Abstract
Apathy is a reduction in goal-directed activity in the cognitive, behavioral, emotional, or social domains of a patient’s life and occurs in one out of three patients after stroke. Despite this, apathy is clinically under-recognized and poorly understood. This overview provides a contemporary introduction to apathy in stroke for researchers and practitioners, covering topics including diagnosis, neurobiological mechanisms, associated consequences, and potential treatments for apathy. Apathy is often misdiagnosed as other post-stroke conditions such as depression. Accurate differential diagnosis of apathy, which manifests as reductions in initiative, and depression, which manifests as negative emotionality, is important as it informs prognosis. Research on the neurobiology of apathy suggests that there are few consistent associations between stroke lesion location and the development of apathy. These may be resolved by adopting a network neuroscience approach, which models apathy as a pathology arising from structural or functional damage to brain networks underlying motivated behavior. Importantly, networks can be affected by physiological changes related to stroke, including the acute infarct but also diaschisis and neurodegeneration. Aside from neurobiological changes, apathy is also associated with other negative outcome measures such as functional disability, cognitive impairment, and emotional distress, suggesting that apathy is indicative of a worse prognosis following stroke. Unfortunately, high-quality trials aimed at treating apathy are scarce. Antidepressants may have limited effects on apathy. Acetylcholine and dopamine pharmacotherapy, behavioral interventions, and transcranial magnetic stimulation may be more promising avenues for treatment.
Keywords: Apathy, depression, stroke, diagnosis, outcomes, neurobiology, treatment
Introduction
Apathy is a behavioral syndrome characterized by a loss of motivation that occurs in one-third of patients after stroke.1,2 Post-stroke patients with apathy suffer from greater functional impairment and demonstrate slower recovery times to normal functioning.3,4 Furthermore, apathy is a risk factor for incident vascular disease, dementia, and mortality.5,6 Despite high prevalence and an impact on outcomes after stroke, apathy remains poorly understood. It is also under-recognized, although the extent of this is unknown. This leads to a dearth of treatment approaches. This overview provides a contemporary introduction to apathy in stroke for researchers and practitioners, covering topics including diagnosis, neurobiological mechanisms, associated consequences, and potential treatments for apathy. The search strategy and selection criteria for papers referenced in this overview can be found after the “Discussion” section.
Diagnostic criteria for apathy
Apathy can be defined as a quantitative reduction in goal-directed behaviors (GDB) occurring in the cognitive/behavioral, emotional, or social domains of an individual’s life (Box 1). 7 Reductions are relative to an individual’s previous level of functioning and can be reported by the individual or others. A previous version of these diagnostic guidelines has been validated in patients with a range of neurological disorders, including those with cerebrovascular damage, showing good inter-rater reliability. 8
Box 1.
Diagnostic criteria for apathy.
| CRITERION A: A quantitative reduction of goal-directed activity either in behavioral, cognitive, emotional or social dimensions in comparison to the patient’s previous level of functioning in these areas. These changes may be reported by the patient themselves or by observation of others. |
| CRITERION B: The presence of at least two of the three following dimensions for a period of at least four weeks and present most of the time: |
| B1. BEHAVIOR AND COGNITION |
| Loss of, or diminished, goal-directed behavior or cognitive activity as evidenced by at least one of the following: |
| General level of activity: The patient has a reduced level of activity either at home or work, makes less effort to initiate or accomplish tasks spontaneously or needs to be prompted to perform them. |
| Persistence of activity: They are less persistent in maintaining an activity or conversation, finding solutions to problems or thinking of alternative ways to accomplish them if they become difficult. |
| Making choices: They have less interest or take longer to make choices when different alternatives exist. |
| Interest in external issue: They have less interest in or reacts less to news, either good or bad, or has less interest in doing new things. |
| Personal wellbeing: They are less interested in their own health and wellbeing or personal image. |
| B2. EMOTION |
| Loss of, or diminished, emotion as evidenced by at least one of the following: |
| Spontaneous emotions: The patient shows less spontaneous (self-generated) emotions regarding their own affairs or appears less interested in events that should matter to them or to people that they know well. |
| Emotional reactions to environment: They express less emotional reaction in response to positive or negative events in their environment that affect them or people they know well. |
| Impact on others: They are less concerned about the impact of their actions or feelings on the people around them. |
| Empathy: They show less empathy to the emotions or feelings of others. |
| Verbal or physical expressions: They show less verbal or physical reactions that reveal their emotional states. |
| B3. SOCIAL INTERACTION |
| Loss of or diminished engagement in social interaction as evidenced by at least one of the following: |
| Spontaneous social initiative: The patient takes less initiative in spontaneously proposing social or leisure activities to family or others. |
| Environmentally stimulated social interaction: They participate less or are less comfortable or more indifferent to social or leisure activities suggested by people around them. |
| Relationship with family members: They show less interest in family members. |
| Verbal interaction: They are less likely to initiate a conversation or withdraw soon from it. |
| Homebound: They prefer to stay at home more frequently or longer than usual and show less interest in getting out to meet people. |
| CRITERION C: These symptoms (A–B) cause clinically significant impairment in personal, social, occupational, or other important areas of functioning. |
| CRITERION D: The symptoms (A–B) are not exclusively explained or due to physical disabilities, to motor disabilities, to a diminished level of consciousness, to the direct physiological effects of a substance, or to major changes in the patient’s environment. |
Adapted with permission. 7
Apathy can be suspected in routine clinical practice during the history taking and examination from an observed loss of motivation. Informant histories may also reveal symptoms of apathy, such as loss of interest in previous activities and hobbies or doing little when left alone, which can be valuable as patients may underplay symptoms. Apathy assessments can be supplemented with semi-structured interviews or questionnaires (Table 1, Supplementary Table 1). These are less thorough than clinical examinations but can be administered more flexibly, which can be useful for research and screening.
Table 1.
Commonly used apathy scales.
| Scale | Administration | Questions | Subscales |
|---|---|---|---|
| Apathy Evaluation Scale | Self-report, Informant-rated, Clinician-rated | 18 | Behavior, Cognition, Emotion, Other |
| Apathy Inventory | Rated based on informant or patient interview | 3 | Lack of Initiative, Lack of Interest, Emotional Blunting |
| Apathy-Motivation Index | Self-report | 18 | Behavioral, Social, Emotional |
| Dimensional Apathy Scale | Self-report, Informant-rated | 24 | Behavioral/Cognitive Initiation, Executive, Emotional |
| Lille Apathy Rating Scale | Clinician-rated based on patient self-report | 33 | Action Initiation, Self-awareness, Intellectual Curiosity, Emotion |
| Starkstein Apathy Scale | Examiner reads questions and responses to patient | 14 | None |
Some scales have not been validated in stroke patients; see references in Supplementary Table 1.
Apathy is described as a symptom in the International Classification of Diseases, Eleventh Revision under code MB24.4. Similarly, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition acknowledges apathy as a symptom of other disorders, such as mood and neurocognitive disorders. 9 Unfortunately, neither classification system describes apathy as a syndrome, potentially limiting its recognition.
Differential diagnosis of post-stroke apathy
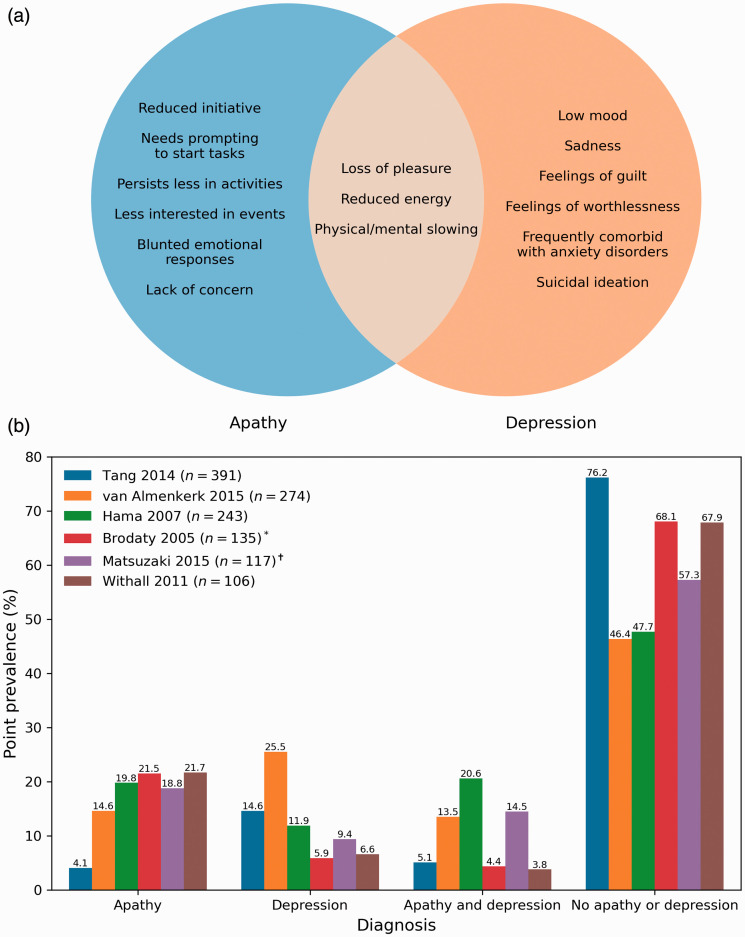
Diagnosing apathy may be complicated by other post-stroke neuropsychiatric symptoms, particularly depression. Core symptoms of a depressive episode, which can be triggered by stroke, include low mood and diminished pleasure (anhedonia). 9 Depressive symptoms such as anhedonia can appear behaviorally similar to apathy and may have a similar neurobiological basis, making them difficult to distinguish in practice (Figure 1(a)).
Figure 1.
Apathy and depression are dissociable syndromes: (a) distinct and overlapping symptoms of apathy and depression. Apathy manifests as a reduction in goal-directed behaviors while depression is marked by negative emotionality. Overlapping symptoms include loss of pleasure and energy; (b) prevalence rates of post-stroke apathy and depression in studies with n ≥ 100. Patients present with either apathy or depression, with a minority showing comorbid symptoms. Distinguishing between the two informs prognosis and treatment.
Despite shared symptoms, post-stroke apathy and depression are dissociable syndromes with different prevalence rates (Figure 1(b)). Stroke patients tend to develop either apathy or depression, with a minority showing symptoms of both (Figure 1(b)). Furthermore, apathy and depression have different trajectories10,11 and effects on outcomes such as functional disability12,13 and cognition.6,14,15
Negative emotionality is a key characteristic of depression that distinguishes it from apathy. Depressed patients may present with pessimism and hopelessness, while those with apathy show a lack of emotional distress. 16 Depressed patients can also actively engage in avoidant behavior, resisting socializing and treatment attempts, while apathetic patients are passive and indifferent to these activities. 16
Post-stroke fatigue may also be a potential comorbidity with post-stroke apathy. Fatigue can be defined as a subjective feeling of extreme and persistent physical/mental tiredness, weakness, or exhaustion. 17 Although fatigue has a similar behavioral manifestation to apathy (i.e. less energy), preliminary findings indicate that they are not correlated and do not interact, 18 suggesting that the two are independent.
Apathy itself may present differently based on the underlying neurological disease. More patients with mixed dementia, which includes individuals with cerebrovascular disease, may show deficits in behavioral initiation compared to patients with other neurocognitive diseases. 8 Conversely, mixed dementia patients also show the lowest proportion of emotional impairment compared to other disorders except major depression. 8 Although these patients did not have stroke per se, stroke pathology is highly prevalent in mixed dementia. 19
Prevalence and natural history of post-stroke apathy
Apathy presents in approximately one-third of stroke patients, with symptoms beginning as early as four days post-stroke. 20 Longitudinal research suggests that most post-stroke patients have a constant level of high (7%), moderate (33%), or low/no (50%) apathy for up to a year after stroke, with a minority improving (7%) or worsening (7%). 4 The prevalence of apathy increases by ∼10% five years following stroke, 21 although this may be an underestimate as patients with apathy may be more likely to drop out of longitudinal studies. 4
Neurobiological mechanisms underlying apathy
Post-stroke apathy is increasingly recognized as a consequence of neurobiological changes triggered by a stroke. Apathy is traditionally described as the result of damage to specific brain structures related to GDB such as the basal ganglia and prefrontal cortex. 22 If this lesion-deficit view of apathy was true, one would expect a clear relationship between lesion location and apathy. However, no common localizations across stroke studies have been found,2,23 suggesting that relationships between structural damage and functional deficits are more complex than initially thought.
A recent theoretical approach has recontextualized apathy in cerebrovascular disease as the product of damage to brain networks underlying GDB. 24 This network-based framework suggests that post-stroke apathy follows focal lesions in key network regions or diffuse cerebrovascular pathology disrupting connections within networks. Acute infarcts to core brain regions underlying GDB can result in apathy, recapitulating the lesion-deficit view. Alternatively, diffuse white matter damage due to cerebral small vessel disease can lead to network disruption, 25 explaining associations between MRI markers of small vessel disease and apathy. 26
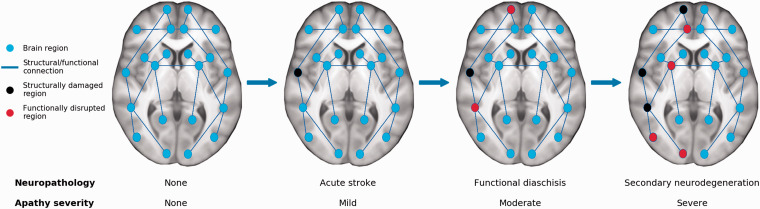
This framework also suggests that post-stroke phenomena such as functional diaschisis and secondary neurodegeneration drive increases in apathy over time. 27 These phenomena can occur distal to an acute infarct, leading to clinical symptoms that appear inconsistent or unexpected if only considering a focal lesion. Importantly, secondary neurobiological changes can propagate through structural and functional connections within the brain, potentially leading to apathy if affecting GDB-related networks (Figure 2). As a corollary, apathy may improve in response to restorative mechanisms such as adaptive plasticity and functional remapping. 27
Figure 2.
A network-based model of how stroke leads to apathy. Apathy can result from focal lesions disrupting key network regions or from diffuse damage such as white matter ischemia. Acute strokes may not immediately lead to apathy symptoms if not occurring in brain regions supporting goal-directed behaviors but can result in the delayed onset of apathy. Infarcts may lead to disrupted functioning in connected areas, known as diaschisis. Over time, secondary neurodegenerative processes lead to atrophy in regions structurally connected to the infarct, which propagates diaschisis-related deficits further throughout the brain. This network-based spreading of stroke-related pathology may explain why apathy can occur in individuals who do not have acute infarcts in motivation-related regions, and why apathy symptoms worsen in some patients over time.
The anterior cingulate cortex (ACC) and nucleus accumbens may be core network regions supporting GDB, as damage to these structures is associated with apathy across neurological disorders. 28 These core regions are embedded in large-scale functionally connected networks that underlie GDB-related cognitive functions such as reward-based decision-making, attentional control, and reinforcement learning.24,28 Disruption to these networks may lead to cognitive deficits that manifest behaviorally as apathy (Supplementary Table 2). 24
This network-based conceptualization of apathy explains a wide range of findings in patients with post-stroke apathy, although further testing of it is necessary. Future work could investigate time since stroke in conjunction with more precise localizations of the acute infarct and any white matter ischemia, given the specificity of the neuroanatomical networks and neurophysiological processes that may be related to post-stroke apathy. 24
Effects of apathy on functional outcomes
Post-stroke apathy is associated with functional disability, including reductions in basic activities of daily living such as eating or dressing and slower functional recovery over time.2,3,12 Factors underlying these relationships remain unexplored, although a potential explanation is that motivational deficits impair recovery by reducing engagement in rehabilitation programs. Alternatively, apathy could precede sedentary behavior, explaining associations between apathy and incident vascular disease. 5
Apathy is associated with general cognitive deficits, with post-stroke apathy patients scoring ∼2–3 points lower on the Mini-Mental State Examination. 2 Apathy has also been associated with impairments in specific cognitive domains such as verbal learning, short- and long-term verbal recall, semantic fluency, abstract reasoning, and attention and concentration.11,14,15,29 This suggests that some post-stroke patients with apathy suffer from cognitive impairment, particularly in executive and memory-related domains, supporting the notion that similar neurobiological networks underlie cognitive function and motivated behavior.24,26 These cognitive deficits may have functional consequences. Apathetic patients show diminished instrumental activities of daily living, which are tasks that require planning such as shopping and housekeeping.29,30 Furthermore, apathy is associated with worse scores on dementia scales and a higher risk of incident dementia, suggesting that apathy may be symptomatic of prodromal vascular dementia.6,31
Finally, although apathy can co-occur with depression, apathy may be a risk factor for developing subsequent depression. 10 Apathy is also associated with suicidal ideation independently of depression three months after stroke. 32 This suggests that apathy can not only occur in tandem with depression but also exacerbate it.
Treatments for post-stroke apathy
There is a lack of high-quality evidence to guide management of post-stroke apathy. Treatment can be considered under pharmacological, behavioral, and other approaches. Given the importance of treatment, we have included all trials that were found (Search strategy and selection criteria).
Pharmacological approaches for treating apathy
Antidepressants such as selective serotonin reuptake inhibitors (SSRIs) may be prescribed for apathy in clinical practice due to shared symptomatology with depression. Unfortunately, little evidence suggests that antidepressants are effective for treating apathy in the absence of additional depressive symptoms, with some research suggesting that certain antidepressants exacerbate effort-based decision-making deficits. 33
One double-blind placebo-controlled trial examined the effects of the SSRI escitalopram (10 mg/day in patients ≤65 or 5 mg/day in patients >65) in apathy-free stroke patients. 34 After 12 months, the 51 participants receiving escitalopram were 3.47 times less likely to develop apathy compared to the 47 on placebo. 34 This suggests that escitalopram reduces apathy risk, though future trials are needed to determine whether it ameliorates existing post-stroke apathy.
Two double-blind placebo-controlled trials have evaluated the efficacy of nefiracetam, which enhances monoaminergic, cholinergic, and GABAergic signaling to treat post-stroke apathy. The first was conducted in patients with post-stroke depression and apathy and assigned patients to placebo (n = 22), 600 mg/day nefiracetam (n = 26), or 900 mg/day nefiracetam (n = 22). 35 After 12 weeks, the patients receiving 900 mg nefiracetam had a greater reduction in apathy compared to those receiving 600 mg nefiracetam or placebo. The second study examined post-stroke apathy patients and assigned participants 900 mg/day nefiracetam (n = 6) or placebo (n = 7), but did not find a statistically significant decrease in apathy after 12 weeks. 36 The conflicting results of these studies should be interpreted with caution due to small samples.
One open-label trial examined the acetylcholinesterase inhibitors galantamine and donepezil in treating apathy in 26 cognitively impaired stroke patients. 37 Thirteen patients were administered galantamine on a dosing regimen starting at 4 mg twice per day (b.i.d.) before increasing to 8 mg b.i.d. and then 12 mg b.i.d. in four-week increments. Remaining patients were administered donepezil starting at 5 mg/day before increasing to 10 mg/day in six-week increments. After 12 weeks, the entire sample showed a non-statistically significant decrease in apathy compared to baseline. 37 Acetylcholinesterase inhibitors warrant further consideration, however, given the small sample in this study and positive results in dementia studies. 38
These inconclusive results preclude the recommendation of any pharmacological treatment for apathy in stroke, although research in other neurological diseases may provide avenues for future investigation. For instance, acetylcholinesterase inhibitors show promise in treating apathy in dementia, 38 while dopamine has been suggested as a treatment for apathy in Parkinson’s disease given the role of this neurotransmitter in motivated decision-making. 39 Theoretical work predicts that dopamine-based improvements in apathy are paralleled by increased reward sensitivity during behavioral tasks and improved functional connectivity within fronto-striatal networks. 24
Behavioral approaches for treating apathy
Neuropsychological advice can be provided to patients with apathy in the context of more general rehabilitation procedures and can be delivered individually or in formal group settings. Patients can be engaged in goal setting with an emphasis on planning future goals and evaluating success to help re-establish GDB. 40 Complimentary approaches include problem-solving, wherein a patient selects an activity and makes a plan to achieve it while self-monitoring the process and outcome. 34 Behavioral activation can be combined with cognitive-behavioral therapy which explores psychological issues that may prevent GDB engagement. Approaches should foster a sense of the self, belonging, and respect and be tailored toward settings, where patients can derive enjoyment from exercises and activities such as planned outings consistent with personal backgrounds. 41
Few trials have evaluated behavioral approaches for treating apathy after stroke. One study on post-stroke patients without apathy found that 56 patients undergoing problem-solving therapy were 1.84 times less likely to developing apathy compared to 47 patients on placebo, though risk was even lower for patients receiving escitalopram. 34 A randomized trial examined strategy training, wherein patients are coached to focus on self-selected activity goals and encouraged to derive strategies to address performance in pursuit of those goals, to treat apathy in cognitively impaired stroke patients. 40 After three months, 15 patients undergoing strategy training had significantly lower apathy compared to 15 in a control condition. 40 Group differences were also found after six months, although these were not statistically significant. Another randomized trial examined the efficacy of a group-based approach to promote activity for treating post-stroke symptoms including apathy in 186 patients. 41 Groups met in a community-based setting twice a week for 3 h each, during which they engaged in exercise, project-based activities, and planned outings. Apathy decreased over the course of the 12-month intervention compared to baseline, although changes were not statistically significant. Apathy was significantly lower after a 15-month follow-up, however, suggesting that motivation continued to improve after the intervention.
Other approaches for treating post-stroke apathy
Repetitive transcranial magnetic stimulation (rTMS) has been used to treat post-stroke apathy. One randomized sample of chronic stroke patients showed that high-frequency rTMS over the ACC and medial prefrontal cortex improved apathy after five days in those receiving treatment (n = 7) compared to sham stimulation (n = 6). 42 A case study suggested that rTMS-based decreases in apathy are paralleled by increasing interhemispheric connectivity. 43 Both studies utilized small samples, however, necessitating replications in larger trials.
Discussion
Apathy is a quantitative reduction in GDB and is a common but under-studied syndrome following stroke. We provided an overview on apathy in stroke, highlighting contemporary issues on definitions, diagnosis, neurobiology, consequences, and treatments. A diagnosis of apathy may be complicated by symptoms of fatigue and especially depression, which may appear behaviorally similar to apathy. Apathy may be the result of damage to neural networks underlying cognitive functions that support motivated GDB. Post-stroke neurophysiological processes may lead to structural and functional network changes, leading to longitudinal changes in apathy. Post-stroke changes to these networks may explain why some patients show improving or worsening apathy over time, although further testing is required.
Finally, apathy is associated with numerous behavioral, cognitive, and emotional concomitants, impacting quality of life and functional outcomes. These emphasize the importance of treating apathy, but unfortunately, pharmacological and behavioral interventions have yielded inconclusive results. Although some treatments, such as dopamine pharmacotherapy and rTMS, show promise for treating apathy, no treatment can be fully recommended.
Clinical recognition of post-stroke apathy is important, as it informs outcomes and treatment approaches. Patients with apathy may recover functional abilities more slowly and could be at-risk for future vascular events, dementia, and mortality, stressing the importance of early detection and continued monitoring. Little evidence suggests that SSRIs effectively treat apathy and should only be used in patients with concomitant depression. It should be noted, however, that our non-systematic review may have led to bias, such as in article inclusion, and further systematic analyses may be required to assess treatment efficacy.
Research on neurobiological mechanisms of apathy should consider adopting the network-based framework for investigating the presentation and development of apathy. Focal lesions or peripheral degeneration in brain networks should be examined in conjunction with different behavioral manifestations of apathy. Epidemiological and outcome research on apathy could also benefit from better stroke and apathy subtyping. Finally, validating apathy measures in stroke patients would improve study reliability and help characterize specific motivational deficits in post-stroke patients. These could then inform approaches for treating apathy, such as pharmacological interventions to target neurotransmitter systems, behavioral interventions to target cognitive-behavioral symptoms, and brain stimulation approaches that target distinct brain networks.
Search strategy and selection criteria
PubMed was searched for articles published in English between 1 January 1970 and 31 December 31 using the following terms: (apath* OR indifferen* OR abuli* OR motivat*) AND (stroke OR infarct OR cerebrovascular* OR lacun* OR infarct* OR small vessel disease* OR white matter hyperintens* OR white matter lesion* OR white matter disease* OR microbleed* OR ischemia OR ischaemia OR haemorrhag* OR hemorrhag* OR perivascular space* OR leukoaraiosis OR leukoencephalopath* OR age-related white matter damage OR vascular dementia). This returned 3210 results. Titles and abstracts were screened by one of the authors (JT) for relevance to the topics covered in this review. Full-text articles of relevant articles were retrieved and reviewed. Reference lists from these articles were screened for other eligible studies. Further relevant studies were also taken from the authors’ own published works.
Supplemental Material
Supplemental material, sj-pdf-1-wso-10.1177_1747493021990906 for Apathy after stroke: Diagnosis, mechanisms, consequences, and treatment by Jonathan Tay, Robin G Morris and Hugh S Markus in International Journal of Stroke
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article:This work is funded by a Priority Programme Grant from the Stroke Association (2015-02) and National Institute of Health Research (NIHR) Biomedical Research Centre Dementia and Neurodegeneration Theme (146281). JT is supported by a Cambridge International Scholarship from the Cambridge Trust. HSM is supported by the NIHR Cambridge Biomedical Research Centre and an NIHR Senior Investigator Award.
ORCID iD: Jonathan Tay https://orcid.org/0000-0003-0598-0004
Supplemental material: Supplemental material for this article is available online.
References
- 1.Caeiro L, Ferro JM, Costa J. Apathy secondary to stroke: a systematic review and meta-analysis. Cerebrovasc Dis 2013; 35: 23–39. [DOI] [PubMed] [Google Scholar]
- 2.van Dalen JW, Moll van Charante EP, Nederkoorn PJ, van Gool WA, Richard E. Poststroke apathy. Stroke 2013; 44: 851–860. [DOI] [PubMed] [Google Scholar]
- 3.Santa N, Sugimori H, Kusuda K, Yamashita Y, Ibayashi S, Iida M. Apathy and functional recovery following first-ever stroke. Int J Rehabil Res 2008; 31: 321–326. [DOI] [PubMed] [Google Scholar]
- 4.Mayo NE, Fellows LK, Scott SC, Cameron J, Wood-Dauphinee S. A longitudinal view of apathy and its impact after stroke. Stroke 2009; 40: 3299–3307. [DOI] [PubMed] [Google Scholar]
- 5.Eurelings LS, van Dalen JW, Ter Riet G. Apathy and depressive symptoms in older people and incident myocardial infarction, stroke, and mortality: a systematic review and meta-analysis of individual participant data. Clin Epidemiol 2018; 10: 363–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tay J, Morris RG, Tuladhar AM, Husain M, de Leeuw FE, Markus HS. Apathy, but not depression, predicts all-cause dementia in cerebral small vessel disease. J Neurol Neurosurg Psychiatry 2020; 91: 953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert P, Lanctôt KL, Agüera-Ortiz L, et al. Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur Psychiatry 2018; 54: 71–76. [DOI] [PubMed] [Google Scholar]
- 8.Mulin E, Leone E, Dujardin K, et al. Diagnostic criteria for apathy in clinical practice. Int J Geriatr Psychiatry 2011; 26: 158–165. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5, 5th ed. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 10.Withall A, Brodaty H, Altendorf A, et al. A longitudinal study examining the independence of apathy and depression after stroke: the Sydney Stroke Study. Int Psychogeriatr 2011; 23: 264–273. [DOI] [PubMed] [Google Scholar]
- 11.Caeiro L, Ferro JM, Pinho e Melo T, et al. Post-stroke apathy: an exploratory longitudinal study. Cerebrovasc Dis 2013; 35: 507–513. [DOI] [PubMed] [Google Scholar]
- 12.Hama S, Yamashita H, Shigenobu M, et al. Depression or apathy and functional recovery after stroke. Int J Geriatr Psychiatry 2007; 22: 1046–1051. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzaki S, Hashimoto M, Yuki S, et al. The relationship between post-stroke depression and physical recovery. J Affect Disord 2015; 176: 56–60. [DOI] [PubMed] [Google Scholar]
- 14.Fishman KN, Ashbaugh AR, Lanctôt KL, et al. Apathy, not depressive symptoms, as a predictor of semantic and phonemic fluency task performance in stroke and transient ischemic attack. J Clin Exp Neuropsychol 2018; 40: 449–461. [DOI] [PubMed] [Google Scholar]
- 15.Fishman KN, Ashbaugh AR, Lanctôt KL, et al. The role of apathy and depression on verbal learning and memory performance after stroke. Arch Clin Neuropsychol 2018; 34: 327–336. [DOI] [PubMed] [Google Scholar]
- 16.Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry 1990; 147: 22–30. [DOI] [PubMed] [Google Scholar]
- 17.Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. J Psychosomatic Res 2004; 56: 157–170. [DOI] [PubMed] [Google Scholar]
- 18.Douven E, Köhler S, Schievink SH, et al. Temporal associations between fatigue, depression, and apathy after stroke: results of the cognition and affect after stroke, a prospective evaluation of risks study. Cerebrovasc Dis 2017; 44: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimer’s Res Ther 2014; 6: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caeiro L, Ferro JM, Figueira M. Apathy in acute stroke patients. Eur J Neurol 2012; 19: 291–297. [DOI] [PubMed] [Google Scholar]
- 21.Brodaty H, Liu Z, Withall A, et al. The longitudinal course of post-stroke apathy over five years. J Neuropsychiatry Clin Neurosci 2013; 25: 283–291. [DOI] [PubMed] [Google Scholar]
- 22.Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex–basal ganglia circuits. Cereb Cortex 2006; 16: 916–928. [DOI] [PubMed] [Google Scholar]
- 23.Douven E, Köhler S, Rodriguez MM, et al. Imaging markers of post-stroke depression and apathy: a systematic review and meta-analysis. Neuropsychol Rev 2017; 27: 202–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tay J, Lisiecka-Ford DM, Hollocks MJ, et al. Network neuroscience of apathy in cerebrovascular disease. Prog Neurobiol 2020; 188: 101785. [DOI] [PubMed] [Google Scholar]
- 25.Shen J, Tozer DJ, Markus HS, et al. Network efficiency mediates the relationship between vascular burden and cognitive impairment: a DTI study in UK Biobank. Stroke 2020; 51: 1682–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tay J, Tuladhar AM, Hollocks MJ, et al. Apathy is associated with large-scale white matter network disruption in small vessel disease. Neurology 2019; 92: e1157–e1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sist B, Jesudasan SJB, Winship IR. Diaschisis, degeneration, and adaptive plasticity after focal ischemic stroke. In: Rodríguez JC. (eds). Acute ischemic stroke, Rijeka, Croatia: InTech, 2011, pp. 1–28. [Google Scholar]
- 28.Le Heron C, Apps M, Husain M. The anatomy of apathy: a neurocognitive framework for amotivated behaviour. Neuropsychologia 2018; 118: 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brodaty H, Sachdev PS, Withall A, et al. Frequency and clinical, neuropsychological and neuroimaging correlates of apathy following stroke – the Sydney Stroke Study. Psychol Med 2005; 35: 1707–1716. [DOI] [PubMed] [Google Scholar]
- 30.Castellanos-Pinedo F, Hernández-Pérez JM, Zurdo M, et al. Influence of premorbid psychopathology and lesion location on affective and behavioral disorders after ischemic stroke. J Neuropsychiatry Clin Neurosci 2011; 23: 340–347. [DOI] [PubMed] [Google Scholar]
- 31.Onoda K, Kuroda Y, Yamamoto Y, et al. Post-stroke apathy and hypoperfusion in basal ganglia: SPECT study. Cerebrovasc Dis 2011; 31: 6–11. [DOI] [PubMed] [Google Scholar]
- 32.Tang WK, Caeiro L, Lau CG, et al. Apathy and suicide-related ideation 3 months after stroke: a cross-sectional study. BMC Neurol 2015; 15: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yohn SE, Collins SL, Contreras-Mora HM, et al. Not all antidepressants are created equal: differential effects of monoamine uptake inhibitors on effort-related choice behavior. Neuropsychopharmacology 2016; 41: 686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikami K, Jorge RE, Moser DJ, et al. Prevention of poststroke apathy using escitalopram or problem-solving therapy. Am J Geriatr Psychiatry 2013; 21: 855–862. [DOI] [PubMed] [Google Scholar]
- 35.Robinson RG, Jorge RE, Clarence-Smith K, et al. Double-blind treatment of apathy in patients with poststroke depression using nefiracetam. J Neuropsychiatry Clin Neurosci 2009; 21: 144–151. [DOI] [PubMed] [Google Scholar]
- 36.Starkstein SE, Brockman S, Hatch KK, et al. A randomized, placebo-controlled, double-blind efficacy study of nefiracetam to treat poststroke apathy. J Stroke Cerebrovasc Dis 2016; 25: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 37.Whyte EM, Lenze EJ, Butters M, et al. An open-label pilot study of acetylcholinesterase inhibitors to promote functional recovery in elderly cognitively impaired stroke patients. Cerebrovasc Dis 2008; 26: 317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berman K, Brodaty H, Withall A, et al. Pharmacologic treatment of apathy in dementia. Am J Geriatr Psychiatry 2012; 20: 104–122. [DOI] [PubMed] [Google Scholar]
- 39.Chong T-J and Husain M. The role of dopamine in the pathophysiology and treatment of apathy. In: Studer B and Knecht S (eds.) Progress in brain research. Amsterdam, Netherlands: Elsevier, 2016, pp.389–426. [DOI] [PubMed]
- 40.Skidmore ER, Whyte EM, Butters MA, et al. Strategy training during inpatient rehabilitation may prevent apathy symptoms after acute stroke. PMR 2015; 7: 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayo NE, Anderson S, Barclay R, et al. Getting on with the rest of your life following stroke: a randomized trial of a complex intervention aimed at enhancing life participation post stroke. Clin Rehabil 2015; 29: 1198–1211. [DOI] [PubMed] [Google Scholar]
- 42.Sasaki N, Hara T, Yamada N, et al. The efficacy of high-frequency repetitive transcranial magnetic stimulation for improving apathy in chronic stroke patients. Eur Neurol 2017; 78: 28–32. [DOI] [PubMed] [Google Scholar]
- 43.Mitaki S, Onoda K, Abe S, et al. The effectiveness of repetitive transcranial magnetic stimulation for poststroke apathy is associated with improved interhemispheric functional connectivity. J Stroke Cerebrovasc Dis 2016; 25: e219–e221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-wso-10.1177_1747493021990906 for Apathy after stroke: Diagnosis, mechanisms, consequences, and treatment by Jonathan Tay, Robin G Morris and Hugh S Markus in International Journal of Stroke