Abstract
Purpose
To investigate the effect of different FSH concentrations on human oocyte maturation in vitro and its impact on gene expression of key factors in the surrounding cumulus cells.
Methods
The study included 32 patients who underwent unilateral oophorectomy for ovarian tissue cryopreservation (OTC) (aged 28 years on average). Immature oocytes were collected from surplus medulla tissue. A total of 587 immature oocytes were divided into three categories according to the size of the cumulus mass: large (L-COCs), small (S-COCs), and naked oocytes (NOs), and submitted to 44-h IVM with one of the following concentrations of recombinant FSH: 0 IU/L, 20 IU/L, 40 IU/L, 70 IU/L, or 250 IU/L. After IVM, oocyte nuclear maturation stage and diameter were recorded. The relative gene expression of FSHR, LHCGR, and CYP19A1 in cumulus cells before (day 0; D0) and after IVM were evaluated.
Results
Addition of 70 or 250 IU/L FSH to the IVM medium improved oocyte nuclear maturation compared to 0, 20, and 40 IU/L FSH by upregulating LHCGR and downregulating FSHR in the cumulus cells.
Conclusion
FSH improved oocyte nuclear maturation at concentrations above 70 IU/L suggesting a threshold for FSH during IVM of ex vivo collected human oocytes from small antral follicles. Moreover, current results for the first time highlight that FSH function in vitro is mediated via cumulus cells by downregulating FSHR and upregulating LHCGR, which was also observed when the immature oocytes progressed in meiosis from the GV to the MII stage.
Keywords: FSH, IVM, Ex vivo collected oocytes, Small antral follicles, Fertility preservation
Introduction
In vitro maturation (IVM) of oocytes has remained an attractive solution to simplify assisted reproduction, reducing both treatment complications and costs. Even though IVM is now the mainstream method for assisted reproduction in domestic farm animals, the use of human IVM is still limited. Methods of IVM have mainly focused on women with PCOS, who are particularly prone to side effects of conventional ovarian stimulation, such as ovarian hyperstimulation syndrome [1–6]. However, current IVM methods have not yet resulted in a reproductive outcome that is comparable to conventional IVF treatment, and oocytes matured in vitro are still considered to have lower competence than their in vivo counterparts [5, 7, 8]. In a clinical setting, IVM is most often used in connection with an hCG trigger with or without a short prior stimulation with exogenous follicle-stimulating hormone (FSH) [9, 10] followed by aspiration of oocytes from follicles with a diameter of 10–14 mm [4]. More recently, IVM of oocytes collected ex vivo from small antral follicles (SAFs) in connection with ovarian tissue cryopreservation (OTC) for fertility preservation has gained interest [11–14]. In both scenarios, maturation is completed in vitro in a medium containing FSH [15].
FSH is a central component of all IVM media as it has been shown to improve cumulus expansion [16], fertilization, and early embryonic development [17] in animal models. Nevertheless, its role in oocyte maturation is poorly understood [18], although evidence in mice demonstrated that it acts via cumulus cells [19]. For human IVM, there is no consensus on which FSH concentration is the optimal. The most used FSH concentrations range from 75 to 100 IU/L [3, 13, 20], but it is not uncommon to find concentrations as high as 1000 IU/L [21–23], or studies using media with no addition of FSH [24, 25].
To our knowledge, no previous studies have defined neither the most suitable FSH concentration nor its role in human IVM, perhaps because the oocyte cohort aspirated from antral follicles with diameters > 6 mm after ovarian stimulation and human chorionic gonadotropin (hCG) triggering is heterogeneous, with some of these oocytes already having resumed meiosis in vivo by the time of aspiration [26]. Moreover, major changes occur when follicles reach diameters of around 8 to 11 mm, which corresponds to the transition from the recruitment stage to the selection of the dominant follicle, as indicated by changes in both protein levels and gene expression of multiple key factors in follicular fluid and granulosa cells, respectively [27]. Nonetheless, our group has previously demonstrated that a remarkably high number of immature oocytes can be recovered ex vivo from SAFs (not visible macroscopically, < 2–3 mm in diameter) of unstimulated patients in association with OTC (on average 15 to 36 oocytes per patient) [14, 20]. This more homogenous cohort of immature oocytes that has neither been exposed to exogenous gonadotropin stimulation nor entered the follicle selection phase may represent a better starting point to study the role of FSH during human IVM. Hence, the present study aimed to investigate the effect of different FSH concentrations on human oocyte maturation in vitro and its impact on gene expression of key factors in the surrounding cumulus cells.
Materials and methods
Study approval
The project (H-2-2011-044) was approved by the Scientific Ethical Committee for the Capital Region of Denmark. All patients gave informed consent to donate their surplus ovarian tissue for research purposes.
Patients
A total of 32 patients (aged 28 years on average; range 17–37) undergoing unilateral oophorectomy and OTC for fertility preservation [28] from December 2019 to August 2020, were included in the study. The indications for fertility preservation were Hodgkin lymphoma (n = 5), non-Hodgkin lymphoma (n = 1), breast cancer (n = 16), cervix cancer (n = 2), colorectal cancer (n = 2), sarcoma (n = 2), neuroendocrine tumor (n = 1), choriocarcinoma (n = 1), and other benign diseases (n = 2). The fertility history of the patients and the phase of their menstrual cycle were not recorded.
Ovary transport and oocyte collection
Ovaries were transported to the laboratory in IVF flushing medium (Origio A/S, Måløv, Denmark) either at 37 °C from the local hospital (10 min transport) or on crushed ice from collaborating hospitals in Odense and Aarhus (2–5 h transport). On arrival, fluids from all visible antral follicles were aspirated and used for other research purposes. After isolating the ovarian cortex for cryopreservation, dishes containing the surplus medulla tissue in HEPES-buffered HTF medium (Invitrogen, GIBCO™) were thoroughly examined for the presence of immature oocyte under a stereomicroscope (Leica MZ12, Germany) within a flow hood with heated tabletop at 37 °C. Most oocytes were derived from follicles with diameters below 3 mm. Recovered oocytes were placed in holding medium which consisted of McCoy’s 5α plus 25 mM HEPES (Invitrogen, GIBCO™) with 5 mg/mL human serum albumin (HSA; CSL Behring 20%, Germany), 10 μg/mL insulin, 5.5 μg/mL transferrin, 6.7 ng/mL selenium (ITS; Invitrogen Co., GIBCO™), 2 mM Glutamax (GIBCO™), and 0.05 mg/mL penicillin/streptomycin (GIBCO™) [14]. Only oocytes that showed clear signs of degeneration, such as darkened cytoplasm, were excluded from the study.
Oocyte in vitro maturation
Oocytes were divided into three categories according to the size of the cumulus mass: large cumulus mass (L-COCs), small cumulus mass (S-COCs), and naked oocytes (NOs) (Fig. 1). Naked oocytes were included as control. The oocytes were washed twice in IVM medium which consisted of MediCult IVM system (Origio A/S, Denmark) supplemented with 100 IU/L human recombinant luteinizing hormone (rLH) (Luveris, Serono, Germany), 10 mg/mL HSA, and 1 μg/mL human recombinant Midkine (SRP3114, Sigma-Aldrich, USA) [14], and then individually transferred to 25-μL drops of IVM medium supplemented with different concentrations of human rFSH (Rekovelle, Ferring, Copenhagen, Denmark); 0 IU/L (No FSH), 20 IU/L (FSH20), 40 IU/L (FSH40), 70 IU/L (FSH70), or 250 IU/L (FSH250). These concentrations were based on a previous study [19]. All oocytes were incubated under paraffin oil (Origio A/S, Denmark) for 44 h at 37 °C with 5% CO2 in air. Fresh medium was prepared weekly and pre-equilibrated overnight prior to use. For each patient, oocytes from each category (L-COCs, S-COCs, and NOs) were equally distributed among the different treatments. After 44 h, COCs were mechanically denuded using a 130–133 μm denudation pipette (Vitrolife, Gothenburg, Sweden) and visualized under an inverted microscope (Carl Zeiss Axiovert 135, Germany; ×20 magnification) [20]. Oocytes were classified as germinal vesicle (GV), metaphase I (MI), metaphase II (MII), or degenerated (DEG) (Fig. 2). Oocyte nuclear maturity was determined by the presence of the first polar body (i.e., MII oocyte). Pictures of all oocytes were taken, and oocyte diameters were recorded using the AxioVision software (SE64 Rel.4.9.1). Oocyte diameter was calculated as the mean of two perpendicular measures of each oocyte from the internal part of the zona pellucida (thus, zona pellucida was not included).
Fig. 1.
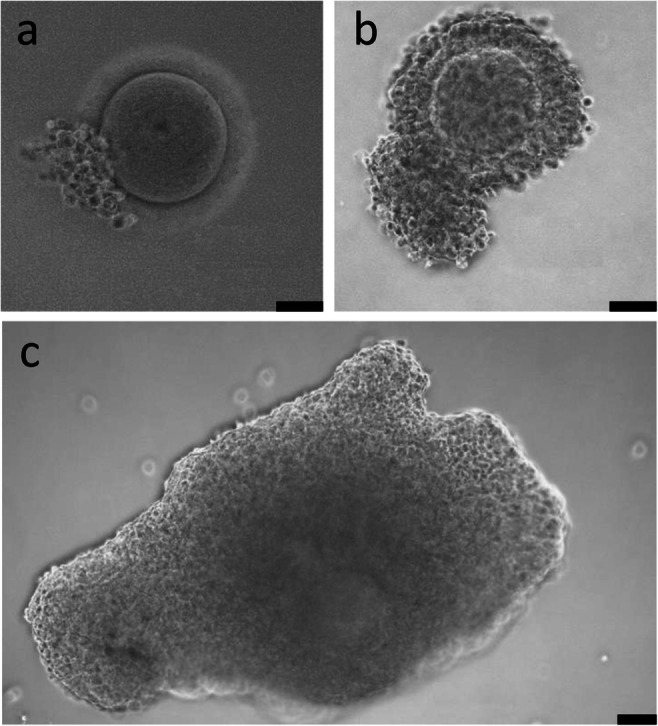
Immature cumulus-oocyte complexes (COCs) collected from the surplus medulla tissue during OTC. a Naked oocyte (NO). b COC with small cumulus mass (S-COC). c COC with large cumulus mass (L-COC). Scale bars: 50 μm
Fig. 2.
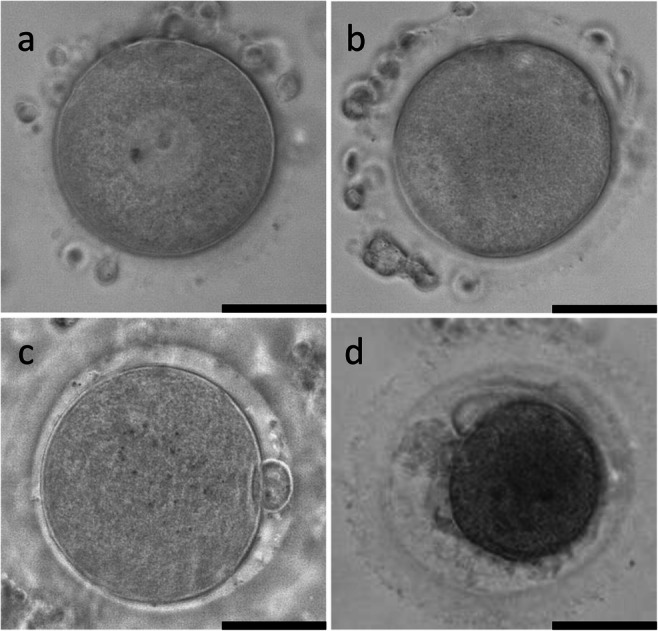
a–d Oocyte classification after IVM: GV (a), MI (b), MII (c), or DEG (d). Scale bars: 50 μm
Quantitative real-time PCR analysis
Cumulus cells from COCs before (day 0; D0) and after IVM were evaluated. Total RNA was individually extracted and purified from cumulus cells of each COC (only L-COCs and S-COCs; NOs were evidently not included) with TRIzol® reagent (Ambion, Life Technologies, USA) and 1-bromo-3-chloropropane (Sigma-Aldrich, USA) and subsequently, with RNeasy® Minikit 250 (Qiagen, Denmark) according to manufacturer’s instructions. All steps were performed on ice. RNA from 37 cumulus samples was isolated. The quality and quantity of the isolated RNA were evaluated using Agilent RNA 6000 Pico kit and Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA). Due to limited amounts of RNA and quality variations among samples, only samples from L-COCs with an RNA integrity value (RIN) ≥ 5 were included in the study (n = 35; 94.6% RNA isolation efficiency). For each selected sample, first-strand cDNA was prepared using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s instructions. The quantitative RT-PCR analysis was carried out by TaqMan® technology using the TaqMan™ Fast Advanced Master Mix (Applied Biosystems, Foster City, CA, USA). The following TaqMan probes were used: FSH receptor (FSHR; #Hs01019695_m1), aromatase cytochrome p450 family 19 subfamily a polypeptide 1 (CYP19A1, #Hs00903412_m1), LH receptor (LHCGR; #Hs00174885_m1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; #Hs02786624_g1) and large ribosomal protein P0 (RPLP0; #Hs00420895_gH) as the reference genes. All samples were run in duplicates and normalized to GAPDH and RPLP0. The relative expression levels were quantified according to the comparative cycle threshold method (LightCycler480 Software, Roche).
Statistical analysis
The impact of FSH on the maturation of the oocyte after IVM was modeled as a mixed logistic regression with maturation outcome (MII yes or no) as outcome and FSH levels (four levels: No FSH, FSH20/40, FSH70, and FSH250), COC size (NO, S-COC, and L-COC), and diameter of oocyte (linear effect) as explanatory variables. Patient effect was included as a random intercept.
The impact of FSH or maturation stage on expression of the target genes (FSHR, LHCGR, and CYP19A1) was modeled as a mixed linear regression with expression of genes as outcome and FSH levels (five levels: No FSH, FSH20, FSH40, FSH70, and FSH250) or maturation outcome (MII yes/no) as explanatory variables. Furthermore, a post hoc test (Tukey) was performed to identify differences among the groups. Patient effect was included as a random intercept. All analysis was done using R version 3.4.3. All P-values below 0.05 were regarded as significant.
Results
Oocyte recovery and in vitro maturation
A total of 875 oocytes were recovered ex vivo from 32 patients in connection with OTC (average 27.3 ± 3.14 oocytes per patient; mean ± SEM, range 8–76), of which 587 were used for IVM, including, 194 L-COCs, 230 S-COCs, and 163 NOs. The remaining, 288 oocytes, were allocated to a different project, although, 20 L-COCs from these groups were denuded and frozen for quantitative RT-PCR analysis (day 0; D0).
The overall statistical analysis showed a significant positive impact of both treatment (FSH addition) (P < 0.0009) and COC size (NO, S-COC, or L-COC) (P < 0.001) on oocyte maturation. In contrast, patient age did not affect oocyte maturation rates (P > 0.05).
As shown in Table 1, the overall average oocyte diameters were similar (P > 0.05) among all treatments and COC categories after IVM, ranging from 115.5 ± 1.2 to 117.9 ± 1.2 μm (mean diameter ± SEM). The overall maturation rate was 31.2% (range 8–48%). Since oocytes are a scarce resource and no differences were observed between No FSH, FSH20, and FSH40, we decided to combine FSH20 and FSH40 (FSH20/40) and stop allocating oocytes to these treatments to increase the number of oocytes in the No FSH treatment instead. The percentages of immature oocytes that reached MII within each FSH concentration were 24%, 19%, 39%, and 37% in 0, 20–40 combined, 70, and 250 IU/L, respectively (Table 1). Compared to the No FSH treatment, oocyte maturation rates significantly increased when the FSH concentrations were 70 IU/L (P = 0.01) and 250 IU/L (P = 0.03), while FSH 20 and 40 IU/L did not differ from 0 IU/L (Table 1).
Table 1.
Mean oocyte diameter and maturation rate of oocytes recovered ex vivo from surplus medulla tissue according to different FSH concentrations used in the IVM medium
| Treatment | Overall | MII-rate according to COC size (%) | |||
|---|---|---|---|---|---|
| Average oocyte diameter (mean ± SEM) |
MII-rate (%)a | NOs | S-COCs | L-COCs | |
| No FSH | 115.5 ± 1.2 | 24.4 (57/234) | 18.8 (13/69) | 28.4 (25/88) | 24.7 (19/77) |
| FSH20/40 | 116.1 ± 2.0 | 19.2 (10/52) | 12.5 (1/8) | 17.4 (4/23) | 23.8 (5/21) |
| FSH70 | 117.9 ± 1.2 | 39.4 (67/170)** | 19.1 (9/47) | 45.5 (30/66) | 49.1 (28/57) |
| FSH250 | 116.7 ± 1.3 | 37.4 (49/131)* | 12.8 (5/39) | 41.5 (22/53) | 56.4 (22/39) |
| Overall | 116.3 ± 0.6 | 31.2 (183/587) | 17.2 (28/163)*** | 35.2 (81/230) | 38.1 (74/194) |
| Average oocyte diameter (mean ± SEM) | 117.1 ± 1.5 | 116.4 ± 1.2 | 117.7 ± 1.4 | ||
aThe impact of FSH on the maturation of the oocyte after IVM was modeled as a mixed logistic regression with maturation outcome (MII yes or no) as outcome and FSH levels (four levels: No FSH, FSH20/40, FSH70, and FSH250), COC size (NO, S-COC, and L-COC), and diameter of oocyte (linear effect) as explanatory variables. *P = 0.01; **P = 0.03 within a column and compared to control treatment (No FSH). ***P < 0.001 within a row and compared to S-COCs and L-COCs
Independent of the treatment, when all oocytes were individually evaluated, the presence of cumulus cells and oocyte diameter were highly and positively correlated with oocyte maturation. Hence, higher maturation rates were found in L-COCs and S-COCs (38% and 35%, respectively) compared to NOs (17%) (P < 0.001) (Table 1, Fig. 3). Likewise, the probability of reaching MII increased with increasing oocyte diameter (P < 0.001).
Fig. 3.
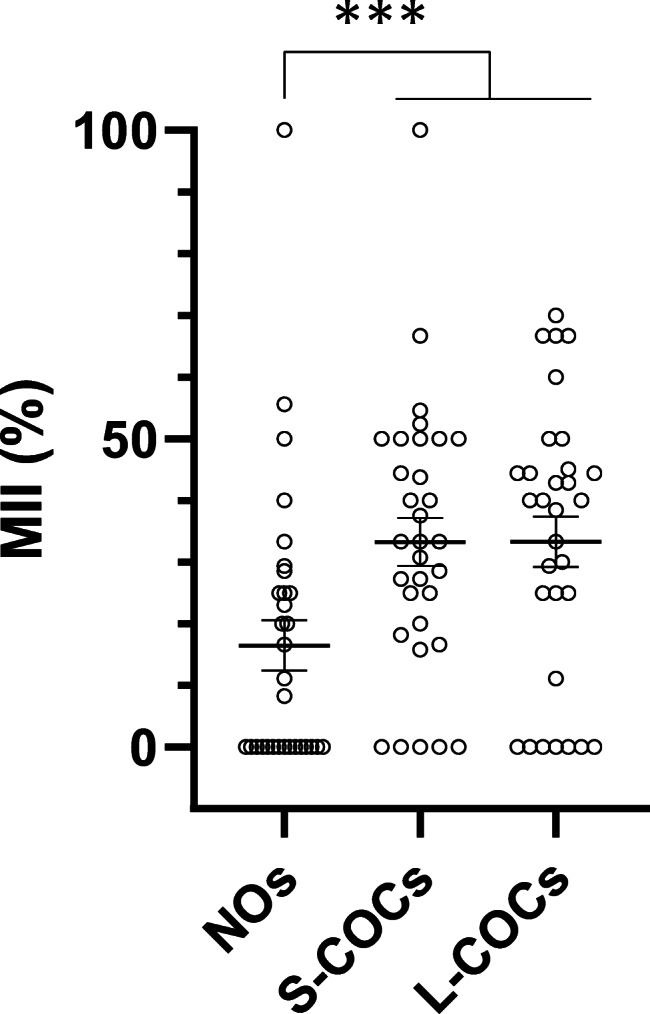
Scatter dot plot representation of maturation rates in naked oocytes (NOs), and cumulus-oocyte complexes with small cumulus mass (S-COCs) and large cumulus mass (L-COCs). (Error bars: mean ± SEM). Each circle of the graph represents one single patient. ***P < 0.001
Relative expression of FSHR, LHCGR, and CYP19A1 in cumulus cells before and after IVM
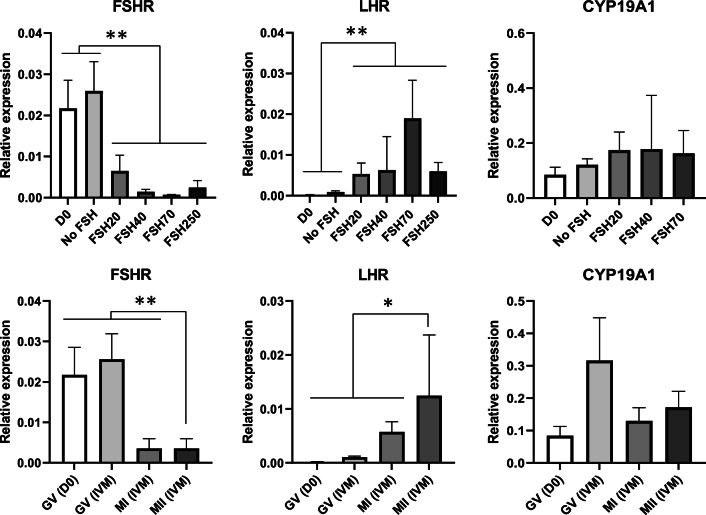
Thirty-five cumulus samples from seven patients were included. Overall, the addition of FSH to the IVM medium downregulated FSHR (P < 0.001) and upregulated LHCGR (P < 0.001). Also, cumulus cells from MII oocytes expressed higher levels of LHCGR (P = 0.01) and lower levels of FSHR (P < 0.001) than cumulus cells from non-MII oocytes both before (D0) and after IVM (GV and MI) (Fig. 4). Cumulus cells from COCs before IVM (all of them at GV stage) presented similar expression to cumulus cells from both COCs non-exposed to FSH (No FSH) and GV oocytes after IVM, regardless of the treatment, for all evaluated genes (P > 0.05). Likewise, the expression of CYP19A1 was not affected by either FSH concentration or oocyte maturation stage (Fig. 4).
Fig. 4.
Relative expression of FSHR, LHCGR, and CYP19A1 in cumulus cells from L-COCs before (D0, n = 4) and after IVM in the presence of increasing concentrations of FSH (0 IU/L, n = 6; 20 IU/L, n = 6; 40 IU/L, n = 7; 70 IU/L, n = 6; and 250 IU/L, n = 6) (upper figures), and according to maturation stage before IVM (GV (D0), n = 4) and after IVM (GV, n = 6; MI, n = 13; and MII, n = 12) (lower figures) (error bars: mean ± SEM). All samples were run in duplicates and normalized to GAPDH and RPLP0. *P = 0.01; **P < 0.001
Discussion
To our knowledge, this is the first study to link the presence of FSH in the IVM medium to both the maturation rate of human oocytes and the expression of FSHR and LHCGR in their corresponding somatic cells, hereby confirming that cumulus cells are intimately involved in the regulation of oocyte maturation in vitro. Cumulus-oocyte complexes that respond to FSH stimulation in vitro by increasing LHCGR expression are more prone to support the resumption of meiosis than those which only develop a modest LHCGR expression. A further characteristic of oocytes that resumed meiosis is a concomitant FSHR downregulation. A previous study also correlated the downregulation of FSHR in cumulus cells to an increased maturation rate after IVM, supporting our findings [29]. Thus, the present data agrees with the interpretation that oocyte maturation could be induced by LH (present in a concentration of 100 IU/L) in those COCs which have developed a yet undefined threshold level of LHCGR expression as a result of FSH stimulation. Further, the present data suggest that an FSH concentration of at least 70 IU/L is required to promote enough LHCGR expression for the induction of oocyte maturation, confirming and extending previous studies with a smaller number of oocytes included [29–31]. It is also possible that the optimal FSH concentration in IVM media could be proportional to the volume of the cumulus cell mass. Even though differences were not statistically significant, slightly higher maturation rates were obtained in L-COCs with FSH 250 IU/L compared to 70 IU/L (56% and 49% MII, respectively). Further investigations are needed to evaluate this hypothesis.
Previous studies from our group have evaluated the expression of FSHR in mural granulosa cells from SAFs and found it on average around 30 times higher than in cumulus cells as found in the present study [27, 32]. The expression of LHCGR at modest levels in cumulus cells of the present study before IVM corresponds to that previously determined in granulosa cells from follicles of similar size [32]. It is, however, interesting to notice that after an IVM period, where the COCs were exposed to FSH stimulation, the expression of LHCGR in those COCs that supported MII transition was increased to a magnitude similar to that observed in granulosa cells at its maximum in pre-ovulatory follicles shortly before the initiation of the midcycle surge of gonadotropins [32].
The much higher FSHR expression in mural granulosa cells as compared to cumulus cells of SAFs indicates that FSH signaling in SAFs is more pronounced in mural granulosa cells than in cumulus cells. In vivo, the mural granulosa cells will probably shield the COCs from FSH action as the hormone will need to penetrate these layers with a high expression of FSHR in order to reach the COCs and exert its effect. This is supported by measurements of FSH in follicular fluid from small antral follicles (4–8 mm in diameter), which on average reached a modest 2.5 IU/L (N=26 follicles, N=17 women with no ovarian stimulation) (unpublished data). Obviously, in connection with IVM, in which only cumulus cells unsheltered by mural granulosa cells are present, an altered FSH signal transduction may take place within the cumulus cells, and effects that would not be activated in the intact follicle may be induced and vice versa. This may explain the accelerated maturation of the immature oocytes in vitro and it can be hypothesized that during IVM in connection with relatively high FSH concentrations exceeding 70 IU/L, the relatively low expression of FSHR in cumulus cells will still provide sufficient signal to induce LHCGR expression and oocyte maturation. Likely, the COC will not receive such stimulation during the in vivo situation. Evidence in the mouse model demonstrated that the signal transduction of gonadotropins in vivo is predominantly exerted via mural granulosa cells and propagated by second messengers of the EGF-like family members to COC [33]. Further studies are needed to evaluate the combined roles of gonadotropins and the EGF-network during human IVM.
The overall mean oocyte diameter after IVM in the present study is almost similar to that seen during normal IVF treatment (104–121 μm, excluding zona pellucida) [34], with no differences between L-COCs, S-COCs, and NOs, and yet maturation rates were higher when cumulus cells were present. This raises the intriguing possibility that the cumulus cells are FSH responsive but are shielded from FSH exposure by the mural granulosa cells, which need to be present in large numbers to secrete sufficient sex-steroids to prepare reproductive organs for the possible arrival of an embryo rather than a direct stimulation of the oocyte. In fact, the oocyte diameter as observed in this study suggests that the COC already at this developmental stage has most of the machinery to support MII transition after proper stimulation.
Only a few studies have previously described the effect of gonadotropins in human IVM [30, 31, 35, 36]. Unlike ours, these studies used either a fixed FSH concentration in a very limited number of oocytes (around 60) [30, 35], or oocytes from patients that had received stimulation with gonadotropins before oocyte retrieval [30, 31, 36], and therefore had been triggered to resume meiosis in vivo [26]. In this study, on the other hand, we have used a cohort of 583 GV oocytes that had not been exposed to any exogenous FSH in vivo prior to IVM.
The results of the present study may also have implications for women undergoing regular IVF. Women with PCOS often require only modest stimulation with exogenous FSH to produce many preovulatory follicles. A sub-group of these patients, or other patients in general, in whom a relatively high number of immature oocytes are aspirated may only have reduced chances for success following ART. One explanation for the low maturation rate could be that the COCs in these patients have been exposed to too little FSH stimulation in vivo for them to develop sufficient LHCGR expression to induce oocyte maturation. Clinically, this group of patients is often difficult to manage, but the present findings suggest that results may be improved by adding FSH and LH to the culture medium in an attempt to augment the maturation rate and thereby the number of available embryos for treatment.
It is noticeable that a maturation rate of 24% was observed with no FSH in the medium. This may be explained by spontaneous luteinization during the in vitro period, with LHCGR being expressed on the cumulus, which then, in the presence of LH (100 IU/L), may have triggered oocyte maturation. Alternatively, some of the oocytes may derive from follicles that have embarked on atresia, and therefore, may have escaped intrafollicular control and started to become luteinized. It would have been interesting to observe the maturation rate of oocytes non-exposed to either FSH or LH during the IVM period. However, this treatment was not included in the present study. Our results are in line with previous findings that have described a similar spontaneous maturation rate of human oocytes in vitro, without gonadotropins or growth factors in the culture media [24, 25]. According to these authors, meiotic resumption can be mechanically triggered by releasing GV oocytes from antral follicles.
The present study has several limitations. Gene expression analysis was performed in a limited number of samples. Unfortunately, most of the stored cumulus cells were lost due to a technical problem, and only samples from seven patients were used for quantitative RT-PCR. Nonetheless, the results were still statistically significant. Also, measuring the expression of more genes would strengthen the study, such as 3β-HSD, CYP11A, and AR, which are involved in steroidogenesis. However, it was only possible to extract a limited amount of mRNA from each COC to perform the quantitative RT-PCR analysis.
The clinical relevance of this study would be increased if the MII oocytes were fertilized and evaluated for aneuploidies and reproductive potential, but the Danish authorities consider IVM an experimental procedure and do not allow it in a clinical setting.
In conclusion, the present study showed that the nuclear maturation of human COCs collected ex vivo from SAFs was significantly improved by adding at least 70 IU/L FSH to the IVM medium. Moreover, FSH stimulation downregulated the expression of FSHR while upregulating LHCGR in cumulus cells. Our data suggest that rather than directly, FSH and LH enhanced oocyte maturation first by stimulating the expression of LHCGR in cumulus cells, making cumulus cells responsive to LH. Whether the FSH concentration may affect oocyte developmental capacity and reproductive outcome still needs further investigation. We believe these findings will be useful for the development of future human IVM protocols for oocytes retrieved from SAF of unstimulated patients, potentially by stimulating the expression of LHCGR in cumulus cells with FSH before exposing the COCs to LH.
Acknowledgements
The authors would like to give special thanks to the colleagues of the LRB, to the patients who actively donated their surplus tissue, and to the doctors and nurses at the operation theaters of the University Hospital of Copenhagen, Rigshospitalet, University Hospital of Aarhus, and University Hospital of Odense.
Author contribution
JC, DN, LAZ, YW, and ZG collected oocytes and executed the IVM. JC performed the qPCR, wrote the manuscript, and produced the figures. SEP was responsible for data analysis. JC, DN, SEP, LAZ, YW, ZG, LSM, SGK, and CYA participated in critical discussion of the findings and revision of the manuscript. CYA was responsible for the concept and the final revision of the manuscript. All authors approved the final version of the manuscript.
Funding
This research was supported by the University Hospital of Copenhagen, Rigshospitalet, the ReproUnion collaborative study, co-financed by the European Union, Interreg V ÖKS, and the Danish Medical Research Council.
Declarations
Ethical approval
The project (H-2-2011-044) was approved by the Scientific Ethical Committee for the Capital Region of Denmark. All patients gave informed consent to donate their surplus ovarian tissue for research purposes.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Le Du A, Kadoch IJ, Bourcigaux N, Doumerc S, Bourrier MC, Chevalier N, et al. In vitro oocyte maturation for the treatment of infertility associated with polycystic ovarian syndrome: the French experience. Hum Reprod. 2005;20(2):420–424. doi: 10.1093/humrep/deh603. [DOI] [PubMed] [Google Scholar]
- 2.Gulekli B, Kovali M, Aydiner F, Dogan S, Dogan SS. IVM as an alternative for patients with PCO after failed conventional IVF attempt. J Assist Reprod Genet. 2011;28:495–499. doi: 10.1007/s10815-011-9591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Junk SM, Yeap D. Improved implantation and ongoing pregnancy rates after single-embryo transfer with an optimized protocol for in vitro oocyte maturation in women with polycystic ovaries and polycystic ovary syndrome. Fertil Steril. 2012;98(4):888–892. doi: 10.1016/j.fertnstert.2012.06.055. [DOI] [PubMed] [Google Scholar]
- 4.Gremeau AS, Andreadis N, Fatum M, Craig J, Turner K, McVeigh E, et al. In vitro maturation or in vitro fertilization for women with polycystic ovaries? A case-control study of 194 treatment cycles. Fertil Steril. 2012;98(2):355–360. doi: 10.1016/j.fertnstert.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez F, Lolicato F, Romero S, De Vos M, Van Ranst H, Verheyen G, et al. An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield. Hum Reprod. 2017;32(10):2056–2068. doi: 10.1093/humrep/dex262. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez F, Le AH, Ho VNA, Romero S, Van Ranst H, De Vos M, et al. Biphasic in vitro maturation (CAPA-IVM) specifically improves the developmental capacity of oocytes from small antral follicles. J Assist Reprod Genet. 2019;36(10):2135–2144. doi: 10.1007/s10815-019-01551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imesch P, Scheiner D, Xie M, Fink D, Macas E, Dubey R, et al. Developmental potential of human oocytes matured in vitro followed by vitrification and activation. J Ovarian Res. 2013;6(30). 10.1186/1757-2215-6-30 [DOI] [PMC free article] [PubMed]
- 8.Walls ML, Hunter T, Ryan JP, Keelan JA, Nathan E, Hart RJ. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries: a comparative analysis of fresh, frozen and cumulative cycle outcomes. Hum Reprod. 2015;30(1):88–96. doi: 10.1093/humrep/deu248. [DOI] [PubMed] [Google Scholar]
- 9.Fadini R, Dal Canto MB, Mignini Renzini M, Brambillasca F, Comi R, Fumagalli D, et al. Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: a prospective randomized study. Reprod BioMed Online. 2009;19(3):343–351. doi: 10.1016/s1472-6483(10)60168-x. [DOI] [PubMed] [Google Scholar]
- 10.De Vos M, Ortega-Hrepich C, Albuz FK, Guzman L, Polyzos NP, Smitz J, et al. Clinical outcome of non-hCG-primed oocyte in vitro maturation treatment in patients with polycystic ovaries and polycystic ovary syndrome. Fertil Steril. 2011;96(4):860–864. doi: 10.1016/j.fertnstert.2011.07.1108. [DOI] [PubMed] [Google Scholar]
- 11.Prasath EB, Chan ML, Wong WH, Lim CJ, Tharmalingam MD, Hendricks M, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29:276–278. doi: 10.1093/humrep/det420. [DOI] [PubMed] [Google Scholar]
- 12.Uzelac PS, Delaney AA, Christensen GL, Bohler HC, Nakajima ST. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil Steril. 2015;104:1258–1260. doi: 10.1016/j.fertnstert.2015.07.1148. [DOI] [PubMed] [Google Scholar]
- 13.Segers I, Bardhi E, Mateizel I, Van Moer E, Schots R, Verheyen G, et al. Live births following fertility preservation using in-vitro maturation of ovarian tissue oocytes. Hum Reprod. 2020;35(9):2026–2036. doi: 10.1093/humrep/deaa175. [DOI] [PubMed] [Google Scholar]
- 14.Nikiforov D, Junping C, Cadenas J, Shukla V, Blanshard R, Pors SE, Kristensen SG, Macklon KT, Colmorn L, Ernst E, Bay-Bjørn AM, Ghezelayagh Z, Wakimoto Y, Grøndahl ML, Hoffmann E, Andersen CY. Improving the maturation rate of human oocytes collected ex vivo during the cryopreservation of ovarian tissue. J Assist Reprod Genet. 2020;37:891–904. doi: 10.1007/s10815-020-01724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walls ML, Hart RJ. In vitro maturation. Best Pract Res Clin Obstet Gynaecol. 2018;53:60–72. doi: 10.1016/j.bpobgyn.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Calder MD, Caveney AN, Smith LC, Watson AJ. Responsiveness of bovine cumulus-oocyte-complexes (COC) to porcine and recombinant human FSH, and the effect of COC quality on gonadotropin receptor and Cx43 marker gene mRNAs during maturation in vitro. Reprod Biol Endocrinol. 2003;1:14. doi: 10.1186/1477-7827-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izadyar F, Zeinstra E, Bevers MM. Follicle-stimulating hormone and growth hormone act differently on nuclear maturation while both enhance developmental competence of in vitro matured bovine oocytes. Mol Reprod Dev. 1998;51:339–345. doi: 10.1002/(SICI)1098-2795(199811)51:3<339::AID-MRD14>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 18.Sirard MA, Desrosier S, Assidi M. In vivo and in vitro effects of FSH on oocyte maturation and developmental competence. Theriogenology. 2007;68S:S71–S76. doi: 10.1016/j.theriogenology.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 19.Byskov AG, Andersen CY, Hossaini A, Guoliang X. Cumulus cells of oocyte–cumulus complexes secrete a meiosis-activating substance when stimulated with FSH. Mol Reprod Dev. 1997;46:296–305. doi: 10.1002/(SICI)1098-2795(199703)46:3<296::AID-MRD8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 20.Yin H, Jian H, Kristensen SG, Andersen CY. Vitrification of in vitro matured oocytes collected from surplus ovarian medulla tissue resulting from fertility preservation of ovarian cortex tissue. J Assist Reprod Genet. 2016;33:741–746. doi: 10.1007/s10815-016-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderiesz C, Ferraretti AP, Magli C, Fiorentino D, Fortini D, Gianaroli L, et al. Effect of recombinant human gonadotrophins on human, bovine and murine oocyte meiosis, fertilization and embryonic development in vitro. Hum Reprod. 2000;15(5):1140–1148. doi: 10.1093/humrep/15.5.1140. [DOI] [PubMed] [Google Scholar]
- 22.Yang SH, So WY, Yoon SY, Ko Y, Lim JH. Correlation between in vitro maturation and expression of LH receptor in cumulus cells of the oocytes collected from PCOS patients in HCG-primed IVM cycles. Hum Reprod. 2005;20(8):2097–2103. doi: 10.1093/humrep/dei045. [DOI] [PubMed] [Google Scholar]
- 23.Magli MC, Ferraretti AP, Crippa A, Lappi M, Feliciani E, Gianaroli L. First meiosis errors in immature oocytes generated by stimulated cycles. Fertil Steril. 2006;86(3):629–635. doi: 10.1016/j.fertnstert.2006.02.083. [DOI] [PubMed] [Google Scholar]
- 24.Escrich L, Grau N, Mercader A, Rubio C, Pellicer A, Escribá MJ. Spontaneous in vitro maturation and artificial activation of human germinal vesicle oocytes recovered from stimulated cycles. J Assist Reprod Genet. 2011;28:111–117. doi: 10.1007/s10815-010-9493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escribá MJ, Grau N, Escrich L, Novella-Maestre E, Sánchez-Serrano M. Spontaneous in vitro maturation of oocytes prior to ovarian tissue cryopreservation in natural cycles of oncologic patients. J Assist Reprod Genet. 2012;29:1261–1265. doi: 10.1007/s10815-012-9860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Son WY, Tan SL. Laboratory and embryological aspects of hCG-primed in vitro maturation cycles for patients with polycystic ovaries. Hum Reprod Update. 2010;16:675–689. doi: 10.1093/humupd/dmq014. [DOI] [PubMed] [Google Scholar]
- 27.Kristensen SG, Mamsen LS, Jeppesen JV, Bøtkjær JA, Pors SE, Borgbo T, Ernst E, Macklon KT, Andersen CY. Hallmarks of human small antral follicle development: implications for regulation of ovarian steroidogenesis and selection of the dominant follicle. Front Endocrinol. 2018;8:376. doi: 10.3389/fendo.2017.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen CY, Mamsen LS, Kristensen SG. Freezing of ovarian tissue and clinical opportunities. Reproduction. 2019;158:F27–F34. doi: 10.1530/REP-18-0635. [DOI] [PubMed] [Google Scholar]
- 29.Guzman L, Adriaenssens T, Ortega-Hrepich C, Albuz FK, Mateizel I, Devroey P, de Vos M, Smitz J. Human antral follicles <6 mm: a comparison between in vivo maturation and in vitro maturation in non-hCG primed cycles using cumulus cell gene expression. Mol Hum Reprod. 2013;19(1):7–16. doi: 10.1093/molehr/gas038. [DOI] [PubMed] [Google Scholar]
- 30.Prins GS, Wagner C, Weidel L, Gianfortoni J, Marut EL, Scommegna A. Gonadotropins augment maturation and fertilization of human immature oocytes cultured in vitro. Fertil Steril. 1987;47(6):1035–1037. doi: 10.1016/s0015-0282(16)59243-7. [DOI] [PubMed] [Google Scholar]
- 31.Yan J, Yang Y, Liying Y, Zichuan L, Ping L, Huailiang F, Qi Z, Jie Q. In vitro maturation of cumulus-partially enclosed immature human oocytes by priming with gonadotropin. Fertil Steril. 2011;96(3):629–634. doi: 10.1016/j.fertnstert.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 32.Jeppesen JV, Kristensen SG, Nielsen ME, Humaidan P, Dal Canto M, Fadini R, Schmidt KT, Ernst E, Yding Andersen C. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab. 2012;97(8):E1524–E1531. doi: 10.1210/jc.2012-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richani D, Gilchrist B. The epidermal growth factor network: role in oocyte growth, maturation and developmental competence. Hum Reprod Update. 2018;24(1):1–14. doi: 10.1093/humupd/dmx029. [DOI] [PubMed] [Google Scholar]
- 34.Romão GS, Araújo MCPM, De Melo AS, Navarro PAAS, Ferriani RA, Dos Reis RM. Oocyte diameter as a predictor of fertilization and embryo quality in assisted reproduction cycles. Fertil Steril. 2010;93(2):621–625. doi: 10.1016/j.fertnstert.2008.12.124. [DOI] [PubMed] [Google Scholar]
- 35.Durinzi KL, Wentz AC, Saniga EM, Johnson DE, Lanzendorf SE. Follicle stimulating hormone effects on immature human oocytes: in vitro maturation and hormone production. J Assist Reprod Genet. 1997;14:199–204. doi: 10.1007/BF02766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu YW, Peng YT, Wang B, Zeng YH, Zhuang GL, Zhou CQ. High follicle-stimulating hormone increases aneuploidy in human oocytes matured in vitro. Fertil Steril. 2011;95(1):99–104. doi: 10.1016/j.fertnstert.2010.04.037. [DOI] [PubMed] [Google Scholar]