Abstract
Oocyte in vitro maturation (IVM) is an assisted reproductive technology designed to obtain mature oocytes following culture of immature cumulus–oocyte complexes collected from antral follicles. Although IVM has been practiced for decades and is no longer considered experimental, the uptake of IVM in clinical practice is currently limited. The purpose of this review is to ensure reproductive medicine professionals understand the appropriate use of IVM drawn from the best available evidence supporting its clinical potential and safety in selected patient groups. This group of scientists and fertility specialists, with expertise in IVM in the ART laboratory and/or clinic, explore here the development of IVM towards acquisition of a non-experimental status and, in addition, critically appraise the current and future role of IVM in human ART.
Keywords: In vitro maturation (IVM), Oocyte maturation, Onco-fertility, Fertility preservation, Polycystic ovary syndrome (PCOS)
Introduction
Human ARTs (assisted reproductive technologies), while initiated to provide family building opportunities to subfertile or infertile patients, have now expanded into an important branch of contemporary medicine serving a far wider segment of the global population than anticipated following the birth of Louise Brown in 1978. From its humble beginnings, having access to human gametes and embryos has expanded the knowledge base upon which ARTs have flourished, providing for the first time the opportunity to do original research in human reproductive biology. Coming at a time of unprecedented technological breakthroughs, the ability to obtain and analyze the molecular and physiological basis of gametogenesis and embryogenesis in humans has both enriched the practice of ARTs for the benefit of patients and opened new opportunities for clinical and basic scientists alike. After many years of foundational study, in vitro maturation (IVM) has now reached a point in the history of human ARTs where its clinical utilization will both broaden and enrich the hopes of practitioners and patients in the decades ahead. It is the singular purpose of this review to provide a platform for understanding how, why, and when IVM has entered the spectrum of ARTs available to reproductive and regenerative medicine.
Historical landmarks in the development of human IVM
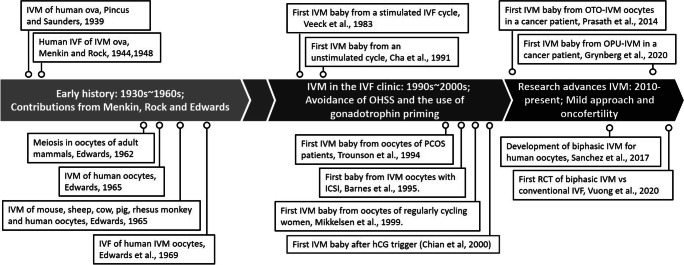
In an ironic twist of fate, the first reports of IVF in the human were the result of IVM using ovarian oocytes. Menkin and Rock harnessed the potential of maturing oocytes in vitro based on the long-standing collaboration between Rock and Pincus. The latter first reported IVM using rabbit ova [1] and then subsequently using human oocytes [2]. Buoyed by their collaboration, Menkin and Rock reported successful fertilization of in vitro matured human ova [3, 4] (Fig. 1). In hindsight, these first reports of human IVF using IVM oocytes were notable for two reasons: they established the concept of spontaneous oocyte maturation in mammals when cumulus–oocyte masses released from Graafian follicles displayed meiotic and developmental competence over a certain time interval and, importantly, made the earliest stages of human development experimentally tractable.
Fig. 1.
Landmarks in the development of human IVM. Major stages in the development of IVM beginning with studies using human oocytes and initial attempts at IVF and during 1990–2000 decades, first uses in the clinic, are shown. The last decade has witnessed modest expansion of clinical usage, especially in areas of onco-fertility and fertility preservation
Fast forward to the 1960s, Edwards made significant inroads into IVM in the human (Fig. 1) (reviewed in [5]). The kinetics and culture requirements for IVM were established for several different mammalian species [6], including for human oocytes [7]. His landmark work on human oocyte IVM established the kinetics of nuclear maturation showing metaphase 2 (MII) stage was reached at about 36 h [8]. And within a few short years, his work with Bavister led to the successful IVF of human IVM oocytes [9, 10]. The impetus to move in the direction of clinical IVF, while not to draw upon IVM as a step in the process, was realized from this pioneering work. Moreover, that mammalian oocytes from many species shared the properties implicit to spontaneous maturation provided a research platform for the next 40 years of progress in oocyte biology, maturation being the final act.
While the next 20 years (1970–1990; Fig. 1) would bring clinical IVM closer to translation, it was a pivotal period during which fundamental insights into oocyte physiology were made using animal models. The first IVM baby resulting from immature oocytes derived from oocyte donors was reported in 1991 by Cha and colleagues [11]. Since that report, IVM gained attention during the 1990s [12–14], primarily as a treatment option to reduce the risk of ovarian hyperstimulation syndrome (OHSS) associated with ovarian stimulation (OS), especially for polycystic ovary syndrome (PCOS) patients [15]. During this same time period, IVM became an experimental platform for the discovery of how the dialogue between cumulus cells and the oocyte regulate both nuclear and cytoplasmic maturation. At the heart of the debates bridging clinical and basic science for the next 20 years was the need to understand the relationship between hormonal control of oocyte maturation, its coupling to ovulation, and the molecular and cellular underpinnings of meiotic arrest and cell cycle progression to MII.
From the initial efforts of Chian, Tan, and colleagues at McGill University [16], various gonadotropin priming strategies coupled with differences in IVM protocols between clinics clouded the field [17], prompting calls for clarity regarding a simple and logical definition of oocyte IVM [18, 19]. Central to current and future efforts to bring IVM into the realm of everyday human ARTs are major research advances into the physiology of ovulation in mammals. From the earliest days implicating a single gonadotropin signal, capable of blocking the delivery of meiotic arresting factors like cGMP and cAMP, to the more recent elaboration of multiparametric signaling cascades downstream of LH reception during ovulation, the complexities and nuances emergent from 20 years of research have led to the development of a laboratory platform designed to mimic, to the best of our current capabilities, what happens naturally in vivo (reviewed in [20, 21]). Translation to the human has progressed [22, 23] due to three central physiological parameters we now have a deeper understanding of:
-
A.
Maintenance of the cumulus–oocyte dialogue is essential to assure bioenergetic and metabolic support required to achieve cytoplasmic maturation.
-
B.
Signaling reciprocity between oocyte-secreted factors and cumulus define temporal parameters for maturation that establish developmental competence.
-
C.
Coordination of ovulation with meiotic maturation involves a series of metabolic and gene expression changes in somatic granulosa cells and enclosed oocytes that can now be reproducibly manipulated under in vitro conditions.
Given these extraordinary advances in reproductive physiology (as summarized below), it is imperative that IVM assumes its role in human infertility treatment, prompting the stated objective of this paper to raise the awareness from clinicians, embryologists, and patients and recognize IVM as a patient-friendly and efficient ART technique.
The challenge: to mimic in vivo oocyte maturation in vitro
The central challenge facing the IVM field was the discovery that IVM oocytes displayed reduced developmental competence compared to their in vivo matured counterparts. This held true even for IVM in agricultural animals, where IVM is used routinely for in vitro embryo production and has been studied far more extensively than in humans [24]. In all mammals, the follicular origin of the oocyte has a major impact on its subsequent development potential [25, 26] (Fig. 2). It appears that once the oocyte is removed from its follicle, its developmental potential is curtailed [26], which was recognized early on as an obstacle that would have to be overcome for IVM to succeed. During follicular development, few antral follicles gain dominant follicle status, most being subordinate and likely in varying states of atresia; heterogeneity of oocytes retrieved for IVM is thus due to their derivation from follicles at varying stages of development. Despite this, most oocytes from most species resume meiosis spontaneously once freed from follicles; human oocytes do this too although at a notably lower frequency than most mammals [8]. Although IVM oocytes are able to complete nuclear maturation, their ability to be fertilized and support subsequent embryo development is reliant on the inherent developmental competence of the oocyte acquired in vivo (traditionally called cytoplasmic maturation). Mammalian oocytes are largely transcriptionally quiescent during meiotic maturation, depending on processing of stored transcripts for protein synthesis and post-translational mechanisms to complete maturation, and acquire the competence to support early embryo development before embryo zygote genome activation [27]. Other aspects of cytoplasmic maturation including organelle redistribution, epigenetic and membrane modifications [28], are essential for fertilization and embryo development to proceed. Loss of synchrony between nuclear and cytoplasmic maturation, a common occurrence under in vitro conditions, is attributable to precocious meiotic resumption in vitro of an oocyte which was still acquiring developmental competence in vivo. This factor constitutes a major obstacle to the goal of generating high quality blastocysts from IVM oocytes.
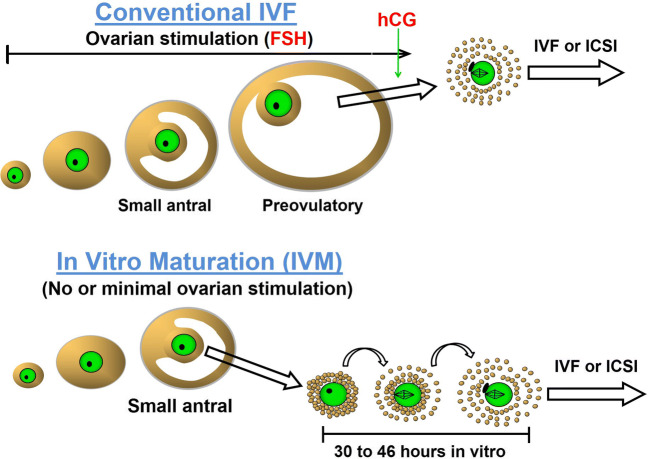
Fig. 2.
Differences between conventional IVF and IVM. The principal differences between conventional IVF and IVM are that in IVM cycles, patients receive minimal or no ovarian stimulation prior to OPU, oocytes are collected from small-medium sized antral follicles, and oocytes are meiotically matured in vitro from the germinal vesicle (GV) to metaphase II (MII) stage. Thereafter, mature IVM oocytes are treated exactly as per mature oocytes from conventional IVF. Adapted from [25]
Extraction from the follicular environment is inevitable for oocyte IVM and with it, the loss of all somatic cell and follicular fluid influences. However, several approaches can be taken to mitigate the impact of in vitro culture on the oocyte. The first is to try to mimic in vitro, as far as is possible, the in vivo follicular environment for the oocyte including retention of the architectural integrity of the COC and maintenance of meiotic arrest [29] (Fig. 3). Follicles prevent oocyte meiotic resumption by maintaining high levels of intra-oocyte cAMP through the infusion of natriuretic peptides and cGMP from the follicular somatic cells to the oocyte, as demonstrated in mouse and cow oocytes [30–33]. Hence, meiotic resumption can be readily prevented, and the COC structure retained, when oocytes from most mammalian species are cultured in the presence of natriuretic peptides (NPPC) or cAMP hydrolysis inhibitors (e.g., IBMX) [34]. The second approach is to include growth factors present in the follicular environment, including those like the EGF-like peptides, that are physiologically upregulated during oocyte maturation in vivo [21]. Notably, IVM media formulations have not changed substantially for decades. COCs, or naked oocytes, are cultured in various media supplemented with protein sources (serum or albumin) and gonadotropins. Most growth factors present in the follicular environment are not utilized in current standard IVM systems. As suggested by recent mouse and cow oocyte studies [35, 36], addition of growth factors into the IVM medium may yield oocytes with improved quality. These media modifications will continue to follow the findings from future research regarding the coordination of somatic cell–oocyte interactions.
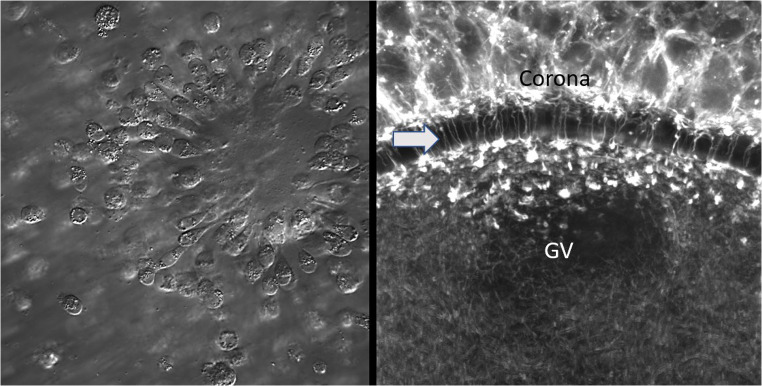
Fig. 3.
Oocyte–cumulus cell communication is fundamental to IVM success. Left, image of human MII oocyte collected after conventional ovarian stimulation and prior to removal of cumulus cells; note extensions from corona cells towards the oocyte surface. Confocal projection on the right is of an immature germinal vesicle (GV) stage bovine oocyte illustrating compact corona cells (top) sending numerous transzonal projections (arrow) that terminate on the oocyte cell surface. Alexa 555-phalloidin was used to label actin filaments in confocal image
Laboratory and clinical approaches to IVM
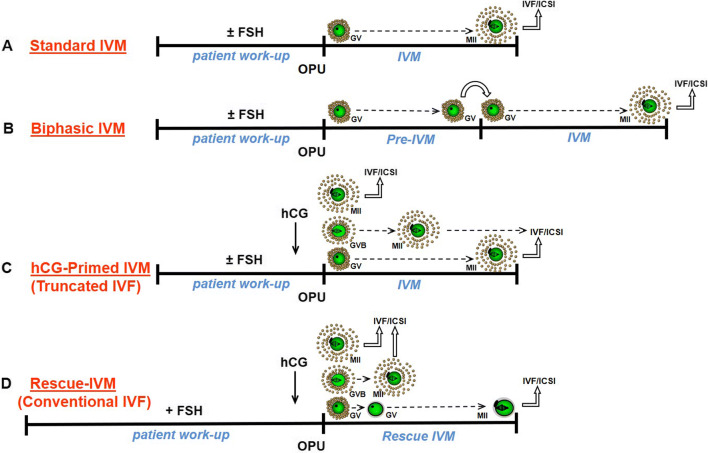
The introduction of a variety of clinical and laboratory approaches to IVM has led to considerable confusion and debate in the literature [18]. However, the traditional and broadly accepted definition of IVM [37], as originally described by Edwards [8], is the maturation of immature cumulus–oocyte complexes (COCs) collected from antral follicles that progress from the germinal vesicle (GV) stage through to MII in vitro over a species-specific time period (Fig. 4A). Variations on IVM theme are principally based on the administration of gonadotropins to the patient with the aim of obtaining oocytes with the highest developmental potential from larger antral follicles (stimulation with FSH) and/or to enhance the proportion of MII oocytes (through administration of a bolus of hCG before oocyte retrieval).
Fig. 4.
Major IVM protocols. A The original IVM protocol [8], where immature, GV-stage COCs are matured in vitro in one step to MII. Patients may or may not receive prior FSH priming as in either case all oocytes are at the GV stage at OPU. B A biphasic IVM protocol is a small variation on standard IVM, the notable difference being the additional pre-IVM culture step. Here, meiosis of immature cumulus-enclosed oocytes is deliberately arrested for ~ 24 h, before moving COCs into a meiosis promoting medium. Examples include the SPOM- and CAPA-IVM protocols. Patients may receive prior FSH priming, but not hCG priming, as the latter is incompatible with the need for intact compact COCs in this platform. C Patients receive a bolus of hCG prior to OPU, +/− prior FSH priming. A proportion (~ 10–20%) of oocytes are collected at the MII stage, some resume meiosis in vivo but are not mature (germinal vesicle breakdown (GVB) or MI), and the majority of oocytes are at the GV stage. The different stages of meiosis at OPU necessitate differing treatment in the laboratory: MII require fertilization on the day of OPU, whereas the maturing and immature oocytes require IVM culture. D This is the maturation in vitro of immature GV-stage oocytes collected from conventional IVF cycles after OS and ovulation triggering, mostly with hCG [38]. These are commonly regarded as medically unusable oocytes and are usually discarded in most IVF clinics [39]. These oocytes are usually naked, as oocytes are denuded of cumulus cells after OPU prior to ICSI; hence, rescue IVM oocytes are invariably cultured in a denuded state from the GV to MII stage in vitro
In simple terms, the laboratory component of IVM involves collection and culture of intact COCs over a time course expected to yield MII oocytes. Thereafter, mature oocytes and resultant embryos are treated exactly as they would be in a conventional IVF cycle. COCs are typically cultured in complex tissue culture-like medium with supplementation of a protein source and hormones (e.g., FSH +/− hCG), for 30–48 h, usually under atmospheric oxygen. In this review, we will not review the myriad of hormone, growth factor, and other additives that have been tested in IVM media. However, it is worth noting that there are three main recognized clinical IVM laboratory protocols [37], the choice of which, to some extent, is dictated by the clinical preparation of the patient prior to oocyte collection (Fig. 4A–C). Rescue IVM of GV oocytes from conventional IVF cycles is an additional consideration [38] (Fig. 4D), although it is not considered a clinical IVM procedure as it is a non-recommended and unconventional clinical practice [39, 40]. Table 1 briefly outlines the pros and cons of these different approaches to IVM.
Table 1.
Advantages and disadvantages of the major IVM protocols
| Advantages | Disadvantages | |
|---|---|---|
| Standard IVM |
• Simple one-step culture system [8, 18] • All oocytes at the same immature meiotic stage at the start of culture [8, 41] |
|
| Biphasic IVM |
• Relatively high MII rates at ~ 70% [22] |
• Additional laboratory burden due the extra day of culture [22] • Has only recently been introduced in a limited number of IVM labs [23, 45] |
| hCG-primed IVM | • Relatively high MII rates at ~ 70% [46] |
• Oocytes are collected at a mixture of meiotic stages [47] • Additional laboratory burden due to at least two rounds of ICSI per OPU [18] • IVM of GVB oocyte cohort is suboptimal as the exact extent of their meiotic progression at start cannot be determined [47] • Modest live birth rates [41, 47] • Prohibitive to the use of any pre-IVM culture approach [34] |
| Rescue IVM |
• GV oocytes are common in conventional IVF patients • May generate additional embryos for transfer [38] |
• Oocytes commonly have meiotic defects [48] • IVM in the absence of cumulus cell support → poor oocyte quality [20, 27, 40] |
The role of FSH priming
Ovarian stimulation (OS) before oocyte retrieval in IVM has been applied with different protocols, including the use of clomiphene citrate, letrozole, or recombinant or urinary FSH. The most common OS protocol used and studied for IVM is a short course of FSH administered to the patient (FSH priming). FSH priming does not induce oocyte meiotic resumption in vivo, so this protocol always yields immature compact COCs after OPU (Fig. 4A, B). Based on extensive animal studies, FSH priming enhances follicular development and the meiotic and developmental competence of immature oocytes in vivo [49, 50]. Nevertheless, when priming with FSH results in follicle growth to a diameter of ~ 10 mm or more, the recovery rate of COCs from these follicles using a single lumen needle may be reduced [51]. Although the use of FSH priming in IVM cycles is based on solid rationale from animal studies and is quite common in clinical practice, there is no strong evidence from clinical trials for its use in human IVM [14, 41].
The first study suggesting that mild stimulation with FSH may improve oocyte yield and maturation rates was reported by Wynn et al. [52]. In this study, a course of 600 IU was used for 5 days, starting from day 2 of the cycle. A small RCT in 28 women with PCOS showed that a course of recombinant FSH for 3 days (150 IU daily from day 3 of the cycle), improved oocyte MII competence and implantation rate of cleaved embryos [42]. Nevertheless, FSH priming did not result in better clinical outcomes in women who did not have PCOS [14, 41]. There has been no consensus on the dose and duration of FSH priming for IVM, yet the common dosage is 150 IU of FSH daily for 2 or 3 days, starting from day 2 or 3 of the cycle or after a progestin withdrawal bleed [23, 53, 54]. Data from a small RCT by Mikkelsen et al. [55] showed no difference in apoptosis of granulosa cells and no difference in developmental competence of oocytes obtained, when oocyte retrieval was done 3 days, compared with 2 days, after the last FSH injection.
Recapitulating the follicle environment: the evolution of biphasic IVM
Perhaps the most significant development in clinical IVM in recent years (Fig. 1) is the introduction of biphasic IVM (also called pre-IVM) into clinical practice (Fig. 4B). Although this represents a significant new direction for human IVM, the concept of pre-IVM has been in the animal literature for decades [56–58]. The basic principles of biphasic IVM culture systems are to (a) maintain the oocyte in vitro in a meiotically arrested (GV) stage, (b) retain intact the physical contact and paracrine signaling system of communication between oocyte and cumulus cells (Fig. 3), (c) foster and maintain an environment that allows the acquisition of developmental competence for the oocyte over ~ 24 h in the pre-IVM step, and (d) elicit resumption of and progression of meiosis under conditions mimicking the post-LH surge follicular environment (Fig. 4B) (reviewed in [34]). Refinement of these concepts eventually led to the breakthrough application of biphasic IVM to human oocytes in an IVM center in Belgium [22]. This proof-of-principle study in 30 oocyte donors represents a seminal contribution to the clinical IVM field [22], as it demonstrated the effectiveness of NPPC in human IVM, it demonstrated that biphasic IVM is superior to standard IVM, and it established the media formulation for “capacitation-IVM” (CAPA-IVM) which has been used in subsequent clinical trials. This study was followed by a safety study [59] and pilot RCTs in an IVM center of expertise in Vietnam of standard IVM versus biphasic IVM with favorable clinical outcomes for the biphasic approach [43, 60]. This culminated in a large RCT comparing the efficacy of biphasic IVM with conventional IVF, in which patients in the IVM arm received 150 IU of hMG daily for just 2 days, which was 5.5-fold less FSH than patients received in the IVF arm of the trial [23]. Although the difference in live birth rate after the first embryo transfer between biphasic IVM (35%) and conventional IVF (43%) was only 8% [23], which illustrates the potential of biphasic IVM to narrow the efficiency gap between IVM and IVF, the higher number of usable embryos after conventional IVF resulted in an almost 19% lower cumulative ongoing pregnancy rate at 12 months after randomization per started IVM cycle compared to conventional IVF.
Triggering before OPU (hCG priming)
hCG and GnRHa have been used for triggering before oocyte retrieval in IVM, although most of the available data are with hCG triggering. The first successful application of hCG triggering before oocyte retrieval in IVM was reported in 1999 [61]. Since then, the use of hCG triggering in IVM has been adopted by many centers. Often hCG triggering in IVM is combined with a short course of FSH priming, except in the context of urgent fertility preservation [62]. Nevertheless, OS with FSH followed by a bolus of hCG may in many respects be considered a “truncated IVF” cycle [18] (Fig. 4C).
The hypothesis is that hCG may promote the initiation of oocyte maturation in vivo and the time course of oocyte maturation in vitro is hastened, and hence, the mature oocyte rate is increased. Therefore, it is possible that pregnancy rates may be improved by priming with hCG prior to immature oocyte retrieval [16]. The largest study of IVM using hCG triggering, combined with FSH priming, included 921 PCOS women. The oocyte maturation rate was 71% and the cumulative live birth rate over 12 months after one IVM cycle was 33.4% [46]. However, a Cochrane review recently found no conclusive evidence that hCG triggering before oocyte retrieval and IVM would have an effect on clinical pregnancy or live birth rates [63]. The authors provided low quality evidence suggesting that hCG priming may reduce clinical pregnancy rates, although these findings were limited by the small size of the data set. A recent RCT conducted on 172 cancer patients having IVM for fertility preservation verified the dubious role of ovulation triggering in IVM treatment. The authors found no difference in number of retrieved oocytes or the number of mature oocytes between the three interventions of no triggering, hCG triggering, or GnRH agonist triggering [64].
Fresh transfer or freeze-only for IVM?
In IVM cycles, the follicular phase and duration of endometrial exposure to adequate estradiol levels are much shorter than in stimulated IVF/ICSI cycles. Yet, according to retrospective data, fresh embryo transfer (ET) was feasible for the majority of IVM treatment cycles after a short FSH-priming protocol for a least 3 days, with satisfactory pregnancy outcomes, either using a non-triggered IVM protocol [65] or hCG-triggered IVM protocol [66]. Nevertheless, based on the observation, again with retrospective data, that live birth rates after frozen ET (FET) were higher compared to fresh ET [53, 54], it was suggested that endometrial quality in non-hCG-triggered IVM cycles may be suboptimal for normal embryo implantation. In a prospective cohort study of 68 women who underwent endometrial sampling, an aberrant expression pattern of steroid receptors in endometrium in non-hCG-triggered IVM cycles was observed as well as a deficient mid-luteal histological signature of endometrial receptivity, possibly due to the combination of a short phase of endometrial proliferation and exposure of the endometrium to insufficient levels of progesterone [67]. Therefore, a freeze-only strategy was proposed for non-hCG IVM cycles [44]. The only RCT comparing live birth rates between fresh transfer or freeze-only strategies in non-hCG IVM cycles was published recently, which showed that frozen transfer provided a significantly higher live birth rate than fresh transfer [68]. In parallel, there is also emerging evidence that success rates after conventional OS and IVF in high responders [69] may be improved when a freeze-all approach is adopted instead of fresh embryo transfer. Although embryo cryopreservation has gained widespread acceptance, a recent concern in the approach of women with PCOS who undergo FET is the observation that FET may be associated with an increased risk of early pregnancy loss [70] and hypertensive disorders of pregnancy (HDP) [71], if FET is performed in hormonal replacement therapy (HRT) cycles. This is possibly due to inadequate progestin support [72]. In view of this, it seems mandatory to develop efficient clinical protocols for FET that are associated with a lower risk of adverse obstetric events in general and, in particular, in women with PCOS who undergo IVM.
Selection of suitable patients for IVM
IVM of oocytes has been advocated as a safer alternative for conventional OS because of the ability to avoid the side effects and risks that are associated with OS in women with elevated functional ovarian reserve [12], including OHSS and ovarian torsion. In recent years, strategies have been developed to reduce OHSS risk, and the adoption of GnRH agonist triggering in combination with a policy of freeze-all embryos can eliminate the severe type of OHSS [73]. Therefore, clinicians have become less concerned about hyperresponse after OS. Furthermore, the observed relationship between high oocyte yield and favorable cumulative live birth rates per IVF cycle may be used as an argument by IVF practitioners to stimulate the ovaries of predicted high responders with higher doses than before the “freeze-all” era. Consequently, severe OHSS has been diminished, but moderate OHSS persists in many European countries [74] and in the USA [75]. Although the majority of predicted high responders accept the inherent risk of OS, a considerable proportion of women would embark on a less efficient fertility treatment if the burden of the treatment would be lower [76]. IVM could have an emerging role in this specific group of patients, although it is currently unknown to which extent women would accept a lower chance of pregnancy in return. Subfertile women with PCOS who are eligible for ART are probably the best candidates for IVM. PCOS is the most common endocrine condition in women and has an overall prevalence of approximately 10% according to the diagnostic criteria. These women may expect to have sufficiently high numbers of immature oocytes to make up for the inherently lower efficiency of IVM compared to conventional OS for IVF [77, 78]. Not only can women with PCOS exhibit excessive response to OS, a subset of them, especially those with hyperandrogenism and/or obesity, may have a narrow window of optimal ovarian response: OS in these patients requires frequent monitoring and may result in suboptimal outcome if hypo-response is observed [79]. For these patients, IVM may be an attractive option because monitoring of follicular growth can be kept to a minimum and oocyte retrieval for IVM can be scheduled at the patient’s convenience. In view of this, after patient counseling and discussion of pros and cons of conventional OS and IVM, a subset of patients with PCOS may embrace IVM as an alternative, simplified, and low-burden ART.
Anti-Müllerian hormone (AMH) correlates with the severity of the PCOS phenotype [80], with the highest AMH serum levels found in women with the classical PCOS phenotype A, characterized by PCO-like morphology of the ovaries, ovulatory dysfunction, and hyperandrogenism. Previous research has shown that AMH as a proxy of oocyte yield is a strong predictor of pregnancy after IVM [77]. In a retrospective cohort study encompassing 320 women with PCOS who underwent IVM, Mackens et al. [81] illustrated the importance of assigning a specific phenotype to women with PCOS, based on a combination of Rotterdam criteria. Indeed, after adjusting for potential confounders, the PCOS phenotype significantly correlated with cumulative live birth rate (CLBR) after IVM; patients with the classical PCOS phenotype A had the highest CLBR (40% per started cycle) [81]. Although no prospective studies have compared clinical outcome after IVM and conventional OS in women with PCOS type A, such a comparative study could be a valuable future endeavor.
IVM for fertility preservation
Cryopreservation of embryos or oocytes after conventional OS is currently the most established technique for fertility preservation (FP) in women who have not recently received gonadotoxic treatment [82]. However, OS is not possible in prepubertal girls and may be contraindicated in women with estrogen receptor positive breast cancer, although FSH-induced hyperestradiolemia may be counteracted or avoided with adjuvant therapies like selective estrogen receptor modulators or aromatase inhibitors [83]. In these cases, IVM of oocytes harvested via transvaginal follicular aspiration or, alternatively, derived from extracorporeal ovarian tissue may be considered as suitable techniques for FP, although the inherently lower meiotic and developmental potential of IVM oocytes will inevitably mitigate the prospective chances of a live birth in cancer survivors who return to use their vitrified IVM oocytes, when compared to a similar number of oocytes that are cryopreserved after conventional ovarian stimulation.
Data from a center of expertise in IVM for FP illustrate the feasibility of transvaginal egg retrieval for IVM in the follicular or luteal phase in 248 breast cancer patients included in a FP program before neoadjuvant chemotherapy [62]. In this largest series of cancer patients undergoing IVM for FP so far, with a mean age of 31.5 ± 0.3 years, a mean number of 6.4 ± 0.3 mature oocytes were cryopreserved after IVM. The feasibility and safety of performing IVM in emergency settings has also been shown in women diagnosed with hematologic diseases [84]. In cancer patients, the administration of a bolus of hCG before oocyte retrieval has shown to not improve the total number of mature oocytes for cryopreservation [64]. The first livebirth following vitrification of in vitro matured oocytes harvested transvaginally demonstrates the utility of IVM in the overall strategy of female FP [85] (Fig. 1). Sonigo et al. [86] observed that antral follicle count and AMH values above 28 follicles and 3.9 ng/mL, 20 follicles and 3.7 ng/mL, and 19 follicles and 3.5 ng/mL were required to obtain at least 15, 10, or 8 cryopreserved oocytes, respectively, after transvaginal egg retrieval for standard IVM. Based on these data, the concept of double IVM, implying the repetition of IVM cycles even within a very short time frame (< 10 days), may emerge as a viable and safe option for increasing the number of mature eggs available for FP [87].
In order to expand the sources of cryopreserved material in cancer patients, IVM can be combined with oophorectomy or ovarian biopsies from the contralateral ovary for cryopreservation of ovarian cortex [88, 89]. Cumulus–oocytes complexes recovered during processing of extracorporeal ovarian tissue adds to the pool of available material [90–92]. Patients with high risk of malignant invasion of the ovary, such as borderline ovarian carcinoma [93], leukemia, neuroblastoma, and Burkitt lymphoma, may be most suitable for IVM of oocytes from excised ovarian tissue (OTO-IVM), given that ovarian tissue transplantation poses inherent risks for tumor reintroduction. However, OTO-IVM remains experimental because the long-term safety studies have yet to be conducted. Moreover, lower maturation rates were observed after OTO-IVM [91], not unexpectedly, due to the fact that COCs derived from non-selected antral follicles from patients of different ages are unlikely to have completed key stages of folliculogenesis and would not be well supported by standard IVM protocols. The biphasic IVM platform described above has already shown promising results for OTO-IVM [45].
IVM for resistant ovary syndrome
Resistant ovary syndrome (ROS) is a rare endocrine condition characterized by hypergonadotropic anovulation (WHO group 3) and infertility. Patients often suffer from primary or secondary amenorrhea with timely and spontaneous onset of secondary sexual characteristics [94, 95]. Serum levels of FSH and LH are elevated, in spite of normal levels of AMH and normal antral follicle counts [96]. The pathophysiology of this syndrome relies on the inability of antral follicles to respond to both endogenous and exogenous FSH. Genetic or immunologic abnormalities may explain antral follicle unresponsiveness to FSH although the etiology remains often unexplained [97, 98]. Mutations with loss of function [99, 100] and polymorphisms of the FSH receptor [101, 102] have been described. For ROS patients, IVM is currently the only viable alternative option to egg donation, with several live births reported [103, 104].
IVM for poor responders
Women exhibiting a poor response to exogenous gonadotropins have a reduced chance of achieving pregnancy, and the optimal management of these patients remains a matter of debate. Among the approaches considered, the practice of using high doses of gonadotropins in poor responders has not been supported by evidence [105]. Despite reports of livebirths obtained after rescue IVM [38, 106, 107] or unprimed immature oocyte retrieval [108], IVM as practiced yielded limited success in poor prognosis patients of either advanced reproductive age and/or low ovarian reserve. Indeed, the success of IVM relies heavily on the number of oocytes retrieved from a patient, even more than in a conventional OS cycle for IVF, due to the unpredictable recovery of COCs, suboptimal meiotic maturation rates from IVM of ~ 50% [109], and higher embryo attrition rate using IVM compared to conventional OS [110]. In addition, the fact that AFC, AMH, and total testosterone are the only independent predictors of oocyte yield in PCOS patients undergoing IVM [77] suggests that poor prognosis patients are not well suited to IVM.
IVM in women with oocyte maturation defects
A small subset of infertile women exhibit oocyte meiotic maturation defects, in which immature (GV, M1 stages) oocytes are obtained after repeated conventional OS cycles [111]. These women have few alternatives available to them for infertility treatment. Some patients have underlying genetic defects in their oocytes [112] and IVM is unlikely to help their infertility. In other patients the pathophysiological basis for the disorder is unknown. The few case series deploying IVM for these patients have yielded disappointing results [103, 113]. Future developments with the IVM platform, including the use of biphasic IVM systems where meiosis is induced in vitro during the IVM phase (reviewed in [34]), could offer new opportunities for patients affected by such conditions.
Safety aspects of IVM
All information and data available to date suggest that IVM is safe for both patients treated and children born from the technique [37, 114]. Indeed, for women with PCOS, IVM is less hazardous than treatment with conventional OS and IVF as the risk of OHSS is eliminated in IVM pregnancies [23, 54]. For some time, there were concerns about the higher rate of miscarriage in IVM cycles relative to conventional IVF [115], which is now know to be attributable to the use of fresh ET in IVM cycles [54], and hence with the adoption of a freeze-all strategy for IVM, miscarriage rates are the same as in conventional IVF [23]. In terms of pregnancy and obstetric complications, outcomes are not different for IVM and conventional IVF pregnancies in terms of rates of ectopic pregnancies, gestational diabetes, and antepartum hemorrhage [23, 114]. One study found a significant increase in hypertensive disorders of pregnancy in IVM compared to IVF pregnancies [116], but this was not found in the subsequent prospective RCT of biphasic IVM versus conventional IVF [23].
In terms of fetal and neonatal development, some concerns have been expressed about possible epigenetic risks for IVM children as oocyte meiosis occurs in vitro. The two studies to date that have examined the status of key imprinted genes in human IVM oocytes [59, 117] suggest that IVM does not interfere with genomic imprinting establishment. This is corroborated by imprinting studies using chorionic villus and cord blood samples from children born from IVM [118]. Consistent with findings from these epigenetic studies, recent conclusions from a meta-analysis [114] and from a RCT [23] found that the major measures of neonatal outcomes are not different between IVM and IVF babies, including preterm birth, spontaneous preterm birth, iatrogenic preterm birth, low or high birth weight, large for gestational age birth, congenital anomalies, and admission to the neonatal intensive care unit.
Follow-up studies of 2-year-old IVM children show normal growth and body weight compared to OS-IVF children, and there is no evidence of a delay in mental development in IVM children [119–121]. A long-term follow-up study of IVM children and adolescents up the age of 19 found no increased risk associated with IVM compared to IVF [122]. Collectively, these studies provide a degree of reassurance that outcomes for mothers and children born from IVM do not differ from conventional OS-IVF [114]; however, to date, IVM numbers remain low and ongoing follow-up studies of IVM children are warranted.
Divergent regional perspectives on the role of IVM
Access to reproductive health care is excellent in European countries. Large numbers of reproductive medicine centers continue to contribute high level of safety and efficiency measures in current ART treatment options. Despite the linear relationship between cumulative birth rates and ovarian response after conventional OS and IVF/ICSI, even while the risk of iatrogenic complications and side effects has been reduced during the past decade, the perceived burden by patients of conventional OS remains high, especially in predicted high responders [76]. In women with excessive antral follicle counts, IVM has a better safety profile and may be advocated as a minimal-burden treatment option [123]. After balanced counseling of pros and cons of IVM compared to conventional OS, the tendency towards mild stimulation treatment with relatively lower success rates may appeal to a subset of high responders in countries where the out-of-pocket cost for the patient undergoing ART is relatively low (Table 2).
Table 2.
Empirical analysis of factors that may modulate the uptake of IVM in different regions
| Regions | Availability of reproductive care services | Out-of-pocket costs for patients | Incentive for IVM for infertility treatment | Incentive for IVM for onco-fertility preservation |
|---|---|---|---|---|
| Europe | +++ | + |
Low incentive: - Reduced efficiency compared to OS - Utilization of freeze-all strategies in high responders - Limited cost savings for the patient |
High incentive: - Utilization will grow as more centers develop expertise in IVM - Focus on OTO-IVM in spite of experimental nature |
| USA and Canada | +++ | +++ |
Low incentive: - High cost of ART for patients means prioritizing treatments with maximal efficiency |
High incentive: - Utilization will increase as more centers develop expertise in IVM - Focus on OTO-IVM in spite of experimental nature |
| Russia | +++ | ++ |
Low incentive: - Relatively high cost of ART for patients means prioritizing treatments with maximal efficiency |
High incentive: - Utilization will grow as more centers develop expertise in IVM - Focus on OTO-IVM in spite of experimental nature |
| Middle East and Maghreb | ++ | ++ |
High incentive: - High incidence of patients with severe PCOS and underutilized safety measures in high responders - Limited uptake because of lack of experienced centers in the region and perceived complexity of clinical and laboratory IVM procedures |
Low incentive: - Limited availability of onco-fertility programs in the region |
| India | +++ | ++ |
High incentive: - High incidence of patients with severe PCOS and underutilized safety measures in high responders - Limited uptake because of lack of experienced centers in the region and perceived complexity of clinical and laboratory IVM procedures |
High incentive: - Utilization will grow as more centers develop expertise in IVM |
| Southeast Asia | ++ | ++ |
High incentive: - High relative cost of gonadotropins - Increasing uptake in view of emerging number of centers in the region developing a high level of expertise in IVM |
High incentive: - Utilization will grow as more centers develop expertise in IVM - Focus on OTO-IVM in spite of experimental nature |
| China | ++ | ++ |
Low incentive: - High cost of ART for patients means prioritizing treatments with maximal efficiency |
Low incentive: - Limited availability of onco-fertility programs in the region |
| Japan and South Korea | +++ | + |
Low incentive: - Reduced efficiency compared to OS - Utilization of freeze-all strategies in high responders - Limited cost savings for the patient |
High incentive: - Utilization will grow as more centers develop expertise in IVM - Focus on OTO-IVM in spite of experimental nature |
| Australia and New Zealand | +++ | + |
Low incentive: - Reduced efficiency compared to OS - Utilization of freeze-all strategies in high responders - Limited cost savings for the patient |
High incentive: - Utilization will grow as more centers develop expertise in IVM - Focus on OTO-IVM in spite of experimental nature |
| Central and South America | ++ | ++ |
Low incentive: - Lack of experienced centers in the region and perceived complexity of clinical and laboratory IVM procedures |
Low incentive: - Limited availability of onco-fertility programs in the region |
| Middle and Southern Africa | + | ++ |
Low incentive: - Lack of experienced centers in the region and perceived complexity of clinical and laboratory IVM procedures |
Low incentive: - Limited availability of onco-fertility programs in the region |
The potential adoption of IVM in the ART clinics in Europe, and to a large extent in Australia and New Zealand, is in sharp contrast with prospects in the USA and Canada (Table 2). In North America, the projected uptake of IVM in ART clinics will be lower unless the efficiency of IVM culture systems can be markedly improved. ART practice patterns in the USA continue to follow ASRM guidelines in most clinics according to recent SART data. Historically, US clinics have been quick to adopt variations in protocols that increase marketing potential in a competitive atmosphere expected to be driven by capitalism. Hence, the rapid evolution of conventional OS strategies, freeze-all cycles, oocyte banking, and genetic testing approaches, all of which move into daily practice at a rate far in excess of more discriminating countries around the world. Given this backdrop, IVM has received little attention in the USA, admittedly in part because the latest advances in ovarian physiology that have formed the cornerstone of, for example, biphasic IVM, have yet to be fully appreciated. The much-needed change in attitude for the future will hopefully be prompted by this contribution to this special issue of JARG.
IVM was developed decades ago in South Korea and Japan [11], with many IVM programs continuing today. While the actual costs associated with one IVM cycle may be less than for one IVF cycle [124–126] in many Western countries, a major portion of IVF treatment or medication costs are often covered by public health and/or health insurance; therefore, IVM is not necessarily less expensive for infertile couples. Moreover, as long as IVM is less efficacious compared to conventional IVF, the cost for a baby using IVM may not be lower in many health care settings. In contrast, in countries with emerging economies, there is often no reimbursement system for infertility treatment. Therefore, IVM can be a more affordable ART lowering the out-of-pocket expenses for patients. In this sense, IVM could be even more attractive to infertile patients in lower income countries in SE Asia (Table 2). In fact, this is likely one of the principal drivers of the uptake of IVM in the many Asian ART centers with ongoing active IVM programs in China, Vietnam, India, Indonesia, Malaysia, and Thailand. By contrast, patients in South Korea and Japan are less likely to be motivated by the lower costs of IVM, even though they partly pay out-of-pocket for ART treatment, such that patients in these two countries have similar motivators to do IVM as patients in Europe and Australia.
The need to develop a consortium of centers of expertise in IVM
As with any emerging ART, an anticipated barrier to clinical uptake of IVM will be access to clinical and laboratory knowhow of how to perform IVM. As IVM has been practiced for decades (but with low cycle numbers), there is in fact considerable literature on IVM. However, more recently, a major shortcoming of the human IVM literature is the enormous degree of confusion about what it is, and what it is not, and what constitutes clinically acceptable IVM practice [18], e.g., rescue IVM is very often conflated with standard IVM, when in fact rescue IVM has questionable safety and should probably not be practiced [37]. Consequently, many clinical practitioners are unclear about the current clinical status of IVM. A major gap for the field has been the need for a consensus statement on the clinical practice of IVM, something that in many respects has been addressed by the recent ASRM Committee Opinion on IVM declaring IVM non-experimental [37]. In addition, clinics are likely to be unsure how to implement an IVM practice. Importantly, IVM is substantially less technically demanding for embryologists to learn, compared to learning ICSI or embryo biopsy, and requires no additional laboratory equipment, although it does represent an extra procedure in the laboratory. Oocyte retrieval is more challenging in an IVM cycle compared to an IVF cycle; however, adaptations to standard retrieval technique enable any clinician to perform oocyte retrieval for IVM in most patients [127]. Moreover, clinical management and cycle monitoring of patients is greatly simplified compared to conventional OS or even ovulation induction cycles. Nonetheless, a misguided and insufficient understanding across the ART sector of what constitutes IVM, coupled with lack of sufficient centers of expertise that offer training in IVM, comprises current barriers to uptake and progress in IVM. In the past, pioneering clinical IVM hubs, such as those in Melbourne, Seoul, Osaka, Copenhagen, Monza, and Montreal, were instrumental in driving developments in IVM and accepting visitors from other clinics for training. These days the centers of expertise have shifted, to some extent, to Brussels, Paris, Tel Aviv, Ho Chi Minh City, and multiple locations in China including Guangzhou, Beijing, and Nanjing. It is imperative that these leading centers continue to pass on their expertise by offering training, including in the form of workshops, and that these are supported by industry and academic societies, such as the large and highly successful 2018 ASPIRE Masterclass in IVM, hosted by My Duc Hospital in Ho Chi Minh City, Vietnam. It is only through such concerted and collaborative effort by leading clinicians, scientists, academia and industry that IVM will obtain a foothold in routine ART practice, such that patients, in particular PCOS and cancer patients, will gain from the health and financial benefits that comes from infertility treatment with IVM.
Gaps for future IVM research
Improving embryo yield from IVM: Despite recent improvements, a recognized need will be to improve blastocyst rates from IVM oocytes. This requires innovative and sophisticated new IVM culture systems, built on the latest scientific advances guided by continued animal oocyte biology research.
Improving the recovery rate of oocytes in an IVM OPU: The efficiency of IVM would be significantly enhanced if a higher proportion of oocytes could be recovered per follicle aspirated. This will require development of novel and sophisticated needle technology.
New clinical approaches to patient management: Can we further simplify patient management prior to IVM, such that cycle monitoring/management may not be needed at all? Further refinements on approaches such as random-start and the use of hormonal pretreatment are warranted.
IVM as part of standard practice in fertility preservation: IVM should be routinely integrated into the repertoire of technologies needed to maximize fertility preservation prospects for cancer patients. Research is needed on how to integrate the technology options of conventional OS with OPU-IVM, OTO-IVM, and ovarian tissue cryopreservation into clinical practice. In addition, studies integrating follicle in vitro growth technologies with pre-IVM are needed [128].
IVM and planned oocyte cryopreservation: Due to the convenient, lower cost, and mild stimulation nature of IVM, it provides an alternative for women seeking planned oocyte cryopreservation, especially younger women with a high AFC and AMH. To date, this approach has been poorly exploited and further research is needed.
Towards zero-stimulation ART: Furthering the development of sophisticated culture systems capable of supporting the growth and differentiation of oocytes prior to meiotic maturation, followed by IVM, will position the ART field to a point where minimal or zero stimulation is used.
Summary points
IVM of oocytes refers to the in vitro culture of cumulus-enclosed oocytes retrieved at the GV stage in medium that supports oocyte developmental potential.
IVM is a non-experimental procedure as it has been practiced for several decades and the evidence to date suggests it is safe for women and offspring.
IVM is a low intervention, mild approach to ART, well suited to patients with an excessive antral follicle count and is not suitable for poor prognosis patients.
Although the technology is not new, IVM is not widely used, and incentives leading to enhanced uptake of its use in the ART clinic vary widely across the globe.
With currently available IVM systems, clinical outcomes are lower than those after conventional ovarian stimulation in most women, but for some infertile women and after appropriate counseling, the improved safety and a simplified clinical approach will outweigh lower efficacy.
Fertility preservation (FP) has enlarged the spectrum of fertility disrupting conditions, such as cancer, by offering a range of treatments that have evolved over the past decade. IVM has been and will continue to serve this population of patients in need of strategies to become parents precluded by conventional ARTs.
Acknowledgements
The authors wish to acknowledge the organizational support provided by CooperSurgical for the preparation of this paper.
Funding
IVM Research at Vrije Universiteit Brussel is supported by a grant from the Fonds National de la Recherche Scientifique de Belgique–the Excellence of Science (FNRS–EOS), number 30443682, awarded to M.D.V. T.M.H.’s IVM research program is funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED; grant number FWO.106-YS.2017.02). Y.Y.’s IVM research is funded by internal research funds provided by the Colorado Center for Reproductive Medicine. R.B.G.’s IVM research program in Sydney is funded by a grant (APP1139763) and fellowship (APP1117538) from the National Health and Medical Research Council of Australia and by support from City Fertility Global and Open Philanthropy. D.F.A.’ research has been supported by the ESHE Fund.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pincus G, Enzmann EV. The comparative behavior of mammalian eggs in vivo and in vitro: I. the activation of ovarian eggs. J Exp Med. 1935;62:665–675. doi: 10.1084/jem.62.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pincus G, Saunders B. The comparative behavior of mammalian eggs in vivo and in vitro. VI. The maturation of human ovarian ova. Anat Rec. 1939;75:537–545. [Google Scholar]
- 3.Rock J, Menkin MF. In vitro fertilization and cleavage of human ovarian eggs. Science. 1944;100:105–107. doi: 10.1126/science.100.2588.105. [DOI] [PubMed] [Google Scholar]
- 4.Menkin MF, Rock J. In vitro fertilization and cleavage of human ovarian eggs. Am J Obstet Gynecol. 1948;55:440–452. doi: 10.1016/s0002-9378(15)32963-x. [DOI] [PubMed] [Google Scholar]
- 5.Thompson JG, Gilchrist RB. Pioneering contributions by Robert Edwards to oocyte in vitro maturation (IVM) Mol Hum Reprod. 2013;19:794–798. doi: 10.1093/molehr/gat075. [DOI] [PubMed] [Google Scholar]
- 6.Edwards RG. Meiosis in ovarian oocytes of adult mammals. Nature. 1962;196:446–450. [Google Scholar]
- 7.Edwards RG. Maturation in vitro of human ovarian oocytes. Lancet. 1965;2:926–929. doi: 10.1016/s0140-6736(65)92903-x. [DOI] [PubMed] [Google Scholar]
- 8.Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature. 1965;208:349–351. doi: 10.1038/208349a0. [DOI] [PubMed] [Google Scholar]
- 9.Edwards RG, Bavister BD, Steptoe PC. Early stages of fertilization in vitro of human oocytes matured in vitro. Nature. 1969;221:632–635. doi: 10.1038/221632a0. [DOI] [PubMed] [Google Scholar]
- 10.Bavister BD, Edwards RG, Steptoe PC. Identification of the midpiece and tail of the spermatozoon during fertilization of human eggs in vitro. J Reprod Fertil. 1969;20:159–160. doi: 10.1530/jrf.0.0200159. [DOI] [PubMed] [Google Scholar]
- 11.Cha KY, Koo JJ, Ko JJ, Choi DH, Han SY, Yoon TK. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil Steril. 1991;55:109–113. doi: 10.1016/s0015-0282(16)54068-0. [DOI] [PubMed] [Google Scholar]
- 12.Trounson A, Wood C. A Kausche. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil Steril. 1994;62:353–362. doi: 10.1016/s0015-0282(16)56891-5. [DOI] [PubMed] [Google Scholar]
- 13.Barnes FL, Crombie A, Gardner DK, Kausche A, Kaplan OL, Suikkari AM, et al. Blastocyst development and birth after in-vitro maturation of human primary oocytes, intracytoplasmic sperm injection and assisted hatching. Hum Reprod. 1995, 10:3243–7. [DOI] [PubMed]
- 14.Mikkelsen AL, Smith SD, Lindenberg S. In-vitro maturation of human oocytes from regularly menstruating women may be successful without follicle stimulating hormone priming. Hum Reprod. 1999;14:1847–1851. doi: 10.1093/humrep/14.7.1847. [DOI] [PubMed] [Google Scholar]
- 15.Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update. 2002;8:559–577. doi: 10.1093/humupd/8.6.559. [DOI] [PubMed] [Google Scholar]
- 16.Chian RC, Buckett WM, Tulandi T, Tan SL. Prospective randomized study of human chorionic gonadotrophin priming before immature oocyte retrieval from unstimulated women with polycystic ovarian syndrome. Hum Reprod. 2000;15:165–170. doi: 10.1093/humrep/15.1.165. [DOI] [PubMed] [Google Scholar]
- 17.Dahan MH, Tan SL, Chung J, Son WY. Clinical definition paper on in vitro maturation of human oocytes. Hum Reprod. 2016;31:1383–1386. doi: 10.1093/humrep/dew109. [DOI] [PubMed] [Google Scholar]
- 18.De Vos M, Smitz J, Thompson JG. RB Gilchrist. The definition of IVM is clear-variations need defining. Hum Reprod. 2016;31:2411–2415. doi: 10.1093/humrep/dew208. [DOI] [PubMed] [Google Scholar]
- 19.Coticchio G. IVM in need of clear definitions. Hum Reprod. 2016;31:1387–1389. doi: 10.1093/humrep/dew110. [DOI] [PubMed] [Google Scholar]
- 20.Gilchrist RB. Recent insights into oocyte-follicle cell interactions provide opportunities for the development of new approaches to in vitro maturation. Reprod Fertil Dev. 2011;23:23–31. doi: 10.1071/RD10225. [DOI] [PubMed] [Google Scholar]
- 21.Richani D, Gilchrist RB. The epidermal growth factor network: role in oocyte growth, maturation and developmental competence. Hum Reprod Update. 2018;24:1–14. doi: 10.1093/humupd/dmx029. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez F, Lolicato F, Romero S, De Vos M, Van Ranst H, Verheyen G, et al. An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield. Hum Reprod. 2017;32:2056–2068. doi: 10.1093/humrep/dex262. [DOI] [PubMed] [Google Scholar]
- 23.Vuong LN, Ho VNA, Ho TM, Dang VQ, Phung TH, Giang NH, le AH, Pham TD, Wang R, Smitz J, Gilchrist RB, Norman RJ, Mol BW. In-vitro maturation of oocytes versus conventional IVF in women with infertility and a high antral follicle count: a randomized non-inferiority controlled trial. Hum Reprod. 2020;35:2537–2547. doi: 10.1093/humrep/deaa240. [DOI] [PubMed] [Google Scholar]
- 24.Lonergan P, et al. Maturation of oocytes in vitro. Annu Rev Anim Biosci. 2016;4:255–268. doi: 10.1146/annurev-animal-022114-110822. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JG, RB Gilchrist, Improving oocyte maturation in vitro., in Biology and Pathology of the oocyte: role in fertility, medicine, and nuclear reprogramming, Second edition, G.R. Trounson AO, Eichenlaub-Ritter U., Editor. 2013, Cambridge University Press: Cambridge, UK 212-223.
- 26.Luciano AM, Sirard MA. Successful in vitro maturation of oocytes: a matter of follicular differentiation. Biol Reprod. 2018;98:162–169. doi: 10.1093/biolre/iox149. [DOI] [PubMed] [Google Scholar]
- 27.Conti M, Franciosi F. Acquisition of oocyte competence to develop as an embryo: integrated nuclear and cytoplasmic events. Hum Reprod Update. 2018;24:245–266. doi: 10.1093/humupd/dmx040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGinnis LK, Albertini DF. Dynamics of protein phosphorylation during meiotic maturation. J Assist Reprod Genet. 2010;27:169–182. doi: 10.1007/s10815-010-9391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coticchio G, Canto MD, Renzini MM, Guglielmo MC, Brambillasca F, Turchi D, et al. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update. 2015;21:427–454. doi: 10.1093/humupd/dmv011. [DOI] [PubMed] [Google Scholar]
- 30.Vaccari S, JL Weeks, 2nd, M Hsieh, FS Menniti, M Conti Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81:595–604. doi: 10.1095/biolreprod.109.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, et al. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136:1869–1878. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–369. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franciosi F, Coticchio G, Lodde V, Tessaro I, Modina SC, Fadini R, et al. Natriuretic peptide precursor C delays meiotic resumption and sustains gap junction-mediated communication in bovine cumulus-enclosed oocytes. Biol Reprod. 2014;91:61. doi: 10.1095/biolreprod.114.118869. [DOI] [PubMed] [Google Scholar]
- 34.Gilchrist RB, Luciano AM, Richani D, Zeng HT, Wang X, Vos MD, et al. Oocyte maturation and quality: role of cyclic nucleotides. Reproduction. 2016;152:R143–R157. doi: 10.1530/REP-15-0606. [DOI] [PubMed] [Google Scholar]
- 35.Sugimura S, Ritter LJ, Sutton-McDowall ML, Mottershead DG, Thompson JG, Gilchrist RB. Amphiregulin co-operates with bone morphogenetic protein 15 to increase bovine oocyte developmental competence: effects on gap junction-mediated metabolite supply. Mol Hum Reprod. 2014;20:499–513. doi: 10.1093/molehr/gau013. [DOI] [PubMed] [Google Scholar]
- 36.Stocker WA, Walton KL, Richani D, Chan KL, Beilby KH, Finger BJ, et al. A variant of human growth differentiation factor-9 that improves oocyte developmental competence. J Biol Chem. 2020;295:7981–7991. doi: 10.1074/jbc.RA120.013050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Practice Committees of the American Society for Reproductive Medicine tSoRB, Technologists, jao the Society for Assisted Reproductive Technology. Electronic address In vitro maturation: a committee opinion. Fertil Steril. 2021;115:298–304. [Google Scholar]
- 38.Veeck LL, Wortham JW, Jr, Witmyer J, Sandow BA, Acosta AA, Garcia JE, et al. Maturation and fertilization of morphologically immature human oocytes in a program of in vitro fertilization. Fertil Steril. 1983;39:594–602. doi: 10.1016/s0015-0282(16)47052-4. [DOI] [PubMed] [Google Scholar]
- 39.Jie H, Zhao M, Alqawasmeh OAM, Chan CPS, Lee TL, Li T, et al. In vitro rescue immature oocytes - a literature review. Hum Fertil (Camb). 2021:1–20. [DOI] [PubMed]
- 40.Jones GM, Cram DS, Song B, Magli MC, Gianaroli L, Lacham-Kaplan O, et al. Gene expression profiling of human oocytes following in vivo or in vitro maturation. Hum Reprod. 2008;23:1138–1144. doi: 10.1093/humrep/den085. [DOI] [PubMed] [Google Scholar]
- 41.Fadini R, Canto MBD, Renzini MM, Brambillasca F, Comi R, Fumagalli D, et al. Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: a prospective randomized study. Reprod BioMed Online. 2009;19:343–351. doi: 10.1016/s1472-6483(10)60168-x. [DOI] [PubMed] [Google Scholar]
- 42.Mikkelsen AL, Lindenberg S. Benefit of FSH priming of women with PCOS to the in vitro maturation procedure and the outcome: a randomized prospective study. Reproduction. 2001;122:587–592. doi: 10.1530/rep.0.1220587. [DOI] [PubMed] [Google Scholar]
- 43.Vuong LN, Le AH, Ho VNA, Pham TD, Sanchez F, Romero S, et al. Live births after oocyte in vitro maturation with a prematuration step in women with polycystic ovary syndrome. J Assist Reprod Genet. 2020;37:347–357. doi: 10.1007/s10815-019-01677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortega-Hrepich C, Stoop D, Guzman L, Van Landuyt L, Tournaye H, Smitz J, et al. A “freeze-all” embryo strategy after in vitro maturation: a novel approach in women with polycystic ovary syndrome? Fertil Steril. 2013;100:1002–1007. doi: 10.1016/j.fertnstert.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 45.Kirillova A, Bunyaeva E, Van Ranst H, Khabas G, Farmakovskaya M, Kamaletdinov N, et al. Improved maturation competence of ovarian tissue oocytes using a biphasic in vitro maturation system for patients with gynecological malignancy: a study on sibling oocytes. J Assist Reprod Genet. 2021. [DOI] [PMC free article] [PubMed]
- 46.Ho VNA, Pham TD, Le AH, Ho TM, Vuong LN. Live birth rate after human chorionic gonadotropin priming in vitro maturation in women with polycystic ovary syndrome. J Ovarian Res. 2018;11:70. doi: 10.1186/s13048-018-0445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Son WY, Chung JT, Chian RC, Herrero B, Demirtas E, Elizur S, et al. A 38 h interval between hCG priming and oocyte retrieval increases in vivo and in vitro oocyte maturation rate in programmed IVM cycles. Hum Reprod. 2008;23:2010–2016. doi: 10.1093/humrep/den210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nogueira D, Staessen C, Van de Velde H, Van Steirteghem A. Nuclear status and cytogenetics of embryos derived from in vitro-matured oocytes. Fertil Steril. 2000;74:295–298. doi: 10.1016/s0015-0282(00)00642-7. [DOI] [PubMed] [Google Scholar]
- 49.Eppig JJ, Schroeder AC, O’Brien MJ. Developmental capacity of mouse oocytes matured in vitro: effects of gonadotrophic stimulation, follicular origin and oocyte size. J Reprod Fertil. 1992;95:119–127. doi: 10.1530/jrf.0.0950119. [DOI] [PubMed] [Google Scholar]
- 50.Gilchrist RB, Nayudu PL, Hodges JK. Maturation, fertilization, and development of marmoset monkey oocytes in vitro. Biol Reprod. 1997;56:238–246. doi: 10.1095/biolreprod56.1.238. [DOI] [PubMed] [Google Scholar]
- 51.Rose BI, Laky D. A comparison of the Cook single lumen immature ovum IVM needle to the Steiner-Tan pseudo double lumen flushing needle for oocyte retrieval for IVM. J Assist Reprod Genet. 2013;30:855–860. doi: 10.1007/s10815-013-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wynn P, Picton HM, Krapez JA, Rutherford AJ, Balen AH, Gosden RG. Pretreatment with follicle stimulating hormone promotes the numbers of human oocytes reaching metaphase II by in-vitro maturation. Hum Reprod. 1998;13:3132–3138. doi: 10.1093/humrep/13.11.3132. [DOI] [PubMed] [Google Scholar]
- 53.De Vos M, Ortega-Hrepich C, Albuz FK, Guzman L, Polyzos NP, Smitz J, et al. Clinical outcome of non-hCG-primed oocyte in vitro maturation treatment in patients with polycystic ovaries and polycystic ovary syndrome. Fertil Steril. 2011;96:860–864. doi: 10.1016/j.fertnstert.2011.07.1108. [DOI] [PubMed] [Google Scholar]
- 54.Walls ML, Hunter T, Ryan JP, Keelan JA, Nathan E, Hart RJ. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries: a comparative analysis of fresh, frozen and cumulative cycle outcomes. Hum Reprod. 2015;30:88–96. doi: 10.1093/humrep/deu248. [DOI] [PubMed] [Google Scholar]
- 55.Mikkelsen AL, Host E, Blaabjerg J, Lindenberg S. Time interval between FSH priming and aspiration of immature human oocytes for in-vitro maturation: a prospective randomized study. Reprod BioMed Online. 2003;6:416–420. doi: 10.1016/s1472-6483(10)62160-8. [DOI] [PubMed] [Google Scholar]
- 56.Funahashi H, Cantley TC, Day BN. Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilization. Biol Reprod. 1997;57:49–53. doi: 10.1095/biolreprod57.1.49. [DOI] [PubMed] [Google Scholar]
- 57.Nogueira D, Cortvrindt R, De Matos DG, Vanhoutte L, Smitz J. Effect of phosphodiesterase type 3 inhibitor on developmental competence of immature mouse oocytes in vitro. Biol Reprod. 2003;69:2045–2052. doi: 10.1095/biolreprod.103.021105. [DOI] [PubMed] [Google Scholar]
- 58.Thomas RE, Thompson JG, Armstrong DT, Gilchrist RB. Effect of specific phosphodiesterase isoenzyme inhibitors during in vitro maturation of bovine oocytes on meiotic and developmental capacity. Biol Reprod. 2004;71:1142–1149. doi: 10.1095/biolreprod.103.024828. [DOI] [PubMed] [Google Scholar]
- 59.Saenz-de-Juano MD, Ivanova E, Romero S, Lolicato F, Sanchez F, Van Ranst H, et al. DNA methylation and mRNA expression of imprinted genes in blastocysts derived from an improved in vitro maturation method for oocytes from small antral follicles in polycystic ovary syndrome patients. Hum Reprod. 2019;34:1640–1649. doi: 10.1093/humrep/dez121. [DOI] [PubMed] [Google Scholar]
- 60.Sanchez F, Le AH, Ho VNA, Romero S, Van Ranst H, De Vos M, et al. Biphasic in vitro maturation (CAPA-IVM) specifically improves the developmental capacity of oocytes from small antral follicles. J Assist Reprod Genet. 2019;36:2135–2144. doi: 10.1007/s10815-019-01551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chian RC, Gulekli B, Buckett WM, Tan SL. Priming with human chorionic gonadotropin before retrieval of immature oocytes in women with infertility due to the polycystic ovary syndrome. N Engl J Med. 1999;341:1624–6. [DOI] [PubMed]
- 62.Grynberg M, Poulain M, le Parco S, Sifer C, Fanchin R, Frydman N. Similar in vitro maturation rates of oocytes retrieved during the follicular or luteal phase offer flexible options for urgent fertility preservation in breast cancer patients. Hum Reprod. 2016;31:623–629. doi: 10.1093/humrep/dev325. [DOI] [PubMed] [Google Scholar]
- 63.Reavey J, Vincent K, Child T, Granne IE. Human chorionic gonadotrophin priming for fertility treatment with in vitro maturation. Cochrane Database Syst Rev. 2016;11:CD008720. doi: 10.1002/14651858.CD008720.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sonigo C, Le Conte G, Boubaya M, Ohanyan H, Presse M, El Hachem H, et al. Priming before in vitro maturation cycles in cancer patients undergoing urgent fertility preservation: a randomized controlled study. Reprod Sci. 2020;27:2247–2256. doi: 10.1007/s43032-020-00244-0. [DOI] [PubMed] [Google Scholar]
- 65.Junk SM, Yeap D. Improved implantation and ongoing pregnancy rates after single-embryo transfer with an optimized protocol for in vitro oocyte maturation in women with polycystic ovaries and polycystic ovary syndrome. Fertil Steril. 2012;98:888–892. doi: 10.1016/j.fertnstert.2012.06.055. [DOI] [PubMed] [Google Scholar]
- 66.Ho VNA, Braam SC, Pham TD, Mol BW, Vuong LN. The effectiveness and safety of in vitro maturation of oocytes versus in vitro fertilization in women with a high antral follicle count. Hum Reprod. 2019;34:1055–1064. doi: 10.1093/humrep/dez060. [DOI] [PubMed] [Google Scholar]
- 67.Ortega-Hrepich C, Drakopoulos P, Bourgain C, Van Vaerenbergh I, Guzman L, Tournaye H, et al. Aberrant endometrial steroid receptor expression in in-vitro maturation cycles despite hormonal luteal support: a pilot study. Reprod Biol. 2019;19:210–217. doi: 10.1016/j.repbio.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 68.Vuong LN, Nguyen LK, Le AH, Pham HH, Ho VN, Le HL, et al. Fresh embryo transfer versus freeze-only after in vitro maturation with a pre-maturation step in women with high antral follicle count: a randomized controlled pilot study. J Assist Reprod Genet. 2021. [DOI] [PMC free article] [PubMed]
- 69.Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update. 2019;25:2–14. doi: 10.1093/humupd/dmy033. [DOI] [PubMed] [Google Scholar]
- 70.Tomas C, Alsbjerg B, Martikainen H, Humaidan P. Pregnancy loss after frozen-embryo transfer--a comparison of three protocols. Fertil Steril. 2012;98:1165–1169. doi: 10.1016/j.fertnstert.2012.07.1058. [DOI] [PubMed] [Google Scholar]
- 71.von Versen-Hoynck F, Schaub AM, Chi YY, Chiu KH, Liu J, Lingis M, et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension. 2019;73:640–649. doi: 10.1161/HYPERTENSIONAHA.118.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Labarta E, Mariani G, Paolelli S, Rodriguez-Varela C, Vidal C, Giles J, et al. Impact of low serum progesterone levels on the day of embryo transfer on pregnancy outcome: a prospective cohort study in artificial cycles with vaginal progesterone. Hum Reprod. 2021;36:683–692. doi: 10.1093/humrep/deaa322. [DOI] [PubMed] [Google Scholar]
- 73.Ioannidou PG, Bosdou JK, Lainas GT, Lainas TG, Grimbizis GF, Kolibianakis EM. How frequent is severe ovarian hyperstimulation syndrome after GnRH agonist triggering in high-risk women? A systematic review and meta-analysis. Reprod BioMed Online. 2021;42:635–650. doi: 10.1016/j.rbmo.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 74.Calhaz-Jorge C, De Geyter C, Kupka MS, de Mouzon J, Erb K, Mocanu E, et al. Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE. Hum Reprod. 2017;32:1957–1973. doi: 10.1093/humrep/dex264. [DOI] [PubMed] [Google Scholar]
- 75.Rotshenker-Olshinka K, Badeghiesh A, Volodarsky-Perel A, Steiner N, Suarthana E. MH Dahan. Trends in ovarian hyperstimulation syndrome hospitalization rates in the USA: an ongoing concern. Reprod BioMed Online. 2020;41:357–360. doi: 10.1016/j.rbmo.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 76.Braam SC, de Bruin JP, Mol BWJ, van Wely M. The perspective of women with an increased risk of OHSS regarding the safety and burden of IVF: a discrete choice experiment. Hum Reprod Open. 2020;2020:hoz034. doi: 10.1093/hropen/hoz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guzman L, Ortega-Hrepich C, Polyzos NP, Anckaert E, Verheyen G, Coucke W, et al. A prediction model to select PCOS patients suitable for IVM treatment based on anti-Mullerian hormone and antral follicle count. Hum Reprod. 2013;28:1261–1266. doi: 10.1093/humrep/det034. [DOI] [PubMed] [Google Scholar]
- 78.Siristatidis C, Sergentanis TN, Vogiatzi P, Kanavidis P, Chrelias C, Papantoniou N, et al. In Vitro maturation in women with vs. without polycystic ovarian syndrome: a systematic review and meta-analysis. PLoS One. 2015;10:e0134696. doi: 10.1371/journal.pone.0134696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Vos M, Pareyn S, Drakopoulos P, Raimundo JM, Anckaert E, Santos-Ribeiro S, et al. Cumulative live birth rates after IVF in patients with polycystic ovaries: phenotype matters. Reprod BioMed Online. 2018;37:163–171. doi: 10.1016/j.rbmo.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26:3123–3129. doi: 10.1093/humrep/der297. [DOI] [PubMed] [Google Scholar]
- 81.Mackens S, Pareyn S, Drakopoulos P, Deckers T, Mostinckx L, Blockeel C, et al. Outcome of in-vitro oocyte maturation in patients with PCOS: does phenotype have an impact? Hum Reprod. 2020;35:2272–2279. doi: 10.1093/humrep/deaa190. [DOI] [PubMed] [Google Scholar]
- 82.Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377:1657–1665. doi: 10.1056/NEJMra1614676. [DOI] [PubMed] [Google Scholar]
- 83.Rodgers RJ, Reid GD, Koch J, Deans R, Ledger WL, Friedlander M, et al. The safety and efficacy of controlled ovarian hyperstimulation for fertility preservation in women with early breast cancer: a systematic review. Hum Reprod. 2017;32:1033–1045. doi: 10.1093/humrep/dex027. [DOI] [PubMed] [Google Scholar]
- 84.Sonigo C, Bajeux J, Boubaya M, Eustache F, Sifer C, Levy V, et al. In vitro maturation is a viable option for urgent fertility preservation in young women with hematological conditions. Hematol Oncol. 2020;38:560–564. doi: 10.1002/hon.2724. [DOI] [PubMed] [Google Scholar]
- 85.Grynberg M, A Mayeur Le Bras, L Hesters, V Gallot, N Frydman. First birth achieved after fertility preservation using vitrification of in vitro matured oocytes in a woman with breast cancer. Ann Oncol. 2020. [DOI] [PubMed]
- 86.Sonigo C, Simon C, Boubaya M, Benoit A, Sifer C, Sermondade N, et al. What threshold values of antral follicle count and serum AMH levels should be considered for oocyte cryopreservation after in vitro maturation? Hum Reprod. 2016;31:1493–1500. doi: 10.1093/humrep/dew102. [DOI] [PubMed] [Google Scholar]
- 87.Sermondade N, Grynberg M, Comtet M, Valdelievre C, Sifer C, Sonigo C. Double-in vitro maturation increases the number of vitrified oocytes available for fertility preservation when ovarian stimulation is unfeasible. Sci Rep. 2020;10:18555. doi: 10.1038/s41598-020-75699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Gook DA, Walters KA, Anazodo A, Ledger WL, Gilchrist RB. Improving fertility preservation for girls and women by coupling oocyte in vitro maturation with existing strategies. Women Health. 2016;12(3):275–278. doi: 10.2217/whe-2016-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Delattre S, Segers I, Van Moer E, Drakopoulos P, Mateizel I, Enghels L, et al. Combining fertility preservation procedures to spread the eggs across different baskets: a feasibility study. Hum Reprod. 2020;35:2524–2536. doi: 10.1093/humrep/deaa193. [DOI] [PubMed] [Google Scholar]
- 90.Revel A, Koler M, Simon A, Lewin A, Laufer N, Safran A. Oocyte collection during cryopreservation of the ovarian cortex. Fertil Steril. 2003;79:1237–1239. doi: 10.1016/s0015-0282(02)04963-4. [DOI] [PubMed] [Google Scholar]
- 91.Segers I, Bardhi E, Mateizel I, Van Moer E, Schots R, Verheyen G, et al. Live births following fertility preservation using in-vitro maturation of ovarian tissue oocytes. Hum Reprod. 2020;35:2026–2036. doi: 10.1093/humrep/deaa175. [DOI] [PubMed] [Google Scholar]
- 92.Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, et al. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32:1221–1231. doi: 10.1007/s10815-015-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang JY, Buckett WM, Gilbert L, Tan SL, Chian RC. Retrieval of immature oocytes followed by in vitro maturation and vitrification: a case report on a new strategy of fertility preservation in women with borderline ovarian malignancy. Gynecol Oncol. 2007;105:542–544. doi: 10.1016/j.ygyno.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 94.Talbert LM, Raj MH, Hammond MG, Greer T. Endocrine and immunologic studies in a patient with resistant ovary syndrome. Fertil Steril. 1984;42:741–744. doi: 10.1016/s0015-0282(16)48200-2. [DOI] [PubMed] [Google Scholar]
- 95.Huhtaniemi I, Alevizaki M. Gonadotrophin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20:561–576. doi: 10.1016/j.beem.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 96.Jones GS, De Moraes-Ruehsen M. A new syndrome of amenorrhae in association with hypergonadotropism and apparently normal ovarian follicular apparatus. Am J Obstet Gynecol. 1969;104:597–600. doi: 10.1016/s0002-9378(16)34255-7. [DOI] [PubMed] [Google Scholar]
- 97.Conway GS, Conway E, Walker C, Hoppner W, Gromoll J, Simoni M. Mutation screening and isoform prevalence of the follicle stimulating hormone receptor gene in women with premature ovarian failure, resistant ovary syndrome and polycystic ovary syndrome. Clin Endocrinol. 1999;51:97–99. doi: 10.1046/j.1365-2265.1999.00745.x. [DOI] [PubMed] [Google Scholar]
- 98.Haller-Kikkatalo K, Salumets A, Uibo R. Review on autoimmune reactions in female infertility: antibodies to follicle stimulating hormone. Clin Dev Immunol. 2012;2012:762541. doi: 10.1155/2012/762541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aittomaki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–968. doi: 10.1016/0092-8674(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 100.Layman LC, Lee EJ, Peak DB, Namnoum AB, Vu KV, van Lingen BL, Gray MR, McDonough PG, Reindollar RH, Jameson JL. Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone beta-subunit gene. N Engl J Med. 1997;337:607–611. doi: 10.1056/NEJM199708283370905. [DOI] [PubMed] [Google Scholar]
- 101.Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Poor ovarian response to gonadotrophin stimulation is associated with FSH receptor polymorphism. Reprod BioMed Online. 2009;18:509–515. doi: 10.1016/s1472-6483(10)60127-7. [DOI] [PubMed] [Google Scholar]
- 102.Huang X, Li L, Hong L, Zhou W, Shi H, Zhang H, et al. The Ser680Asn polymorphism in the follicle-stimulating hormone receptor gene is associated with the ovarian response in controlled ovarian hyperstimulation. Clin Endocrinol. 2015;82:577–583. doi: 10.1111/cen.12573. [DOI] [PubMed] [Google Scholar]
- 103.Galvao A, Segers I, Smitz J, Tournaye H, De Vos M. In vitro maturation (IVM) of oocytes in patients with resistant ovary syndrome and in patients with repeated deficient oocyte maturation. J Assist Reprod Genet. 2018;35:2161–2171. doi: 10.1007/s10815-018-1317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y, Pan P, Yuan P, Qiu Q, Yang D. Successful live birth in a woman with resistant ovary syndrome following in vitro maturation of oocytes. J Ovarian Res. 2016;9:54. doi: 10.1186/s13048-016-0263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lensen SF, Wilkinson J, Leijdekkers JA, La Marca A, Mol BWJ, Marjoribanks J, et al. Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI) Cochrane Database Syst Rev. 2018;2:CD012693. doi: 10.1002/14651858.CD012693.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu J, Lu G, Qian Y, Mao Y, Ding W. Pregnancies and births achieved from in vitro matured oocytes retrieved from poor responders undergoing stimulation in in vitro fertilization cycles. Fertil Steril. 2003;80:447–449. doi: 10.1016/s0015-0282(03)00665-4. [DOI] [PubMed] [Google Scholar]
- 107.Braga DP, Figueira Rde C, Ferreira RC, Pasqualotto FF, Iaconelli A, Jr, Borges E., Jr Contribution of in-vitro maturation in ovarian stimulation cycles of poor-responder patients. Reprod BioMed Online. 2010;20:335–340. doi: 10.1016/j.rbmo.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 108.Chian RC, Lim JH, Tan SL. State of the art in in-vitro oocyte maturation. Curr Opin Obstet Gynecol. 2004;16:211–219. doi: 10.1097/00001703-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 109.Nogueira D, Sadeu JC, Montagut J. In vitro oocyte maturation: current status. Semin Reprod Med. 2012;30:199–213. doi: 10.1055/s-0032-1311522. [DOI] [PubMed] [Google Scholar]
- 110.Walls ML, Ryan JP, Keelan JA, Hart R. In vitro maturation is associated with increased early embryo arrest without impairing morphokinetic development of useable embryos progressing to blastocysts. Hum Reprod. 2015;30:1842–1849. doi: 10.1093/humrep/dev125. [DOI] [PubMed] [Google Scholar]
- 111.Rudak E, Dor J, Kimchi M, Goldman B, Levran D, Mashiach S. Anomalies of human oocytes from infertile women undergoing treatment by in vitro fertilization. Fertil Steril. 1990;54:292–296. doi: 10.1016/s0015-0282(16)53706-6. [DOI] [PubMed] [Google Scholar]
- 112.Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, et al. Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med. 2016;374:223–232. doi: 10.1056/NEJMoa1510791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hourvitz A, Maman E, Brengauz M, Machtinger R, Dor J. In vitro maturation for patients with repeated in vitro fertilization failure due to “oocyte maturation abnormalities”. Fertil Steril. 2010;94:496–501. doi: 10.1016/j.fertnstert.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 114.Strowitzki T, Bruckner T, Roesner S. Maternal and neonatal outcome and children’s development after medically assisted reproduction with in-vitro matured oocytes-a systematic review and meta-analysis. Hum Reprod Update. 2021;27:460–473. doi: 10.1093/humupd/dmaa056. [DOI] [PubMed] [Google Scholar]
- 115.Buckett WM, Chian RC, Dean NL, Sylvestre C, Holzer HE, Tan SL. Pregnancy loss in pregnancies conceived after in vitro oocyte maturation, conventional in vitro fertilization, and intracytoplasmic sperm injection. Fertil Steril. 2008;90:546–550. doi: 10.1016/j.fertnstert.2007.06.107. [DOI] [PubMed] [Google Scholar]
- 116.Mostinckx L, Segers I, Belva F, Buyl R, Santos-Ribeiro S, Blockeel C, et al. Obstetric and neonatal outcome of ART in patients with polycystic ovary syndrome: IVM of oocytes versus controlled ovarian stimulation. Hum Reprod. 2019;34:1595–1607. doi: 10.1093/humrep/dez086. [DOI] [PubMed] [Google Scholar]
- 117.Kuhtz J, Romero S, De Vos M, Smitz J, Haaf T, Anckaert E. Human in vitro oocyte maturation is not associated with increased imprinting error rates at LIT1, SNRPN, PEG3 and GTL2. Hum Reprod. 2014;29:1995–2005. doi: 10.1093/humrep/deu155. [DOI] [PubMed] [Google Scholar]
- 118.Pliushch G, Schneider E, Schneider T, El Hajj N, Rosner S, Strowitzki T, et al. In vitro maturation of oocytes is not associated with altered deoxyribonucleic acid methylation patterns in children from in vitro fertilization or intracytoplasmic sperm injection. Fertil Steril. 2015;103:720–7.e1. doi: 10.1016/j.fertnstert.2014.12.096. [DOI] [PubMed] [Google Scholar]
- 119.Roesner S, von Wolff M, Elsaesser M, Roesner K, Reuner G, Pietz J, et al. Two-year development of children conceived by IVM: a prospective controlled single-blinded study. Hum Reprod. 2017;32:1341–1350. doi: 10.1093/humrep/dex068. [DOI] [PubMed] [Google Scholar]
- 120.Soderstrom-Anttila V, T Salokorpi, M Pihlaja, S Serenius-Sirve AM Suikkari. Obstetric and perinatal outcome and preliminary results of development of children born after in vitro maturation of oocytes. Hum Reprod. 2006. [DOI] [PubMed]
- 121.Shu-Chi M, Jiann-Loung H, Yu-Hung L, Tseng-Chen S, Ming I, Tsu-Fuh Y. Growth and development of children conceived by in-vitro maturation of human oocytes. Early Hum Dev. 2006;82:677–682. doi: 10.1016/j.earlhumdev.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 122.Yu EJ, Yoon TK, Lee WS, Park EA, Heo JY, Ko YK, Kim J. Obstetrical, neonatal, and long-term outcomes of children conceived from in vitro matured oocytes. Fertil Steril. 2019;112:691–699. doi: 10.1016/j.fertnstert.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 123.Vesztergom D, Segers I, Mostinckx L, Blockeel C, De Vos M. Live births after in vitro maturation of oocytes in women who had suffered adnexal torsion and unilateral oophorectomy following conventional ovarian stimulation. J Assist Reprod Genet. 2021. [DOI] [PMC free article] [PubMed]
- 124.Paulson RJ, Fauser B, Vuong LTN, Doody K. Can we modify assisted reproductive technology practice to broaden reproductive care access? Fertil Steril. 2016;105:1138–1143. doi: 10.1016/j.fertnstert.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 125.Braam SC, Ho VNA, Pham TD, Mol BW, van Wely M, Vuong LN. In-vitro maturation versus IVF: a cost-effectiveness analysis. Reprod BioMed Online. 2021;42:143–149. doi: 10.1016/j.rbmo.2020.09.022. [DOI] [PubMed] [Google Scholar]
- 126.Rose BI, Laky D, Miller B. The case for in vitro maturation lower cost and more patient friendly. J Reprod Med. 2014;59:571–578. [PubMed] [Google Scholar]
- 127.Rose BI. Approaches to oocyte retrieval for advanced reproductive technology cycles planning to utilize in vitro maturation: a review of the many choices to be made. J Assist Reprod Genet. 2014;31:1409–1419. doi: 10.1007/s10815-014-0334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bertoldo MJ, Smitz J, Wu LE, Lee HC, Woodruff TK, Gilchrist RB, et al. Trends Endocrinol Metab. 2020;31:708–711. doi: 10.1016/j.tem.2020.07.004. [DOI] [PubMed] [Google Scholar]