Abstract
Background
Autonomic dysregulation may lead to blunted sympathetic reactivity in chronic pain states. Autonomic responses are controlled by the central autonomic network (CAN). Little research has examined sympathetic reactivity and associations with brain CAN structures in the presence of chronic pain; thus, the present study aims to investigate how chronic pain influences sympathetic reactivity and associations with CAN brain region volumes.
Methods
Sympathetic reactivity was measured as change in skin conductance level (ΔSCL) between a resting reference period and walking periods for typical and complex walking tasks (obstacle and dual-task). Participants included 31 people with (n = 19) and without (n = 12) chronic musculoskeletal pain. Structural 3 T MRI was used to determine gray matter volume associations with ΔSCL in regions of the CAN (i.e., brainstem, amygdala, insula, and anterior cingulate cortex).
Results
ΔSCL varied across walking tasks (main effect p = 0.036), with lower ΔSCL in chronic pain participants compared to controls across trials 2 and 3 under the obstacle walking condition. ΔSCL during typical walking was associated with multiple CAN gray matter volumes, including brainstem, bilateral insula, amygdala, and right caudal anterior cingulate cortex (p’s < 0.05). The difference in ΔSCL from typical-to-obstacle walking were associated with volumes of the midbrain segment of the brainstem and anterior segment of the circular sulcus of the insula (p’s < 0.05), with no other significant associations. The difference in ΔSCL from typical-to-dual task walking was associated with the bilateral caudal anterior cingulate cortex, and left rostral cingulate cortex (p’s < 0.05).
Conclusions
Sympathetic reactivity is blunted during typical and complex walking tasks in persons with chronic pain. Additionally, blunted sympathetic reactivity is associated with CAN brain structure, with direction of association dependent on brain region. These results support the idea that chronic pain may negatively impact typical autonomic responses needed for walking performance via its potential impact on the brain.
Keywords: chronic pain, neuroimaging, mobility, central autonomic network, sympathetic nervous system
Introduction
Chronic pain disrupts the lives of more than 1.5 billion people worldwide, decreasing quality of life and overall well-being. 1 Chronic pain leads to diminished physical and cognitive function through a number of mechanisms, including emerging evidence for a role of autonomic nervous system dysfunction.
In healthy people, a nociceptive stimulus provides a stressor that activates the sympathetic nervous system as part of the ‘fight or flight’ response. By activating the sympathetic nervous system, the acute pain stimulus influences cardiovascular parameters such as heart rate, heart rate variability, and blood pressure.2,3 Because sympathetic nerve endings are found in the skin, increased sympathetic activity will also increase skin electrodermal activity, or skin conductance. 2
In chronic pain states, the autonomic nervous system may be dysregulated, often leading to blunted autonomic reactivity to nociceptive or stressful stimuli. For example, heart rate variability is significantly decreased in patients with irritable bowel syndrome compared to healthy controls. 4 Similarly, galvanic skin response reactivity tends to be lower in these same patients. 4 In low back pain, sympathetic response latency increases, and amplitudes tend to decrease compared to healthy controls. 5 Rheumatoid arthritis 6 and fibromyalgia 3 may also lead to autonomic dysregulation.
The sympathetic nervous system is important for priming the body for action. While commonly known for its role in the ‘fight or flight’ response, the sympathetic nervous system is also upregulated in response to lesser threats such as walking in complex environments.7,8 Blunted sympathetic nervous system response may have negative implications on attention and readiness for action under such conditions, which could increase fall risk. However, it is not known whether chronic pain patients exhibit similar sympathetic nervous system responses to simple and complex walking task engagement.
Autonomic responses to both internal and external stimuli are controlled by the central autonomic network (CAN), 9 which consists of three levels that form complex structural and functional relationships. First, the spinal cord controls reflexive actions of the autonomic nervous system. Next, areas of the brainstem, including the nucleus tractus soltarius (NTS), ventrolateral medulla (VLM), and parabrachial nucleus of the dorsolateral pons, integrate afferent autonomic and nociceptive signals to immediately modulate cardiovascular, respiratory, and digestive function. Also at the brainstem level, the periaqueductal grey (PAG) incorporates nociceptive and autonomic signals to proceed to cortical regions of the brain to be processed as pain or to modulate descending pathways. 10 Other functions, such as autonomic, endocrine, sleep, bodily sensations, pain, and emotional stimuli are integrated in the forebrain regions (anterior cingulate cortex (ACC), amygdala, and insular cortices). 9
Neuroimaging techniques are useful in evaluating the complex structural and functional relationships between different brain centers in the CAN (for in depth review see Sklerov et al. 11 ). Many studies have examined the normal structural and functional relationships of the CAN using fMRI; however, there is a critical need for studies examining autonomic dysfunction of the CAN in disease states. One study showed reduced grey matter volume in the CAN with neurological disease and respiratory dysfunction. 12 While chronic pain patients exhibit grey matter reductions in several brain areas belonging to the nociceptive system, 13 it is not known whether chronic pain patients exhibit maladaptive structural plasticity in the CAN and whether grey matter volume is related to autonomic function in persons with chronic pain. Therefore, the first aim of the present study was to investigate chronic pain differences in sympathetic nervous system reactivity during simple and complex mobility tasks. The second aim was to determine relationships between sympathetic nervous system reactivity and brain volume in regions of the CAN (i.e., brainstem, amygdala, insula, and the anterior circulate cortex). We hypothesized sympathetic reactivity would be blunted in individuals that report chronic pain compared to those that do not. Further, we hypothesized lower grey matter volume in brain regions of the CAN would be associated with baseline levels of sympathetic reactivity and changes from this baseline.
Methods
Participants
This investigation is a secondary analysis of thirty-one individuals (ages 18–93) enrolled in a project at the University of Florida to evaluate the neurobiology of age-related differences in pain modulation and its impact on function (Neuromodulatory Examination of Pain and Mobility Across the Lifespan [NEPAL]). Potential NEPAL participants were recruited through posted fliers, newspaper ads, and word of mouth referral. Initial screening occurred over the phone, with a secondary screening in person. Persons were excluded based on the following criteria: (1) Alzheimer’s, Parkinson’s, or other condition directly impacting the brain; (2) serious psychiatric conditions (e.g. schizophrenia, major depression, bipolar disorder), (3) uncontrolled hypertension (blood pressure of >150/95 mmHg), heart failure, or history of acute myocardial infarction; (4) systemic rheumatic disorders (i.e. rheumatoid arthritis, systemic lupus erythematosus, fibromyalgia); (5) chronic opioid use; (6) MRI contraindications (i.e., claustrophobia, metal anywhere on the body); (7) excessive anxiety regarding protocol procedures; (8) hospitalization within the preceding year for psychiatric illness; (9) HIV or AIDS; and (10) cognitive impairment (Modified Mini-Mental State Examination [3 MS] score ≤ 77). All procedures were reviewed and approved by the University of Florida's Institutional Review Board, and all participants provided verbal and written informed consent. For the current study, data presented are from 3 separate laboratory visits: (1) a health assessment session (i.e. demographic, general health, pain, and psychological information), (2) a physical function session including the walking tasks, and (3) a neuroimaging session as detailed below. Other measures including data from other study visits are not included in the present investigation.
Health Assessment Session
After obtaining verbal and written consent, general health and demographic information were collected via questionnaires. Additionally, domains related to self-reported pain and other potential covariates were assessed using the following instruments:
Self-Reported Pain
Participants completed a standardized pain history interview regarding the presence of pain across several body regions (i.e., head/face, neck, shoulders, arms, hands, chest, stomach, upper and lower back, leg, knees, and feet) using a validated body mannequin; participants also reported pain intensity on average.14,15 Participants reporting musculoskeletal pain on most days during daily activities during the past three months were categorized as having chronic pain. This is consistent with the Task Force for the Classification of Chronic Pain Consensus for the 11th version of the International Classification of Diseases (ICD-11) of the World Health Organization (WHO). 16
Psychological and Emotional Function
The frequency of depressive symptoms was measured using the 20-item Center for Epidemiologic Studies Depression Scale (CES-D) questionnaire. 17
Walking Tasks Session
Walking Tasks
During the walking tasks part of the physical function visit, participants completed three tasks; one simple and two complex. The order of the walking tasks remained the same for all participants. Skin conductance measures were collected simultaneously. At the start of each walking task, participants were asked to stand quietly for 40 seconds (resting period) before beginning to walk (performance period). First, participants were asked to walk across a 10-meter walkway at a steady-state comfortable speed (“typical” walking). Next, 3 foam blocks were placed on the same walkway and same distance apart (2.5, 5, and 7.5 meters), and participants were asked to walk at a comfortable speed across the walkway while stepping over the obstacles (“obstacle” walking). This complex task requires more attention, task planning, and execution. Finally, participants completed a cognitive task while walking across the 10-meter walkway. Here, participants were asked to list as many words as possible beginning with a specific letter while walking at a normal pace (“dual-task” walking). Each walking task was repeated three times presenting the letters F, A and S consecutively.
Skin Conductance Level
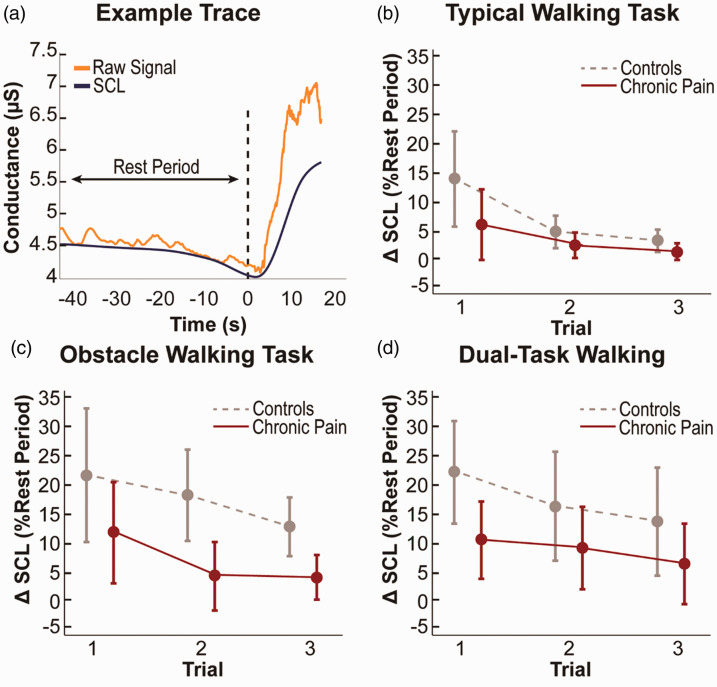
Sympathetic activity was measured via skin conductance during each walking task using a commercially available portable data acquisition unit (Flexcomp Infiniti, Thought Technologies Ltd., QC, Canada). At the palm side of the proximal phalanx on the index and ring finger, adhesive electrodes with conductive electrode gel were adhered to both hands. Electrodes were then plugged into an encoder worn on the participant’s waist, and to prevent the wires from dangling during the tasks, wires were secured to the arm using pre-wrap. During recording, the environment was kept quiet and the participants were asked not to engage in conversation. Because skin conductance is a measure of cognitive arousal, any activity in the environment could potentially increase arousal level and contaminate the data. An example trace is shown in Figure 2.
Figure 2.
Sympathetic reactivity as change in skin conductance level (ΔSCL) from resting to performance periods of walking tasks. (a) Example trace of skin conductance. (b) Typical walking task. (c) Obstacle walking task. (d) Dual-task walking. Data are presented as mean ± 95% confidence interval.
Raw skin conductance data were separated into the tonic component (skin conductance level, or SCL) and the phasic component (skin conductance response) using Ledalab v3.4.9 18 and custom analysis programs in MATLAB version R2018b (The Mathworks, Natick MA). Continuous decomposition analysis 18 was used, with skin conductance responses separated out using an amplitude criterion of 0.01 microsiemens (μS). After removing the skin conductance response, the SCL was used for further data analysis. For each trial, the minimum SCL value during the resting period was extracted, along with the maximum SCL value during the performance period. These values were used to calculate the percent change in SCL (denoted ΔSCL) between resting and performance period of each task:
Calculating the percent change from rest reduces the confounding influence of inter-individual differences in the absolute amplitude of skin conductance.
Neuroimaging Session
Data Collection
Structural brain images were acquired at the University of Florida McKnight Brain Institute with a 3 T Philips Achieva MR Scanner (Philips Medical Systems, Best, The Netherlands) using a 32-channel head coil. We used a MP-RAGE T1-weighted (T1w) sequence 1 mm isotropic voxel, TR = 7.1 ms, TE = 3.2 ms and flip angle of 8 degrees.
Data Analysis
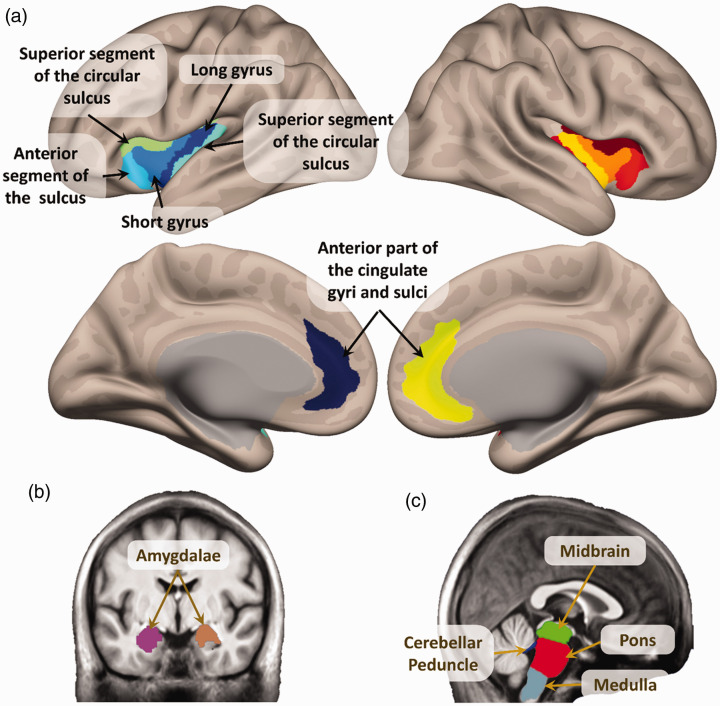
Each T1w image was preprocessed using “recon-all” in Freesurfer 7.1.0. 19 This procedure calculated the volumes of the main areas belonging to the CAN. These are shown in Figure 1 and were all the sulci and gyri the insulae, 20 the anterior cingulate cortices, 20 the amygdalae, 21 and the subdivisions of the brainstem. 22
Figure 1.
Main structures of the Central Autonomic Network (CAN), used in the volumetric analysis. (a) Insulae and anterior cingulate cortices.19,20 (b) Amygdalae.19,21 (c) Areas of the brainstem.22
Statistical Analyses
A repeated-measures ANCOVA with Greenhouse-Geisser correction was conducted to assess whether there were pain differences between ΔSCL measures across walking tasks controlling for age, sex, race, pain, education, and the baseline SCL measure with Bonferronic post-hoc comparisons. Partial correlation coefficients were used to examine associations between ΔSCL during typical and complex walking tasks with volume in brain regions part of the CAN controlling for age, sex, race, pain intensity, education, and the baseline SCL measure. Given the exploratory nature of this correlational analysis, we employed the less strict false discovery rate correction to control for multiple comparisons. 23
Results
Our sample is a subset of NEPAL participants that underwent measurement of skin conductance during mobility testing. Detailed information about the NEPAL participants have been previously reported.24–27 Table 1 summarizes demographic characteristics of the study participants (n = 31). Chronic pain participants experienced musculoskeletal pain of at least 3 months duration that significantly impacted their life on most days; pain was most commonly reported on the back and the knee/leg region. The pain-free control group was significantly younger than the chronic pain group; thus, age was included as a covariate in all analyses. Further, given the known differences in pain and/or autonomic reactivity by sex, race, and education, these variables were included in the analyses. Finally, since pain intensity during walking may confound performance in the walking tasks, this variable was also included in the analyses.
Table 1.
Characteristics of the study participants.
| Chronic pain (n = 19) | No pain controls (n = 12) | p-value | |
|---|---|---|---|
| Age, mean (SD) | 66.8 (17.7) | 46.4 (27.9) | 0.018 |
| Males, N (%) | 9 (47.4) | 5 (41.7) | 0.756 |
| Race, N (%) | 0.399 | ||
| Non-Hispanic White | 16 (84.2) | 10 (83.3) | |
| Asian/Pacific Islander | 1 (5.3) | 1 (8.3) | |
| African American | 2 (10.6) | 0 | |
| Hispanic | 0 | 1 (8.3) | |
| Education (yrs), mean (SD) | 15.5 (2.5) | 14.6 (3.6) | 0.441 |
| CES-D, mean ± SD years | 6.2 (3.2) | 6.1 (6.3) | 0.941 |
boldface values represent a statistical significance between chronic pain and no pain controls (p<0.05).
Chronic Pain Is Related to Blunted Sympathetic Reactivity During Simple and Complex Mobility Tasks
Sympathetic reactivity was measured as ΔSCL between resting and walking periods of a control walking task (typical) and two complex walking tasks (obstacle and dual-task). The repeated measures analysis revealed a main effect of pain group on ΔSCL across walking tasks (F (1, 23)=5.0, p = 0.036, partial eta squared = 0.178), with no other significant interaction effects of interest (p’s > 0.05) controlling for age, sex, race, pain intensity, education, and the baseline SCL measure. Participants reporting chronic pain had significantly lower changes in ΔSCL compared to the controls (Figure 2). Post-hoc comparisons revealed ΔSCL differences during the obstacle walking tasks in trials 2 and 3 (Bonferroni corrected p = 0.016 and p = 0.020, respectively).
Sympathetic Reactivity Is Associated With Regions of the Central Autonomic Network
Associations between ΔSCL during typical and complex walking tasks with volume in brain regions part of the CAN were assessed using partial correlation coefficients. The following variables were controlled for in the statistical model: age, sex, race, pain intensity, education, baseline SCL, and estimated total intracranial volume. First, associations between CAN brain region volumes and ΔSCL during typical walking were determined. For the brainstem regions, ΔSCL during the typical walking task was significantly associated with the medulla (r(24) = 0.493, p = 0.022, q = 0.062) and the midbrain (r(24) = 0.439, p = 0.039, q = 0.062), but not the pons (r(24)=0.309, p = 0.114, q = 0.167) or the superior cerebral peducle (r(24) = −0.122, p = 0.320, q = 0.167). For higher regions of the brain, ΔSCL during typical walking was significantly associated with the right and left anterior segments of the circular sulcus of the insula (Right: r(24) = 0.483, p = 0.029, q = 0.067, Left: r(24)=0.902, p = 0.000, q = 0.000), the left inferior segment of the circular sulcus of the insula (r(24) = 0.605, p = 0.006, q = 0.037), the left superior segment of the circular sulcus of the insula (r(24) = 0.478, p = 0.031, q = 0.067), the right and left amygdala (Right: r(24) = 0.465, p = 0.035, q = 0.067, Left: r(24)=0.507, p = 0.022, q = 0.062), the right caudal anterior cingulate cortex (r(24) = 0.616, p = 0.004, q = 0.032), and the right rostral anterior cingulate cortex (r(24) = 0.412, p = 0.050, q = 0.067).
Finally, changes in sympathetic reactivity from typical to complex walking tasks were used to determine the influence of walking task on associations between ΔSCL and CAN brain region volumes. ΔSCL changes from typical to obstacle walking task were significantly associated with the midbrain segment of the brainstem (r(24) = −0.491, p = 0.027, q = 0.123) and the anterior segment of the circular sulcus of the insula (r(24) = 0.778, p = 0.000, q = 0.000), with no other significant associations. ΔSCL changes from typical to dual-task walking were also significantly associated with the right and left caudal anterior cingulate cortex (Right: r(24) = 0.491, p = 0.027, q = 0.188, Left: r(24) = −0.446, p = 0.042, q = 0.188) and the left rostral cingulate cortex (r(24) = −0.524, p = 0.019, q = 0.188).
Discussion
The present preliminary study examined whether sympathetic reactivity during walking tasks differed by chronic musculoskeletal pain status, and whether sympathetic reactivity was associated with grey matter volume in regions comprising the brain’s central autonomic network (CAN). Two main findings emerged: first, sympathetic reactivity is blunted in persons reporting chronic musculoskeletal pain compared to pain-free controls during simple and complex walking tasks. Second, blunted sympathetic reactivity was significantly associated with grey matter volume in CAN brain regions. Although CAN brain regions are expected to control autonomic responses to both internal and external stimuli, to our knowledge, this is the first investigation testing such associations in persons with and without chronic musculoskeletal pain during simple and complex walking tasks.
Both acute and chronic pain can act as powerful stressors, causing adaptive or maladaptive changes in various physiologic systems, including the autonomic nervous system. The significantly blunted sympathetic reactivity in the participants with chronic pain compared to pain-free controls is consistent with the idea that prolonged stress states (e.g., chronic pain) can reduce the dynamic flexibility of the autonomic nervous system and result in poor adaptation to altered internal or external demands. While acute pain increases sympathetic reactivity, chronic musculoskeletal pain conditions are associated with autonomic dysfunction28–30 with potential detrimental consequences on task performance. 31 Our findings are also consistent with Gaab and colleagues, 32 who showed reduced reactivity of the hypothalamic–pituitary–adrenal axis, a closely interacting system to the autonomic nervous system, in a small sample of participants with chronic whiplash associated disorder. Future research is needed to replicate our findings in larger samples of individuals with chronic musculoskeletal pain.
A novel part of our study was having structural neuroimaging in our participants and, to our knowledge, this is the first attempt to examine associations between sympathetic reactivity and brain structure in persons with and without chronic musculoskeletal pain. Our findings align with a few neuroimaging studies reporting activation in insular cortex, anterior cingulate cortex (ACC), and amygdala, among others, after pain-related sympathetic reactivity.33–35 Maihofner and colleagues 36 also reported an increased activity in ACC and anterior insula that was associated with pain-evoked sympathetic vasoconstrictor reflexes. Seifert 37 also reported that activity in anterior insula correlated to sympathetic reactivity during the pain experience and pain anticipation. ACC activity is also correlated with pain-induced sympathetic vasoconstrictor responses, heat-pain related skin conductance reactivity, and pain anticipation, supporting a potential role for ACC in the generations of autonomic arousal. 38 Further, there are maladaptive autonomic profiles in persons with spinal cord injury (SCI) and neuropathic pain compared to persons with SCI but no neuropathic pain to able-bodied controls 39 suggesting that chronic pain may change the interactions between brain regions of the pain network and those that subserve autonomic control. At the central level, autonomic activation is strongly connected to nociception.40,41 Given the close anatomical and functional overlap between cortical and sub-cortical structures involved in pain processing and those controlling autonomic regulation (e.g., the brainstem, thalamus, hypothalamus, amygdala and insular, anterior cingulate, prefrontal and somatosensory cortices),42–46 our findings are not surprising, but rather confirm these associations in vivo using an ecologically valid model (i.e., walking tasks of various complexicities). Future research in larger samples using additional neuroimaging modalities may further clarify the central mechanisms underlying autonomic dysfunction in chronic musculoskeletal pain.
This study is not without limitations. First, while we used walking tasks to elicit a sympathetic response, it is difficult to discern between responses due to stress versus pain-related responses. However, our findings were robust in controlling for the pain intensity experienced by the participants while walking, thus implying our findings are a result of the stress from the tasks being performed. Future studies should be designed to test this assertion explicitly. Additionally, due to our small sample size, only a few regions of the CAN were explored; accordingly, future studies should include more complex evaluations of relationships in the brain. Additionally, the pain-free group was significantly younger than the chronic pain group making it difficult to discern age-related versus pain-related changes in sympathetic reactivity and CAN grey matter volumes. Although age was included as a covariate, future studies should include age-matched subgroups. Finally, because this is a secondary analysis of a small sample from a larger study, this study was not designed to test for causality between chronic pain, sympathetic reactivity, and grey matter volume of CAN regions. Therefore, chronic pain may be affecting sympathetic reactivity, autonomics may be affecting chronic pain, or the relationship may be bi-directional. Future studies should be designed and powered appropriately to test these hypotheses directly through mediation analysis.
Conclusions
In summary, our study provides further support for a role for chronic musculoskeletal pain in autonomic nervous system dysfunction, or vice versa, during simple and complex walking tasks. Chronic pain is associated with blunted sympathetic reactivity, and this blunted sympathetic reactivity is correlated to grey matter volume in regions of the CAN. Our findings were robust to controlling for multiple factors known to be associated with the complex pain experience and the autonomic nervous system. Future studies should include a larger sample size, which will allow for inclusion of more brain regions, perform more complex neuroimaging measurements (e.g., EEG, functional connectivity, BOLD), and test for causal relationships between chronic pain and sympathetic reactivity.
Acknowledgements
The authors are grateful to our volunteers for their participation and the NEPAL study team. The authors would also like to acknowledge the COVID-19 pandemic, which led to the necessity to work from home and allowed more time to focus on the data analysis of this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Institutes of Health by the following grants: National Institute on Aging (NIA) (grant number K01AG048259) and National Intitute of Arthritis & Musculoskeletal & Skin Diseases (NIAMS) (grant numbers R01AR071431 and F31 AR077996).
ORCID iDs: Taylor D. Yeater https://orcid.org/0000-0002-9078-3066
Julio A. Peraza https://orcid.org/0000-0003-3816-5903
References
- 1.Pfeifer GM. Transforming pain care: an IOM report. Am J Nurs. 2011; 111(9): 18. [DOI] [PubMed] [Google Scholar]
- 2.Kyle BN, Ichola, McNeil DW. Autonomic arousal and experimentally induced pain: a critical review of the literature. Pain Res Manag. 2014; 19(3): 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reyes del Paso GA, Garrido S, Pulgar Á.and Duschele, S. Autonomic cardiovascular control and responses to experimental pain stimulation in fibromyalgia syndrome. J Psychosom Res. 2011; 70(2): 125–134. [DOI] [PubMed] [Google Scholar]
- 4.Tousignant-Laflamme Y, Goffaux P, Bourgault P.and Marchand, S. Different autonomic responses to experimental pain in IBS patients and healthy controls. J Clin Gastroenterol. 2006; 40(9): 814–820. [DOI] [PubMed] [Google Scholar]
- 5.El-Badawy MA, El Mikkawy DME. Sympathetic dysfunction in patients with chronic low back pain and failed back surgery syndrome. Clin J Pain. 2016; 32(3): 226–231. [DOI] [PubMed] [Google Scholar]
- 6.Adlan AM, Paton JFR, Lip GYH.Kitas, GD, and Fisher JP. Increased sympathetic nerve activity and reduced cardiac baroreflex sensitivity in rheumatoid arthritis. J Physiol. 2017; 595(3): 967–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark DJ, Rose DK, Ring SA.and Porges, E. Utilization of central nervous system resources for preparation and performance of complex walking tasks in older adults. Brain Rehabil Res Cent. 2014; 6: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadjistavropoulos T, Carleton NR, Delbaere K, et al. The relationship of fear of falling and balance confidence with balance and dual tasking performance. Psychol Aging. 2012; 27(1): 1–13. doi: 10.1037/a0024054 [DOI] [PubMed] [Google Scholar]
- 9.Benarroch EE. The Central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993; 68(10): 988–1001. [DOI] [PubMed] [Google Scholar]
- 10.Cortelli P, Giannini G, Favoni V.Cevoli S, and Pierangeli G. Nociception and autonomic nervous system. Neurol Sci. 2013; 34(S1): S41–S46. [DOI] [PubMed] [Google Scholar]
- 11.Sklerov M, Dayan E, Browner N. Functional neuroimaging of the central autonomic network: recent developments and clinical implications. Clin Auton Res. 2019; 29(6): 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S-Y, Chen M-H, Chiang P-L, et al. Reduced gray matter volume and respiratory dysfunction in Parkinson’s disease: a voxel-based morphometry study. BMC Neurol. 2018; 18(1): 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruscheweyh R, Deppe M, Lohmann H, et al. Pain is associated with regional grey matter reduction in the general population. Pain. 2011; 152(4): 904–911. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Almeida Y, Martinez-Arizala A, Widerstrm-Noga EG. Chronicity of pain associated with spinal cord injury: a longitudinal analysis. J Rehabil Res Dev. 2005; 42(5): 585–594. [DOI] [PubMed] [Google Scholar]
- 15.Margolis RB, Chibnall JT, Tait RC. Test-retest reliability of the pain drawing instrument. Pain. 1988; 33(1): 49–51. [DOI] [PubMed] [Google Scholar]
- 16.Treede R-D, Rief W, Barke A., et al. A classification of chronic pain for ICD-11. Pain. 2015; 156(6): 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radloff LS. The CES-D scale. Appl Psychol Meas. 1977; 1(3): 385–401. [Google Scholar]
- 18.Benedek M, Kaernbach C. A continuous measure of phasic electrodermal activity. J Neurosci Methods. 2010; 190(1): 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FreeSurfer. https://surfer.nmr.mgh.harvard.edu/. Accessed May 13, 2021.
- 20.Destrieux C, Fischl B, Dale A.and Halgen E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010; 53(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002; 33(3): 341–355. [DOI] [PubMed] [Google Scholar]
- 22.Iglesias JE, Van Leemput K, Bhatt P, et al.; Alzheimer’s Disease Neuroimaging Initiative. Bayesian segmentation of brainstem structures in MRI. Neuroimage. 2015; 113: 184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995; 57(1): 289–300. [Google Scholar]
- 24.Lysne P, Cohen R, Hoyos L.Fillingim RB, Riley JL, and Cruz-Almeida Y. Age and pain differences in non-verbal fluency performance: associations with cortical thickness and subcortical volumes. Exp Gerontol. 2019; 126: 110708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz-Almeida Y, Fillingim RB, Riley JL, et al. Chronic pain is associated with a brain aging biomarker in community-dwelling older adults. Pain. 2019; 160(5): 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montesino-Goicolea S, Valdes-Hernandez PA, Hoyos L, et al. Cortical thickness mediates the association between self-reported pain and sleep quality in community-dwelling older adults. J Pain Res. 2020; 13: 2389–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardoso J, Apagueno B, Lysne P, et al. Pain and the Montreal cognitive assessment (MoCA) in aging [published online ahead of print March 15, 2021]. Pain Med. doi:10.1093/pm/pnab003 [DOI] [PMC free article] [PubMed]
- 28.Gockel M, Lindholm H, Niemistö L.and Hurri H. Perceived disability but not pain is connected with autonomic nervous function among patients with chronic low back pain. J Rehabil Med. 2008; 40(5): 355–358. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Lavin M. Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia. Arthritis Res Ther. 2007; 9(4): 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greening J, Lynn B, Leary R. Sensory and autonomic function in the hands of patients with non-specific arm pain (NSAP) and asymptomatic office workers. Pain. 2003; 104(1–2): 275–281. [DOI] [PubMed] [Google Scholar]
- 31.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010; 141(2): 122–131. [DOI] [PubMed] [Google Scholar]
- 32.Gaab J, Baumann S, Budnoik A.Gmünder H, Hottinger N, and Ehlert U. Reduced reactivity and enhanced negative feedback sensitivity of the hypothalamus–pituitary–adrenal axis in chronic whiplash-associated disorder. Pain. 2005; 119(1–3): 219–224. [DOI] [PubMed] [Google Scholar]
- 33.Dubé AA, Duquette M, Roy M.Lepore F, Duncan G, and Rainville P. Brain activity associated with the electrodermal reactivity to acute heat pain. Neuroimage. 2009; 45(1): 169–180. [DOI] [PubMed] [Google Scholar]
- 34.Mobascher A, Brinkmeyer J, Warbrick T, et al. Fluctuations in electrodermal activity reveal variations in single trial brain responses to painful laser stimuli—a fMRI/EEG study. Neuroimage. 2009; 44(3): 1081–1092. [DOI] [PubMed] [Google Scholar]
- 35.Piché M, Arsenault M, Rainville P. Dissection of perceptual, motor and autonomic components of brain activity evoked by noxious stimulation. Pain. 2010; 149(3): 453–462. [DOI] [PubMed] [Google Scholar]
- 36.Maihöfner C, Seifert F, DeCol R. Activation of central sympathetic networks during innocuous and noxious somatosensory stimulation. Neuroimage. 2011; 55(1): 216–224. [DOI] [PubMed] [Google Scholar]
- 37.Seifert F, Schuberth N, De Col R.Peltz E, Nickel FT, and Maihofner C. Brain activity during sympathetic response in anticipation and experience of pain. Hum Brain Mapp. 2013; 34(8): 1768–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Critchley HD. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. Int J Psychophysiol. 2009; 73(2): 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karri J, Li S, Chen Y.Stampas A, and Li S. Observations of autonomic variability following central neuromodulation for chronic neuropathic pain in spinal cord injury. Neuromodulation Technol Neural Interface. 2021; 24(3): 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miao FJP, Jänig W, Jasmin L.and Levine JD. Blockade of nociceptive inhibition of plasma extravasation by opioid stimulation of the periaqueductal gray and its interaction with vagus-induced inhibition in the rat. Neuroscience. 2003; 119(3): 875–885. [DOI] [PubMed] [Google Scholar]
- 41.Schlereth T, Birklein F. The sympathetic nervous system and pain. NeuroMolecular Med. 2008; 10(3): 141–147. [DOI] [PubMed] [Google Scholar]
- 42.Apkarian AV, Bushnell MC, Treede RD.and Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005; 9(4): 463–484. [DOI] [PubMed] [Google Scholar]
- 43.Thunberg J, Lyskov E, Korotkov A, et al. Brain processing of tonic muscle pain induced by infusion of hypertonic saline. Eur J Pain. 2005; 9(2): 185–194. [DOI] [PubMed] [Google Scholar]
- 44.Benarroch EE. Pain-autonomic interactions. Neurol Sci. 2006; 27(S2): s130–s133. [DOI] [PubMed] [Google Scholar]
- 45.Critchley HD, Corfield DR, Chandler MP.Mathias CJ, and Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol. 2000; 523(1): 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000; 288(5472): 1769–1772. [DOI] [PubMed] [Google Scholar]