Abstract
Objective:
To determine the optimal combination of imaging and biochemical biomarkers to predict knee osteoarthritis (OA) progression.
Methods:
Nested case-control study from the FNIH OA Biomarkers Consortium of participants with Kellgren-Lawrence grade 1–3 and complete biomarker data (n=539 to 550). Cases were knees with radiographic and pain progression between 24–48 months from baseline. Radiographic progression only was assessed in secondary analyses. Biomarkers (baseline and 24-month changes) with p<0.10 in univariate analysis were selected, including MRI (quantitative (Q) cartilage thickness and volume; semi-quantitative (SQ) MRI markers; bone shape and area; Q meniscal volume), radiographic (trabecular bone texture (TBT)), and serum and/or urine biochemical markers. Multivariable logistic regression models were built using three different step-wise selection methods (complex vs. parsimonious models).
Results:
Among baseline biomarkers, the number of locations affected by osteophytes (SQ), Q central medial femoral and central lateral femoral cartilage thickness, patellar bone shape, and SQ Hoffa-synovitis predicted progression in most models (C-statistics 0.641–0.671). 24-month changes in SQ MRI markers (effusion-synovitis, meniscal morphology, and cartilage damage), Q central medial femoral cartilage thickness, Q medial tibial cartilage volume, Q lateral patellofemoral bone area, horizontal TBT (intercept term), and urine NTX-I predicted progression in most models (C-statistics 0.680–0.724). A different combination of imaging and biochemical biomarkers (baseline and 24-month change) predicted radiographic progression only, with higher C-statistics (0.716–0.832).
Conclusion:
This study highlights the combination of biomarkers with potential prognostic utility in OA disease-modifying trials. Properly qualified, these biomarkers could be used to enrich future trials with participants likely to progress.
Keywords: knee osteoarthritis, biochemical markers, predictive validity, MRI
Introduction
There are currently no pharmacologic therapies approved by regulatory agencies to prevent or halt knee osteoarthritis (OA) progression (1), although some therapies have recently been found to beneficially modify structural progression (2, 3). Half of knee OA patients are estimated to progress to end-stage disease requiring total knee replacement (TKR) (4). Improvements in clinical trial design are critically needed to overcome barriers to the development of disease-modifying treatments to improve OA care. Biomarkers may enhance the success of every phase of the drug development process; they can improve predictability by identifying those more likely to benefit, those most likely to incur adverse events, or help better understand drug mechanisms and actions (5, 6).
Further refinement and improvement of measures of joint structural change based on imaging and/or biochemical markers are needed to identify individuals likely to progress radiographically and symptomatically and to overcome the limited responsiveness of existing imaging biomarkers (e.g. radiographic joint space width (JSW) loss) (7). To overcome these obstacles, the Foundation for the National Institutes of Health (FNIH) OA Biomarkers Consortium undertook an extensive phase 1 biomarker validation study from 2012 to 2015 using a nested case-control sample of symptomatic and/or radiographic knee OA progression within the Osteoarthritis Initiative (OAI) (8). The overarching project objective was to establish the prognostic validity of several imaging and biochemical biomarkers for knee OA progression. Some results of this study have been published in papers focusing on individual biomarker domains (9–13).
As some of these biomarkers may be highly correlated with each other, the specific purpose of the current work and ultimate aim of the FNIH phase 1 study was to determine the optimal combination of imaging and biochemical biomarkers in multivariable analyses. This final step will allow the development of a multifactorial model of biomarkers that best predict the risk of OA progression for further validation in the phase 2 of the OA Biomarkers Consortium. To this end, we evaluated the association and prognostic validity between biomarkers (assessed either at baseline or change over 24 months) with radiographic and pain progression over the longer-term (baseline to 48 months) in knees with mild to moderate tibiofemoral (TF) OA.
Methods
Study Design
Six hundred participants in the OAI were selected for the FNIH Biomarkers Consortium based on presence of at least one knee with frequent pain and Kellgren Lawrence grade (KLG) of 1, 2 or 3 on knee radiograph at baseline (8). Selected participants were required to have baseline and 24 months of radiographic minimum medial TF JSW data (measured using automated software (14)), knee MRI, stored serum and urine specimens and clinical data.
A pre-determined number of index knees were selected based on outcome assessment at 48 months (one knee per participant) in four mutually exclusive groups: 1) knees with both radiographic and pain progression (n=194); 2) knees with radiographic but not pain progression (n=103); 3) knees with pain but not radiographic progression (n=103); and 4) knees with neither radiographic nor pain progression (n=200).
The main analysis compared knees with both radiographic and pain progression (n=194) with all other knees (n=406). We took this approach to ensure the two main OA outcome domains (structural and symptomatic) were represented in the main progression definition. Radiographic and pain progression were determined as previously described (9). Briefly, radiographic progression was defined as minJSW loss of ≥0.7mm and pain progression was defined as a persistent (sustained at ≥2 time points) increase of ≥9 points on the WOMAC pain subscale (0–100 scale) (8, 15, 16). Knees were excluded if progression criteria were met by 12 months to enable the study of change in biomarker before the progression definition was met, if radiographic lateral joint space narrowing (JSN) grade 2 or 3 was present at baseline (17), or if TKR or THR had occurred prior to 24 months due to possible effects on biochemical markers. The complete flow diagram is provided in Figure 1.
Figure 1.
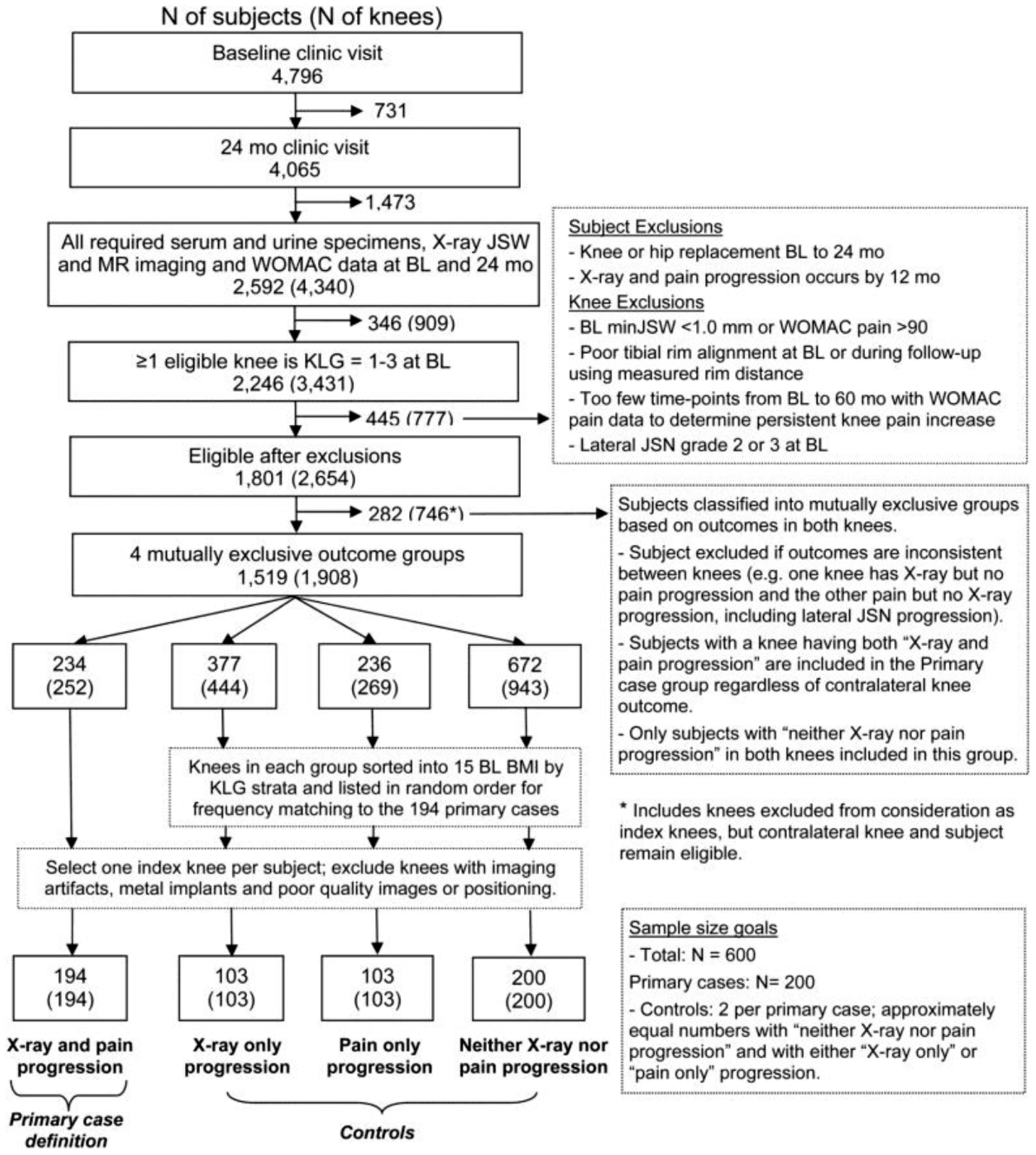
Participant’s flow diagram.
BL, baseline; BMI, body mass index; JSN, joint space narrowing; JSW, joint space width; KLG, Kellgren and Lawrence grade; WOMAC, the Western Ontario and McMaster Universities Arthritis Index.
Knees and participants were frequency matched for baseline KLG and body mass index (BMI) (kg/m2) categories, respectively (10).
Knee MRI Acquisition
MRI acquisition was performed using a 3 Tesla MRI system (Trio, Siemens Healthcare, Erlangen, Germany) at the four OAI clinical sites. Additional parameters of the full OAI pulse sequence protocol and sequence parameters have been published in detail elsewhere (18) (Supplementary Methods).
Biomarkers
Biomarkers included MRI (quantitative (Q) cartilage thickness and volume; semi-quantitative (SQ) MRI markers; bone shape and area; Q meniscal volume), radiographic (trabecular bone texture (TBT)), and serum and/or urine biochemical markers, described in detail previously (10–13) (see Supplementary Methods for further details). The reproducibility of the biomarker measurements was overall satisfactory and has been previously reported (10, 12, 13, 19).
Semi-Quantitative Analyses
Semi-quantitative scoring of MRI included assessment of cartilage and meniscal damage, bone marrow lesions, osteophytes and effusion/synovitis using water-sensitive conventional MRI acquisitions (20–23). MRIs were read according to the MRI Osteoarthritis Knee Score (MOAKS) system (24) in sequential order and without blinding to the time point of acquisition. The readers were blinded to clinical characteristics and case/control status.
Quantitative Cartilage Morphometry
Cartilage thickness analysis relied on sagittal double-echo steady-state (DESS) imaging (9). Segmentation of the femorotibial cartilage surfaces at the medial and lateral tibia and weight-bearing femur were processed as triplets by the same reader. The readers were blinded to case/control status and image acquisition order.
Bone shape and area
Femur, tibia and patella bone surfaces were automatically segmented from DESS-we images using active appearance models (AAM) (10). Two measures were used: i) subchondral bone area (tAB) (mm2) on the medial and lateral femur, tibia and patella; and, ii) position on 3D shape vectors for the femur, tibia and patella (Supplementary Figure 1). Shape measures were normalized to a z-scale with the mean non-OA shape represented as +1 and the mean OA shape as −1.
Meniscal volume
Medial and lateral meniscus volumes were automatically quantified using the computer-based Knee Imaging Quantification framework (KIQ). The framework combines multi-atlas registration and supervised classification to segment the knee tissues (25).
Radiographic Trabecular Bone Texture (TBT)
Trabecular bone texture (TBT) is a way of representing the state of the vertical and horizontal bone trabeculae. Quantification of TBT is a two-step process (Supplementary Methods) using a semi-automated software (12, 26).
Biochemical markers
Biochemical markers were quantified in both serum and/or urine (13). All urinary markers were normalized to urinary creatinine (Cr) concentration. Inter-assay coefficients of variation (CVs) ranged from 3.0% to 12.3% (13).
Patient and public involvement
Consumers are part of the steering committee guiding the design and ongoing conduct of the study. Once published, the results will be disseminated through advocacy groups, twitter and other mainstream media to engage with the wider public.
Statistical Analysis
All variables with p<0.10 in univariate analysis were advanced to multivariable modeling. In total, 27 and 43 biomarkers were tested in the baseline and change in biomarker over 24 months analyses, respectively. Models were fit separately for baseline and change in biomarkers. For both sets of models, we first considered models with imaging parameters only (models 1 to 3) and then added the biochemical markers in a second step (models 4 to 6) in order to assess the additional prognostic value of adding biochemical markers to imaging parameters only. Three different stepwise selection methods were used to determine the best subset of predictors: 1) Akaike Information Criterion (AIC) (models 1 and 4); 2) Schwarz Bayesian Criterion (SBC) (models 2 and 5); and 3) p-value (models 3 and 6) (p=0.2 for entry/0.1 for retention). Results were compared across the three types of selection procedures in order to assess the robustness of the results. Multivariable logistic regression was used for the analysis including participants with complete data on all biomarker parameters.
To assess the prognostic ability of each multivariable model, we present the Area Under (AUC) the Receiver Operating Characteristic (ROC) curve (C-statistic), the integrated discrimination improvement (IDI) and the category-less net reclassification (NRI) for each model (27, 28). The AUCs are presented for the unadjusted, adjusted for covariates (sex, race, and the following baseline measures: minJSW, WOMAC pain score, age, BMI, KLG, use of pain medications), and adjusted with 10-fold cross-validation. The IDI and NRI are calculated as improvement vs. the model with covariates only and are calculated under 10-fold cross-validation (28) (Supplementary Methods).
For the TBT and biochemical markers, change over 24 months was quantified as time-integrated concentration (TICs). TICs are equivalent to the area under the curve defined by the individual values for the specific time interval (13).
Sensitivity Analyses
Outcome:
We used structural (i.e. radiographic) progression, irrespective of pain progression (n=297), as the progression definition in secondary analysis using all radiographic non-progressors as controls (n=303).
Definition of change in TBT and biochemical markers:
As a sensitivity analysis, we ran models using absolute change of biomarkers over 24 months (24-month value minus baseline value) for TBT and biochemical markers.
Missing Data:
Because most missing data was in TBT parameters, we ran a sensitivity analysis excluding the TBT parameters (n=600 and 596 in the baseline and 24-month change analysis, respectively).
Results
Study sample
Of the 600 participants included in the FNIH study, 46 participants were missing TBT data. Initial univariate analyses were run in the cohort of n=554 with TBT data. The results of the univariate analysis using baseline and 24-month change in biomarkers are provided in Appendix 1 and 2, respectively. After further excluding participants that did not have complete data on all selected biomarkers, 550 (92%; 173 cases and 377 controls) and 539 (90%; 171 cases and 368 controls) participants were included in the baseline and 24-month change multivariable analysis, respectively. The demographic characteristics of the study sample included in the baseline and 24-month change analysis are provided in the Table 1 and Supplementary Table 1, respectively.
Table 1.
Characteristics for analytic sample included in the baseline analysis to predict radiographic and pain progression (n=550).
| Label | Level | Cases (radiographic and pain progression) | Controls | |||
|---|---|---|---|---|---|---|
| All three groups | Radiographic progression only | Pain progression only | Neither radiographic or pain progression | |||
| Age | 61.9 (8.8) | 61.6 (8.8) | 63.5 (8.2) | 59.3 (8.7) | 61.9 (9.0) | |
| Sex | Male | 73 (42%) | 150 (40%) | 50 (54%) | 34 (35%) | 66 (35%) |
| Female | 100 (58%) | 227 (60%) | 43 (46%) | 64 (65%) | 120 (65%) | |
| BMI | 30.8 (4.9) | 30.7 (4.8) | 30.7 (4.7) | 31.1 (5.0) | 30.5 (4.7) | |
| Baseline KLG | 1 | 22 (13%) | 50 (13%) | 14 (15%) | 13 (13%) | 23 (12%) |
| 2 | 76 (44%) | 209 (55%) | 44 (47%) | 59 (60%) | 106 (57%) | |
| 3 | 75 (43%) | 118 (31%) | 35 (38%) | 26 (27%) | 57 (31%) | |
| White Race | No | 36 (21%) | 76 (20%) | 10 (11%) | 28 (29%) | 38 (20%) |
| Yes | 137 (79%) | 301 (80%) | 83 (89%) | 70 (71%) | 148 (80%) | |
| Baseline pain medications | No | 115 (66%) | 275 (73%) | 74 (80%) | 63 (64%) | 138 (74%) |
| Yes | 58 (34%) | 102 (27%) | 19 (20%) | 35 (36%) | 48 (26%) | |
| Baseline WOMAC Pain | 10.2 (12.7) | 12.5 (16.3) | 15.1 (18.6) | 9.3 (13.1) | 13.0 (16.3) | |
| Baseline minJSW | 3.8 (1.4) | 3.9 (1.0) | 3.9 (1.1) | 4.0 (1.0) | 3.9 (1.0) | |
Baseline biomarkers predicting pain and radiographic progression over 48 months
For the imaging biomarkers, the adjusted AUCs with 10-fold cross-validation ranged from 0.641 to 0.669 with inclusion of number of locations affected by osteophyte (SQ) and patella shape in all models (Table 2). Quantitative central medial femoral cartilage thickness (external) (ecMF), central lateral femoral cartilage thickness (internal) (icLF.ThCtAB) and Hoffa-synovitis (SQ) were associated with case status in two of the three models. When biochemical markers were added to the imaging biomarkers, no biochemical markers were selected using the BIC or p-value based approaches, while the AIC based approach additionally selected serum NTX-1 (AUC 0.671).
Table 2.
Results of multivariable modeling – Baseline biomarker to predict radiographic and pain progression (n=550).
| Imaging Biomarkers Only | Imaging + Biochemical Biomarkers | |||||
|---|---|---|---|---|---|---|
| MODEL | M1 | M2 | M3* | M4 | M5** | M6* |
| Selection Method | Stepwise, AIC | Stepwise, SBC | Stepwise, P-value | Stepwise, AIC | Stepwise, SBC | Stepwise, P-value |
| Model characteristics | ||||||
| AUC (unadjusted) | 0.684 | 0.646 | 0.684 | 0.688 | 0.646 | 0.684 |
| AUC (adjusted)*** | 0.718 | 0.688 | 0.718 | 0.722 | 0.688 | 0.718 |
| AUC (adjusted, 10 fold cross-validation) | 0.669 | 0.641 | 0.669 | 0.671 | 0.641 | 0.669 |
| IDI | 0.0832 | 0.0548 | 0.0832 | 0.0861 | 0.0548 | 0.0832 |
| NRI | 0.4746 | 0.4048 | 0.4746 | 0.5007 | 0.4048 | 0.4746 |
| %cases correctly reclassified1 | 28% | 29% | 28% | 28% | 29% | 28% |
| %controls correctly reclassified2 | 19% | 12% | 19% | 22% | 12% | 19% |
| Biomarkers Included | ||||||
| Semi-quantitative Hoffa-synovitis score | 0.0467 | 0.0467 | 0.0682 | 0.0467 | ||
| 0 | REF | REF | REF | REF | ||
| 1 | 1.67 (1.09, 2.56) | 1.67 (1.09, 2.56) | 1.61 (1.05, 2.48) | 1.67 (1.09, 2.56) | ||
| 2–3 | 1.87 (0.86, 4.05) | 1.87 (0.86, 4.05) | 1.83 (0.84, 3.95) | 1.87 (0.86, 4.05) | ||
| Quantitative mean cartilage thickness - central lateral femur (internal) [mm] z-score | 0.0374 | 0.0374 | 0.0374 | 0.0374 | ||
| OR for each 1 unit increase in SD | 1.29 (1.02, 1.65) | 1.29 (1.02, 1.65) | 1.29 (1.02, 1.65) | 1.29 (1.02, 1.65) | ||
| Quantitative mean cartilage thickness - central medial femur (external) [mm] (z-score) | 0.0013 | 0.0013 | 0.0010 | 0.0013 | ||
| OR for each 1 unit increase in SD | 0.65 (0.50, 0.85) | 0.65 (0.50, 0.85) | 0.65 (0.50, 0.84) | 0.65 (0.50, 0.85) | ||
| Number of locations affected by any osteophyte (semi-quantitative) | 0.0008 | <.0001 | 0.0008 | 0.0008 | <.0001 | 0.0008 |
| 0–2 | REF | REF | REF | REF | REF | REF |
| 3–5 | 1.92 (0.92, 4.02) | 1.92 (0.93, 3.95) | 1.92 (0.92, 4.02) | 1.99 (0.95, 4.18) | 1.92 (0.93, 3.95) | 1.92 (0.92, 4.02) |
| 6+ | 3.47 (1.72, 7.02) | 3.94 (1.99, 7.78) | 3.47 (1.72, 7.02) | 3.55 (1.75, 7.22) | 3.94 (1.99, 7.78) | 3.47 (1.72, 7.02) |
| Patella shape (vector) (z-score) | 0.0028 | 0.0015 | 0.0028 | 0.0029 | 0.0015 | 0.0028 |
| OR for each 1 unit increase in SD | 0.72 (0.58, 0.89) | 0.71 (0.58, 0.88) | 0.72 (0.58, 0.89) | 0.72 (0.59, 0.90) | 0.71 (0.58, 0.88) | 0.72 (0.58, 0.89) |
| Serum NTX-I**** | 0.1197 | |||||
| OR for each 1 unit increase in SD | 1.16 (0.96, 1.41) | |||||
P value and OR (95% CI) are presented in the table for the individual biomarkers.
The biomarkers selected were the same as model 1.
The biomarkers selected were the same as model 2.
Adjusted for age, BMI, sex, race, baseline minimum joint space width, baseline WOMAC pain score, baseline KLG and use of pain medications
Interpolated research value if below lower limit
IDI = integrated discrimination improvement
NRI = category-less net reclassification; 1: %cases correctly reclassified = % of cases with a higher probability of being a case in new model vs. old; 2: %controls correctly reclassified = % of controls with a lower probability of being a case in new model vs. old;
Change in biomarkers over 24 months predicting pain and radiographic progression over 48 months
In models including only imaging markers, worsening in SQ effusion-synovitis and SQ meniscal damage were predictive of progression in all three models, with the addition of the intercept (horizontal) TBT parameter (Table 3). Other markers were significantly associated with case status in two of the three models: increase in the number of areas with worsening SQ cartilage morphology, loss of Q cartilage thickness in the central medial femur (center), loss of Q cartilage volume in the medial tibia, and change in Q lateral patellofemoral bone area. AUCs ranged from 0.680 to 0.713. Increases in serum or urine NTX-I were associated with outcome in at least one model. The AUCs of the models including biochemical markers ranged from 0.683 to 0.724.
Table 3.
Results of multivariable modeling – 24-month change in biomarkers to predict radiographic and pain progression; time-integrated-concentrations used for biochemical and trabecular bone texture biomarkers (n=539).
| Imaging Biomarkers Only | Imaging + Biochemical Biomarkers | |||||
|---|---|---|---|---|---|---|
| MODEL | M1 | M2 | M3 | M4 | M5 | M6 |
| Selection Method | Stepwise, AIC | Stepwise, SBC | Stepwise, p-value | Stepwise, AIC | Stepwise, SBC | Stepwise, p-value |
| Model characteristics | ||||||
| AUC (unadjusted) | 0.765 | 0.713 | 0.755 | 0.774 | 0.713 | 0.764 |
| AUC (adjusted) | 0.777 | 0.730 | 0.770 | 0.788 | 0.731 | 0.781 |
| AUC (adjusted, 10 fold cross-validation) | 0.713 | 0.680 | 0.713 | 0.724 | 0.683 | 0.724 |
| IDI (vs. covariates only model) | 0.1612 | 0.1129 | 0.1510 | 0.1699 | 0.1145 | 0.1616 |
| NRI (vs. covariates only model) | 0.6611 | 0.5164 | 0.6951 | 0.6498 | 0.5482 | 0.6819 |
| %cases correctly reclassified1 | 23% | 10% | 26% | 25% | 15% | 26% |
| %controls correctly reclassified2 | 43% | 41% | 43% | 40% | 40% | 42% |
| Biomarkers Included | ||||||
| Change in SQ effusion-synovitis | 0.0686 | 0.0026 | 0.0424 | 0.0481 | 0.0012 | 0.0310 |
| No Change vs. Improvement | 1.17 (0.59, 2.32) | 1.41 (0.74, 2.68) | 1.33 (0.68, 2.62) | 1.07 (0.54, 2.15) | 1.36 (0.71, 2.60) | 1.22 (0.62, 2.41) |
| Worsen vs. Improvement | 2.01 (0.95, 4.27) | 2.86 (1.41, 5.80) | 2.28 (1.09, 4.78) | 1.97 (0.93, 4.19) | 2.96 (1.46, 6.02) | 2.23 (1.06, 4.69) |
| Number of subregions with worsening in SQ cartilage thickness | 0.0534 | 0.0391 | 0.0462 | 0.0357 | ||
| 1 vs. 0 | 1.38 (0.81, 2.32) | 1.48 (0.89, 2.47) | 1.36 (0.80, 2.30) | 1.45 (0.87, 2.43) | ||
| 2 vs. 0 | 1.44 (0.73, 2.84) | 1.65 (0.86, 3.15) | 1.42 (0.72, 2.79) | 1.60 (0.84, 3.05) | ||
| 3+ vs. 0 | 3.36 (1.39, 8.13) | 3.15 (1.35, 7.36) | 3.53 (1.45, 8.61) | 3.32 (1.41, 7.82) | ||
| Any regions with worsening SQ meniscal morphology | 0.0084 | 0.0014 | 0.0098 | 0.0060 | 0.0027 | 0.0067 |
| Yes vs. No | 2.30 (1.24, 4.27) | 2.47 (1.42, 4.32) | 2.18 (1.21, 3.94) | 2.41 (1.29, 4.50) | 2.33 (1.34, 4.05) | 2.29 (1.26, 4.17) |
| Worsening in SQ Hoffa-synovitis | 0.0620 | 0.0694 | ||||
| Yes vs. No | 1.92 (0.97, 3.81) | 1.90 (0.95, 3.80) | ||||
| Number of subregions with worsening in SQ cartilage surface area across entire knee (include within-grade change) | 0.1063 | 0.0330 | 0.1287 | 0.0512 | ||
| 1 vs. 0 | 1.35 (0.80, 2.28) | 1.33 (0.79, 2.23) | 1.41 (0.83, 2.38) | 1.39 (0.82, 2.34) | ||
| 2 vs. 0 | 1.77 (0.92, 3.43) | 2.00 (1.05, 3.82) | 1.64 (0.84, 3.20) | 1.84 (0.96, 3.53) | ||
| 3+ vs. 0 | 2.27 (1.13, 4.56) | 2.50 (1.27, 4.91) | 2.27 (1.12, 4.57) | 2.48 (1.26, 4.89) | ||
| Maximum change in osteophyte score ≥1 across all locations in knee | 0.0423 | 0.0523 | ||||
| Yes vs. No | 0.51 (0.27, 0.98) | 0.52 (0.27, 1.01) | ||||
| Change in mean quantitative cartilage thickness - central medial femur (center) (ccMF.ThCtAB) (z-score) | 0.0581 | 0.0001 | 0.0711 | 0.0001 | ||
| OR for each 1 unit increase in SD3 | 1.30 (0.99, 1.71) | 1.56 (1.25, 1.96) | 1.29 (0.98, 1.70) | 1.56 (1.24, 1.95) | ||
| Change in quantitative medial tibial cartilage volume (z-score) | 0.0278 | 0.0071 | 0.0152 | 0.0034 | ||
| OR for each 1 unit increase in SD3 | 1.29 (1.03, 1.63) | 1.36 (1.09, 1.69) | 1.33 (1.06, 1.68) | 1.40 (1.12, 1.75) | ||
| Change in quantitative lateral patellofemoral region on femur area, mm2 (z-score) | 0.0785 | 0.0164 | 0.0896 | 0.0209 | ||
| OR for each 1 unit increase in SD | 1.25 (0.98, 1.59) | 1.33 (1.05, 1.68) | 1.24 (0.97, 1.59) | 1.32 (1.04, 1.67) | ||
| Urine NTXI** (z-score) | 0.0094 | 0.0063 | ||||
| OR for each 1 unit increase in SD | 1.34 (1.07, 1.67) | 1.35 (1.09, 1.68) | ||||
| Serum NTX-I** (z-score) | 0.0057 | |||||
| OR for each 1 unit increase in SD | 1.32 (1.08, 1.61) | |||||
| Intercept (Horizontal) TBT parameter (z-score) | 0.0021 | 0.0161 | 0.0011 | 0.0020 | 0.0013 | |
| OR for each 1 unit increase in SD | 1.48 (1.15, 1.89) | 1.30 (1.05, 1.62) | 1.50 (1.18, 1.92) | 1.48 (1.15, 1.90) | 1.50 (1.17, 1.92) | |
| Slope (Horizontal) TBT parameter (z-score) | 0.0080 | 0.0070 | 0.0036 | 0.0031 | ||
| OR for each 1 unit increase in SD | 0.73 (0.58, 0.92) | 0.73 (0.58, 0.92) | 0.70 (0.55, 0.89) | 0.70 (0.56, 0.89) | ||
P value and OR (95% CI) are presented in the table for the individual biomarkers.
Adjusted for age, BMI, sex, race, baseline minimum joint space width, baseline WOMAC pain score, baseline KLG and use of pain medications.
Interpolated research value if below lower limit.
SQ = semi-quantitative; AUC = area under the curve; IDI = integrated discrimination improvement; NRI = category-less net reclassification
%cases correctly reclassified = % of cases with a higher probability of being a case in new model vs. old;
%controls correctly reclassified = % of controls with a lower probability of being a case in new model vs. old;
coded such that increasing OR = increasing change
Sensitivity analyses
Change in biomarkers over 24 months predicting pain and radiographic progression over 48 months (absolute change used for biochemical markers and TBT)
Compared to the model using TICs for biochemical markers and TBT, the same selection of imaging markers was associated with case status, with the main difference that no biochemical marker or TBT parameter was selected when absolute change in these markers was used (Supplementary Table 2). The adjusted 10-fold cross-validated AUCs were slightly lower, ranging from 0.668 to 0.700.
Baseline biomarkers predicting radiographic progression over 48 months
The number of locations affected by SQ osteophytes, medial meniscus volume and Q cartilage thickness at the central lateral femur (internal), medial tibia (external) and lateral tibia (posterior) were associated with case status in all three models (Supplementary Table 3). Semi-quantitative cartilage morphology (maximum full-thickness cartilage loss score) and SQ Hoffa-synovitis were included in two of the three models. The adjusted 10-fold cross-validated AUCs, using imaging markers only, ranged from 0.716 to 0.723. When biochemical markers were added, AUCs ranged from 0.716 to 0.732. The same imaging markers were selected, with the addition of urinary CTXII and serum PIIANP, in two of the three models.
Change in biomarkers over 24 months predicting radiographic progression over 48 months
The adjusted 10-fold cross-validated AUCs were higher in the models predicting radiographic progression only (AUCs 0.793 to 0.832) compared to the models using pain and radiographic progression as the outcome (Supplementary Table 4). A different set of biomarkers was associated with progression in all three models including imaging and biochemical markers: the number of areas of cartilage damage with worsening in surface area (SQ MRI), worsening meniscus extrusion (SQ), reduction in mean cartilage thickness at the central medial femur (ccMF) (Q MRI), and decrease in serum PIIANP. Several other markers were found to be significant in models 1 (AIC) and/or 3 (p-value) including measures of bone shape and area, Q cartilage thickness and volume, SQ effusion-synovitis, SQ cartilage and meniscal damage, the number of locations with osteophytes (SQ) and serum NTX-1 and CTX-1.
Baseline and change in biomarkers predicting pain and radiographic progression, excluding TBT parameters
The results of the analysis using baseline biomarkers as predictors were overall consistent with the main analysis including TBT parameters, with three main exceptions: i. Hoffa-synovitis was not significant in any model; ii. medial meniscus volume was significant in all models; iii) urinary CTX-II was associated with case-status in the AIC and p-value models. The AUCs ranged from 0.668 to 0.694 (Supplementary Table 5). In the 24-month change analysis, the imaging markers were overall consistent with the original analysis; however, a different biochemical marker was significant in all models: serum CTX-I (Supplementary Table 6). The AUCs were similar compared to the main analysis.
Discussion
The baseline biomarkers that predicted subsequent pain and radiographic progression in most models were the number of locations affected by osteophyte (SQ MRI), Q central medial femoral and central lateral femoral cartilage thickness, patellar bone shape, and SQ Hoffa-synovitis. Only the number of locations affected by SQ osteophytes and patella shape were significantly associated with case status in all models. The 24-month change in biomarkers that predicted pain and radiographic progression in all models were worsening in SQ effusion-synovitis (vs. improvement), increase in the number of knee regions with worsening in SQ meniscal damage and the horizontal TBT (intercept term). An increase in the number of areas with worsening SQ cartilage morphology, loss of Q cartilage thickness in the central medial femur (center), loss of cartilage volume in the medial tibial, and change in Q lateral patellofemoral bone area were significantly associated with case-status in two of the models. For TBT parameters and biochemical markers, 24-month TIC values performed better than change scores. The fact that the strongest biochemical predictor in univariate analysis in this cohort, urinary CTX-II, did not contribute to model predictions containing the core set of cartilage MRI markers suggests its collinearity with these imaging parameters, which is in line with previous studies (29, 30). The overall AUCs were similar with the addition of the biochemical markers as compared to the earlier models with the core set of MRI markers only (adjusted AUCs with 10-fold cross-validation 0.669 vs. 0.671 in the baseline analysis and 0.713 vs. 0.724 in the 24-month change analysis, for models 1 and 4, respectively).
Higher AUCs yielded by a different set of imaging and biochemical markers were found in the secondary analysis to predict radiographic progression only. This is important since surrogate endpoints such as radiographic progression might in theory be accepted by the Food and Drug Administration (FDA) for initial drug approval of a disease modifying OA agent, although post-marketing studies showing benefits on clinically important outcomes would be required (31). Imaging and biochemical markers of structural progression are objective and more fully developed than biomarkers of pain, which to date are largely subjective, self-reported measures. A recent genome-wide association study of knee pain identified GDF5 as the primary locus (32); GDF5 is the same gene most strongly and repeatedly associated with OA based on structural diagnoses. Therefore, it may not be a different underlying pathological process driving symptom and structural progression but our ability to measure them with adequate sensitivity.
Although synovitis (Hoffa- or effusion-) was consistently selected in all models, this study demonstrates that the other biomarkers that predict progression vary dependent upon whether baseline biomarkers or changes in biomarkers over 24 months are evaluated for their ability to predict longer-term (48 month) outcomes of radiographic and symptomatic progression. Both biomarker types may be useful for the same clinical trial, but with different purposes, namely 1) participant selection for inclusion (baseline biomarkers), and 2) structural end-point (longitudinal change). These could be particularly important in enhancing the efficiency and shortening the duration of phase 2 and 3 clinical trials, thereby reducing the cost, increasing the likelihood of drug approval (6) and improving time to market (33).
Other studies have also developed models to predict OA progression using baseline and/or longitudinal biomarker data. A recent study has used a machine learning approach in the same FNIH dataset to identify differences in a variety of baseline characteristics between progressors and non-progressors (34). Similar to our study, the number of locations with osteophytes was a strong contributor to the progressor phenotype which supports previous findings showing the role of osteophytes in OA progression (35). BMLs and uCTXII were also highlighted as prognostic biomarkers, which is in line with our findings, although BMLs were not included in the final multivariable model. However, synovitis did not differentiate progressors and non-progressors in that study despite robust evidence indicating that inflammation plays an important role in OA progression (36). It is of note that their control definition was different (knees with neither clinical nor radiographic progression). In this study, we utilized logistic regression because our focus was not only on the variable selection but also on computing interpretable effect estimates (i.e., odds ratios) for each parameter. Another study tested different models to predict moderate to severe OA (clinical and/or radiographic) over 8 years and found that adding MRI biomarkers (cartilage morphology and T2 and meniscal tear) significantly improved the prognostic ability of the model compared to clinical and radiographic characteristics only (37). The AUCs were similar to this study (0.71–0.72 for the models including biomarkers). We have used a shorter follow up (4 years) in order to make the results more informative for clinical trial design. Although OA progression is typically slow, a large epidemiological study has shown that radiographic progression over a 5 year-interval occurs in 12% to 23% of knees with radiographic OA (38). Other more sophisticated methodological approaches have also been tested such as different machine learning and regression algorithms but to date, no prediction model has been sufficiently validated and qualified for use in trials (39, 40).
There are a few limitations related to this study. Firstly, these analyses were performed on a subsample of the FNIH cohort for which all biomarkers were available; missing data were largely related to missing TBT biomarker, mostly secondary to poor radiographic positioning. The sensitivity analyses excluding TBT showed similar results for the imaging markers and a different selection of biochemical markers. Secondly, the results may not be generalizable to race/ethnicities not represented in the OAI, which mostly included Caucasians. Thirdly, there are no reproducibility data for meniscal volume on scan-rescan images. Fourthly, the analyses were conducted first with imaging parameters only, with subsequent addition of biochemical biomarkers; because the order of addition can affect the incremental explanatory power of the variable, results could vary with a different approach. In addition, participants with radiographic and pain progression by 12 months were excluded, which may have excluded a small number of cases with very fast progression. It is also worth noting that the control definition used in the main analysis included knees with pain only and radiographic only progression, which may have reduced the strength of the association between biomarkers and case status. The approach we used has been pre-defined for the overall FNIH project and used in previous papers studying individual biomarker domains (9–13). Finally, we did not explicitly control for multiple testing. Instead, we sought to examine the robustness of the models by comparing the variables selected across the different selection methods. Machine learning methods that can assess enormous numbers of predictors could be an alternative strategy to variable selection and model fitting.
In conclusion, our study highlights the combination of biomarkers that could provide prognostic utility in the context of OA disease-modifying trials. At baseline, SQ (osteophytes and Hoffa-synovitis) and Q (cartilage thickness and patella shape) imaging markers were selected. Different biomarkers were selected in the 24-month change analysis including SQ (effusion-synovitis, meniscal and cartilage morphology) and Q measures of cartilage thickness and volume, radiographic TBT and urinary or serum NTX-I. The phase 2 of the OA Biomarkers Consortium is currently underway to externally validate these findings and enable the submission of these biomarkers for regulatory review and formal qualification for use as prognostic biomarkers in disease-modifying OA trials.
Supplementary Material
Key messages:
What is already known about this subject?
Several imaging and biochemical markers have been shown to have prognostic validity for knee osteoarthritis progression.
What does this study add?
This study evaluated biomarkers from all biomarker domains (i.e. MRI, radiograph and biochemical) in multivariable models and demonstrated the biomarkers (measured at baseline and change over 24 months) with prognostic value for knee OA progression.
How might this impact on clinical practice or future developments?
These findings indicate the most promising biomarkers that could be used in future structure-modifying OA trials to select participants more likely to progress (baseline biomarkers) and for use as structural end-point (longitudinal change biomarkers), if properly qualified.
Acknowledgements and financial support
Scientific and financial support for the FNIH OA Biomarkers Consortium and the study are made possible through grants, direct and in-kind contributions provided by: AbbVie; Amgen Inc.; Arthritis Foundation; Bioiberica S.A.; DePuy Mitek, Inc.; Flexion Therapeutics, Inc.; GlaxoSmithKline; Merck Serono; Rottapharm | Madaus; Sanofi; Stryker; The Pivotal OAI MRI Analyses (POMA) Study, NIH HHSN2682010000. We thank the Osteoarthritis Research Society International (OARSI) for their leadership and expertise on the FNIH OA Biomarker Consortium project. The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health. Funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the Consortium and OAI is managed by the FNIH.
Disclosures
David Hunter is funded by an NHMRC Practitioner Fellowship and received a grant from the FNIH OA Biomarkers Consortium. He reports consulting fees from Merck Serono, TLCBio, Pfizer and Lilly (<$10,000 for each).
Leticia Deveza is funded by the Royal Australasian College of Physicians and Australian Rheumatology Association & D.E.V Starr Research Establishment Fellowship and receives royalties from UptoDate. She has received partial reimbursement of conference registration cost from Pfizer (<$10,000).
Jamie Collins is funded by the Rheumatology Research Foundation Investigator Award and reports personal fees from Boston Imaging Core Labs (<$10,000).
Elena Losina received a grant from the FNIH OA Biomarkers Consortium and is funded by grants from NIAMS R01 AR064320, K24 AR057827, P30AR072577. She reported grants from Samumed and Flexion Therapeutics and consulting fees from Pfizer (<$10,000).
Michael C. Nevitt has no conflicts of interest to report.
Frank Roemer is a co-owner of Boston Imaging Core Lab (BICL), LLC. and reports personal fees from California Institute for Biomedical Research –CALIBR (<$10,000).
Ali Guermazi is a shareholder to Boston Imaging Core Lab (BICL), LLC. (>$10,000). He is a consultant to Pfizer, MerckSerono, TissueGene (>$10,000 for each), and Roche, Galapagos and AstraZeneca (<$10,000 for each).
Mike Bowes is an employee of Imorphics Ltd, a wholly owned subsidiary of Stryker Corp.
Erik Dam is co-owner of Biomediq. The commercial rights to the Knee Imaging Quantification (KIQ) software used for meniscus quantification is with Biomediq
Felix Eckstein is CEO/CMO and shareholder of Chondrometrics GmbH. He reports consulting fees from MerckKGaA, Abbvie, Galapagos, Novartis, Kolon-Tissuegene, Servier, Roche (<$10,000 each) and grants from Orthotrophix, MerckKGaA, Samumed, Tissuegene, Boston Imaging Core Lab, Galapagos, Novartis, National Institute of Health (NIH), Foundation of the NIH (FNIH), European Union, Paracelsus Medical University Research Fund and BMBF (Fed. Ministry of Education and Res.).
John Lynch reports personal fees from Boston Imaging Core Lab (<$10,000).
Jeffrey N. Katz, is funded by NIH/NIAMS P30AR072577, U01AR071658, R21AR076156, and received grants from Samumed and Flexion Therapeutics.
C.Kent Kwoh reports grants from Merck Serono, and has provided consulting services to Thusane, TLC, GSK, Regeneron, Amzell, Astellas, Regulus (<$10,000 each) and Express Scripts (>$10,000). He reports personal fees from Focus Medical Communications and Prime Education LLC (<$10,000 each).
Steve Hoffmann has no conflicts of interest to report.
Virginia Kraus is funded by grants from the FNIH, the NIH/NIA Claude D Pepper 5P30 AG028716 and NIH R01AR071450. She has a patent related to methods of reduction of the complex fractal data to TBT parameters (no revenue involved).
References
- 1.Matthews GL, Hunter DJ. Emerging drugs for osteoarthritis. Expert Opin Emerg Drugs. 2011;16:479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochberg MC, Guermazi A, Guehring H, Aydemir A, Wax S, Fleuranceau-Morel P, et al. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: the FORWARD randomized clinical trial. Jama. 2019;322:1360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conaghan PG, Bowes MA, Kingsbury SR, Brett A, Guillard G, Rizoska B, et al. Disease-modifying effects of a novel cathepsin K inhibitor in osteoarthritis: a randomized, placebo-controlled study. Ann Intern Med. 2020. 21;172:86–95. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein AM, Rome BN, Reichmann WM, Collins JE, Burbine SA, Thornhill TS, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95:385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lotz M, Martel-Pelletier J, Christiansen C, Brandi ML, Bruyere O, Chapurlat R, et al. Value of biomarkers in osteoarthritis: current status and perspectives. Ann Rheum Dis. 2013;72:1756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas D, Burns J, Audette J, Carroll A, Dow-Hygelund C, Hay M. Clinical development success rates 2006–2015: Biotechnology Innovation Organization (BIO), Biomedtracker and Amplion; 2016. p. 1–26.
- 7.Hunter DJ, Losina E, Guermazi A, Burstein D, Lassere MN, Kraus V. A pathway and approach to biomarker validation and qualification for osteoarthritis clinical trials. CurrDrug Targets. 2010;11:536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter DJ, Nevitt M, Losina E, Kraus V. Biomarkers for osteoarthritis: current position and steps towards further validation. Best Pract Res Clin Rheumatol. 2014;28:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckstein F, Collins JE, Nevitt MC, Lynch JA, Kraus VB, Katz JN, et al. Brief Report: Cartilage thickness change as an imaging biomarker of knee osteoarthritis progression: data from the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol. 2015;67:3184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter D, Nevitt M, Lynch J, Kraus VB, Katz JN, Collins JE, et al. Longitudinal validation of periarticular bone area and 3D shape as biomarkers for knee OA progression? Data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis. 2016;75:1607–14. [DOI] [PubMed] [Google Scholar]
- 11.Collins JE, Losina E, Nevitt MC, Roemer FW, Guermazi A, Lynch JA, et al. Semiquantitative imaging biomarkers of knee osteoarthritis progression: data from the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol. 2016;68:2422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraus VB, Collins JE, Charles HC, Pieper CF, Whitley L, Losina E, et al. Predictive validity of radiographic trabecular bone texture in knee osteoarthritis: the Osteoarthritis Research Society International/Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol. 2018;70:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraus VB, Collins JE, Hargrove D, Losina E, Nevitt M, Katz JN, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis. 2017;76:186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neumann G, Hunter D, Nevitt M, Chibnik LB, Kwoh K, Chen H, et al. Location specific radiographic joint space width for osteoarthritis progression. Osteoarthritis Cartilage. 2009;17:761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ornetti P, Brandt K, Hellio-Le Graverand MP, Hochberg M, Hunter DJ, Kloppenburg M, et al. OARSI-OMERACT definition of relevant radiological progression in hip/knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol. 2002;29:131–8. [PubMed] [Google Scholar]
- 17.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15 Suppl A:A1–56. [DOI] [PubMed] [Google Scholar]
- 18.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roemer FW, Guermazi A, Collins JE, Losina E, Nevitt MC, Lynch JA, et al. Semi-quantitative MRI biomarkers of knee osteoarthritis progression in the FNIH biomarkers consortium cohort − Methodologic aspects and definition of change. BMC Musculoskelet Disord. 2016;17:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. [DOI] [PubMed] [Google Scholar]
- 21.Biswal S, Hastie T, Andriacchi TP, Bergman GA, Dillingham MF, Lang P. Risk factors for progressive cartilage loss in the knee: a longitudinal magnetic resonance imaging study in forty-three patients. Arthritis Rheumatol. 2002;46:2884–92. [DOI] [PubMed] [Google Scholar]
- 22.Hunter D, Gale D, Grainger G, Lo G, Conaghan P. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score). Ann Rheum Dis. 2008;67:206–11. [DOI] [PubMed] [Google Scholar]
- 23.Kornaat PR, Ceulemans RY, Kroon HM, Riyazi N, Kloppenburg M, Carter WO, et al. MRI assessment of knee osteoarthritis: Knee Osteoarthritis Scoring System (KOSS)--inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skeletal Radiology. 2005;34:95–102. [DOI] [PubMed] [Google Scholar]
- 24.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage. 2011;19:990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dam EB, Lillholm M, Marques J, Nielsen M. Automatic segmentation of high- and low-field knee MRIs using knee image quantification with data from the osteoarthritis initiative. J Med Imaging (Bellingham). 2015;2:024001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraus VB, Feng S, Wang S, White S, Ainslie M, Brett A, et al. Trabecular morphometry by fractal signature analysis is a novel marker of osteoarthritis progression. Arthritis Rheumatol. 2009;60:3711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu W, Pepe M. Measures to summarize and compare the predictive capacity of markers. Int J Biostat. 2009;5:Article 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pencina MJ, D’Agostino RB Sr., D’Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27:157–72. [DOI] [PubMed] [Google Scholar]
- 29.Eckstein F, Le Graverand MP, Charles HC, Hunter DJ, Kraus VB, Sunyer T, et al. Clinical, radiographic, molecular and MRI-based predictors of cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2011;70:1223–30. [DOI] [PubMed] [Google Scholar]
- 30.Deveza LA, Kraus VB, Collins JE, Guermazi A, Roemer FW, Bowes M, et al. Association between biochemical markers of bone turnover and bone changes on imaging: data from the Osteoarthritis Initiative. Arthritis Care Res. 2017;69:1179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraus VB, Simon LS, Katz JN, Neogi T, Hunter D, Guermazi A, et al. Proposed study designs for approval based on a surrogate endpoint and a post-marketing confirmatory study under FDA’s accelerated approval regulations for disease modifying osteoarthritis drugs. Osteoarthritis Cartilage. 2019;27:571–9. [DOI] [PubMed] [Google Scholar]
- 32.Meng W, Adams MJ, Palmer CNA, Agee M, Alipanahi B, Bell RK, et al. Genome-wide association study of knee pain identifies associations with GDF5 and COL27A1 in UK Biobank. Communications Biology. 2019;2:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berndt E, Gottschalk A, Strobeck M. Opportunities for improving the drug development process: results from a survey of industry and FDA. MIT-FDA-Industry White Paper. http://webmitedu/cbi/docs/berndt-et-al6-3-05pdf [Internet]. 2006 March/17/2009. Available from: http://web.mit.edu/cbi/docs/berndt-et-al6-3-05.pdf.
- 34.Nelson AE, Fang F, Arbeeva L, Cleveland RJ, Schwartz TA, Callahan LF, et al. A machine learning approach to knee osteoarthritis phenotyping: data from the FNIH Biomarkers Consortium. Osteoarthritis Cartilage. 2019;27:994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis and cartilage. 2007;15(3):237–44. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Hunter DJ, Jin X, Ding C. The importance of synovial inflammation in osteoarthritis: current evidence from imaging assessments and clinical trials. Osteoarthritis Cartilage. 2018;26:165–74. [DOI] [PubMed] [Google Scholar]
- 37.Joseph GB, McCulloch CE, Nevitt MC, Neumann J, Gersing AS, Kretzschmar M, et al. Tool for osteoarthritis risk prediction (TOARP) over 8 years using baseline clinical data, X-ray, and MRI: data from the Osteoarthritis Initiative. J Magn Reson imaging. 2018;47:1517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leyland KM, Hart DJ, Javaid MK, Judge A, Kiran A, Soni A, et al. The natural history of radiographic knee osteoarthritis: a fourteen-year population-based cohort study. Arthritis Rheumatol. 2012;64:2243–51. [DOI] [PubMed] [Google Scholar]
- 39.Widera P, Welsing PMJ, Ladel C, Loughlin J, Lafeber FPFJ, Petit Dop F, et al. Multi-classifier prediction of knee osteoarthritis progression from incomplete imbalanced longitudinal data. Sci Rep. 2020;10:8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halilaj E, Le Y, Hicks JL, Hastie TJ, Delp SL. Modeling and predicting osteoarthritis progression: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2018;26:1643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


