Abstract
Objectives
Human papillomavirus (HPV) is a risk factor for head and neck squamous cell carcinoma (HNSCC), which is currently increasing worldwide. We evaluated the prevalence of HPV DNA and p16 expression in HNSCC patients age <45 years compared with patients aged ≥45 years.
Methods
Thirty-nine patients aged <45 years who presented at Besançon University Hospital with HNSCC since 2005 were included in this retrospective study. HPV DNA was detected by HPV genotyping and p16 expression was determined by immunohistochemistry using paraffin-embedded tissues. A matched-group of 38 patients aged ≥45 years from Besançon University Hospital was included.
Results
The overall prevalence of HPV infection was 11.7%. HPV16 was the only genotype detected in 4/39 and 5/38 patients, and p16 was expressed in 6/39 and 4/38 patients aged <45 years and ≥45 years, respectively.
Conclusions
HPV-positivity and p16 expression were similar in both age groups. The results suggest that p16 immunohistochemistry may provide a prognosis biomarker for all HNSCCs, not only oropharyngeal cancers, and this should be addressed in large clinical trials.
Keywords: Head and neck squamous cell carcinoma, human papillomavirus, p16 expression, young patient, biomarker, virus DNA
Introduction
More than 90% of all head and neck cancers are squamous cell carcinomas (SCCs), and head and neck SCCs (HNSCCs) constitute approximately 4% of all cancers, with more than 800,000 new cases diagnosed each year worldwide. 1 HNSCC arises in various anatomical sites, including the oral cavity, oropharynx, hypopharynx, and larynx, and mainly affects men in their fifth and sixth decades of life. However, the incidence of these tumors has tended to increase in developing countries since the 1980s, 2 , 3 especially in patients <45 years old.4–6
Universally, the main risk factors for HNSCC remain tobacco exposure (either smoking or chewing tobacco) and, to a lesser extent, alcohol consumption. In 2007, the International Agency for Research on Cancer also recognized an increasing incidence of high-risk human papillomavirus (hrHPV)-associated HNSCC, and de Martel and collaborators estimated that HPV was present in approximately 7% of all HNSCCs worldwide and was responsible for 30% of oropharyngeal SCCs (OPSCCs) and 2.2% and 2.4% of oral cavity and laryngeal cancers, respectively. 7 HPV16 accounts for most (85%) HPV+ HNSCCs. 7 HPV-associated HNSCC are also more frequent in younger individuals, associated with an increased number of oral-sex partners and minimal or no exposure to tobacco. 8 , 9
HPV-related and HPV-unrelated HNSCCs present with different molecular carcinogenic pathways 10 and show different treatment responses, 8 , 11 and patients with HPV-related OPSCC having better survival than those with HPV-unrelated tumors. 12 , 13 Consequently, the 2017 World Health Organization (WHO) Classification of Head and Neck Tumors included a new chapter on OPSCC related to HPV. 14 The better outcome of HPV-associated HNSCC raises the issue of how best to distinguish between HPV- and non-HPV-associated SCCs. 15 However, there is currently no consensus regarding the optimal method of detection of HPV, 15 involving in situ hybridization to detect HPV DNA or RNA, HPV polymerase chain reaction (PCR) assays, reverse transcription-PCR, DNA/RNA microarrays, and immunohistochemical detection of p16. 16 The WHO recently defined diffuse cytoplasmic and nuclear immunoreactivity for p16 as a reliable surrogate marker for the presence of hrHPV in OPSCC. 14 The College of American Pathologists also recommends that pathologists test for hrHPV by p16 immunohistochemistry in all cases diagnosed as OPSCC. 17 However, although the scoring system for defining p16 expression is well established in OPSCC, 13 this is not the case for non-OPSCC.
Given that the epidemiology of HNSCC is changing, with an ever-increasing incidence in young patients, it is important to conduct bioclinical studies in this population. The present study thus aimed to evaluate and compared the prevalence of HPV DNA and p16 expression in young (<45 years) and old (≥45 years) patients with HNSCC.
Materials and methods
The reporting of this study conformed to the REMARK statement. 18
Patients
This retrospective study included all patients aged <45 years who were diagnosed with HNSCC and treated at the Otorhinolaryngology Department of the University Hospital of Besançon from January 2005 to June 2018. Oropharyngeal cancers were localized at the base of the tongue, the tonsils, and included one patients with regional lymph node metastasis of an unknown primary. 19 Non-oropharyngeal cancers were localized at the mobile tongue, floor of mouth, retromolar trigone, and larynx. Patients with cancer of the nasopharynx, salivary glands, parotid glands, nasal cavity, middle ear, or sinus, and patients with non-SCC of the head and neck were excluded.
Information on patient characteristics and treatment methods were collected. Clinicopathologic data, including age at diagnosis, sex, tobacco and alcohol consumption, histopathological data, WHO performance status, tumor size, nodal stage, metastases, and anatomic site (oropharynx vs. other) were obtained by reviewing medical records. Data on the number of sexual partners were not available. All patient details were de-identified.
The age threshold for defining young and old patients was set at 45 years. Patients aged <45 years were compared with a matched group of patients aged ≥45 years (from University Hospital of Besançon), according to tumor location, except for one patient aged <45 years with regional lymph node metastasis of an unknown primary, for whom no matched patient aged ≥45 years could be retrieved from the medical records.
Archival formalin-fixed paraffin-embedded (FFPE) tumor samples consisting of core biopsies or resections were provided by the regional biobank of Franche-Comté.
DNA extraction
DNA was extracted from FFPE tissue sections using a QIAamp DNA FFPE Tissue Kit® (Qiagen, Courtaboeuf, France) according to the manufacturer’s instructions. Briefly, the paraffin was dissolved in xylene and samples were digested by proteinase K at 56°C overnight in ATL buffer. The lysates were then filtered through the columns provided in the kit, and DNA was eluted using 100 µL of elution solution and was quantified by spectrophotometry.
HPV DNA testing
HPV genotyping was performed using an INNO-LiPA® HPV Genotyping Extra II assay (Fujirebio, Gent, Belgium), which allowed the identification of 32 different HPV genotypes based on PCR amplification followed by reverse hybridization. The amplicons were genotyped by hybridization to HPV type-specific oligonucleotide probes targeting the HPV L1 gene, and detected by alkaline-streptavidin conjugation and colorimetric analysis, according to the manufacturer’s instructions. The strips were scanned and interpreted using Line Reader and Analysis Software (LiRAS®) (Fujirebio).
Immunohistochemical detection of p16
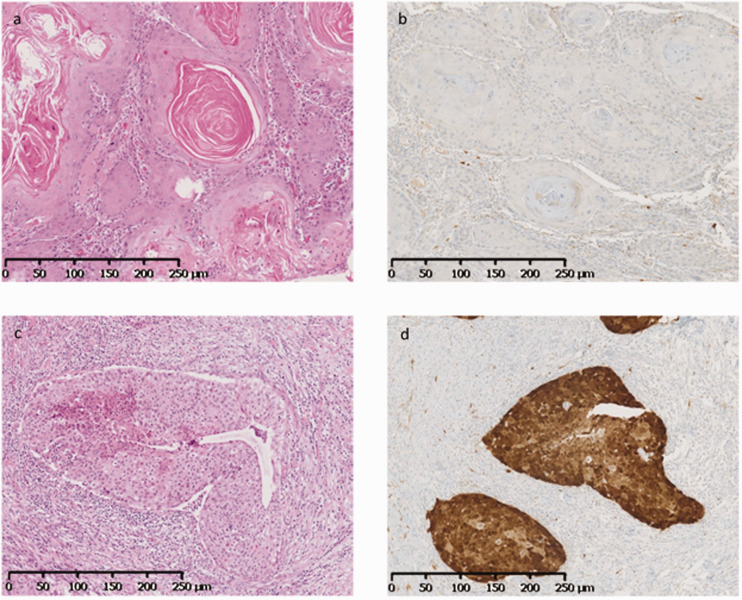
Immunohistochemical detection of p16 was performed using 3-µm FFPE tissue sections, with the monoclonal antibody clone E6H4 (Roche Diagnostics, Meylan, France) and a Benchmark automated system (Roche Diagnostics, Meylan, France), following the manufacturer’s instructions. According to the guidelines from the College of American Pathologists, p16 was defined as positive when ≥70% of tumor cells showed diffuse and strong cytoplasmic and nuclear staining 17 (Figure 1). Staining in the cytoplasm alone, diffuse but weak staining, and/or focal/patchy staining were scored as negative.
Figure 1.
Immunostaining for p16. (a) Oral tongue squamous cell carcinoma with keratinization (hematoxylin and eosin (HE) ×100); (b) lack of p16 expression in tumor cells (immunohistochemistry ×100). (c) Regional lymph node metastasis of unknown primary, with non-keratinizing squamous cell carcinoma (HE ×100); (d) expression of p16 in tumor cells indicated by diffuse nuclear and strong cytoplasmic staining (immunohistochemistry ×100).
Statistical analysis
Differences between the two age groups were evaluated by χ2 test, Yates correction, and Fisher’s test. Concordance between HPV test presence/absence and p16 expression results was assessed by kappa score. All tests were two-tailed and the statistical significance threshold was set as P<0.05. Statistical analysis was performed using R software version 3.5.1 (https://www.r-project.org) with the Survival Analysis package version 2.42-6.
Ethics
The regional biobank of Franche-Comté, France (registration number BB-0033-00024, Tumorothèque Régionale de Franche-Comté) ensured that all patients provided written informed consent. The project was approved by the scientific board of the biobank on 20 October 2018.
Results
Patient characteristics
The sociodemographic and clinicopathologic characteristics of the patients are shown in Table 1. There were 39 patients aged <45 years and 38 aged ≥45 years. The overall male to female ratio was 0.97 (0.95 for younger patients and 1.1 for older patients). Data on tobacco and alcohol consumption were available for 70 and 68 patients, respectively, but there was no difference in tobacco or alcohol consumption between the two groups.
Table 1.
Sociodemographic and clinicopathologic characteristics of patients with head and neck squamous cell carcinoma.
| Characteristic | Age <45 years | Age ≥45 years | P-value |
|---|---|---|---|
| (n = 39) | (n = 38) | ||
| Age, years | |||
| Median | 39 | 65.3 | |
| Range | 25–44 | 48–85 | |
| Sex | |||
| Male | 19 | 20 | 0.731 |
| Female | 20 | 18 | |
| Tobacco consumption | |||
| No | 7 | 7 | 0.904 |
| Yes | 29 | 27 | |
| Unknown | 3 | 4 | |
| Alcohol consumption | |||
| No | 17 | 12 | 0.418 |
| Yes | 19 | 20 | |
| Unknown | 3 | 6 | |
| WHO Performance status | |||
| 0 | 27 | 12 | 0.004 |
| 1 | 10 | 17 | |
| 2–3 | 2 | 8 | |
| Unknown | 0 | 1 | |
| T classification | |||
| 1 | 7 | 5 | 0.178 |
| 2 | 11 | 16 | |
| 3 | 9 | 3 | |
| 4 | 10 | 14 | |
| Unknown | 2 | 0 | |
| N classification | |||
| 0 | 11 | 14 | 0.464 |
| 1–3 | 27 | 24 | |
| Unknown | 1 | 0 | |
| M classification | |||
| 0 | 28 | 33 | not evaluable |
| 1 | 0 | 0 | |
| Unknown | 11 | 5 | |
| Anatomic site | |||
| Oropharyngeal | 8 | 8 | 0.953 |
| Non-oropharyngeal | 31 | 30 | |
| Treatment | |||
| Surgery | 9 | 13 | 0.279 |
| Chemotherapy and/or radiotherapy | 15 | 12 | 0.527 |
| Surgery + adjuvant treatment | 15 | 11 | 0.377 |
| No treatment | 0 | 2 | 0.240 |
| HPV status | |||
| HPV16+ | 4 | 5 | 0.967 |
| HPV16− | 35 | 33 | |
| p16 expression | |||
| p16+ | 6 | 4 | 0.768 |
| p16− | 33 | 34 |
HPV, human papillomavirus, WHO, World Health Organization.
Most patients had non-OPSCC (79%). There were no significant differences in T and N classifications between the groups, but older patients had a significantly worse WHO performance status than younger patients (P=0.004). There was no difference in the treatments received by patients in the two age groups.
Regarding the histopathological characteristics of the tumors, keratinizing carcinoma, well-differentiated carcinoma, positive margins, perineural invasion, and vascular invasion occurred in 82% vs. 89%, 54% vs. 42%, 35% vs. 16%, 20.5% vs. 10.5%, and 20.5% vs. 13% of younger and older patients, respectively.
HPV status and p16 expression
The overall prevalence of HPV infection was 11.7%. HPV DNA was detected in 4 of 39 (10.2%) tumors in patients aged <45 years and in 5 of 38 (13.1%) tumors in patients aged ≥45 years. Genotyping revealed that HPV16 was the only genotype in positive cases. Among the nine HPV16+ cases, six patients (66.7%) had oropharyngeal tumors, two (22.3%) had oral cavity tumors, and one (11.1%) had a laryngeal tumor (Table 1). HPV-positivity was significantly associated with oropharyngeal compared with non-oropharyngeal tumors (P=0.002). Tobacco and alcohol consumption were similar between patients with HPV+ and HPV− tumors.
Ten patients were positive for p16, including six younger and four older patients. Five p16+ cases corresponded to oropharyngeal tumors (50%) and five to oral cavity tumors (50%) (Table 2). There was no significant correlation between p16 expression and anatomical site.
Table 2.
Number of HPV16+ and p16+ tumors according to patient age group and localization.
| Tumor location |
Age <45 years |
Age ≥45 years |
||||
|---|---|---|---|---|---|---|
| n = 39 | HPV16 | p16 | n = 38 | HPV16 | p16 | |
| Oropharynx | ||||||
| Tonsil | 7 | 1 | 1 | 7 | 3 | 2 |
| Base of tongue | 1 | 1 | 1 | 1 | 0 | 0 |
| Regional lymph node metastasis of unknown primary | 1 | 1 | 1 | 0 | 0 | 0 |
| Non-oropharynx | ||||||
| Mobile tongue | 17 | 0 | 2 | 14 | 1 | 1 |
| Floor of mouth | 6 | 0 | 1 | 8 | 1 | 1 |
| Retromolar trigone | 3 | 0 | 0 | 2 | 0 | 0 |
| Larynx | 5 | 1 | 0 | 6 | 0 | 0 |
HPV, human papillomavirus.
Five tumors were HPV16+/p16+, four were HPV16+/p16−, and five were HPV16−/p16+. Given that p16 expression represents a surrogate marker of HPV infection, we calculated the agreement between HPV presence/absence and p16 detection, and showed a concordance of 88.3%. The kappa score was 0.46, indicating moderate agreement according to the Landis–Koch reference value. The detailed characteristics of the HPV16/p16-concordant and non-concordant cases are presented in Table 3.
Table 3.
Clinicopathological characteristics of HPV/p16 concordant and non-concordant cases.
| Case | HPV status | p16 staining | Anatomic site | Age (years) | Sex | Tobacco consumption | Alcohol consumption | Year of diagnosis |
|---|---|---|---|---|---|---|---|---|
| # 1 | + | + | Base of tongue | 44 | F | Yes | No | 2014 |
| # 2 | + | + | Tonsil | 43 | M | Yes | Yes | 2009 |
| # 3 | + | + | RLNMUP | 43 | F | Yes | No | 2014 |
| # 8 | + | + | Tonsil | 67 | F | No | No | 2015 |
| # 9 | + | + | Tonsil | 53 | M | Yes | Yes | 2015 |
| # 4 | + | − | Larynx | 32 | M | Yes | No | 2016 |
| # 10 | + | − | Floor of mouth | 52 | M | NA | NA | 2011 |
| # 11 | + | − | Mobile tongue | 64 | F | No | No | 2014 |
| # 12 | + | − | Tonsil | 63 | M | No | No | 2014 |
| # 5 | − | + | Mobile tongue | 28 | M | No | No | 2015 |
| # 6 | − | + | Mobile tongue | 44 | M | Yes | Yes | 2014 |
| # 7 | − | + | Floor of mouth | 40 | M | Yes | No | 2013 |
| # 13 | − | + | Floor of mouth | 65 | M | Yes | Yes | 2012 |
| # 14 | − | + | Mobile tongue | 70 | F | No | No | 2016 |
HPV, human papillomavirus; F, female; M, male; NA, not available; RLNMUP: regional lymph node metastasis of unknown primary.
Finally, the proportion of HPV+ samples in this small series of HNSCC increased from 7% (2/30) in 2005 to 2011 to 15% (7/47) in 2012 to 2018.
Discussion
We conducted a retrospective study including 39 patients aged <45 years and a matched group of 38 patients aged ≥45 years with HNSCC treated at our University Hospital over a period of 13 years. There is currently no consensus in the literature regarding the threshold for differentiating between young and old patients, with previous authors using either 40 or 45 years; 6 ,20–22 We therefore chose 45 years as the age threshold to define young and old patients.
HPV DNA was detected in 11.7% of HNSCCs in the present study, and HPV16 was the only genotype detected. This low prevalence may be explained by the localization of the tumors, which were mostly in the oral cavity (79% of cases). The distribution of HPV+ tumors has accordingly been previously associated with specific anatomic sites in the head and neck and geographic regions. 23 In addition, HPV16 is the most common HPV type in these tumors worldwide. 23 The overall HPV prevalence in Europe is 41.4% for oropharyngeal cancers, 17.5% for oral cavity cancers, and 20.9% for laryngeal cancers; 23 However, the prevalence in France is lower, including 34% of HPV+ oropharyngeal cancers and 4% of HPV+ oral cavity and laryngeal cancers. 24 In the current study, HPV DNA was detected in 5% of non-oropharyngeal tumors and 37.5% of oropharyngeal tumors, in line with the French data. 24 , 25 It has been postulated that HPV would be more prevalent in younger patients or in patients with less exposure to other known carcinogens, such as tobacco smoke or ethanol; 26 however, we found no difference in age at diagnosis or sex between HPV+ and HPV− patients. The clinicopathologic characteristics were also similar in both age groups, except for WHO performance status, which was significantly better in younger patients, as expected. 27 The current study found no difference in tobacco and alcohol consumption, as the two major risk factors for HNSCC development, 26 between the two groups of patients. However, tobacco (number of cumulative pack-years) and alcohol (quantity, frequency) consumption were not quantified in the medical records, and we therefore cannot exclude the possibility that the older patients had longer/higher exposures to tobacco and alcohol than the younger patients. 26
In this study, the proportion of HPV+ tumors increased approximately two-fold after 2011, in line with previous observations. 28 , 29 However, this result must be interpreted cautiously because of the small number of HPV+ cases in this small series.
The cellular tumor suppressor protein p16INK4a (p16) has been identified as a biomarker for transforming HPV infections. 30 In addition, p16 immunostaining has previously been assessed as a method for improving the interpretation of cervical lesions, triaging women with atypical squamous cell cytology of undetermined significance or low-grade squamous intraepithelial lesions, and for increasing the specificity of HPV testing. 31 Overexpression of p16 has been widely accepted as a surrogate marker for HPV-positivity in OPSCC. 32 In the present study, positive p16 immunostaining (defined by the College of American Pathologists 17 ) was observed in 13% of HNSCCs, with an overall concordance of 88.3% between HPV detection and p16 expression in the analyzed samples. This high concordance was consistent with the findings of a meta-analysis by Albers et al., which showed a concordance of 86%. 33 However, Cohen’s kappa coefficient only revealed a moderate agreement in the current study, possibly because of the high number of HPV−/p16− samples and the small number of HPV+ and/or p16+ samples. Interestingly, the five HPV+/p16+ cases had tumors in the oropharynx, which were caused by HPV. 34 Indeed, p16-positivity in this case is considered as a good marker of transcriptionally active HPV in oropharyngeal carcinogenesis. 35 In contrast, all but one HPV−/p16+ and one HPV+/p16− case were non-oropharyngeal tumors, for which the role of HPV infection in the carcinogenic process remains questionable, especially in HPV+/p16− cases.
Although HPV-related HNSCCs are associated with a better prognosis than HPV-unrelated HNSCCs, there is no consensus on the routine methods that should be used to identify HPV-induced HNSCC, 15 or if HPV-based tests are more relevant than p16 staining. 36 Detection of HPV E6 and E7 mRNA by reverse transcriptase-PCR is currently considered to be the reference test for detecting transcriptionally active hrHPV infection. 15 However, although the INNO-LiPA assay allows the detection of HPV DNA, it provides information about the virus presence but not about the infection state. 15 HPV DNA-positivity may thus be less clinically valuable for identifying HPV-related HNSCC compared with E6/E7 oncogene mRNA, which stimulates cancerous lesion development. 15 , 37 Nevertheless, Mena and collaborators 34 proposed that double positivity for HPV DNA and p16 was the optimal prognostic biomarker for HNSCC in clinical practice.
A preliminary analysis showed that younger patients with p16+ or HPV+ HNSCC tended to have a better prognosis in terms of overall and progression-free survival compared with patients with p16− or HPV− HNSCC (data not shown). This observation is consistent with the validation of p16 as a biomarker of good prognosis in OPSCC, 17 and to a lesser extent in non-OPSCC.38–40
This study had some limitations. First, it included a small series of patients from a single center, and this cohort may not be representative of French patients presenting with HNSCC overall. Second, this was a retrospective study, and follow-up of prospectively recruited patients would increase the robustness of this preliminary observation. In addition, further large clinical studies are needed, especially in patients <45 years, to determine if these biomarkers have a prognostic value regardless of the tumor location.
This study found no difference in HPV/p16 prevalence in HNSCC tumors between old and young patients. However, the high sensitivity of p16 immunostaining means that it would be interesting to extend its use for the clinical management of all HNSCCs (OPSCC and non-OPSCC), with subsequent confirmation of the viral etiology by the detection of HPV DNA.
Acknowledgments
The authors would like to thank the technicians and engineers from the HPV National Reference Center and the Cellular and Molecular Biology Laboratory and Pathology Department of the University Hospital of Besançon for excellent technical assistance. They also acknowledge the Tumorothèque Régionale de Franche-Comté for biobanking. Dr Julie Guillet is gratefully acknowledged for her critical comments on the manuscript.
Footnotes
Declaration of conflicting interest: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CMO, VL, AO, CS, MPA, DG, OM, and JLP declared that they have no competing interests. CM has received honoraria from MSD unrelated to this work.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Jean-Luc Prétet https://orcid.org/0000-0002-8430-214X
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. Doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 2013; 31: 4550–4559. Doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep 2018; 67: 918–924. Doi: 10.15585/mmwr.mm6733a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers JN, Elkins T, Roberts D, et al. Squamous cell carcinoma of the tongue in young adults: increasing incidence and factors that predict treatment outcomes. Otolaryngol Head Neck Surg 2000; 122: 44–51. Doi: 10.1016/S0194-5998(00)70142-2. [DOI] [PubMed] [Google Scholar]
- 5.Majchrzak E, Szybiak B, Wegner A, et al. Oral cavity and oropharyngeal squamous cell carcinoma in young adults: a review of the literature. Radiol Oncol 2014; 48: 1–10. Doi: 10.2478/raon-2013-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussein AA, Helder MN, De Visscher JG, et al. Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: A systematic review. Eur J Cancer 2017; 82: 115–127. Doi: 10.1016/j.ejca.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 7.De Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017; 141: 664–670. Doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 2008; 100: 407–420. Doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 9.Timbang MR, Sim MW, Bewley AF, et al. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother 2019; 15: 1920–1928. Doi: 10.1080/21645515.2019.1600985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi K, Hisamatsu K, Suzui N, et al. A review of HPV-related head and neck cancer. J Clin Med 2018; 7: 241. Doi: 10.3390/jcm7090241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You EL, Henry M, Zeitouni AG. Human papillomavirus-associated oropharyngeal cancer: review of current evidence and management. Curr Oncol 2019; 26: 119–123. Doi: 10.3747/co.26.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacau St Guily J, Rousseau A, Baujat B, et al. Oropharyngeal cancer prognosis by tumour HPV status in France: The multicentric Papillophar study. Oral Oncol 2017; 67: 29–36. Doi: 10.1016/j.oraloncology.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363: 24–35. Doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katabi N, Lewis JS. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: What is new in the 2017 WHO Blue Book for tumors and tumor-like lesions of the neck and lymph nodes. Head Neck Pathol 2017; 11: 48–54. Doi: 10.1007/s12105-017-0796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KY, Lewis JS, Chen Z. Current status of clinical testing for human papillomavirus in oropharyngeal squamous cell carcinoma. J Pathol Clin Res 2018; 4: 213–226. Doi: 10.1002/cjp2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venuti A, Paolini F. HPV detection methods in head and neck cancer. Head Neck Pathol 2012; 6: S63–S74. Doi: 10.1007/s12105-012-0372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis JS, Beadle B, Bishop JA, et al. Human Papillomavirus Testing in Head and Neck Carcinomas: Guideline from the College of American Pathologists. Arch Pathol Lab Med 2018; 142: 559–597. Doi: 10.5858/arpa.2017-0286-CP. [DOI] [PubMed] [Google Scholar]
- 18.Altman DG, McShane LM, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. BMC Med 2012; 10: 51. Doi: 10.1186/1741-7015-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeder L, Boscolo-Rizzo P, Dal Cin E, et al. Human papillomavirus as prognostic marker with rising prevalence in neck squamous cell carcinoma of unknown primary: A retrospective multicentre study. Eur J Cancer 2017; 74: 73–81. Doi: 10.1016/j.ejca.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Martinez RCP, Sathasivam HP, Cosway B, et al. Clinicopathological features of squamous cell carcinoma of the oral cavity and oropharynx in young patients. Br J Oral Maxillofac Surg 2018; 56: 332–337. Doi: 10.1016/j.bjoms.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Miranda Galvis M, Freitas Jardim J, Kaminagakura E, et al. Expression of cell cycle proteins according to HPV status in oral squamous cell carcinoma affecting young patients: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol 2018; 125: 317–325. Doi: 10.1016/j.oooo.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Young D, Xiao CC, Murphy B, et al. Increase in head and neck cancer in younger patients due to human papillomavirus (HPV). Oral Oncol 2015; 51: 727–730. Doi: 10.1016/j.oraloncology.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol 2014; 15: 1319–1331. Doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 24.Shield KD, Marant Micallef C, De Martel C, et al. New cancer cases in France in 2015 attributable to infectious agents: a systematic review and meta-analysis. Eur J Epidemiol 2018; 33: 263–274. Doi: 10.1007/s10654-017-0334-z. [DOI] [PubMed] [Google Scholar]
- 25.Mirghani H, Bellera C, Delaye J, et al. Prevalence and characteristics of HPV-driven oropharyngeal cancer in France. Cancer Epidemiol 2019; 61: 89–94. Doi: 10.1016/j.canep.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Dhull AK, Atri R, Dhankhar R, et al. Major risk factors in head and neck cancer: a retrospective analysis of 12-year experiences. World J Oncol 2018; 9: 80–84. Doi: 10.14740/wjon1104w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Monsjou HS, Lopez-Yurda MI, Hauptmann M, et al. Oral and oropharyngeal squamous cell carcinoma in young patients: the Netherlands Cancer Institute experience. Head Neck 2013; 35: 94–102. Doi: 10.1002/hed.22935. [DOI] [PubMed] [Google Scholar]
- 28.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 2008; 26: 612–619. Doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 29.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29: 4294–4301. Doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16INK4a as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer 2001; 92: 276–284. [DOI] [PubMed] [Google Scholar]
- 31.Carozzi F, Confortini M, Dalla Palma P, et al. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2008; 9: 937–945. Doi: 10.1016/S1470-2045(08)70208-0. [DOI] [PubMed] [Google Scholar]
- 32.Khanal S, Ferraris ED, Zahin M, et al. Targeting synthetic Human Papillomavirus (HPV) L2 disulfide-induced N-terminus conformational epitopes for pan-HPV vaccine development. Exp Mol Pathol 2015; 99: 330–334. Doi: 10.1016/j.yexmp.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Albers AE, Qian X, Kaufmann AM, et al. Meta analysis: HPV and p16 pattern determines survival in patients with HNSCC and identifies potential new biologic subtype. Sci Rep 2017; 7: 16715. Doi: 10.1038/s41598-017-16918-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mena M, Taberna M, Tous S, et al. Double positivity for HPV-DNA/p16ink4a is the biomarker with strongest diagnostic accuracy and prognostic value for human papillomavirus related oropharyngeal cancer patients. Oral Oncol 2018; 78: 137–144. Doi: 10.1016/j.oraloncology.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Taberna M, Mena M, Pavón MA, et al. Human papillomavirus-related oropharyngeal cancer. Ann Oncol 2017; 28: 2386–2398. Doi: 10.1093/annonc/mdx304. [DOI] [PubMed] [Google Scholar]
- 36.Craig SG, Anderson LA, Schache AG, et al. Recommendations for determining HPV status in patients with oropharyngeal cancers under TNM8 guidelines: a two-tier approach. Br J Cancer 2019; 120: 827–833. Doi: 10.1038/s41416-019-0414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Augustin J, Outh-Gauer S, Mandavit M, et al. Evaluation of the efficacy of the 4 tests (p16 immunochemistry, polymerase chain reaction, DNA, and RNA in situ hybridization) to evaluate a human papillomavirus infection in head and neck cancers: a cohort of 348 French squamous cell carcinomas. Hum Pathol 2018; 78: 63–71. Doi: 10.1016/j.humpath.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Vitzthum LK, Mell LK. The role of p16 as a biomarker in nonoropharyngeal head and neck cancer. Oncotarget 2018; 9: 33247–33248. Doi: 10.18632/oncotarget.26053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bryant AK, Sojourner EJ, Vitzthum LK, et al. Prognostic role of p16 in nonoropharyngeal head and neck cancer. J Natl Cancer Inst 2018; 110: 1393–1399. Doi: 10.1093/jnci/djy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris SL, Thorne LB, Seaman WT, et al. Association of p16(INK4a) overexpression with improved outcomes in young patients with squamous cell cancers of the oral tongue. Head Neck 2011; 33: 1622–1627. Doi: 10.1002/hed.21650. [DOI] [PubMed] [Google Scholar]