the decreased potential of OTO to maturation and development could be in part associated with the suboptimal in vitro culture conditions of cumulus-oocyte complexes (COCs) from the dissected smaller unstimulated follicles during the ovarian cortex preparation. When immature COCs are retrieved from small and mid-sized follicles, only part of the oocytes can resume meiosis [10]. Not all COCs retrieved from the higher mentioned follicle classes have yet acquired full developmental competence by structural and functional modifications, i.e., “capacitation” [11]. Such oocytes lack molecular and cellular machinery and might therefore still lack the competence to maturation and embryo development [10]. This problem of nuclear and cytoplasmic asynchrony may be solved by the implementation of a biphasic in vitro maturation system. The first step of the system keeps all oocytes under a temporary meiosis arrest and enhances a prolonged interaction between oocyte and cumulus cells [10, 12]. The capacitation of the oocyte depends on the tight interaction with the cumulus cells [13]. During the second step of IVM, the meiosis resumes and the COCs reach final maturation.
It has been demonstrated for porcine [14], bovine [15], murine [16, 17], and goat [18] animal models that modulation of oocytes’ spontaneous maturation improves oocytes developmental competence. In the first step, inhibition of spontaneous meiotic maturation has been achieved by using phosphodiesterase inhibitors, for which—in human oocytes—a specific phosphodiesterase 3-inhibitor (PDE3) was used for the first time [19]. Instead of including synthetic PDE3 inhibitors, C-type natriuretic peptide (CNP), which is the natural molecule inhibiting the PDE3 action in the oocyte, is effective and turned out to better retain the cumulus oocyte communication [20, 21]. This pre-IVM step which was called “capacitation” or “CAPA” [22, 23] aims to mimic the process of capacitation that occurs in vivo.
It was demonstrated that in vitro maturation with the capacitation (CAPA-IVM) system (using CNP as a temporary meiosis inhibitor) improved embryo development over standard IVM systems for COCs from 2- to 10-mm follicles and yielded good clinical pregnancy rates in minimally stimulated women with polycystic ovarian syndrome (PCOS) [21]. However, the efficiency of the biphasic in vitro maturation approach in ovarian tissue oocytes in oncological patients who were unstimulated has never been studied before.
In this pilot study, we demonstrate that the biphasic in vitro maturation system significantly improves the maturation potential of ovarian tissue oocytes in oncological patients by 21%, has a tendency to result in a lower degeneration rate after IVM, and demonstrated blastocyst formation capacity.
Methods
Patients
Ten patients were selected for this study from October 2019 to August 2020. They underwent ovariectomies for neoplasia of the reproductive system organs. The inclusion criteria were as follows: whole ovariectomy, gynecological cancer or borderline ovarian tumor, age 16–37, normal ovarian cycle, desire for fertility preservation. The exclusion criteria were as follows: partial ovarian resection during the surgery, chemo- or radiotherapy in the past, advanced reproductive age, irregular cycle. All women got consultations by a reproductive physician and an oncologist. Four of them were diagnosed with stage IIIa–VIb ovarian cancer (according to the FIGO classification), three with cervical cancer Ib–IIb, two with stage Ia endometrial cancer, and 1 with borderline ovarian tumor stage 2c. The FIGO (International Federation of Gynecology and Obstetrics) staging system is used most often for cancers of the female reproductive organs [24]. It involves information on the location of tumors, the cell type, size, grade, and whether or not it has spread to nearby lymph nodes or other parts of the body [25]. According to this classification, the lower the number, the less cancer has spread [24].
None of the patients in our study had a smoking habit. In 9 patients, the AMH serum levels were analyzed (Supplementary Table 1). One patient preferred not to have a gynecological exam for personal reasons. BMI, the age of menarche, cycle length, and menstrual duration were recorded for all patients. Two patients (patient №2 and patient №5, Supplementary Table 1) reported that they had previously undergone an ovariectomy without fertility preservation counseling or intervention. Six patients with ovarian cancer, endometrial cancer, and cervical cancer had a hysterectomy in the course of the surgeries. None of the patients had radiation or chemotherapy before the surgery.
Surgical procedures and ovaries transportation
Nine women underwent surgeries at our medical center and 1 at the children's clinical hospital. As part of the study, 2 laparotomies in the lower midline access and 8 laparoscopies were performed. Six patients had hysterectomies during the surgery, 6 ovariectomies were unilateral, and 4 were bilateral. Intact ovaries were transported on ice in a sterile container filled with Flushing Medium with 10 IU/ml heparin (Origio, CooperSurgical) within 15 min in our center and within 1 h from the children’s hospital.
Ovarian tissue preparation
Each ovary was cut into two even halves (Fig. 1). One of the halves was immediately placed in the Petri dish with Sydney IVF Gamete Buffer (Cook, Australia) supplemented with 50 nM CNP (Tocris Bioscience; Abingdon, UK) and 20 nM Estradiol (Sigma; Schnelldorf, Germany). All the COCs found in this half of the ovary were attributed to the CAPA-IVM group. The other half was placed in the Petri dish with Flushing Medium with 10 IU/ml heparin (Origio, CooperSurgical) for standard IVM (Fig. 1) and all the COCs found in this half of the ovary were attributed to the standard IVM group. Two embryologists were simultaneously dissecting each half of the ovary. The cortical layer was separated from the medulla with scalpels. Then, ovarian tissue was chopped into small pieces to allow follicles of all sizes to rupture. Then, the Petri dishes were inspected for the presence of COCs. The average searching time to collect the COCs from the chopped ovarian tissue was 40 min (20–60 min range).
Fig. 1.
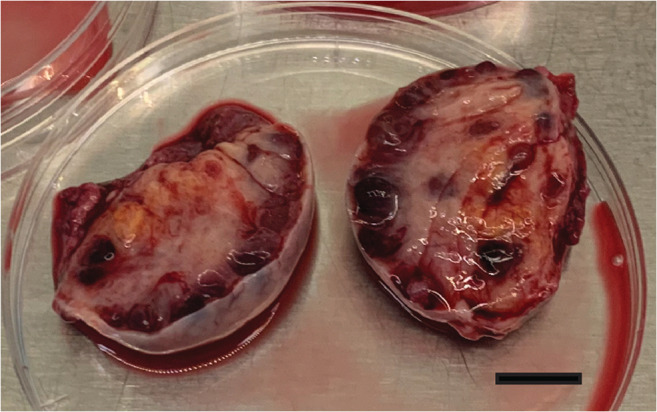
Two even halves of the ovary during ovarian tissue preparation. Scale bar 1 cm
In vitro maturation
For the standard IVM, identified COCs were first washed in the Sydney IVF Gamete Buffer (Cook, Australia) and then placed in the IVM medium (Sage, CooperSurgical) supplemented with 0.75 IU/ml HP-hMG (Menopur, Ferring) and 10% HSA (FUJIFILM Irvine Scientific). The in vitro maturation time (48 h) was chosen on the basis of earlier studies done in 1998 by Cha and Chian [26] and in 2000 by Smith and coauthors [27]. These authors studied non-PCOS patients which were not stimulated by gonadotropins (no FSH and no HCG ovulatory stimulus); thus, conditions were comparable to ours. In Cha and Chian [26], it was indicated that maturation times were 36 to 48 h depending on the size and expansion of the cumulus. In our case, none of the COCs showed any expansion and were thus kept for 48 h before cumulus cell denudation and insemination. In Smith et al., the COCs were matured for 28 or 36 h, and the results showed no strikingly different outcomes in fertilization and embryo formation [27]. We believe that 48 h was the best compromise for ending the maturation for OTO in the standard protocol.
For the biphasic CAPA-IVM system, all components to be added to the Sage media for the capacitation IVM procedure were kindly donated by Follicle Biology Laboratory from the Vrije Universiteit Brussel, to be integrated as published by Sánchez et al. [20]. Briefly, COCs were first placed in the medium, containing CNP peptide for 22 h, and then were cultured for 30 h in the IVM medium (the same basal medium from Sage as used in the routine arm of this study). COCs were cultured in groups of 5 per 500 μL well with the 350-μL oil overlay (OVOIL; Vitrolife, Gothenburg, Sweden).
Embryological procedures
All oocytes were denuded after IVM with SynVitro Hyadase (Origio, CooperSurgical). Five patients decided to fertilize their (fresh) oocytes with their husband’s sperm by ICSI and five patients decided to have all their matured oocytes vitrified. Embryos were cultured in GTL medium (Vitrolife, Gothenburg, Sweden) for 5–6 days. Oocytes and blastocysts were cryopreserved using the standard vitrification method (Kitazato Corporation, Japan). In one patient, we performed trophectoderm biopsy on day 6 with the OCTAX laser. The development of this embryo is presented in Fig. 2 (b, d, f). Preimplantation genetic testing for aneuploidies (PGT-A) was performed as previously described [28].
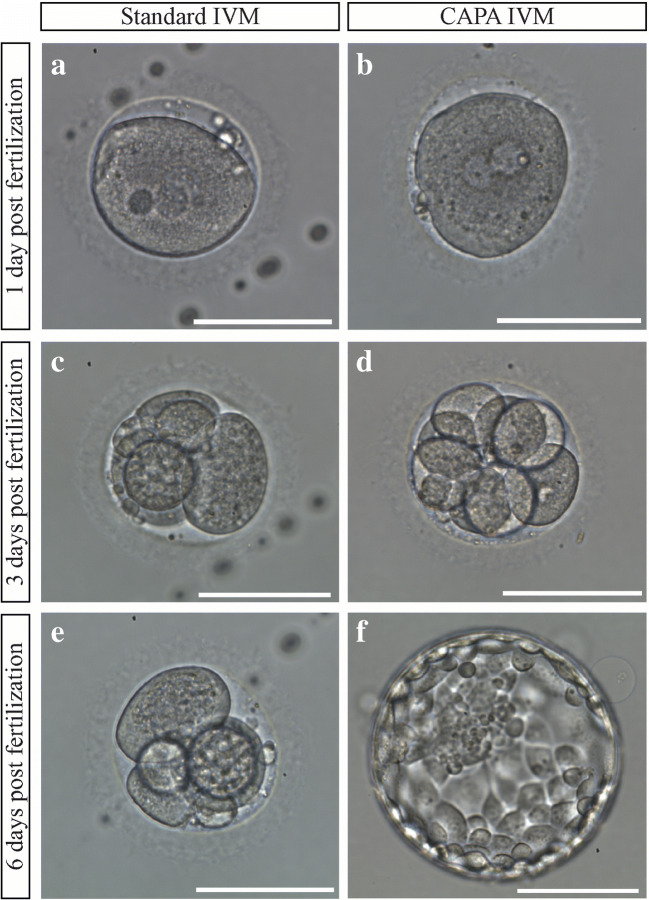
Fig. 2.
Embryological development of embryos in standard (a, c, e) and CAPA (b, d, f) IVM media. a, b Zygotes. c, d Embryos on day 3 of development. e Arrested embryo in the standard IVM group on day 6 post-fertilization. f Blastocyst in the CAPA IVM group on day 6 post-fertilization. Scale bar 100 μm
Images were taken at the Olympus IX73 microscope. The mean oocyte diameter (MOD) was determined using OCTAX EyeWare 2.2.2.318 software (Vitrolife, Gothenburg, Sweden) as described in Romao et al. [29] for all viable oocytes after maturation.
Statistical analyses
For OTO IVM programs efficiency evaluation, oocytes from using each IVM protocol were combined. Any covariates were not included in the analysis since the group of patients was homogeneous. IVM outcome rates were compared with the two-tailed Fisher exact test using R software (R Core Team, 2016). Statistical significance was defined as p < 0.05. Ninety-five percent Clopper-Pearson confidence intervals were calculated for all IVM outcome rates with the binom.test function in R. COCs with different types of thicknesses of the cumulus layer (thick, thin, or denuded) were compared by Mann-Whitney U test due to the non-normality of the data. Mean diameters of oocytes were compared between standard and CAPA groups by Student’s t test. Since in GV the data were not normal and the sample sizes were not so high, we additionally performed Mann-Whitney U test.
Results
Patients’ characteristics
In our study, we included a homogeneous group of patients with gynecological tumors. The patient’s characteristics are presented in Table 1. The mean age of patients was 29.4 ± 1.76 years (mean, SE); the mean body mass index (BMI) was 26.8 ±2.45 kg/m2. All the patients had a normal menstrual cycle with a mean length of 31.67 ± 1.59, a mean bleeding duration of 5.5 ± 0.37, and had their menarche at 12.7 ± 0.36. The AMH serum level ranged from 0.7 to 19.8 with the mean 5.14 ± 1.91.
Table 1.
Patient characteristics who underwent the OTO IVM
| No of patients | 10 |
|---|---|
| Age (years) | |
| Range | 16–36 |
| Mean ± SE | 29.40 ± 1.76 |
| BMI (kg/m2) | |
| Range | 18–44 |
| Mean ± SE | 26.80 ± 2.45 |
| Diagnosis, n (%) | |
| Ovarian cancer | 4 (40%) |
| Cervical cancer | 3 (30%) |
| Endometrial cancer | 2 (20%) |
| Borderline ovarian tumor | 1 (10%) |
| Menarche | |
| Range | 11–14 |
| Mean ± SE | 12.70 ± 0.36 |
| Menstrual cycle | |
| Range | 27–37 |
| Mean ± SE | 31.67 ± 1.59 |
| Bleeding duration | |
| Range | 4–8 |
| Mean ± SE | 5.50 ± 0.37 |
| Pregnancies in the past | 2 (20%) |
| Ovariectomy in the past | 2 (20%) |
| Hysterectomy | 6 (60%) |
| AMH serum level, (ng/mL) | |
| Range | 0.70–19.8 |
| Mean ± SE | 5.14 ± 1.91 |
BMI body mass index, AMH anti Müllerian hormone, OTO IVM ovarian tissue oocyte in vitro maturation, SE standard error
OTO IVM programs efficiency
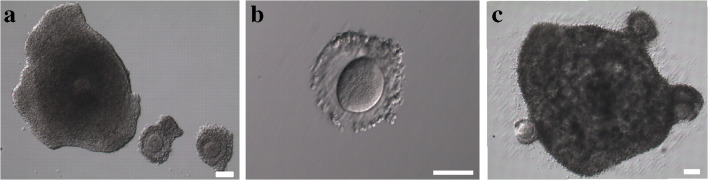
In this study, we used sibling oocytes in order to compare the efficiency of the biphasic IVM system with the standard protocol. The main outcomes of the IVM programs are presented in Table 2. Upon retrieval, none of the COCs had any sign of degeneration. Thus, all two hundred and one COCs were included in the study and the mean number of oocytes per patient did not differ between the groups (10.5±3.47 vs 9.65±2.84, p= 0.8785). The obtained COCs had either a thick layer of cumulus cells (Fig. 4a), a thin layer of cumulus cells (Fig. 4a), or were (partially) denuded (Fig. 4b). No statistical difference was found between the CAPA- and Standard IVM groups for the mean number of COCs with different cumulus types (Table 2). The mean MOD of MII, MI, or GV oocytes also did not differ statistically between the groups and is presented in Table 3. Thus, it was rightful to compare the two pools of COCs in CAPA- and standard IVM groups.
Table 2.
Outcomes of IVM programs, n=10
| Main outcomes | CAPA-IVM | 95% CI | Standard IVM | 95% CI | P value |
|---|---|---|---|---|---|
| Total number of COCs | 105 | 96 | |||
| Mean number of COCs per patient (± SE) | 10.5±3.47 | 9.65±2.84 | 0.8785 | ||
| Total number of COCs with a thick layer of cumulus cells | 71 | 61 | |||
| Mean number of COCs with a thick layer of cumulus (± SE) | 7.1±2.92 | 6.7±2.14 | 0.8421 | ||
| Total number of COCs with a thin layer of cumulus cells | 28 | 26 | |||
| Mean number of COCs with a thin layer of cumulus cells (± SE) | 2.8±0.71 | 2.89±0.82 | 0.9682 | ||
| Total number of (partially) denuded oocytes | 6 | 9 | |||
| Mean number of (partially) denuded oocytes (± SE) | 0.6±0.34 | 1±0.33 | 0.2775 | ||
| Maturation (MII) rate, % (n) | 56% (59) | 46.2–56.9% | 35% (34) | 26.0–48.6% | 0.0045 |
| Degeneration rate after IVM, % (n) | 2% (2) | 0.2–6.7% | 11% (11) | 5.9–19.6% | 0.2298 |
| Spontaneous cleavage after IVM | 0% (0) | 0.0–3.5% | 2% (2) | 0.3–7.3% | N/A |
| Number of vitrified MII oocytes | 19 | 15 | |||
| Number of vitrified MII oocytes used for ICSI | 40 | 19 | - | ||
| Fertilization rate per ICSI, % (n) | 80% (32) | 64.4–91.1% | 68.4% (13) | 43.5–87.4% | 0.3453 |
| Degeneration rate after ICSI, % (n) | 10% (4) | 2.8–23.7% | 11% (2) | 0.070328178 | 1.0 |
| Blastocyst formation rate, % (n) | 16% (5) | 5.3–32.8% | 0% (0) | 0–24.8% | 0.2086 |
| Arrested embryos, % (n) | 84% (27) | 67.2–94.7% | 100% (13) | 75.2–100% |
IVM in vitro maturation, CAPA capacitation prematuration, COC cumulus-oocyte complex, SE standard error, MII metaphase II, ICSI intracytoplasmic sperm injection
Fig. 4.
a 1 COC with a thick layer of cumulus cells (left) and 2 COCs with thin layers of cumulus cells (right) upon retrieval. b Denuded oocyte. c 4 COCs merged together after IVM culture. Scale bar 100 μm
Table 3.
Comparison of mean oocyte diameters in CAPA- and standard IVM groups
| CAPA-IVM | Standard IVM | P value (t test) | P value (M-W-test) | |
|---|---|---|---|---|
| Total number of MII | 59 | 34 | ||
| Mean MOD of MII oocytes (± SE), μm | 109.81±0.55 | 110.16±0.83 | 0.7149 | |
| Total number of MI | 15 | 19 | ||
| Mean MOD of MI oocytes (± SE), μm | 109.34±0.89 | 106.99±1.09 | 0.1200 | |
| Total number of GV | 29 | 30 | ||
| Mean MOD of GV oocytes (± SE), μm | 104.47±1.5 | 103.33±1.33 | 0.5732 | 0.3537 |
IVM in vitro maturation, CAPA capacitation prematuration, MOD mean oocyte diameter, SE standard error, MII metaphase II, MI metaphase I, GV germinal vesicle, M-W Mann-Whitney
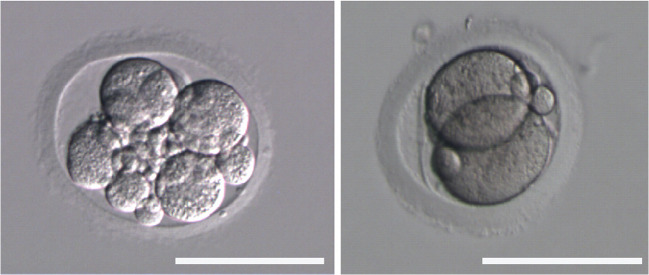
OTO IVM with capacitation culture resulted in higher maturation rate (56 vs 35%, p=0.0045) and had a tendency for lower degeneration rate after IVM (2 vs 11%, p=0.2298), higher fertilization rate (80 vs 68.4%, p=0.3454), and higher blastocyst formation rate (16 vs 0%, p=0.2086). All the blastocysts formed in this study were obtained using the biphasic IVM protocol (Table 4). Thus, the CAPA-IVM program resulted in the vitrification of 5 embryos for 3 patients. We performed PGT-A for one patient with T4bNxM1b ovarian cancer and the molecular karyotype of the embryo was normal ((seq(1-22)x2,(X,Y)x1)). This patient had only 2 embryos: 1 in the standard IVM group which arrested and 1 in the CAPA IVM group which formed a good quality euploid blastocyst (Fig. 2). Moreover, in standard IVM protocol, we observed 2 spontaneously cleaving parthenogenetic embryos after COC denudation (Fig. 3). One of these patients was a young patient of 16 years of age with a Sertoli-Leydig ovarian tumor cell and another was a 29-year-old woman with a low-grade ovarian cancer.
Table 4.
Outcomes of IVM programs with fertilization, n=5
| Obtained COCs | No of MII oocytes | No of 2pn | No of vitrified blastocysts | Embryo quality | |||||
|---|---|---|---|---|---|---|---|---|---|
| Patient number | CAPA-IVM | Standard IVM | CAPA-IVM | Standard IVM | CAPA-IVM | Standard IVM | CAPA-IVM | Standard IVM | CAPA-IVM |
| 1 | 3 | 2 | 1 | 2 | 1 | 2 | 1 | 0 | 4AA |
| 4 | 2 | 4 | 0 | 2 | - | 2 | - | 0 | - |
| 6 | 8 | 7 | 5 | 1 | 4 | 0 | 1 | 0 | 3BB |
| 7 | 38 | 27 | 23 | 11 | 16 | 7 | 0 | 0 | - |
| 8 | 18 | 20 | 11 | 3 | 11 | 2 | 3 | 0 | 2BA, 2BB, 2BB |
IVM in vitro maturation, CAPA capacitation prematuration, COCs cumulus-oocyte complexes, 2pn two pronuclei zygotes
Fig. 3.
Spontaneously cleaving parthenogenetic embryos after COC denudation in the standard IVM group. Scale bar 100 μm
Discussion
OTO IVM is a valuable method pioneered here in a very homogenous group of patients with gynecological malignancies. These first data suggest that it is a reliable option for fertility preservation for women with ovarian cancers and for types of malignancies that metastasize into ovaries. These are the most vulnerable patients since the routine methods of fertility preservation such as COS or ovarian tissue cryopreservation cannot be applied to them due to the high risk of malignant cell transmission. Moreover, patients with gynecological cancers often have to go through radical ovariectomies or panhysterectomies as part of their treatment [1]. The limited number of antral follicles present on the day of the surgery is the only source of immature COCs available for fertility preservation programs. Therefore, it is of high importance to optimize the OTO IVM protocol in order to grant patients the best chances for fertility preservation and restoration after oncological treatment.
Several protocols for prematuration and maturation have been attempted for the improvement of human IVM. In 2000, Anderiesz and coauthors showed that 6-dimethylaminopurine (DMAP) reversibly inhibited oocyte meiotic maturation, but fertilization and cleavage rates were not improved using this system which had indicated that more specific meiotic inhibitors were required [30]. Later, Nogueira et al. (2006) demonstrated that IVM with a specific phosphodiesterase type 3 (PDE3) inhibitor could lead to a higher maturation rate for COCs. However, the cleavage rate was not improved in the PDE3 inhibitor-treated group compared to the control [19]. C-type natriuretic peptide (CNP) is the physiological oocyte maturation inhibitor produced by mural granulosa cells and secreted into follicular fluid [18]. IVM systems with capacitation (CAPA-IVM) using CNP turned out to improve not only maturation but also the developmental potential of immature COCs in PCOS patients [20, 21].
We demonstrate here for the first time that the embryological outcomes of OTO IVM can be improved by the implementation of CAPA-IVM. In our study, the maturation potential of ovarian tissue oocytes improved by 21% in the biphasic system. Our data corroborate the data on the implication of CAPA-IVM for transvaginally aspirated COCs which resulted in 21% (70 vs 49%) [20] and 14.6% (63.6 vs 49%) [31] increase in maturation rate. It had been demonstrated already in PCOS volunteers that IVM with capacitation improved the good blastocyst formation rate [20].
Currently, there are no data available on the potential of OTO for in vitro blastocyst formation in untreated oncofertility patients: in all previously reported cases, the embryos were only cultured up to the cleavage stage and vitrified [3, 4, 32]. In order to predict the efficiency of OTO IVM programs and to provide more profound information to oncological patients as to their chance for live birth, the study of blastocyst formation capacity is essential. OTO are derived mostly from small to mid-sized unstimulated follicles and therefore lack the competence to support early embryogenesis [10, 33]. This sibling study shows that CAPA-IVM might improve the developmental potential of OTO to blastocyst formation. OTO matured using standard IVM protocol failed to form blastocysts while in contrast, the protocol with capacitation resulted in vitrification of 5 blastocysts.
In our study, five patients did not have male partners for fertilization and thus left with the only option of vitrifying their matured oocytes. Though mature oocyte vitrification is a routine and efficient method in clinical practice, matured in vitro vitrified metaphase II oocytes have lower survival rates compared to mature oocytes originated from large follicles after conventional ovarian stimulation [34]. Further studies are needed in order to determine the ovarian tissue oocytes cryosurvival ability.
Interestingly, we observed here and in a previous study [28] spontaneous oocyte parthenogenetic activation in the standard IVM group (Fig. 3.). After denudation of the COCs post-IVM, we observed a 2- and a 7-cell parthenogenetic embryo with fragmentation. Parthenogenesis is the production of an embryo by a female gamete without contribution from a male gamete [35]. The pathological analyses of human ovaries demonstrated in rare cases the presence of cleavage stage embryos in unruptured follicles [36, 37]. Based on this finding, Krafka suggested that an oocyte might continue to develop inside a follicle up to the cleavage stage in case of failed ovulation [36]. Though the certain mechanisms, underlying spontaneous oocyte activation are not known; it was suggested that the incidence of parthenogenetic oocyte activation may increase with the age of the oocytes and might be due to the disturbed oocyte maturation [38]. Such oocytes fail to maintain the MII arrest and prematurely enter the interphase. The analyses of oocytes in a patient with a history of spontaneous activation demonstrated that the patient’s oocytes had cytoarchitectural characteristics similar to the fertilized egg cells such as pronuclear chromatin and cytoplasmic microtubules [39]. The in vitro studies of human oocytes after rescue IVM showed that parthenogenetically activated oocytes fail to maintain M-phase and completely lose phosphorylation in histone-3 [40]. Most likely, that parthenogenetic activation results from the defects in the c-Mos/MAPK pathway, which is the main regulator of the normal MII arrest [39, 40]. In vitro maturation system with capacitation might prevent parthenogenetic oocyte activation since CNP inhibits MAPK cascade activation [41] and does not allow MII exit.
Furthermore, we observed that oocytes after CAPA-IVM had a very low degeneration rate (2%) in comparison with the standard protocol (11%). It was suggested that follicle atresia is linked to the oocyte-cumulus contacts removal and thus disturbance of the maturation inhibition [30, 42]. We suppose that prevention of the dehiscence of cumulus-oocyte cell contacts by avoiding cumulus expansion and inhibiting meiotic reinitiation can rescue the oocytes from degeneration by preserving the shuttling of metabolic factors between the oocyte and cumulus [43].
In our study, we cultured COCs with different types of cumulus morphology (thick, thin, or denuded oocytes) together in groups. In PCOS patients, naked or partially denuded oocytes usually are not considered for IVM [20, 44] and only COCs with a good cumulus morphology are used. Indeed, it was previously demonstrated that nude ovarian tissue oocytes have an extremely low maturation potential [6]. However, denuded oocytes might benefit from the coculture with the COCs with a complete layer of cumulus cells during IVM. For instance, it was demonstrated that coculture of denuded germinal vesicle oocytes with the cumulus cells from mature oocytes of the same patient can improve maturation rate and correct oocytes gene expression [45]. In this study, during IVM, cumulus cells of different COCs merged together forming one cluster (Fig. 4c). Yet, the developmental potential of COCs with different cumulus morphology remains to be determined in future studies.
The main limitation of this study is the small number of enrolled patients; a strength is the homogeneous group of oncofertility patients with gynecological cancer, where few other opportunities are available for fertility preservation. Only 59 oocytes were available for fertilization (40 in the CAPA-IVM group and only 19 in the standard IVM group) in our study. Further study with a larger sample size might help to evaluate better the developmental potential of ovarian tissue oocytes after CAPA IVM. As of today, the patients have not returned for their vitrified oocytes and blastocysts and so we cannot know the potential of cryopreserved genetic material for surviving after thawing and implantation. Further study is needed in order to determine the efficiency of CAPA OTO IVM for pregnancy and live births. Nevertheless, our pilot study demonstrates that the CAPA-IVM system led to higher meiotic maturation and thus might be more efficient as a fertility preservation method for oncological patients than the standard ovarian tissue oocytes in vitro maturation programs.
Conclusions
This is the first study demonstrating that the biphasic in vitro maturation system with capacitation improved the competence of OTO in comparison to the standard IVM method. Oocytes after CAPA-IVM are likely to degenerate less, yet leading to higher fertilization and developmental rates. Thus, fertility preservation programs could become more efficient using a capacitation culture step prior to IVM. These data are encouraging to apply OTO IVM especially for the patients with ovarian malignancies where almost no other alternatives are currently available.
Supplementary Information
(DOCX 24 kb)
Acknowledgements
The authors are grateful to Dr. Dmitriy Ovodenko, Dr. Shakhin Mamedov, Dr. Stanislav Pronin, Dr. Yulia Nosova, Dr. Polina Sheshko, Dr. Muslim Malcagov, Dr. Aleksandr Hizhnikov, and Dr. Aleksey Vinokurov for the oncological consultations of the patients and surgical assistance. We want to thank Dr. Dmitriy Trofimov, Dr. Aleksey Ekimov, and Dr. Olga Ritcher for performing preimplantation genetic testing. The authors thank Cerridwen E. McQueen for her critical reading of themanuscript.
Author contribution
J.S, AK, conceived and designed the study; AK, MF, and NK performed embryological procedures; HVR prepared the components for the capacitation medium; TN, AA, and EB recruited and managed the patients; GK performed the surgeries; GS supervised the clinical and laboratory activities; EB, HVR, and AK collected the data; AK, HVR, EB, and MF analyzed and interpreted the data; AK, JS, and EB and wrote the manuscript. All authors approved the manuscript.
Funding
AK and EB were supported by the Grant of the President of the Russian Federation—for state support of young Russian scientists – К 3244.2019.7.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of our institution (protocol №11 from 13.12.2018). A written informed consent was obtained from all patients.
Conflict of interest
J.S. reports lecture fees from Ferring Pharmaceuticals, Biomerieux, Besins Female Healthcare and Merck, and is co-inventor on granted patents on CAPA-IVM methodology in the USA (US10392601B2) and Europe (EP3234112B1). Other authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anastasia Kirillova, Email: anastasia.kirillova@zoho.com.
Johan E. J. Smitz, Email: johan.smitz@uzbrussel.be
References
- 1.Park CW, Lee SH, Yang KM, Lee IH, Lim KT, Lee KH, Kim TJ. Cryopreservation of in vitro matured oocytes after ex vivo oocyte retrieval from gynecologic cancer patients undergoing radical surgery. Clin Exp Reprod Med. 2016;43:119–125. doi: 10.5653/cerm.2016.43.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hourvitz A, Yerushalmi GM, Maman E, Raanani H, Elizur S, Brengauz M, Orvieto R, Dor J, Meirow D. Combination of ovarian tissue harvesting and immature oocyte collection for fertility preservation increases preservation yield. Reprod BioMed Online. 2015;31:497–505. doi: 10.1016/j.rbmo.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Uzelac PS, Delaney AA, Christensen GL, Bohler HCL, Nakajima ST. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil Steril. 2015;104:1258–1260. doi: 10.1016/j.fertnstert.2015.07.1148. [DOI] [PubMed] [Google Scholar]
- 4.Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, et al. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32:1221–1231. doi: 10.1007/s10815-015-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segers I, Bardhi E, Mateizel I, Van Moer E, Schots R, Verheyen G, et al. Live births following fertility preservation using in-vitro maturation of ovarian tissue oocytes. Hum Reprod. 2020;35:2026–2036. doi: 10.1093/humrep/deaa175. [DOI] [PubMed] [Google Scholar]
- 6.Wilken-Jensen HN, Kristensen SG, Jeppesen JV, Andersen CY. Developmental competence of oocytes isolated from surplus medulla tissue in connection with cryopreservation of ovarian tissue for fertility preservation. Acta Obstet Gynecol Scand. 2014;93:32–37. doi: 10.1111/aogs.12264. [DOI] [PubMed] [Google Scholar]
- 7.Yin H, Jiang H, Kristensen SG, Andersen CY. Vitrification of in vitro matured oocytes collected from surplus ovarian medulla tissue resulting from fertility preservation of ovarian cortex tissue. J Assist Reprod Genet. 2016;33:741–746. doi: 10.1007/s10815-016-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abir R, Ben-Aharon I, Garor R, Yaniv I, Ash S, Stemmer SM, Ben-Haroush A, Freud E, Kravarusic D, Sapir O, Fisch B. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum Reprod. 2016;31:750–762. doi: 10.1093/humrep/dew007. [DOI] [PubMed] [Google Scholar]
- 9.Kedem A, Yerushalmi GM, Brengauz M, Raanani H, Orvieto R, Hourvitz A, Meirow D. Outcome of immature oocytes collection of 119 cancer patients during ovarian tissue harvesting for fertility preservation. J Assist Reprod Genet. 2018;35:851–856. doi: 10.1007/s10815-018-1153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilchrist RB, Thompson JG. Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology. 2007;67:6–15. doi: 10.1016/j.theriogenology.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Hyttel P, Fair T, Callesen H, Greve T. Oocyte growth, capacitation and final maturation in cattle. Theriogenology. 1997;47:23–32. [Google Scholar]
- 12.Smitz J, Thompson J, Gilchrist R. The promise of in vitro maturation in assisted reproduction and fertility preservation. Semin Reprod Med. 2011;29:024–037. doi: 10.1055/s-0030-1268701. [DOI] [PubMed] [Google Scholar]
- 13.Sugimura S, Kobayashi N, Okae H, Yamanouchi T, Matsuda H, Kojima T, Yajima A, Hashiyada Y, Kaneda M, Sato K, Imai K, Tanemura K, Arima T, Gilchrist RB. Transcriptomic signature of the follicular somatic compartment surrounding an oocyte with high developmental competence. Sci Rep. 2017;7:6815. doi: 10.1038/s41598-017-07039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funahashi H, Cantley TC, Day BN. Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilization. Biol Reprod. 1997;57:49–53. doi: 10.1095/biolreprod57.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Thomas RE, Thompson JG, Armstrong DT, Gilchrist RB. Effect of specific phosphodiesterase isoenzyme inhibitors during in vitro maturation of bovine oocytes on meiotic and developmental capacity. Biol Reprod. 2004;71:1142–1149. doi: 10.1095/biolreprod.103.024828. [DOI] [PubMed] [Google Scholar]
- 16.Nogueira D, Cortvrindt R, De Matos DG, Vanhoutte L, Smitz J. Effect of phosphodiesterase type 3 inhibitor on developmental competence of immature mouse oocytes in vitro. Biol Reprod. 2003;69:2045–2052. doi: 10.1095/biolreprod.103.021105. [DOI] [PubMed] [Google Scholar]
- 17.Romero S, Sanchez F, Lolicato F, Van Ranst H, Smitz J. Immature oocytes from unprimed juvenile mice become a valuable source for embryo production when using C-type natriuretic peptide as essential component of culture medium. Biol Reprod. 2016;95:64. doi: 10.1095/biolreprod.116.139808. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Wei Q, Cai J, Zhao X, Ma B. Effect of C-type natriuretic peptide on maturation and developmental competence of goat oocytes matured in vitro. PLoS One. 2015;10:e0132318. [DOI] [PMC free article] [PubMed]
- 19.Nogueira D, Ron-El R, Friedler S, Schachter M, Raziel A, Cortvrindt R, et al. Meiotic arrest in vitro by phosphodiesterase 3-inhibitor enhances maturation capacity of human oocytes and allows subsequent embryonic development. Biol Reprod. 2006;74:177–184. doi: 10.1095/biolreprod.105.040485. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez F, Lolicato F, Romero S, De Vos M, Van Ranst H, Verheyen G, et al. An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield. Hum Reprod. 2017;32:2056–2068. doi: 10.1093/humrep/dex262. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez F, Le AH, Ho VNA, Romero S, Van Ranst H, De Vos M, et al. Biphasic in vitro maturation (CAPA-IVM) specifically improves the developmental capacity of oocytes from small antral follicles. J Assist Reprod Genet. 2019;36:2135–2144. doi: 10.1007/s10815-019-01551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugimura S, Yamanouchi T, Palmerini MG, Hashiyada Y, Imai K, Gilchrist RB. Effect of pre-in vitro maturation with cAMP modulators on the acquisition of oocyte developmental competence in cattle. J Reprod Dev. 2018;64:233–241. doi: 10.1262/jrd.2018-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, Liao X, Krysta AE, Bertoldo MJ, Richani D, Gilchrist RB. Capacitation IVM improves cumulus function and oocyte quality in minimally stimulated mice. J Assist Reprod Genet. 2020;37:77–88. doi: 10.1007/s10815-019-01610-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Cancer Society. [Internet]. 2018. Available from: https://www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/staging.html. Accessed Feb 2021.
- 25.FIGO. International Federation of Gynecology and Obstetrics. [Internet]. 2018. Available from: https://www.figo.org/news/staging-ovftp-malignancies. Accessed Feb 2021.
- 26.Cha K-Y, Chian R-C. Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Update. 1998;4:103–120. doi: 10.1093/humupd/4.2.103. [DOI] [PubMed] [Google Scholar]
- 27.Smith SD, Mikkelsen A-L, Lindenberg S. Development of human oocytes matured in vitro for 28 or 36 hours. Fertil Steril. 2000;73:541–544. doi: 10.1016/s0015-0282(99)00574-9. [DOI] [PubMed] [Google Scholar]
- 28.Kirillova A, Kovalskaya E, Brovkina O, Ekimov A, Bunyaeva E, Gordiev M, Mishieva N, Nazarenko T, Abubakirov A, Sukikh G. Cryopreservation of euploid blastocysts obtained after fertilization of in vitro matured ovarian tissue oocytes: a case report. J Assist Reprod Genet. 2020;37:905–911. doi: 10.1007/s10815-020-01729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romão GS, Araújo MCPM, de Melo AS, Navarro PA d AS, Ferriani RA, dos Reis RM. Oocyte diameter as a predictor of fertilization and embryo quality in assisted reproduction cycles. Fertil Steril. 2010;93:621–625. doi: 10.1016/j.fertnstert.2008.12.124. [DOI] [PubMed] [Google Scholar]
- 30.Anderiesz C, Fong C-Y, Bongso A, Trounson AO. Regulation of human and mouse oocyte maturation in vitro with 6-dimethylaminopurine. Hum Reprod. 2000;15:379–388. doi: 10.1093/humrep/15.2.379. [DOI] [PubMed] [Google Scholar]
- 31.Vuong LN, Le AH, Ho VNA, Pham TD, Sanchez F, Romero S, et al. Live births after oocyte in vitro maturation with a prematuration step in women with polycystic ovary syndrome. J Assist Reprod Genet. 2020;37:347–357. doi: 10.1007/s10815-019-01677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prasath EB, Chan MLH, Wong WHW, Lim CJW, Tharmalingam MD, Hendricks M, Loh SF, Chia YN. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29:276–278. doi: 10.1093/humrep/det420. [DOI] [PubMed] [Google Scholar]
- 33.Thompson JG, Gilchrist RB. Improving oocyte maturation in vitro. In: Trounson A, Gosden R, Eichenlaub-Ritter U, editors. Biology and Pathology of the Oocyte. 2. Cambridge: Cambridge University Press; 2013. pp. 212–223. [Google Scholar]
- 34.Son W-Y, Chung J-T, Demirtas E, Holzer H, Sylvestre C, Buckett W, Chian RC, Tan SL. Comparison of in-vitro maturation cycles with and without in-vivo matured oocytes retrieved. Reprod BioMed Online. 2008;17:59–67. doi: 10.1016/s1472-6483(10)60294-5. [DOI] [PubMed] [Google Scholar]
- 35.Beatty RA. Parthenogenesis and polyploidy in mammalian development. Cambridge Monographs in Experimental Biology, No. 7. Cambridge: At the University Press; 1957. [Google Scholar]
- 36.Krafka J. Parthenogenic cleavage in the human ovary. Anat Rec. 1939;75:19–21. [Google Scholar]
- 37.Shettles LB. Parthenogenetic cleavage of the human ovum. Obstet Gynecol Surv. 1958;13:252–253. [Google Scholar]
- 38.Bałakier H, Casper RF. A morphologic study of unfertilized oocytes and abnormal embryos in human in vitro fertilization. J In Vitro Fert Embryo Transf. 1991;8:73–79. doi: 10.1007/BF01138658. [DOI] [PubMed] [Google Scholar]
- 39.Combelles CMH, Kearns WG, Fox JH, Racowsky C. Cellular and genetic analysis of oocytes and embryos in a human case of spontaneous oocyte activation. Hum Reprod. 2011;26:545–552. doi: 10.1093/humrep/deq363. [DOI] [PubMed] [Google Scholar]
- 40.Combelles CMH, Cekleniak NA, Racowsky C, Albertini DF. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod. 2002;17:1006–1016. doi: 10.1093/humrep/17.4.1006. [DOI] [PubMed] [Google Scholar]
- 41.Yasoda A, Komatsu Y, Chusho H, Miyazawa T, Ozasa A, Miura M, Kurihara T, Rogi T, Tanaka S, Suda M, Tamura N, Ogawa Y, Nakao K. Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med. 2004;10:80–86. doi: 10.1038/nm971. [DOI] [PubMed] [Google Scholar]
- 42.Gougeon A, Testart J. Germinal vesicle breakdown in oocytes of human atretic follicles during the menstrual cycle. Reproduction. 1986;78:389–401. doi: 10.1530/jrf.0.0780389. [DOI] [PubMed] [Google Scholar]
- 43.Conti M, Franciosi F. Acquisition of oocyte competence to develop as an embryo: integrated nuclear and cytoplasmic events. Hum Reprod Update. 2018;24:245–266. doi: 10.1093/humupd/dmx040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dal Canto M, Brambillasca F, Mignini Renzini M, Coticchio G, Merola M, Lain M, de Ponti E, Fadini R. Cumulus cell-oocyte complexes retrieved from antral follicles in IVM cycles: relationship between COCs morphology, gonadotropin priming and clinical outcome. J Assist Reprod Genet. 2012;29:513–519. doi: 10.1007/s10815-012-9766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Virant-Klun I, Bauer C, Ståhlberg A, Kubista M, Skutella T. Human oocyte maturation in vitro is improved by co-culture with cumulus cells from mature oocytes. Reprod BioMed Online. 2018;36:508–523. doi: 10.1016/j.rbmo.2018.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 24 kb)