Abstract
Objective
To investigate the expression levels and mechanisms of microRNA (miRNA) 26a (miR-26a) and phosphatase and tensin homolog (PTEN) in patients with human papillomavirus (HPV)-induced condyloma acuminatum (CA) and penile squamous cell carcinoma (PSCC).
Methods
Thirty-one patients with HPV-positive CA and 28 with HPV-positive PSCC were included in this retrospective, cross-sectional study. PTEN mRNA and miR-26a levels in lesion tissues, blood, and urine were analyzed by quantitative reverse transcription polymerase chain reaction, and PTEN protein was detected by western blot and enzyme-linked immunosorbent assay. Cell proliferation was assessed by MTT assay. The interaction between miR-26a and PTEN was predicted by bioinformatics analysis and confirmed by dual luciferase reporter assay
Results
PTEN mRNA and protein levels were significantly lower and miR-26a levels were significantly higher in all samples from patients with PSCC compared with the CA group. Bioinformatics analysis and luciferase reporter assay confirmed PTEN as a target gene of miR-26a. Up-regulation of miR-26a significantly increased the proliferation of Penl1 PSCC cells.
Conclusions
PTEN expression is down-regulated and miR-26a levels are up-regulated in PSCC compared with CA. PTEN is a direct target gene of miR-26a. These results suggest that miR-26a might regulate HPV-positive progression from CA to PSCC through modulating PTEN.
Keywords: MicroRNA, miR-26a, phosphatase and tensin homolog, condyloma acuminatum, penile squamous cell carcinoma, human papillomavirus
Introduction
Condyloma acuminatum (CA) is a common sexually transmitted disease (STD) caused by human papillomavirus (HPV) infection. The incidence of CA has been gradually increasing. 1 The disease may represent a pre-malignant lesion with the potential to develop into invasive cancer. 2 CA is characterized clinically by multiple squamous epithelial inflammatory papilloma-like proliferative lesions near the external genitalia and perianal area. 3 HPV is a non-enveloped double-stranded virus with a monomolecular ring structure, with a genomic DNA length of 7.5 to 8.0 kb, encoding the early proteins E1 to E7 and the capsid proteins L1 and L2. Among these viruses, HPV-6, HPV-11, HPV-16, and HPV-18 are closely related to the pathogenesis of CA, and HPV-16 and HPV-18 are associated with genital squamous cell carcinomas, such as cervical, penile, and vaginal cancers, 4 and have been classified as carcinogenic factors in human reproductive tract tumors. 5
The development of penile squamous cell carcinoma (PSCC) results from interactions among multiple pathways, involving many kinds of messenger RNAs (mRNAs) and microRNAs (miRNAs). Wnt-1 and TSLC1 were found to be abnormally expressed in CA development, 6 and p16INK4a was associated with HPV expression and was found to be highly expressed in penile cancer. 7 Furthermore, many miRNAs have been implicated in the development of CA and penile cancer. 8 , 9 HPV has also been recognized as a causative factor in the pathogenesis and development of cervical and penile cancers. 10
The occurrence of these cancers is related to mutations of tumor suppressor genes and activation of oncogenes. Phosphatase and tensin homolog (PTEN) is a tumor suppressor gene, the mutational inactivation of which is closely related to the occurrence and development of many malignant tumors in humans. 11 PTEN also plays important roles in apoptosis, cell cycle, and cell migration, 12 and is thus closely linked with cancer development. PTEN expression has been found to be down-regulated in many malignancies, including prostate cancer, breast cancer, brain tumors, 13 endometrioma, 14 glioblastoma, 15 melanoma, 16 cervical cancer, 17 breast cancer, 18 and colon cancer. 19 PTEN has also been closely linked with CA and penile cancer, and PTEN-mediated phosphoinositide 3-kinase/Akt signaling may be involved in the pathogenesis of CA. 20 PTEN is also involved in the development, progression, and metastasis of penile cancer. 21 However, the regulatory role of PTEN upstream factors in HPV-positive CA and penile cancer has not been fully defined.
Abnormal expression of miRNA-26a (miR-26a) has been implicated in various biological processes, including the natural immune response against pathogen invasion, the development and differentiation of organ tissues, and the pathogenesis of various solid tumors and hematopoietic malignancies. 22 However, the regulation of PTEN by miR-26a has not been reported.
We conducted a retrospective, cross-sectional study to investigate the roles and molecular mechanisms of miR-26a and PTEN in patients with HPV-positive CA and penile squamous cell carcinoma (PSCC), focusing on the regulatory role of miR-26a. We detected PTEN mRNA and protein expression levels in lesion tissue, and in blood and urine samples from patients with CA and PSCC by quantitative real-time polymerase chain reaction (PCR), western blot analysis, and enzyme-linked immunosorbent assay (ELISA). We also predicted and validated the relationship and interaction between PTEN and miR-26a by bioinformatics analysis and dual luciferase reporter assay. The results of this study clarify the regulatory relationship between miR-26a and PTEN, mechanisms that lead to the development of HPV-positive CA and PSCC.
Materials and methods
Study subjects
Patients with HPV-positive CA or HPV-positive PSCC who were admitted to and diagnosed at Taizhou Municipal Hospital, Taizhou, China, from December 2015 to December 2018 were included in this study. The inclusion criteria were: carcinoma with no distant metastasis, with or without lymph nodes; no chemotherapy; no other tumors or immune diseases; and resection for HPV-positive CA or HPV-positive PSCC. The exclusion criteria were: patients with other tumors or immunological diseases; prior long-term radiotherapy (chemotherapy); and distant metastases or metastatic cancers. Lesion tissue and blood and urine samples were collected from the patients before resection. Prior written informed consent was obtained from all patients and the study was approved by the ethics review board of Taizhou Municipal Hospital (Taizhou, Zhejiang, China) (approval no.: IACUC-20160815-58).
For sample collection, CA skin lesions and surrounding normal tissues were collected, and tumor and paracancerous tissues were resected and stored in liquid nitrogen until further processing. Peripheral blood and urine samples were obtained from patients in the morning after overnight fasting. Blood samples were anticoagulated with EDTA. Blood and urine samples were centrifuged at 1200 ×g at 4°C for 10 minutes, and the supernatant was collected and stored at −20°C until further processing.
Quantitative reverse transcription PCR (RT-qPCR)
Total RNA was extracted with TRIzol reagent and cDNA was obtained by reverse transcription. Quantitative real-time PCR was performed using a miRcute miRNA kit (EP401; Tiangen, Beijing, China) containing SuperReal PreMix (SYBR Green) (FP204; Tiangen) with a PCR-iQ5 qRT-PCR detection system (Bio-Rad, Hercules, CA, USA). The primer sequences were as follows: PTEN, forward 5′-TTGAAGACCATAACCCACCACAG-3′ and reverse 5′-CATTACACCAGTTCGTCCCTTTC-3′; and glyceraldehyde phosphate dehydrogenase (internal reference), forward 5′-AAGGCTGTGGGCAAGG-3′ and reverse 5′-TGGAGGAGTGGGTGTCG-3′. The 20-µL reaction system consisted of 10 µL RT-qPCR-Mix, 0.5 µL each primer, 2 µL cDNA, and 7 µL double distilled H2O. The PCR conditions were set as follows: 95°C for 2 minutes, 95°C for 25 s, 55°C for 30 s, and 72°C for 30 s, for a total of 40 cycles. Target gene expression levels were calculated using the 2−ΔΔCt method. 23
miR-26a was detected using the following primer sequences: miR-26a, forward 5′-CTGTCAACGATACGCTAC-3′ and reverse 5′-GTAATCCAGGATAGGCTG-3′; and U6 (internal reference), forward 5′-CTTCGGCAGCACATATAC-3′ and reverse 5′-GAACGCTTCACGAATTTGC-3′, with the following reaction conditions: 90°C for 60 s, 95°C for 15 s, and 60°C for 30 s, for a total of 40 cycles.
Western blot analysis
The tissues were lysed using RIPA buffer (P0013B; Beyotime, Shanghai, China), according to the manufacturer’s manual, and the protein concentration was determined using a bicinchoninic acid kit (RTP7102; Real-times, Beijing, China). Protein (20 µg) was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. After blocking with 5% non-fat milk at room temperature for 1 hour, the membrane was incubated with rabbit anti-human anti-PTEN polyclonal antibody (1:500 dilution; ab32199; Abcam, Cambridge, MA, USA) or rabbit anti-mouse anti-β-actin primary antibody (1:2500 dilution; ab8227; Abcam), at 4°C overnight. The membrane was then treated with goat anti-rabbit secondary antibody (1:3000 dilution; ab6721; Abcam) at room temperature for 1 hour. Color was developed using an enhanced chemiluminescence kit (ab65623; Abcam), and the images were observed and analyzed using Image Lab 3.0 software (Bio-Rad, Hercules, CA, USA). β-actin was used as internal reference.
ELISA
Serum and urine samples were analyzed by ELISA (sE95822Hu; USCN, Wuhan, China). Ten microliters of sample plus 40 µL of diluting solution, or 50 µL standard solution at the indicated concentrations, were added into the wells. Blank wells had no additions. Horseradish peroxidase-conjugated detection antibody (1:1000, 100 µL; USCN) was added into the standard and sample wells, and the plates were sealed and incubated in the dark for 1 hour. After washing, substrates A and B (50 µL each) were added into each well and incubated at 37°C for 15 minutes, followed by the addition of 50 µL stop solution. The optical density (OD) values at 450 nm were then read within 15 minutes.
Bioinformatics analysis
Based on a literature search for upstream miRNAs of PTEN, we used miRanda target gene prediction software (http://34.236.212.39/microrna/home.do) to predict the possible regulators of PTEN.
Dual luciferase reporter assay
PTEN 3′-untranslated region (UTR) sequences with wild-type and mutant seed regions for miR-26a were synthesized by Sangon, Shanghai, China, with the addition of Spe-1 and HindIII restriction sites at both ends. These two DNA fragments were cloned into the pMIR-REPORT luciferase reporter plasmid (E1980; Promega, Madison, WI, USA), and 0.8 µg plasmids carrying the wild-type and mutant 3′-UTR sequences, respectively, were transfected into 293T cells (Cell Bank, Chinese Academy of Sciences, Shanghai, China), using liposomes, followed by transfection with 100 nM agomiR-26a (Sangon Biotech). After 24 hours, the cells were lysed and luciferase was determined using a GloMax 20/20 luminometer (Promega), with Renilla luciferase as an internal reference.
Cell transfection
For cell transfection, Penl1 PSCC cells (Cell Bank, Chinese Academy of Sciences) in logarithmic growth phase were inoculated onto a 24-well plate at a density of 3 × 105 cells/well and cultured with Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) containing 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA), without antibiotics. The cells were transfected when they reached 70% confluence. Plasmid/small interfering RNA/agomiR 1 µL and Lipofectamine 2000 1 µL were added into 50 µl Opti Memi medium (Thermo Fisher Scientific) separate Eppendorf tubes (Thermo Fisher Scientific) for 5 minutes. The solutions in the two tubes were then mixed together for 20 minutes, and the mixture was incubated with the cells for 6 hours. The cultured medium was then replaced with DMEM/F12 containing 10% fetal bovine serum for another 48 hours before further analysis. The agomiR-26a and agomiR-NC (Sangon Biotech) sequences were as follows: agomiR-26a, forward 5′-UUCAAGUAAUCCAGGAUAGGCU-3′ and reverse 3′-AAGUUCAUUAGGUCCUAUCCGA-5′; and agomiR-NC, forward 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse 3′-TTAAGAGGCUUGCACAGUGCA-5′.
MTT assay
Cell proliferation after transfection was detected by MTT assay. The cells were inoculated onto a 96-well plate at a density of 2 × 103 cells/well and, 20 µL MTT (5 g/L; Beyotime) was added at 24, 48, and 72 hours, respectively, followed by incubation at 37°C for 4 hours. Dimethyl sulfoxide 150 µL was then added into each well. The OD values at 490 nm were determined and proliferation curves were plotted.
Statistical analysis
Data were expressed as mean ± standard deviation. Statistical analyses were carried out using SPSS Statistics for Windows, Version 18.0 (SPSS Inc., Chicago, IL, USA). No sample size calculation was performed. Group comparisons were performed by one-way analysis of variance and t-tests. P < 0.05 was considered statistically significant.
Results
Patients
Thirty-one men with HPV-positive CA and 28 men with HPV-positive PSCC admitted to and diagnosed at our hospital from December 2015 to December 2018 were included in this study. The median age of the patients with HPV-positive CA was 38.6 years (range, 17–56 years) and the median age of the patients with HPV-positive PSCC was 39.2 years (range, 20–60 years).
mRNA expression levels of PTEN in tumor tissues and in urine and blood samples
We investigated the mRNA expression levels of PTEN in tumor tissues and in urine and blood samples by RT-qPCR. PTEN mRNA levels in tumor, blood, and urine samples from patients with PSCC were all significantly lower compared with patients with CA group (P < 0.05) (Figure 1). These results suggest that PTEN might be involved in the HPV-induced pathological process from CA to PSCC.
Figure 1.
Analysis of phosphatase and tensin homolog (PTEN) mRNA expression levels. mRNA expression levels of PTEN in (a) lesion tissue, (b) blood, and (c) urine samples detected by quantitative reverse transcription polymerase chain reaction. *P < 0.05, **P < 0.01 compared with condyloma acuminatum (CA) group.
PSCC, penile squamous cell carcinoma.
Protein expression levels of PTEN in lesions
We also investigated PTEN protein expression levels in lesion tissues by western blot. Compared with the CA group, PTEN protein levels were significantly lower in lesion tissues from patients with PSCC compared with the CA group (P < 0.05) (Figure 2), in line with the mRNA results. These results suggest that down-regulation of PTEN at both transcriptional and translational levels might be involved in the HPV-induced pathological progression from CA to PSCC.
Figure 2.
Analysis of phosphatase and tensin homolog (PTEN) protein expression levels. PTEN protein expression levels in (a) lesion tissues detected by western blot analysis, and in (b) blood and (c) urine detected by enzyme-linked immunosorbent assay. *P < 0.05, **P < 0.01 compared with condyloma acuminatum (CA) group.
PSCC, penile squamous cell carcinoma.
PTEN levels in blood and urine samples
We also investigated PTEN protein levels in blood and urine samples by ELISA. PTEN levels in the blood and urine were significantly lower in patients with PSCC compared with the CA group (P < 0.05) (Figure 2).
miR-26a expression in tumor tissues and in urine and blood samples
Bioinformatics analysis identified miR-26a as a possible regulator of PTEN, and UACUUGA as the potential conserved seed region in miR-26a (Figure 3). We therefore investigated the expression levels of miR-26a in tumor tissues and in urine and blood samples by RT-qPCR. miR-26a expression levels in tumor tissues and in urine and blood samples were all significantly higher in patients with PSCC compared with those with CA (P < 0.05) (Figure 4). Combined with the predicted relationship between PTEN and miR-26a, these results suggest that miR-26a might play a regulatory role in the HPV-induced pathological progression from CA to PSCC by affecting the transcription and translation of PTEN.
Figure 3.
Bioinformatics analysis of target mRNA for microRNA-26a.
Figure 4.
Analysis of microRNA(miR)-26a expression levels. miR-26a expression levels in (a) lesion tissue, (b) blood, and (c) urine detected by quantitative reverse transcription polymerase chain reaction. *P < 0.05, **P < 0.01 compared with condyloma acuminatum (CA) group.
PSCC, penile squamous cell carcinoma.
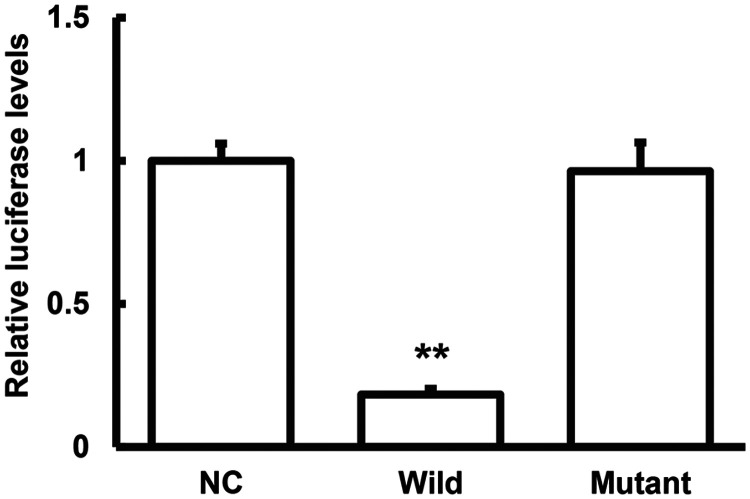
Dual luciferase reporter assay
We validated the relationship and interaction between miR-26a and PTEN by dual luciferase reporter assay. Luciferase expression was significantly reduced following co-transfection of agomiR-26a and pMIR-REPORT luciferase reporter plasmids (P < 0.05). However, there was no significant difference in luciferase expression in the mutant plasmid group (Figure 5). These results suggested that miR-26a could bind to the PTEN 3′-UTR to regulate its gene expression.
Figure 5.
Dual luciferase reporter assay. pMIR-REPORT luciferase reporter plasmids carrying wild-type and mutant phosphatase and tensin homolog 3′-untranslated region sequences were co-transfected into 293T cells with agomiR-26a. The cells were lysed and luciferase expression was determined 24 hours later. **P < 0.01 compared with the negative control (NC) group.
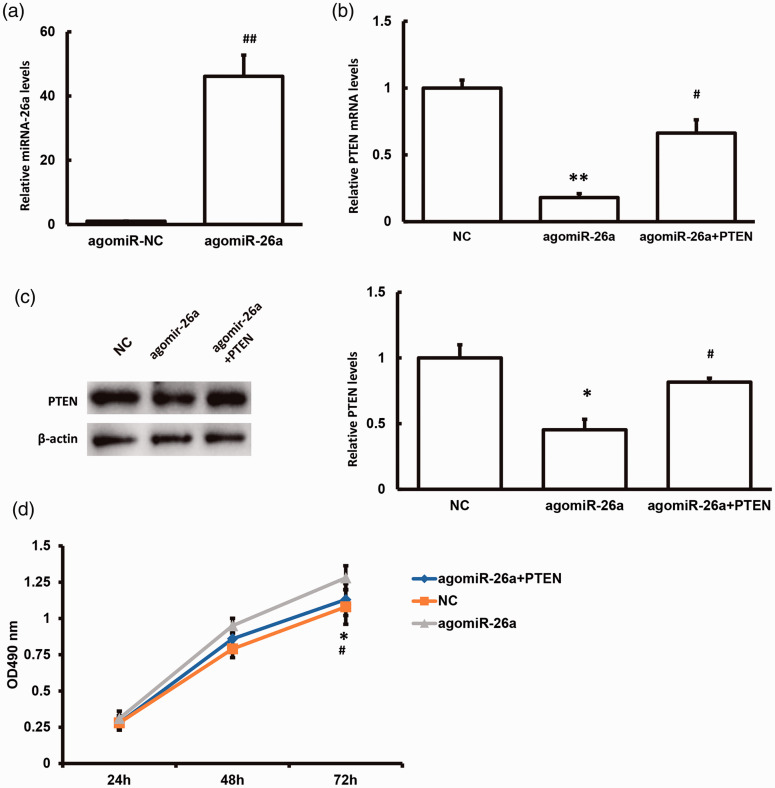
Effect of miR-26a on PTEN expression and cell proliferation
We investigated the effects of miR-26a on the expression levels of PTEN. miR-26a was over-expressed and PTEN levels were down-regulated in Penl1 PSCC cells following transfection with agomiR-26a. Cell proliferation activity was also significantly increased (P < 0.05). However, PTEN mRNA and protein expression levels recovered in cells co-transfected with agomiR-26a and plasmid contemporary DNA (pcDNA)-PTEN (Figure 6). These results suggest that miR-26a could regulate PTEN expression and affect the proliferation PSCC cells.
Figure 6.
Effects of microRNA(miR)-26a on phosphatase and tensin homolog (PTEN) expression and cell proliferation. (a) miR-26a expression levels in Penl1 cells were detected by quantitative reverse transcription polymerase chain reaction (PCR) after transfection with agomiR-26a. PTEN (b) mRNA and (c) protein expression levels in Penl1 cells were detected by quantitative reverse transcription PCR and western blot analysis, respectively, after transfection with agomiR-26a. (d) Cell viability was detected by MTT assay at the indicated time points. NC, agomiR-NC+pcDNA3.1 empty vector; agomiR-26a, agomiR-26a+ pcDNA3.1 empty vector; agomiR-26a+PTEN, agomiR-26a+ pcDNA-PTEN. *P < 0.05, **P < 0.01 compared with the NC group; #P < 0.05 compared with the agomiR-26a group.
OD, optical density.
Discussion
In this study, we measured the mRNA and protein expression levels of PTEN and of the upstream regulator miR-26a in lesion tissues and in blood and urine samples from patients with HPV-positive CA and PSCC. We also investigated the relationship and interaction between miR-26a and PTEN in preliminarily cell experiments to determine the possible mechanism by which miR-26a regulates downstream PTEN to affect the pathogenesis and development of HPV-positive CA and PSCC.
CA is ranked as the most common STD in Europe and the United States, and recent surveys in China have also suggested an increased incidence of genital HPV infections (such as around the vulva, anus, and cervix). 24 CA is currently ranked as the second most common STD in China, with a significantly greater annual increase than other STDs. CA is characterized by a high recurrence rate, and some persistent high-risk HPV infections may also cause malignant transformation, making clinical treatment difficult and imposing heavy psychological and economic burdens on the patients and their families.25,26 There is thus an urgent need to understand the pathogenesis of HPV-associated CA and PSCC, to aid the discovery of effective treatment and prevention methods.
The tumor suppressor gene PTEN, located at 10q23.3 and including nine exons, was first discovered in 1997.27,28 Down-regulation/deletion of PTEN has been closely related to the development and progression of many malignant tumors in humans, and plays a key role in apoptosis, cell cycle, and cell migration. 29 However, most studies on the effects of PTN on tumor formation have focused on its down-regulated expression in endometrial cancer, glioma, prostate cancer, breast cancer, and melanoma,13–19 and its roles in the pathogenesis of CA and PSCC remain unclear. The current study showed that expression levels of PTEN were significantly lower in skin lesions and in blood and urine samples from patients with PSCC compared with patients with CA. Considering that, in addition to local infiltration, tumors may also be disseminated through the blood circulation, changes in PTEN levels in the blood suggest the need to pay attention to the role of PTEN mutations in tumor dissemination. Furthermore, blood and urine levels of PTEN may serve as potential biomarkers for HPV-positive CA and PSCC.
miRNAs are important gene-regulatory factors involved in various pathophysiological processes, such as tumor cell proliferation, invasion, and metastasis, hypertension, diabetes, and atherosclerosis.30,31 miR-26a has been shown to control the secretion of various inflammatory chemokines and activate the innate immune response.32,33 It also plays an important role in the differentiation of stem cells via regulating the Smad transcription factor family, and is thus involved in the differentiation of liver stem cells into mature hepatocytes and biliary cells, and of adipose-derived stem cells into osteoblasts.34,35 Expression levels of miR-26a are decreased in various cancer cells and cancer tissues, leading to inhibition of proliferation of nasopharyngeal carcinoma, breast cancer, and hepatocellular carcinoma cells.36–40 miR-26a has been predicted to be closely related to PTEN, as an upstream miRNA regulating PTEN.41,42 The current results showed that expression levels of miR-26a were significantly elevated in lesion tissues and in blood and urine samples from patients with PSCC compared with patients with HPV-positive CA. Combined with the abnormal PTEN expression levels in the fluid samples, we speculated that up-regulation of miR-26a might be responsible for down-regulating PTEN expression. We further confirmed the direct interaction between miR-26a and the PTEN 3′-UTR by dual luciferase reporter assay. Using cell transfection experiments, we showed that over-expression of miR-26a reduced PTEN expression and increased proliferation of PSCC cells, suggesting that expression of PTEN in PSCC affects cell proliferation, and that this process is regulated by miR-26a. We therefore speculated that the regulation of PTEN by miR-26a might play an important biological role in the pathogenesis of HPV-positive CA and PSCC.
This study had some limitations. Penl1 cells are derived from HPV-negative penile cancer, but HPV-positive diseases were also considered. Moreover, the sample size was relatively small due to the specific inclusion criteria. Further in-depth studies need to be conducted with larger sample sizes.
In conclusion, the results of this study indicate that up-regulation of miR-26a caused enhanced cleavage of PTEN mRNA, thereby attenuating the pro-apoptotic effects of PTEN and resulting in uncontrolled cell proliferation, further inducing pathological changes in patients with HPV-positive CA and PSCC. These findings suggest that disease occurrence and development are determined by the balance between miR-26a and PTEN. In addition, the stability of miR-26a in blood and urine suggests that it might be a potential genetic biomarker for early tumor diagnosis.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funds from the Medical Scientific Research Foundation of Zhejiang Province, China [grant number: 2021KY398], and the Taizhou Science and Technology Bureau [grant number: 15yw04].
ORCID iD: Yayu Hu https://orcid.org/0000-0002-2588-8144
References
- 1.Cong X, Sun R, Zhang X, et al. Correlation of human papillomavirus types with clinical features of patients with condyloma acuminatum in China. Int J Dermatol 2016; 55: 775–780. [DOI] [PubMed] [Google Scholar]
- 2.Fernández-Nestosa MJ, Guimerà N, Sanchez DF, et al. Human papillomavirus (HPV) genotypes in condylomas, intraepithelial neoplasia, and invasive carcinoma of the penis using laser capture microdissection (LCM)-PCR: a study of 191 lesions in 43 patients. Am J Surg Pathol 2017; 41: 820–832. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Zhang Y, Wang S, et al. Loss of miR-143 and miR-145 in condyloma acuminatum promotes cellular proliferation and inhibits apoptosis by targeting NRAS. R Soc Open Sci 2018; 5: 172376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz N, Bosch FX, De Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348: 518–527. [DOI] [PubMed] [Google Scholar]
- 5.Nebesio CL, Mirowski GW, Chuang TY. Human papillomavirus: clinical significance and malignant potential. Int J Dermatol 2001; 40: 373–379. [DOI] [PubMed] [Google Scholar]
- 6.Yin GW, Xia XX, Song FJ, et al. Expression of Wnt-1 and TSLC1 in condyloma acuminatum. Clin Exp Dermatol 2019; 44: 620–624. [DOI] [PubMed] [Google Scholar]
- 7.Martins VA, Pinho JD, Teixeira Junior AAL, et al. P16INK4a expression in patients with penile cancer. PLoS One 2018; 13: e0205350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peta E, Cappellesso R, Masi G, et al. Down-regulation of microRNA-146a is associated with high-risk human papillomavirus infection and epidermal growth factor receptor overexpression in penile squamous cell carcinoma. Hum Pathol 2017; 61: 33–40. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Fang R, Gong Q, et al. miR-99b suppresses IGF-1R expression and contributes to inhibition of cell proliferation in human epidermal keratinocytes. Biomed Pharmacother 2015; 75: 159–164. [DOI] [PubMed] [Google Scholar]
- 10.Lie AK. [Human papillomavirus as a risk factor in carcinogenesis]. Tidsskr Nor Laegeforen 2000; 120: 2771–2776. [PubMed] [Google Scholar]
- 11.Ghafouri-Fard S, Abak A, Shoorei H, et al. Regulatory role of microRNAs on PTEN signaling. Biomed Pharmacother. 2021; 133: 110986. [DOI] [PubMed] [Google Scholar]
- 12.Squarize CH, Castilho RM, Santos Pinto D Jr. Immunohistochemical evidence of PTEN in oral squamous cell carcinoma and its correlation with the histological malignancy grading system. J Oral Pathol Med 2002; 31: 379–384. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997; 275: 1943–1947. [DOI] [PubMed] [Google Scholar]
- 14.Mutter GL, Lin MC, Fitzgerald JT, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst 2000; 92: 924–930. [DOI] [PubMed] [Google Scholar]
- 15.Duerr EM, Rollbrocker B, Hayashi Y, et al. PTEN mutations in gliomas and glioneuronal tumors. Oncogene 1998; 16: 2259–2264. [DOI] [PubMed] [Google Scholar]
- 16.Tsao H, Zhang X, Benoit E, et al. Identification of PTEN/MMAC1 alterations in uncultured melanomas and melanoma cell lines. Oncogene 1998; 16: 3397–3402. [DOI] [PubMed] [Google Scholar]
- 17.Rizvi MM, Alam MS, Ali A, et al. Aberrant promoter methylation and inactivation of PTEN gene in cervical carcinoma from Indian population. J Cancer Res Clin Oncol 2011; 137: 1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang XF, Xin Y, Mao LL. Clinicopathological significance of PTEN and Caspase-3 expressions in breast cancer. Chin Med Sci J 2008; 23: 95–102. [DOI] [PubMed] [Google Scholar]
- 19.Bowen KA, Doan HQ, Zhou BP, et al. PTEN loss induces epithelial–mesenchymal transition in human colon cancer cells. Anticancer Res 2009; 29: 4439–4449. [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Ma YB, Mao YQ, et al . Dehydrocostus lactone suppresses cell growth and induces apoptosis in recombinant human papilloma virus18 HaCaT cells via the PI3K/Akt signaling pathway. Mol Med Rep 2018; 17: 7925–7930. [DOI] [PubMed] [Google Scholar]
- 21.Pilarski R, Burt R, Kohlman W, et al. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst 2013; 105: 1607–1616. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Zhang K, Xu Y, et al. The role of microRNA-26a in human cancer progression and clinical application. Tumour Biol 2016; 37: 7095–7108. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 24.Buechner SA. Common skin disorders of the penis. BJU Int 2002; 90: 498–506. [DOI] [PubMed] [Google Scholar]
- 25.Sir E, Gungor M, Ucer O, et al. Invasive squamous cell carcinoma originating from a giant penile condyloma. Int J STD AIDS 2017; 28: 619–622. [DOI] [PubMed] [Google Scholar]
- 26.Ferrándiz-Pulido C, De Torres I, García-Patos V. [ Penile squamous cell carcinoma]. Actas Dermosifiliogr 2012; 103: 478–487. [DOI] [PubMed] [Google Scholar]
- 27.Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997; 15: 356–362. [DOI] [PubMed] [Google Scholar]
- 28.Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res 1997; 57: 2124–2129. [PubMed] [Google Scholar]
- 29.Alvarez-Garcia V, Tawil Y, Wise HM, et al. Mechanisms of PTEN loss in cancer: It's all about diversity. Semin Cancer Biol 2019; 59: 66–79. [DOI] [PubMed] [Google Scholar]
- 30.Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta 2016; 1859: 169–176. [DOI] [PubMed] [Google Scholar]
- 31.Varshney J, Subramanian S. MicroRNAs as potential target in human bone and soft tissue sarcoma therapeutics. Front Mol Biosci 2015; 2: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witwer KW, Sisk JM, Gama L, et al. MicroRNA regulation of IFN-beta protein expression: rapid and sensitive modulation of the innate immune response. J Immunol 2010; 184: 2369–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones MR, Quinton LJ, Blahna MT, et al. Zcchc11-dependent uridylation of microRNA directs cytokine expression. Nat Cell Biol 2009; 11: 1157–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogler CE, Levoci L, Ader T, et al. MicroRNA-23b cluster microRNAs regulate transforming growth factor-beta/bone morphogenetic protein signaling and liver stem cell differentiation by targeting Smads. Hepatology 2009; 50: 575–584. [DOI] [PubMed] [Google Scholar]
- 35.Luzi E, Marini F, Sala SC, et al. Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J Bone Miner Res 2008; 23: 287–295. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Zheng J, Zhang Y, et al. Tumor-specific expression of microRNA-26a suppresses human hepatocellular carcinoma growth via cyclin-dependent and -independent pathways. Mol Ther 2011; 19: 1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu J, He ML, Wang L, et al. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res 2011; 71: 225–233. [DOI] [PubMed] [Google Scholar]
- 38.Zhang B, Liu XX, He JR, et al. Pathologically decreased miR-26a antagonizes apoptosis and facilitates carcinogenesis by targeting MTDH and EZH2 in breast cancer. Carcinogenesis 2011; 32: 2–9. [DOI] [PubMed] [Google Scholar]
- 39.Kota J, Chivukula RR, O'Donnell KA, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell 2009; 137: 1005–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciarapica R, Russo G, Verginelli F, et al. Deregulated expression of miR-26a and Ezh2 in rhabdomyosarcoma. Cell Cycle 2009; 8: 172–175. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Jiang Y, Wu X, et al. Targeted regulation of miR-26a on PTEN to affect proliferation and apoptosis of prostate cancer cells. Cancer Biother Radiopharm 2019; 34: 480–485. [DOI] [PubMed] [Google Scholar]
- 42.Ding K, Wu Z, Wang N, et al. MiR-26a performs converse roles in proliferation and metastasis of different gastric cancer cells via regulating of PTEN expression. Pathol Res Pract 2017; 213: 467–475. [DOI] [PubMed] [Google Scholar]