Abstract
Purpose
The aim of this study is to investigate the mechanisms by which the testis specific Na,K-ATPase ion transport system (Atp1a4) controls sperm morphology and shape.
Methods
Sperm from wild-type (WT) and Atp1a4 knockout (Atp1a4 KO) mice were analyzed morphologically, using light, transmission, and scanning electron microscopy; and functionally, applying sperm osmotic challenge and viability tests. In addition, a sperm proteomic study was performed.
Results
Light microscopy confirmed that sperm lacking Atp1a4 present a bend at the junction of the mid- and principal piece of the flagellum. This bend had different degrees of angulation, reaching occasionally a complete flagellar retroflexion. The defect appeared in sperm collected from the cauda epididymis, but not the epididymal caput or the testis. Transmission and scanning electron microscopy revealed a dilation of the cytoplasm at the site of the bend, with fusion of the plasma membrane in overlapping segments of the flagellum. This was accompanied by defects in the axoneme and peri-axonemal structures. Sperm from Atp1a4 KO mice showed an abnormal response to hypoosmotic challenge with decreased viability, suggesting reduced capacity for volume regulation. Exposure to Triton X-100 only partially recovered the flagellar bend of Atp1a4 KO sperm, showing that factors other than osmotic regulation contribute to the flagellar defect. Interestingly, several key sperm structural proteins were expressed in lower amounts in Atp1a4 KO sperm, with no changes in their localization.
Conclusions
Altogether, our results show that Atp1a4 plays an important role in maintaining the proper shape of the sperm flagellum through both osmotic control and structurally related mechanisms.
Keywords: Na,K-ATPase α4; Isoform; Sperm flagellum; Sperm volume; Outer dense fiber protein; Dynein intermediate chain I; Glutathione peroxidase
Introduction
Most animal cells maintain an uneven distribution of Na+ and K+ across the cell plasma membrane thanks to the continuous activity of Na,K-ATPase. Na,K-ATPase is an enzyme that couples the energy obtained from the hydrolysis of ATP to the transmembrane exchange of cytoplasmic Na+ for extracellular K+ in a 3:2 relationship [1]. In somatic cells, the Na+ and K+ gradients generated by Na,K-ATPase are vital for maintaining cell ion homeostasis, cell resting membrane potential, and the sodium dependent secondary transport of various solutes and water across the cell surface [2–4].
Na,K-ATPase is composed of two major membrane embedded proteins, the α or catalytic and the β or glycosylated subunits [5]. The catalytic subunit is involved in the binding and transport of Na+ and K+ across the cell membrane and also contains the binding sites for ATP and the Na,K-ATPase inhibitor ouabain [6]. The β subunit is important in helping with the proper folding of the α subunit during its synthesis, targeting the whole transporter to the plasma membrane, and mediating cell-cell interactions between cells [7–9].
In mammalian cells, Na,K-ATPase consist of a family of isozymes, which result from the association of different molecular forms of the α subunit (Atp1a1, Atp1a2, Atp1a3, and Atp1a4) and the β polypeptide (Atp1b1, Atp1b2 and Atp1b3). Each Na,K-ATPase αβ pair is expressed and regulated in a cell- and tissue-specific manner and has particular functional properties which are suited to the specific needs of each cell [10, 11]. Among the molecular variants of the Na,K-ATPase α subunit, Atp1a4 is the isoform with the most restricted pattern of expression, localizing to the male germ cells of the testis [12]. Expression of Atp1a4 is highly regulated and its levels raise after meiosis, to become abundant in the differentiated sperm. In mouse sperm, Atp1a4 predominates in the midpiece of the sperm flagellum [13, 14].
We have previously shown that Atp1a4 is essential for sperm function and that its deletion in mice results in complete infertility of male, but not female mice [15]. The main phenotype of sperm from Atp1a4 knock out mice is their inability to properly swim, showing decreased total motility and a reduction in most parameters of sperm kinematics. Sperm from Atp1a4 KO mice also have a serious defect in hypermotility, the pattern of movement typical of capacitated sperm, which is required for fertilization [15, 16]. Sperm lacking Atp1a4 or where Atp1a4 was inhibited with ouabain show alterations that are secondary to a disbalance in cytoplasmic ion levels, including increased intracellular Na+, depolarization of the plasma membrane, cytoplasmic acidification, and elevated calcium levels [15, 17].
In addition to the mentioned defects, sperm from mice null in Atp1a4 present a bend in the sperm flagellum [15]. However, the reasons for this morphological abnormality have remained unclear. Two main factors are known to be involved in sperm flagellar alterations; one relates to deficiencies in osmoregulation and cell volume control; the other includes primary structural defects, secondary to alteration in expression and function of proteins directly involved with sperm morphology [18]. Commonly, sperm osmotic disbalance presents with flagellar angulation as has been reported in several mouse models associated with swollen spermatozoa [19]. Control of osmotic balance is particularly important for sperm, since these cells are exposed to media with changing ionic strength and face osmotic challenges as they move through the male genital tract and after, when leaving the testis. Thus, while stored in the epididymis sperm are subjected to a medium that has high osmolarity [20]. Once released into the female genital tract and during their journey to find the egg, sperm face an environment where osmolarity is drastically reduced to levels that are isosmotic with respect to plasma [21]. These extreme osmotic variations threaten the stability of the cells by inducing volume and morphological changes. In particular, low osmolarity leading to volume increases impose mechanical stress to the sperm plasma membrane, which leads to bending of the sperm flagellum in an effort to accommodate the cell geometry to the new dimension of the cells. Eventually, cell swelling can disrupt the cell membrane and affect sperm functionality [20]. To counteract cell shrinkage or swelling, sperm turn on mechanisms known as regulatory volume increase (RVI), or regulatory volume decrease (RVD) respectively. These mechanisms rely on the movement of ions across the plasma membrane, which are secondarily accompanied by water shifts to restore cell volume to normal levels [22, 23]. While RVI and RVD have been well documented in somatic cells, less is known regarding these volume protective mechanisms in sperm. However, it has been shown that K-channels [24], chloride channels, such as ClC3 [25], and several aquaporin water channels [26] have been implicated as mechanisms operating in sperm to adjust cell volume. Failure of the mechanisms controlling cell volume results in sperm with abnormal motility characteristics, inability to properly progress through the fallopian tube mucus, or in failure to fertilize eggs [27, 28].
As mentioned above, another factor leading to changes in sperm shape are primary axonemal morphology defects. A variety of anomalies of the sperm flagellum have been observed in mice after deletion of genes that are specifically involved in the generation or maintenance of different components of the axoneme. The resulting flagellar phenotypes are accompanied by asthenospermia, dyskinesia, or complete sperm immobility (reviewed in [18]).
At present, a characterization of the morphological changes underlying the structural defect that accompanies the Atp1a4 KO phenotype, as well as the potential mechanisms by which Atp1a4 controls sperm flagellar morphology are unknown. Our findings here show that sperm lacking Atp1a4 present deficiencies in osmoregulation and volume control, as well as a reduction in the expression levels, but no changes in localization, of several proteins that are key for maintaining the structural stability of the sperm flagellum.
Material and methods
Animals
All experimental protocols involving animals in this work were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee. Spermatozoa from wild-type (WT) and Atp1a4 KO mice were obtained directly from the testis and the caput and caudal regions of the epididymis. Cells were collected by swim-up as previously described [15]. Sperm was resuspended in modified Whitten’s medium, containing 100 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5.5 mM glucose, 0.8 mM pyruvic acid, 4.8 mM lactic acid, an 20 mM Hepes (pH 7.4). For the experiments in which sperm were exposed to Triton X-100, cells were treated with the detergent at a final concentration of 0.1% for 30 min.
Immunoblot analysis
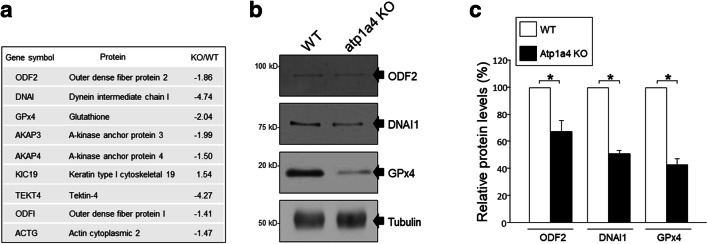
Protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, SDS/PAGE (10% gel), and expression was determined by immunoblot, as previously described [13]. To identify ODF2, DNAI1, GPx4, and tubulin, the following antibodies were used: rabbit anti-ODF2 (1:2000 dilution; a kind gift from Prof. Abraham Kierszenbaum, City University of New York Medical School), mouse anti-DNAI1 (1:10000 dilution; a generous gift from Prof. Larry Ostrowski, University of North Carolina Medical School), rabbit anti-GPx4 (1:1000 dilution; from Abcam; Cambridge, UK), and mouse anti-beta tubulin (1:1000 dilution; Sigma-Aldrich; St. Louis, MO). Horseradish peroxidase conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.) and chemiluminescence were used for detection.
Immunocytochemistry studies
Immunocytochemistry of caudal epididymal spermatozoa was performed as previously described. Briefly, cells were placed on glass slides and fixed in 10% formalin. Samples were permeabilized with 0.3% Triton X100 in 25 mM Hepes, pH 7.4, 150 mM NaCl, and 1 mM ethylene glycol-bis (2 aminoethyl-ether)-N,N,N′,N′-tetraacetic acid (EGTA; HBS). Blocking of unspecific epitopes was performed for 2 h at room temperature with 0.1% bovine serum albumin and 5% normal goat serum in phosphate-buffered saline (PBS). Primary antibodies against GPx4 was the same as that used for immunoblotting. The ODF2 and DNAI1 antibodies were obtained from Thermo-Fisher (Waltham, MA). All antibodies were applied at a dilution of 1:500 and incubated overnight at 4 °C. Then, samples were washed three times, 15 min each, and treated with secondary antisera conjugated to Alexa Fluor 594 (Molecular Probes) at a 1:1000 dilution. After washing as described above, samples were mounted on slides using SlowFade mounting solution (Molecular Probes; Eugene, OR) containing 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) to stain the cell nuclei. Fluorescent digital images were obtained using a Nikon Eclipse 80i microscope.
Osmolarity challenge tests
The sperm hypoosmotic swelling (HOS) test was used to investigate the capacity of the cells to respond to hypoosmotic conditions in response to cell swelling. This assay predicts the capacity of sperm to maintain fluid balance with the environment [29, 30]. In addition, we also tested the capacity of the cells to cope with not only hypoosmotic but also hyperosmotic solutions. Briefly, 10 μl of sperm sample (containing 3 × 104 cells) was added to a 100 μl of modified HOS solution containing 5.5 mM glucose, 0.8 mM pyruvic acid, 4.8 mM lactic acid, 20 mM Hepes (pH 7.4), with an osmolality of 53 Osm/kg. This was supplemented with different amounts of a 1 M sucrose solution (containing an osmolality of 1415 Osm/kg) to achieve the various osmotic insult conditions desired (53, 150, 250, and 450 mosmol/kg). Osmolality values were all verified using a Vapro 5520 vapor pressure osmometer (Wescor; Logan, Utah). Samples were incubated at 37 °C for 30 min, fixed in 200 μl of buffered formalin (Fisher Scientific, Waltham, MA, and analyzed under a phase contrast microscope. The number of swollen cells, which corresponded to those presented ballooning and coiling of the tail, were counted and expressed as the percent of the total cells in the field.
Cell viability assay
Viability of sperm was determined using the LIVE/DEAD Sperm Viability Kit and flow cytometry of the SYBR green fluorescence/propidium iodide label as suggested by the manufacturer (Molecular Probes; Eugene, OR).
Light microscopy morphology analysis
Epididymal and testicular spermatozoa were viewed under a Nikon 80i microscope using a × 100 objective and images were captured using an Olympus DP72 digital camera. Spermatozoa were categorized and scored into two main groups: (1) cells with a straight extended and smooth flagellum was considered morphologically “normal”; (2) sperm with flagellar angulation of any degree, including complete retroflexion of the flagellum, was considered as “bent” sperm. Cells were counted and expressed as percent of the total number of cells.
Ultrastructural studies
Spermatozoa from wild-type and Atp1a4 KO mice were collected in Whitten’s modified medium and fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer at pH 7.4 for 1 h. After rinsing three times with 0.1 M cacodylate buffer for 5 min each, samples were incubated in 1% osmium tetroxide in 0.1 M cacodylate buffer plus 0.1% potassium ferricyanide for 1 h. Samples were then rinsed with deionized distilled water and dehydrated, using a graded series of ethanol solutions (30%, 70%, 80%, 95%, and 100%) for 10 min each. Cells were equilibrated with propylene oxide for 15 min, followed by a 1:1 propylene oxide-Embed resin mixture overnight. For some experiments, testes and epididymides were cut and squeezed onto a 13 mm Thermanox coverslip (NUNC, Thermo Scientific) to release sperm. Samples were allowed to air dry on ice for 5 min and were fixed and processed as described above. For transmission electron microscopy, samples were embedded with fresh Embed resin and allowed to polymerize overnight and sectioned in 80 nm sections, using a Diatome diamond knife on a Leica EM UC-7 ultra-microtome. Samples were contrasted with 3% uranyl acetate and Sato’s Lead stain and examined in a J.E.O.L. JEM-1400 TEM equipped with a LAB6 gun and AMT 601 hi resolution camera.
For scanning electron microscopy, samples were fixed and processed as described above. Then, they were point dried with an EMS 850 critical point dryer, mounted on a stub, and sputter coated with gold using a Pelco SC-6 sputter coater and were viewed using a Hitachi S-2700 SEM.
Sperm proteomic analysis
Sperm samples were purified from contaminants by gradient centrifugation using Percoll [31] and subjected to difference gel electrophoresis (2D-DIGE) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI TOF/TOF). This analysis was performed at Applied Biomics (Hayward, CA). Briefly, proteins were extracted from sperm and adjusted to the desired concentration. Equal amounts of protein from paired samples were labeled by CyDye DIGE fluors (size and charge matched) respectively, and the spectrally resolvable dyes enabled the simultaneous co-separation and analysis of samples on a single multiplexed gel. Samples were separated on a single 2D gel, using isoelectric focusing (IEF) in the first dimension and SDS polyacrylamide gel electrophoresis (SDS-PAGE) in the second dimension. After electrophoresis, the gel was scanned using a Typhoon image scanner. ImageQuant software was used to generate the single and overlay images. Comparative analysis of all protein spots was performed using DeCyder “in-gel” or “cross-gel” analysis software to generate protein expression ratios between the samples. Protein spots of interest were automatically selected from the 2D gels with the Ettan Spot Picker. Samples were then dried to absorb the digestion buffer and rehydrated in digestion buffer containing sequencing grade modified trypsin. After digestion at 37 °C, peptides were extracted from the gel with TFA extraction buffer and shaking. Tryptic peptides were desalted using C-18 Zip-tips (Millipore-Sigma; St. Louis, MO), mixed with CHCA matrix (alpha-cyano-4-hydroxycinnamic acid), and spotted into the wells of a MALDI plate. Ten to twenty of the most abundant peptides in each sample were further subjected to fragmentation and tandem mass spectrometry (MS/MS) analysis. Protein identification was based on peptide fingerprint mass mapping and peptide fragmentation mapping, using MS/MS spectra. Combined MS and MS/MS spectra are submitted for database search using GPS Explorer software equipped with the MASCOT search engine to identify proteins from primary sequence databases. Gene ontology was performed using the Panther Gene Ontology database to help classify proteins based on their molecular function.
Results
Spermatozoa from Atp1a4 KO mice present a flagellar angulation
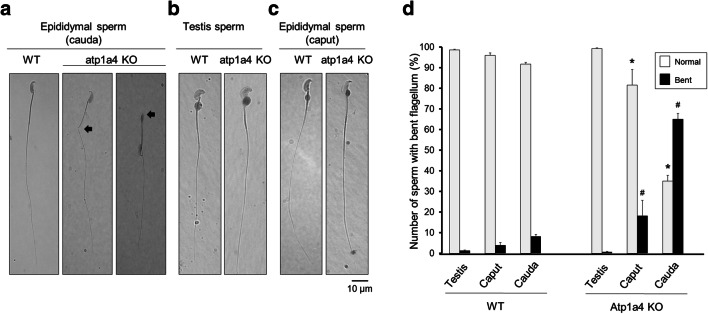
An unexpected finding of sperm obtained from the cauda epididymis of Atp1a4 knockout mice is that they exhibit a morphological phenotype consisting of a bend in the flagellum [15]. Analysis under light microscopy showed that, different from WT sperm which have a straight and smooth flagellum, sperm from the Atp1a4 KO mice exhibited an angulation between the mid- and principal piece of the flagellum (Fig. 1a, middle panel). This bend included different degrees of angularity, ranging from obtuse and acute angles to serious retroflexion into a complete 180° folding of the head and mid-piece over the principal piece and tail of the sperm flagellum (indicated by arrows in Fig. 1a). Interestingly, when sperm from Atp1a4 KO mice was obtained from the testis or the epididymal caput, the “bent” phenotype was not present and cells showed a normal smooth flagellum, similar in morphology to sperm from WT animals (Fig. 1b and c).
Fig. 1.
Sperm from Atp1a4 knockout mice exhibit a bent in the flagellum. Bright field microscopy of sperm from wild type (WT) and Atp1a4 knockout (Atp1a4 KO) mice, obtained from a the cauda epididymis, b the testis, and c the caput caput of the epididymis. Sperm was collected in modified Whitten’s medium, placed on coverslips and images were obtained under a regular light microscope. The arrows show the different degree of angulation of the flagellum in sperm taken from the cauda epididymis. d Quantification of sperm flagellar bending. Sperm from the cauda epididymis of WT and Atp1a4 KO mice was collected in modified Whitten’s medium, placed on coverslips and sperm with angulated flagella were counted. All type of angulation, including retroflexed flagella, were considered as bent flagella. Results are expressed as a percent of the total number of cells. Values are the mean + SEM of 3 different experiments. Asterisks indicate statistical differences with respect WT controls and # shows differences with normal straight sperm flagella, with P < 0.05
Quantification of the defective flagellar morphology was performed under light microscopy by counting the number of cells that presented a flagellar bend, expressed as a fraction of all the cells present in the sample. Normal sperm was considered as that presenting a smooth flagellum; sperm that presented any type of angulation, including retroflexion, was considered “bent” sperm. This showed that sperm from WT mice, independent from its origin (testis, or epididymal caput and cauda), always had < 10% of sperm with a bent flagellum (Fig. 1d, WT). This agrees with the observed presence of a small number of sperm with abnormal morphology in WT mice [32]. For sperm from Atp1a4 KO mice, no flagellar bending was observed for cells from the testis; however, in the epididymis, the number of cells with altered sperm flagellar morphology was significantly higher than in WT. While the amount of cells with the bent flagellum was small in sperm from the epididymal caput (~ 20% of the total cell population), it drastically increased in samples from the epididymal cauda, in which almost 70% of the sperm exhibited the flagellar defect (Fig. 1d, Atp1a4 KO). Therefore, deletion of Atp1a4 is accompanied by a bend in the sperm flagellum, which is not present in all the cells and becomes more apparent in sperm that already left the testis and has reached the cauda epididymis.
Sperm from Atp1a4 KO mice exhibits abnormal ultrastructural characteristics
To more closely look at the morphological alterations of sperm from Atp1a4 KO mice, we analyzed sperm from WT and Atp1a4 KO mice obtained from the cauda epididymis under scanning electron microscopy. Sperm from WT mice showed the normal straight flagellum (Fig. 2a). In contrast, sperm from Atp1a4 KO mice presented at the site of angulation, a dilation of the cytoplasm with the plasma membrane bulging at the site of the bend (Fig. 2b, white arrows). In some cases, the angulation did not show the dilation, but rather the disorganization of the axonemal microtubules and their exposure out of the cell. This is probably due to the rupture of the plasma membrane secondary to uncontrolled cell swelling (indicated by white arrows in Fig. 2c). In the extreme cases in which the flagellum showed a complete retroflexion, the plasma membrane was ruptured at the site of the bend and there was swelling of the sperm flagellum (white arrows in Fig. 2d). This dilation of the cytoplasm and detachment of the plasma membrane could be also observed in the sperm head (yellow arrow in Fig. 2d).
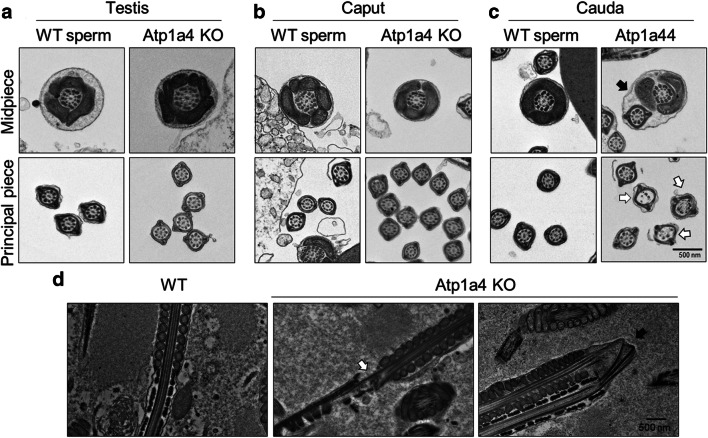
Fig. 2.
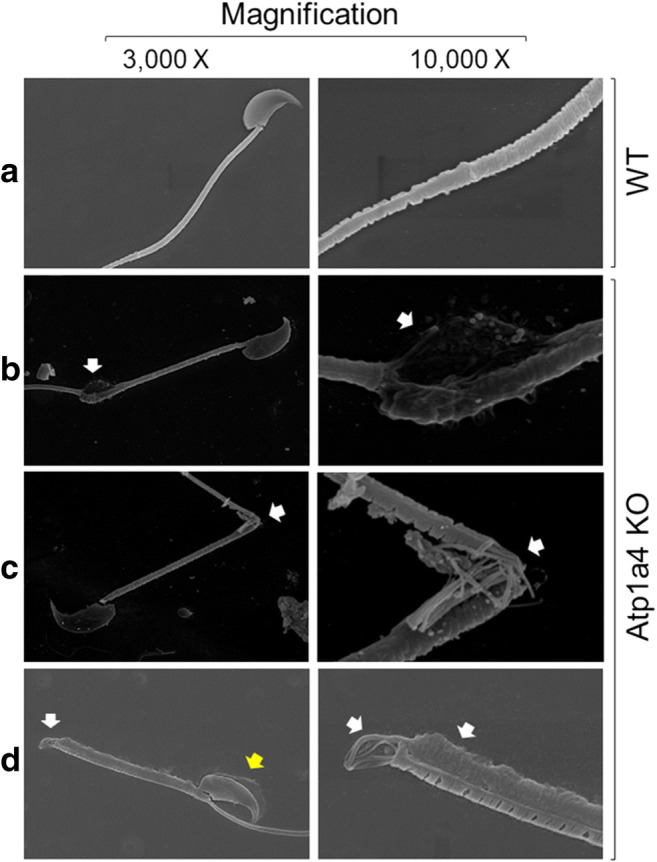
Sperm null in Atp1a4 show a series of ultrastructural defects under SEM. a Micrographs of sperm from WT mice. b–d Images of sperm from Atp1a4 KO mice obtained from the cauda epididymis showing different degree of angulation, dilation of the cell flagellum (white arrows) and detachment of the cell plasma membrane at the sperm flagellum and head (yellow arrow)
When analyzed under transmission electron microscopy (TEM), sperm from Atp1a4 KO mice, collected from either the testis or the epididymal caput, showed a flagellar cross-section morphology at the mid-piece and principal piece that was similar to that of WT sperm (Fig. 3a and b). In contrast, sperm from Atp1a4 KO mice taken from the cauda epididymis presented ultrastructural abnormalities in the axoneme at the place of the flagellar bend. In the most prominent cases, when the sperm presented a complete retroflexion of the flagellum, a fusion of the plasma membrane between the mid- and principal piece was observed (indicated by a black arrow in the upper right panel of Fig. 3c). In addition, absence of axonemal microtubular doublets and a disorganization of the outer dense fibers were found at different points of the flagellum (indicated by white arrows in Fig. 3c, lower right panel).
Fig. 3.
Sperm lacking Atp1a4 present flagellar ultrastructural abnormalities under TEM. Electron micrographs of WT and Atp1a4 KO sperm, obtained from a testis, b epididymal caput, and c epididymal cauda. Cell morphology was analyzed after collection in modified Whitten’s medium. The fusion of the principal piece with the midpiece is shown with a black arrow. The white arrow shows absence of microtubular doublets. d Electron microscopy analysis of WT and Atp1a4 KO sperm directly collected from the caput and cauda of the epididymis
To examine sperm morphology without the potential effects of exposing the sample to the collection media, samples were directly taken from the cauda epididymis, smeared on a charged coverslip, and fixed instantly to preserve their native morphology. TEM showed that epididymal caudal sperm from Atp1a4 KO mice showed the flagellar bend at the midpiece-tail junction with various degrees of angulation, discontinuity of the cell plasma membrane, and microtubular and outer dense fiber defects (Fig. 3d, white arrow in middle panel). In the cases of extreme bending, there was a complete fracturing of the axonemal structures (Fig. 3d, black arrow in the right-side panel). As mentioned before, WT and null sperm from the caput and corpus of the epididymis appeared normal (data not shown). These experiments provided a better understanding of the ultrastructural changes that sperm lacking Atp1a4 exhibit at the site of the flagellar bend and show that the defect is already apparent in sperm directly collected from the caudal portion of the epididymis.
Sperm null in Atp1a4 do not properly respond to hypoosmotic shock
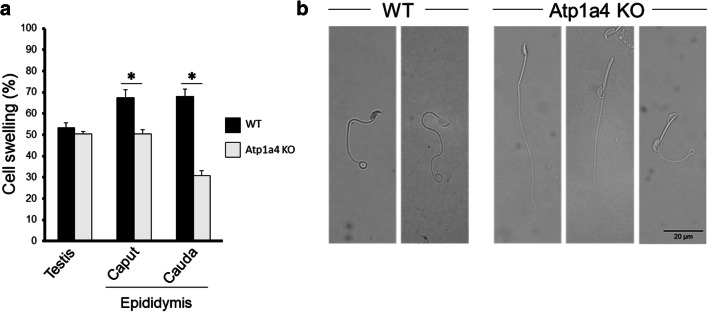
The abnormality in cell shape that we observe in α4 null sperm could be a consequence of deficiencies in osmoregulation and volume control by spermatozoa, which could result in disruption of the integrity of the sperm plasma membrane [19]. To explore this possibility, we subjected sperm from the testis and epididymal caput and cauda to the hypoosmotic swelling (HOS) test, by exposing the cells to a solution with low osmolarity [29]. Normal spermatozoa respond to this challenge by expanding their volume and coiling their tail, due to the influx of fluid into the cell cytoplasm. This will not happen if sperm are unable to regulate fluid movement across the cell plasma membrane or if the cell plasma membrane is disrupted by excessive swelling [29]. As shown in Fig. 4, sperm showed cell swelling in response to the hypotonic challenge that was similar for WT and Atp1a4 KO mice when the sperm was collected from the testis. In contrast, when epididymal sperm was tested, there was a significant decrease in swelling in sperm from Atp1a4 than WT mice. This difference was more pronounced for sperm taken from the cauda than from the caput epididymis, with a reduction in swelling of approximately 28% and 57% respectively (Fig. 4a). Figure 4b shows representative pictures of sperm from the WT and Atp1a4 KO mice subjected to hypoosmotic shock. As shown, WT sperm showed the typical swelling of the flagellum (notice the swelling at the tip of the sperm tail). In contrast, sperm from Atp1a4 KO mice lack the expected swollen response (Fig. 4b first two cells in the panel labeled Atp1a4 KO). Figure 4b, third panel, also shows that some sperm from Atp1a4 KO did undergo hypoosmotic shock swelling (third panel labeled Atp1a4 KO). Therefore, the lower swelling of sperm from Atp1a4 KO mice compared to WT sperm indicates that sperm devoid of Atp1a4 are less capable of handling hypoosmotic conditions and present impaired plasma membrane integrity. In addition, this reduced capacity to swell is more pronounced in sperm taken from the epididymal cauda.
Fig. 4.
Sperm lacking Atp1a4 does not properly respond to hypoosmotic shock. a Sperm from WT and Atp1a4 KO mice was collected from the indicated tissue sources and subjected to a medium with an osmolarity of 53 mOsmol/kg for 60 min. Sperm swelling was analyzed morphologically under phase contrast microscopy. The number of cells presenting signs of swelling were quantified and expressed as percent of the total cells in the sample. Values represent the mean + SEM of 3 different experiments. Asterisks indicate statistical differences, with P < 0.05. b Representative pictures of caudal epididymal sperm from WT and Atp1a4 KO mice subjected to the hypoosmotic test
Sperm lacking Atp1a4 are less susceptible to high than low osmotic conditions
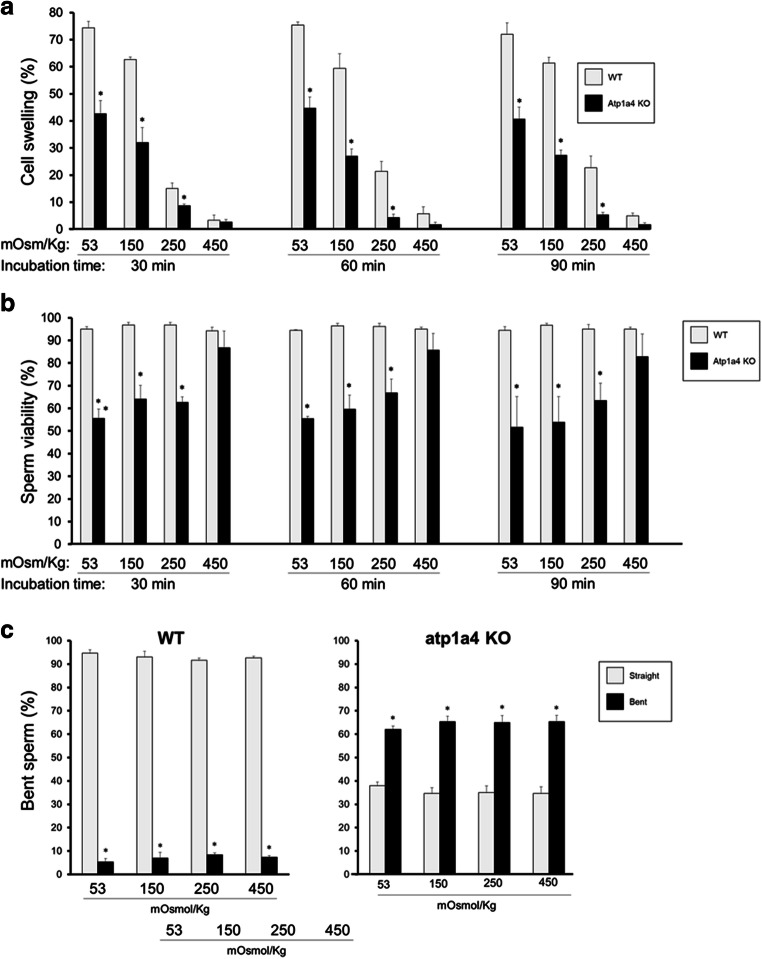
To more completely assess the capacity of Atp1a4 KO sperm to regulate osmotic balance, we subjected the cells not only to hypotonic (53 and 150 Osm) but also isotonic (250 Osm) and hypertonic conditions (450 Osm). Concomitantly, we examined the viability and morphology of the cells. As expected, sperm from WT mice showed a decrease in swelling as the cells were exposed from hypoosmotic to hyperosmotic solutions (Fig. 5a). Similarly, sperm from Atp1a4 KO mice showed a decrease in swelling as the osmolality in the medium increased; however, cell swelling was always significantly lower than in WT sperm, until an osmolarity of 450 mOsm/kg was reached (Fig. 5a). To ensure that water and salt equilibrium across the cell plasma membrane was reached, we repeated the experiments at different time points (30, 60, and 90 min). This showed that increasing the time of exposure to the media did not significantly affect the results, indicating that 30 min was enough for sperm to reach steady state conditions for swelling (Fig. 5a).
Fig. 5.
a Sperm from Atp1a4 KO mice are unable to properly handle osmotic challenges. Caudal epididymal sperm from WT and Atp1a4 KO mice was collected in medium with the indicated osmolarity (53, 150, 250, and 450 mOsmol/kg). After incubation of the samples for various times (30, 60, and 90 min), the number of sperm showing swelling was quantified under phase contrast microscopy. Swollen sperm counts were expressed as percent of the total cells in the sample. b Osmotic challenge affects the viability of Atp1a4 KO sperm. Sperm was collected as in (a) and after incubation for the indicated times, the number of viable (live) sperm was quantified using the LIVE/DEAD viability kit and expressed as percent of the total cells in the sample. c Sperm from Atp1a4 KO mice show higher bending of the sperm flagellum than wild type despite the osmolarity of the medium. After collection of the cells as above and incubation for 60 min, the number of sperm with a straight or different degree of angulation of the flagellum was separately scored and expressed as percent of the total cells in the sample. In (a) and (b), values represent the mean + SEM of 3 different experiments. Asterisks indicate statistical differences compared to the WT controls, with P < 0.05
When cell viability was determined, sperm from WT mice maintained over 95% viability at all osmotic conditions and for all time points tested (Fig. 5b). In contrast, the viability of sperm from Atp1a4 KO mice was improved as the osmolarity of the solution was raised and in a similar manner for all the incubation times tested. This suggests that Atp1a4 sperm can better accommodate to hyperosmotic than hypoosmotic media (Fig. 5b).
To determine the effect of varying osmolarity on sperm morphology, we analyzed flagellar bending in caudal epididymal sperm from WT and Atp1a4 KO mice after subjecting the cells to the different hypo- and hyperosmotic conditions. Flagellar bending was quantified as the percent of the total cells in the sample showing any degree of angularity. At all osmolarities tested, sperm from WT mice always showed a minimal number of cells with a bent flagellum. In contrast, sperm from Atp1a4 KO mice presented a significantly higher (with more than half of the cells) showing flagellar angulation (Fig. 5c). Therefore, despite changes in osmolarity of the medium, the flagellar bent pattern of Atp1a4 KO sperm was present and while an improvement in cell viability was observed with increasing osmolarity of the medium, the abnormal flagellar bend could not be reverted. This suggests the possibility that basal structural defects, which are independent from the deficiencies in cell osmotic regulation, are involved in impeding the complete recovery of the sperm back to its normal cell shape.
Treatment with detergent does not fully rescue the morphology of Atp1a4 KO sperm
As shown above, sperm from Atp1a4 KO mice have a diminished capacity to respond to osmolarity changes in the medium. This leads to alterations in sperm volume, which causes deformation of the plasma membrane and sperm shape. When the main defect is due to osmotic disbalance, the addition of detergents to sperm to dissolve the plasma membrane can restore the sperm morphology, with the flagellum straightening and recovering its shape after the cells are demembranated [33]. To determine whether we could relieve the tension created by the expanded plasma membrane and rescue the “bent” phenotype of sperm from the Atp1a4 KO mice, we treated caudal epididymal sperm from Atp1a4 KO mice in the absence and presence of 0.1% of Triton-X100 for 10 min. Then, we quantified the number of sperm with a straight or bent flagellum, separating flagellar angulation from total retroflexion. This approach showed that in the absence of detergent, the number of cells with straight, bent, or retroflexed flagellum was similar, corresponding to approximately one-third of the total cell population in the sample (white bars in Fig. 6). In the presence of detergent, the number of sperm with a straight flagellum remained constant, those with a bent flagellum increased, and the ones with complete flagellar retroflexion significantly decreased (Fig. 6, dark bars). This shift from the most severe retroflexed shape into the milder bent profile suggests that treatment with Triton-X100 only partially recovered the shape of Atp1a4 KO sperm, not being able to fully restore flagellar straightness. Therefore, these results suggest that defects in osmotic regulation only partially account for the morphological defect of sperm from Atp1a4 knockout mice and that additional underlying structural factors are responsible for the defect.
Fig. 6.
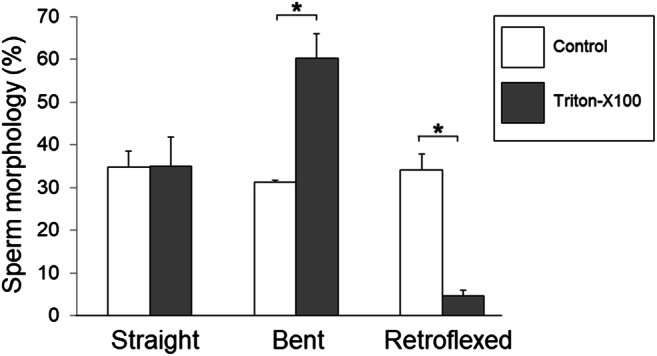
Detergent treatment partially recovers flagellar angulation of sperm from Atp1a4 KO mice. Caudal epididymal sperm from Atp1a4 KO mice was collected in medium with an osmolarity of 250 mOsmol/kg and were incubated in the absence and presence of 0.1% of Triton-X100 for 10 min. Then, the number of sperm with a straight or bent flagellum was quantified, identifying angulation from total retroflexion. Cells were scored and expressed as percent of the total cells in the sample. Values represent the mean + SEM of 3 different experiments. Asterisks show statistically significant differences, with P < 0.05
Several structural proteins show altered expression in Atp1a4 KO sperm
To investigate potential changes in structural proteins that could account for the morphological defects in sperm from the Atp1a4 KO mice, we performed 2D-DIGE and MALDI TOF/TOF mass spectrometry and compared the proteome profile of sperm from WT and Atp1a4 KO mice. This was aided by gene ontology analysis, using the Panther Gene Ontology database, which identified that within the category of structural molecular proteins, there were several candidates with abnormal expression levels. Seven proteins were shown to be downregulated, while two were upregulated (Fig. 7a). To validate these results, we performed immunoblots for some of those proteins and estimated their amounts by densitometry of the specific protein bands. As anticipated by the proteomic analysis, we found a significant decrease in expression for glutathione peroxidase 4 (GPx4), outer dense fiber protein 2 (ODF2), and dynein intermediate chain 1 (DNAI1) in sperm from Atp1a4 KO compared to WT mice (Fig. 7b and c).
Fig. 7.
Proteomic analysis of structural proteins in sperm from WT and Atp1a4 KO mice. Caudal epididymal sperm from Atp1a4 KO mice was collected using Percoll separation. Samples were subjected to 2D-gel electrophoresis for quantification and Maldi-TOF/TOF for identification. a Gene classification obtained from proteome analysis with cutoff values of 1.4-fold and P < 0.1. Proteins were functionally categorized based on the PANTHER GO Molecular Function. Listed proteins correspond to the “structural molecular activity” category. b Immunoblot analysis of selected structural proteins (ODF2, DNAI, and GPx4). c Relative protein levels as determined by densitometry from the immunoblot analysis. Protein levels were normalized using tubulin as a control and expressed relative to the level of the corresponding protein from WT sperm. Values represent the mean + SEM of 3 different experiments. Asterisks show statistically significant differences, with P < 0.001
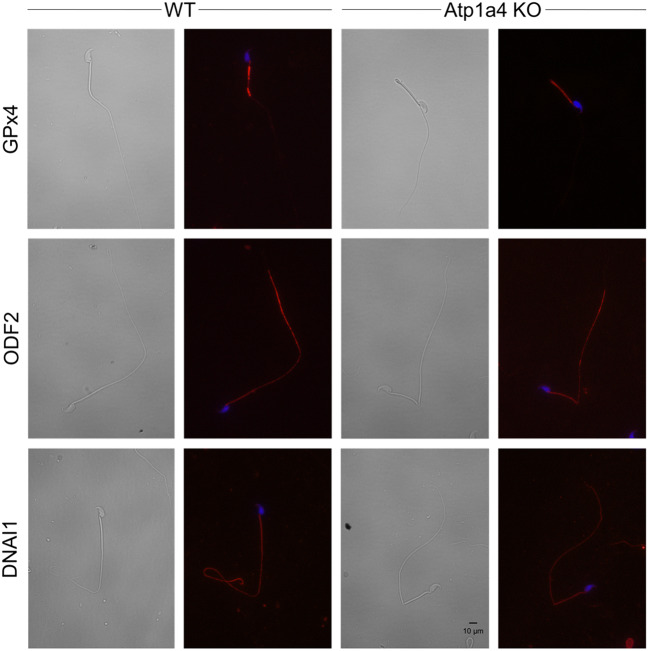
To determine if the changes in expression of the structural proteins found to be associated with Atp1a4 deletion affected their cell distribution, we determined their localization by immunocytochemistry. Figure 8 presents representative images of cells, captured in brightfield or under UV light. Samples not treated with the primary antibody was used as a control for the background, showing no significant signal (data not shown). We did not detect differences in the localization pattern for GPx4, ODF2, and DNAI1 in sperm from Atp1a4 compared to WT mice. Therefore, absence of Atp1a4 is accompanied by downregulation of the expression, but no differences in the distribution of several proteins that are key for maintenance of the structure and shape of sperm.
Fig. 8.
Immunochemical analysis of selected structural proteins in sperm from WT and Atp1a4 KO mice. Caudal epididymal sperm were fixed and labelled with antibodies against GPx4, ODF2, and DNAI1. Alexa Fluor 594 was used as the secondary antibody
Discussion
In this work, we have studied the flagellar morphological alterations that forms part of the phenotype of sperm null in Atp1a4, with the idea of better understanding the role of this ion transporter in the biology of the male gamete and gain insight into general mechanisms of sperm function. The main changes in shape that we find in sperm lacking Atp1a4 includes an angulation of the sperm flagellum between the mid- and principal piece, bulging of the cells, fusion of the plasma membrane at areas in which the plasma membrane becomes juxtaposed, and defects in the axonemal microtubular pairs and peri-axonemal structures, such as deformations in the outer dense fibers. The flagellar angulation is reminiscent of sperm that contain deficiencies in osmoregulation and cell volume control, such as those occurring after treatment of WT sperm with ion channel blockers [19], or in sperm from genetically modified mice in which the SLO3 potassium channel was knocked out [34], the c-ros tyrosine kinase receptor was deleted [35], or the SV-40 T-antigen was over-expressed in the epididymis under the glutathione peroxidase promoter [36]. In line with this, sperm from Atp1a4 KO mice have an abnormal response to osmotic challenge and they do not undergo the normal swelling exhibited by sperm from WT mice when subjected to a hypoosmotic shock.
Supporting the idea that sperm from Atp1a4 KO mice suffer from an osmotic disbalance is the fact that the defect does not occur in sperm isolated from the testes and is minimal in the caput of the epididymis, but it is apparent in sperm isolated from the epididymal cauda. In this last region of the male genital tract, sperm are exposed to an environment with high osmolarity [20]. Thus, when placed in the isosmotic solutions of the collection medium, sperm face an important osmotic challenge that makes cells swell and if this change in volume remains uncontrolled, as occurs in sperm from Atp1a4 KO mice, it will affect cell shape and lead to the eventual disruption of the sperm plasma membrane. It is possible that lack of Atp1a4 results in reduced movement of Na+ out of the cell and the resulting increase of this cation in the cytoplasm leads to water retention and failure of the cells to properly adjust volume. In favor of this idea is our previous finding showing that intracellular Na+ level in sperm from Atp1a4 KO mice is increased [17]. This emphasizes the unique role that Atp1a4 has in sperm, which cannot be compensated by the somatic Na,K-ATPase isoform Atp1a1, also expressed in these cells [11]. The Atp1a4 isoform is the main contributor to maintenance of intracellular Na+ levels in sperm. This is possible due to the higher affinity that Atp1a4 has for Na+ binding and transport compared to Atp1a1 [11]. In addition to Na+, which importantly contributes to the osmotic pressure of biological fluids, previous work has shown that K+ plays an important role in sperm volume regulation control. Movement of K+ out of the cell, via K channels, represents a mechanism for RVD that prevents cell swelling [24]. The transport of K+ out of the cell is a passive mechanism driven by the transmembrane gradient of K+ created by Na,K-ATPase. The lack of Atp1a4 in the null mice is expected to reduce the driving force for the efficient movement of K+ outside of the cell. In this manner, not only the increase in intracellular Na+ level but also the dissipation of the transmembrane K+ gradient can lead to the impaired capacity of sperm from Atp1a4 KO mice to regulate cell volume and shape. Altogether, our results place Atp1a4 as part of the machinery involved in regulation of cell volume and osmolarity in sperm. Interestingly, the midpiece of the flagellum and the cytoplasmic droplet has been found to be the region of the sperm where the mechanisms involved in volume control are operating [37]. The Atp1a4 is mainly localized to those flagellar regions, which is ideal for its participation in sperm volume control along with other cell plasma membrane transport mechanisms.
While lack of Atp1a4 has important consequences for sperm exposure to hypoosmotic conditions and swelling, its deletion appears to be less detrimental when cells are subjected to hyperosmotic conditions and for cell shrinking. This is supported by the lack of difference in cell swelling or cell viability between sperm from WT and Atp1a4 KO mice when the cells are incubated in high osmotic medium (450 mOsm/kg). This shows that absence of Atp1a4 makes the cells more vulnerable to hypoosmotic than hyperosmotic shock, suggesting that Atp1a4 plays a more significant role in the mechanisms involved in RVD than those implicated in RVI.
Whereas cell viability was improved by placing the cells in medium with increased osmolarity, bending of the sperm flagellum could not be reverted and the number of cells with this defect remained relatively constant at the various osmolarity conditions tested. In addition, we observed that even when taken directly from the cauda epididymis and not exposed to isosmotic medium, sperm null in Atp1a4 exhibited the flagellar bend. Moreover, treatment of the cells with detergent was unable to fully recover cell straightness. Altogether, this suggests that additional factors, besides the osmotic changes, are involved in the morphological phenotype of Atp1a4 sperm. Through our proteomic analysis, we unexpectedly found that ablation of Atp1a4 is accompanied by differential expression of a series of structural proteins that are important for maintaining sperm shape. Among these proteins, we confirmed the downregulation of several of these proteins, including the following: (1) GPx4, an anti-oxidant selenoenzyme that has been shown to prevent flagellar angulation by protecting against the oxidation of protein sulfhydryl groups and disulfide bond formation. Disruption of GPx4 in mice results in severe structural abnormalities of the sperm midpiece, including a flagellar bend at the midpiece that is similar to the one we observe in the Atp1a4 KO mice [38, 39]. (2) ODF 2, which belongs to a family of proteins that participate in stabilizing the sperm tail and helps with its elastic recoil to support flagellar beat. Targeted deletion of this protein in mice causes male infertility due to abnormalities in the head to tail coupling and first portion of the flagellum, where we locate the morphological defects of sperm devoid in Atp1a4 [40]. (3) The axonemal protein DNAI1, which forms part of the outer dynein arms. Mutations of this protein result in impairment of primary cilium function and abnormal sperm flagellar axoneme morphology and kinematics [41, 42]. Supporting the relevance of these changes in proteomics are our electron micrographs, which show axonemal defects, including the absence of the microtubular doublets and alterations in the shape of the outer dense fibers in sperm of Atp1a4 KO mice. At the present time, it is unclear how abnormal expression of these critical structural proteins is associated with the Atp1a4 gene. In addition, it is unclear if the reduced protein levels correspond to a decrease in their expression, or an increase in their degradation. Our results also show that ablation of Atp1a4 does not influence the cell distribution of GPx4, ODF2, and DNAI1. Similar studies have shown that knocking out these individual genes is detrimental to the integrity and stability of the flagellum when compared with WT mouse sperm [38–42]. While future studies will be needed to assess the correlation between Atp1a4 and sperm structural proteins, it is clear that an important component of the morphological alterations of sperm devoid of Atp1a4 can be attributed to the changes in expression of sperm flagellar proteins.
In this manner, it appears that both structural defects, as well as osmotic imbalance account for the morphological phenotype of Atp1a4 KO sperm. It is possible that the primary morphological deficiency due to abnormal expression of axonemal and periaxonemal proteins makes the cells structurally unstable and that, as soon as sperm are exposed to osmotically challenging situations, the flagellar malformation becomes apparent and permanent. The relative contribution of both of these factors may also explain the heterogeneity in the morphological phenotype of sperm lacking Atp1a4, with approximately a third of the cells showing the complete retroflexion of the flagellum; another third showing variable bending; and the remaining sperm exhibiting what appears to be a relatively straight flagellum. The defects in sperm structure and volume control that we find have been shown to have important consequences for the physiology of sperm, affecting multiple parameters of sperm function [43]. The combination of these defects contribute specifically to the male infertility phenotype of our Atp1a4 KO mouse model.
In conclusion, the results from this work indicate that Atp1a4 plays a key role in sperm structural stability. On the one hand, Atp1a4 is important to maintain osmotic balance of the cells by representing one of the mechanisms that operate in sperm RVD. On the other hand, Atp1a4 expression is linked to the expression of several proteins involved in the formation of axonemal and peri-axonemal sperm structures. These actions on cell morphology, combined with the functional defects that we have previously described for sperm null in Atp1a4 [15], highlights the complex nature of the sperm phenotype that results from Atp1a4 ablation.
Acknowledgments
We would like to thank the University of Kansas Medical Center Electron Microscopy Research lab and especially Barbara Fegley for her assistance and expertise with the ultrastructural assessment of spermatozoa. Also, we appreciate the generosity of Drs. A. Kierszenbaum (City University of New York) and L. Ostrowski (University of North Carolina) in providing us with antibodies used in this study. Finally, we thank the National Institutes of Health grant HD102623 for supporting this work.
Funding
This work was supported by the National Institutes of Health grant HD102623.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 2.Feraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev. 2001;81(1):345–418. doi: 10.1152/physrev.2001.81.1.345. [DOI] [PubMed] [Google Scholar]
- 3.Gloor SM. Relevance of Na,K-ATPase to local extracellular potassium homeostasis and modulation of synaptic transmission. FEBS Lett. 1997;412(1):1–4. doi: 10.1016/s0014-5793(97)00774-6. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann EK, Simonsen LO. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- 5.Nyblom M, Poulsen H, Gourdon P, Reinhard L, Andersson M, Lindahl E, Fedosova N, Nissen P. Crystal structure of Na+, K(+)-ATPase in the Na(+)-bound state. Science. 2013;342(6154):123–127. doi: 10.1126/science.1243352. [DOI] [PubMed] [Google Scholar]
- 6.Apell HJ, Schneeberger A, Sokolov VS. Partial reactions of the Na,K-ATPase: kinetic analysis and transport properties. Acta Physiol Scand Suppl. 1998;643:235–245. [PubMed] [Google Scholar]
- 7.Vagin O, Sachs G, Tokhtaeva E. The roles of the Na,K-ATPase beta 1 subunit in pump sorting and epithelial integrity. J Bioenerg Biomembr. 2007;39(5-6):367–372. doi: 10.1007/s10863-007-9103-0. [DOI] [PubMed] [Google Scholar]
- 8.Ueno S, Takeda K, Noguchi S, Kawamura M. Significance of the beta-subunit in the biogenesis of Na+/K(+)-ATPase. Biosci Rep. 1997;17(2):173–188. doi: 10.1023/a:1027333529412. [DOI] [PubMed] [Google Scholar]
- 9.Geering K. Functional roles of Na,K-ATPase subunits. Curr Opin Nephrol Hypertens. 2008;17(5):526–532. doi: 10.1097/MNH.0b013e3283036cbf. [DOI] [PubMed] [Google Scholar]
- 10.Mobasheri A, Avila J, Cozar-Castellano I, Brownleader MD, Trevan M, Francis MJ, et al. Na+, K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci Rep. 2000;20(2):51–91. doi: 10.1023/a:1005580332144. [DOI] [PubMed] [Google Scholar]
- 11.Blanco G. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin Nephrol. 2005;25(5):292–303. doi: 10.1016/j.semnephrol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Syeda SS, Sanchez G, McDermott JP, Hong KH, Blanco G, Georg GI. The Na+ and K+ transport system of sperm (ATP1A4) is essential for male fertility and an attractive target for male contraceptiondagger. Biol Reprod. 2020;103:343–356. doi: 10.1093/biolre/ioaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagoner K, Sanchez G, Nguyen AN, Enders GC, Blanco G. Different expression and activity of the alpha1 and alpha4 isoforms of the Na,K-ATPase during rat male germ cell ontogeny. Reproduction. 2005;130(5):627–641. doi: 10.1530/rep.1.00806. [DOI] [PubMed] [Google Scholar]
- 14.McDermott JP, Sanchez G, Chennathukuzhi V, Blanco G. Green fluorescence protein driven by the Na,K-ATPase alpha4 isoform promoter is expressed only in male germ cells of mouse testis. J Assist Reprod Genet. 2012;29(12):1313–1325. doi: 10.1007/s10815-012-9876-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jimenez T, McDermott JP, Sanchez G, Blanco G. Na,K-ATPase alpha4 isoform is essential for sperm fertility. Proc Natl Acad Sci U S A. 2011;108(2):644–649. doi: 10.1073/pnas.1016902108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stival C, del C Puga Molina L, Paudel B, Buffone MG, Visconti PE, Krapf D. Sperm capacitation and acrosome reaction in mammalian sperm. Adv Anat Embryol Cell Biol. 2016;220:93–106. doi: 10.1007/978-3-319-30567-7_5. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez T, Sanchez G, Wertheimer E, Blanco G. Activity of the Na,K-ATPase alpha4 isoform is important for membrane potential, intracellular Ca2+, and pH to maintain motility in rat spermatozoa. Reproduction. 2010;139(5):835–845. doi: 10.1530/REP-09-0495. [DOI] [PubMed] [Google Scholar]
- 18.Escalier D. Knockout mouse models of sperm flagellum anomalies. Hum Reprod Update. 2006;12(4):449–461. doi: 10.1093/humupd/dml013. [DOI] [PubMed] [Google Scholar]
- 19.Cooper TG, Yeung CH, Wagenfeld A, Nieschlag E, Poutanen M, Huhtaniemi I, Sipilä P. Mouse models of infertility due to swollen spermatozoa. Mol Cell Endocrinol. 2004;216(1-2):55–63. doi: 10.1016/j.mce.2003.10.076. [DOI] [PubMed] [Google Scholar]
- 20.Yeung CH, Barfield JP, Cooper TG. Physiological volume regulation by spermatozoa. Mol Cell Endocrinol. 2006;250(1-2):98–105. doi: 10.1016/j.mce.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 21.Yeung CH, Anapolski M, Depenbusch M, Zitzmann M, Cooper TG. Human sperm volume regulation. Response to physiological changes in osmolality, channel blockers and potential sperm osmolytes. Hum Reprod. 2003;18(5):1029–1036. doi: 10.1093/humrep/deg204. [DOI] [PubMed] [Google Scholar]
- 22.Lang F. Mechanisms and significance of cell volume regulation. J Am Coll Nutr. 2007;26(5 Suppl):613S–623S. doi: 10.1080/07315724.2007.10719667. [DOI] [PubMed] [Google Scholar]
- 23.Lang F, Busch GL, Volkl H. The diversity of volume regulatory mechanisms. Cell Physiol Biochem. 1998;8(1-2):1–45. doi: 10.1159/000016269. [DOI] [PubMed] [Google Scholar]
- 24.Yeung CH, Cooper TG. Potassium channels involved in human sperm volume regulation--quantitative studies at the protein and mRNA levels. Mol Reprod Dev. 2008;75(4):659–668. doi: 10.1002/mrd.20812. [DOI] [PubMed] [Google Scholar]
- 25.Cooper TG, Yeung CH. Involvement of potassium and chloride channels and other transporters in volume regulation by spermatozoa. Curr Pharm Des. 2007;13(31):3222–3230. doi: 10.2174/138161207782341240. [DOI] [PubMed] [Google Scholar]
- 26.Chen Q, Peng H, Lei L, Zhang Y, Kuang H, Cao Y, Shi QX, Ma T, Duan E. Aquaporin3 is a sperm water channel essential for postcopulatory sperm osmoadaptation and migration. Cell Res. 2011;21(6):922–933. doi: 10.1038/cr.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mordel N, Dano I, Epstein-Eldan M, Shemesh A, Schenker JG, Laufer N. Novel parameters of human sperm hypoosmotic swelling test and their correlation to standard spermatogram, total motile sperm fraction, and sperm penetration assay. Fertil Steril. 1993;59(6):1276–1279. doi: 10.1016/s0015-0282(16)55989-5. [DOI] [PubMed] [Google Scholar]
- 28.Rossato M, Balercia G, Lucarelli G, Foresta C, Mantero F. Role of seminal osmolarity in the reduction of human sperm motility. Int J Androl. 2002;25(4):230–235. doi: 10.1046/j.1365-2605.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- 29.Jeyendran RS, Van der Ven HH, Zaneveld LJ. The hypoosmotic swelling test: an update. Arch Androl. 1992;29(2):105–116. doi: 10.3109/01485019208987714. [DOI] [PubMed] [Google Scholar]
- 30.Kasimanickam RK, Kasimanickam VR, Arangasamy A, Kastelic JP. Associations of hypoosmotic swelling test, relative sperm volume shift, aquaporin7 mRNA abundance and bull fertility estimates. Theriogenology. 2017;89:162–168. doi: 10.1016/j.theriogenology.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Furimsky A, Vuong N, Xu H, Kumarathasan P, Xu M, Weerachatyanukul W, Bou Khalil M, Kates M, Tanphaichitr N. Percoll gradient-centrifuged capacitated mouse sperm have increased fertilizing ability and higher contents of sulfogalactosylglycerolipid and docosahexaenoic acid-containing phosphatidylcholine compared to washed capacitated mouse sperm. Biol Reprod. 2005;72(3):574–583. doi: 10.1095/biolreprod.104.036095. [DOI] [PubMed] [Google Scholar]
- 32.Albert M, Roussel C. Strain differences in the concentration, motility and morphology of epididymal sperm in relation to puberty in mice. Int J Androl. 1984;7(4):334–347. doi: 10.1111/j.1365-2605.1984.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 33.Yeung CH, Sonnenberg-Riethmacher E, Cooper TG. Infertile spermatozoa of c-ros tyrosine kinase receptor knockout mice show flagellar angulation and maturational defects in cell volume regulatory mechanisms. Biol Reprod. 1999;61(4):1062–1069. doi: 10.1095/biolreprod61.4.1062. [DOI] [PubMed] [Google Scholar]
- 34.Santi CM, Martinez-Lopez P, de la Vega-Beltran JL, Butler A, Alisio A, Darszon A, et al. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010;584(5):1041–1046. doi: 10.1016/j.febslet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeung CH, Anapolski M, Cooper TG. Measurement of volume changes in mouse spermatozoa using an electronic sizing analyzer and a flow cytometer: validation and application to an infertile mouse model. J Androl. 2002;23(4):522–528. [PubMed] [Google Scholar]
- 36.Sipila P, Cooper TG, Yeung CH, Mustonen M, Penttinen J, Drevet J, et al. Epididymal dysfunction initiated by the expression of simian virus 40 T-antigen leads to angulated sperm flagella and infertility in transgenic mice. Mol Endocrinol. 2002;16(11):2603–2617. doi: 10.1210/me.2002-0100. [DOI] [PubMed] [Google Scholar]
- 37.Cooper TG, Yeung CH. Acquisition of volume regulatory response of sperm upon maturation in the epididymis and the role of the cytoplasmic droplet. Microsc Res Tech. 2003;61(1):28–38. doi: 10.1002/jemt.10314. [DOI] [PubMed] [Google Scholar]
- 38.Schneider M, Forster H, Boersma A, Seiler A, Wehnes H, Sinowatz F, Neumüller C, Deutsch MJ, Walch A, Angelis MH, Wurst W, Ursini F, Roveri A, Maleszewski M, Maiorino M, Conrad M. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 2009;23(9):3233–3242. doi: 10.1096/fj.09-132795. [DOI] [PubMed] [Google Scholar]
- 39.Imai H, Hakkaku N, Iwamoto R, Suzuki J, Suzuki T, Tajima Y, Konishi K, Minami S, Ichinose S, Ishizaka K, Shioda S, Arata S, Nishimura M, Naito S, Nakagawa Y. Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J Biol Chem. 2009;284(47):32522–32532. doi: 10.1074/jbc.M109.016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang K, Grzmil P, Meinhardt A, Hoyer-Fender S. Haplo-deficiency of ODF1/HSPB10 in mouse sperm causes relaxation of head-to-tail linkage. Reproduction. 2014;148(5):499–506. doi: 10.1530/REP-14-0370. [DOI] [PubMed] [Google Scholar]
- 41.Loges NT, Olbrich H, Fenske L, Mussaffi H, Horvath J, Fliegauf M, Kuhl H, Baktai G, Peterffy E, Chodhari R, Chung EMK, Rutman A, O'Callaghan C, Blau H, Tiszlavicz L, Voelkel K, Witt M, Ziętkiewicz E, Neesen J, Reinhardt R, Mitchison HM, Omran H. DNAI2 mutations cause primary ciliary dyskinesia with defects in the outer dynein arm. Am J Hum Genet. 2008;83(5):547–558. doi: 10.1016/j.ajhg.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guichard C, Harricane MC, Lafitte JJ, Godard P, Zaegel M, Tack V, Lalau G, Bouvagnet P. Axonemal dynein intermediate-chain gene (DNAI1) mutations result in situs inversus and primary ciliary dyskinesia (Kartagener syndrome) Am J Hum Genet. 2001;68(4):1030–1035. doi: 10.1086/319511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu NH, Zhao WL, Wang GS, Sun F. Comparative analysis of mammalian sperm ultrastructure reveals relationships between sperm morphology, mitochondrial functions and motility. Reprod Biol Endocrinol. 2019;17(1):66. doi: 10.1186/s12958-019-0510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]