Abstract
Meningioma is the most common central nervous system tumor that usually behaves benignly and has a good prognosis after treatment with tumor gross resection and with or without adjuvant therapy. Malignancy in meningioma is very rare and extracranial metastasis to cervical lymph nodes is even rarer. We report a case of a 40-year-old woman diagnosed with metastatic rhabdoid meningioma. She had recurrent primary disease and metastasis to bilateral cervical lymph nodes. She previously had intracranial tumor twice resected. We also review relevant, previously published cases in the literature. I hope you find these suggestions helpful.
Keywords: meningioma, rhabdoid meningioma, cervical lymph nodes
Introduction
Meningioma is the most common central nervous system (CNS) tumor with a wide range of histopathological variants, grouped into 3 grades by their microscopic appearances, according to the World Health Organization (WHO) classification. Grade III (malignant) meningiomas, including rhabdoid meningiomas (RMs), are unusual neoplasms that proliferate quickly, behave aggressively, and have a poor prognosis. Most RM tend to recur intracranially after initial treatment, but it presents as an extracranial metastasis in very rare cases.
Case Presentation
A 40-year-old female was present with a complaint of bilateral lymph node enlargement lasting for 3 months. Fifteen years ago, she was admitted for the first time with a frontal meningioma and underwent gross tumor resection. The tumor had recurred locally and had been totally resected for the second time 1 year before her last presentation. The postoperative histopathology confirmed the presence of an atypical meningioma. However, that patient refused adjuvant irradiation after her second surgery.
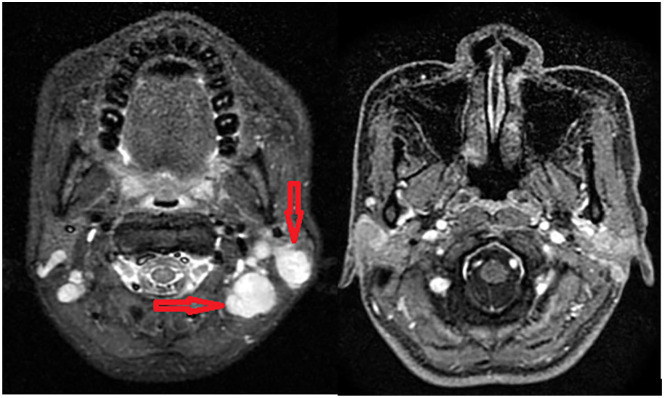
Physical examination revealed bilateral submandibular and upper cervical lymph nodes. The largest one was measured approximately 3 cm in diameter. In addition, she has persistent right-sided hemiplegia since the first surgery. Other examinations were normal with no neuropathic deficiency. Sonography and magnetic resonance imaging revealed bilateral lymphadenopathy of upper jugular, middle jugular, and parotid groups (levels II, III, and VIII, according to Robbins modified system). Some of those showed necrosis center (Figure 1).
Figure 1.
Magnetic resonance imaging demonstrated enlargement of bilateral neck node of upper jugular, middle jugular, and parotid groups (arrow). These nodes lost their normal architecture and some showed necrosis center. The largest one measured 21 × 17 mm.
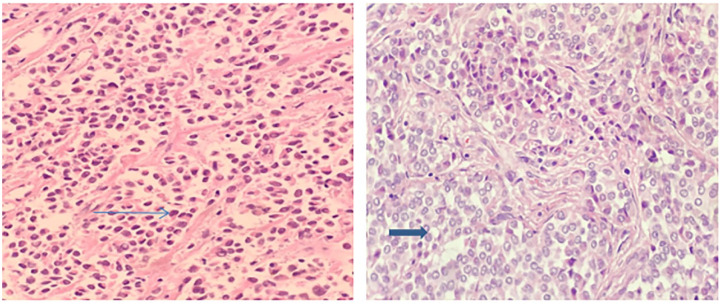
Careful upper gastrointestinal endoscopy and flexible endoscopy of ear, nose, and throat yielded no abnormality. An excisional neck node biopsy was performed, resecting the left level II node. Microscopically, the node was composed of spindle cells with abundant eosinophilic cytoplasm. They had large and pleomorphic nuclei with prominent nucleoli (Figure 2).
Figure 2.
Results of pathology: Tumor cells are clustered, with round or oval nuclear, and unclear nucleoli. Mitosis activities in those cells are low with eosinophilic cytoplasm (arrow).
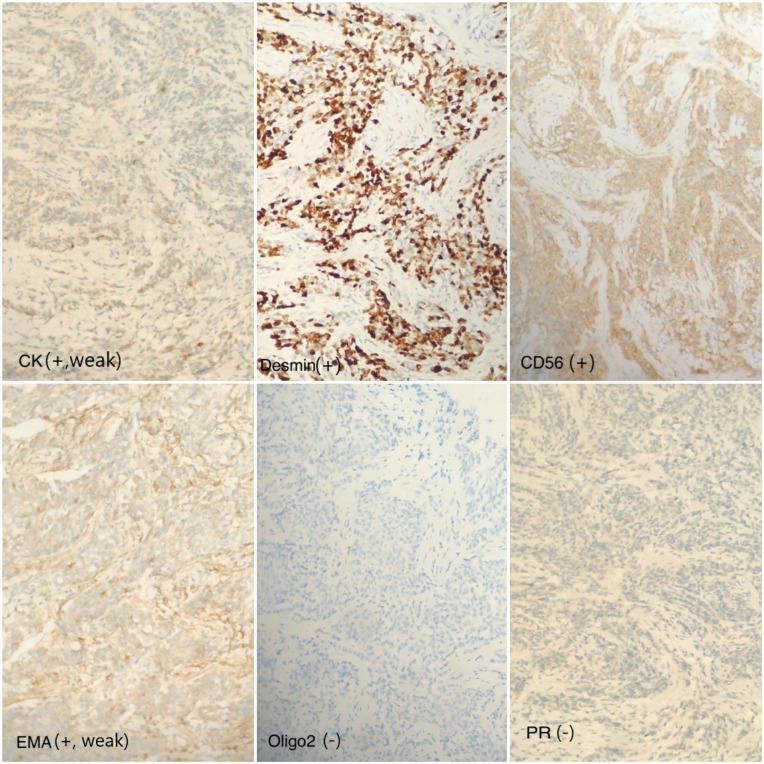
In terms of immunohistochemical features, the tumor cells were positive for epithelial membrane antigen (EMA), vimentin-1, and negative for the cluster of differentiation markers including TTF-1, CDX-2, S100, and PR (Figure 3).
Figure 3.
Results of immunohistochemistry: marker CK (+) sporadic, weak; marker desmin (+); marker CD56 (+); marker epithelial membrane antigen (+) weak; marker Oligo2 (−); marker PR (−).
The clinical and histological evidence above was consistent with a diagnosis of metastatic neck nodes from meningioma. Subsequently, 18F-fluorodeoxyglucose positron emission tomography/computed tomography was carried out for further evaluation. The result demonstrated no abnormality but the fluorodeoxyglucose-avid lesions corresponding to previously found neck nodes. We decided to treat our patient with total neck node dissection and adjuvant radiation therapy because of the localized nature of the disease to the neck and the absence of extranodal involvement. Our patient underwent a cervical lymphadenectomy that removed bilateral parotid gland and level Ib-V lymph nodes. Following surgery, she received radiation therapy with a dose of 60 Gy to the resected tumor bed. Histopathological findings of resected nodes confirmed the diagnosis of metastatic RM. Twenty-six months after treatment completion, the patient is currently disease-free, without any sign of recurrence.
Discussion
Meningiomas are known to arise from arachnoid meningothelial cells of meninges, and they also are the most common CNS tumors that cause one third of all intracranial and spinal neoplasms. 1 The cause of meningioma is unclear, but ionizing radiation exposure, genetic predisposition, hormonal factors, and obesity have been proved to play a potential role.2,3 Diagnosis of this entity is usually based on clinical evidence, mainly by neuroimaging that typically presents as an extra-axial, dural-based mass. However, due to unspecific clinical appearance that may mimic a wide range of other benign and malignant diseases, the definite diagnosis should be based on the postoperative histological findings.
By morphologic features, meningioma is classified into 3 grades, according to the WHO classification system. 4 Approximately 81.1% of cases are WHO grade I, defined as the benign neoplasm that lacks anaplastic features seen in higher grades. WHO grade II (atypical, clear cell, choroid meningiomas) and grade III (malignant meningiomas) account for only 16.9% and 1.7%. 5 The higher grade diseases relate to a higher risk of recurrence and distal metastasis. The reported recurrence rates for patients diagnosed with grades I, II, and III are 7% to 25%, 30% to 50%, and 50% to 94%, respectively.6-8
Although WHO grade III is known as a malignant disease, most of the reported cases recurred in the primary site, while extracranial metastatic cases are uncommon. Proposed mechanisms for the low probability of metastasis are the strong connection between tumor cells and the lack of an intracranial lymphatic drainage system. 9 Malignant meningioma (WHO grade III) is subdivided into 3 subgroups: anaplastic, papillary, and RMs. While anaplastic meningioma, the most common subgroup, has been reported in several studies recently, other categories including RM are still poorly understood.
In the literature, fewer than 20 cases were found to be diagnosed with RM with extracranial spread via PubMed search for “rhabdoid meningioma” and “metastasis” in case reports. Therefore, we present here 6 published cases with detailed patient information in comparison with our patient (Table 1).
Table 1.
Comparison of Clinical Features of Documented RM Case Reports.
| Source | Gender/age (years) | Metastatic site | Number of recurrences up to now (with time interval) | Previous treatment | Current treatment | Outcome | |
|---|---|---|---|---|---|---|---|
| Surgical treatment | Other therapy | ||||||
| Kakkar et al 10 | Male/31 | Lung, lymph nodes, bone | 6 (13 years), atypical meningioma | Resection/GKR | CT | 7 months | |
| Zhao et al 11 | Female/39 | Lung, cerebellum, kidney, bone | Concurrent with primary tumor | Primary tumor and pulmonary metastasis resection | Radiation therapy/chemotherapy | 1 year | |
| Nair et al 12 | Male/59 | Parotid gland | 3 (7 years) | Resection/RT | Parotidectomy | Alive | |
| Wang et al 13 | Male/16 | Liver | 2 (22 months) | Resection/RT/CT | CT | Progression after 5 months | |
| Hussein et al 14 | Female/16 | Lung | Concurrence with primary tumor | Tumor resection | RT/CT | 3 months | |
| Filho et al 9 | Female/21 | Lung, liver | Several times | Resection | Palliative surgery | 4 months | |
| Our case | Female/40 | Cervical lymph nodes | 3 (15 years) | Resection | Lymphadenectomy | RT | 26 months |
Abbreviations: RM, rhabdoid meningioma; GKR, gamma knife radiation; CT, computed tomography; RT, radiation therapy.
Distinguished from benign meningioma whose incidence increases with age, the average onset age of metastatic RM was 27.33 years (65 for benign meningioma), and a high proportion of patients were diagnosed in the age of adolescence and young adulthood (Table 1). Most cases were identified metastasis with a history of several recurrences; otherwise, 2 patients were diagnosed with simultaneous primary tumor and distal progression. When present, meningioma metastases most commonly occur in the lung (48.1%), followed by the spine (18.7%), skeletal bone (11.4%), and the liver (9.5%). 1 The exact mechanism of metastasis is still unclear, but it is believed mostly through the hematogenous pathway, by microembolization or invasion of large vessels, and venous sinuses. 9 Other pathways investigated include skull trauma, surgery, and cerebrospinal fluid route.15,16
In general, clinical presentations of metastatic malignant meningioma (and RM particularly) vary and depend on the metastatic site concurrence in CNS. Definite diagnosis is made by confirmation of histopathology finding of resected or biopsied tissue. WHO criteria to identify RM are the presentation of predominant rhabdoid morphology of tumor cells in the accessed sample. Typically, these cells are characterized by abundant eosinophilic cytoplasm, cytoplasmic hyaline inclusions, and eccentric nuclei. Other features such as the mitotic index, high cellularity, sheeting architecture, or nuclear atypia are met in most cases but just considered as supporting criteria. 4 From 4 first published cases using the term “rhabdoid meningioma,” Kepes et al 17 suggested that rhabdoid morphology related to malignant transformation and poorer prognosis. In general, the majority of RM behave aggressively. However, not all of the meningioma patients with rhabdoid displaying malignant characteristics; histological features of malignancy were useful in these cases.18,19
Immunohistochemical staining may be helpful not only in diagnosis but also in exclusion of other different diagnoses and evaluation of tumor aggressiveness. On immunohistochemistry, immunoprofile of meningioma is a group of diseases with positiveness to EMA, vimentin, and claudin-1. Desmin and INI-1 are valuable markers to identify malignant rhabdoid variants. 9 In addition, Ki-67 index (MIB-1), an identification of cell proliferate activity, appears as a promising predictor of survival and disease recurrence in meningioma. 20
An optimal therapeutic approach for metastatic meningioma is still controversial; however, it seems like a single modality treatment is inadequate to prevent the malignant progression. Current treatment is based on consensus guidelines of atypical and anaplastic meningioma (WHO grades II and III), that surgery and radiotherapy are employed. Complete tumor resection, both primary tumor and metastatic disease if feasible, is the preferred initial treatment. The addition of irradiation to resected tumor bed is a standard component in treatment of patients with malignant meningioma in an effort to improve local control. In the literature, we found 14 reported cases with cervical lymph node metastases from meningioma. Noticeably, there was a case of a 58-year-old male with anaplastic meningioma recurrence intracranially and in the neck nodes after tumor resection twice. He then was successfully treated with radical neck dissection and adjuvant radiotherapy to the primary site and involved neck with 2 years follow-up of no recurrence. 21
Limited therapeutic options still challenge treatment of unresectable malignant meningioma. Alternative choices as systematic therapy and immunotherapy are being investigated in ongoing studies. In a retrospective study, Kessler et al 22 reviewed 168 patients diagnosed with WHO grades II and III meningiomas, in those 6 patients with histologically confirmed metastatic disease. 22 Although 5 of those 6 patients received at least one type of chemotherapy and a wide range of agents were used, with or without combined treatment, it was supposed that there was no significant improvement or benefit obtained. Interferon α2-b and hormonal therapy, which was suggested to be able to exhibit marked regression in aggressive meningioma, provided no positive response in the described metastatic cases.23,24 In addition, it was recently shown that the breast cancer (BRCA)1-associated protein-1 tumor suppressor gene (BAP1) is inactivated in a subset of meningiomas that display rhabdoid histomorphology. Patients with BAP1-deficient meningiomas were clinically more aggressive and more likely to relapse compared with BAP1-intact cases. Thus, this group of patients may require closer posttreatment surveillance and have more benefit from adjuvant therapies. Regrettably, BAP1 immunohistochemical staining was not performed for this case due to our institute’s limited laboratory resource, but it is recommended for suspected cases with RM.2,3
Overall, the prognosis of meningioma patients diagnosed with extra-CNS involvement is poor, with an expected overall survival of only few months to few years. Most deaths were due to uncontrolled CNS tumor rather than metastasis.
Conclusion
We highlight here a rare case of RM with pathological confirmation of cervical node metastases. In general, diagnosis and treatment of metastatic meningioma are controversial, due to its rarity, aggressive nature, and unspecific appearance. Further studies should be conducted in order to establish a consensus guidance, but surgical resection with adjuvant radiation therapy may be a promising treatment for these cases.
Footnotes
Authors’ Note: The datasets used during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: The study was approved by our research committee, Hanoi Medical University, Hanoi, Vietnam, and Vietnam National Cancer Hospital, Hanoi, Vietnam.
Informed Consent: The publication of this study has been consented by the patient.
ORCID iD: Dang Van Nguyen  https://orcid.org/0000-0002-3370-478X
https://orcid.org/0000-0002-3370-478X
References
- 1. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99:307-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braganza MZ, Kitahara CM, de González AB, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 2012;14:1316-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asgharian B, Chen YJ, Patronas NJ, et al. Meningiomas may be a component tumor of multiple endocrine neoplasia type 1. Clin Cancer Res. 2004;10:869-880. [DOI] [PubMed] [Google Scholar]
- 4. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803-820. [DOI] [PubMed] [Google Scholar]
- 5. Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016;18(suppl 5):v1-v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang SY, Park CK, Park SH, Kim DG, Chung YS, Jung HW. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry. 2008;79:574-580. [DOI] [PubMed] [Google Scholar]
- 7. Pasquier D, Bijmolt S, Veninga T, et al. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2008;71:1388-1393. [DOI] [PubMed] [Google Scholar]
- 8. Palma L, Celli P, Franco C, Cervoni L, Cantore G. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J Neurosurg. 1997;86:793-800. [DOI] [PubMed] [Google Scholar]
- 9. Filho PMM, Silva RMS, Annes RD, et al. Multiple extracranial metastases from a rhabdoid meningioma: a case report. Arq Bras Neurocir. 2015;34:232-236. [Google Scholar]
- 10. Kakkar A, Baghmar S, Garg A, et al. Recurrent rhabdoid meningioma with lymph node, pulmonary and bone metastases: a diagnostic and therapeutic challenge. Brain Tumor Pathol. 2016;33:228-233. [DOI] [PubMed] [Google Scholar]
- 11. Zhao P, Li N, Cao J, et al. Rhabdoid meningioma arising concurrent in pulmonary and intracranial with a rare malignant clinical progression: case report and literature review. World Neurosurg. 2017;107:1046.e17-1046.e22. [DOI] [PubMed] [Google Scholar]
- 12. Nair RP, Vinod Sarma Y, Nayal B, Dil KS, Tripathi PK. Metastatic rhabdoid meningioma of the parotid—mimicking primary salivary gland neoplasm. Int J Surg Case Rep. 2014;6:104-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Z, Kong M, Li J, Xiao W, Zheng S. Intraspinal rhabdoid meningioma metastasis to the liver. J Clin Neurosci. 2011;18:714-716. [DOI] [PubMed] [Google Scholar]
- 14. Kheshaifati H, Alhindi H, Homoud MM. Rhabdoid meningioma with lung metastasis in a paediatric patient: a case report and literature review. J Health Specialties. 2016;4:301-305. [Google Scholar]
- 15. Ng JJ, Teo KA, Shabbir A, Yeo TT. Widespread intra-abdominal carcinomatosis from a rhabdoid meningioma after placement of a ventriculoperitoneal shunt: a case report and review of the literature. Asian J Neurosurg. 2018;13:386-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chuang HC, Lee HC, Cho DY. Intracranial malignant meningioma with multiple spinal metastases—a case report and literature review: case report. Spine (Phila Pa 1976). 2006;31:E1006-E1010. [DOI] [PubMed] [Google Scholar]
- 17. Kepes JJ, Moral LA, Wilkinson SB, Abdullah A, Llena JF. Rhabdoid transformation of tumor cells in meningiomas: a histologic indication of increased proliferative activity: report of four cases. Am J Surg Pathol. 1998;22:231-238. [DOI] [PubMed] [Google Scholar]
- 18. Cooper WA, Shingde M, Lee VK, et al. “Rhabdoid meningioma” lacking malignant features. Report of two cases. Clin Neuropathol. 2004;23:16-20. [PubMed] [Google Scholar]
- 19. Mardi K, Thakur RC, Biswas B. Rhabdoid meningioma lacking malignant features: report of a rare case with review of literature. Asian J Neurosurg. 2015;10:172-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Torp SH, Lindboe CF, Grønberg BH, Lydersen S, Sundstrøm S. Prognostic significance of Ki-67/MIB-1 proliferation index in meningiomas. Clin Neuropathol. 2005;24:170-174. [PubMed] [Google Scholar]
- 21. Moubayed SP, Guertin L, Lambert C, Desrochers P, Nehmé J, Coulombe G. Successful treatment of anaplastic meningioma metastatic to cervical lymph nodes. Head Neck. 2013;35:E115-E118. [DOI] [PubMed] [Google Scholar]
- 22. Kessler RA, Garzon-Muvdi T, Yang W, et al. Metastatic atypical and anaplastic meningioma: a case series and review of the literature. World Neurosurg. 2017;101:47-56. [DOI] [PubMed] [Google Scholar]
- 23. Sioka C, Kyritsis AP. Chemotherapy, hormonal therapy, and immunotherapy for recurrent meningiomas. J Neurooncol. 2009;92:1-6. [DOI] [PubMed] [Google Scholar]
- 24. Omidvari S, Nasrolahi H, Daneshbod Y, et al. Cervical lymph node metastases from meningioma: report of two cases and treatment outcome. Middle East J Cancer. 2010;1:45-49. [Google Scholar]