Abstract
Background:
Coronary artery disease (CAD) is the leading cause of death in advanced kidney disease. However, its best treatment has not been determined.
Methods:
We searched PubMed and Cochrane databases and scanned references to related articles. Studies comparing the different treatments for patients with CAD and advanced CKD (estimated glomerular filtration rate <30 ml/min/1.73 m2 or dialysis) were selected. The primary result was all-cause death, classified according to the follow-up time: short-term (<1 month), medium-term (1 month-1 year), and long-term (>1 year).
Results:
A total of 32 studies were selected to enroll 84,498 patients with advanced kidney disease. Compared with medical therapy (MT) alone, percutaneous coronary intervention (PCI) was associated with low risk of short-, medium-term and long-term all-cause death (more than 3 years). For AMI patients, compared with MT, PCI was not associated with low risk of short- and medium-term all-cause death. For non-AMI patients, compared with MT, PCI was associated with low risk of long-term mortality (more than 3 years). Compared with MT, coronary artery bypass surgery (CABG) had no significant advantages in each follow-up period of all-cause death. Compared with PCI, CABG was associated with a high risk of short-term death, but low risk of long-term death: 1–3 years; more than 3 years. CABG could also reduce the risk of long-term risk of cardiac death, major adverse cardiovascular events (MACEs), myocardial infarction (MI), and repeat revascularization.
Conclusions:
In patients with advanced kidney disease and CAD, PCI reduced the risk of short-, medium- and long- term (more than 3 years) all-cause death compared with MT. Compared with PCI, CABG was associated with a high risk of short-term death and a low risk of long-term death and adverse events.
Keywords: coronary artery bypass graft, coronary artery disease, kidney disease, medical therapy, percutaneous coronary intervention, revascularization
Introduction
Coronary artery disease (CAD) is the main cause of death in patients with chronic kidney disease (CKD), and the risk of death will gradually increase with the deterioration of renal function. 1 The mortality of dialysis patients with acute coronary syndrome (ACS) is more than 70% at 2 years. 2 However, CAD patients with CKD rarely receive the treatment recommended by standard guidelines (coronary angiography or revascularization).3,4 This result is caused by a variety of factors: a high risk of acute kidney injury after revascularization, a high risk of acute complications, a high risk of restenosis and repeated revascularization, and an increased risk of bleeding.5,6
Many large-scale studies exclude patients with chronic kidney disease, resulting in a lack of evidence support for treatment. 7 Earlier studies believed that revascularization could reduce the risk of cardiovascular death and acute myocardial infarction (AMI) and improve the quality of life.6,8,9 However, the latest ISCHEMIA-CKD study showed that an invasive strategy was not superior to osteopathic manipulation (OMT) for end-stage kidney disease patients with stable CAD. 10 At present, no unified conclusion has been reached: in patients with advanced kidney disease, can early high risks after revascularization be offset by late gains?11–13
This study aims to explore the best treatment for coronary heart disease in patients with advanced kidney disease. We control the three comparison groups separately [percutaneous coronary intervention (PCI) versus medical therapy (MT); coronary artery bypass grafting (CABG) versus MT; and CABG versus PCI] in addition to the different CAD types, different follow-up time, and types of stents for subgroup analysis. We hope that this study will provide evidence support for the treatment of coronary heart disease in patients with advanced kidney disease.
Methods
Search strategy
We searched the PubMed and Cochrane library database to find relevant studies though 22 April 2020. We used the search terms such as ‘chronic kidney disease, renal failure, end-stage renal disease, advanced kidney disease, CKD’ and ‘percutaneous coronary intervention, coronary revascularization PCI, coronary artery bypass grafting, CABG’, and ‘medical therapy, MT, drug treatment, optimal medication therapy, OMT’. We also manually screened the included manuscript reference list as a supplement to the first search. These studies were independently retrieved by two authors (J.W.Y and J.F.T). The inconsistencies were resolved with the third author (X.T.S). This systematic review protocol has been registered with the PROSPERO (CRD42020201788).
Selection criteria
The selected studies were required to meet the following criteria: (1) included patients with advanced kidney disease, that is, patients with CKD stage IV and stage V, defined as an estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73 m2 or receiving dialysis. 10 The eGFR was estimated by the Modification of Diet in Renal Disease Study formula (MDRD); (2) and diagnosed with coronary artery disease; (3) at least compared the two treatments (MT, PCI or CABG); (4) provided at least one of the following outcomes: all cause death, cardiac death, major cardiovascular events, myocardial infarction (MI), and recurrent myocardial infarction, repeat revascularization at follow-up; (5) RCT or observational study.
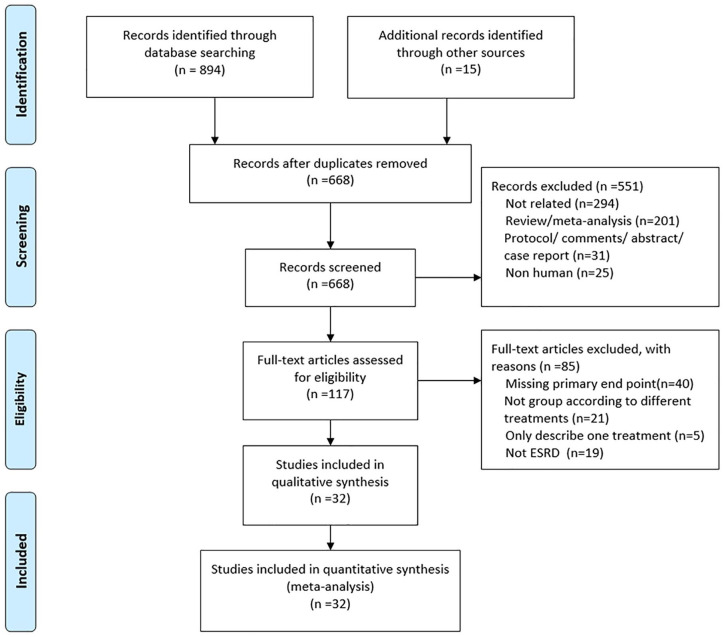
We removed the duplicates, conducted headline review, and conducted abstract screening. The reasons for exclusion were as follows: (1) failure to meet the diagnosis of advanced kidney disease and coronary heart disease; (2) failure to provide at least one valid outcome; (3) no treatment comparison; (4) meta-analysis, review, study protocol, comment, abstract, case report, or letter. Figure 1 shows the search and screen protocol.
Figure 1.
Flowchart of study selection.
Data extraction and outcomes
Data were extracted independently by two authors (J.W.Y and J.F.T) and verified by a third author (X.T.S). The extracted data included: study type, diagnosis of CAD, diagnosis of CKD, comparison groups (PCI versus MT; CABG versus MT; and CABG versus PCI), patient characteristics, and results at different follow-up times. The primary outcome was all-cause death. The secondary outcomes included cardiac death, major adverse cardiac events (MACEs), MI, and unplanned coronary revascularization. MACEs were defined as all-cause death/cardiac death, non-fatal MIs, re-hospitalization because of congestive heart failure, or repeat revascularization. The short-term follow-up time was less than 30 days, the medium-term follow-up time was 1 month–1 year, and the long-term follow-up time was more than 1 year (1–3 years and more than 3 years).
Statistical analysis
Stata11.0 (StataCorp, College Station, Tex) was used for analysis. Firstly, we performed the meta-analysis based on different endpoints and different treatment comparison groups. Secondly, we conducted subgroup analysis based on different follow-up times. Respecting to the all-cause death, individuals of AMI, non-AMI, and multivessel disease (MVD) were analyzed separately. Regarding the second endpoint, AMI and non-AMI were analyzed separately. We calculated the odds ratio (OR) of the 95% confidence interval (CI). The I2 test was used to assess heterogeneity between studies (low heterogeneity: I2 0–25%; moderate heterogeneity: I2 25–50%; severe heterogeneity: I2 greater than 50%). Assuming I2 < 50%, the M-H fixed effects model was used, and if I2 > 50%, the M-H random effects model is used in the statistical analysis process. A p-value < 0.05 was considered statistically significant.
Sensitivity analysis and quality assessment
When heterogeneity is high, sensitivity analysis was performed by excluding all end points of each study one by one. Random effects are used to recalculate set estimates. We used the Egger’s linear regression test and funnel chart visual inspection to assess publication bias. We used the Cochrane risk bias rating scale to assess the quality of randomized controlled trials (RCTs) and the Newcastle-Ottawa Scale (NOS) for retrospective studies.
Result
A total of 894 references were identified from the database search analysis, and 15 references were identified from other sources. Among them, 551 were excluded from the screening process at the title and summary levels (Figure 1). Of the remaining 117 studies, 85 were excluded for the following reasons: missing the primary endpoint (n = 40), not group studies according to different treatment (n = 21); only describe one treatment (n = 5); and not meet the diagnostic criteria for end-stage renal disease (n = 19). The remaining 32 studies reported all-cause mortality and did not meet any other exclusion criteria. Four studies14–17 were from the USRDS database, but the time was different. Three of them: Shroff et al. 14 (2004–2009) Herzog et al. 16 (1978–1995), and Herzog 15 (1995–1998), were used for CAD meta-analysis, while Chang et al. 17 (1997–2009) was only used for MVD meta-analysis.
Baseline characteristics
The characteristics of the study are listed in Table 1. The 32 selected studies included 84,598 patients, two of which were randomized controlled trials and 30 were observational studies. There are four studies on patients with acute myocardial infarction (AMI), five studies on patients with non-AMI, and four studies on MVD. Twenty-six studies reported on the results of dialysis patients. Regarding each endpoint, we separately analyzed the population with AMI and non-AMI. However, due to the lack of reports on secondary endpoints in the AMI study, only all CAD and non-AMI classifications were included in the secondary endpoints. In addition, we also distinguished the types of stents used in PCI: bare metal stent (BMS) and drug-eluting stent (DES). However, many articles do not completely distinguish BMS from DES. Only eight studies clearly indicated that DES-PCI was compared with CABG in patients with non-AMI (Figure 2), and none compared DES-PCI with MT. The follow-up period ranges from in-hospital to 8 years. The average age ranges were from 41 to 77 years old. Most of the patients were men, >60 years old, more than two thirds had hypertension, and more than one third had diabetes.
Table 1.
Study characteristics.
| Author | Study design | CAD diagnosis | CKD diagnosis | Follow-up | Mean age (year) | Male (%) | HT (%) | DM (%) | HC (%) | Smoke (%) | Previous MI (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Manske et al. 8 | RCT | CAD | ESRD wait for renal transplantation | 1 year, 3 years | 41 | 46.15 | NR | 100 | NR | 34.62 | 0 |
| Szummer et al. 13 | Prospective | NSTEMI | eGFR < 15 ml/min/1.73 m2/dialysis | 1 year | 67 | 67.30 | 42 | 23 | NR | 24.40 | 18.80 |
| Hemmelgarn et al. 18 | Prospective | CAD | Dialysis; Ser > 2.3 mg/dL | 8 years | 56.64 | 72.01 | 76.82 | 46.27 | 44.62 | NR | 56.17 |
| Charytan et al. 4 | Respective | AMI | Dialysis | In hospital | 67.45 | 55 | NR | 57.00 | NR | NR | NR |
| Chertow et al. 19 | Respective | AMI | ESRD (Ser > 5 mg/dL, dialysis) | 30 days, 12 months | NR | 59 | 81 | 52.00 | NR | NR | 30 |
| Medi et al. 20 | Prospective | STE/LBBB | eGFR < 30 ml/min/1.73 m2 | In hospital, 6 months | 77.00 | 47 | 74 | 41.00 | 36.00 | 38 | 34 |
| Yasuda 21 | Prospective | CAD (MVD, SVD) | Dialysis | 10 months, 30 months, 60 months | 63.28 | 64 | 59.18 | 41.48 | NR | 45 | 0 |
| Agirbasli et al. 22 | Retrospective | CAD | Dialysis | In hospital, 1 year | 59.03 | 64 | 87.70 | 48.41 | 36.51 | 65 | 35.32 |
| Marui et al. 23 | Retrospective | MVD and/or LM CAD | Dialysis | 30 days, 1 year, 3 years, 5 years | 66.30 | 75 | 86.60 | 62.89 | NR | 17 | 17.78 |
| Herzog et al. 16 | Retrospective | CAD | Dialysis | In hospital, 1 year, 2 years, 5 years | NR | 61 | 27.07 | 34.87 | NR | NR | NR |
| Herzog 15 | Retrospective | CAD | Dialysis | In hospital, 1 year, 2 years, 3.5 years | NR | 57.37 | 24.85 | 45.96 | NR | NR | NR |
| Ivens et al. 24 | Retrospective | CAD | Dialysis | 30 days, 1 year, 2 years | 53 | 79.05 | 94.43 | 20.10 | 71.76 | 65.62 | 33.30 |
| Ohmoto et al. 25 | Retrospective | IHD | ESRD | In hospital, 38–51 months | 61 | 80.58 | 86.33 | 46.76 | 72.66 | 58.27 | 27.34 |
| Shroff et al. 14 | Retrospective | CAD | Dialysis | 30 days, 1 year, 2 years, 5 years | NR | 57.28 | 26.64 | 75.68 | NR | NR | NR |
| Sunagawa et al. 26 | Retrospective | CAD without MI | Dialysis | 30 days, 2 years | 64.56 | 76.90 | 77.84 | 43 | 20 | NR | NR |
| Terazawa et al. 27 | Retrospective | CAD without AMI | Dialysis | 30 days, 1 year, 3 years, 5 years | 64.25 | 77 | 77.60 | 58.40 | NR | NR | NR |
| Chang et al. 17 | Retrospective | MVD | Dialysis | 1.7 years | 63.73 | 60.63 | 80.05 | 64.56 | 25.37 | NR | 19.13 |
| Kumada et al. 28 | Retrospective | CAD without AMI | Dialysis | 30 days, 20 months, 40 months | 66 | 70.5 | 61.50 | 59.50 | 23.30 | 27.40 | 7.30 |
| Baek et al. 29 | Propensity-matched | CAD without AMI | Dialysis | 1 year, 3 years, 6 years | 60.59 | 63.22 | 89.66 | 68.87 | NR | 80.46 | NR |
| Ashirth et al. 30 | Retrospective | MVD | Dialysis; eGFR < 15 ml/min/1.73 m2 | 30 days, 2.5 years | NR | 30.38 | NR | 33.76 | 54.31 | 42.72 | 24 |
| Yeats et al. 31 | Prospective | CAD | Dialysis; eGFR < 15 ml/min/1.73 m2 | 30 days, 1 year, 2 years | 60.92 | 60 | 97 | 50 | 73 | 61.11 | NR |
| Manabe et al. 32 | Retrospective | CAD | Dialysis | In hospital, 1.5 years | 62.55 | 71.43 | 60.71 | 48.21 | 21.43 | 35.71 | NR |
| Aoki et al. 33 | Retrospective | CAD | Dialysis | In hospital, 20 months | 62.59 | 79.20 | 86.40 | 56 | 36.80 | 50.40 | 29.60 |
| Szczech et al. 34 | Retrospective | CAD | Dialysis | 3 years | 61.1 | 64.90 | NR | 48.40 | NR | NR | NR |
| Simsir et al. 35 | Retrospective | CAD | Dialysis | 30 days, 20 months | 64 | 51 | 95.12 | 73.17 | 26.83 | NR | 19.51 |
| Koyanagi et al. 36 | Retrospective | CAD | Dialysis | In hospital, 26 months | 55.93 | 88.37 | NR | NR | NR | NR | 51.26 |
| Rinehart et al. 37 | Retrospective | CAD | Dialysis | 30 days, 2 years, 66 months | 62.53 | 71.43 | NR | 41.67 | NR | NR | NR |
| Bangalore et al. 10 | RCT | SCAD | eGFR < 30 ml/min/1.73 m2 | 2.2 years | 63 | 68.90 | 92 | 57.10 | NR | 10.80 | 17.10 |
| Fujimoto et al. 38 | Retrospective | CAD | Dialysis | In hospital, 5 years | NR | NR | NR | NR | NR | NR | NR |
| Takeshita et al. 39 | Retrospective | CAD | Dialysis | In hospital, 35 months | 57 | 84 | NR | NR | NR | NR | NR |
| Sakakibara et al. 40 | Retrospective | CAD | Dialysis | 44 months | 63.90 | 71 | NR | NR | NR | NR | NR |
| Baldovinos et al. 41 | Retrospective | CAD | Dialysis | 30 days, 24 months | 63 | 68 | NR | NR | NR | NR | NR |
AMI, acute myocardial infarction; CAD, coronary artery disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HC, hyper-cholesterol; HT, hypertension; IHD, ischemic heart disease; MVD, multivessel disease; NR, not reported; PCI, percutaneous coronary intervention; SCAD, stable coronary artery disease; Ser, serum creatinine; STE/LBBB, ST-segment elevation/left bundle branch block.
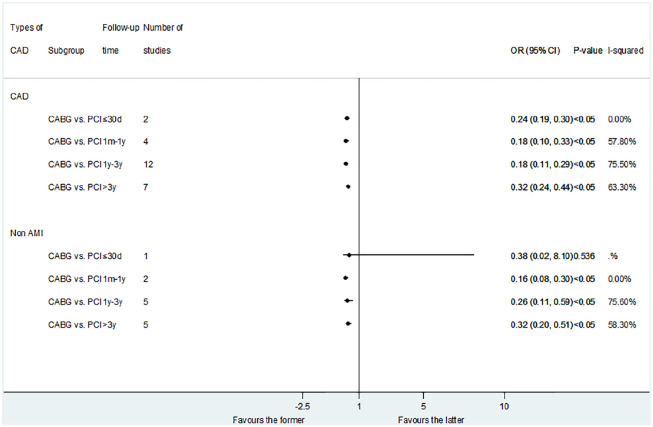
Figure 2.
Comparison of CABG and PCI with DES for advanced kidney disease patients with non-AMI.
AMI, acute myocardial infarction; CABG, coronary artery bypass surgery; DES, drug-eluting stent; PCI, percutaneous coronary intervention.
All-cause death
Percutaneous coronary intervention versus medical therapy
A total of ten studies compared PCI with MT, including 1480 people receiving PCI and 4422 receiving MT. None of them compared DES-PCI with MT. Compared with MT, PCI was associated with reduced short- and medium-term mortality and long-term mortality (more than 3 years): 0–1 month (OR: 0.60; 95% CI: 0.43–0.82, p < 0.05); 1 month–1 year (OR: 0.60; 95% CI: 0.44–0.82, p < 0.05); more than 3 years (OR: 0.64; 95% CI: 0.48– 0.85, p < 0.05). During 1–3 years of follow-up, there was no significant difference in all-cause mortality between PCI and MT (OR: 0.82; 95% CI: 0.62–1.09, p = 0.182). Different follow-up time subgroups had mild to moderate heterogeneity (I2 = 26.80%, 20.30%, 37.10%, and 48.50%, respectively) (Table 2).
Table 2.
Comparison of different treatments in advanced kidney disease patients with coronary artery disease: all-cause death.
| Types of CAD | Subgroup | Follow-up time | No. of studies | OR (95% CI) | p-value for meta-analysis | I-squared (%) | p-value for heterogeneity |
|---|---|---|---|---|---|---|---|
| CAD | PCI versus MT | ⩽1 month | 4 | 0.6 (0.43, 0.82) | <0.05 | 26.80 | p = 0.251 |
| 1 month–1 year | 7 | 0.6 (0.44, 0.82) | <0.05 | 20.30 | p = 0.275 | ||
| 1–3 years | 4 | 0.82 (0.62, 1.09) | 0.182 | 37.10 | p = 0.190 | ||
| >3 years | 3 | 0.64 (0.48, 0.85) | <0.05 | 48.50 | p = 0.143 | ||
| CABG versus MT | ⩽1 month | 3 | 1.25 (0.83, 1.88) | 0.392 | 10.50 | p = 0.327 | |
| 1 month–1 year | 3 | 0.67 (0.21, 2.09) | 0.079 | 51.70 | p = 0.126 | ||
| 1–3 years | 2 | 0.80 (0.06, 10.68) | 0.536 | 63.60 | p = 0.097 | ||
| >3 years | 1 | 1.17 (0.80, 1.73) | 0.415 | / | / | ||
| CABG versus PCI | ⩽1 month | 19 | 2.24 (1.80, 2.80) | <0.05 | 60.30 | p < 0.001 | |
| 1 month–1 year | 10 | 0.91 (0.81, 1.02) | 0.116 | 66.50 | p = 0.001 | ||
| 1–3 years | 20 | 0.81 (0.74, 0.88) | <0.05 | 38.70 | p = 0.040 | ||
| >3 years | 11 | 0.85 (0.75, 0.96) | <0.05 | 73.10 | p < 0.001 | ||
| AMI | PCI versus MT | ⩽1 month | 3 | 0.65 (0.41, 1.05) | 0.079 | 50.00 | p = 0.135 |
| 1 month–1 year | 4 | 0.70 (0.42, 1.15) | 0.157 | 51.70 | p = 0.102 | ||
| CABG versus MT | ⩽1 month | 2 | 1.18 (0.78, 1.80) | 0.437 | 0 | p = 0.344 | |
| 1 month–1 year | 1 | 0.37 (0.16, 0.82) | <0.05 | / | / | ||
| CABG versus PCI | ⩽1 month | 2 | 1.95 (0.69, 5.47) | 0.206 | 58.20 | p = 0.122 | |
| 1 month–1 year | 1 | 0.54 (0.20, 1.42) | 0.211 | / | / | ||
| Non AMI | PCI versus MT | 1 month–1 year | 2 | 0.54 (0.24, 1.25) | 0.151 | 0 | p = 0.645 |
| 1–3 years | 3 | 0.63 (0.30, 1.31) | 0.213 | 57 | p = 0.095 | ||
| >3 years | 2 | 0.46 (0.30, 0.72) | <0.05 | 0 | p = 0.608 | ||
| CABG versus MT | 1 month–1 year | 1 | 0.42 (0.02, 10.28) | 0.594 | / | / | |
| 1–3 years | 1 | 0.14 (0.01, 3.08) | 0.213 | / | / | ||
| MVD | PCI versus MT | 1 month–1 year | 1 | 0.55 (0.21, 1.44) | 0.221 | / | / |
| 1–3 years | 1 | 0.29 (0.11, 0.77) | <0.05 | / | / | ||
| >3 years | 1 | 0.33 (0.12, 0.93) | <0.05 | / | / | ||
| CABG versus PCI | ⩽1 month | 2 | 2.01 (0.76, 5.31) | 0.158 | 0 | p = 0.950 | |
| 1 month–1 year | 2 | 1.15 (0.69, 1.91) | 0.598 | 0 | p = 0.714 | ||
| 1–3 years | 3 | 0.91 (0.54, 1.55) | 0.74 | 42.90 | p = 0.174 | ||
| >3 years | 2 | 1.03 (0.72, 1.49) | 0.854 | 0 | p = 0.579 |
AMI, acute myocardial infarction; CABG, coronary artery bypass surgery; CAD, coronary artery disease; CI., confidence interval; MT, medical therapy; MVD, multivessel disease; PCI, percutaneous coronary intervention; OR, odd’s ratio.
However, for AMI patients, PCI did not reduce the short- and medium-term all-cause mortality compared with MT: 0–1 month (OR: 0.65; 95% CI: 0.41–1.05, p = 0.079); 1 month–1 year (OR: 0.70; 95% CI: 0.42–1.15, p = 0.157) (Table 2).
For non-AMI patients, there was no significant difference in medium-term and long-term (1–3 years) mortality between PCI and MT: 1 month–1 year (OR: 0.54; 95% CI: 0.24–1.25, p = 0.151); 1–3 years (OR: 0.63; 95% CI: 0.30–1.31, p = 0.213). However, PCI was associated with a reduction in long-term mortality (more than 3 years) (OR: 0.46; 95% CI: 0.30–0.72, p < 0.05).
As for MVD patients, PCI was only associated with a reduction in long-term mortality (more than 1 year): 1 month–1 year (OR: 0.55; 95% CI: 0.21–1.44, p = 0.221); 1–3 years (OR: 0.29; 95% CI: 0.11–0.77, p < 0.05); more than 3 years (OR: 0.33; 95% CI: 0.12–0.93, p < 0.05) (Table 2).
Coronary artery bypass surgery versus medical therapy
Five studies compared CABG with MT, including 341 people receiving CABG and 2896 receiving MT. There was no statistical difference between CABG and MT in each subgroup of follow-up time: less than 1 month (OR: 1.25; 95% CI: 0.83–1.88, p = 0.392); 1 month–1 year (OR: 0.67; 95% CI: 0.21–2.09, p = 0.079); 1–3 years (OR: 0.80; 95% CI: 0.06–10.68, p = 0.536); and more than 3 years (OR: 1.17; 95% CI: 0.80–1.73, p = 0.415) (Table 2). There were mild heterogeneities in the subgroup with less than 1 month follow-up time (I2 = 10.50%), and severe heterogeneity in the subgroups with 1 month–1 year and 1–3 years (I2 is 51.70% and 63.60%, respectively).
For patients with AMI, CABG did not reduce the short-term all-cause mortality compared with medical treatment (OR: 1.18; 95% CI: 0.77–1.80, p = 0.437); however, CABG was associated with the reduction in the medium-term all-cause mortality (OR: 0.37; 95% CI: 0.16–0.82, p < 0.05). In patients with non-AMI, compared with MT, CABG did not have a statistically significant difference in the subgroup of medium and long follow-up time: 1 month–1 year (OR: 0.42; 95% CI: 0.02–10.28, p = 0.594); 1–3 years (OR: 0.14; 95% CI: 0.01–3.08, p = 0.213) (Table 2).
Coronary artery bypass surgery versus percutaneous coronary intervention
A total of twenty-seven studies compared CABG with PCI. Compared with CABG, PCI was associated with the reduction in short-term all-cause mortality (OR: 2.24; 95% CI: 1.80–2.80, p < 0.05). CABG was associated with a reduction in long-term all-cause mortality: 1–3 years (OR: 0.81; 95% CI: 0.74–0.88, p < 0.05); more than 3 years (OR: 0.85; 95% CI: 0.75–0.96, p < 0.05). There was no statistically significant difference in medium term all-cause mortality (OR: 0.91; 95% CI: 0.81–1.02, p = 0.116) (Table 2). There were moderate heterogeneities in the subgroup with 1–3 years follow-up time (I2 = 38.70%), and severe heterogeneity in the other three subgroups (I2 is 60.30% 66.50%, and 73.10%, respectively) (Table 2).
For patients with AMI, CABG did not reduce the short- and medium-term all-cause mortality compared with PCI: less than 1 month (OR: 1.95; 95% CI: 0.69–5.47, p = 0.206); 1 month–1 year (OR: 0.54; 95% CI: 0.20–1.42, p = 0.211) (Table 2).
In patients with non-AMI, PCI was associated with a reduction in short-term mortality compared with CABG (OR: 3.02; 95% CI: 1.67–5.46, p < 0.05). CABG was associated with a reduction in long-term mortality compared with PCI: more than 3 years (OR: 0.82; 95% CI: 0.77–0.88, p < 0.05). But there was no statistical difference in 1 month– years follow-up times subgroups: 1 month–1 year (OR: 1.05; 95% CI: 0.98–1.13, p = 0.133); 1–3 years (OR: 0.90; 95% CI: 0.71–1.14, p = 0.400) (Table 2). When we performed a subgroup analysis of DES-PCI versus CABG, we found the same results: DES-PCI was associated with a reduced risk of short-term death (OR: 3.17; 95% CI: 2.75–3.65, p < 0.05), while CABG was associated with a reduced risk of long-term death (more than 3 years) (OR: 0.82; 95% CI: 0.77–0.88, p < 0.05) (Figure 2).
For patients with MVD, no benefit of PCI was found compared with CABG: less than 1 month (OR: 2.01; 95% CI: 0.76–5.31, p = 0.158); 1 month–1 year (OR: 1.15; 95% CI: 0.69–1.91, p = 0.598); 1–3 years (OR: 0.91; 95% CI: 0.54–1.55, p = 0.74); and more than 3 years (OR: 1.03; 95% CI: 0.72–1.49, p = 0.854) (Table 2).
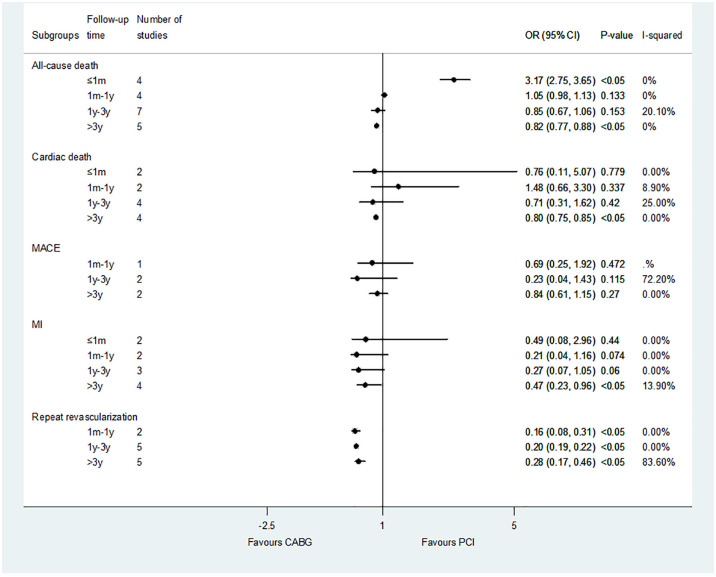
Cardiac death
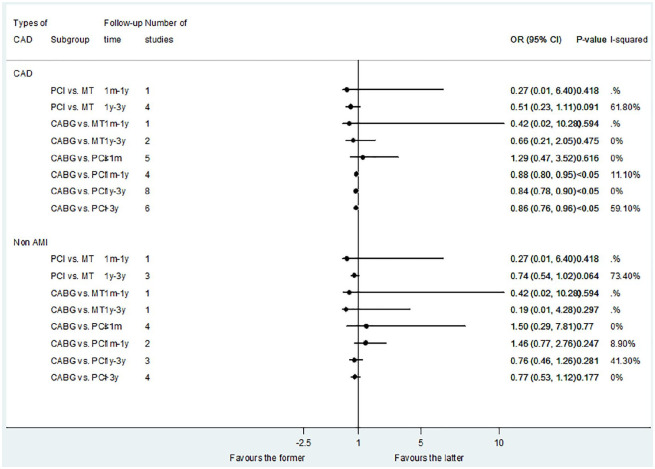
Sixteen studies reported the results of cardiac death. The results showed no difference between the PCI versus MT and CABG versus MT (Figure 3). Compared with PCI, CABG had no difference in short-term cardiac death, but was associated with a reduction in the risk of medium- and long-term cardiac death: less than 1 month (OR: 1.29; 95% CI: 0.47–3.52, p = 0.616); 1 month–1 year (OR: 0.88; 95% CI: 0.80–0.95, p < 0.05); 1–3 years (OR: 0.84; 95% CI: 0.78–0.90, p < 0.05); and more than 3 years (OR: 0.86; 95% CI: 0.76–0.96, p < 0.05).
Figure 3.
Comparison of different treatments for advanced kidney disease patients with CAD: cardiac death.
CAD, coronary artery disease.
For patients with non-AMI, there was no statistical difference between the PCI versus MT, CABG versus MT, and CABG versus PCI in all the follow-up times subgroups (Figure 3). However, when we compared CABG with DES-PCI, we found that CABG can obtain long-term benefits of cardiac death: more than 3 years (OR: 0.80; 95% CI: 0.75–0.85, p < 0.05) (Figure 2).
Major adverse cardiovascular events
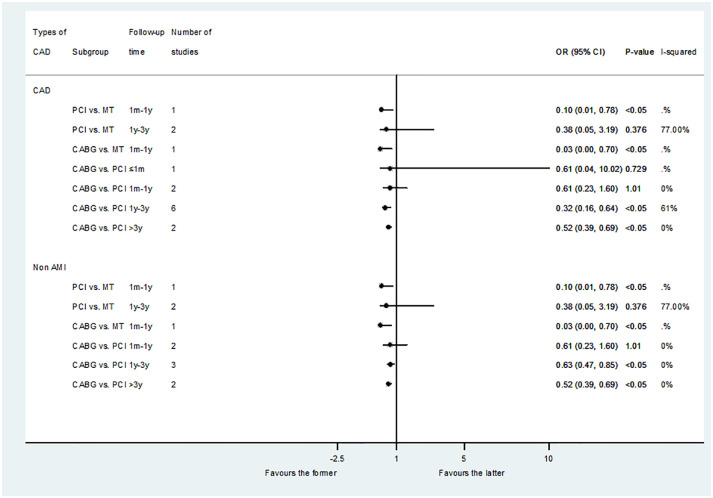
A total of eight studies reported the results of MACEs. The results showed that the revascularization group (PCI and CABG) was associated with a reduced risk of medium-term MACEs compared with MT: PCI versus MT (OR: 0.10; 95% CI: 0.01–0.78, p < 0.05); CABG versus MT (OR: 0.03; 95% CI: 0.00–0.70, p < 0.05). Compared with PCI, CABG had no difference in the risk of short- and medium-term MACEs, but was related to reducing the risk of long-term MACEs: less than 1 month (OR: 0.61; 95% CI: 0.04–10.02, p = 0.729); 1 month–1 year (OR: 0.61; 95% CI: 0.23–1.60, p = 1.01); 1–3 years (OR: 0.32; 95% CI: 0.16–0.64, p < 0.05); and more than 3 years (OR: 0.52; 95% CI: 0.39–0.69, p < 0.05) (Figure 4).
Figure 4.
Comparison of different treatments for advanced kidney disease patients with CAD: MACEs.
CAD, coronary artery disease; MACEs, major adverse cardiovascular events.
The result of MACEs in non-AMI was similar (Figure 4). However, in the subgroup analysis of CABG versus DES-PCI, there was no significant difference between CABG and DES-PCI in the medium and long-term follow-up time: 1 month–1 year (OR: 0.69; 95% CI: 0.25–1.92, p = 0.472); 1–3 years (OR: 0.23; 95% CI: 0.04–1.43, p = 0.115); and more than 3 years (OR: 0.84; 95% CI: 0.61–1.15, p = 0.27) (Figure 2).
Myocardial infarction
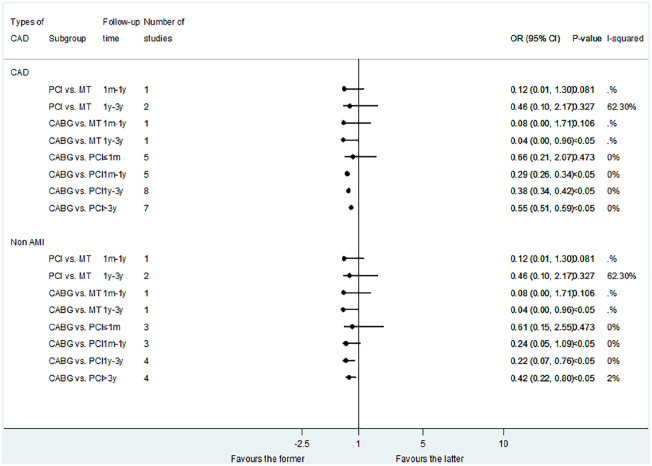
A total of 15 studies reported the results of MI shown in Figure 5. There was no significant difference in the risk of MI between PCI and MT. CABG was associated with a reduced risk of long-term (1–3 years) MI compared with MT (OR: 0.04; 95% CI: 0.00–0.96, p < 0.05). Compared with PCI, CABG had no difference in short-term results of MI, but was associated with a reduction in the risk of MI in the medium and long-term results: less than 1 month (OR: 0.66; 95% CI: 0.21–2.07, p = 0.473); 1 month–1 year (OR: 0.29; 95% CI: 0.26–0.34, p < 0.05); 1–3 years (OR: 0.38; 95% CI: 0.34–0.42, p < 0.05); and more than 3 years (OR: 0.55; 95% CI: 0.51–0.59, p < 0.05).
Figure 5.
Comparison of different treatments for advanced kidney disease patients with CAD: MI.
CAD, coronary artery disease; MI, myocardial infarction.
The result of MI in non-AMI patients was similar to that in CAD patients when comparing PCI with CABG. However, when compared to DES-PCI in non-AMI patients, CABG was only associated with a reduction in the risk of MI in long-term results: more than 3 years (OR: 0.47; 95% CI: 0.23–0.96, p < 0.05) (Figure 2).
Repeat revascularization
Fifteen studies reported repeat revascularization outcomes in the CABG versus PCI control group (Figure 6). The results showed that CABG was associated with lower risk of repeat revascularization compared with PCI : less than 1 month (OR: 0.24; 95% CI: 0.19–0.30, p < 0.05); 1 month–1 year (OR: 0.18; 95% CI: 0.10–0.33, p < 0.05); 1–3 years (OR: 0.18; 95% CI: 0.11–0.29, p < 0.05); and more than 3 years (OR: 0.32; 95%CI: 0.24–0.44, p < 0.05). The result of CABG versus DES-PCI was consistent with CABG versus PCI (Figure 2).
Figure 6.
Comparison of different treatments for advanced kidney disease patients with CAD: repeat revascularization.
CAD, coronary artery disease.
For the non-AMI patients, CABG was just associated with lower risk of medium- and long-term repeat revascularization compared with PCI: less than 1 month (OR: 0.38; 95% CI: 0.02–8.10, p = 0.536); 1 month–1 year (OR: 0.16; 95% CI: 0.08–0.30, p < 0.05); 1–3 years (OR: 0.26; 95% CI: 0.11–0.59, p < 0.05); and more than 3 years (OR: 0.32; 95% CI: 0.20–0.51, p < 0.05).
Sensitivity analysis, quality of studies and publication bias
In sensitivity analysis, the conclusions from forest plots for cardiac death, MACEs, MI, and repeat revascularization, were in consistent with the primary analyses when each trial was individually excluded. In terms of all-cause mortality, the statistical heterogeneity of PCI versus MT and CABG versus MT measured by I2 is 20.3–48.5%, and 10.5–43.6%, indicating a low to moderate heterogeneity (p = 0.145; 0.086). CABG versus PCI considered the potential high degree of heterogeneity between studies (I2: 74.0–85.6%, p < 0.05), and performed a subgroup analysis based on follow-up time. When each study was excluded individually, the conclusion of recalculating the set estimate was consistent with the original analysis (Supplemental Figures 1–3).
The quality assessment of RCT and observational studies is shown in Supplemental Table 1. A preliminary assessment of the quality of the two randomized studies was conducted. These two studies were considered low-risk randomization because they specified the randomization method applied; but they both reported no blindness or unclear blindness. Both of these studies fully reported data on each major outcome indicator (including lost to follow-up and dropped out). Therefore, follow-up deviations are considered low-risk. In the observational study, according to the NOS scale, 10 studies scored 7 points, and the remaining 20 studies scored 8 points.
We found evidence of publication bias based on the funnel plot (Supplemental Figures 4, Figure 5) and the Begg test (t = 2.05; p = 0.045).
Discussion
A total of 32 studies involving 84,498 patients were selected for this study. This study showed that PCI reduced the risk of short-, medium- and long- term (more than 3 years) all-cause death compared with MT. CABG showed no significant difference in all-cause death compared with MT. Compared with PCI, CABG had higher risk of short-term mortality and lower long-term mortality.
Patients with advanced kidney disease and CAD are a large population, 42 and they are all facing the question of whether to accept revascularization, which is inconclusive. So this issue is very urgent and difficult. 43
Previous META analyses also studied similar content, but there were several limitations:44–46 First, the targeted patient was patients with chronic kidney disease (eGFR < 60 ml/min/1.73 m2), which included patients with mild to moderate renal failure. And the guidelines recommend mild to moderate renal failure patients with CAD undergo revascularization (PCI or CABG). Secondly, only one of the control groups (PCI versus MT; CABG versus PCI) was studied. Finally, most research follow-up time was divided into two groups: short-term (less than 1 month) and long-term (more than 1 year).
Our research has been improved: Firstly, we are targeting patients with advanced kidney disease (eGFR < 30 ml/min/1.73 m2), which was usually ignored in research and guidelines. Secondly, we have a more comprehensive comparison, including pairwise comparisons of three treatment options. Finally, we subdivided the follow-up time, especially we added a long-term follow-up time of more than 3 years.
PCI versus MT
Our study found that PCI had low short-term, medium-term, and long-term (more than 3 years) all-cause mortality, and low risk of medium-term MACEs compared with MT.
Some studies reached the same result as us. A study included 151 diabetes patients with chronic renal failure and asymptomatic coronary stenosis found that PCI can reduce the incidence of cardiac events and mortality. 8 The APPROACH study including 662 dialysis patients, showed that PCI had low risk of death compared with conservative treatment. 18 A 5-year follow-up study of 259 dialysis patients with ischemic heart disease found that the 5-year all-cause survival rate in the MT group was 19.3%, and that in the PCI group was 48.4% (p < 0.0001). 21 It was recommended that dialysis patients with CAD should undergo PCI. This maybe because PCI can treat myocardial ischemia and improve heart function. And with the extension of follow-up time, this difference is gradually significant.
When we distinguished CAD type, we found different results. For patients with AMI, PCI cannot improve short- and medium-term all-cause mortality compared with MT. This result is consistent with Chan et al., 47 Medi et al., 20 and Szummer et al.: 13 for patients with advanced kidney disease accompanied by non-ST segment elevated MI (NSTEMI) and ST segment elevated MI (STEMI), PCI does not reduce the risk of death and AMI. For non-AMI patients, it can reduce the risk of medium-term MACEs and death greater than 3 years. Interestingly, we speculate that CAD can benefit from PCI is driven by non-AMI. However, for non-AMI, PCI did not reduce the risk of death in 1 month–3 years, which is consistent with the result of Bangalore et al. 10
At present, we are not fully aware of the mechanism that causes the difference between AMI/non-AMI on PCI versus MT. But for non-AMI patients, PCI is still recommended, which can bring long-term benefits.
Moreover, when patients with MVD were taken into consideration, PCI was associated with a reduction in long-term mortality compared with conservative treatment. However, there are few studies on MVD patients with end-stage renal disease. More studies, especially RCT, are needed to confirm whether PCI or MT is better for these patients.
We regret that we have not been able to compare BMS and DES separately, which may be a very important influencing factor. In addition, the improvement of guideline-based MT over the last decades (e.g., DAPT) also may cause deviations.
CABG versus MT
Few studies compared CABG and drug therapy. Some found that CABG can reduce the risk of death and improve the quality of life compared with conservative treatment in dialysis patients.18,31 We found that in patients with CAD, CABG did not reduce all-cause mortality and end-stage renal disease, but it did reduce the risk of medium-term MACEs and 1–3 years MI. It is assumed that the reduced MACEs maybe driven by the reduced MI rate. The result for non-AMI patients and CAD patients is consistent with each other. However, when AMI patients are considered, CABG is associated with the reduced medium-term mortality in dialysis compared with drug alone. This result is in consistency with Charytan et al. 4 and Chertow et al. 19 The Chertow et al. 19 study showed that for AMI patients with end-stage renal disease, the 1-year survival rates of drugs alone group and CABG group were 45% and 69%, respectively. Unfortunately, only 5% of these patients received CABG treatment, and 88% of them received conservative treatment. 19 Therefore, for patients with CAD, or AMI, the existence of kidney disease or dependence on dialysis should not become an obstacle to revascularization therapy, especially CABG.
CABG versus PCI
Our study found that among patients with CAD, compared with PCI, CABG was associated with high risk of short-term death, and low risk of long-term death. CABG can also reduce the risk of long-term cardiac death, MACEs, MI and repeat revascularization. In addition, previous retrospective studies have reached similar conclusions,14–16 which revealed that dialysis patients have better long-term survival rates after CABG than after PCI.
For non-AMI patients, compared with PCI, CABG was also associated with high risk of short-term death, and low risk of long-term death, long-term MACEs, medium- and long-term MI, and medium- and long-term repeat revascularization. The risk of cardiac death was not affected by CABG or PCI. When we performed a subgroup analysis of DES-PCI, we found the similar result except for cardiac death and MACE. Compared with DES-PCI, CABG reduced both long-term (more than 3 years) all-cause death and cardiac death. It suggested that the reduction of cardiac death by CABG lead to the reduction of all-cause death on the long-term. With respect to MACE, the benefit of CABG disappeared compared with DES-PCI. It could be explanted by the fact that the advance of stents reducing MACEs.
For patients with AMI, CABG did not reduce the short- and medium-term all-cause mortality compared with PCI. This is different from previous research results. Previous studies showed that, for patient with AMI, CABG was superior to PCI. Chang et al. 17 studied the 5-year survival rate of 21,981 patients undergoing dialysis and demonstrated that, from 1994 to 1995, 640 patients with ESRD and AMI were studied and found that CABG may be the best treatment for AMI in ESRD. 19 The reason why CABG cannot reduce the risk of short-term death could be explained by the fact that patients with single-vessel and double-vessel disease are more likely to use PCI treatment, while patients with higher-risk left main and three-vessel disease are treated with CABG. 48 As to the better long-term survival rates for CABG compared with PCI, the following reasons may contribute to this result. Firstly, CABG surgery can provide complete revascularization, while PCI often cannot, 18 which is why CABG reduce the risk of long-term death. And secondly, CKD patients undergoing PCI increased risk of kidney injury, recurrent myocardial infarction, and revascularization, 49 which leads to poor long-term prognosis for PCI patients.
Limitations
First of all, different studies included different drug treatment; PCI includes both PTCA and BMS and DES. Therefore, we separated BMS and DES into subgroups. But many studies cannot clearly distinguish the types of stents used. We only have eight studies on DES-PCI, all comparing DES-PCI with CABG, without comparison between DES-PCI and MT. This may cause the deviations in the results of PCI versus MT. Secondly, the characteristics of different treatment groups were different. In CABG group, the types of lesions were more multi vessel and left main trunk lesions. Thirdly, only two RCT studies were included, and more than 60% of them were retrospective studies. This may be the main source of heterogeneity. But it also shows the necessity of our research. We need stronger evidence to guide clinical practice. Finally, advanced kidney disease includes CKD stage 4 and stage 5 (dialysis patients). The physiological and clinical characteristics of the two groups were different. Nevertheless, the ISCHEMIA-CKD trial showed that the clinical results of the two groups were similar. As a result, we grouped them according to ISCHEMIA-CKD trial. 10 In addition, there is no subdivision of chronic coronary syndrome and acute coronary syndrome. Moreover, the lack of clinical endpoints for acute kidney injury is of great significance for patients with advanced kidney disease. Finally, there is publication bias in this study.
Conclusion
In patients with advanced kidney disease and coronary artery disease, PCI reduced the risk of short-, medium- and long- term (more than 3 years) all-cause death compared with MT. CABG showed no significant difference in all-cause death compared with MT/compared with PCI, CABG was associated with a higher risk of short-term death and a lower risk of long-term death and adverse events, which were also observed in non-AMI patients. These correlations should be tested in future randomized trials.
Supplemental Material
Supplemental material, sj-pdf-1-taj-10.1177_20406223211024367 for Optimal treatment strategies for coronary artery disease in patients with advanced kidney disease: a meta-analysis by Jingwen Yong, Jinfan Tian, Xin Zhao, Xueyao Yang, Haoran Xing, Yi He and Xiantao Song in Therapeutic Advances in Chronic Disease
Footnotes
Author’s contribution: Xiantao Song and Yi He helped to conceive the theme and revise the manuscript. Jingwen Yong and Jinfan Tian participated in the research selection, data extraction, analysis, and manuscript drafting. Xin Zhao, Xueyao Yang, and Haoran Xing contributed data collation and manuscript revision.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Capital Health Development Research Project (No. 2018-2-2063), National Natural Science Foundation of China (NO. 81971569, NO. 81670324 and NO. 81671650), Beijing Lab for Cardiovascular Precision Medicine (PXM2018_014226_000013), Beijing Municipal Science and Technology Project (Z161100000516139), and 2018 Beijing Excellent Talent Fund (NO. 2018000021469G241).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical approval: This is a Meta-analysis of the studies involving human participants.
Informed consent: Not applicable.
ORCID iD: Xiantao Song  https://orcid.org/0000-0002-2114-2230
https://orcid.org/0000-0002-2114-2230
Data availability: All data included in this study are available upon request by contact with the corresponding author.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jingwen Yong, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China.
Jinfan Tian, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China.
Xin Zhao, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China.
Xueyao Yang, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China.
Haoran Xing, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China.
Yi He, Department of Radiology, Beijing Friendship Hospital, Capital Medical University, Yongan Road 95, Beijing City, 100050, China.
Xiantao Song, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Chaoyang District, Anzhen Road No. 2, Beijing City, 100029, China.
References
- 1. Sarnak M, Amann K, Bangalore S, et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol 2019; 74: 1823–1838. [DOI] [PubMed] [Google Scholar]
- 2. Keeley EC, Kadakia R, Soman S, et al. Analysis of long-term survival after revascularization in patients with chronic kidney disease presenting with acute coronary syndromes. Am J Cardiol 2003; 92: 509–514. [DOI] [PubMed] [Google Scholar]
- 3. Fox CS, Muntner P, Chen AY, et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the national cardiovascular data acute coronary treatment and intervention outcomes network registry. Circulation 2010; 121: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Charytan D, Mauri L, Agarwal A, et al. The use of invasive cardiac procedures after acute myocardial infarction in long-term dialysis patients. Am Heart J 2006; 152: 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States renal data system 2002 annual data report: atlas of end-stage renal disease in the United States. Am J Kidney Dis 2003; 41(4Suppl. 2): v–ix, S7-254. [DOI] [PubMed] [Google Scholar]
- 6. Huang HD, Alam M, Hamzeh I, et al. Patients with severe chronic kidney disease benefit from early revascularization after acute coronary syndrome. Int J Cardiol 2013; 168: 3741–3746. [DOI] [PubMed] [Google Scholar]
- 7. Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int 2006; 70: 2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manske CL, Wang Y, Rector T, et al. Coronary revascularisation in insulin-dependent diabetic patients with chronic renal failure. Lancet 1992; 340: 998–1002. [DOI] [PubMed] [Google Scholar]
- 9. Sedlis SP, Jurkovitz CT, Hartigan PM, et al. Health status and quality of life in patients with stable coronary artery disease and chronic kidney disease treated with optimal medical therapy or percutaneous coronary intervention (post hoc findings from the COURAGE trial). Am J Cardiol 2013; 112: 1703–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bangalore S, Maron DJ, O’Brien SM, et al. Management of coronary disease in patients with advanced kidney disease. N Engl J Med 2020; 382: 1608–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zannad F, Rossignol P. Cardiovascular outcome trials in patients with advanced kidney disease. Circulation 2017; 135: 1769–1771. [DOI] [PubMed] [Google Scholar]
- 12. Shavadia JS, Southern DA, James MT, et al. Kidney function modifies the selection of treatment strategies and long-term survival in stable ischaemic heart disease: insights from the Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease (APPROACH) registry. Eur Heart J Qual Care Clin Outcomes 2018; 4: 274–282. [DOI] [PubMed] [Google Scholar]
- 13. Szummer K, Lundman P, Jacobson SH, et al. Influence of renal function on the effects of early revascularization in non-ST-elevation myocardial infarction: data from the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Circulation 2009; 120: 851–858. [DOI] [PubMed] [Google Scholar]
- 14. Shroff GR, Solid CA, Herzog CA. Long-term survival and repeat coronary revascularization in dialysis patients after surgical and percutaneous coronary revascularization with drug-eluting and bare metal stents in the United States. Circulation 2013; 127: 1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herzog CA. Comparative survival of dialysis patients in the United States after coronary angioplasty, coronary artery stenting, and coronary artery bypass surgery and impact of diabetes. Circulation 2002; 106: 2207–2211. [DOI] [PubMed] [Google Scholar]
- 16. Herzog CA, Ma JZ, Collins AJ. Long-term outcome of dialysis patients in the United States with coronary revascularization procedures. Kidney Int 1999; 56: 324–332. [DOI] [PubMed] [Google Scholar]
- 17. Chang TI, Shilane D, Kazi DS, et al. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol 2012; 23: 2042–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hemmelgarn BR, Southern D, Culleton BF, et al. Survival after coronary revascularization among patients with kidney disease. Circulation 2004; 110: 1890–1895. [DOI] [PubMed] [Google Scholar]
- 19. Chertow GM, Normand SL, Silva LR, et al. Survival after acute myocardial infarction in patients with end-stage renal disease: results from the cooperative cardiovascular project. Am J Kidney Dis 2000; 35: 1044–1051. [DOI] [PubMed] [Google Scholar]
- 20. Medi C, Montalescot G, Budaj A, et al. Reperfusion in patients with renal dysfunction after presentation with ST-segment elevation or left bundle branch block: GRACE (Global Registry of Acute Coronary Events). JACC Cardiovasc Interv 2009; 2: 26–33. [DOI] [PubMed] [Google Scholar]
- 21. Yasuda K. Comparison of percutaneous coronary intervention with medication in the treatment of coronary artery disease in hemodialysis patients. J Am Soc Nephrol 2006; 17: 2322–2332. [DOI] [PubMed] [Google Scholar]
- 22. Agirbasli M, Weintraub WS, Chang GL, et al. Outcome of coronary revascularization in patients on renal dialysis. Am J Cardiol 2000; 86: 395–399. [DOI] [PubMed] [Google Scholar]
- 23. Marui A, Kimura T, Nishiwaki N, et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with end-stage renal disease requiring dialysis (5-year outcomes of the CREDO-Kyoto PCI/CABG Registry Cohort-2). Am J Cardiol 2014; 114: 555–561. [DOI] [PubMed] [Google Scholar]
- 24. Ivens K, Gradaus F, Heering P, et al. Myocardial revascularization in patients with end-stage renal disease: comparison of percutaneous transluminal coronary angioplasty and coronary artery bypass grafting. Int Urol Nephrol 2001; 32: 717–723. [DOI] [PubMed] [Google Scholar]
- 25. Ohmoto Y, Ayabe M, Hara K, et al. Long-term outcome of percutaneous transluminal coronary angioplasty and coronary artery bypass grafting in patients with end-stage renal disease. Jpn Circ J 1999; 63: 981–987. [DOI] [PubMed] [Google Scholar]
- 26. Sunagawa G, Komiya T, Tamura N, et al. Coronary artery bypass surgery is superior to percutaneous coronary intervention with drug-eluting stents for patients with chronic renal failure on hemodialysis. Ann Thorac Surg 2010; 89: 1896–1900. [DOI] [PubMed] [Google Scholar]
- 27. Terazawa S, Tajima K, Takami Y, et al. Early and late outcomes of coronary artery bypass surgery versus percutaneous coronary intervention with drug-eluting stents for dialysis patients. J Card Surg 2012; 27: 281–287. [DOI] [PubMed] [Google Scholar]
- 28. Kumada Y, Ishii H, Aoyama T, et al. Long-term clinical outcome after surgical or percutaneous coronary revascularization in hemodialysis patients. Circ J 2014; 78: 986–992. [DOI] [PubMed] [Google Scholar]
- 29. Baek CH, Kim SO, Park SJ, et al. Propensity-matched comparison of drug-eluting stent implantation and coronary artery bypass graft surgery in chronic hemodialysis patients. J Nephrol 2014; 27: 87–93. [DOI] [PubMed] [Google Scholar]
- 30. Ashrith G, Lee VV, Elayda MAA, et al. Short- and long-term outcomes of coronary artery bypass grafting or drug-eluting stent implantation for multivessel coronary artery disease in patients with chronic kidney disease. Am J Cardiol 2010; 106: 348–353. [DOI] [PubMed] [Google Scholar]
- 31. Yeates A, Hawley C, Mundy J, et al. Treatment outcomes for ischemic heart disease in dialysis-dependent patients. Asian Cardiovasc Thorac Ann 2012; 20: 281–291. [DOI] [PubMed] [Google Scholar]
- 32. Manabe S, Shimokawa T, Fukui T, et al. Coronary artery bypass surgery versus percutaneous coronary artery intervention in patients on chronic hemodialysis: does a drug-eluting stent have an impact on clinical outcome? J Card Surg 2009; 24: 234–239. [DOI] [PubMed] [Google Scholar]
- 33. Aoki J, Ikari Y, Sugimoto T, et al. Clinical outcome of percutaneous transluminal coronary rotational atherectomy in patients with end-stage renal disease. Circ J 2003; 67: 617–621. [DOI] [PubMed] [Google Scholar]
- 34. Szczech LA, Reddan DN, Owen WF, et al. Differential survival after coronary revascularization procedures among patients with renal insufficiency. Kidney Int 2001; 60: 292–299. [DOI] [PubMed] [Google Scholar]
- 35. Simsir SA, Kohlman-Trigoboff D, Flood R, et al. A comparison of coronary artery bypass grafting and percutaneous transluminal coronary angioplasty in patients on hemodialysis. Cardiovasc Surg 1998; 6: 500–505. [DOI] [PubMed] [Google Scholar]
- 36. Koyanagi T, Nishida H, Kitamura M, et al. Comparison of clinical outcomes of coronary artery bypass grafting and percutaneous transluminal coronary angioplasty in renal dialysis patients. Ann Thorac Surg 1996; 61: 1793–1796. [DOI] [PubMed] [Google Scholar]
- 37. Rinehart AL, Herzog CA, Collins AJ, et al. A comparison of coronary angioplasty and coronary artery bypass grafting outcomes in chronic dialysis patients. Am J Kidney Dis 1995; 25: 281–290. [DOI] [PubMed] [Google Scholar]
- 38. Fujimoto Y, Ishiwata S, Dohi T, et al. Long-term prognosis after coronary revascularization in patients with end-stage renal disease on dialysis: comparison of percutaneous coronary intervention and coronary artery bypass grafting. J Cardiol 2007; 50: 11–20. [PubMed] [Google Scholar]
- 39. Takeshita S, Yamaguchi T, Isshiki T, et al. Percutaneous transluminal coronary angioplasty and coronary bypass grafting for refractory angina in chronic dialysis patients. J Cardiol 1992; 22: 383–390. [PubMed] [Google Scholar]
- 40. Sakakibara T, Ishii H, Yoneda K, et al. Percutaneous coronary intervention is beneficial for better survival compared with medication alone in chronic hemodialysis patients with single-vessel disease. Circulation 2011; 124(Suppl. 21): Abstract 12514. [Google Scholar]
- 41. Baldovinos G, Petraglia A, Larre Borges P, et al. Ischemic cadiopathy in patients undergoing chronic hemodialysis. Nefrologia 2002; 22: 60–65. [PubMed] [Google Scholar]
- 42. Hoerger TJ, Simpson SA, Yarnoff BO, et al. The future burden of CKD in the United States: a simulation model for the CDC CKD initiative. Am J Kidney Dis 2015; 65: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shroff G, Chang T. Risk stratification and treatment of coronary disease in chronic kidney disease and end-stage kidney disease. Semin Nephrol 2018; 38: 582–599. [DOI] [PubMed] [Google Scholar]
- 44. Volodarskiy A, Kumar S, Amin S, et al. Optimal treatment strategies in patients with chronic kidney disease and coronary artery disease. Am J Med 2016; 129: 1288–1298. [DOI] [PubMed] [Google Scholar]
- 45. Wu P, Luo F, Fang Z. Multivessel coronary revascularization strategies in patients with chronic kidney disease: a meta-analysis. Cardiorenal Med 2019; 9: 145–159. [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Zhu S, Gao P, et al. Comparison of coronary artery bypass grafting and drug-eluting stents in patients with chronic kidney disease and multivessel disease: a meta-analysis. Eur J Intern Med 2017; 43: 28–35. [DOI] [PubMed] [Google Scholar]
- 47. Chan MY, Becker RC, Sim LL, et al. Reperfusion strategy and mortality in ST-elevation myocardial infarction among patients with and without impaired renal function. Ann Acad Med Singap 2010; 39: 179–184. [PubMed] [Google Scholar]
- 48. Komiya T, Ueno G, Kadota K, et al. An optimal strategy for coronary revascularization in patients with severe renal dysfunction. Eur J Cardiothorac Surg. Epub ahead of print 21 November 2014. DOI: 10.1093/ejcts/ezu426. [DOI] [PubMed] [Google Scholar]
- 49. Ix JH, Mercado N, Shlipak MG, et al. Association of chronic kidney disease with clinical outcomes after coronary revascularization: the Arterial Revascularization Therapies Study (ARTS). Am Heart J 2005; 149: 512–519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-taj-10.1177_20406223211024367 for Optimal treatment strategies for coronary artery disease in patients with advanced kidney disease: a meta-analysis by Jingwen Yong, Jinfan Tian, Xin Zhao, Xueyao Yang, Haoran Xing, Yi He and Xiantao Song in Therapeutic Advances in Chronic Disease