Abstract
Premature or primary ovarian insufficiency (POI) affects approximately 1% of women and can be due to a variety of causes. Genetic causes include syndromic and non-syndromic POI. There are several promising candidate genes for whom a clear Mendelian association with non-syndromic POI has not yet been conclusively established, including GDF9. GDF9 is an oocyte-secreted factor and is part of the TGF-beta superfamily of morphogens. It has an important role in follicular development and granulosa cell maturation. We report the case of two siblings with primary ovarian insufficiency (POI) and a homozygous truncating variant in GDF9 (c.604C>T; p.(Gln202*). This report helps establish a clear gene-disease association between GDF9 and POI and argues for routine evaluation for GDF9 variants in patients undergoing genomic investigation for POI.
Keywords: GDF9, Primary ovarian insufficiency
Introduction
Premature or primary ovarian insufficiency (POI) is commonly defined as primary or secondary amenorrhoea in women under the age of 40 years [1, 2] due to the absence, lack of function, or early attrition of ovarian follicles [3]. POI affects approximately 1% of women [4]. The condition usually manifests clinically after ovarian reserve is already severely depleted and results in premature menopause.
The clinical presentation of POI is highly heterogeneous, reflecting the various aetiologies and mechanisms that can cause POI, including the absence of ovarian follicles, follicles that are present but lack function, and abnormally rapid attrition of follicles leading to depletion of ovarian reserve prematurely [5]. There are syndromic and non-syndromic primary forms of POI, with differing genetic aetiologies. Causes of syndromic POI include Turner syndrome (45XO), other X chromosome abnormalities such as deletions, translocations and isochromosome X, autoimmune polyendocrinopathy syndrome type I, pseudo-hypoparathyroidism type 1a, progressive external ophthalmoplegia and ovarioleucodystrophy [5]. Established non-syndromic causes include fragile X syndrome, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) receptor gene mutations (FSHR and LHCGR respectively) and TGF-beta superfamily factor mutations [5]. In patients without a secondary cause of POI (e.g., radiotherapy, chemotherapeutic agents and thyroid dysfunction), initial clinical evaluation involves assessment of hormone status with the aim of determining the cause of hypogonadism. Identification of anatomic abnormalities such as gonadal dysgenesis or rudimentary ovaries through gynaecologic ultrasound points towards specific genetic aetiologies such as Turner Syndrome. Despite comprehensive biochemical, radiological and genetic analysis, a cause of POI remains elusive in up to 70% of cases[3, 5, 6].
Growth differentiation factor 9 (GDF9) and its ortholog bone morphogenetic protein 15 (BMP-15) are oocyte-expressed proteins that are important for follicular development and granulosa cell maturation[5]. Biallelic variants in GDF9 have been reported twice previously in patients with primary amenorrhoea [7, 8]. Here we present a case of a homozygous truncating variant in GDF9 in an individual with primary amenorrhoea, identified also in homozygous form in her sister who is similarly affected.
Patients and methods
Case report
The proband (individual II:1 on the family pedigree) is a 23-yearold born to non-consanguineous Caucasian parents, referred to our service by an endocrinologist for genetic assessment. She had never experienced spontaneous menarche but had normal breast and pubertal development. Apart from borderline hypertension (BP 140/90 mmHg), there was no significant past medical history. She was taking the oral contraceptive pill for bone protection. Her sister is 19 years of age and had experienced two brief spontaneous menstrual periods a year apart at the age of 14 years, but no menstrual activity since. She had no other medical history. There was no family history of menstrual or reproductive abnormalities. Their mother had suffered one miscarriage in the first trimester but otherwise had uncomplicated pregnancies with both sisters (G3P2).
Both the proband and her sister showed normal secondary sexual characteristics and normal external genitalia on examination, and both had hypergonadotrophic hypogonadism on biochemical investigation; alternative causes of POI were ruled out through additional clinical testing (see Table 1). The proband consented to whole exome sequencing with analysis targeted to a pre-specified gene list (see ‘Molecular Analyses’), with the intention of finding a precise molecular diagnosis that would explain the familial POI. Both individuals provided written informed consent, and approval from the institutional review board was obtained prior to publication.
Table 1.
Baseline genetic, biochemical and radiological investigations
| Investigations | Individual | Reference range | |
|---|---|---|---|
| II.1 | II.2 | ||
| Gynaecologic ultrasound† | Normal | Normal | |
| Fragile X PCR | Normal allele sizes | Normal allele sizes | |
| Karyotype | Normal | Normal | |
| FSH | 69.1 IU/L | 138.0 IU/L | 1.5–33.4 IU/L‡ |
| LH | 59.1 IU/L | 80 IU/L | 0.5–76.3 IU/L‡ |
| Oestradiol | 84 pmol/L | 93 pmol/L | 102–1300 pmol/L‡ |
| Anti-adrenal antibodies | Negative | Negative | |
| Coeliac antibodies | Negative | Negative | |
| TSH | 1.22 mIU/L | 1.70mIU/L | 0.30–4.00 mU/L |
| Anti-TPO and anti-TG antibodies | Negative | Negative | |
| Bone Mineral Density (Z-score hip) | −1.0 | −0.7 | |
†Ultrasound in both patients revealed small inactive ovaries with otherwise normal reproductive organs.
‡These hormone reference ranges vary with the menstrual cycle phase; the reference ranges provided here encompass this full spectrum
Molecular analyses
DNA extraction, sequencing and reporting of results were performed in National Association of Testing Authorities (NATA) accredited diagnostic laboratories in Melbourne, Australia. Genomic DNA was extracted from peripheral blood leucocytes as per standard laboratory protocol. Genetic testing was performed by an accredited commercial laboratory (Victorian Clinical Genetics Services, Victoria, Australia) with a target mean coverage of 100x and a minimum of 90% of bases sequenced to at least 15x. Alignment was to the GRCh38 reference genome. A literature search spanning review articles and primary reports was conducted to develop a list of 43 genes definitively or putatively associated with POI (see appendix for gene list); analysis of variants was limited to those identified within these genes only. The variant of interest was classified according to modified American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG/AMP) 2015 guidelines [9]. Subsequently, PCR amplification and Sanger sequencing were utilised to identify the variant in the proband’s sister and parents.
Results
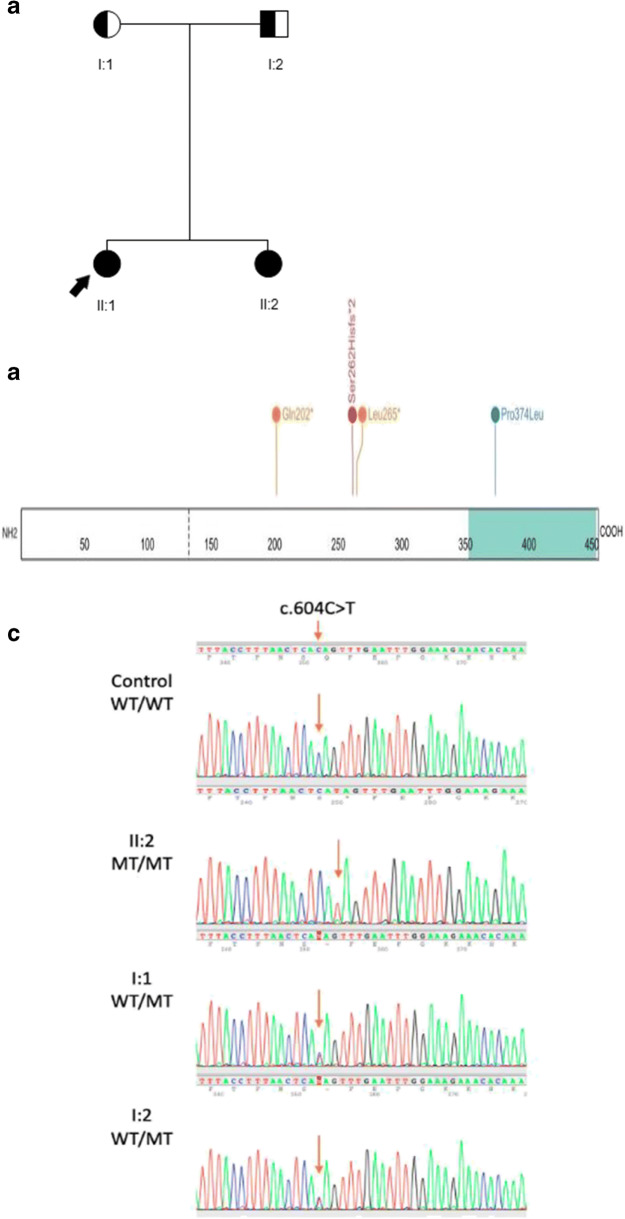
A homozygous nonsense variant in GDF9 (NM_005260.5:c.604C>T; p.(Gln202*)) was identified in the proband. This variant is present in 18 heterozygotes in the genome aggregation database (allele frequency 0.006%, 17 alleles in non-Finnish Europeans) (gnomAD v2.1.1[10]) and has not previously been reported in the literature. It is predicted to introduce a premature stop codon within the last exon (exon 2 of 2 coding exons), and while not expected to undergo nonsense-mediated decay [11], it is predicted to truncate the final protein product by more than 50%, including loss of the TGF-beta domain [12]. This variant was classified as likely pathogenic as both previously reported truncating variants are downstream of our variant, its allele frequency is compatible with pathogenicity for an autosomal recessive condition, and the variant segregated with disease within our patient’s family. We subsequently identified the same variant, also in homozygous form, in the proband’s sister, and in heterozygous form in both parents (see Figure 1). Both the proband and her sister have subsequently been offered referral to an assisted reproduction specialist to discuss their fertility options should they wish to family plan in the future.
Fig. 1.
A. Family pedigree showing unaffected heterozygote parents (partially shaded in individuals) and affected homozygous children (completely shaded in individuals); proband is indicated with the black arrow. B. Location of hitherto published truncating and loss-of-function missense mutations in the GDF9 protein, including our variant (Gln202*). Orange represents nonsense variants, red represents frameshift variants, and blue missense variants. Teal area of gene represents the transforming growth factor (TGF) betalike domain region. C. Sanger sequencing plots of the control, the proband’s sister and the proband’s parents. WT denotes the wildtype and MT indicates the c.604C>T variant. Orange arrow highlights the position of interest on each electropherogram
Discussion
Growth differentiation factor-9 (GDF9 or GDF-9) is a member of the TGF-beta superfamily of morphogens that are highly conserved across mammals and signal by activating transmembrane serine-threonine kinase receptors [12]. It is expressed only in oocytes from the primary one-layer follicle stage until after ovulation [13, 14] and plays a key role in granulosa cell development and ovarian folliculogenesis [15, 16]. Gdf9-null mice have been demonstrated to be infertile, with histological analysis of ovaries revealing failure to develop oocytes beyond the primary one-layer follicle stage; in contrast, Gdf9 heterozygous female mice (Gdf9+/-) and homozygous male mice (Gdf9-/-) were both fertile [16].
Missense substitutions in the propeptide region of GDF9 have been reported in several international cohorts with premature ovarian insufficiency; the patients in whom these substitutions were identified had developed secondary amenorrhoea (cessation of menstruation before the age of 40 years after spontaneous menarche) [17–22]. In contrast, loss-of-function variants on both alleles of GDF9 in a patient with primary amenorrhoea (absence of spontaneous menarche) have only been reported in two other probands (see Figure 1). In the first reported proband with primary amenorrhoea [7], the family was consanguineous; in our family there was no reported consanguinity, although we did not perform a molecular karyotype to evaluate for long contiguous stretches of homozygosity. No homozygous loss-of-function variants in GDF9 are present in population databases (gnomAD v2.1[10]). In the second case, a truncating and a missense variant were detected in compound heterozygous form in a proband with primary amenorrhoea, with functional studies suggesting significant alterations in protein formation, expression and dimerisation with Bmp15 [8]. Taken together, our cases alongside the published literature suggest that the severity of the POI phenotype correlates with the number of GDF9 alleles affected, and the type of mutation in each allele. A single heterozygous missense variant probably contributes to the development of premature menopause, whilst compound heterozygous or homozygous variants that are demonstrated or predicted to have a significant loss-of function effect can cause primary ovarian insufficiency.
In summary, our report of a homozygous nonsense variant in GDF9 segregating with disease in sisters with POI provides additional evidence for establishing a Mendelian gene-disease association between GDF9 and POI. Given the body of evidence, from mechanistic and functional studies to clinical data, published hitherto on GDF9, clinicians investigating patients with POI should consider GDF9, alongside other autosomal genes like FSHR and LHCGR, as a rare but clear monogenic cause of this condition. This case highlights the developing role of genomic sequencing in the diagnostic work-up of patients with otherwise unexplained POI.
Appendix
| Gene List | |
| AIRE | |
| AMH | |
| AMHR2 | |
| BMP15 | |
| C10ORF2 | |
| CLPP | |
| CYP17A1 | |
| CYP19A1 | |
| EIF2B2 | |
| EIF2B4 | |
| EIF2B5 | |
| ESR1 | |
| FGFR1 | |
| FIGLA | |
| FMR1 | |
| FOXL2 | |
| FSHR | |
| GALT | |
| GDF9 | |
| GNAS | |
| HSD17B4 | |
| KISS1 | |
| KISS1R | |
| LARS2 | |
| LHB | |
| LHCGR | |
| LMNA | |
| MCM8 | |
| MCM9 | |
| NOBOX | |
| NR5A1 | |
| NUP107 | |
| POLG | |
| POR | |
| PROK2 | |
| PROKR2 | |
| PSMC3IP | |
| SEMA3A | |
| STAR | |
| TAC3 | |
| TACR3 | |
| WDR11 | |
| WT1 |
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shelling AN. Premature ovarian failure. Reproduction. 2010;140(5):633–641. doi: 10.1530/REP-09-0567. [DOI] [PubMed] [Google Scholar]
- 2.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fortuno C, Labarta E. Genetics of primary ovarian insufficiency: a review. J Assist Reprod Genet. 2014;31(12):1573–1585. doi: 10.1007/s10815-014-0342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67(4):604–606. [PubMed] [Google Scholar]
- 5.Rossetti R, Ferrari I, Bonomi M, Persani L. Genetics of primary ovarian insufficiency. Clin Genet. 2017;91(2):183–198. doi: 10.1111/cge.12921. [DOI] [PubMed] [Google Scholar]
- 6.Chapman C, Cree L, Shelling AN. The genetics of premature ovarian failure: current perspectives. Int J Women's Health. 2015;7:799–810. doi: 10.2147/IJWH.S64024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franca MM, et al. Identification of the first homozygous 1-bp deletion in GDF9 gene leading to primary ovarian insufficiency by using targeted massively parallel sequencing. Clin Genet. 2018;93(2):408–411. doi: 10.1111/cge.13156. [DOI] [PubMed] [Google Scholar]
- 8.Jaillard S, Bell K, Akloul L, Walton K, McElreavy K, Stocker WA, Beaumont M, Harrisson C, Jääskeläinen T, Palvimo JJ, Robevska G, Launay E, Satié AP, Listyasari N, Bendavid C, Sreenivasan R, Duros S, van den Bergen J, Henry C, Domin-Bernhard M, Cornevin L, Dejucq-Rainsford N, Belaud-Rotureau MA, Odent S, Ayers KL, Ravel C, Tucker EJ, Sinclair AH. New insights into the genetic basis of premature ovarian insufficiency: novel causative variants and candidate genes revealed by genomic sequencing. Maturitas. 2020;141:9–19. doi: 10.1016/j.maturitas.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Richards S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karczewski KJ, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abou Tayoun AN, Pesaran T, DiStefano MT, Oza A, Rehm HL, Biesecker LG, Harrison SM, ClinGen Sequence Variant Interpretation Working Group (ClinGen SVI) Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum Mutat. 2018;39(11):1517–1524. doi: 10.1002/humu.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McPherron AC, Lee SJ. GDF-3 and GDF-9: two new members of the transforming growth factor-beta superfamily containing a novel pattern of cysteines. J Biol Chem. 1993;268(5):3444–3449. doi: 10.1016/S0021-9258(18)53714-5. [DOI] [PubMed] [Google Scholar]
- 13.Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13(6):1035–1048. doi: 10.1210/mend.13.6.0310. [DOI] [PubMed] [Google Scholar]
- 14.McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9(1):131–136. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- 15.Elvin JA, Yan C, Matzuk MM. Oocyte-expressed TGF-beta superfamily members in female fertility. Mol Cell Endocrinol. 2000;159(1-2):1–5. doi: 10.1016/S0303-7207(99)00185-9. [DOI] [PubMed] [Google Scholar]
- 16.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383(6600):531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 17.Laissue P, Christin-Maitre S, Touraine P, Kuttenn F, Ritvos O, Aittomaki K, Bourcigaux N, Jacquesson L, Bouchard P, Frydman R, Dewailly D, Reyss AĆ, Jeffery L, Bachelot A, Massin N, Fellous M, Veitia RA. Mutations and sequence variants in GDF9 and BMP15 in patients with premature ovarian failure. Eur J Endocrinol. 2006;154(5):739–744. doi: 10.1530/eje.1.02135. [DOI] [PubMed] [Google Scholar]
- 18.Dixit H, Rao LK, Padmalatha V, Kanakavalli M, Deenadayal M, Gupta N, Chakravarty B, Singh L. Mutational screening of the coding region of growth differentiation factor 9 gene in Indian women with ovarian failure. Menopause. 2005;12(6):749–754. doi: 10.1097/01.gme.0000184424.96437.7a. [DOI] [PubMed] [Google Scholar]
- 19.Kovanci E, et al. Growth differentiating factor-9 mutations may be associated with premature ovarian failure. Fertil Steril. 2007;87(1):143–146. doi: 10.1016/j.fertnstert.2006.05.079. [DOI] [PubMed] [Google Scholar]
- 20.Juarez-Rendon KJ, Garcia-Ortiz JE. Evaluation of four genes associated with primary ovarian insufficiency in a cohort of Mexican women. J Assist Reprod Genet. 2018;35(8):1483–1488. doi: 10.1007/s10815-018-1232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuiko O, et al. Copy number variation analysis detects novel candidate genes involved in follicular growth and oocyte maturation in a cohort of premature ovarian failure cases. Hum Reprod. 2016;31(8):1913–1925. doi: 10.1093/humrep/dew142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B, Wen Q, Ni F, Zhou S, Wang J, Cao Y, Ma X. Analyses of growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) mutation in Chinese women with premature ovarian failure. Clin Endocrinol. 2010;72(1):135–136. doi: 10.1111/j.1365-2265.2009.03613.x. [DOI] [PubMed] [Google Scholar]