Abstract
Insufficiency of oocyte activation impairs the subsequent embryo development in assisted reproductive technology (ART). Intracellular Ca2+ concentration ([Ca2+]i) oscillations switch the oocytes to resume the second meiosis and initiate embryonic development. However, the [Ca2+]i oscillation patterns in oocytes are poorly characterized. In this study, we investigated the effects of various factors, such as the oocytes age, pH, cumulus cells, in vitro or in vivo maturation, and ER stress on [Ca2+]i oscillation patterns and pronuclear formation after parthenogenetic activation of mouse oocytes. Our results showed that the oocytes released to the oviduct at 17 h post-human chorionic gonadotrophin (hCG) displayed a significantly stronger [Ca2+]i oscillation, including higher frequency, shorter cycle, and higher peak, compared with oocytes collected at earlier or later time points. [Ca2+]i oscillations in acidic conditions (pH 6.4 and 6.6) were significantly weaker than those in neutral and mildly alkaline conditions (pH from 6.8 to 7.6). In vitro-matured oocytes showed reduced frequency and peak of [Ca2+]i oscillations compared with those matured in vivo. In vitro-matured oocytes from the cumulus-oocyte complexes (COCs) showed a significantly higher frequency, shorter cycle, and higher peak compared with the denuded oocytes (DOs). Finally, endoplasmic reticulum stress (ER stress) severely affected the parameters of [Ca2+]i oscillations, including elongated cycles and lower frequency. The pronuclear (PN) rate of oocytes after parthenogenetic activation was correlated with [Ca2+]i oscillation pattern, decreasing with oocyte aging, cumulus removal, acidic pH, and increasing ER stress. These results provide fundamental but critical information for the mechanism of how these factors affect oocyte activation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02100-9.
Keywords: Oocytes, Parthenogenesis, Calcium oscillations, Endoplasmic reticulum stress, In vitro oocyte maturation techniques
Introduction
Approximately 9–15% of couples worldwide suffer from infertility, and the number is rising steadily [1]. Human assisted reproductive technology (ART) can help infertile couples [2]. Intracytoplasmic sperm injection (ICSI) is the most effective technique used for patients with failed fertilization after conventional in vitro fertilization (IVF) [3]. However, fertilization failure after ICSI still occurs in many cases [4]. Oocyte activation failure is one of the primary reasons for the poor fertilization after ICSI [5]. Assisted oocyte activation is often used to treat patients with low ICSI fertilization rate or fertilization failure [6]. Thus, it is critical to understand the factors affecting the activation process of oocytes.
Under natural conditions, the oocyte is activated by sperm, which further triggers the periodical fluctuation of intracellular calcium concentration ([Ca2+]i) [7]. It is found that the enhanced [Ca2+]i oscillations are closely associated with the activation of oocytes. For example, downregulation of mitogen-activated protein kinase and pronucleus formation is highly correlated with the frequency of [Ca2+]i oscillations [8–10]. In addition, a study by Yazawa et al. on the measurements of Ca2+ oscillations revealed that repetitive spike-shaped [Ca2+]i increases in response to oocyte activation with intervals at 2–10 min [11]. Intracellular Ca2+ oscillations are mediated by inositol 1,4,5-trisphosphate (IP3) [12] and protein kinase C (PKC), decreasing the level of the maturation promoting factor (MPF) for the resumption of meiosis [13] and initiation of embryonic development. [Ca2+]i oscillations affect oocyte activation and subsequent developmental potential of embryos [14]. As reported by Ducibella et al. [14], cellular events depend on the level or duration of an [Ca2+]i-dependent enzyme activity. Ozil et al. [8] also revealed that the process of oocyte activation by Ca2+ stimulation determines several developmental events such as pronuclear formation, embryo compaction, and blastocyst formation. Clinically, when the oocyte is insufficiently activated during ICSI, assisted oocyte activation can be used to artificially activate the oocytes by facilitating the influx of Ca2+, improving reproductive outcomes [8]. The importance of Ca2+ activity in oocyte activation and embryo development provides a promising therapeutic possibility to rescue failed fertilization after ICSI. Oocytes can be activated artificially in a variety of ways. There are physical and chemical methods for parthenogenetic activation of mouse oocytes. Physical methods include electrical, temperature, and mechanical stimulation. The most widely used method is electrical stimulation that is simple, easy, and controllable. Ozil et al. [8] studied the relationship between an electric pulse and oocyte activation by controlling the electric pulse’s intensity and frequency. Chemical methods [15] such as ion modulators and protein synthesis inhibitors, osmotic pressure stimulation were also used. The parthenogenetic activation method used in this experiment is to activate mouse oocytes with strontium chloride (SrCl2). We observed the effect of different treatments on the calcium oscillation patterns of oocytes activated via parthenogenetic activation. Strontium ion is a divalent cation similar to calcium ion and is not consumed during cell development. Parthenogenetic activation provides a unique method to study the early development of oocytes during fertilization without the male gamete’s contribution. It can be accomplished by exposing unfertilized oocytes to a strontium-based culture medium to observe the biochemical and morphological changes [16]. By using parthenogenetic activation, we can accurately record the timing of Ca2+ changes, while we cannot accurately record these changes when employing ICSI and IVF, which are difficult to make activation of all examined oocytes synchronously. It is, therefore, an ideal in vitro model for studying [Ca2+]i oscillations during fertilization.
Many factors affect the efficiency of oocyte activation and embryo development [17]. The age of a post-ovulatory oocyte affects its developmental capacity, and a series of intracellular signaling pathways are involved in the post-hCG injection aging process. Conformational changes in endoplasmic reticulum (ER) and mitochondria occur naturally in aging cells, which impacts their function in supplying ATP for Ca2+ pumps in the ER membrane [18, 19]. The ER functions like a factory for protein synthesis and serves as an intracellular Ca2+ pool to maintain the [Ca2+]i. Glucose-regulated protein 78 (GRP78) and Chop gene expression are two important regulatory factors in ER stress reaction. When ER stress inducers are present, GRP78 will be upregulated to ameliorate the cell stress. Meanwhile, GRP78 dissociates with phosphor-extracellular-signal-regulated kinase (pERK). The free pERK will then be translocated into the nucleus to trigger the expression of Chop gene to induce programmed apoptosis [20, 21]. The production, maturation, and activation of oocytes require proper pH in the environment. Although oocytes can maintain the balance of intracellular pH in response to the change of environment to a certain degree, highly acidic or alkaline conditions significantly increases cell death rate and decreases fertilization efficiency [22]. This evidence suggests that oocytes are particularly sensitive to the pH in the environment. A study demonstrates that oocytes tightly attaching to cumulus cells can promote the quality of matured oocytes, which will further improve the fertilization and embryo development [23].
Nevertheless, whether and how [Ca2+]i oscillations are affected by these factors during maturation and activation of oocytes remains mostly unknown. In our study, considering the limited availability of human oocytes, we utilized parthenogenetic mouse oocytes to perform systematic recordings of the [Ca2+]i oscillation patterns in different conditions mentioned above. We found that [Ca2+]I oscillation patterns changed in oocytes in a time-dependent manner during development. The strongest [Ca2+]i oscillation pattern occurred at 17 h post-hCG injection. Furthermore, multiple factors, including culture medium, oocyte age, pH change, oocyte maturation in vivo or in vitro, presence of cumulus cells, and ER stress, can impact [Ca2+]i oscillation patterns during oocyte activation. These results provide fundamental, and critical information to better understand how these factors affect oocyte activation and the percentage of pronuclei formed, which could positively correlate with the subsequent embryo development [24].
Materials and methods
Animals
ICR mice (6–8 weeks old; female; Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) were maintained under a 12/12-h light/dark cycle with standard mouse diet and water at the laboratory animal center of the institute.
Oocyte collection and in vitro maturation
All oocytes collected from mouse oviducts were incubated at 37 °C with 5% CO2 and 95% air. For studying the effects of post-ovulatory age of oocyte on [Ca2+]i oscillation patterns, mice were super-ovulated by injecting 10 IU of pregnant mare serum gonadotropin (PMSG, Ningbo Hormone Product Co. Ltd., Cixi, China), and 44 h later 10 IU of hCG (Ningbo Hormone Product Co. Ltd., Cixi, China). Five groups of ovulated COCs were collected from oviducts by blunt dissection at 13, 15, 17, 19, and 21 h after the hCG administration. The COCs were denuded in 1 mg/ml hyaluronidase (Sigma-Aldrich, USA). Twenty-five to 30 oocytes were distributed in each experimental group described below.
For studying the effects of surrounding cumulus presence on [Ca2+]i oscillation patterns during in vitro oocyte maturation, the COCs and denuded oocytes were collected from mice stimulated with 10 IU PMSG after 44 h. The COCs and denuded germinal vesicle (GV) oocytes were cultured in M2 culture medium (M7167, Sigma-Aldrich, USA) covered with sterile mineral oil (M8410, Sigma-Aldrich, USA) for 17 h. The COCs were then denuded in 1 mg/ml hyaluronidase before parthenogenetic activation.
To study the effect of ER stress on [Ca2+]i oscillation patterns, female mice were intraperitoneally injected with 10 IU of PMSG followed by 10 IU hCG treatment 44 h later. After 15.5 h, the mice were sacrificed by cervical dislocation, and the oocytes were collected following the identical procedure described above and discharged into M2 solution. The ER stress inducer tunicamycin (TM, Greensky, China) at concentrations of 0, 2.5, 5, 10, 20, 30, and 50 μg/ml dissolved in M2 medium was added to M2 medium in which oocytes were cultured for 1.5 h before parthenogenetic activation. Control groups received an equal volume of M2 medium treatment.
To study the effect of gradient pH medium on [Ca2+]i oscillation patterns, oocytes collected 17 h post-hCG treatment were placed in SrCl2 medium (10025-70-4, Sangon Biotech, Shanghai) in Ca2+-free Chatot Ziomek Bavister (CZB) medium with the pH adjusted to pH 6.8, pH 7.0, pH 7.2, pH 7.4, and pH 7.6 by NaOH and HCl. The pH was monitored with a pH meter (Horiba, B-212, Kyoto, Japan). Control groups were treated with an equal amount of Ca2+ -free CZB medium.
Parthenogenetic activation
To induce parthenogenetic activation, the oocytes were incubated in 10 mM SrCl2 for 3.5 h activation. The oocytes were then cultured in potassium simplex optimized medium (KSOM) (Sigma-Aldrich, USA) after activation. After 1 h, the pronucleus (PN) formation ratio was observed.
Real-time [Ca2+]i recording
To record Ca2+ changes in cultured cells, Fluo-4AM fluorescent agent (2.5 μM, Beyotime) was added to the culture medium to visualize Ca2+ signals. Oocyte [Ca2+]i oscillation images were obtained every 20 s and captured for up to 3.5 h using a time-lapse confocal laser microscope (UltraVIEW-VoX; Perkin-Elmer Life Sciences, Waltham, MA, USA). The fluorescent signal was collected using an argon laser at 488 nm (Fig. S1). The peak of Ca2+ concentration, Ca2+ move speed, and each cycle’s duration were analyzed for each group. As shown in Supplemental Figure S1, we measured the maximum fluorescent intensity to obtain the peak value. The relative peak was calculated by subtracting the baseline intensity from the peak. The cycle of [Ca2+]i oscillation was defined as the interval between two adjacent peaks. The change of fluorescent intensity every 20 s was used to calculate the Ca2+ moving speed. The difference of amplitude between two adjacent peaks was measured to calculate the Δpeak.
RNA isolation and RT-PCR
The GRP78 and Chop genes relative mRNA levels were assessed by quantitative real-time PCR (qRT-PCR). Total RNA was isolated from 100 oocytes using the RNeasy Micro kit (QIAGEN, Valencia, CA) according to the manufacturer’s instruction. RNA was reverse-transcribed with the Fast Quant RT Kit (Tiangen Biotech Co., LTD, Beijing, China). The oligonucleotide sequences of the primers used for qRT-PCR were GRP78 forward (F) 5'-ACT TGG GGA CCA CCT ATT CCT-3' and reverse (R) 5'-ATC GCC AAT CAG ACG CTC C-3', Chop F 5'-CTG GAA GCC TGG TAT GAG GAT-3' and R 5'-CAG GGT CAA GAG TAG TGA AGG T-3', and GAPDH F 5'-CCC CAA TGT GTC CGT CGT G-3' and R 5'-TGC CTG CTT CAC CAC CTT CT-3'. Real-time PCR was carried out using the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and a real-time thermocycler under the following conditions: (1) 3 min at 95 °C; (2) 40 cycles of 15 s at 95 °C, 20 s at 60 °C, and 20 s at 72 °C; and (3) 5 min at 72 °C. The relative gene expression was determined using the 2ΔΔCt method normalized to GAPDH.
Statistical analysis
All data were analyzed using SPSS V22.0 software (IBM, Armonk, NY, USA). Data were shown in the format of mean ± standard deviation. The differences in relative and PN rates among all groups were assessed using the chi-square test. One-way ANOVA was used to analyze the relative expression difference in all groups with the LSD post hoc test. Error bars indicate standard deviations. P-values < 0.05 were considered statistically significant. All experiments were repeated at least three times independently.
Results
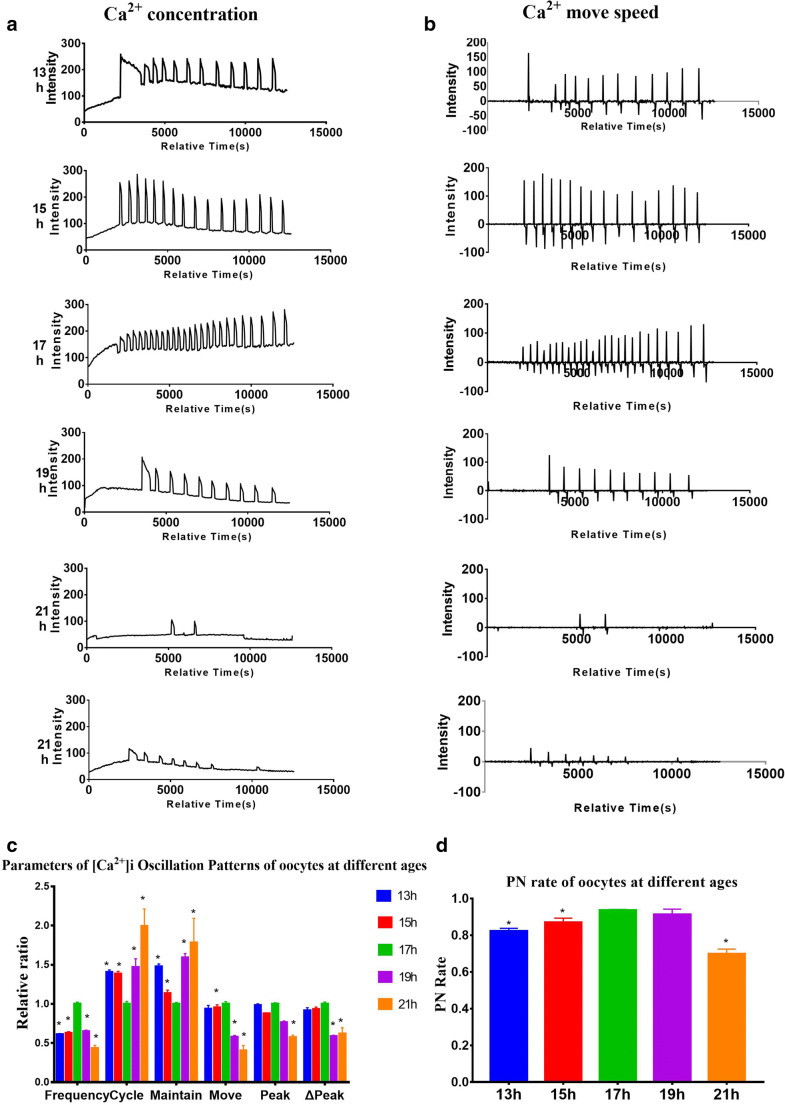
[Ca2+]i oscillation patterns of oocytes collected at 17 h post-hCG showed a higher frequency and shorter cycle than the other groups
To examine the [Ca2+]i oscillation pattern changes in oocytes during the activation of oocytes, we measured the fluorescent intensity of Ca2+ signals, which reflects the intracellular Ca2+ concentration, cycle frequency, and duration, Ca2+ speed, peak, and Δpeak at 13, 15, 17, 19, and 21 h post-hCG after parthenogenetic activation of mouse oocytes. As shown in Fig. 1a, the level of Ca2+ concentration gradually increased from 13 to 17 h post-hCG activation, following by a dramatic decrease from 19 to 21 h. Similarly, the measurement of Ca2+ move speed was firstly increased to the highest level at 17-h time point, then gradually decreased at the following time points (Fig. 1b). For statistical analyses, we further normalized the values of various parameters including frequency, cycle, maintain, move, peak, and Δpeak in oocytes collected at 13, 15, 19, and 21 h post-hCG to those in oocytes collected at 17 h post-hCG (Fig. 1c). We found that [Ca2+]i oscillation patterns of the oocytes collected at 17 h post-hCG showed a higher frequency and shorter cycle than the other groups (P < 0.05) (Fig. 1c). The [Ca2+]i oscillations of the 21 h post-hCG group were the weakest among all time points (Fig. 1c), showing as the lowest frequency (0.43 ± 0.04, P < 0.05), longest cycle (1.99 ± 0.22, P < 0.05), and smallest peak (0.57 ± 0.03, P < 0.05). Only a 2-h difference post-hCG caused a significant difference in the frequency and cycle, whereas there was no significant difference in the maintain, move, peak, and Δpeak (Fig. 1c). As shown in Fig. 1c, post-ovulatory aging oocytes collected at 21 h post hCG failed to maintain long-term high-intensity Ca2+ oscillations.
Fig. 1.
Intracellular free calcium ([Ca2+]i) oscillation patterns after parthenogenetic activation in oocytes collected at different times post-human chorionic gonadotropin (hCG) injection. a, b [Ca2+]i oscillation patterns at 13-21-h post-hCG. c Relative [Ca2+]i oscillation parameters including “frequency,” “cycle,” “maintain,” “move,” “peak,” and “Δpeak” for statistical analyses; each ratio of different groups was compared with the 17-h group. d Rates of pronucleus (PN) formation of oocytes collected at different post-hCG time points after parthenogenetic activation. N = 25 to 30 for each group. Data represent mean ± SD, and chi-square test and one-way ANOVA are used for statistical analysis. *P < 0.05 versus the 17-h group
To test whether the difference of SrCl2-induced oscillations observed in mouse oocytes with different post-hCG times affected the PN rate, we recorded the PN rates of the different groups. The highest PN rate was found in the 17 h post-hCG group (93.7 ± 0.2%), while there was no significant difference in the PN rates between the 17 h and 19 h post-hCG (91.3 ± 2.3%, P > 0.05) (Fig. 1d). The PN rate of the 13 h post-hCG group (82.3 ± 1.2%, P < 0.05) and 15 h post-hCG group (87.0 ± 1.9%, P < 0.05) was lower than that of the 17 h post-hCG group. The 21 h post hCG group’s PN rate was also significantly lower compared to the 17 h post-hCG group. Also, some embryos developed abnormally in the 21 h post-hCG group (data not shown). These results show that the [Ca2+]i changes followed an inverted U pattern after hCG activation, which matches the time course of pronuclear formation, suggesting that post-ovulatory oocyte age correlates with the intracellular Ca2+ activity, which is important for the activation of oocytes.
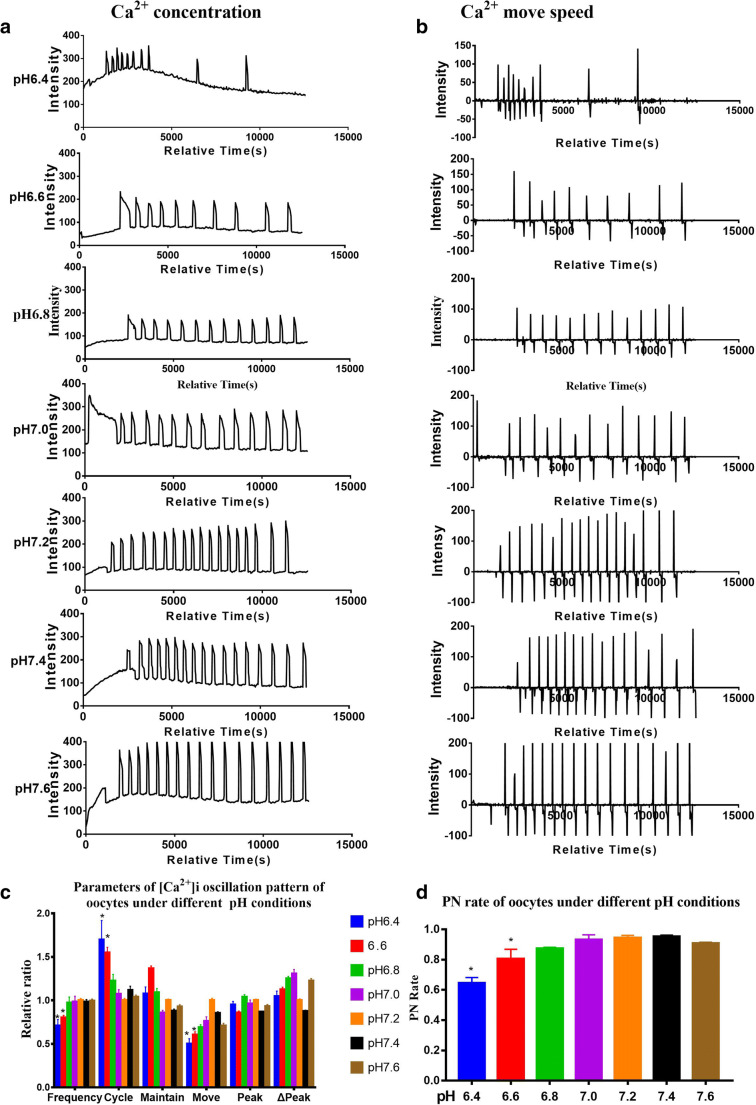
The [Ca2+]i oscillations of the pH 7.2 group showed the highest frequency and shortest cycle
We investigated the effects of pH values on [Ca2+]i oscillation patterns in oocytes collected at 17 h post-hCG during parthenogenetic activation. Ca2+ signals were recorded for 3.5 h (Fig. 2a, b) while the pH value was continuously measured by a pH meter, which was confirmed to be within 0.1 of standard error.
Fig. 2.
Intracellular free calcium ([Ca2+]i) oscillation patterns during parthenogenetic activation under different pH conditions at 17 h post-hCG. a, b [Ca2+]i oscillation patterns after parthenogenetic activation in gradient pH conditions from 6.4 to 7.6. c Relative [Ca2+]i oscillation parameters in gradient pH conditions in parthenogenetic activation medium. Each group was compared with the pH 7.2 group. The [Ca2+]i oscillation parameters, “frequency” and “cycle,” were significantly different between pH 7.2 and pH 6.4 or pH 6.6 groups. d Pronucleus (PN) rate in gradient pH conditions of the parthenogenetic activation system. PN ratios of pH 6.4 and pH 6.6 treatments were significantly lower compared with the other groups. N = 25 to 30 for each group. Data represent mean ± SD, and chi-square test and one-way ANOVA are used for statistical analysis. *P < 0.05 versus pH 7.2 group
The [Ca2+]i oscillations of the pH 7.2 group showed the highest frequency and the shortest cycle (Fig. 2c). There were no significant differences among pH 6.8, pH 7.0, pH 7.2, pH 7.4, and pH 7.6 groups (Fig. 2c). The [Ca2+]i oscillation patterns of the pH 6.4 (Fig. 2a) and pH 6.6 (Fig. 2c) groups showed significantly lower frequency and longer cycle compared to that of the pH 7.2 group.
We counted the PN formation rates in different pH conditions to explore whether the activation medium’s pH influences the PN formation rate. The PN rates among the groups of pH 6.8, pH 7.0, pH 7.2, pH 7.4, and pH 7.6 were not significantly different (all P > 0.05), ranging from 86.4 to 96.9% (Fig. 2d). The PN rate in the pH 6.4 (64.5 ± 3.0%, P < 0.05) or pH 6.6 (80.1 ± 5.2%, P < 0.05) group was significantly lower compared to that of the pH 7.2 group (Fig. 2d).
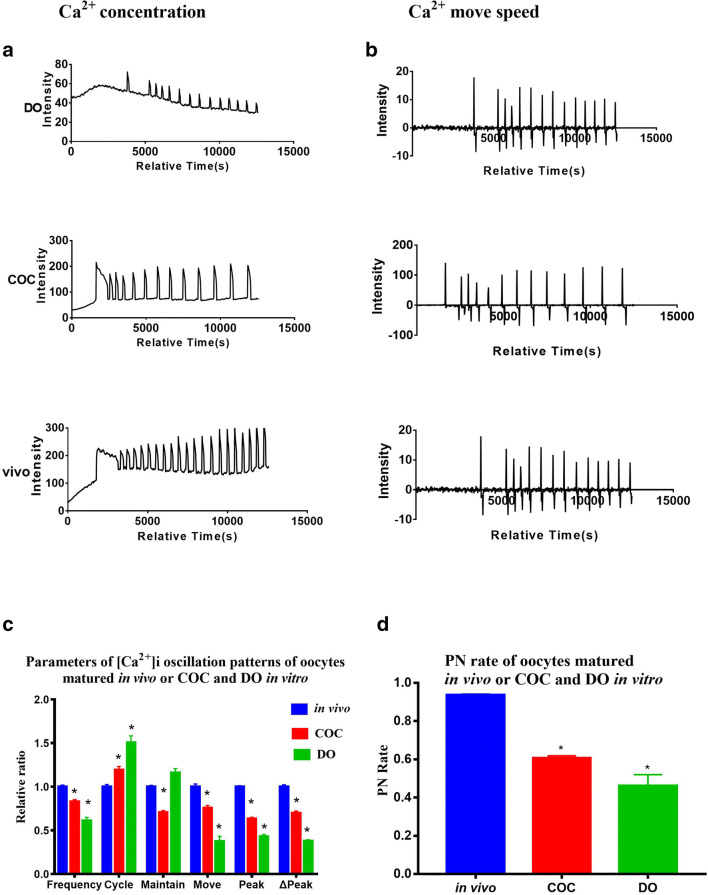
The [Ca2+]i oscillations of the COCs group showed significantly higher frequency, shorter cycle, and higher peak
We compared the [Ca2+]i oscillations after parthenogenetic activation of the oocytes matured in vitro. We explored whether there was a difference in [Ca2+]i oscillations between oocytes derived from COCs and DOs matured in vitro and COCs matured in vivo (Fig. 3a, b).
Fig. 3.
Intracellular free calcium ([Ca2+]i) oscillation patterns of in vivo oocytes and in vitro matured cumulus-denuded oocytes (DOs) and cumulus-enclosed oocyte complexes (COCs) in vitro-matured for 17 h. a, b [Ca2+]i oscillation patterns after parthenogenetic activation. c Relative [Ca2+]i oscillation parameters of oocytes matured in vivo or in vitro. The in vivo group was compared with the in vitro groups. d Pronucleus (PN) rate of in vivo and in vitro matured oocytes. N = 25 to 30 for each group. Data represent mean ± SD, and chi-square test and one-way ANOVA are used for statistical analysis. *P < 0.05 versus the control group
The [Ca2+]i oscillations of the COCs group showed significantly higher frequency (1.36 ± 0.04, P < 0.05), shorter cycle (0.79 ± 0.02, P < 0.05), and higher peak (1.47 ± 0.04, P < 0.05) than the DOs group (Fig. 3c). Moreover, the PN rate of the COCs group (60.6 ± 1.0%) was significantly higher when compared to the DOs group (46.2 ± 4.7%, P < 0.05), but both in vitro groups had lower PN rates than the in vivo group (Fig. 3d). The frequency and peak of the [Ca2+]i oscillations (Fig. 3c) in the in vitro groups were lower than the in vivo group.
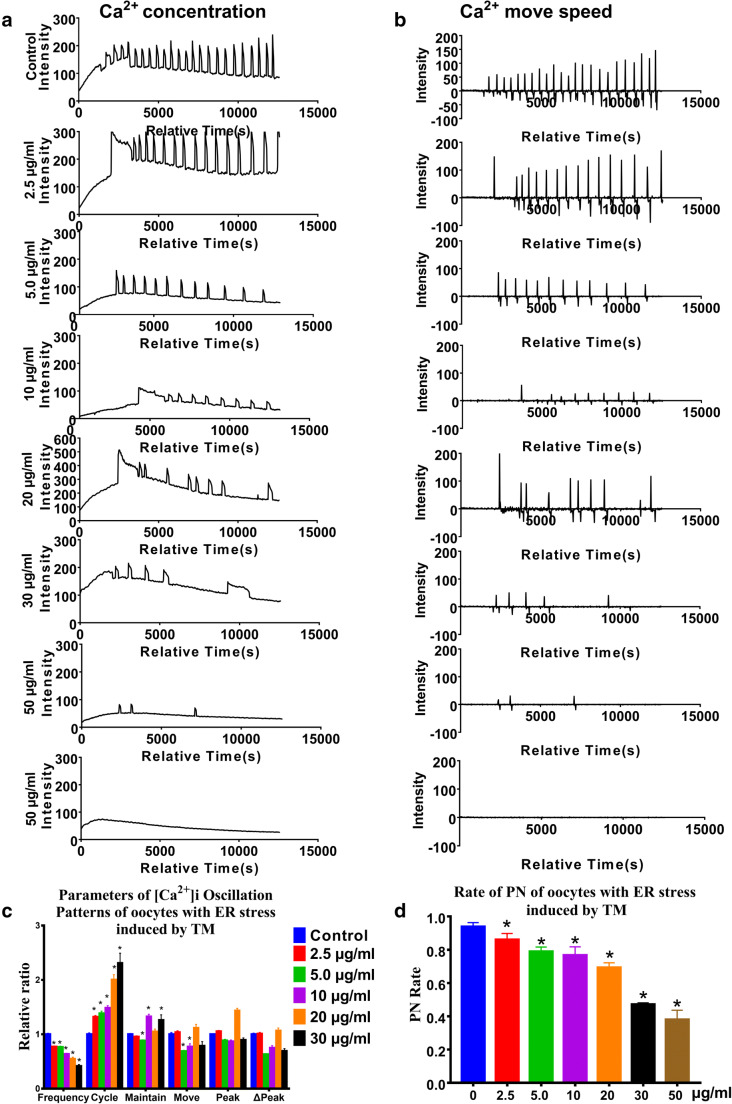
[Ca2+]i oscillations of the ER stress group showed significantly lower frequency and elongated cycle
To determine whether ER stress was induced by TM in mouse oocytes, qRT-PCR was performed to examine the mRNA level of gene GRP78 and gene Chop in the different groups, two classic ER stress markers. Oocytes treated with TM showed significantly higher mRNA levels of GRP78 and Chop compared with the control group (Supplemental Figure S2). The results indicated that the 10 μg/ml TM group’s oocytes had the highest expression levels of ER stress markers.
We observed that TM treatment affected [Ca2+]i oscillations in a dose-dependent manner, including elongated cycles and lower frequency (Fig. 4a–c). The effect became more prominent, with an increase in TM concentrations. Treatment with TM at 5–30 μg/ml severely affected the parameters of [Ca2+]i oscillations. In contrast, treatment with 50 μg/ml TM resulted in the near disappearance of [Ca2+]i oscillations, and some of the oocytes lysed. Correspondingly, the PN rate linearly decreased with increasing TM concentrations (Fig. 4d).
Fig. 4.
Intracellular free calcium ([Ca2+]i) oscillation patterns after parthenogenetic activation of oocytes treated with the endoplasmic reticulum stress inducer tunicamycin (TM). a, b Oocytes were treated with tunicamycin (TM) for 1.5 h. The 0-μM group was the control group, and 2.5, 5, 10, 20, 30, and 50 μg/ml TM were respectively added to the M2 medium. c [Ca2+]i oscillation parameters in oocytes treated with gradient TM concentrations ranging from 0 to 50 μg/ml. d The pronucleus (PN) rate of oocytes with ER stress induced by TM. N = 25 to 30 for each group. Data represent mean ± SD, and chi-square test and one-way ANOVA are used for statistical analysis. *P < 0.05 versus the control group
Discussion
Oocyte activation and embryo development are affected by many factors such as culture medium, oocyte age, pH change, and oocyte maturation in vivo or in vitro, the presence/absence of cumulus cells, and environmental stress [17]. A large number of studies have found that the enhanced [Ca2+]i oscillations are associated with the activation of oocytes. Whether and how [Ca2+]i oscillations are affected by these factors during maturation and activation of oocytes remains mostly unknown. To address this question, we utilized parthenogenetic mouse oocytes in this study to perform systematic recordings of the [Ca2+]i oscillation patterns in different conditions mentioned above. We found that [Ca2+]i oscillation patterns changed in oocytes in a time-dependent manner during development. The strongest [Ca2+]i oscillation pattern appeared 17 h post-hCG injection. Meanwhile, multiple factors, including culture medium, oocyte age, pH change, and oocyte maturation in vivo or in vitro, cumulus cells, and ER stress, can impact the [Ca2+]i oscillation during oocyte activation.
Post-ovulatory age and [Ca2+]i oscillations pattern in oocytes
An early study by Kubiak et al. [25] found that oocytes collected at 12 h post-hCG were difficult to be activated by SrCl2, suggesting that oocytes need sufficient time to reach post-ovulatory maturation for subsequent development. Ma et al. [26] reported that the blastocyst rate of oocytes collected at 18 h post-hCG was the highest after being activated by SrCl2. Recent studies found that the enhanced [Ca2+]i oscillations are associated with the activation of oocytes. For example, downregulation of mitogen-activated protein kinase and pronucleus formation is positively correlated with the frequency of [Ca2+]i oscillations [8–10]. Our results showed that the oocyte activation capacity and [Ca2+]i oscillation pattern was regulated by post-ovulatory time. The [Ca2+]i oscillations of fully mature oocytes (17 h group) showed the highest frequency, smallest cycle, and largest peak, suggesting a higher degree of oocytes activation of 17 h post hCG injection, which may further lead to a higher PN rate. The mechanism underlying the higher rate of PN is still unclear. We speculate that it might due to the cytoplasm of the oocytes collected at 17 h post-hCG being fully mature [27]. The Ca2+ channel receptors’ sensitivity might be increased in oocytes collected at 17 h post-hCG [28]. Furthermore, the decline of proteins needed for metaphase II arrest might make the Ca2+ signal more effective in the fully mature oocytes, and the Ca2+ pump of the ER is more frequent in the fully mature oocytes [29].
High rates of cell fragmentation and death are observed in aged oocytes, and some of the surviving embryos exhibit birth defects [30]. Indeed, changes in chromosomal segregation errors and mitochondrial DNA arrangements have been reported in aged oocytes [31]. This study reported a maternal age-related deterioration in [Ca2+]i oscillations in oocytes collected 21 h post-hCG. The study suggests that the aged oocytes cannot properly handle Ca2+ loading into the cytosol, resulting in a dramatic decrease of Ca2+ concentration, reduced [Ca2+]i oscillation frequency and Ca2+ moving speed, and prolonged cycle. These may further lead to failure to produce periodic [Ca2+]i oscillations.
Furthermore, the mitochondrial function is impaired, and ATP gradually decreases due to age-related oxidative stress [32]. As a result, mitochondria provide insufficient energy for Ca2+ oscillations and subsequent development. These results might have guiding significance for fertilization time to improve the pregnancy rates of IVF-ET. For patients with repeated failure of oocyte activation, the fertilization rate might be improved by appropriately prolonging the of time window for collecting oocytes post hCG injection. This will allow higher frequency and concentration of intracellular Ca2+ during oocyte “capacitation,” improving oocyte activation and fertilization. We should avoid using aging oocytes. Bjercke et al. [33] reported that for some patients with unexplained fertilization failure and poor embryo quality and repeated failure, the cleavage rate and embryo quality of oocyte retrieval at 38 h after hCG injection were higher than that of the 36-h group. This may provide a temporal-scale reference for prolonging the time window to collect oocytes from patients in clinical cases. Nevertheless, a large-scale, prospective, randomized controlled study is still needed to determine the specific limit of prolonged fertilization time.
Acidic pH alters [Ca2+]i oscillations pattern
The pH critically influences every biological process at the cellular, tissue, and whole-body level. Maintaining normal pH in an intracellular environment sets the foundation for regulating tissue and whole-body function [30]. It has been reported that the optimal pH of whole embryo culture ranges from 7.5 to 8.2, while in vitro culture medium falls between 7.2 and 7.4 [34]. This evidence suggests that oocytes are particularly sensitive to an acidic environment. By exposing the oocytes to a conditioned medium under different pH conditions, we found that the frequency of [Ca2+]i oscillations increased in a neutral or slightly alkaline pH medium, suggesting a mildly alkaline or neutral medium was beneficial to the success of parthenogenetic activation. It has been reported that the elevation of the medium pH may inhibit plasma membrane Ca2+-ATPase, which leads to the frequent InsP3-induced Ca2+ transients [35]. It is widely known that an acidic pH can suppress the cell cycle [36]. The mammalian oocytes can maintain pH homeostasis within certain ranges through Na+/H+ and HCO3-/Cl- exchangers against acidosis and alkalosis, respectively [37]. Therefore, [Ca2+]i oscillations and PN rate of oocytes exposed in pH ranging from 6.8 to 7.6 was not significantly changed compared with control group. However, an acidic environment reduces the success of parthenogenetic activation. Our study demonstrates the importance of stable pH values of the activation solution and CO2 concentration of the incubator during oocyte activation in clinical practice [34, 38]. Too high a concentration of CO2 in the incubator will reduce the pH value and thus affects the effective oscillation of Ca2+ oscillations and oocyte activation [39, 40].
IVM oocytes shows reduced [Ca2+]i oscillation capacity
We next explored the difference between oocytes matured in vivo or in vitro. In vitro maturation (IVM) of oocytes is an important option in the clinic. However, there are several disadvantages compared with in vivo maturation, including poor oocyte quality and poor embryo development [35]. A previous study also revealed that in vivo matured oocyte exhibits a specific pattern of calcium oscillations affected by in vitro maturation, in vitro aging, and cryopreservation [41]. Our study alluded that the in vitro matured oocytes’ PN rate was lower compared to that of the ovulated oocytes. The decreased activation was related to the reduced frequency and peak of [Ca2+]i oscillations after parthenogenetic activation. This could explain why the average fertilization rate and pregnancy rate of oocytes matured in vitro are reduced clinically [42].
Cumulus cells improve oocyte capacity to produce [Ca2+]i oscillations
Cumulus cells play an important role in oocyte maturation. They are important in resuming the meiosis of oocytes [21] and facilitating the development of oocytes through close communication [43]. In this study, the PN rate of the COCs group significantly increased compared to the DOs group. Moreover, the [Ca2+]i oscillations of the oocyte in the COCs group showed significantly higher frequency, shorter cycle, and higher peak than the DOs group. The cumulus cells benefit oocyte maturation in vitro, as reported in a previous study [39]. Cumulus cells provide nutrition and directly regulate oocyte metabolism by supplying energy via gap junctions, required for [Ca2+]i oscillations in oocytes [40]. If the gap junctions are damaged prematurely, oocytes may develop into poor-quality embryos [21]. Thus, cumulus cells during IVM culture are important for producing normal [Ca2+]i oscillations and improving oocyte quality.
ER stress affects [Ca2+]i oscillations pattern
Oocytes and embryos in vitro are vulnerable to external stresses such as shearing, temperature changes, altered pH, and higher oxygen pressure [43]. The endoplasmic reticulum is a primary intracellular calcium storage compartment that plays a vital role in maintaining Ca2+ homeostasis [44, 45]. IP3Rs expressed on the ER membranes are physically and functionally linked with voltage-dependent anionic channels (VDAC) located on the mitochondria through GRP75 [46]. The IP3R-GRP75-VDAC directly transfer Ca2+ between ER and mitochondria to maintain Ca2+ homeostasis [47]. It has been reported that TM-induced ER stress caused a disturbance in the Ca2+ equilibrium due to a decrease in IP3R-GRP75-VDAC interactions [48]. Our study treated oocytes with TM to induce ER stress and explore the relation between ER stress and [Ca2+]i oscillations. We showed that ER stress affected oocyte parthenogenetic activation and embryo development through impairment of mouse oocyte [Ca2+]i oscillations. The oocytes with EM stress of varying degrees showed disrupted [Ca2+]i oscillations pattern and reduced PN rate after parthenogenetic activation. Therefore, during the ART procedures, reducing ER stress of oocytes could be one of the critical factors for the oocytes’ successful activation.
GRP78 and Chop gene expression are two important regulatory factors in the ER stress reaction. When endoplasmic reticulum stress occurs, the expression of GRP78 will be increased to alleviate ER stress response and maintain ER homeostasis. GRP78 is isolated from pERK, IRE1 (inositol essential enzyme-1), and ATF6 (activating transcription factor 6) under ER stress and binds unfolded folding protein in ER to regulate calcium ion [43]. Therefore, GRP78 is an important regulator in endoplasmic reticulum stress. When external stimulation exceeds ER stress, it activates the apoptosis signal. Multiple studies found that when ER stimulation exceeds the self-regulatory range, Chop gene expression will be significantly increased and transferred to the nucleus, inducing apoptosis [44]. Thus, pERK plays a crucial role in the expression of Chop gene. When the ER stress is prolonged, pERK is isolated from GRP78, resulting in the phosphorylation of eIF2 α (eukaryotic initiation factor 2 α), thus upregulating the expression of Chop gene [21]. The pERK signaling pathway can promote the high expression of Chop gene and reverse the survival signal to apoptosis signal. Simultaneously, after ER stress, other factors can also promote Chop gene activation, thus promoting oocyte apoptosis [21, 49]. In our study, utilizing RT-qPCR, we found a dramatic increase of GRP78 and Chop gene expression in responding to TM treatment at 10 ug/ml and above. The upregulation of GRP78 might function to ameliorate the stress reaction in cells. The elevated Chop expression was likely a consequence of the dissociation of the pERK with GRP78. The free pERK will then be translocated into the nucleus to trigger Chop gene expression to induce programmed apoptosis, a protective physiological process in response to stress.
In conclusion, we found that various factors influence oocyte parthenogenetic activation and embryo development through impairment of mouse oocyte [Ca2+]i oscillations. [Ca2+]i oscillations of 17-h post-hCG oocytes in a pH of 6.8 to 7.6 showed the highest frequency and the shortest cycle. The presence of cumulus cells is beneficial to producing normal [Ca2+]i oscillations. In contrast, the frequency and peak of the [Ca2+]i oscillations of oocytes matured in vitro were not as high as in the oocytes matured in vivo. ER stress severely affected the parameters of [Ca2+]i oscillations, including elongated cycles and lower frequency. Continuing to develop a better system by optimizing the culture environment via precise control of pH, cumulus cells, oocyte age, and excluding ER stress inducers is essential for a higher success rate of in vitro oocyte parthenogenetic activation. These findings may have important implications for improving human ART.
Supplementary information
Intracellular free calcium ([Ca2+]i) oscillations and parameters. A to E indicate different stages of two continuous [Ca2+]i oscillations. “Ca2+ move speed" was the changing fluorescence value every 20 s. We defined the length from the beginning of first [Ca2+]i rise to the beginning of next [Ca2+]i rise as one "cycle"; time from beginning to end of an [Ca2+]i rise as “maintain”; the maximum fluorescence value and maximum changing fluorescence value as “peak” and “move”; and “△peak" was defined as maximum [Ca2+]i fluorescence value minus baseline values. The baseline was defined as the stable minimum [Ca2+]i fluorescence value. The bright field and fluorescent embryo image specifications were as follows: magnification: 200X, time-point: 8008.867s - 9732.370s, wavelength: 191s. The embryos were derived from the 17 h group, and Fluo-4AM fluorescent dye was used. (JPG 160 kb)
Relative gene expression was determined using the 2ΔΔCt method normalized to GAPDH. Expression of GRP78 and Chop in oocytes with endoplasmic reticulum stress-induced by tunicamycin (TM) in a dose-dependent manner. RNA was extracted from 100 mouse oocytes for each group. Data represent mean±SD, and Chi-square test and one-way ANOVA are used for statistical analysis. *P<0.05 versus the control group. (JPG 137 kb)
Code availability
Not applicable.
Funding
This work was sponsored by the National Natural Science Foundation of China (No 81671425 and No 81901477).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
The study was approved by the Laboratory Animal Care and Use Committee of the Institute of Zoology, Chinese Academy of Sciences.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Human reproduction (Oxford, England) 2007;22(6):1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 2.Kamel RM. Management of the infertile couple: an evidence-based protocol. Reprod Biol Endocrinol. 2010;8:21. doi: 10.1186/1477-7827-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324.e1–1331.e1. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Intracytoplasmic sperm injection (ICSI) for non-male factor infertility: a committee opinion. Fertility and sterility. 2012;98(6):1395–9. 10.1016/j.fertnstert.2012.08.026. [DOI] [PubMed]
- 5.Rawe VY, Olmedo SB, Nodar FN, Doncel GD, Acosta AA, Vitullo AD. Cytoskeletal organization defects and abortive activation in human oocytes after IVF and ICSI failure. Molecular human reproduction. 2000;6(6):510–516. doi: 10.1093/molehr/6.6.510. [DOI] [PubMed] [Google Scholar]
- 6.Neri QV, Lee B, Rosenwaks Z, Machaca K, Palermo GD. Understanding fertilization through intracytoplasmic sperm injection (ICSI) Cell calcium. 2014;55(1):24–37. doi: 10.1016/j.ceca.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanden Meerschaut F, Nikiforaki D, Heindryckx B, De Sutter P. Assisted oocyte activation following ICSI fertilization failure. Reproductive biomedicine online. 2014;28(5):560–571. doi: 10.1016/j.rbmo.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Ozil JP, Markoulaki S, Toth S, Matson S, Banrezes B, Knott JG, Schultz RM, Huneau D, Ducibella T. Egg activation events are regulated by the duration of a sustained [Ca2+]cyt signal in the mouse. Developmental biology. 2005;282(1):39–54. doi: 10.1016/j.ydbio.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Ozil JP. Role of calcium oscillations in mammalian egg activation: experimental approach. Biophysical chemistry. 1998;72(1-2):141–152. doi: 10.1016/s0301-4622(98)00130-6. [DOI] [PubMed] [Google Scholar]
- 10.Ozil JP, Swann K. Stimulation of repetitive calcium transients in mouse eggs. The Journal of physiology. 1995;483(Pt 2):331–346. doi: 10.1113/jphysiol.1995.sp020589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yazawa H, Yanagida K, Sato A. Oocyte activation and Ca(2+) oscillation-inducing abilities of mouse round/elongated spermatids and the developmental capacities of embryos from spermatid injection. Human reproduction (Oxford, England) 2001;16(6):1221–1228. doi: 10.1093/humrep/16.6.1221. [DOI] [PubMed] [Google Scholar]
- 12.Wakai T, Fissore RA. Ca(2+) homeostasis and regulation of ER Ca(2+) in mammalian oocytes/eggs. Cell calcium. 2013;53(1):63–67. doi: 10.1016/j.ceca.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malcuit C, Kurokawa M, Fissore RA. Calcium oscillations and mammalian egg activation. Journal of cellular physiology. 2006;206(3):565–573. doi: 10.1002/jcp.20471. [DOI] [PubMed] [Google Scholar]
- 14.Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Developmental biology. 2002;250(2):280–291. doi: 10.1006/dbio.2002.0788. [DOI] [PubMed] [Google Scholar]
- 15.Chan C, Martin P, Liptrott NJ, Siccardi M, Almond L, Owen A. Incompatibility of chemical protein synthesis inhibitors with accurate measurement of extended protein degradation rates. Pharmacol Res Perspect. 2017;5(5):e00359. doi: 10.1002/prp2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman MH. Parthenogenetic activation of oocytes. Cold Spring Harbor protocols. 2018;2018(1). 10.1101/pdb.prot094409. [DOI] [PubMed]
- 17.Sfontouris IA, Nastri CO, Lima ML, Tahmasbpourmarzouni E, Raine-Fenning N, Martins WP. Artificial oocyte activation to improve reproductive outcomes in women with previous fertilization failure: a systematic review and meta-analysis of RCTs. Human reproduction (Oxford, England) 2015;30(8):1831–1841. doi: 10.1093/humrep/dev136. [DOI] [PubMed] [Google Scholar]
- 18.Wang X. The expanding role of mitochondria in apoptosis. Genes & development. 2001;15(22):2922–2933. [PubMed] [Google Scholar]
- 19.Chi MM, Manchester JK, Yang VC, Curato AD, Strickler RC, Lowry OH. Contrast in levels of metabolic enzymes in human and mouse ova. Biology of reproduction. 1988;39(2):295–307. doi: 10.1095/biolreprod39.2.295. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta biochimica et biophysica Sinica. 2014;46(8):629–640. doi: 10.1093/abbs/gmu048. [DOI] [PubMed] [Google Scholar]
- 21.Hu H, Tian M, Ding C, Yu S. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Frontiers in immunology. 2018;9:3083. doi: 10.3389/fimmu.2018.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng JM, Li J, Tang JX, Chen SR, Deng SL, Jin C, et al. Elevated intracellular pH appears in aged oocytes and causes oocyte aneuploidy associated with the loss of cohesion in mice. Cell cycle (Georgetown, Tex) 2016;15(18):2454–2463. doi: 10.1080/15384101.2016.1201255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanghe S, Van Soom A, Nauwynck H, Coryn M, de Kruif A. Minireview: Functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Molecular reproduction and development. 2002;61(3):414–424. doi: 10.1002/mrd.10102. [DOI] [PubMed] [Google Scholar]
- 24.Eriani K. Fertilization and development of mice (Mus musculus) embryo in vitro after supplementing the extract of Pandanus conoideus. Nusantara Bioence. 2017;9(2).
- 25.Kubiak JZ. Mouse oocytes gradually develop the capacity for activation during the metaphase II arrest. Developmental biology. 1989;136(2):537–545. doi: 10.1016/0012-1606(89)90279-0. [DOI] [PubMed] [Google Scholar]
- 26.Ma SF, Liu XY, Miao DQ, Han ZB, Zhang X, Miao YL, Yanagimachi R, Tan JH. Parthenogenetic activation of mouse oocytes by strontium chloride: a search for the best conditions. Theriogenology. 2005;64(5):1142–1157. doi: 10.1016/j.theriogenology.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Bomsel-Helmreich O, Huyen LV, Durand-Gasselin I, Salat-Baroux J, Antoine JM. Timing of nuclear maturation and cumulus dissociation in human oocytes stimulated with clomiphene citrate, human menopausal gonadotropin, and human chorionic gonadotropin. Fertil Steril. 1987;48(4):586–595. doi: 10.1016/s0015-0282(16)59469-2. [DOI] [PubMed] [Google Scholar]
- 28.Vincent C, Cheek TR, Johnson MH. Cell cycle progression of parthenogenetically activated mouse oocytes to interphase is dependent on the level of internal calcium. Journal of Cell Science. 1992;103(2):389. doi: 10.1242/jcs.103.2.389. [DOI] [PubMed] [Google Scholar]
- 29.Miao YL, Williams CJ. Calcium signaling in mammalian egg activation and embryo development: the influence of subcellular localization. Mol Reprod Dev. 2012;79(11):742–756. doi: 10.1002/mrd.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Occhipinti R, Boron WF. Mathematical modeling of acid-base physiology. Progress in biophysics and molecular biology. 2015;117(1):43–58. doi: 10.1016/j.pbiomolbio.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barritt JA, Cohen J, Brenner CA. Mitochondrial DNA point mutation in human oocytes is associated with maternal age. Reproductive biomedicine online. 2000;1(3):96–100. doi: 10.1016/s1472-6483(10)61946-3. [DOI] [PubMed] [Google Scholar]
- 32.Eichenlaub-Ritter U, Wieczorek M, Luke S, Seidel T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion. 2011;11(5):783–796. doi: 10.1016/j.mito.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Bjercke S, Tanbo T, Dale PO, Abyholm T. Comparison between two hCG-to-oocyte aspiration intervals on the outcome of in vitro fertilization. J Assist Reprod Genet. 2000;17(6):319–322. doi: 10.1023/a:1009401027251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriwaki K, Nakagawa T, Nakaya F, Hirohashi N, Chiba K. Arrest at metaphase of meiosis I in starfish oocytes in the ovary is maintained by high CO2 and low O2 concentrations in extracellular fluid. Zoological science. 2013;30(11):975–984. doi: 10.2108/zsj.30.975. [DOI] [PubMed] [Google Scholar]
- 35.Ata B, Shalom-Paz E, Chian RC, Tan SL. In vitro maturation of oocytes as a strategy for fertility preservation. Clinical obstetrics and gynecology. 2010;53(4):775–786. doi: 10.1097/GRF.0b013e3181f9718f. [DOI] [PubMed] [Google Scholar]
- 36.Taylor IW, Hodson PJ. Cell cycle regulation by environmental pH. Journal of cellular physiology. 1984;121(3):517–525. doi: 10.1002/jcp.1041210310. [DOI] [PubMed] [Google Scholar]
- 37.FitzHarris G, Baltz JM. Regulation of intracellular pH during oocyte growth and maturation in mammals. Reproduction. 2009;138(4):619–627. doi: 10.1530/REP-09-0112. [DOI] [PubMed] [Google Scholar]
- 38.Musa-Aziz R, Occhipinti R, Boron WF. Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes. American Journal of Physiology-Cell Physiology. 2014;307(9):C814–CC40. doi: 10.1152/ajpcell.00050.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Animal reproduction science. 2004;82-83:431–446. doi: 10.1016/j.anireprosci.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Mori T, Amano T, Shimizu H. Roles of gap junctional communication of cumulus cells in cytoplasmic maturation of porcine oocytes cultured in vitro. Biology of reproduction. 2000;62(4):913–919. doi: 10.1095/biolreprod62.4.913. [DOI] [PubMed] [Google Scholar]
- 41.Nikiforaki D, Vanden Meerschaut F, Qian C, De Croo I, Lu Y, Deroo T, et al. Oocyte cryopreservation and in vitro culture affect calcium signalling during human fertilization. Human reproduction (Oxford, England) 2014;29(1):29–40. doi: 10.1093/humrep/det404. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Yuan RY, Li L, Meng TG, Fan LH, Jing Y, Zhang RR, Li YY, Liang QX, Dong F, Hou Y, Schatten H, Sun QY, Ou XH. Mitochondrial regulation of [Ca2+]i oscillations during cell cycle resumption of the second meiosis of oocyte. Cell Cycle. 2018;17(12):1471–1486. doi: 10.1080/15384101.2018.1489179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michalak M, Gye MC. Endoplasmic reticulum stress in periimplantation embryos. Clinical and experimental reproductive medicine. 2015;42(1):1–7. doi: 10.5653/cerm.2015.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krebs J, Agellon LB, Michalak M. Ca(2+) homeostasis and endoplasmic reticulum (ER) stress: an integrated view of calcium signaling. Biochemical and biophysical research communications. 2015;460(1):114–121. doi: 10.1016/j.bbrc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Kaufman RJ, Scheuner D, Schröder M, Shen X, Lee K, Liu CY, Arnold SM. The unfolded protein response in nutrient sensing and differentiation. Nature reviews Molecular cell biology. 2002;3(6):411–421. doi: 10.1038/nrm829. [DOI] [PubMed] [Google Scholar]
- 46.Giorgi C, De Stefani D, Bononi A, Rizzuto R, Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41(10):1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szabadkai G, Bianchi K, Várnai P, De Stefani D, Wieckowski MR, Cavagna D, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175(6):901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pauly M, Angebault-Prouteau C, Dridi H, Notarnicola C, Scheuermann V, Lacampagne A, Matecki S, Fauconnier J. ER stress disturbs SR/ER-mitochondria Ca(2+) transfer: Implications in Duchenne muscular dystrophy. Biochimica et biophysica acta Molecular basis of disease. 2017;1863(9):2229–2239. doi: 10.1016/j.bbadis.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 49.Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Current molecular medicine. 2016;16(6):533–544. doi: 10.2174/1566524016666160523143937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intracellular free calcium ([Ca2+]i) oscillations and parameters. A to E indicate different stages of two continuous [Ca2+]i oscillations. “Ca2+ move speed" was the changing fluorescence value every 20 s. We defined the length from the beginning of first [Ca2+]i rise to the beginning of next [Ca2+]i rise as one "cycle"; time from beginning to end of an [Ca2+]i rise as “maintain”; the maximum fluorescence value and maximum changing fluorescence value as “peak” and “move”; and “△peak" was defined as maximum [Ca2+]i fluorescence value minus baseline values. The baseline was defined as the stable minimum [Ca2+]i fluorescence value. The bright field and fluorescent embryo image specifications were as follows: magnification: 200X, time-point: 8008.867s - 9732.370s, wavelength: 191s. The embryos were derived from the 17 h group, and Fluo-4AM fluorescent dye was used. (JPG 160 kb)
Relative gene expression was determined using the 2ΔΔCt method normalized to GAPDH. Expression of GRP78 and Chop in oocytes with endoplasmic reticulum stress-induced by tunicamycin (TM) in a dose-dependent manner. RNA was extracted from 100 mouse oocytes for each group. Data represent mean±SD, and Chi-square test and one-way ANOVA are used for statistical analysis. *P<0.05 versus the control group. (JPG 137 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.