Abstract
Background:
To report and compare the characteristics and outcomes of COVID-19 patients on extracorporeal membrane oxygenation (ECMO) to non-COVID-19 acute respiratory distress syndrome (ARDS) patients on ECMO.
Methods:
We performed an international retrospective study of COVID-19 patients on ECMO from 13 intensive care units from March 1 to April 30, 2020. Demographic data, ECMO characteristics and clinical outcomes were collected. The primary outcome was to assess the complication rate and 28-day mortality; the secondary outcome was to compare patient and ECMO characteristics between COVID-19 patients on ECMO and non-COVID-19 related ARDS patients on ECMO (non-COVID-19; January 1, 2018 until July 31, 2019).
Results:
During the study period 71 COVID-19 patients received ECMO, mostly veno-venous, for a median duration of 13 days (IQR 7-20). ECMO was initiated at 5 days (IQR 3-10) following invasive mechanical ventilation. Median PaO2/FiO2 ratio prior to initiation of ECMO was similar in COVID-19 patients (58 mmHg [IQR 46-76]) and non-COVID-19 patients (53 mmHg [IQR 44-66]), the latter consisting of 48 patients. 28-day mortality was 37% in COVID-19 patients and 27% in non-COVID-19 patients. However, Kaplan-Meier curves showed that after a 100-day follow-up this non-significant difference resolves. Non-surviving COVID-19 patients were more acidotic prior to initiation ECMO, had a shorter ECMO run and fewer received muscle paralysis compared to survivors.
Conclusions:
No significant differences in outcomes were found between COVID-19 patients on ECMO and non-COVID-19 ARDS patients on ECMO. This suggests that ECMO could be considered as a supportive therapy in case of refractory respiratory failure in COVID-19.
Keywords: survival, ECMO, COVID-19, ARDS
Introduction
After in December 2019 the first case of pneumonia caused by the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was reported, it has since spread and became a pandemic of Coronavirus Disease-2019 (COVID-19). 1 On September 22nd 2020, over 31 million infected cases and over 965,000 SARS-CoV-2 related deaths have been confirmed. 2 Infected individuals can differ in presentation from asymptomatic carriers to severe respiratory failure and acute respiratory distress syndrome (ARDS). Up to 35% of the hospitalized patients with COVID-19 have to be treated in an intensive care unit (ICU). 3 In these cases, the cornerstones of supportive therapy include high flow nasal oxygen, as well as non-invasive and invasive mechanical ventilation.
Extracorporeal membrane oxygenation (ECMO) is recommended as a “last resource” supportive therapy in case of cardiac and/or respiratory failure, including ARDS, refractory to other conventional therapies. As the extracorporeal circuit provides both oxygenation and carbon dioxide clearance, it can facilitate protective mechanical ventilation. 4,5 Following the influenza A (H1N1) pandemic in 2009, worldwide application, expertise and experience with the use of ECMO as a supportive treatment in severe ARDS increased. 6,7 During the Middle East respiratory syndrome (MERS) outbreak, an association with improved outcome in patients with ARDS on ECMO was demonstrated. 8 However, in COVID-19 the first results from small Chinese cohorts were disappointing, showing a very high mortality. 9 Recently, more promising results were presented, including a French study reporting an estimated probability of day-60 mortality of 31%. 10
The WHO interim guidelines recommends the administration of ECMO to eligible patients with COVID-19 related ARDS in expert centers. 11 The Society of Critical Care Medicine (SCCM) has agreed with this statement and released guidelines regarding the management of COVID-19 patients in the ICU, providing criteria regarding the use of ECMO in COVID-19. 12 Although some research has been carried out on ECMO in COVID-19, these studies are limited due to small sample sizes, single-center design or the lack of control group. 13 –15 As a result, no robust conclusion can be drawn about the added value of ECMO in patients with severe respiratory failure due to COVID-19.
Therefore, this study aimed to provide additional insight on the role of ECMO in refractory ARDS due to COVID-19 by describing patient and ECMO characteristics of COVID-19 patients on ECMO, and comparing their outcomes with patients with non-COVID-19 ARDS on ECMO.
Methods
This retrospective observational study was performed in 13 ICUs providing ECMO: 8 from the Netherlands, 3 from Belgium, 1 from Sweden and 1 from Spain. This study was approved by the institutional review board (IRB) of the Amsterdam University Medical Centers (Amsterdam UMC; W20_199#20.230), and, thereafter, from local Ethics Committees. Data were collected retrospectively from electronic patient records of all consecutive COVID-19 patients receiving ECMO admitted to the ICU from March 1 to April 30, 2020, which corresponds with the timing of the first COVID-19 peak in most European countries. Patients were included if they were aged 18 years and older and received ECMO during their ICU admission due to RT-PCR confirmed COVID-19. As comparative non-COVID-19 group, data were collected of patients with who received ECMO for ARDS between January 1, 2018, and July 31, 2019 from the same ICUs. Patients were eligible for ECMO according to applicable guidelines provided by the extracorporeal life support organization (ELSO). 16 ECMO is considered a last resource, in case of reversible cardiac and/or pulmonary failure, refractory to other therapies. Therefore, for example, in case of ARDS, it is encouraged to have applied several interventions such as prone positioning and neuromuscular blockage prior initiation of ECMO. 17,18 During ECMO, standard care included systemic anticoagulation using unfractionated heparin in all 13 centers. More details on the anticoagulation practices can be found in the Additional File (E1. Anticoagulation practices). Data collection included demographics, comorbidities, laboratory values, ECMO characteristics, complications and 28-day mortality (Additional File: E2. Definitions used). The primary outcome was the complication rate and 28-day mortality of patients on ECMO due to COVID-19. The secondary outcome consisted of a comparison of patient and ECMO characteristics between patients with COVID-19 on ECMO to patients with non-COVID-19 ARDS on ECMO.
Statistical Analysis
Statistical analysis was performed using R statistics (version 3.6.1) with the R Studio interface (The R Foundation, Lucent Technologies, Inc., Murray Hill, NJ, USA, www.r-project.org). Baseline and outcome parameters were summarized using simple descriptive statistics. Non-normal distributed continuous variables were presented as a median (with interquartile range (IQR)). Categorical variables were presented as percentages and frequencies. Data were compared between groups of COVID-19 patients and non-COVID-19 ARDS patients using the Mann-Whitney U test for numerical data and Chi square test for categorical data. Survival rates of COVID-19 and non-COVID-19 patients were compared using Kaplan-Meier analyses and the log-rank test for equality of survival curves using the R survival package. A P value <.05 was considered significant.
Results
COVID-19 Patients on ECMO
During the study period, a total of 71 patients received ECMO due to refractory respiratory failure related to COVID-19. The majority of 57 patients (80%) were male and median age was 52 years (IQR 46-57). The patients were predominantly overweight with a median body mass index (BMI) of 29.2 kg/m2 (IQR 26.1-32.1). A minority of 29 patients (40%) had a medical history of cardiovascular or pulmonary disease, of which most frequently scored comorbidities consisted of hypertension (n = 15, 21%), asthma (n = 7, 10%) and diabetes (n = 6, 8%).
An overview of the patient baseline characteristics prior to the initiation of ECMO is shown in Table 1. The arterial blood gas (ABG) values prior to initiation of ECMO reported a pH of 7.35 (IQR 7.22-7.42), PaCO2 of 8.0 kPa (IQR 6.6-10.1), bicarbonate of 32 mmol/L (IQR 26-37) and PaO2 of 8.0 kPa (IQR 6.8-9.3). Controlled ventilation mode was mostly used: pressure-controlled ventilation in 31 patients (44%) and volume-controlled ventilation in 31 patients (44%). Median ventilation parameters consisted of PEEP of 12 cm H2O (IQR 8-16), FiO2 of 100% (IQR 80-100), resulting in a median PaO2/FiO2 of 58 mmHg (IQR 46-76). In a large part of the patients, prone positioning (79%) and muscle paralysis (77%) had been applied prior to initiation of ECMO. Acute kidney injury (AKI) was already present in 17 patients (24%), of which 12 (17%) were also receiving renal replacement therapy (RRT).
Table 1.
Patient Baseline Characteristics.
| n = 71 | ||
|---|---|---|
| Demographics | ||
| Age, median (IQR), y | 52 | (47-57) |
| Male gender, No. (%) | 57 | (80) |
| Body mass index, median (IQR), kg/m2 | 29.2 | (26.1-32.1) |
| Comorbidities, No. (%) | ||
| Cardiovascular disease | 21 | (30) |
| Hypertension | 15 | (21) |
| Diabetes | 6 | (8) |
| Myocardial infarction | 1 | (2) |
| Pulmonary disease | 11 | (14) |
| Pulmonary hypertension | 1 | (2) |
| Asthma | 7 | (10) |
| COPD | 4 | (6) |
| Chronic Kidney Disease | 3 | (4) |
| Malignancy | 1 | (2) |
| Liver cirrhosis | 1 | (2) |
| Values prior to initiation ECMO a, median (IQR) | ||
| SOFA | 9 | (7-12) |
| Lactate, mmol/L | 1.7 | (1.2-2.8) |
| CRP, mg/L | 260 | (145-384) |
| Procalcitonin, ng/mL | 2.15 | (0.61-6.76) |
| Arterial blood gas, median (IQR) | ||
| PaO2/FiO2-ratio, mmHg | 58 | (46-76) |
| pH | 7.35 | (7.22-7.42) |
| PCO2, kPa | 8 | (6.6-10.1) |
| bicarbonate, mmol/L | 32 | (26-37) |
| PO2, kPa | 8 | (6.8-9.3) |
| Mechanical ventilation settings prior to initiation ECMO | ||
| Type of mechanical ventilation, No. (%) | ||
| Pressure supported ventilation | 4 | (6) |
| Volume controlled mechanical ventilation | 31 | (44) |
| Pressure controlled mechanical ventilation | 31 | (44) |
| Other | 1 | (1) |
| Missing | 4 | (6) |
| FiO2, median (IQR), % | 100 | (80-100) |
| Peak pressure, median (IQR), cm H2O | 34 | (31-39) |
| PEEP, median (IQR), cm H2O | 12 | (8-16) |
| Rescue therapies applied prior to initiation ECMO, No. (%) | ||
| Prone positioning | 56 | (79) |
| Muscle paralysis | 55 | (77) |
| Complications prior to initiation ECMO, No. (%) | ||
| Pulmonary embolism | 5 | (7) |
| Venous thromboembolism | 5 | (7) |
| Acute kidney injury | 17 | (24) |
| Renal replacement therapy | 12 | (17) |
Abbreviations: COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; SOFA, sequential organ failure assessment; CRP, C-reactive protein; PaO2, partial oxygen pressure; PCO2, partial carbon dioxide pressure; FiO2, fraction inspired oxygen; PEEP, positive end expiratory pressure.
a Values prior to initiation of ECMO include worst values within 12 hours prior to initiation.
After the first occurrence of symptoms, hospital admission followed at a median of 13 days (IQR 7-16), and ICU admission 1 day later. ECMO was started at a median of 18 days (IQR 15-25) after disease onset and at a median of 5 days (IQR 3-10) after start of invasive mechanical ventilation. Almost all patients received veno-venous ECMO (VV-ECMO: 93%, n = 66), 3 patients received veno-arterial ECMO (VA-ECMO), one veno-veno-arterial ECMO (VVA-ECMO) and one extracorporeal CO2-removal (ECCO2R). The main indication for VV-ECMO was combined refractory hypoxemia and hypercapnia, which occurred in 34 patients (52%), followed by refractory hypoxemia in 32 patients (49%). To be able to offer hemodynamic support in the presence of right ventricular failure, 3 patients received VA-ECMO and one VVA-ECMO. The median duration of ECMO was 13 days (IQR 7-20); 3 patients were in need of a second run of ECMO. Muscle paralysis was the most commonly applied rescue therapy, which was applied in 51 patients (72%), followed by prone positioning (n = 27, 38%). The variance in pharmacotherapeutic drugs was high; hydroxychloroquine (n = 47, 66%) and lopinavir/ritonavir (n = 14, 20%) were most frequent (Table 2: ECMO Characteristics).
Table 2.
ECMO Characteristics.
| n = 71 | ||
|---|---|---|
| ECMO indication, No. (%) | ||
| Refractory hypercapnia | 1 | (1) |
| Refractory hypoxemia | 33 | (46) |
| Combined refractory hypoxemia and hypercapnia | 36 | (51) |
| Other | 1 | (1) |
| ECMO mode, No. (%) | ||
| Veno-Venous ECMO | 66 | (93) |
| Veno-Arterial ECMO | 3 | (4) |
| Veno-Veno-Arterial ECMO | 1 | (1.5) |
| Extracorporeal CO2 Removal | 1 | (1.5) |
| ECMO run duration, median, d | 13 | (7-20) |
| Second run, No. (%) | 3 | (4) |
| Time from start symptoms until hospital admission, median, d | 13 | (7-16) |
| Time from start symptoms until ICU admission, median, d | 14 | (10-18) |
| Time from start symptoms until ECMO initiation, median, d | 18 | (15-25) |
| Time from intubation until start ECMO, median, d | 5 | (3-10) |
| Rescue therapies applied during ECMO, No. (%) | ||
| Prone positioning | 27 | (38) |
| Muscle paralysis | 51 | (72) |
| Nitric oxide | 9 | (13) |
| Beta blockade | 11 | (15) |
| Upgrade ECMO: additional cannula | 3 | (4) |
| Upgrade ECMO: larger diameter cannula | 3 | (4) |
| Upgrade ECMO-membrane | 2 | (3) |
| Pharmacotherapeutic therapies during ECMO, No (%) | ||
| Anti-IL 1 | 3 | (4) |
| Hydroxychloroquine | 47 | (66) |
| Lopinavir/ritonavir (Kaletra®) | 14 | (20) |
| Remdesivir | 5 | (7) |
| Imatinib | 1 | (1) |
| Convalescent plasma | 0 | (0) |
| Tocilizumab | 9 | (13) |
| Intravenous immunoglobulins | 2 | (3) |
| Plasmapheresis | 1 | (1) |
| Cytokine absorber (CytoSorb®) | 13 | (18) |
Abbreviations: ECMO, extracorporeal membrane oxygenation; CO2, carbondioxide; ICU, Intensive Care Unit; IL, interleukine.
During ECMO, 60 patients (85%) suffered one or more complications, mostly a new infection (n = 40, 56%), AKI (n = 39, 55%) and hemorrhagic event (n = 38, 54%) as shown in Table 3. From the 39 patients with AKI, 22 out of 39 developed during ECMO, and 37 out of 39 patients (55%) received RRT. More than half of the patients (n = 37, 52%) were successfully weaned from ECMO. The 3 patients who received a second run were successfully weaned and survived. Within 28 days after ECMO initiation, 26 patients had died (37%). In those non-survivors a lower median pH and higher median PCO2 was shown in comparison to survivors (respectively: 7.28 versus 7.38 [P = .04] and 9.82 versus 7.33 [P = .002]). Moreover, muscle paralysis had been applied in fewer non-survivors (non-survivors 68% versus survivors 97%, P = .002). The median ECMO run was 6 days shorter in non-survivors (non-survivors 8 days [IQR 5-17] versus survivors 14 days [IQR 10-23]). A table showing survivors versus non-survivors can be found in the Additional File (E3. Survivors versus non-survivors).
Table 3.
ECMO Outcome and Complications.
| n = 71 | ||
|---|---|---|
| Complications, No. (%) | ||
| Hemorrhagic event | 38 | (54) |
| Cannula | 10 | (14) |
| Hemorrhagic stroke | 7 | (10) |
| Gastro-intestinal | 3 | (4) |
| Other | 19 | (27) |
| Pulmonary embolism | 2 | (3) |
| Arterial thrombotic event | 3 | (4) |
| Ischemic stroke | 1 | (1.5) |
| Leg ischemia | 0 | (0) |
| Other | 2 | 3) |
| Venous thrombotic event | 8 | (11) |
| Upper extremity | 4 | (6) |
| Lower extremity | 2 | (3) |
| Heart | 0 | (0) |
| Other | 2 | (3) |
| Mechanical thrombotic event | 10 | (14) |
| Cannula | 1 | (1.5) |
| Oxygenator | 9 | (13) |
| Pump | 0 | (0) |
| Infection | 40 | (56) |
| Ventilator-associated pneumonia | 23 | (32) |
| Catheter-related bloodstream infection | 9 | (13) |
| Superinfection/second infection | 6 | (8) |
| Other | 10 | (14) |
| Type of infection | ||
| Bacterial | 30 | (42) |
| Fungus (e.g. Aspergillus) | 5 | (7) |
| Yeast | 7 | (10) |
| Viral | 5 | (7) |
| Unknown | 3 | (4) |
| Acute kidney injury | 39 | (55) |
| Renal replacement therapy | 37 | (52) |
| Outcome, No. (%) | ||
| Successful weaning | 37 | (52) |
| 28-day mortalitya | 26 | (37) |
| Location of decease | ||
| ICU, unanticipated during ECMOb | 5 | (14) |
| ICU, anticipated during ECMO | 24 | (67) |
| ICU, within 48 hours after ECMO decannulation | 2 | (6) |
| ICU, after 48 hours after ECMO decannulation | 5 | (14) |
| Hospital, not on ECMO | 0 | (0) |
| Different hospital, after referral | 0 | (0) |
| Non-hospital | 0 | (0) |
Abbreviations: ECMO, Extracorporeal Membrane Oxygenation; ICU, Intensive Care Unit.
a 28-day mortality: death within 28 days after initiation ECMO.
b Causes of death are described in the Additional File (E4. Unanticipated Death).
Non-COVID-19 ARDS
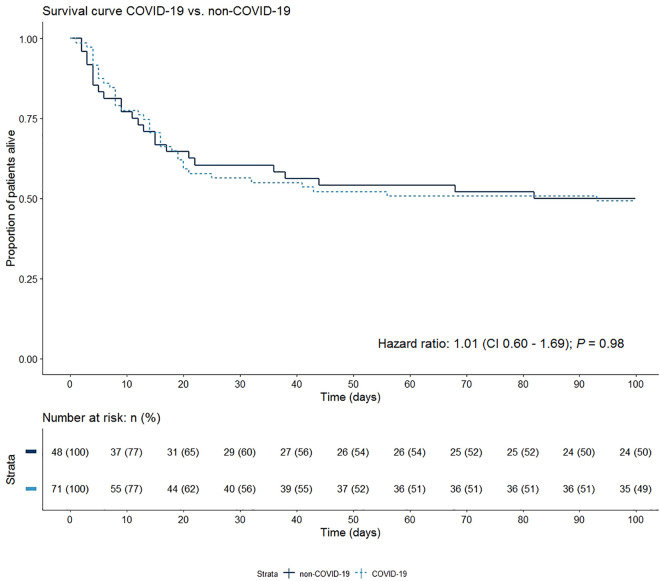
A total of 48 out of 440 patients received ECMO due to ARDS unrelated to COVID-19. In this consecutive subgroup, median age was 55 years (IQR 40-61), sex was equally distributed, and median BMI was 28.3 kg/m2 (IQR 24.7-33). When compared to the patients with COVID-19 related respiratory failure, the COVID-19 group had a significantly higher proportion of males (80% versus 50%; P = .001). No other significant differences were found in patient baseline characteristics, comorbidities, and disease severity-related variables including sequential organ failure assessment (SOFA)-score and PaO2/FiO2-ratio. Also, complication rate and ECMO support duration did not differ significantly (Table 4). 28-day mortality was 37% in COVID-19 patients and 27% in non-COVID-19 patients. However, this difference was not significant, as shown in the Kaplan-Meier curves in Figure 1 (Hazard Ratio 1.01 [95% CI 0.60-1.69]; P = .98). In this figure it is apparent that this non-significant difference in mortality resolves after a prolonged 100-day follow-up.
Table 4.
Comparison COVID-19 on ECMO and Non-COVID-19 on ECMO.
| COVID-19 (n = 71) |
Non-COVID ARDS (n = 48) |
P value | ||||
|---|---|---|---|---|---|---|
| Pre-ECMO patient characteristics | ||||||
| Age, median (IQR), years | 52 | (47-57) | 55 | (40-61) | .49 | |
| Male gender, No (%) | 57 | (80) | 24 | (50) | .001 | |
| Body mass index, median (IQR), kg/m2 | 29.2 | (26.1-32.1) | 28.3 | (24.7-33) | .84 | |
| SOFA-score, median (IQR) | 9 | (7-12) | 10 | (9-13) | .13 | |
| PaO2/FiO2-ratio, median (IQR), mmHg | 58 | (46-76) | 53 | (44-67) | .84 | |
| Lactate, median (IQR), mmol/L | 1.7 | (1.2-2.8) | 1.4 | (1.1-2.7) | .27 | |
| Medical history, No. (%) | ||||||
| Hypertension | 15 | (21) | 13 | (27) | >.99 | |
| Diabetes | 6 | (8%) | 4 | (8%) | >.99 | |
| Asthma | 7 | (10) | 3 | (6) | .63 | |
| COPD | 4 | (6) | 1 | (2) | .60 | |
| ECMO characteristics and complications | ||||||
| Complications, No. (%) | ||||||
| Hemorrhage | 38 | (54) | 22 | (46) | .53 | |
| Arterial thrombosis | 3 | (4) | 0 | (0) | .27 | |
| Venous thrombosis | 8 | (11) | 4 | (8) | .76 | |
| Mechanic thrombosis | 10 | (14) | 7 | (15) | >.99 | |
| Infection | 40 | (56) | 26 | (54) | .96 | |
| Acute kidney injury | 39 | (55) | 27 | (56) | >.99 | |
| ECMO run duration, median, d | 13 | (7-20) | 9 | (5-17) | .16 | |
| 28-day mortalitya, No. (%) | 26 | (37) | 19 | (27) | .49 | |
Abbreviations: COPD, chronic obstructive pulmonary disease; SOFA, sequential organ failure assessment; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation.
a 28-day mortality, mortality 28 days after initiation of ECMO.
Figure 1.
Kaplan-Meier estimates for patients with COVID-19 on ECMO and patients with ARDS not due to COVID-19 on ECMO. Unadjusted hazard ratio and 95% confidence interval calculated from a Cox proportional hazard model is presented.
Discussion
To date, this study is one of the first multi-center observational studies of COVID-19 patients on ECMO. We reported a 28-day mortality of 37% in patients with COVID-19 on ECMO. This survival rate and the complication rate did not significantly differ from our non-COVID-19 patients on ECMO. Moreover, patient characteristics of our COVID-19 and non-COVID-19 patients were complementary prior to initiation of ECMO, except for gender.
The past decades, ECMO is considered a life-saving therapy. However, its overall beneficial effect in ARDS is not beyond any doubt, resulting in the current role of ECMO as a final resort therapy. 5,19 The rise of the COVID-19 pandemic has reignited the discussion if ECMO should or should not be offered in patients with severe ARDS, 20 and whether such therapy would be associated with acceptable complication and mortality rates. This debate was amplified by a Chinese cohort study where 5 out of 6 patients on ECMO died, 21 after which concerns were expressed. 22 In our study, complication rate and 28-day mortality did not significantly differ in COVID-19 and non-COVID-19 patients. Moreover, our mortality rates were in line with a recent observational study showing an estimated probability of day-60 mortality of 31%. 10 Also, comparable survival rates were found in ICU patients with ARDS due to COVID-19 not on ECMO. 23 This suggests that in case of severe, refractory ARDS due to COVID-19, ECMO could be considered as supportive therapy in case conventional therapies prove insufficient.
One of the questions remains if selection and triage of COVID-19 patients for ECMO differed from other non-COVID-19 ARDS patients. Several respiratory variables emphasize the severity of the respiratory insufficiency in our study population. Rescue therapies to improve oxygenation including prone positioning and muscle paralysis were applied in most patients in our study. Moreover, almost 9 out 10 patients were ventilated using controlled modes. In ARDS, the Surviving Sepsis Campaign has made several recommendations, including (ultra)protective ventilation. Although both ventilation modes have advantages and disadvantages, such as high peek pressure in volume-controlled ventilation, no mode has consistently shown to be advantageous. 12 In general, prior to initiation of ECMO, it is advised to apply best conventional intensive care as possible, including above described rescue therapies. In spite of these attempts, oxygen delivery was compromised, as shown by the low median PaO2/FiO2 ratio of 58 mmHg (IQR 46-76) and the need of a high FiO2 (median 100% [IQR 80%-100%]). When compared to previous ARDS groups on ECMO these values confirm the severity in the COVID-19 ECMO population. 19,24,25 Not only the characteristics prior to initiation of ECMO were in line with previous ECMO non-COVID-19 ARDS groups, but also the ECMO characteristics themselves. 5 This could suggest that no large differences in patient selection have occurred, e.g. no different timing of cannulation and no earlier discontinuation of treatment.
In comparison with general COVID-19 patients in the ICU, our group on ECMO support appears relatively young. 24 This can be explained by the selection criteria used for initiation of ECMO: among others the interim guideline of ELSO advises to apply age above 65 years old as a relative contraindication. 26 Given the expected rise in COVID-19 of patients administered to the hospital with COVID-19, discussions arose which patients had to be selected in case capacity was insufficient, in which age was a common topic. 27 Although triage with a limit on age was not applied in all participating hospitals of this study during the first peak, it is possible that age discrimination has occurred unwittingly. The sex discrepancy (more males) has been described in large Italian and German groups of patients with COVID-19 as well. 23,24 In contrast to general hospitalized COVID-19 patients, where an incidence of up to 90% has been described, this study population was relatively healthy prior to COVID-19 as only 40% had comorbidities. 3 No differences in comorbidities were found between survivors and non-survivors in our COVID-19 group on ECMO. However, interestingly, the arterial blood gas of non-survivors reported a significantly lower pH and higher PCO2. This finding is confirmed by the descriptive study of Yang et al. 28
At last, one main consideration should remain the availabilities of sufficient resources, including personnel and equipment. Concerns are still raised whether ECMO is justifiable in times of a pandemic, or if saving few lives would decrease the quality of care in other patients. 20 As stated by the SSCM and WHO, this is not the time to start with implementing ECMO in centers who do not yet have the experience and resources for ECMO. However, in case personnel, equipment, facilities and systems apply, our results suggest that ECMO could be considered as a supportive therapy in case conventional therapies are insufficient.
This study has several strengths. It is one of the largest multicenter observational studies presenting data on the use of ECMO from multiple countries during the first peek of the COVID-19 pandemic. Moreover, it is the first multicenter study comparing COVID-19 patient characteristics with a previous non-COVID-19 ARDS group from the same participating centers. It gives an extensive overview of COVID-19 ECMO characteristics including applied therapies. Some limitations should however be recognized. Due to its observational design, some biases cannot be excluded. Hence, it is unknown what the outcome would be in the absence of ECMO support. Furthermore, the time frames of patients with and without COVID-19 on ECMO were different. It cannot be excluded that the level of care for patients on ECMO prior COVID-19 was different compared with the period during COVID-19. Finally, no data were collected regarding functional outcomes.
Conclusions
To conclude, we found an acceptable survival rate in ECMO patients with COVID-19, not differing significantly from our non-COVID-19 ARDS patients on ECMO. ECMO could be considered as a supportive therapy in case of COVID-19 related respiratory failure, in case conventional therapies are insufficient.
Supplemental Material
Supplemental Material, sj-docx-1-jic-10.1177_08850666211007063 for Extracorporeal Membrane Oxygenation in Patients With COVID-19: An International Multicenter Cohort Study by Senta Jorinde Raasveld, Thijs S. R. Delnoij, Lars M. Broman, Annemieke Oude Lansink-Hartgring, Greet Hermans, Erwin De Troy, Fabio S. Taccone, Manuel Quintana Diaz, Franciska van der Velde, Dinis Dos Reis Miranda, Erik Scholten, ETALON Study Group and Alexander P. J. Vlaar in Journal of Intensive Care Medicine
Supplemental Material, sj-docx-2-jic-10.1177_08850666211007063 for Extracorporeal Membrane Oxygenation in Patients With COVID-19: An International Multicenter Cohort Study by Senta Jorinde Raasveld, Thijs S. R. Delnoij, Lars M. Broman, Annemieke Oude Lansink-Hartgring, Greet Hermans, Erwin De Troy, Fabio S. Taccone, Manuel Quintana Diaz, Franciska van der Velde, Dinis Dos Reis Miranda, Erik Scholten, ETALON Study Group and Alexander P. J. Vlaar in Journal of Intensive Care Medicine
Appendix
ETALON Study Group (in alphabetical order): Walter M. van den Bergh, MD, PhD (Department of Critical Care, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands); Judith M. D. van den Brule, MD, PhD (Department of Intensive Care, Radboud University Nijmegen Medical Centre, Nijmegen, the Netherlands); Dieter F. Dauwe, MD, PhD (Surgical Adult Intensive Care Unit, Department of Intensive Care Medicine, University Hospitals Leuven, KU Leuven, Leuven, Belgium); Dave A. Dongelmans, MD, PhD (Department of Intensive Care, Amsterdam University Medical Centers, location Academic Medical Center [AMC], Amsterdam, the Netherlands); Elisa Bogossian Guvea, MD (Department of Intensive Care, Université Libre de Bruxelles, Hôpital Erasme Bruxelles, Brussels, Belgium); Marijn Kuijpers, MD (Department of Intensive Care, Isala Hospital, Zwolle, the Netherlands); Wim K. Lagrand, MD, PhD (Department of Intensive Care, Amsterdam University Medical Centers, location Academic Medical Center [AMC], Amsterdam, the Netherlands); Jacinta J. Maas, MD, PhD (Department of Intensive Care, Leids University Medical Center, Leiden, the Netherlands); Philippe Meersseman, MD, PhD (Medical Intensive Care Unit, Department of General Internal Medicine, University Hospitals Leuven, KU Leuven, Herestraat, Leuven, Belgium; Laboratory of Intensive Care Medicine, Department of Cellular and Molecular Medicine, KU Leuven, Herestraat, Leuven, Belgium); Marcel C. G. van de Poll, MD, PhD (Department of Intensive Care, Maastricht University Medical Center, Maastricht, the Netherlands).
Footnotes
Authors’ Note: This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the institutional review board (IRB) of the Amsterdam University Medical Centers (Amsterdam UMC; W20_199#20.230), and, thereafter, from local Ethics Committees. After final data collection has occurred in the end of 2020, encrypted data can be requested by contacting the corresponding author. Reasonable requests will be taken in consideration. Raasveld and Vlaar had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Raasveld, Vlaar, van den Bergh, Broman. Acquisition, analysis or interpretation of data: All authors. Drafting of the manuscript: All Authors. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Raasveld, Vlaar. Supervision: Vlaar, van den Bergh. Trial registration: Netherlands trial registry, NL8706, date of registration: June 9, 2020. https://www.trialregister.nl/trial/8706.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Lars M. Broman, MD, PhD  https://orcid.org/0000-0003-4124-4581
https://orcid.org/0000-0003-4124-4581
Annemieke Oude Lansink-Hartgring, MD  https://orcid.org/0000-0003-3641-9658
https://orcid.org/0000-0003-3641-9658
Alexander P. J. Vlaar, MD, PhD  https://orcid.org/0000-0002-7398-3445
https://orcid.org/0000-0002-7398-3445
Supplemental Material: Supplemental material for this article is available online.
References
- 1. WHO—Novel Coronavirus 2019—Events as they happen. Accessed April 27, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- 2. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University (JHU). Accessed September 22, 2020. https://coronavirus.jhu.edu/map.html [DOI] [PMC free article] [PubMed]
- 3. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi:10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 4. Brogan T V, Lequier L, Lorusso R, MacLaren G, Peek G. Extracorporal Life Support: The ELSO Red Book. ELSO; 2017. [Google Scholar]
- 5. Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi:10.1056/NEJMoa1800385 [DOI] [PubMed] [Google Scholar]
- 6. Hong X, Xiong J, Feng Z, Shi Y. Extracorporeal membrane oxygenation (ECMO): does it have a role in the treatment of severe COVID-19? Int J Infect Dis. 2020;94:78–80. doi:10.1016/j.ijid.2020.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. 2015;61(1):31–36. doi:10.1097/MAT.0000000000000160 [DOI] [PubMed] [Google Scholar]
- 8. Alshahrani MS, Sindi A, Alshamsi F, et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018;8(1):3. doi:10.1186/s13613-017-0350-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmidt M, Hajage D, Lebreton G, et al. Articles extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8(11):1121–1131. doi:10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. WHO. Clinical management of severe acute respiratory infection when COVID-19 is suspected. Interim Guidance V1.2: 1–21. Accessed March 13, 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
- 12. Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854–887. doi:10.1007/s00134-020-06022-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sultan I, Habertheuer A, Usman AA, et al. The role of extracorporeal life support for patients with COVID-19: preliminary results from a statewide experience. J Card Surg. 2020;35(7):1410–1413. doi:10.1111/jocs.14583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Loforte A, Dal Checco E, Gliozzi G, et al. Veno-venous extracorporeal membrane oxygenation support in Covid-19 respiratory distress syndrome: initial experience. ASAIO J. 2020;66(7):734–738. doi:10.1097/MAT.0000000000001198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng Y, Cai Z, Xianyu Y, Yang BX, Song T, Yan Q. Prognosis when using extracorporeal membrane oxygenation (ECMO) for critically ill COVID-19 patients in China: a retrospective case series. Crit Care. 2020;24(1):8–10. doi:10.1186/s13054-020-2840-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Extracorporeal Life Support Organization (ELSO). Guidelines for adult respiratory failure. Version 1.4. 2017. Accessed May 2020. https://www.elso.org/Portals/0/ELSO%20Guidelines%20For%20Adult%20Respiratory%20Failure%201_4.pdf [Google Scholar]
- 17. Guérin C, Reignier J, Richard J-C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi:10.1056/nejmoa1214103 [DOI] [PubMed] [Google Scholar]
- 18. Papazian L, Forel JM, Gacouin A, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1117. doi:10.1056/NEJMoa1901686 [DOI] [PubMed] [Google Scholar]
- 19. Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi:10.1016/S0140-6736(09)61069-2 [DOI] [PubMed] [Google Scholar]
- 20. Abrams D, Lorusso R, Vincent J-L, Brodie D. ECMO during the COVID-19 pandemic: when is it unjustified? Crit Care. 2020;24(1):507. doi:10.1186/s13054-020-03230-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi:10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henry BM. COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8(4):e24. doi:10.1016/S2213-2600(20)30119-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853–862. doi:10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. doi:10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmidt M, Pham T, Arcadipane A, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome an international multicenter prospective cohort. Am J Respir Crit Care Med. 2019;200(8):1002–1012. doi:10.1164/rccm.201806-1094OC [DOI] [PubMed] [Google Scholar]
- 26. Shekar K, Badulak J, Peek G, et al. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an International Group of Interdisciplinary Extracorporeal Membrane Oxygenation Providers. ASAIO J. 2020;66(7):707–721. doi:10.1097/MAT.0000000000001193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. British Medical Association. COVID-19—Ethical Issues. A guidance note. 2020;(March):1–9.
- 28. Yang X, Cai S, Luo Y, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019-induced acute respiratory distress syndrome. Crit Care Med. 2020;48(9):1289–1295. doi:10.1097/ccm.0000000000004447 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-docx-1-jic-10.1177_08850666211007063 for Extracorporeal Membrane Oxygenation in Patients With COVID-19: An International Multicenter Cohort Study by Senta Jorinde Raasveld, Thijs S. R. Delnoij, Lars M. Broman, Annemieke Oude Lansink-Hartgring, Greet Hermans, Erwin De Troy, Fabio S. Taccone, Manuel Quintana Diaz, Franciska van der Velde, Dinis Dos Reis Miranda, Erik Scholten, ETALON Study Group and Alexander P. J. Vlaar in Journal of Intensive Care Medicine
Supplemental Material, sj-docx-2-jic-10.1177_08850666211007063 for Extracorporeal Membrane Oxygenation in Patients With COVID-19: An International Multicenter Cohort Study by Senta Jorinde Raasveld, Thijs S. R. Delnoij, Lars M. Broman, Annemieke Oude Lansink-Hartgring, Greet Hermans, Erwin De Troy, Fabio S. Taccone, Manuel Quintana Diaz, Franciska van der Velde, Dinis Dos Reis Miranda, Erik Scholten, ETALON Study Group and Alexander P. J. Vlaar in Journal of Intensive Care Medicine