Abstract
Purpose
In vitro maturation (IVM) is an alternative to in vitro fertilization (IVF) for women at high risk of developing ovarian hyperstimulation syndrome (OHSS). This study determined the effectiveness and safety of a freeze-only strategy versus fresh embryo transfer (ET) after IVM with a pre-maturation step (CAPA-IVM) in women with a high antral follicle count (AFC).
Methods
This randomized, controlled pilot study (NCT04297553) was conducted between March and November 2020. Forty women aged 18–37 years with a high AFC (≥24 follicles in both ovaries) undergoing one cycle of CAPA-IVM were randomized to a freeze-only strategy with subsequent frozen ET (n = 20) or to fresh ET (n = 20). The primary endpoint was ongoing pregnancy resulting in live birth after the first ET of the started treatment cycle.
Results
The ongoing pregnancy rate in the freeze-only group (65%) was significantly higher than that in the fresh ET group (25%; p = 0.03), as was the live birth rate (60% versus 20%; p = 0.02). Clinical pregnancy rate was numerically, but not significantly, higher after frozen versus fresh ET (70% versus 35%; p = 0.06), while the number of day 3 or good quality embryos, endometrial thickness on the day of oocyte pick-up, implantation rate, and positive pregnancy test rate did not differ significantly between groups. No cases of OHSS were observed, and miscarriage and multiple pregnancy rates were similar in the two groups.
Conclusions
These findings suggest that the effectiveness of CAPA-IVM could be improved considerably by using a freeze-only strategy followed by frozen ET in subsequent cycles.
Trial registration number:
Keywords: In vitro maturation, Frozen embryo transfer, Fresh embryo transfer, Infertility, Ongoing pregnancy, Live birth, Polycystic ovary syndrome
Background
In vitro maturation (IVM) is an alternative to conventional in vitro fertilization (IVF) for women at high risk of ovarian hyperstimulation syndrome (OHSS), such as those with polycystic ovary syndrome (PCOS) [1, 2]. In addition to the absence of (or minimal) controlled ovarian stimulation, other advantages of IVM include a shorter treatment duration, lower medication costs, and improved patient convenience (including a lower monitoring burden) [2, 3].
There are currently a variety of options with respect to IVM strategies [2, 4]. At My Duc Hospital in Vietnam, IVM with a pre-maturation step, known as capacitation IVM (CAPA-IVM) [5, 6], has routinely been used instead of IVM with human chorionic gonadotropin (hCG) priming because of better synchronization of oocyte maturation state, and better embryology and pregnancy outcomes [7]. The current approach to CAPA-IVM includes frozen embryo transfer (ET) for all patients, which has been shown to be a feasible alternative to conventional IVF [6, 8] for patients with a high antral follicle count, although the cumulative live birth rate was lower in the CAPA-IVM group compared with conventional IVF [6]. However, the use of a freeze-only strategy with transfer of frozen embryo(s) in subsequent cycles in this setting increases the treatment duration by several weeks at the minimum, places a burden on patients to return during multiple cycles, adds the cost of embryo vitrification, and potentially increases the time to achievement of pregnancy. Therefore, the possibility of performing CAPA-IVM with fresh ET might be more convenient for patients.
Previously at our center, the protocol used for IVM with fresh embryo transfer used included human chorionic gonadotropin (hCG) administration 36 h before oocyte pick-up (OPU), with endometrial preparation using estradiol and progesterone. Given our good results with the hCG-IVM protocol and fresh day 3 embryo transfer [9], one of the aims of the current pilot study was to determine whether it was possible to transfer fresh day 3 embryos after CAPA-IVM (without hCG priming). In addition, in the setting of IVM, there is a lack of data from prospective, comparative trials regarding clinical outcomes after fresh versus frozen ET. Although previous studies have reported comparatively low success rates with fresh ET in IVM [10, 11], it is possible that different IVM methodologies and different endometrial preparation regimens might result in a different outcome. Therefore, this randomized, controlled pilot study was designed to compare the effectiveness and safety of a freeze-only strategy versus fresh ET in women with a high antral follicle count (AFC) undergoing CAPA-IVM.
Methods
Study design
This randomized, open-label study was conducted at IVFMD, My Duc Hospital in Ho Chi Minh City, Vietnam (NCT04297553) from 6 March 2020 to 30 Nov 2020. The study was approved by the hospital ethics committee and conducted according to Good Clinical Practice and Declaration of Helsinki 2002 principles, including oversight by an independent data and safety monitoring committee. All participants provided written informed consent. The full study protocol can be accessed at www.clinicaltrials.gov (NCT04297553).
Study population
Women aged 18–37 years with a high AFC (≥24 antral follicles in both ovaries as defined previously [12]; including PCOS, polycystic ovarian morphology) with an indication for assisted reproductive technologies who had undergone ≤2 previous IVM or IVF attempts and agreed to have ≤2 embryos transferred were eligible for inclusion in this study. Oocyte donation and preimplantation genetic diagnosis cycles were excluded.
Randomization
After providing written informed consent, on day 2 of the menstrual cycle, women were randomized (1:1) to the fresh transfer or freeze-only group using block randomization by an independent study coordinator via telephone and a computer-generated random list (block size 2 or 4).
Treatments and assessments
At first study visit, patients were screened for eligibility and provided with information about the study. They were asked to return to the clinic on the second day of their menstrual cycle (amenorrheic women were treated with oral contraceptives for 2 weeks to induce bleeding). At this visit, those who provided informed consent were randomized to one of the two study groups. Patients had an ultrasound scan and levels of the following hormones were determined: luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (E2), progesterone, anti-Müllerian hormone (AMH), sex hormone-binding globulin (SHBG), testosterone, prolactin, thyroid-stimulating hormone (TSH), anti-thyroid peroxidase, 17-hydroxyprogesterone, and dehydroepiandrosterone sulfate (DHEAS).
All patients received highly purified human menopausal gonadotropin 150 IU/day (hp-hMG; Menopur®, Ferring, Switzerland) for 2 days from the third day of the menstrual cycle (this treatment was given to improve follicular growth and oocyte health, and reduce the variability in response between patients). OPU took place 42 h after the last injection of hp-hMG. OPU was performed using a double needle system, with a 17G external needle and a 19G internal needle (Kinder Needle, Biopsy Bell, Italy). The pressure used for collection was 100–120 mmHg. After aspiration, follicular fluid was transferred to the lab where immature oocytes were identified under a stereomicroscope using the sliding technique; subsequently, then all fluid was poured into a strainer to check for any missed oocytes.
All cumulus-oocyte complexes (COCs) were placed in a meiotic arresting capacitation medium (based on Medicult IVM medium (Origio, Denmark), supplemented with recombinant FSH (rFSH) 1 mIU/mL (Puregon®; MSD, Australia), insulin 5 ng/mL (Sigma, Schnelldorf, Germany), E2 10 nmol/L (Sigma, Schnelldorf, Germany), human serum albumin 10 mg/mL (SAGE, Denmark), and C-type natriuretic peptide (CNP) 25 nM (Tocris Bioscience, Abingdon, UK) and incubated under oil for 24 h at 37°C with 6% carbon dioxide in air. After 24 h, COCs were washed and transferred into maturation medium (based on Medicult IVM medium, Origio, Denmark; containing insulin 5 ng/mL, E2 10 nmol/L, human recombinant amphiregulin 100 ng/mL, and rFSH 100 mIU/mL) and incubated under oil for an additional 30 h at 37°C with 6% carbon dioxide in air. Mature oocytes were fertilized using intracytoplasmic sperm injection at 3–4 h after checking maturation under a stereomicroscope. Embryo evaluation was performed at 68 ± 1 h after fertilization based on the Istanbul consensus [13]. Embryo quality was graded by two trained embryologists who had >5 years’ experience. In addition, all embryo images were stored for review and checking if required.
In the freeze-only group, all grade 1 or grade 2 embryos were vitrified. Starting on the day of embryo freezing, patients received oral progesterone (Duphaston® 10 mg; Abbott, Singapore) for 10 days to induce bleeding. In the subsequent cycle, endometrial preparation was performed using oral estradiol valerate (Valiera®; Abbott, Singapore) 2 mg, 4 times daily from day 2 of the menstrual cycle. Progesterone (Cyclogest®; Accord-UK Limited, UK) 200 mg intravaginally 4 times daily was started when patients had received estradiol valerate for ≥10 days and when endometrial thickness was ≥8 mm. Embryo transfer (maximum 2 embryos) was scheduled for 3 days after the initiation of progesterone. Embryos were thawed on the day of transfer and embryo transfer was performed 2 h later as per routine clinical practice at our clinic.
In the fresh ET group, patients received 10,000 IU of hCG (Pregnyl® 5000 IU/amp; MSD, Australia) immediately after OPU. Two days later, treatment with estradiol valerate (Valiera®; Abbott, Singapore) 2 mg, 4 times daily, and progesterone (Cyclogest®; Accord-UK Limited, UK) 400 mg, twice daily, was performed until the day of pregnancy testing. Day 3 embryo transfer was performed 5 days after OPU (including 24 h for capacitation culture, 30 h for maturation culture, intracytoplasmic sperm injection, and 3 days of embryo culture). A maximum of two embryos was transferred. All remaining grade 1 or 2 embryos were vitrified for later use.
In both groups, a pregnancy test was performed 14 days after embryo transfer; a positive pregnancy test was defined as serum beta hCG >5 mIU/mL. Ultrasound was performed 3 weeks after pregnancy testing.
Outcomes
The primary endpoint was ongoing pregnancy resulting in live birth after the first ET of the started treatment cycle. Ongoing pregnancy was defined as pregnancy with a detectable heart rate at ≥12 weeks’ gestation after the completion of the first transfer. Live birth was defined as the birth of ≥1 newborn after 24 weeks’ gestation that exhibits any sign of life (twins were a single count).
A number of predefined secondary outcomes were also evaluated: positive pregnancy test rate; implantation rate; clinical pregnancy rate; ongoing pregnancy; number of embryos on day 3; number of good quality embryos on day 3; time from randomization to ongoing pregnancy; time from randomization to live birth; rate of OHSS; rate of ectopic pregnancy; rate of miscarriage; rate of hypertensive disorders of pregnancy (including pregnancy-induced hypertension, pre-eclampsia, and eclampsia); rate of gestational diabetes; rate of preterm delivery (at <24, <28, <32, and <37 completed weeks’ gestation); multiple pregnancy rate; birth weight (of singletons and twins); and rate of any congenital anomalies.
Implantation was defined as the number of gestational sacs per number of embryos transferred at 3 weeks after ET. Clinical pregnancy was defined as at least one gestational sac on ultrasound at 7 weeks’ gestation with the detection of heart beat activity. Embryo quality was defined using the Istanbul consensus [13]. Routine assessments for OHSS were performed on day 3 post oocyte retrieval in both groups. At other times, OHSS was evaluated if symptoms were reported by the patient. OHSS was classified using the flow diagram developed by Humaidan and colleagues for use in clinical trial settings [14]. Ectopic pregnancy was defined as a pregnancy in which implantation occurred outside the uterine cavity. Miscarriage was defined as pregnancy loss at <12 weeks’ gestation. Gestational diabetes was diagnosed using a 75-g oral glucose tolerance test at 24-28 weeks’ gestation. Multiple pregnancy was confirmed when there was >1 sac at early pregnancy ultrasound (6–8 weeks’ gestation).
Statistical analysis
The planned sample size for this pilot study was 40 patients (20 per group), which should allow a conclusion regarding feasibility outcomes. Given that this is the first time that CAPA-IVM has been performed with fresh embryo transfer, the objective of this pilot study was to estimate the ongoing pregnancies resulting in live birth rate and associated 95% confidence intervals (CI) for the new strategy in clinical practice. The primary statistical analysis was conducted on an intention-to-treat basis, in which all participants remained in their allocated group for analysis. After the first ET, the live birth rate was compared between groups by calculating the risk difference and associated 95% confidence interval. Between-group differences in secondary endpoints were analyzed using parametric methods (normally distributed data), nonparametric methods (skewed data), or Fisher’s exact test (categorical variables), and were reported as relative risks and 95% confidence intervals.
Univariate analysis was performed to determine predictors of live birth, and then multivariate analysis was conducted on all variables with a p-value of <0.25 in the univariate analysis.
Statistical significance was defined as p<0.05. All analyses were performed using the R statistical program (v3.5.0).
Results
Patients
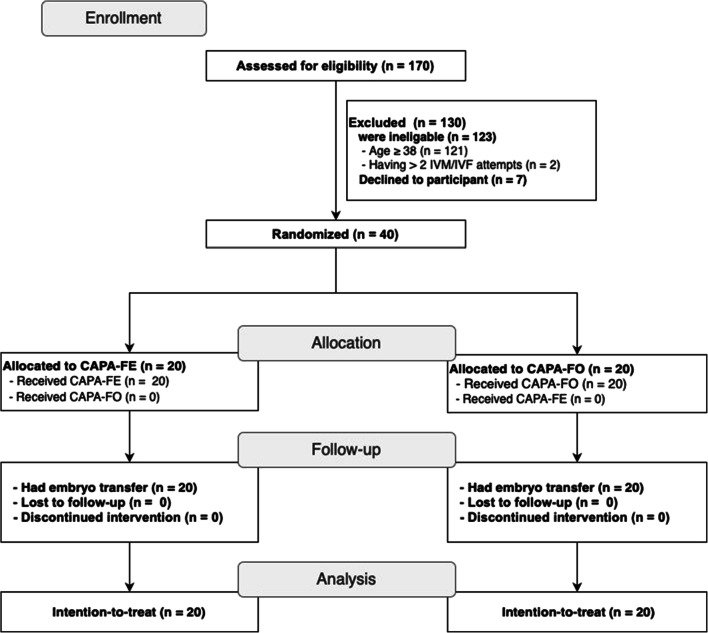
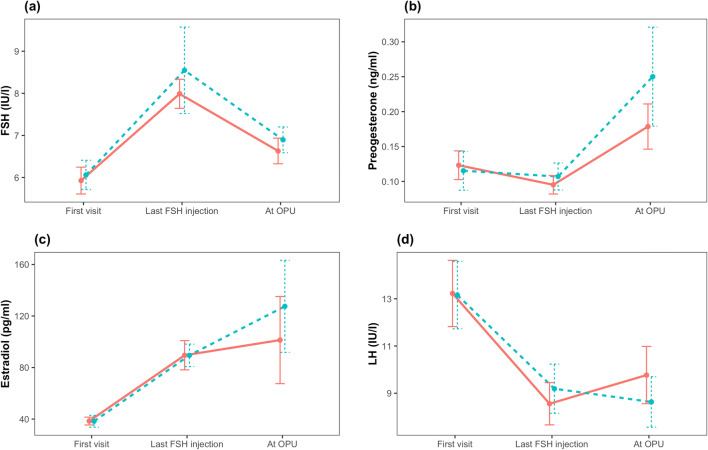
One hundred and seventy patients were assessed for eligibility, of whom 123 were ineligible and seven declined to participate; therefore, the study population included a total of 40 patients (20 per group) (Fig. 1). No patients were lost to follow-up and all were included in the intention-to-treat analysis. The fresh ET and freeze-only groups had comparable clinical and demographic characteristics at baseline (Table 1). The majority of patients in both groups had two embryos transferred (Table 2). Hormonal profiles up to OPU were similar in the fresh ET and freeze-only groups (Fig. 2) and were compatible with the PCOS phenotype. No other endocrine abnormalities were diagnosed. One patient in the freeze-only group had elevated anti-thyroid antibodies (anti-thyroperoxidase level, 600 IU/mL) but this patient had no active disease.
Fig. 1.
CONSORT flowchart. CAPA-FE, CAPA-IVM then fresh embryo transfer; CAPA-FO, CAPA-IVM, and a freeze-only strategy
Table 1.
Patient demographics and clinical characteristics at baseline
| Characteristic | CAPA-IVM | p-value | |
|---|---|---|---|
| Fresh embryo transfer (n = 20) | Freeze-only strategy (n = 20) | ||
| Age, years | 30.1 ± 3.3 | 28.70 ± 3.0 | 0.16 |
| Body mass index, kg/m2 | 24.3 ± 5.0 | 23.7 ± 3.9 | 0.70 |
| Anti-Müllerian hormone, ng/mL | 8.83 ± 3.27 | 8.11 ± 4.51 | 0.57 |
| Antral follicle count, n | 55.6 ± 18.6 | 53.3 ± 25.5 | 0.75 |
| Duration of infertility, years | 2 [1, 3] | 2 [1, 3] | 0.73 |
| Type of infertility, n (%) | 0.73 | ||
| Primary | 13/20 (65.0) | 15/20 (75.0) | |
| Secondary | 7/20 (35.0) | 5/20 (25.0) | |
| Number IVF attempts, n (%) | 0.99 | ||
| 1 | 17/20 (85.0) | 17/20 (85.0) | |
| 2 | 3/20 (15.0) | 3/20 (15.0) | |
| Hormonal profile at first visit | |||
| Prolactin, ng/mL | 19.93 ± 12.03 | 18.18 ± 9.22 | 0.61 |
| Testosterone, nmol/L | 1.33 ± 0.57 | 1.39 ± 0.69 | 0.78 |
| SHBG, nmol/L | 32.94 ± 20.85 | 36.78 ± 20.22 | 0.56 |
| TSH, μIU/mL | 2.60 ± 1.17 | 2.14 ± 0.79 | 0.16 |
| DHEA-SO4, μg/mL | 1.97 ± 0.88 | 2.11 ± 1.08 | 0.65 |
| Anti-thyroperoxidase, IU/mL* | 8.22 ± 2.39 | 10.5 ± 03.87 | 0.29 |
| 17-hydroxyprogesterone, ng/mL | 1.10 ± 0.46 | 1.28 ± 0.78 | 0.38 |
| Number of follicles at last ultrasound | 30.9 ± 7.2 | 33.4 ± 18.9 | 0.58 |
| Number of oocytes retrieved | 18.4 ± 10.2 | 18.2 ± 12.7 | 0.96 |
| Number of MII oocytes | 11.8 ± 6.5 | 12.9 ± 10.7 | 0.71 |
| Number of pronuclear stage | 11.6 ± 6.3 | 12.8 ± 10.8 | 0.79 |
Values are mean ± standard deviation, median [interquartile range], or number of patients (%)
DHEA-SO4 dehydroepiandrosterone sulfate; IVF in vitro fertilization; IVM in vitro maturation; MII metaphase II; SHBG sex hormone-binding globulin; TSH thyroid-stimulating hormone
*Excluding one extreme value of 600 IU/mL
Table 2.
Fertility outcomes and pregnancy complications after first embryo transfer
| CAPA-IVM | Absolute difference (95% CI) | Relative risk (95% CI) | p-value | ||
|---|---|---|---|---|---|
| Fresh embryo transfer (n = 20) | Freeze-only strategy (n = 20) | ||||
| Fertility outcomes | |||||
| Number of embryos on day 3 | 5.7 ± 4.5 | 5.9 ± 5.5 | −0.2 (−3.4, 3.0) | - | 0.9 |
| Number of good quality embryos on day 3 | 4.3 ± 4.3 | 4.6 ± 5.3 | −0.3 (−3.4, 2.7) | - | 0.82 |
| Number of embryos transferred, n (%) | 0.99 | ||||
| 1 | 1/20 (5.0) | 0/20 (0.0) | |||
| 2 | 19/20 (95.0) | 20/20 (100.0) | |||
| Number of good quality embryos transferred, n (%) | 0.693 | ||||
| 1 | 5/20 (25.0) | 3/20 (15.0) | |||
| 2 | 15/20 (75.0) | 17/20 (85.0) | |||
| Endometrial thickness on day of OPU, mm | 10.9 ± 1.2 | 11.4 ± 1.3 | −0.5 (−1.3, 0.3) | - | 0.19 |
| Positive pregnancy test, n (%) | 8/20 (40.0) | 14/20 (70.0) | −30 (−64.4, 4.4) | 0.57 (0.31, 1.05) | 0.11 |
| Clinical pregnancy rate, n (%) | 7/20 (35.0) | 14/20 (70.0) | −35 (−69, −1) | 0.5 (0.26, 0.97) | 0.06 |
| Implantation rate, % | 25.0 ± 38.0 | 45.0 ± 39.4 | −20.0 (−44.8, 4.8) | - | 0.11 |
| Ongoing pregnancy rate, n (%) | 5/20 (25.0) | 13/20 (65.0) | −40 (−73.2, −6.8) | 0.38 (0.17, 0.88) | 0.03 |
| Time to ongoing pregnancy, days | 64.0 [64.0, 69.0] | 110.0 [107.0, 114.0] | - | - | 0.001 |
| Live birth rate, n (%) | 4/20 (20.0) | 12/20 (60.0) | −40 (−72.7, −7.3) | 0.33 (0.13, 0.86) | 0.02 |
| Time to live birth, days | 150.5 [145.0, 155.0] | 194.0 [191.0, 198.3] | - | - | 0.004 |
| Maternal safety | |||||
| OHSS, n (%) | 0/20 (0) | 0/20 (0) | - | - | - |
| Pregnancy complications | |||||
| Ectopic pregnancy, n (%) | 1/20 (5.0) | 0/20 (0.0) | - | - | - |
| Miscarriage, n (%) | 1/20 (5.0) | 1/20 (5.0) | 0 (−13.5, 13.5) | 1 (0.07, 14.9) | 0.99 |
| Multiple pregnancy rate, n (%) | 3/20 (15.0) | 3/20 (15.0) | 0 (−22.1, 22.1) | 1 (0.23, 4.37) | 0.99 |
| Obstetric and perinatal complications | |||||
| Gestational diabetes mellitus, n (%) | 0/20 (0) | 0/20 (0) | - | - | - |
| Hypertensive disorders of pregnancy, n (%) | 0/20 (0) | 0/20 (0) | - | - | - |
| Preterm delivery, n (%) | |||||
| Delivery at <24 weeks’ gestation | 1/20 (5) | 1/20 (5) | 0 (−13.5, 13.5) | 1 (0.1, 14.9) | 0.99 |
| Delivery at <28 weeks’ gestation | 1/20 (5) | 1/20 (5) | 0 (−13.5, 13.5) | 1 (0.1, 14.9) | 0.99 |
| Delivery at <32 weeks’ gestation | 1/20 (5) | 3/20 (15) | −10 (−33.3, 13.3) | 0.3 (0, 2.9) | 0.60 |
| Delivery at <37 weeks’ gestation | 3/20 (15) | 4/20 (20) | −5 (−33.5, 23.5) | 0.7 (0.2, 2.9) | 0.99 |
| Birth weight, grams | |||||
| Singleton | 3300 and 3900* | 2900 [2800, 3050] | - | - | - |
| Twins | 2350 [2275, 2425] | 2200 and 2800** | - | - | - |
| Congenital anomaly, n (%) | 0/20 (0) | 0/20 (0) | - | - | - |
Values are mean ± standard deviation, median [interquartile range], or number of patients (%), unless otherwise stated
CI confidence interval; OHSS ovarian hyperstimulation syndrome
*There were only two singleton births in this group
**There was only one twin birth in this group
Fig. 2.
Hormonal profiles during in vitro maturation with fresh embryo transfer versus a freeze-only strategy. CAPA-FE, CAPA-IVM then fresh embryo transfer; CAPA-FO, CAPA-IVM, and a freeze-only strategy; FSH, follicle-stimulating hormone; LH, luteinizing hormone; OPU, oocyte pick-up. Values are mean with standard error
Fertility outcomes
Ongoing pregnancy and live birth rates were significantly higher in the freeze-only versus fresh ET group (65% versus 25% (p = 0.03) and 60% versus 20% (p = 0.02), respectively) (Table 2). There were no statistically significant differences between the fresh ET and freeze-only groups with respect to the number of day 3 embryos, the number of good quality embryos, the endometrial thickness on the day of OPU, the implantation rate, and the positive pregnancy test rate (Table 2). The between-group difference in clinical pregnancy rate in the freeze-only versus fresh ET group was not statistically significant (70% versus 35%; p = 0.06) (Table 2).
Predictors of live birth
Variables with a p-value of <0.25 in the univariate analysis were treatment group (i.e., fresh ET versus freeze-only and frozen ET), age, anti-Müllerian hormone level, and antral follicle count. Of these, only the treatment group remained as a significant predictor of live birth in the multivariate analysis (rate ratio 0.20, 95% confidence interval 0.04–0.83; p = 0.032).
Complications
No cases of OHSS were documented in either group, and the miscarriage and multiple pregnancy rates were comparable between the freeze-only and fresh ET groups. There were no cases of gestational diabetes mellitus or hypertensive disorders of pregnancy. Two women delivered infants at <24 weeks of gestational age (one in each group). Birth weight was similar in the two groups, and there were no congenital abnormalities.
Discussion
The results of this pilot study suggested that the use of a freeze-only strategy in patients with a high AFC undergoing CAPA-IVM could significantly increase the rate of ongoing pregnancy resulting in live birth compared with fresh ET. Other fertility outcomes and complication rates did not differ between the two groups, although larger studies with longer follow-up are needed to confirm the comparative safety of frozen versus fresh ET in IVM. This is the first randomized controlled comparison of fresh ET with a freeze-all strategy followed by transfer of frozen embryos in patients undergoing IVM, contributing to the call for trials investigating newer methods of IVM [2]. Despite the limitation of a small sample size in this pilot study, a statistically significant difference was found in the ongoing pregnancy and live birth rate in favor of a freeze-only strategy.
A key goal of this study was to determine feasibility, acceptability, and outcome variability to aid in planning a larger, adequately powered efficacy trial. However, given that the ongoing pregnancy and live birth rates in the freeze-only arm were more than double of those in the fresh ET group, we consider that it would be unethical to perform a randomized controlled study with a larger sample size at our center that allocated patients undergoing CAPA-IVM to fresh ET.
Despite the fact that we were able to achieve very respectable live birth rates after day 3 replacement of cleavage embryos with the hCG-IVM protocol previously used in our center [9], the injection of 10,000 units of hCG after OPU in the current study did not seem to improve outcomes after fresh day 3 embryo transfer in CAPA-IVM. There are several potential reasons for this, which require further study using endometrial biopsies. The stimulation phase length in the two IVM methods differs, making the total time of exposure of the ovaries and endometrium to gonadotrophins (FSH or hp-hMG and hCG) significantly shorter in the CAPA-IVM protocol. The shorter exposure time of reproductive tissues to hp-HMG in CAPA-IVM (compared with hCG-IVM with 3 days of FSH) might have resulted in a less progressed endometrial tissue build-up. Injecting 10,000 units of hCG (before OPU in hCG-IVM) might also induce a different mobilization of steroids and other growth factors from small and medium-sized follicles than a similar hCG dose would have on emptied follicles (given post-OPU in CAPA-IVM). It should be noted that the endometrial preparation approach taken for the fresh transfer group in this study was that used routinely for hCG-IVM at our center [9, 15], with an injection of hCG immediately after OPU, followed by estradiol and progesterone administration starting on the same day, then fresh ET 5 days after oocyte retrieval/hCG injection. Due to differences in the IVM protocols between the current study and previous ones, the time from hCG administration to fresh ET was 12 h shorter in the current study because otherwise ET would need to be performed overnight, which is not feasible. However, the timing of progesterone administration in relation to fresh ET was the same. Nevertheless, the time between hCG administration and fresh ET could have contributed to the lower success rate after fresh transfer compared to rates after fresh ET reported in the previous hCG-IVM studies. Alternatively, as has been suggested previously [16], poor clinical outcomes seen after fresh ET in IVM might be due to inappropriate endometrial development, linked to the shorter follicular phase of IVM cycles.
Another potential explanation for our findings of higher ongoing pregnancy and live birth rates in patients undergoing CAPA-IVM with a freeze-only strategy is that frozen embryo transfer can only take place after embryos have survived the vitrification and thawing process, which could select out embryos with borderline viability that may have otherwise been transferred using a fresh ET strategy.
Several limitations need to be taken into account when interpreting the findings of this pilot study. Embryos were transferred or frozen at day 3, and therefore our results only apply to cleavage stage embryos and cannot be generalized to blastocyst (day 5 embryo) transfer. However, currently available data suggest that the use of day 3 versus day 5 embryos would be unlikely to markedly alter the study findings [17–21]. In addition, findings relate to the population in which the study was conducted who were aged <38 years, had undergone <2 previous IVF attempts, had a relatively normal body mass index despite the presence of PCOS, and all were of Vietnamese ethnicity. Outcomes may be different in older patients and those with a worse prognosis. These sample characteristics limit the external validity of our findings. Studies with a longer duration of follow-up are also important to evaluate cumulative outcomes over time and the comparative longer-term safety of the freeze-only and fresh ET approaches.
Our findings of a significantly higher live birth rate after frozen versus fresh ET in patients undergoing IVM contrast with data from a retrospective study by Cohen and colleagues, which reported significantly lower rates of fertilization, clinical pregnancy, and live birth in women with PCOS who underwent transfer of frozen versus fresh embryos in IVM cycles [22]. However, the IVM protocol used was different from that in our study and did not include a pre-maturation step, which has been added to improve oocyte competence [7, 23]. In addition, live birth rates in both the frozen and fresh ET groups were comparatively low in the previous study, and the reliability of the data is limited by the retrospective nature of the analysis.
Another retrospective analysis found that rates of biochemical and clinical pregnancy and live birth were not significantly different between women with polycystic ovarian morphology who underwent IVM versus IVF after transfer of frozen embryos; in contrast, when fresh embryos were transferred, rates were significantly lower with IVM versus IVF [11]. Although these data were obtained retrospectively, they do provide an indication that success rates achieved after IVM might be higher when a freeze-only approach was used rather than transfer of fresh embryos, as was shown in our randomized, controlled pilot study.
In patients undergoing IVF, meta-analysis data indicate that a freeze-only strategy is superior to fresh ET with respect to live birth rate in high responders [24, 25]. Also in IVF, transfer of frozen day 3 (cleavage stage) embryos was associated with a significant higher live birth rate than fresh ET in a randomized controlled trial of patients with polycystic ovary syndrome [26]. Data from another randomized controlled trial showed a significantly higher live birth rate after transfer of frozen versus fresh blastocysts (day 5 embryos) in women with a regular menstrual cycle undergoing IVF [27], but this was not the case in a similar study published more recently that showed similar live birth rates after frozen and fresh day 5 embryo transfer [28]. No significant difference in live birth was also reported after transfer of day 2 or day 3 embryos in women undergoing IVF who had regular cycles [29] or did not have polycystic ovary syndrome [30]. These varying data suggest that factors other than whether day 3 or day 5 embryos are transferred are more likely to contribute to the comparative outcomes after frozen versus fresh ET. Therefore, the use of day 3 embryos is unlikely to be an important confounding variable in our study.
The ongoing pregnancy rate with IVM and frozen embryo transfer in this study (65%) was substantially higher than that reported in previous studies at our center (38.1% and 47.5%) [6, 8]. The same capacitation IVM protocol was used in all three studies, but the sample size was smaller in the current study, especially compared with the large randomized trial comparing IVM and IVF [8]. Another possibility is that technical competence with the procedures of this relatively new technique are improving over time, resulting in better outcomes.
It is important to note that the majority of patients in our study had transfer of two embryos, although the multiple birth rate was only 15%. Transfer of two embryos at our center is usually performed due to patient preference because assisted reproductive technologies are self-funded in Vietnam and patients perceive that transfer of two embryos will maximize their chances of achieving a successful pregnancy. Nevertheless, global practice has moved towards single embryo transfer [31], and additional research is needed to evaluate the use of CAPA-IVM and frozen embryo transfer in this setting. The current study showed that CAPA-IVM was safe and well tolerated. No cases of OHSS were reported in a population of patients who would traditionally be at increased risk of this complication if undergoing controlled ovarian hyperstimulation [32, 33]. The rate of obstetric and perinatal complications was low, consistent with our experience of this IVM strategy [6, 8]. However, additional data from larger number of women and babies followed over the longer term are needed to provide a full picture of the comparative safety of both IVM and the two ET approaches [2]. In addition, studies utilizing different endometrial preparation protocols for fresh ET, and those evaluating transfer of day 5 versus day 3 embryos, would provide valuable information to guide the future use of CAPA-IVM.
Conclusions
These findings suggest that patients with a high AFC undergoing CAPA-IVM could be managed using a freeze-only strategy with transfer of frozen embryo(s) in subsequent cycles, and that this approach might maximize the rates of ongoing pregnancy and live birth.
Author contribution
LNV, LKN, AHL, HHP. VNAH, HLL, TDP, VQD, THP, JS, and TMH designed the study and monitored data collection. The statistical analysis plan was written by TDP and LNV. Data analysis was conducted by TDP, who acts as guarantor of the data and the analyses. Planning for the first draft of the manuscript was undertaken by LNV, LKN, and TDP. The first draft of the paper was written by LNV. All authors were involved in the decision to publish the paper and in critical revisions of the manuscript. LNV acts as overall guarantor and accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding
This work was funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED; grant number FWO.106-YS.2017.02) and by the Fund for Research Flanders (FWO) under grant number G.OD97.18N. The study sponsors were not involved in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and had final responsibility to submit for publication.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy but are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
LNV has received speaker and conference fees from Merck, and grant, speaker, and conference fees from Merck Sharpe & Dohme and Ferring. TMH has received speaker fees from Merck, Merck Sharp & Dohme and Ferring. JS reports lecture fees from Ferring Pharmaceuticals, BioMerieux, Besins Female Healthcare and Merck, grants from Fund for Research Flanders (FWO), and is co-inventor on granted patents on CAPA-IVM methodology in the USA (US10392601B2) and Europe (EP3234112B1).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Child TJ, Abdul-Jalil AK, Gulekli B, Tan SL. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil Steril. 2001;76(5):936–942. doi: 10.1016/S0015-0282(01)02853-9. [DOI] [PubMed] [Google Scholar]
- 2.Practice Committees of the American Society for Reproductive Medicine, the Society of Reproductive Biologists and Technologists, and the Society for Assisted Reproductive Technology In vitro maturation: a committee opinion. Fertil Steril. 2021;115(2):298–304. doi: 10.1016/j.fertnstert.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Paulson RJ, Fauser B, Vuong LTN, Doody K. Can we modify assisted reproductive technology practice to broaden reproductive care access? Fertil Steril. 2016;105(5):1138–1143. doi: 10.1016/j.fertnstert.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Vuong LN, Ho TM, Gilchrist RB, Smitz J. The place of in vitro maturation in assisted reproductive technology. Fertility & Reproduction. 2019;1(01):11–15. doi: 10.1142/s2661318219300022. [DOI] [Google Scholar]
- 5.Sanchez F, Lolicato F, Romero S, De Vos M, Van Ranst H, Verheyen G, et al. An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield. Human reproduction (Oxford, England) 2017;32(10):2056–2068. doi: 10.1093/humrep/dex262. [DOI] [PubMed] [Google Scholar]
- 6.Vuong LN, Le AH, Ho VNA, Pham TD, Sanchez F, Romero S, et al. Live births after oocyte in vitro maturation with a prematuration step in women with polycystic ovary syndrome. J Assist Reprod Genet. 2020;37(2):347–357. doi: 10.1007/s10815-019-01677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilchrist RB, Luciano AM, Richani D, Zeng HT, Wang X, Vos MD, Sugimura S, Smitz J, Richard FJ, Thompson JG. Oocyte maturation and quality: role of cyclic nucleotides. Reproduction. 2016;152(5):R143–R157. doi: 10.1530/rep-15-0606. [DOI] [PubMed] [Google Scholar]
- 8.Vuong LN, Ho VNA, Ho TM, Dang VQ, Phung TH, Giang NH, et al. In-vitro maturation of oocytes versus conventional IVF in women with infertility and a high antral follicle count: a randomized non-inferiority controlled trial. Human reproduction (Oxford, England) 2020;35(11):2537–2547. doi: 10.1093/humrep/deaa240. [DOI] [PubMed] [Google Scholar]
- 9.Ho VNA, Pham TD, Le AH, Ho TM, Vuong LN. Live birth rate after human chorionic gonadotropin priming in vitro maturation in women with polycystic ovary syndrome. Journal of ovarian research. 2018;11(1):70. doi: 10.1186/s13048-018-0445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortega-Hrepich C, Stoop D, Guzmán L, Van Landuyt L, Tournaye H, Smitz J, et al. A “freeze-all” embryo strategy after in vitro maturation: a novel approach in women with polycystic ovary syndrome? Fertil Steril. 2013;100(4):1002–1007. doi: 10.1016/j.fertnstert.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Walls ML, Hunter T, Ryan JP, Keelan JA, Nathan E, Hart RJ. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries: a comparative analysis of fresh, frozen and cumulative cycle outcomes. Human reproduction (Oxford, England) 2015;30(1):88–96. doi: 10.1093/humrep/deu248. [DOI] [PubMed] [Google Scholar]
- 12.Broekmans FJ, de Ziegler D, Howles CM, Gougeon A, Trew G, Olivennes F. The antral follicle count: practical recommendations for better standardization. Fertil Steril. 2010;94(3):1044–1051. doi: 10.1016/j.fertnstert.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 13.ALPHA. Scientists in Reproductive Medicine, ESHRE Special Interest Group Embryology Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod BioMed Online. 2011;22(6):632–646. doi: 10.1016/j.rbmo.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Humaidan P, Nelson SM, Devroey P, Coddington CC, Schwartz LB, Gordon K, et al. Ovarian hyperstimulation syndrome: review and new classification criteria for reporting in clinical trials. Human reproduction (Oxford, England) 2016;31(9):1997–2004. doi: 10.1093/humrep/dew149. [DOI] [PubMed] [Google Scholar]
- 15.Ho VNA, Braam SC, Pham TD, Mol BW, Vuong LN. The effectiveness and safety of in vitro maturation of oocytes versus in vitro fertilization in women with a high antral follicle count. Human reproduction (Oxford, England) 2019;34(6):1055–1064. doi: 10.1093/humrep/dez060. [DOI] [PubMed] [Google Scholar]
- 16.Ortega-Hrepich C, Drakopoulos P, Bourgain C, Van Vaerenbergh I, Guzman L, Tournaye H, et al. Aberrant endometrial steroid receptor expression in in-vitro maturation cycles despite hormonal luteal support: a pilot study. Reprod Biol. 2019;19(2):210–217. doi: 10.1016/j.repbio.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Blake DA, Proctor M, Johnson NP. The merits of blastocyst versus cleavage stage embryo transfer: a Cochrane review. Human reproduction (Oxford, England) 2004;19(4):795–807. doi: 10.1093/humrep/deh104. [DOI] [PubMed] [Google Scholar]
- 18.Coskun S, Hollanders J, Al-Hassan S, Al-Sufyan H, Al-Mayman H, Jaroudi K. Day 5 versus day 3 embryo transfer: a controlled randomized trial. Human reproduction (Oxford, England) 2000;15(9):1947–1952. doi: 10.1093/humrep/15.9.1947. [DOI] [PubMed] [Google Scholar]
- 19.Papanikolaou EG, Camus M, Kolibianakis EM, Van Landuyt L, Van Steirteghem A, Devroey P. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med. 2006;354(11):1139–1146. doi: 10.1056/NEJMoa053524. [DOI] [PubMed] [Google Scholar]
- 20.Papanikolaou EG, D’Haeseleer E, Verheyen G, Van de Velde H, Camus M, Van Steirteghem A, et al. Live birth rate is significantly higher after blastocyst transfer than after cleavage-stage embryo transfer when at least four embryos are available on day 3 of embryo culture. A randomized prospective study. Human reproduction (Oxford, England) 2005;20(11):3198–3203. doi: 10.1093/humrep/dei217. [DOI] [PubMed] [Google Scholar]
- 21.Zech NH, Lejeune B, Puissant F, Vanderzwalmen S, Zech H, Vanderzwalmen P. Prospective evaluation of the optimal time for selecting a single embryo for transfer: day 3 versus day 5. Fertil Steril. 2007;88(1):244–246. doi: 10.1016/j.fertnstert.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 22.Cohen Y, St-Onge-St-Hilaire A, Tannus S, Younes G, Dahan MH, Buckett W, Son WY. Decreased pregnancy and live birth rates after vitrification of in vitro matured oocytes. J Assist Reprod Genet. 2018;35(9):1683–1689. doi: 10.1007/s10815-018-1216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smitz JE, Thompson JG, Gilchrist RB. The promise of in vitro maturation in assisted reproduction and fertility preservation. Semin Reprod Med. 2011;29(1):24–37. doi: 10.1055/s-0030-1268701. [DOI] [PubMed] [Google Scholar]
- 24.Bosdou JK, Venetis CA, Tarlatzis BC, Grimbizis GF, Kolibianakis EM. Higher probability of live-birth in high, but not normal, responders after first frozen-embryo transfer in a freeze-only cycle strategy compared to fresh-embryo transfer: a meta-analysis. Human reproduction (Oxford, England) 2019;34(3):491–505. doi: 10.1093/humrep/dey388. [DOI] [PubMed] [Google Scholar]
- 25.Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update. 2019;25(1):2–14. doi: 10.1093/humupd/dmy033. [DOI] [PubMed] [Google Scholar]
- 26.Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, Yang J, Liu J, Wei D, Weng N, Tian L, Hao C, Yang D, Zhou F, Shi J, Xu Y, Li J, Yan J, Qin Y, Zhao H, Zhang H, Legro RS. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med. 2016;375(6):523–533. doi: 10.1056/NEJMoa1513873. [DOI] [PubMed] [Google Scholar]
- 27.Wei D, Liu JY, Sun Y, Shi Y, Zhang B, Liu JQ, Tan J, Liang X, Cao Y, Wang Z, Qin Y, Zhao H, Zhou Y, Ren H, Hao G, Ling X, Zhao J, Zhang Y, Qi X, Zhang L, Deng X, Chen X, Zhu Y, Wang X, Tian LF, Lv Q, Ma X, Zhang H, Legro RS, Chen ZJ. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393(10178):1310–1318. doi: 10.1016/S0140-6736(18)32843-5. [DOI] [PubMed] [Google Scholar]
- 28.Stormlund S, Sopa N, Zedeler A, Bogstad J, Praetorius L, Nielsen HS, et al. Freeze-all versus fresh blastocyst transfer strategy during in vitro fertilisation in women with regular menstrual cycles: multicentre randomised controlled trial. BMJ. 2020;370:m2519. doi: 10.1136/bmj.m2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, Zhu Y, Deng X, Qi X, Li H, Ma X, Ren H, Wang Y, Zhang D, Wang B, Liu F, Wu Q, Wang Z, Bai H, Li Y, Zhou Y, Sun M, Liu H, Li J, Zhang L, Chen X, Zhang S, Sun X, Legro RS, Chen ZJ. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. 2018;378(2):126–136. doi: 10.1056/NEJMoa1705334. [DOI] [PubMed] [Google Scholar]
- 30.Vuong LN, Dang VQ, Ho TM, Huynh BG, Ha DT, Pham TD, Nguyen LK, Norman RJ, Mol BW. IVF transfer of fresh or frozen embryos in women without polycystic ovaries. N Engl J Med. 2018;378(2):137–147. doi: 10.1056/NEJMoa1703768. [DOI] [PubMed] [Google Scholar]
- 31.Practice Committee of the American Society for Reproductive Medicine Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril. 2017;107(4):901–903. doi: 10.1016/j.fertnstert.2017.02.107. [DOI] [PubMed] [Google Scholar]
- 32.Brinsden PR, Wada I, Tan SL, Balen A, Jacobs HS. Diagnosis, prevention and management of ovarian hyperstimulation syndrome. Br J Obstet Gynaecol. 1995;102(10):767–772. doi: 10.1111/j.1471-0528.1995.tb10840.x. [DOI] [PubMed] [Google Scholar]
- 33.MacDougall MJ, Tan SL, Jacobs HS. In-vitro fertilization and the ovarian hyperstimulation syndrome. Human reproduction (Oxford, England) 1992;7(5):597–600. doi: 10.1093/oxfordjournals.humrep.a137702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy but are available from the corresponding author on reasonable request.