Abstract
Background:
Many countries worldwide reported side effects of the coronavirus disease 2019 (COVID-19) pandemic that have influenced the care of patients with other diseases in both acute and elective settings. Patients with multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) represent the major patient population suffering from an autoimmune inflammatory demyelinating disease of the central nervous system. We aimed to analyze MS and NMOSD hospitalizations, the application of plasmapheresis therapy, and the dynamic during different periods of the COVID-19 pandemic in Germany.
Methods:
We conducted a nationwide retrospective cross-sectional study using the administrative database of all hospitalized patients with the main diagnosis of MS and NMOSD, including the information on the application of plasmapheresis therapy. We included full-year data from 1463 hospitals of all MS and NMOSD patients hospitalized in 2019 and 2020 in Germany (n = 87,453). We compared case numbers and plasmapheresis therapy rates of the different pandemic periods in 2020 with the corresponding periods in 2019.
Results:
We observed a substantial decline of MS and NMOSD patients’ hospitalizations during the different pandemic periods, with the most remarkable decline during the first wave of the pandemic (First diagnosis of MS: −16.8%; relapsing-remitting MS: −34.0%; secondary progressive MS: −48.9%; primary progressive MS: −43.8%; NMOSD: −19.2%). Treatment rates with plasmapheresis increased for MS and NMOSD patients in 2020 compared to 2019 (1.8% versus 1.6%, p = 0.003; 14.0% versus 9.3%, p < 0.001), with a substantial increase during the first wave of the pandemic, especially in NMOSD patients (19.7% versus 8.4%, p < 0.001).
Conclusion:
There was a marked decline of MS and NMOSD patients’ hospitalizations during the different pandemic periods in 2020, with the most substantial reduction during the pandemic’s first wave and in progressive MS patients. MS and NMOSD patients who needed rescue relapse treatment continued to receive plasmapheresis therapy in Germany.
Keywords: COVID-19, Germany, multiple sclerosis, neuromyelitis optica, plasmapheresis, SARS-CoV-2
Introduction
Since the beginning of 2020, we have been experiencing an exceptional time caused by the coronavirus disease 2019 (COVID-19) pandemic. This pandemic has caused innumerable victims worldwide who died from a severe acute respiratory coronavirus 2 (SARS-CoV-2) infection, moreover, numerous side effects have been reported. Lockdown measures, elective treatment postponements, and the fear of people have resulted in a substantial decrease in the number of patients seeking medical care in an acute setting or on a regular basis. This has also affected the care of patients with neurological disorders, which are increasingly recognized as major causes of death and disability worldwide. 1
In Germany, there has been an extensive decline in the number of hospitalized stroke and Parkinson’s disease patients during the first wave of the COVID-19 pandemic.2,3 Patients with a demyelinating disease of the central nervous system (CNS), particularly multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) patients, are commonly treated with disease-modifying and immunosuppressive drugs. The evidence of a higher risk for an aggressive course of COVID-19 in patients on disease-modifying therapies (DMT) is controversial.4–6 However, this discussion has fueled uncertainties in MS and NMOSD patients and their treating physicians, affecting the care of these patients. It is unknown to what extend the COVID-19 pandemic has influenced the inpatient care of MS and NMOSD patients, including the application of invasive treatment procedures for acute exacerbations, such as plasmapheresis therapy.
This study aimed to investigate and quantify the number of patients hospitalized for MS and NMOSD, the presence of an acute exacerbation in relapsing-remitting (RR) and secondary progressive (SP) MS patients, and the application of plasmapheresis therapy in MS and NMOSD patients during the different periods of the COVID-19 pandemic in 2020 using full-year nationwide data from Germany.
Methods
Data source and study sample
The data that support the findings of this study are available from the corresponding author upon reasonable request. This is a German nationwide retrospective cross-sectional study using the administrative diagnosis-related group (DRG) database (Data transmission according to §21 KHEntgG and §24 para. 2 KHG; official data on file, source: Institut für das Entgeltsystem im Krankenhaus, InEK, www.g-drg.de). In Germany, all inpatient cases are encoded according to ICD-10-GM and operating and procedure keys (OPS codes) issued by the Federal Institute for Drugs and Medical Devices (BfArM). We included all patients hospitalized in Germany in the period between January 1, 2019 to December 31, 2020 with the ICD-10 main diagnosis G35.0 (first diagnosis of MS, n = 10,117), G35.1- (relapsing-remitting MS, n = 39,805), G35.2- (primary progressive MS, n = 9836), G35.3- (secondary progressive MS, n = 25,436), and G36.0 (NMOSD, n = 2259). Patients being transferred once or multiple times from one hospital to another were censored appropriately to avoid double and multiple counting cases (excluding ‘discharge key 06’). All case numbers were analyzed up to the codable endpoint.
Outcomes
The primary outcomes were the number and demographics of hospitalized MS and NMOSD patients, the presence of an acute exacerbation in RR-/SPMS patients, and the application of plasmapheresis therapy during the predefined periods of interest: 1 January to 29 February 2020 (pre-first wave); 1 March To 31May 2020 (first wave); 1 June to 30 September 2020 (pre-second wave); and 1 October to 31 December 2020 (second wave). The presence of an acute exacerbation in RRMS and SPMS patients was accessed by their fifth digit of the ICD-10 code (endpoint 1: the presence of acute exacerbation; endpoint 0: without acute exacerbation). Plasmapheresis therapy was accessed using the corresponding Operating and Procedure (OPS) Key for (a) plasma exchange (PE, OPS code 8–820.-) or (b) immunoadsorption (IA, OPS 8–821.-) in combination with the main diagnosis ICD G35.- for MS and G36.0 for NMOSD.
Statistical analysis
Rates are given for categorical variables and means and standard deviations (SDs) for continuous variables. The absolute and relative change of MS and NMOSD patients’ hospitalizations between the different periods of interest is given in numbers and percent. We used the chi-squared test (χ2) for categorical variables and t-test for continuous variables to compare each period of interest’s data with the corresponding previous year period.
Results
Patients with a first diagnosis of multiple sclerosis
Overall, the number of hospitalized patients with the first diagnosis of MS decreased by 4.8% in 2020 (2020: n = 4935; 2019: n = 5182). The proportion of female patients was not different (67.5% versus 67.5%, p = 0.994). Hospitalized patients with the first diagnosis of MS were younger in 2020 compared to 2019 (36.5 ± 3.5 years versus 37.3 ± 3.5 years, p < 0.001).
Regarding admissions dynamics during the different periods of interest, there was a strong decline of these patients during the first wave period compared to the corresponding period in 2019 (−16.8%). Minor differences were present for the other periods of interest (pre-first wave: −3.9%; pre-second wave: −0.4%; second wave: +2.9%). Sex distribution was not different during any period of interest compared to the corresponding period in 2019 (Table 1).
Table 1.
Characteristics of patients.
| January–February [Pre-first Wave] | March–May [First Wave] | June–September [Pre-second Wave] | October–December [Second Wave] | |
|---|---|---|---|---|
| First diagnosis of MS, n | 831 (865) | 1175 (1413) | 1786 (1793) | 1143 (1111) |
| Age (years) mean ± SD | 37.0 ± 3.4 (37.1 ± 3.4) | 36.3 ± 3.5 ** (37.3 ± 3.5) | 37.0 ± 3.5 ** (37.4 ± 3.5) | 36.0 ± 3.4 ** (37.4 ± 3.4) |
| Sex (Female), N, % | 592, 71.2% (581, 67.2%) | 747, 63.5% (947, 67.0%) | 1200, 67.2% (1224, 68.3%) | 790, 69.1% (744, 67.0%) |
| RRMS, n | 3745 (3877) | 3796 (5754) | 6328 (7123) | 4187 (4995) |
| Acute exacerbation, n, % | 2527, 67.5% (2616, 67.5%) | 2505, 66.0% ** (3977, 69.1%) | 4120, 65.1%m * (4767, 66.9%) | 2684, 64.1% * (3304, 66.1%) |
| Age (years) mean ± SD | 43.6 ± 3.6 * (43.3 ± 3.7) | 42.0 ± 3.5 ** (43.3 ± 3.6) | 43.1 ± 3.5 ** (43.8 ± 3.7) | 42.6 ± 3.5 ** (43.5 ± 3.6) |
| Sex (Female), n, % | 1795, 71.0% (1866, 71.3%) | 1753, 70.0% (2806, 70.6%) | 2906, 70.5% (3387, 71.1%) | 1880, 70.0% (2333, 70.6%) |
| Without acute exacerbation, n, % | 1218, 32.5% (1261, 32.5%) | 1291, 34.0%** (1777, 30.9%) | 2208, 34.9% * (2356, 33.1%) | 1503, 35.9% * (1691, 33.9%) |
| Age (years) mean ± SD | 42.2 ± 3.6 (42.3 ± 3.7) | 41.6 ± 3.5 * (41.9 ± 3.7) | 42.2 ± 3.5 (42.5 ± 3.5) | 41.5 ± 3.5 ** (42.9 ± 3.5) |
| Sex (Female), n, % | 824, 67.7% (821, 65.1%) | 873, 67.2% (1203, 67.7%) | 1482, 67.1% (1641, 69.7%) | 1018, 67.7% (1162, 68.7%) |
| SPMS, n | 2385 (2633) | 2015 (3941) | 4067 (4924) | 2225 (3246) |
| Acute exacerbation, n, % | 1508, 63.2% (1643, 62.4%) | 1305, 64.8% (2517, 63.4%) | 2652, 65.2% (3141, 63.8%) | 1395, 62.7% (2045, 63.0%) |
| Age (years) mean ± SD | 58.0 ± 4.8 ** (57.1 ± 5.0) | 57.6 ± 4.8 (57.8 ± 4.9) | 58.5 ± 5.0 * (58.1 ± 5.1) | 58.1 ± 5.3 * (57.6 ± 5.0) |
| Sex (Female), n, % | 1023, 67.8% (1106, 67.3%) | 872, 66.8% (1696, 67.4%) | 1750, 66.0% (2065, 65.7%) | 890, 63.8% (1334, 65.2) |
| Without acute exacerbation, n, % | 877, 36.8% (990, 37.6%) | 710, 35.2% (1424, 36.1%) | 1415, 34.8% (1783, 36.2%) | 830, 37.3% (1201, 37.0%) |
| Age (years) mean ± SD | 58.2 ± 4.9 ** (57.2 ± 5.2) | 58.0 ± 5.0 ** (57.2 ± 5.0) | 57.8 ± 5.1 (57.8 ± 5.0) | 58.2 ± 5.1 ** (57.4 ± 4.9) |
| Sex (Female), n, % | 584, 66.6% (665, 67.2%) | 468, 65.9% (932, 65.4%) | 919, 64.9% (1133, 63.5%) | 533, 64.1% (786, 65.4%) |
| PPMS, n | 913 (938) | 821 (1460) | 1599 (1857) | 976 (1272) |
| Age (years) mean ± SD | 57.8 ± 5.2 (57.7 ± 5.0) | 57.9 ± 5.0 (58.1 ± 4.9) | 58.4 ± 5.1 (58.7 ± 5.0) | 58.3 ± 5.1 (58.1 ± 5.0) |
| Sex (Female), n, % | 554, 60.7% ** (492, 52.5%) | 411, 50.1% (790, 54.1%) | 863, 53.4% (979, 52.7%) | 546, 55.9% (699, 55.0%) |
| NMOSD, n | 193 (205) | 249 (308) | 392 (387) | 255 (270) |
| Age (years) mean ± SD | 48.5 ± 3.4 ** (50.1 ± 3.8) | 47.6 ± 3.4 (47.4 ± 3.5) | 48.4 ± 3.4 ** (49.3 ± 3.5) | 49.0 ± 3.6 * (48.2 ± 3.5) |
| Sex (Female), n, % | 146, 75.6% (150, 73.2%) | 171, 68.7% (221, 71.8%) | 291, 74.2% (272, 70.3%) | 191, 74.9% (196, 72.6%) |
| MS-associated Plasmapheresis therapy, n | 145 (132) | 167 (199) | 231 (251) | 154 (162) |
| NMOSD-associated Plasmapheresis therapy, n | 22 (18) | 49 (26) | 55 (36) | 27 (29) |
p-value < 0.05. **p-value < 0.001. Age is given as mean ± SD and rates are given in percent. Periods in 2020 are defined as Pre-first Wave: January to February, First Wave: March to May, Pre-second Wave: June to September, and Second Wave: October to December 2020. The data of the corresponding control period in 2019 are given in (brackets).
NMOSD, neuromyelitis optica spectrum disorder; PPMS, primary progressive multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SD, standard deviation; SPMS, secondary progressive multiple sclerosis.
Patients with relapsing-remitting multiple sclerosis
The overall number of RRMS patient hospitalizations decreased by 17.0% in 2020 (2020: n = 18,056, 2019: n = 21,749). RRMS patients were younger in 2020 (42.3 ± 3.5 years versus 43.0 ± 3.6 years, p < 0.001), without a significant difference regarding the sex distribution in 2020 compared to 2019 (female patients: 69.4% versus 70.0%, p = 0.214).
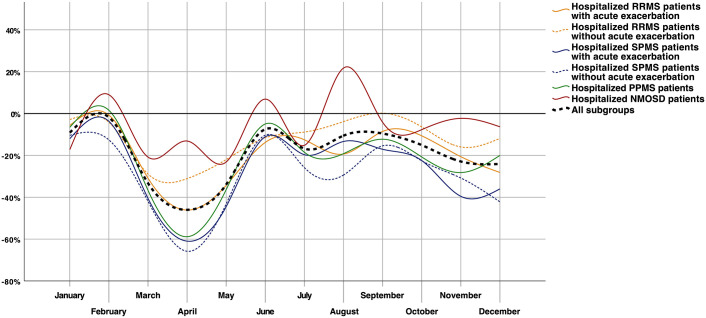
Since March 2020, there was a substantial reduction of hospitalized patients with RRMS for every period of interest, with the most remarkable decline during the first wave period (first wave: −34.0%; pre-second wave: −11.2%; second wave: −16.2%). This decline was mainly driven by a pronounced decrease in RRMS patients with an acute exacerbation, especially during the first wave period (−37.0% versus −27.3%, Figure 2). Hospitalized RRMS patients with an acute exacerbation were significantly younger during all pandemic periods compared to the corresponding previous year periods (Table 1). The age difference of RRMS patients without an acute exacerbation was less remarkable and only significantly different during the two wave periods compared to the corresponding periods in 2019.
Figure 2.
Relative change of MS subtypes and NMOSD patients’ hospitalizations in 2020.
NMOSD, neuromyelitis optica spectrum disorder; PPMS, primary progressive multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis.
Patients with secondary progressive multiple sclerosis
In 2020, the strongest relative decline of hospitalized patients with MS was found for patients with SPMS. In general, the number of hospitalized SPMS patients decreased by 27.5% in 2020 (2020: n = 10,692, 2019: n = 14,744). SPMS patients were slightly older in 2020 (58.0 ± 5.0 years versus 57.6 ± 5.0 years, p < 0.001), without a difference in the sex distribution (female patients: 65.8% versus 65.9%, p = 0.907).
There was a relative decrease in SPMS hospitalizations of 48.9% during the first wave period. The pre-second wave and second wave periods were characterized by a reduction of 17.4% and 31.5%. No significant difference was found in the presence of an acute exacerbation during the different periods of interest. The age of SPMS patients with an acute exacerbation was marginally higher during the pre-first wave, pre-second wave, and second wave period compared to the 2019 periods. SPMS patients without an acute exacerbation were slightly older during the pre-first wave, first wave, and second wave periods (Table 1) compared to the previous year periods. No sex difference was present during any period of interest.
Patients with primary progressive multiple sclerosis
There was also a remarkable decline of hospitalized PPMS patients, with an overall decrease of 22.0% in 2020 (2020: n = 4309, 2019: n = 5527). No significant difference in sex distribution (55.1% versus 55.9%, p = 0.4311) or age (58.1 ± 5.0 years versus 58.2 ± 5.1 years, p = 0.331) was found between PPMS patients in 2020 compared to 2019.
The decline of PPMS hospitalizations was pronounced during the first wave period (−43.8%, Figure 2). The pre-first wave period was characterized by a significantly higher percentage of female patients as compared to the previous year period (60.7% versus 52.5%, p < 0.001), with an opposing finding on female patients for the first wave period (50.1% versus 54.1%, p = 0.063, Table 1).
Patients with neuromyelitis optica spectrum disorder
Overall, the number of hospitalized patients with the main diagnosis of NMOSD decreased by 6.9% (2020: n = 1089, 2019: n = 1170). No difference in sex distribution was found in 2020 compared to 2019 (female patients: 73.4% versus 71.7%, p = 0.377). Hospitalized NMOSD patients in 2020 were slightly younger than NMOSD patients in 2019 (48.4 ± 3.4 years versus 48.7 ± 3.5 years, p = 0.039).
Regarding the dynamic of NMOSD patients’ hospitalizations, there was a decline in hospital admissions during the two wave periods (first wave: −19.2%, second wave: −5.6%; Figure 2). NMOSD patients were the only patient population with increased hospitalizations during any period of interest in 2020 compared to 2019 (pre-second wave period: +1.3%). Hospitalized NMOSD patients were younger during the two pre-wave periods but older during the second wave period compared to the corresponding periods in 2019 (Table 1).
Plasmapheresis therapy
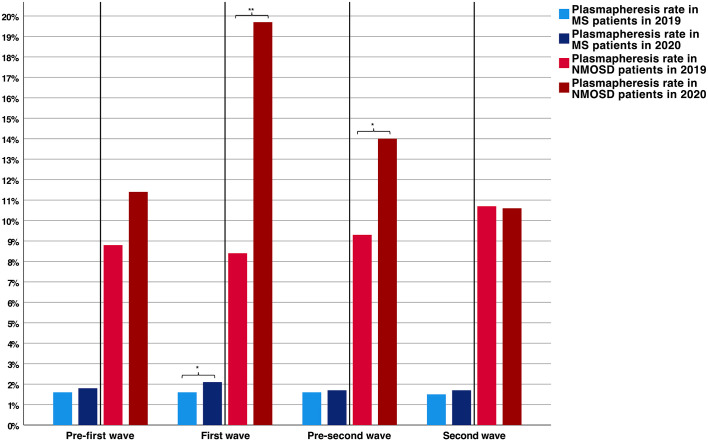
The use of plasmapheresis therapy in MS patients decreased by 6.3% in 2020 (2020: n = 697; 2019: n = 744). The most remarkable decline was found for the first wave period (−16.1%). Treatment rates in MS patients were slightly higher in 2020 compared to 2019 (1.8% versus 1.6%, p = 0.003), with the most significant increase during the first wave of the pandemic as compared to the corresponding period in 2019 (2.1% versus 1.6%, p = 0.006, Figure 3)
Figure 3.
Comparison of MS and NMOSD associated plasmapheresis rates during the different pandemic periods.
*p-value < 0.05. **p-value < 0.001.
MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder.
NMOSD-associated treatment with plasmapheresis did substantially increase in 2020 (2020: n = 153; 2019: n = 109, +40.4%). Analysis of treatment rates in NMOSD patients revealed higher rates in 2020 compared to 2019 (14.0% versus 9.3%, p < 0.001), with the most remarkable increase during the first wave period (19.7% versus 8.4%, p < 0.001, Figure 3).
Discussion
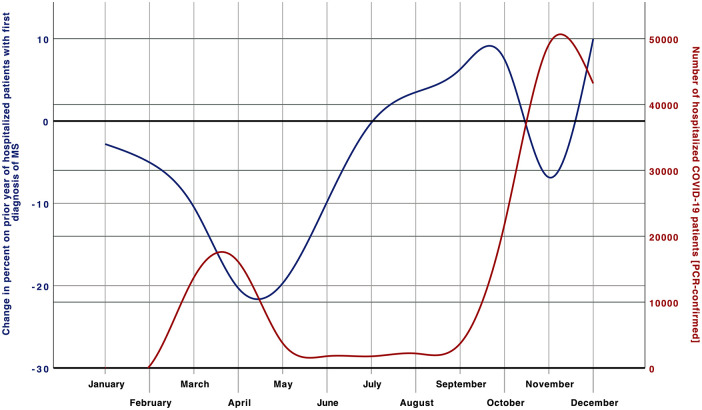
This is the first study analyzing the inpatient care of MS and NMOSD patients during the pandemic year of 2020 using a comprehensive nationwide approach. There was a substantial decline in the hospitalization of these patients in 2020, which was more pronounced in MS (−19.5%) than NMOSD patients (−6.9%). The reasoning for this decrease is speculative, but most likely attributed to a less frequent seek for inpatient medical attention of these patients during the COVID-19 pandemic. Anxiety might be an essential factor for this behavior, as this is frequently present in MS patients in the context of this pandemic. 7 In Germany, studies investigating the reason for patients to avoid hospital stays during the COVID-19 pandemic are lacking, but data from the USA confirm major concerns of people contracting another illness while being at a medical facility during the first wave of the COVID-19 pandemic. 8 Fear as the main driver is also supported by the dynamic of the observed decline seen from March 2020 on, when Germany faced the challenges of this pandemic for the first time, and the German government introduced the first-ever lockdown and strict hygiene measures for strong social distancing to control the spread of COVID-19. After this first wave of the pandemic, case numbers in Germany increased again, to even pre-pandemic levels in the subgroups of NMOSD and first diagnosed MS patients. The second wave period was again characterized by a decline in hospital admissions of patients with NMOSD or known MS. However, the decrease was minor compared to the first wave period, although the incidence of COVID-19 was much higher during this second wave period (Figure 1). In line with this, the national analysis of mobile communication data in Germany revealed differences in the German citizens’ mobility between these two wave periods. There was an average mobility reduction of −20% during the first and −9% during the second wave period compared to the corresponding periods in 2019. 9 This change in the German citizens’ behavior between the two wave periods might be caused by reduced fear of infection. Such anxiety reduction could also account for the lower decline of MS and NMOSD hospitalizations during the second wave period. The routine implementation of hygiene concepts and the hospitals’ well-established infrastructure measures might be important factors for this development.
Figure 1.
Relative change of hospitalizations with the first diagnosis of MS in 2020.
COVID-19, coronavirus disease 2019; MS, multiple sclerosis; PCR, polymerase chain reaction.
Among all patients with MS, inpatient numbers decreased most substantially in progressive MS patients (SPMS, −27.5%; PPMS, −22.0%; RRMS, −17.0%). These patients represent an older patient population with a commonly higher disability than patients with an RRMS disease course. Besides higher age, an increased disability was recently associated with worse outcomes and death from COVID-19 in MS patients. 6 As there are fewer options and strict requirements of DMT use in progressive MS patients,10–13 a considerable amount of these patients is not on DMT. Nevertheless, most progressive MS patients initially present with a relapsing-remitting course 14 and history of DMT that might affect the long-term immune competence of these patients. Such thoughts might have influenced the decision-making of patients and their treating physicians during the COVID-19 pandemic. In general, reasons for hospitalization in progressive MS patients include acute relapses but also elective treatments, such as intrathecal corticosteroid therapy (ICT). ICT reduces spasticity and elongates the patient’s walking distance.15,16 Postponements have strongly compromised such elective inpatient treatments during the pandemic. Together with an assumable decrease in physical activity and other therapy opportunities during the pandemic, the symptomatic therapy approach might have been adversely affected in 2020. Future studies should investigate the impact of lockdown measures and elective treatment postponements on disease progression and quality of life in MS and NMOSD patients, especially in progressive MS patients.
The lowest decrease of hospitalizations in 2020 was observed in NMOSD patients and patients with the first diagnosis of MS (−6.9% and −4.8%, respectively). Both groups commonly need inpatient settings, either for intensive diagnostic workup or invasive relapse therapy. The intensive diagnostic workup that is required to conduct in a suspected case of MS includes at least two specialties (Neurologists, Radiologists). As this pandemic has also compromised the outpatient sector, an ambulatory approach will have been challenging due to the restrictions and lockdown measures of the pandemic. Therefore, we assume that the increase of hospitalized first diagnosed MS patients, seen from post-first wave on, might reflect a delay in diagnosing MS patients during the first wave period (Figure 1).
There was a slight increase in the treatment rates with plasmapheresis in MS patients and a substantial rise in NMOSD patients in 2020 compared to 2019 (+0.2% and 4.7%, respectively). In general, treatment rates with plasmapheresis were significantly higher during the first wave period of the pandemic (Figure 3). In NMOSD patients, plasmapheresis therapy is the current preferred treatment for acute relapses. The early initiation of plasmapheresis within three days of onset has been associated with good outcomes, 17 while there is no evidence of superiority for one of the two apheresis techniques. 18 Due to extensive inflammation, e.g., in the spinal cord, relapses of NMOSD patients are often highly disabling. The decrease of NMOSD patients’ hospitalizations combined with the increasing plasmapheresis rate might reflect a common observation that has also been reported for patients with ischemic stroke during the first wave of the pandemic. 2 Presumably, patients with more severe symptoms presented to the hospital, while primarily patients with minor symptoms did not seek inpatient medical help. In NMOSD, taking into account the absolute increase of plasmapheresis therapy in these patients, increasing expert knowledge in the NMOSD field could be another reasonable explanation, as there is evidence of better relapse recovery by PE or IA in NMOSD patients. 19
This nationwide study has several strengths and limitations. In this study, we accessed comprehensive administrative data from Germany. These data are based on the documented diagnoses and procedures in the G-DRG system, the correctness of which is regularly monitored by insurance companies. There is a lack of available data on confounding factors, such as the severity of symptoms, pre-treatment conditions, DMT use, and serostatus of NMOSD patients, which is the major drawback of this study. Furthermore, we cannot conclude the short- or long-term consequences for disability accumulation in these patients, as there is no follow-up data available.
Nevertheless, this administrative data has high quality and accuracy because registration of all inpatient cases and procedures is a prerequisite to getting financial compensation. The coding is closely controlled by medical services of the medical health insurances. As a result, in contrast to observational registries, our quantitative analysis is robust. It provides almost 100% coverage of all hospitalized patients in Germany with a shallow risk of missing patients or double coding procedures, resulting in high validity and consistency.
In conclusion, there was a marked decline in hospitalized patients with MS and NMOSD during the different pandemic periods, with the most substantial decrease in progressive MS patients and during the first wave period. Our data indicate that provision of rescue relapse therapies in eligible patients with MS or NMOSD was not affected by the COVID-19 pandemic in Germany. Future studies should investigate the impact of these pandemic side effects on disease progression and the patients’ quality of life.
Footnotes
Author’s Note: The Editor-in-Chief of Therapeutic Advances in Neurological Disorders is an author of this paper, therefore, the peer review process was managed by alternative members of the Board and the submitting Editor was not involved in the decision-making process.
Author Contributions: DR and CK participated in the study design. DR drafted the first version of the manuscript and performed the statistical analysis. DB conducted the data abstraction. DR, SF, DB, LT, KH, IA, CK, and RG analyzed and discussed data. SF, LT, KH, IA, CK, and RG revised the manuscript.
Conflict of interest statement: Daniel Richter: receives support from the Medical Faculty of Ruhr-University Bochum (FoRUM grant K136-20).
Simon Faissner: received speaker’s and/or board honoraria from Biogen, BMS, Celgene, Novartis and Roche and grant support from Ruhr-University Bochum, DMSG, Stiftung für therapeutische Forschung and Novartis, none related to this work.
Dirk Bartig: received orders for analysis of the German Diagnosis-Related Groups system from Boehringer Ingelheim and Sanofi Aventis.
Lars Tönges: LT has received travel funding and/or speaker honoraria from Abbvie, Bayer, Bial, Desitin, GE, UCB, Zambon and consulted for Abbvie, Bayer, Bial, Desitin, Stadapharm, UCB, Zambon in the last three years.
Kerstin Hellwig: received speaker’s and/or research support from Biogen, Bayer, Teva, Sanofi Genzyme, Novartis, and Roche and grant support from the Innovation Fond, DMSG and DFG, all not related to this work.
Ilya Ayzenberg: has received travel grants and speaker honoraria from Biogen Idec and Guthy-Jackson Charitable Foundation, Alexion, Santhera, Merck, served on scientific advisory boards for Roche and Alexion, and received research support from Diamed, none related to this study.
Christos Krogias: received speaker honoraria or travel grants for scientific meetings from Bayer Vital and Daichii Sankyo.
Ralf Gold: serves on scientific advisory boards for Teva Pharmaceutical Industries Ltd., Biogen Idec, Bayer Schering Pharma, and Novartis; has received speaker honoraria from Biogen Idec, Teva Pharmaceutical Industries Ltd., Bayer Schering Pharma, and Novartis; serves as editor for Therapeutic Advances in Neurological Diseases and on the editorial boards of Experimental Neurology and the Journal of Neuroimmunology; and receives research support from Teva Pharmaceutical Industries Ltd., Biogen Idec, Bayer Schering Pharma, Genzyme, Merck Serono, and Novartis, none related to this manuscript. Ralf Gold is the Editor-in-Chief of Therapeutic Advances in Neurological Disorders; therefore, the peer review process was managed by alternative members of the Board and the submitting Editor was not involved in the decision-making process.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics statement: No informed consent or ethical approval was required for this study because completely anonymized data were used and provided by the German Federal Statistical Office to comply with the German data protection regulations. The latest Good Practice in Secondary Data Analysis recommendations (version 2; 2008) of the German Working Group for the Survey and Utilization of Secondary Data (with representatives from the German Society for Social Medicine and Prevention and the German Society for Epidemiology) and the Working Group for Epidemiological Methods (with representatives from the German Society for Epidemiology, the German Society for Medical Informatics, Biometry and Epidemiology and the German Society for Social Medicine and Prevention) state in their respective Guideline 1 that “. . . recommendation to consult with an ethics committee need not apply to secondary data analyses if all the data protection provisions on pseudo-anonymization of all personal data are fulfilled. . . and no link to primary data is intended.”
ORCID iDs: Daniel Richter  https://orcid.org/0000-0001-6947-8350
https://orcid.org/0000-0001-6947-8350
Simon Faissner  https://orcid.org/0000-0002-3412-762X
https://orcid.org/0000-0002-3412-762X
Lars Tönges  https://orcid.org/0000-0001-6621-144X
https://orcid.org/0000-0001-6621-144X
Contributor Information
Daniel Richter, Department of Neurology, Ruhr University Bochum, St. Josef-Hospital Bochum, Gudrunstrasse 56, Bochum, 44791 Germany.
Simon Faissner, Department of Neurology, Ruhr-University Bochum, St. Josef-Hospital, Bochum, Germany; Medical Faculty, Ruhr-University Bochum, Germany.
Dirk Bartig, Department of Neurology, Ruhr-University Bochum, St. Josef-Hospital, Bochum, Germany.
Lars Tönges, Department of Neurology, Ruhr-University Bochum, St. Josef-Hospital, Bochum, Germany; Medical Faculty, Ruhr-University Bochum, Germany; Center for Protein Diagnostics (ProDi), Ruhr University Bochum, Bochum, Germany.
Kerstin Hellwig, Department of Neurology, Ruhr-University Bochum, St. Josef-Hospital, Bochum, Germany; Medical Faculty, Ruhr-University Bochum, Germany.
Ilya Ayzenberg, Department of Neurology, Ruhr-University Bochum, St. Josef-Hospital, Bochum, Germany; Medical Faculty, Ruhr-University Bochum, Germany; Department of Neurology, I.M. Sechenov First Moscow State Medical University, Moscow, Russia.
Christos Krogias, Department of Neurology, Ruhr-University Bochum, St. Josef-Hospital, Bochum, Germany; Medical Faculty, Ruhr-University Bochum, Germany.
Ralf Gold, Department of Neurology, Ruhr-University Bochum, St. Josef-Hospital, Bochum, Germany; Medical Faculty, Ruhr-University Bochum, Germany; Center for Protein Diagnostics (ProDi), Ruhr University Bochum, Bochum, Germany.
References
- 1. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richter D, Eyding J, Weber R, et al. Analysis of nationwide stroke patient care in times of COVID-19 pandemic in Germany. Stroke 2021; 52: 716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scherbaum R, Kwon EH, Richter D, et al. Clinical profiles and mortality of COVID-19 inpatients with Parkinson’s disease in Germany. Mov Disord. Epub ahead of print 4 May 2021. DOI: 10.1002/mds.28586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parrotta E, Kister I, Charvet L, et al. COVID-19 outcomes in MS: observational study of early experience from NYU Multiple Sclerosis Comprehensive Care Center. Neurol Neuroimmunol Neuroinflamm 2020; 7: e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zabalza A, Cárdenas-Robledo S, Tagliani P, et al. COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur J Neurol. Epub ahead of print 19 December 2020. DOI: 10.1111/ene.14690. [DOI] [PubMed] [Google Scholar]
- 6. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American Registry of patients with multiple sclerosis. JAMA Neurol. Epub ahead of print 19 March 2021. DOI: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramezani N, Ashtari F, Bastami EA, et al. Fear and anxiety in patients with multiple sclerosis during COVID-19 pandemic; report of an Iranian population. Mult Scler Relat Disord. Epub ahead of print 29 January 2021. DOI: 10.1016/j.msard.2021.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American College of Emergency Physicians COVID-19, https://www.emergencyphysicians.org/globalassets/emphysicians/all-pdfs/acep-mc-Covid19-april-poll-analysis.pdf (accessed 6 May 2021).
- 9. Deutsche statistische Bundesamt. Mobilitätsindikatoren auf Basis von Mobilfunkdaten, https://www.destatis.de/DE/Service/EXDAT/Datensaetze/mobilitaetsindikatoren-mobilfunkdaten.html;jsessionid=30FE5B9E7D43A4999A0B49E9BAE541A6.live741 (accessed 6 May 2021).
- 10. Edan G, Miller D, Clanet M, et al. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry 1997; 62: 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Montalban X, Hauser SL, Kappos L, et al.; ORATORIO Clinical Investigators. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017; 376: 209–220. [DOI] [PubMed] [Google Scholar]
- 12. Kappos L, Bar-Or A, Cree BAC, et al.; EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 2018; 391: 1263–1273. [DOI] [PubMed] [Google Scholar]
- 13. Faissner S, Plemel JR, Gold R, et al. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat Rev Drug Discov 2019; 18: 905–922. [DOI] [PubMed] [Google Scholar]
- 14. Tremlett H, Yinshan Z, Devonshire V. Natural history of secondary- progressive multiple sclerosis. Mult Scler 2008; 14: 314–324. [DOI] [PubMed] [Google Scholar]
- 15. Hoffmann V, Kuhn W, Schimrigk S, et al. Repeat intrathecal triamcinolone acetonide application is beneficial in progressive MS patients. Eur J Neurol 2006; 13: 72–76. [DOI] [PubMed] [Google Scholar]
- 16. Kamin F, Rommer PS, Abu-Mugheisib M, et al. Effects of intrathecal triamincinolone-acetonide treatment in MS patients with therapy-resistant spasticity. Spinal Cord 2015; 53: 109–113. [DOI] [PubMed] [Google Scholar]
- 17. Levy M. Plasmapheresis for acute attacks in neuromyelitis optica spectrum disorders [published correction appears in Neurol Neuroimmunol Neuroinflamm 2018; 6: e518]. Neurol Neuroimmunol Neuroinflamm 2018; 5: e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kleiter I, Gahlen A, Borisow N, et al.; NEMOS (Neuromyelitis Optica Study Group). Apheresis therapies for NMOSD attacks: a retrospective study of 207 therapeutic interventions. Neurol Neuroimmunol Neuroinflamm 2018; 5: e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kleiter I, Gahlen A, Borisow N, et al.; Neuromyelitis Optica Study Group. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol 2016; 79: 206–216. [DOI] [PubMed] [Google Scholar]